Abstract
Background
Previously, we showed experimentally that saturation of slow wave activity provides a potentially individualized neurophysiological endpoint for perception loss during anesthesia. Furthermore, it is clear that induction and emergence from anesthesia are not symmetrically reversible processes. The observed hysteresis is potentially underpinned by a neural inertia mechanism as proposed in animal studies.
Methods
In an advanced secondary analysis of 393 individual electroencephalographic datasets, we used slow wave activity dose-response relationships to parameterize slow wave activity saturation during induction and emergence from surgical anesthesia. We determined whether neural inertia exists in humans by comparing slow wave activity dose-responses on induction and emergence.
Results
Slow wave activity saturation occurs for different anesthetics, and when opioids and muscle relaxants are used during surgery. There was wide inter-patient variability in the hypnotic concentrations required to achieve slow wave activity saturation. Age negatively correlated with power at slow wave activity saturation. On emergence, we observed abrupt decreases in slow wave activity dose-responses coincident with recovery of behavioral responsiveness in ~33% individuals. These patients are more likely to have lower power at slow wave activity saturation, be older, and suffer from short-term confusion on emergence.
Conclusions
Slow wave activity saturation during surgical anesthesia implies that large variability in dosing is required to achieve a targeted potential loss of perception in individual patients. A signature for neural inertia in humans is the maintenance of slow wave activity even in the presence of very low hypnotic concentrations during emergence from anesthesia.
Introduction
It is still unclear when an individual patient experiencing clinical anesthesia stops perceiving the external world and surgery-related stimulation1,2. Previously, we used simultaneous electroencephalography (EEG) and functional magnetic resonance imaging (FMRI) during an ultraslow propofol anesthesia-induced loss of consciousness to track healthy individuals’ responses to external stimuli3. We found that after loss of behavioral responsiveness each individual's slow wave activity (0.5-1.5Hz) - a characteristic waveform seen in sleep and anesthesia4 - rose to saturation, but then remained constant despite increasing anesthetic concentrations. Simultaneous FMRI showed that, at the point of slow wave activity saturation (SWAS), stereotypical thalamocortical responses to nociceptive and auditory inputs were abolished.
Furthermore, we observed a significant correlation between individuals’ maximum slow wave power and their pre-frontal cortex grey matter volume, suggesting a direct neurobiological relationship between slow wave activity saturation and the number of oscillating cortical neurons – the underpinning basis of slow waves5. We hypothesized that slow wave activity saturation is an important individualized electrophysiological end-point that is a manifestation of perception loss to incoming stimuli, and as such reflects isolation from the external world. Therefore, slow wave activity saturation provides a potential brain-based target which would allow optimum anesthetic dosing for perception loss in each individual patient, thus reducing the risk of mortality and morbidity from over- and under-anesthesia, particularly in elderly and vulnerable patients6,7.
Here, we aimed to determine whether slow wave activity saturation occurs in the less well-controlled, clinical environment. We have developed robust analytical methods to parameterize slow wave activity (SWA) dose-response relationships on an individual basis using electroencephalographic data from our previous experimental study and three additional clinical studies3,8–10. We aimed to determine whether slow wave activity saturation occurs for both intravenous and volatile general anesthetics (VGA) on induction to and exists prior to emergence from anesthesia. We also examined how clinically used co-induction agents, such as opioid analgesia and neuromuscular blockades, influence the slow wave activity saturation parameters. As both grey matter volume/density11 and anesthetic dose requirement12 decrease with age, we hypothesised a priori that the slow wave activity saturation parameters for induction and emergence would show similar decreases with age in the adult population.
It has long been thought that induction and emergence from anesthesia are not reversible and symmetric processes13; however, there is little evidence in humans to support this14. Recently, the concept of neural inertia has been suggested to explain the hysteresis observed in anesthetic dose-responses between induction and emergence15. The authors proposed that neural inertia acts to ‘resist behavioral state transitions between unconscious and conscious states’. Specifically, they demonstrated that hysteresis of the anesthetic dose associated with behavioral responsiveness in animals is not simply due to pharmacokinetic differences and can be genetically modulated. Importantly while the authors hypothesized this effect has a neural origin, they do not provide direct evidence of this phenomenon.
We aimed to determine whether neural inertia exists in humans by comparing brain-based differences in the slow wave activity dose-response relationships on induction and emergence. Furthermore, we aimed to identify clinically relevant variables that predict whether an individual is likely to experience neural inertia and show how this can influence short-term confusion/delirium on emergence from anesthesia.
Materials and Methods
Data collection
We analysed electroencephalographic data from adults aged 18-90 years collected in four separate studies with the following information: raw EEG, end-tidal volatile anesthetic gas concentrations and/or estimated propofol concentrations, time of loss and/or recovery of behavioral responsiveness (L/ROBR) and patient demographics. The studies were an experimental 32-channel propofol healthy volunteer (N=16) study that provided the initial description of slow wave activity saturation3 (Study 1), a pre-surgery sevoflurane patient (N=21) study8 (Study 2), a desflurane-fentanyl infusion patient (N=102) study9 (Study 3), and a routine clinical care patient (N=254) study10 (Study 4). Research ethical committee approval was previously obtained for each of the studies separately. Full data collection details for all studies are provided in the Supplementary Material. EEG data for the clinical studies were collected using depth of anesthesia monitors and a standard prefrontal montage (Fp7-Fz). Multichannel EEG data were initially collected using a FCz reference but later re-referenced to common average.
Sigmoid fitting of slow wave activity saturation
A standardized pre-processing and analysis procedure was applied to each individual electroencephalographic dataset on induction and emergence, and at various electrode positions for the multi-channel data using Matlab R2013a (Mathworks, USA) (see Appendix 1 for full details). Pre-processing included: downsampling to 125Hz, 0.25 to 45Hz band-pass filtering using a Butterworth filter, remove blink and eye-movement artefacts using a Whittaker filter, generation of a short-term Fourier transform spectrograms and extraction of mean power in the 0.5-1.5 Hz frequency band. The timeseries of the estimated effect site concentrations (Ce) of propofol, opioids, and volatile anesthetic drugs were calculated using standard population-based pharmacokinetic models16–19.
We then fitted a parametric sigmoid curve using Bayesian inference to identify slow wave activity saturation for each individual electroencephalographic dataset (see Appendix 1). We confirmed the presence of slow wave activity saturation by determining if a plateau in the slow wave activity drug dose-response curve was observed prior to maximum anesthetic dosing (Fig. 1). We formally defined slow wave activity saturation as the power (Pswas) and concentration (Ceswas) that corresponded to 95% of the slow wave activity posterior distribution around the slow wave activity plateau. The following statistics were used to characterize the individual slow wave activity saturation responses:
-
1)
absolute slow wave activity power at saturation (Pswas);
-
2)
concentration required to achieve slow wave activity saturation (Ceswas);
-
3)
absolute slow wave activity power at baseline (PBaseline);
-
4)
gradient of the sigmoid curve; and
-
5)
SWAS-response gap.
