1. Introduction
The next decade will bear witness to a significant increase in the number of individuals living with osteoporosis and experiencing the morbidity consequent upon fragility fractures. These can be defined as fractures which result from a fall from standing height or less; or that present in the absence of trauma. The most common fragility fractures occur at the hip, wrist, spine, humerus and pelvis. It is timely to take stock of the key challenges facing healthcare professionals and policymakers responsible for providing care for populations in relation to bone health and to identify solutions that will reduce fracture rates and ameliorate their personal and societal burden. These challenges broadly fall into five distinct themes: (a) Perceived benefits and risks of therapy; (b) Case finding and management of individuals at high risk of fracture; (c) Public awareness of osteoporosis and fragility fractures; (d) Reimbursement and health system policy; and (e) Epidemiology of fractures in the developing world 1, 2. This review will address these important determinants of the osteoporosis “treatment gap” (the ‘gap’ between those individuals who require treatment and those individuals who actually receive treatment) (Figure 1), and measures that will assist in reducing its magnitude, with the twin objectives of optimising risk assessment and reducing fracture incidence 1, 2.