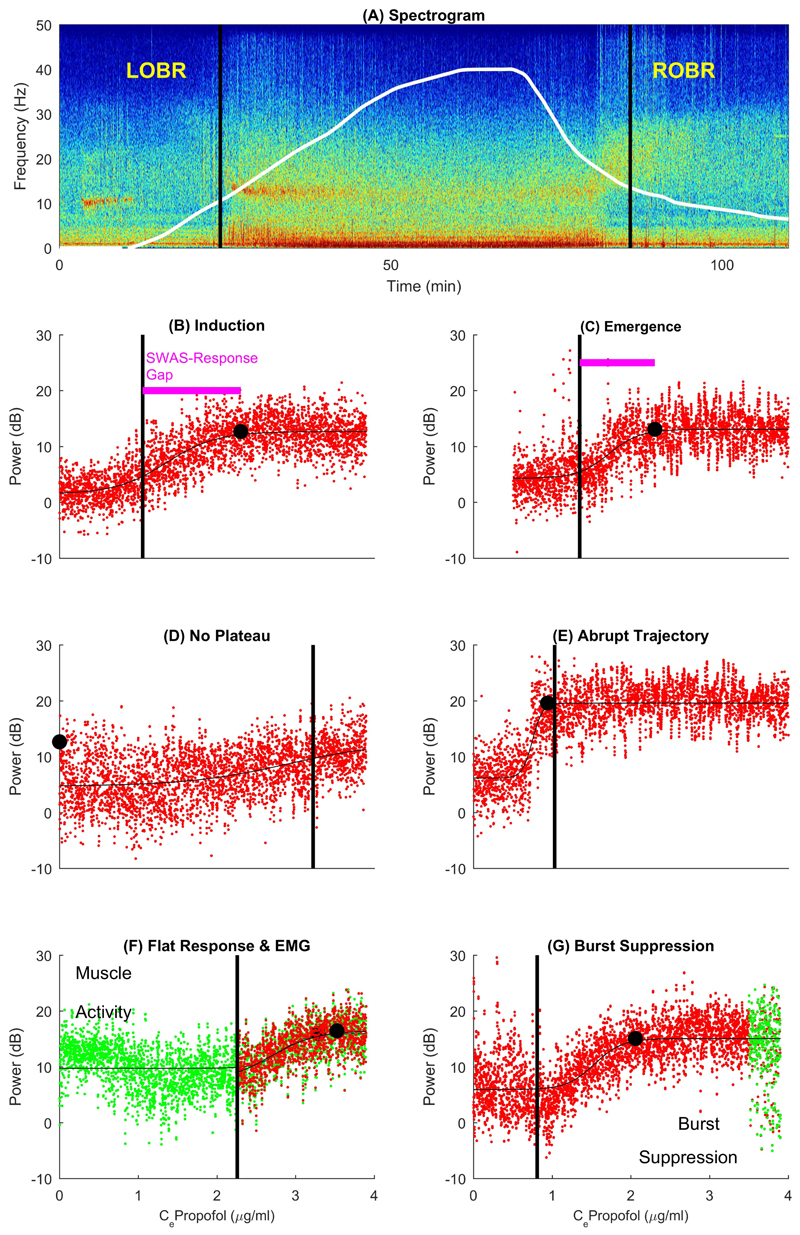
Fig. 1. Estimation of slow wave activity saturation.
(A) An individual EEG time-frequency spectrogram recorded at P7 electrode in Study1, with the propofol effect site concentration (Ce) timecourse shown in white to maximum of 4μg/ml. Corresponding slow wave activity (SWA) -anesthetic dose response curves are shown for induction (B) and emergence (C). Loss and recovery of behavioral responsiveness (L/ROBR) are shown as black vertical lines throughout. SWA data is in red, the thin black line is the sigmoid curve fit and the black dot indicates slow wave activity saturation (SWAS). Sub-figures (D)-(G) show practical issues related to SWAS estimation. Artefactual data are highlighted in green where present. (D) shows a graded pattern but ‘no plateau’, as the anesthetic dose administered was not sufficient to achieve SWAS within this individual. (E) shows an abrupt slow wave trajectory that indicates neural inertia on emergence from anesthesia. It is characterized by the persistence of saturation followed by an abrupt drop in slow wave activity coincident with ROBR, and formally defined by a small SWAS-response gap (shown as pink line). (F) and (G) show examples with electromyographic (EMG)/movement and burst suppression artefactual disturbance, respectively.
As induction and emergence were modeled as two separate processes (see Fig. 1B and 1C), these statistics have slightly different interpretations in each context. For induction, Pbaseline refers to the slow wave activity baseline preceding anesthetic administration, i.e. slow wave activity in the awake state. For emergence, this relates to the slow wave activity level after the initial ROBR i.e. slow wave activity associated with the (partially) awake state. The estimation of slow wave activity saturation and its relationship with loss or recovery of behavioral responsiveness (Cel/robr) is captured by the SWAS-response gap. On emergence, a gentle gradient implies a smoothly changing brain state and good predictability of ROBR.
Statistical analysis
The slow wave activity saturation fitting outcome was expressed as the percentage of datasets with successful and unsuccessful fits. A successful fit indicates that a plateau in the slow wave activity dose-response curve could be identified. Failed/unsuccessful fits were further sub-categorized as either ‘no plateau’ (Fig. 1D) or ‘flat response & EMG’ (Fig. 1F). Summary statistics are used to characterize the variability of the slow wave activity saturation parameters for the successful individual fits. These are presented as mean ± standard deviation (SD) across volunteers for each study unless otherwise specified. After checking for normality (or after the appropriate transformation), paired t-tests were used for within-subject comparisons and unpaired t-tests for between-group comparisons. For multiple groups, we used one or two-way analyses of variance (ANOVA) as appropriate. Chi-squared tests were used for comparison of categorical data. Significance was set at P<0.05 unless otherwise stated.
We hypothesized a priori that at slow wave activity saturation we would observe a negative correlation between power at slow wave activity saturation and age. Therefore, Pswas was compared for induction across all four studies using general linear model (GLM) analyses of covariance (ANCOVA) with age as a covariate. Average values for the frontal electrodes were used for Study 1. In order to assess the influence of co-induction agents, cumulative distribution functions of Ceswas on induction were generated using the ‘ksdensity.m’ Matlab function (Mathworks, USA) with a smoothing of 0.001 for all three studies. For Study 1, this corresponded to average concentration for all channels. Independent t-tests were performed on all slow wave activity saturation parameters on induction for Studies 3 and 4, using repeated-measures ANOVAs with Tukey-Kramer Multiple-Comparison corrections (controlling for age and drug type) to identify significant differences between cases with and without muscle relaxant administration.
Experimental data: effect of channel location and neural inertia
We specified frontal channels as Fp1, Fp2, F3, F4, F7, F8, and Fz; central channels as C3, C4, Cz, Cp1, CP2, FC5, and FC6; and parietal channels as P3, P4, P7, P8, and Pz. Data were averaged for each group of regional channels for each subject. After checking for normality, a two-way repeated-measures ANOVA was applied with channel location (between-region variable) and induction-versus-emergence (within-subject variable) as the explanatory variables.
Associations between slow wave activity saturation parameters and demographic variables
Studies 3 and 4 were used to explore how age and other demographic variables might influence or predict the slow wave activity saturation parameters. Bivariate correlations between age, gender, operation duration and type, volatile and opioid effect-site concentrations, and the slow wave activity saturation parameters were quantified using Pearson’s correlation coefficients (r) for continuous variables.
Predicting graded and abrupt emergence trajectories
After examination of the slow wave activity dose-response patterns from Study 1, it was clear that sub-categorization of the slow wave activity output on emergence was required prior to statistical comparisons. An abrupt slow wave emergence pattern was defined as when the slow wave activity power was maintained by an individual as the hypnotic drug concentration decreased (usually to well below traditional MACawake concentrations), followed by an abrupt decrease in slow wave activity with a steep gradient around the point of ROBR. For Study 1, the criteria for an abrupt trajectory was a SWAS-response gap of <0.4ug/ml. For the clinical studies, abrupt trajectories were defined by a SWAS-response gap of <0.05 MAC (and only positively diagnosed in the absence of significant EMG movement artifact: i.e. only when EMG power <20dB).
We then performed a post-hoc multivariate logistic regression to determine which putative demographic and drug factors might determine whether a particular patient has a graded or an abrupt response on emergence from anesthesia. We focused on explanatory factors that could be plausibly predictive, and therefore only included factors that could be estimated before or early in emergence. These were: age, gender, duration and severity of operation, type of VGA, Pswas on induction, and Pswas, VGA and fentanyl concentrations at start of emergence. We excluded Ceswas from the model because, although by far the strongest predictor, it essentially defines the abrupt pattern and is only accessible after emergence has completed.
Assessment of confusion and delirium
Patients in Study 4 were assessed for delirium (using the Confusion Assessment Method for the ICU (CAM-ICU)20) and pain (using a 0-10 numerical rating scale (NRS)) in the post-anesthesia unit 15 minutes after waking. High levels of pain (i.e. presence of moderate or severe pain) were defined as NRS > 4. As patients could fail the CAM-ICU test if they were sleepy or confused, we investigated the separate components. They were classed as being “confused” if they had an unexplained agitation (CAM-ICU RASS component>0, in absence of moderate or severe pain i.e. NRS>4), or they failed the disordered thinking component of the CAM-ICU test. Fisher’s exact test was used post-hoc to investigate statistical differences in the incidence of high pain, confusion, sleepiness and pass/fail on the CAM-ICU between individuals with abrupt and graded slow wave emergence trajectories.
Results
We successfully identified slow wave activity saturation on induction of anesthesia in 92% of individuals across all four studies (Table 1). We confirmed that slow wave activity saturation occurs for different classes of anesthetic agent, i.e. for inhalational sevoflurane (86% successful fits) as well as intravenous propofol anesthesia (100% successful fits). For sevoflurane, one patient was not administered high sevoflurane levels (maximum 1 MAC) and did not achieve a slow wave activity plateau (see Fig. 1D ‘no plateau’ example). Two other patients had a high Pbaseline caused by excessive muscle artifacts (or movement) that precluded accurate estimation of slow wave activity saturation.
Table 1. Slow wave activity saturation (SWAS) fitting on induction of anesthesia.
The SWAS fitting outcome is expressed as the number (and percentage) of participants for induction of anesthesia in the experimental and clinical electroencephalographic studies. Failed fits were sub-categorized as either ‘no plateau’ (Fig. 1D) or ‘flat response & EMG’ (Fig. 1F). Ceswas is measured in MAC for Study 2 and µg/ml for the other studies. SWAS parameters represent the mean and standard deviation (mean ± SD) across participants. For Study 1, SWAS parameters refer to average data collected from the frontal electrodes.
| Induction | STUDY 1 | STUDY 2 | STUDY 3 | STUDY 4 |
|---|---|---|---|---|
| Study name | Volunteer propofol | Pre-surgery sevoflurane | Desflurane-fentanyl | Routine clinical care |
| Induction agent | Propofol | Sevoflurane | Propofol | Propofol |
| Infusion rate | Slow | Rapid | Rapid | Rapid |
| Co-induction agents | No | No | Yes | Yes |
| Age mean (range) | 29 (18-43) | 38 (18–63) | 42 (17-67) | 59 (18-90) |
| Gender (M:F) | 8M:8F | 18F:3M | 78F:24M | 125F:129M |
| No of datasets | 16 | 21 | 102 | 254 |
| Successful fits | 16 (100%) | 18 (86%) | 97 (95%) | 230 (91%) |
| Failed fits | 0 (0%) | 3 (14%) | 5 (5%) | 24 (9%) |
| 1: No plateau | 0 (0%) | 1 (5%) | 0 (0%) | 0 (0%) |
| 2: Flat response & EMG | 0 (0%) | 2 (9%) | 5 (5%) | 24 (9%) |
| SWAS parameters | ||||
| Pswas (dB) | 19.3±3.8 | 22.3 ±4.0 | 22.1±4.9 | 20.5±4.4 |
| Ceswas | 2.7±0.5(μg/ml) | 0.6±0.3(MAC) | 1.4±0.6(μg/ml) | 0.9±0.3(μg/ml) |
| Pbaseline (dB) | 9.2±3.2 | 6.9±8.5 | 6.8±2.9 | 4.1±10.1 |
| Gradient (degrees) | 83.0±6.0 | 86.2±1.9 | 89.4±0.8 | 89.4±1.6 |
| \ | ||||
The average slow wave activity saturation parameters for sevoflurane were comparable to those for intravenous propofol. The mean Ceswas for sevoflurane is around 0.7MAC, and ranges across volunteers from 0.24-0.9 MAC. The mean propofol Ceswas for Study 1 is at the lower end of the concentrations used for total intravenous anesthesia (TIVA).
We were able to identify slow wave activity saturation in the clinical datasets (successful fits, Study 3: 95% and Study 4: 91%), allowing us to confirm that slow wave activity saturation occurs in routine clinical anesthesia and the operating room environment. The only failed fits on induction in the clinical studies were due to excessive pre-induction movement artifacts.
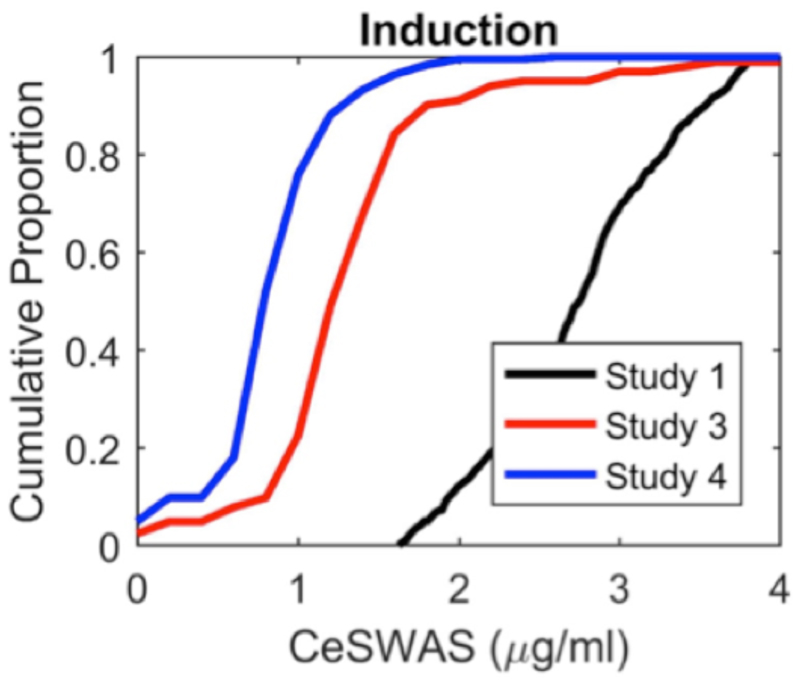
Opioids potentiate hypnotic agents so that reduced concentrations are required to achieve loss of behavioral responsiveness when these drugs are delivered in combination21. Similarly when clinical co-induction agents were used, Ceswas was approximately halved compared with Study 1 (Table 1). We found that 50% of individuals achieved slow wave activity saturation at a propofol Ceswas ≤0.82µg/ml for a rapid infusion (Study 4) compared with 2.7 µg/ml for an ultraslow infusion (Fig. 2). There were no statistically significant differences in the slow wave activity saturation parameters between the patients in Study 4 receiving muscle relaxants (N=145) and those who did not (N=85).
Fig. 2. Opioid analgesia reduces hypnotic concentration required to achieve slow wave activity saturation.
Cumulative distribution function of Ce SWAS on induction of propofol anesthesia. The introduction of opioids (and muscle relaxants) during the clinical administration (Studies 3 and 4) potentiates the hypnotic component so that less propofol is required to achieve slow wave activity saturation across participants than in the experimental study (Study 1).
Despite these observed differences in Ceswas, we found that Pswas was largely consistent across the four separate electroencephalographic studies. Frontal Pswas values for the experimental study were significantly smaller when compared with the clinical studies using a standard prefrontal montage (Fp7-Fz) (P<0.01, GLM ANCOVA with age as a covariate). However, we note that the experimental study also used high-impedance MRI compatible EEG electrodes and different referencing arrangements compared with the other studies.
No significant difference in Pswas was found across the three clinical studies when age was included as a covariate. In particular, no significant differences were found between Pswas for sevoflurane (Study 2) and Pswas for propofol (Studies 3 and 4) using similar electroencephalographic recording equipment. When age was not accounted for, Pswas was smaller for the older cohort in Study 4 when compared with Study 3 (P<0.05, ANOVA Tukey-Kramer Multiple-Comparison Test). Overall, this demonstrates that (for a given recording system) Pswas is not determined by the hypnotic agent used (or any accompanying co-induction agents) but is potentially intrinsic to the individual, suggesting a sound neurobiological basis for slow wave activity saturation. This is consistent with our previous study that identified a significant positive correlation between Pswas and gray matter volume3 since the latter is known to be negatively correlated with age.
We were able to identify slow wave activity saturation at all 31 electrodes for both induction and emergence in Study 1 (Table 2). Out of a total of 496 channels, the slow wave activity saturation model only failed to fit in 36 channels for induction (18 from one subject), and 58 channels during emergence (48 from two subjects). Central channels had more successful fits on induction and emergence across all individuals and channels. Frontal channels had more failed fits due to movement and blink artifacts (P=0.008, Chi-squared test). Central channels also had a less steep sigmoid gradient on emergence (P<0.05, Tukey-Kramer Multiple-Comparison Test). Slow wave activity power was significantly different between channels both at slow wave activity saturation and baseline. In line with previous descriptions of the anteriorization of EEG during general anesthesia3,4, it was largest in frontal channels and smallest in central channels.
Table 2. Variability of slow wave activity saturation across the brain for induction and emergence.
Summary statistics across channel locations for induction and emergence from anesthesia in the experimental study. Slow wave activity saturation (SWAS) parameters are presented as mean ± standard deviation of seven frontal, seven parietal and five central channels for 16 healthy volunteers. After checking for normality, SWAS parameters were analyzed using two-way repeated measures ANOVA with channel location (between-region variable) and induction-versus-emergence (within-subject variable). ** denotes a significant difference between all regions (P<0.001), * between frontal region and the rest (P<0.01), and † between induction and emergence (P<0.001). Abbreviation: SWAS-RG, SWAS-response gap.
| Induction | Emergence | |||||
|---|---|---|---|---|---|---|
| Cel/robr (μg/ml) | 1.5± 0.7 | 1.4± 0.6 | ||||
| Channel Region | Frontal | Parietal | Central | Frontal | Parietal | Central |
| Successful fits (%) * | 87.5 | 93.8 | 94.6 | 80.4 | 87.4 | 89.3 |
| Pswas (dB) * | 19.3±3.8 | 16.5±3.3 | 15.3±3.5 | 19.9±4.0 | 16.8±2.8 | 15.4±3.6 |
| Ceswas (μg/ml) † | 2.7±0.5 | 2.7±0.5 | 2.7±0.5 | 2.1±0.7 | 1.9±0.6 | 1.9±0.6 |
| Pbaseline (dB) **† | 9.1±3.2 | 6.3±2.5 | 4.1±2.5 | 8.6±2.6 | 5.4±2.0 | 4.0±2.1 |
| Gradient (degrees) *† | 83.0±6.0 | 83.9±3.0 | 84.1±2.5 | 83.4±7.5 | 86.2±4.0 | 86.6±3.3 |
| SWAS-RG (μg/ml) | | 1.2±0.5 | 1.1±0.3 | 1.2±0.5 | 0.7±0.6 | 0.5±0.5 | 0.5±0.5 |
Study 1 is the only dataset with the same anesthetic agent for induction and emergence within an individual. Due to the ultraslow induction, the propofol concentration time-courses for induction and emergence are similar, with the obvious proviso that these are derived from population pharmacokinetic models. Despite this we found that the Ceswas, Pbaseline, gradient, and SWAS-response gap were significantly different between induction and emergence (P<0.001, Table 2). However, there was no significant difference in Pswas, adding credence to our hypothesis that slow wave activity saturation (or more specifically Pswas) is intrinsic for an individual.
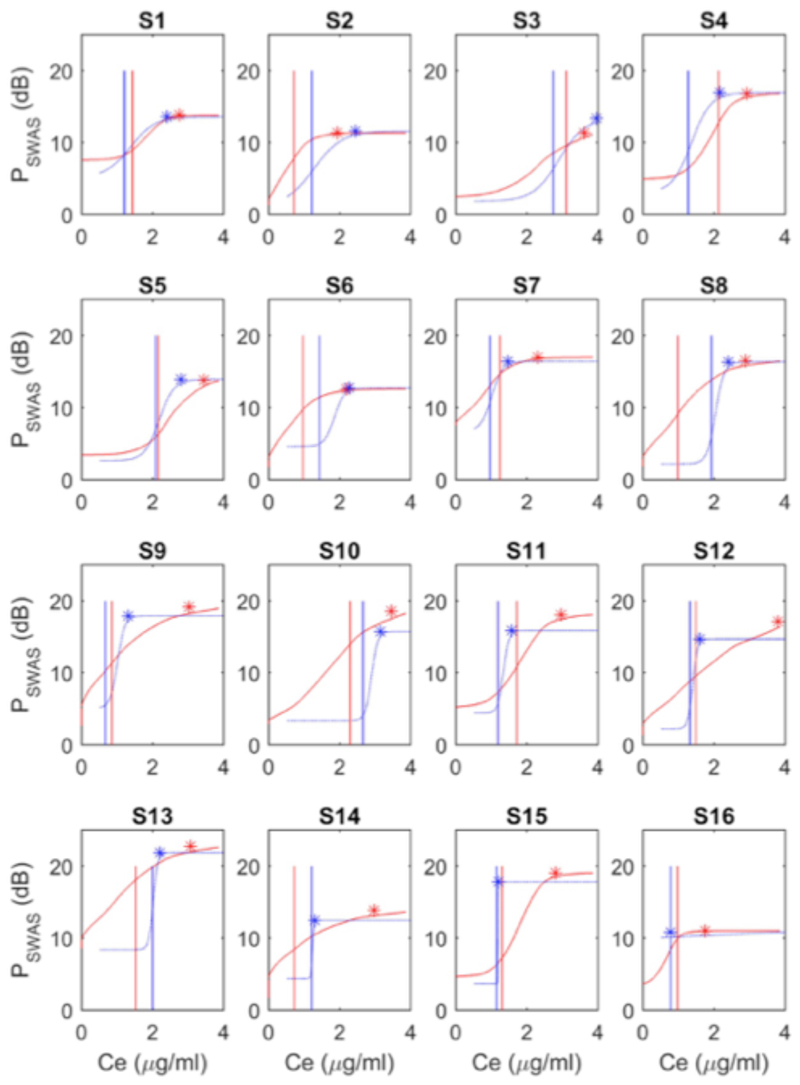
On average, slow wave activity saturation occurred at propofol concentrations of about 1µg/ml greater than those associated with behavioral unresponsiveness (Table 2). When examined on an individual basis, we did not observe any consistent hysteresis in CeLOBR and CeROBR (vertical red and blue lines in Fig. 3 and Fig. 4A). However, we did observe a marked hysteresis in Ceswas across volunteers (see Fig. 4B). In about half the subjects (i.e. the lower 8 subjects in Fig. 3), Ceswas on emergence occurred at lower concentrations than Ceswas on induction, and was often very close to the return of responsiveness.
Fig. 3. Experimental evidence for neural inertia in humans.
Sigmoid regression of absolute slow wave activity power as a function of the estimated propofol C e for induction (red) and emergence (blue) for each individual in Study 1. Asterisks indicate the point of slow wave activity saturation, with the vertical lines indicating loss (LOBR, red line) and recovery of behavioral responsiveness (ROBR, blue line). Subjects (S1-S16) are ordered based on the emergence gradient, from gentlest to steepest. Note that subject 3 (S3) barely achieved saturation in this channel, and subject 16 (S16) had a completely abrupt response on emergence, i.e. they recovered behavioral responsiveness directly from slow wave activity saturation. Examples relate to the P7 electrode.
Fig. 4. Hysteresis of slow wave activity saturation but not behavioral responsiveness .
Cumulative distribution functions of the concentrations associated with A) loss and recovery of behavioral responsiveness (LOBR and ROBR) and B) slow wave activity saturation (SWAS) on induction and emergence from propofol anesthesia. A comparison for 50% of volunteers indicates a significant hysteresis in Ceswas but not for behavioral responsiveness. Data in B) correspond to the mean Ceswas for all 31 EEG channels for each of the 16 individuals who participated in Study 1.
We believe these more abrupt (rather than graded) slow wave emergence trajectories indicate the presence of neural inertia in humans, an effect only previously observed in animals15. Abrupt slow wave emergence trajectories (as defined by a SWAS-response gap < 0.4ug/ml) occurred across the brain. There was no statistical difference in the classification of abrupt or graded slow wave emergence trajectory between channel regions (p=0.14, Chisquared test).
Successful slow wave activity saturation fits for emergence were achieved in 94% of the experimental cases but only about 70% of clinical cases, with approximately a third of these having abrupt slow wave trajectories (Table 3, Fig. 5). Unsuccessful slow wave activity saturation fits were equally distributed between ‘no plateau’ and ‘flat response & EMG’ cases.
Table 3. Slow wave activity saturation on emergence from anesthesia.
Slow wave activity saturation (SWAS) fitting outcome is sub-categorized as graded or abrupt based on the slow wave emergence trajectory gradient. SWAS parameters represent the mean ±SD across participants. The table describes the hypnotic agent, whether any co-induction agents were present and the surgery details. Abbreviations: N/A, not applicable; Gyn, gynaecological; Gen, general surgical; Orth, orthopaedic, Vasc, vascular; Urol, urological.
| Emergence | STUDY 1 | STUDY 3 | STUDY 4 |
|---|---|---|---|
| Anesthetic agent | Propofol | Desflurane | Mixed |
| Co-induction agents | No | Yes | Yes |
| Type of surgery | N/A | Gyn: 54; Gen: 37; Orth: 11 |
Gyn: 129; Gen: 121; Orth: 4;Vasc: 66; Urol: 30; Other: 4 |
| Severity grading of surgery | N/A | 1: 0; 2: 51; 3: 51; 4: 0 | 1: 51; 2: 102; 3: 83; 4: 18 |
| No of datasets | 16 | 102 | 254 |
| Successful fits | 15 (94%) | 71 (71%) | 175 (69%) |
| 1: Graded response | 14 (88%) | 50 (49%) | 110 (43%) |
| 2: Abrupt response | 1 (6%) | 21 (20%) | 65 (26%) |
| Failed fits | 1 (6%) | 31 (31%) | 79 (30%) |
| 1: No plateau | 1 (6%) | 16 (16%) | 42 (16%) |
| 2: Flat response & EMG | 0 (0%) | 15 (15%) | 37 (14%) |
| SWAS parameters | |||
| Pswas (dB) | 19.9±4.0 | 18.1±3.7 | 16.7±4.8 |
| Ceswas | 2.1±0.7 | 0.65±0.39 (MAC) | 0.47±0.33 (MAC) |
| Pbaseline (dB) | 8.6±2.6 | 8.1±4.4 | 6.5±5.9 |
| Gradient (degrees) | 83.4±7.5 | 87.7±2.9 | 87.8±6.4 |
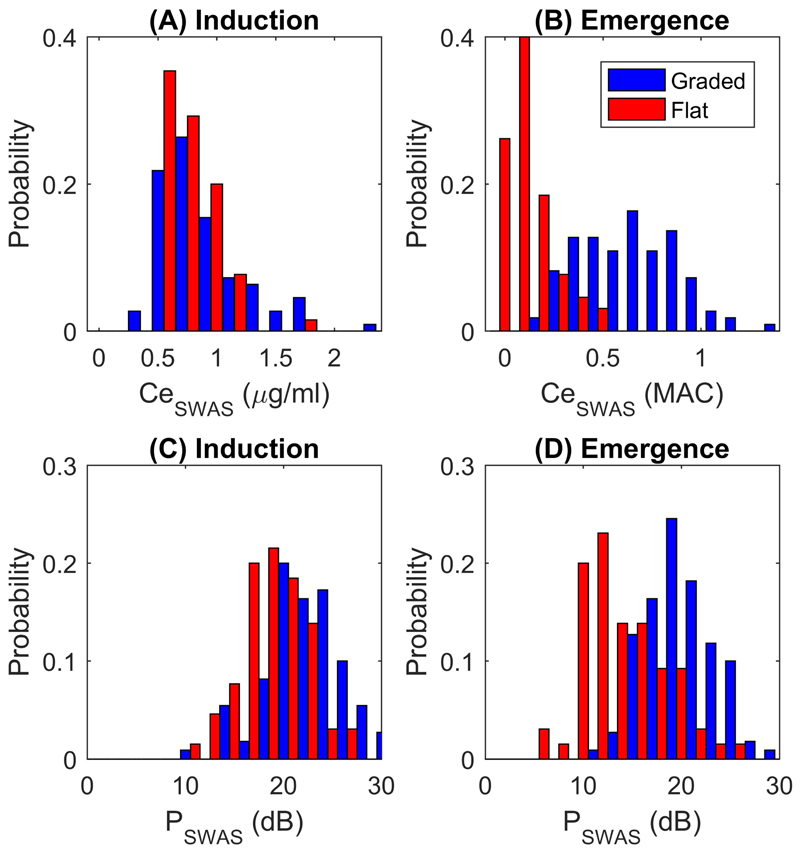
Fig. 5. Slow wave activity saturation parameters reveal clear separation of graded and abrupt slow wave emergence trajectories.
Sub-plots (A)-(D) detail the probability distribution of Ceswas and Pswas on induction and emergence. Clear bimodal distributions in Ceswas and Pswas on emergence can be observed for the graded (red) and abrupt (blue) slow wave trajectories. Data are presented for the N=175 patients in Study 4 that had successful slow wave activity saturation fits both on induction and emergence. Ceswas is in µg/ml for induction and MAC for emergence due to the different hypnotic anesthetic agents used.
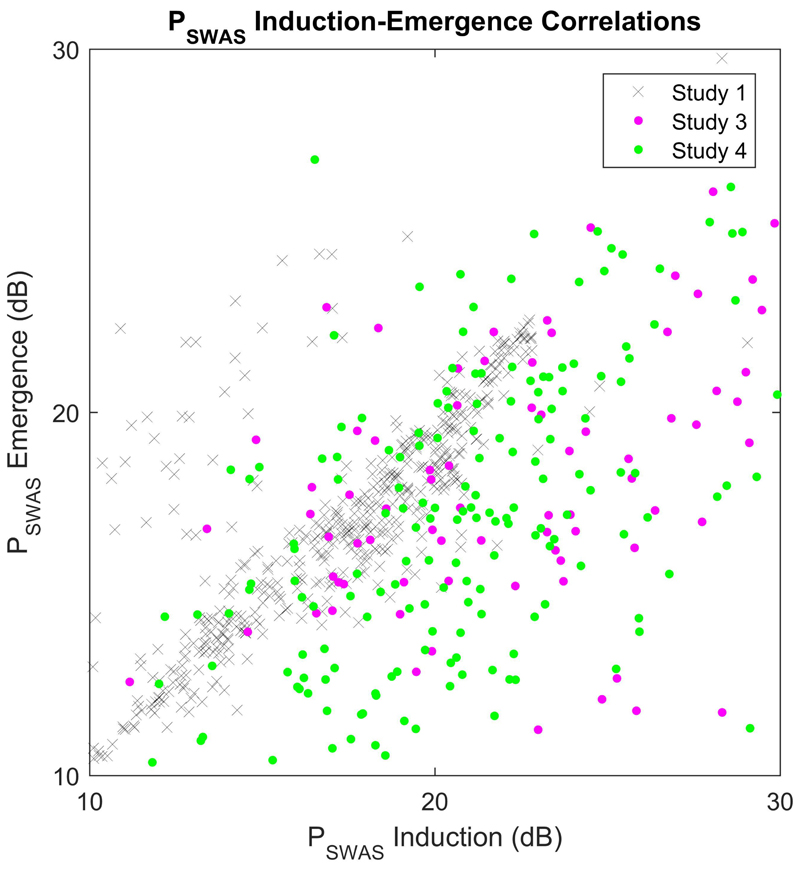
The mean Ceswas on emergence for Studies 3 and 4 both fall within accepted ranges for surgical anesthesia. Again, we observed a wide inter-individual variation with Ceswas, ranging from 0.05-1.6 MAC for Study 3 and 0.03-1.3 MAC for Study 4 (Fig. 5B). We found that Pswas on induction and emergence were correlated across all of the studies (R2 = 0.51, P<0.001, Fig. 6). However, in contrast to the experimental study, Pswas on emergence after surgery was significantly lower than Pswas on induction (of the order of 4-5dB) in both clinical studies (P<0.001, paired t-test, Figs. 5C and 5D for Study 4). The gradient on emergence was steeper compared to induction in the clinical studies (P<0.001, paired t-test).
Fig. 6. Power at slow wave activity saturation is highly correlated between induction and emergence.
Data are presented for the individuals that had successful fits on both induction and emergence for Studies 1 (grey crosses – all 31 EEG channels), 3 (blue dots) and 4 (red dots).
As many of the correlations within the two clinical datasets (Studies 3 and 4) were broadly comparable, we decided to focus on the larger Study 4 dataset. Age was strongly negatively correlated (r ~ -0.6, P<0.001) with Pswas on induction and emergence, and with Ceswas on emergence (Fig. 7, Table 4). From Fig. 7, it is clear that older individuals are more likely to have abrupt slow wave emergence trajectories than graded emergence trajectories.
Fig. 7. Older individuals have lower slow wave activity saturation parameters on induction and emergence.
For both induction and emergence, the sub-plots (A)-(D) detail the correlation of Ceswas and Pswas with age (in years). Individuals with graded and abrupt slow wave trajectories on emergence are shown in red and blue, respectively. Data are presented for the N=175 patients in Study 4 that had successful slow wave activity saturation fits on induction and emergence.
Table 4. Age is negatively correlated with power at slow wave activity saturation on induction and emergence.
Pearson’s correlations between age (in years) and the slow wave activity saturation parameters for Study 4. Age was strongly negatively correlated (r ~ -0.6, P<0.001) with Pswas on induction (P SWAS_I) and emergence (P SWAS_E), and with Ce SWAS on emergence (Ce SWAS_E). Significant correlations at the level of P<0.05 are highlighted in bold.
| Age | P SWAS_I | Ce SWAS_I | P SWAS_E | Ce SWAS_E | ||
|---|---|---|---|---|---|---|
| Age | r | -0.580 | -0.058 | -0.530 | -0.681 | |
| P | <0.001 | 0.471 | <0.001 | <0.001 | ||
| P SWAS_I | r | -0.580 | 0.193 | 0.574 | 0.537 | |
| P | <0.001 | 0.015 | <0.001 | <0.001 | ||
| Ce SWAS_I | r | -0.058 | 0.193 | 0.086 | 0.149 | |
| P | 0.471 | 0.015 | 0.281 | 0.062 | ||
| P SWAS_E | r | -0.530 | 0.574 | 0.086 | 0.550 | |
| P | <0.001 | <0.001 | 0.281 | <0.001 | ||
| Ce SWAS_E | r | -0.681 | 0.537 | 0.149 | 0.550 | |
| P | <0.001 | <0.001 | 0.062 | <0.001 |
There was a good discrimination between the two slow wave emergence trajectories. Graded responders had significantly higher Pswas (19.5±3.4 versus 14.0±4.4 dB, P<0.001) and were younger (48.0±17.3 versus 61.3±16.9 years, P<0.001) than those with abrupt slow wave emergence trajectories. They had also experienced shorter (111±69 versus 137±73 minutes, P=0.005), and less severe operations (74% minor operations for graded versus 48% minor for abrupt, P=0.006). Graded responders were more likely to have received sevoflurane rather than desflurane VGA (58% versus 41%, P=0.002). In general, the differences were more pronounced in Study 4, presumably reflecting the more standardized operations and anesthesia delivery used in Study 3.
Using a multivariate logistic regression model to predict whether a patient having surgical anesthesia is likely to have a graded or abrupt emergence trajectory, we found that age, Pswas on emergence, and VGA type all contributed significantly (with Wald P< 0.001): Ln Odds(abrupt/graded) = 4.31–0.28P SWAS_E –1.6(VGA = Sevoflurane) + 0.42age(decade). With reference to a graded emergence, the odds ratios for an abrupt response are: 0.75/dB Pswas on emergence, 0.20(VGA=Sevoflurane), and 1.5 per decade in age. The model had an overall classification accuracy of 78.4%.
We found that the slow wave activity emergence patterns correlated strongly with cognitive state in the early postoperative period. Patients with abrupt slow wave emergence trajectories experienced increased confusion levels fifteen minutes after waking compared to those with graded trajectories (Fisher’s exact test, P<0.001). Only 2 of the 110 patients who had a graded emergence were confused (i.e. <2%). In contrast, 18% (12/65) of patients who had an abrupt emergence trajectory were confused. The incidence of delirium was also higher in patients with abrupt slow wave emergence trajectories compared with graded responders (14/110 versus 17/65 patients, (CAM-ICU total, Fisher’s exact test, P= 0.02). No significant differences between the slow wave emergence trajectories were found in pain levels (P=0.59) or alertness state (P= 0.4).
Discussion
Neurobiological basis of slow wave activity saturation
Slow wave activity is a manifestation of slow ~1Hz cortical oscillations between a high firing, so-called UP (or active) state, and a quiescent DOWN (or silent) state22. We previously provided evidence that saturation of slow wave activity indicates an individualized state of perception loss and the peak of slow wave activity power was correlated with prefrontal cortical grey matter volume across subjects, suggesting maximal involvement of these cortical neurons due to anesthetic-induced increases in corticothalamic hyperpolarization3. Here, we have demonstrated that slow wave activity saturation occurs clinically on induction of anesthesia in 92% cases, for both volatile and intravenous anesthetic agents (Table 1). The co-administration of fentanyl markedly left-shifts the concentration required to achieve slow wave activity saturation, effectively highlighting the potentiation of hypnotic agents by opioids (Fig. 2).
When parameterized using dose-response models, slow wave activity saturation demonstrates negative correlations with age (Fig. 7). As age is known to be negatively correlated with gray matter volume11, our previous work3 suggested such a relationship with age should exist. Similar adult age-dependent slow wave activity amplitude decreases (but not saturation per se) have been observed during propofol and sevoflurane anesthesia23 and during natural sleep. In the latter case, this has also been linked with age-related reductions in neuronal numbers and synaptic density24. These data lend support to the idea that the slow wave activity saturation observed during anesthesia has a sound neurobiological basis and potentially represents a phenotype of an underlying trait.
In further support of this neurobiological basis, we found that the power at slow wave activity saturation was not significantly different between experimental induction and emergence from anesthesia (Table 2). Additionally, power at slow wave activity saturation on clinical induction of anesthesia was not significantly different between studies when the previously mentioned age-related variability was accounted for (Table 1). During clinical anesthesia, we also demonstrated that power at slow wave activity saturation on emergence was highly correlated with but reduced compared to that on induction (Fig. 6), possibly indicating modification of synaptic scaling through changes in glial ATP handling and interleukin-1 25 due to the inflammatory and stressful effects of surgery.
The goal of surgical anesthesia is to achieve loss of perception/awareness and no pain by targeting anesthetic drug dosing for each individual patient. Despite recent advances in our understanding of the neuroscientific basis of consciousness at a systems-level, a reliable brain-based indicator of unconsciousness under clinical anesthesia is still required2. Importantly, our data presented here further indicate that slow wave activity saturation is potentially a useful individualized biomarker to guide anesthetic drug dosing. Assuming based on our previous work that slow wave activity saturation indicates true perception loss3, the variability in the concentration required to achieve slow wave activity saturation (Fig. 5) implies that around 50% of patients receive twice as much anesthesia as they need, and 20% of patients will not achieve full unconsciousness when given a typical clinical anesthetic of about 0.7MAC.
Evidence of neural inertia in humans
Our findings are in support of the neural inertia framework proposed by Friedman and coworkers15 but have revealed important cross-species differences. Unlike Friedman et al., we did not observe any hysteresis in the average dose associated with behavior in Study 1. However importantly, we did observe clear hysteresis in our brain-based measure of slow wave activity saturation (Fig. 4) that potentially indicates a different arousal state where perception to incoming stimuli is lost3. At an individual level, the observed slow wave activity dose-responses during induction are often markedly different to that during emergence. This was most notable in those subjects who had an abrupt (rather than graded) change in the brain’s slow wave activity that temporally coincided with recovery of the individual’s behavioral responsiveness (Fig. 3).
Clinically, we identified that approximately a third of patients experience abrupt slow wave emergence trajectories after surgery (Table 3). The clear bimodal distributions for Ceswas and Pswas (Fig. 5) for the abrupt or graded slow wave emergence trajectories are indicative of two types of emergence with different associated neural processes. We were able to accurately predict the type of slow wave emergence trajectory in 78% of the individuals using clinically relevant parameters known prior to emergence. Our data suggests that a patient over 60 years of age (Fig. 6), experiencing desflurane anesthesia with a Pswas<16dB at the start of emergence (Fig. 5D), is significantly more likely to have an abrupt slow wave emergence trajectory. It is evident that the patient is ‘stuck’ in this unconscious state when they continue to maintain their slow wave activity saturation level until volatile general anesthetic concentrations are decreased to low levels of less than 0.3MAC.
We suggest that individuals with abrupt slow wave trajectories experience a high degree of neural inertia, as it is evident that (through the assessment of slow wave activity saturation) the individual’s brain is resisting recovery of consciousness. As such, we believe these abrupt slow wave trajectories are an archetypal indicator of neural inertia in humans, as they reveal an inherent resistance of the central nervous system to enter a wakeful state even when the brain concentrations of hypnotic drug have decreased to virtually zero. Based on our findings, we therefore propose a more general definition of neural inertia as: ‘a tendency of the central nervous system to resist transitions between conscious and unconscious states’. This definition could include various different brain state transitions as inferred by electroencephalographic methods, and/or functional brain imaging, and/or behavior.
Importantly, our findings fit well with other investigations of electroencephalographic changes on emergence from anesthesia. When individual emergence trajectories were characterized in alpha-delta frequency space by Chander et al., approximately 30% of cases were found to have a high level of delta power that continued through to just before the recovery of responsiveness26 – similar to the incidence of abrupt trajectories in our study. Lee and coworkers also found two different modes of emergence when examining the connection strength component of global efficiency using graph theory27. Whilst they did not explore frequency band specific changes, our data indicate that these individualized changes in slow wave dose-response could contribute to the gradual and abrupt network-level changes they observed. In light of recent findings in rodents that a series of metastable states exist on recovery from anesthesia that may give rise to this neural inertia28, our findings also have wider reaching implications for recovery in disorders of consciousness patients.
Finally, we provide preliminary evidence that individuals with abrupt slow wave emergence trajectories have more short-term delirium and confusion on recovery from anesthesia. This suggests a link between abrupt slow wave emergence trajectories and disruption of high-level cognitive functioning. Given that these patients are also more likely to be older and have a lower power at slow wave activity saturation, this opens up exciting future directions for the continued exploration of the slow wave activity saturation biomarker, and its neurobiological and clinical significance.
Study limitations and application to depth of anesthesia monitoring
Preliminary evidence we present here suggests that understanding the cause of neural inertia and preventing its occurrence may help reduce short-term post-operative confusion and delirium on recovery from anesthesia. However, further work and larger sample sizes will be required to fully elucidate this link. In the short-term, the accuracy of predicting individuals at risk of neural inertia and abrupt slow wave emergence trajectories could be increased with improved artifact rejection algorithms and slow wave activity saturation model refinements, particularly as failed slow wave activity saturation fits due to increased movement are more likely to occur in those with abrupt slow wave trajectories.
With a view to clinical depth of anesthesia monitoring, the multi-channel dataset indicates that central recording channels rather than the typically used frontal channels could further reduce failed fits due to muscle artifacts/movement. A further advantage is that the sigmoid gradient is comparatively more gradual in these channels, allowing more advance warning of the patient’s recovery of behavioral responsiveness. On emergence we also found equal numbers of failed fits because the model could not identify a plateau in slow wave activity. We believe the main contributing factor here is the reduction of volatile anesthetic concentrations towards the end of surgery to facilitate a quick recovery of the patient, as is common clinical practice.
Conclusions
Our individualized electroencephalographic marker of perception loss, slow wave activity saturation, is readily measurable during induction and emergence of clinical anesthesia and its magnitude negatively relates to age. Furthermore, we have shown that slow wave activity saturation can be observed across the commonly used gamma-amino-butyric-acid-ergic anesthetic drugs, in the presence of co-induction agents, and at various locations across the brain. The concentration of hypnotic drugs required to achieve slow wave activity saturation varies widely between patients; which would suggest that a large percentage of patients are actually under- or over-dosed with conventional, population-based, clinical anesthesia dosing regimens. Finally, slow wave activity saturation on emergence revealed evidence of neural inertia in humans, and a potential link between its occurrence and confusion/delirium shortly after recovery from anesthesia.
Supplementary Material
Brief Summary Statement.
Slow wave activity saturation occurs during surgical anesthesia. On emergence from anesthesia, abrupt slow wave activity emergence trajectories that coincide with recovery of behavior provide evidence of neural inertia in humans.
Acknowledgments
Individuals or organizations whose assistance is acknowledged
We would like to thank everyone involved in data collection.
Sources of financial support for the work
This work was supported by matched funding from a Confidence in Concept (BRR00010-HM01) award from the Medical Research Council of Great Britain and Northern Ireland (MRC), London, UK and the Oxford Invention Fund (OIF 528), Oxford, UK. This work has been partially funded by the LUMINOUS project, which received funding from the European Union's Horizon 2020 research and innovation programme (H2020-FETOPEN-2014-2015-RIA under agreement No. 686764, Brussels, Belgium). CEW was also funded by the Knoop Research Fellowship, St Cross College, University of Oxford, UK. Study 1 was originally funding by the MRC, London, UK; Wellcome Trust, London, UK; National Institute for Academic Anaesthesia, London, UK; and the International Anesthesia Research Society, San Francisco, CA, USA. DH and Study 4 were funded by the James S. McDonnell Foundation, Saint Louis, MO USA, (award 220020346). The results presented were independently derived and do not reflect any endorsement on the part of the James S. McDonnell Foundation.
Abbreviated title
- Neural inertia and slow wave activity saturation
Footnotes
Conflicts of interest
Patent applications were filed by Oxford University Innovation, the technology transfer company of the University of Oxford (formerly Isis Innovation), on perception loss detection. CEW, SJ and IT are listed as inventors. The authors declare no competing interests.
Contributor Information
Catherine E. Warnaby, Nuffield Department of Clinical Neurosciences, University of Oxford, United Kingdom.
Jamie W. Sleigh, Department of Anaesthesia, University of Auckland, Waikato Hospital, Hamilton, New Zealand.
Darren Hight, Department of Anaesthesia, University of Auckland, Waikato Hospital, Hamilton, New Zealand
Saad Jbabdi, Nuffield Department of Clinical Neurosciences, University of Oxford, United Kingdom
Irene Tracey, Nuffield Department of Clinical Neurosciences, University of Oxford, United Kingdom
References
- 1.Sanders RD, Tononi G, Laureys Steven, Sleigh JW. Unresponsiveness ≠ unconsciousness. Anesthesiology. 2012;116:946–59. doi: 10.1097/ALN.0b013e318249d0a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudetz AG, Mashour GA. Disconnecting Consciousness: Is There a Common Anesthetic End Point? Anesth Analg. 2016 doi: 10.1213/ANE.0000000000001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ni Mhuircheartaigh R, Warnaby C, Rogers R, Jbabdi S, Tracey I. Slow-Wave Activity Saturation and Thalamocortical Isolation During Propofol Anesthesia in Humans. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006007. 208ra148-208ra148. [DOI] [PubMed] [Google Scholar]
- 4.Murphy M, Bruno MA, Riedner BA, Boveroux P, Noirhomme Q, Landsness EC, Brichant JF, Phillips C, Massimini M, Laureys S. Propofol anesthesia and sleep: a high-density EEG study. Sleep. 2011;34:283. doi: 10.1093/sleep/34.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Leslie K, Myles PS, Forbes A, Chan MTV. The effect of bispectral index monitoring on long-term survival in the B-aware trial. Anesth Analg. 2010;110:816–22. doi: 10.1213/ANE.0b013e3181c3bfb2. [DOI] [PubMed] [Google Scholar]
- 7.Sessler DI, Sigl JC, Kelley SD, Chamoun NG, Manberg PJ, Saager L, Kurz A, Greenwald S. Hospital stay and mortality are increased in patients having a “triple low” of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia. Anesthesiology. 2012;116:1195–203. doi: 10.1097/ALN.0b013e31825683dc. [DOI] [PubMed] [Google Scholar]
- 8.McKay IDH, Voss LJ, Sleigh JW, Barnard JP, Johannsen EK. Pharmacokinetic-Pharmacodynamic Modeling the Hypnotic Effect of Sevoflurane Using the Spectral Entropy of the Electroencephalogram: Anesth Analg. 2006;102:91–7. doi: 10.1213/01.ane.0000184825.65124.24. [DOI] [PubMed] [Google Scholar]
- 9.Law CJ, Jacobson GM, Kluger M, Chaddock M, Scott M, Sleigh JW. Randomized controlled trial of the effect of depth of anaesthesia on postoperative pain. Br J Anaesth. 2014;112:675–80. doi: 10.1093/bja/aet419. [DOI] [PubMed] [Google Scholar]
- 10.Hight DF, Dadok VM, Szeri AJ, García PS, Voss L, Sleigh JW. Emergence from general anesthesia and the sleep-manifold. Front Syst Neurosci. 2014;8:146. doi: 10.3389/fnsys.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu RSN, Lemieux L, Bell GS, Sisodiya SM, Shorvon SD, Sander JWaS, Duncan JS. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. NeuroImage. 2003;20:22–33. doi: 10.1016/s1053-8119(03)00219-2. [DOI] [PubMed] [Google Scholar]
- 12.Mapleson WW. Effect of age on MAC in humans: a meta-analysis. Br J Anaesth. 1996;76:179–85. doi: 10.1093/bja/76.2.179. [DOI] [PubMed] [Google Scholar]
- 13.Steyn-Ross ML, Steyn-Ross DA, Sleigh JW. Modelling general anaesthesia as a first-order phase transition in the cortex. Prog Biophys Mol Biol. 2004;85:369–85. doi: 10.1016/j.pbiomolbio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Tarnal V, Vlisides PE, Mashour GA. The Neurobiology of Anesthetic Emergence. J Neurosurg Anesthesiol. 2016;28:250–5. doi: 10.1097/ANA.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman EB, Sun Y, Moore JT, Hung H-T, Meng QC, Perera P, Joiner WJ, Thomas SA, Eckenhoff RG, Sehgal A, Kelz MB. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PloS One. 2010;5:e11903. doi: 10.1371/journal.pone.0011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shafer SL, Varvel JR, Aziz N, Scott JC. Pharmacokinetics of fentanyl administered by computer-controlled infusion pump. Anesthesiology. 1990;73:1091–102. doi: 10.1097/00000542-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Mazoit JX, Butscher K, Samii K. Morphine in postoperative patients: pharmacokinetics and pharmacodynamics of metabolites. Anesth Analg. 2007;105:70–8. doi: 10.1213/01.ane.0000265557.73688.32. [DOI] [PubMed] [Google Scholar]
- 18.Stuart-Harris R, Joel SP, McDonald P, Currow D, Slevin ML. The pharmacokinetics of morphine and morphine glucuronide metabolites after subcutaneous bolus injection and subcutaneous infusion of morphine. Br J Clin Pharmacol. 2000;49:207–14. doi: 10.1046/j.1365-2125.2000.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiczling P, Bienert A, Sobczynski P, Hartmann-Sobczynska R, Bieda K, Marcinkowska A, Malatynska M, Kaliszan R, Grzeskowiak E. Pharmacokinetics and pharmacodynamics of propofol in patients undergoing abdominal aortic surgery. Pharmacol Rep PR. 2012;64:113–22. doi: 10.1016/s1734-1140(12)70737-5. [DOI] [PubMed] [Google Scholar]
- 20.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit CARE Med-Baltim. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Bouillon TW. Hypnotic and opioid anesthetic drug interactions on the CNS, focus on response surface modeling. Handb Exp Pharmacol. 2008:471–87. doi: 10.1007/978-3-540-74806-9_22. [DOI] [PubMed] [Google Scholar]
- 22.Riedner BA, Hulse BK, Murphy MJ, Ferrarelli F, Tononi G. Temporal dynamics of cortical sources underlying spontaneous and peripherally evoked slow waves. Prog Brain Res. 2011;193:201–18. doi: 10.1016/B978-0-444-53839-0.00013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purdon PL, Pavone KJ, Akeju O, Smith AC, Sampson AL, Lee J, Zhou DW, Solt K, Brown EN. The Ageing Brain: Age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia. Br J Anaesth. 2015;115(1):i46–57. doi: 10.1093/bja/aev213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ringli M, Huber R. Developmental aspects of sleep slow waves: linking sleep, brain maturation and behavior. Prog Brain Res. 2011;193:63–82. doi: 10.1016/B978-0-444-53839-0.00005-3. [DOI] [PubMed] [Google Scholar]
- 25.Krueger JM, Clinton JM, Winters BD, Zielinski MR, Taishi P, Jewett KA, Davis CJ. Involvement of cytokines in slow wave sleep. Prog Brain Res. 2011;193:39–47. doi: 10.1016/B978-0-444-53839-0.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chander DA-G, AU-MacColl PS, AU-Illing JN, AU-Sleigh S, JW Electroencephalographic Variation during End Maintenance and Emergence from Surgical Anesthesia. PLoS One. 2014;9:e106291. doi: 10.1371/journal.pone.0106291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee U, Müller M, Noh G-J, Choi B, Mashour GA. Dissociable network properties of anesthetic state transitions. Anesthesiology. 2011;114:872–81. doi: 10.1097/ALN.0b013e31821102c9. [DOI] [PubMed] [Google Scholar]
- 28.Hudson AE, Calderon DP, Pfaff DW, Proekt A. Recovery of consciousness is mediated by a network of discrete metastable activity states. Proc Natl Acad Sci. 2014;111:9283–8. doi: 10.1073/pnas.1408296111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy R, McKellow M, French R, Sleigh J. Sevoflurane end-tidal to effect-site equilibration in women determined by response to laryngeal mask airway insertion. Anesth Analg. 2013;117:786–91. doi: 10.1213/ANE.0b013e3182a46d4e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.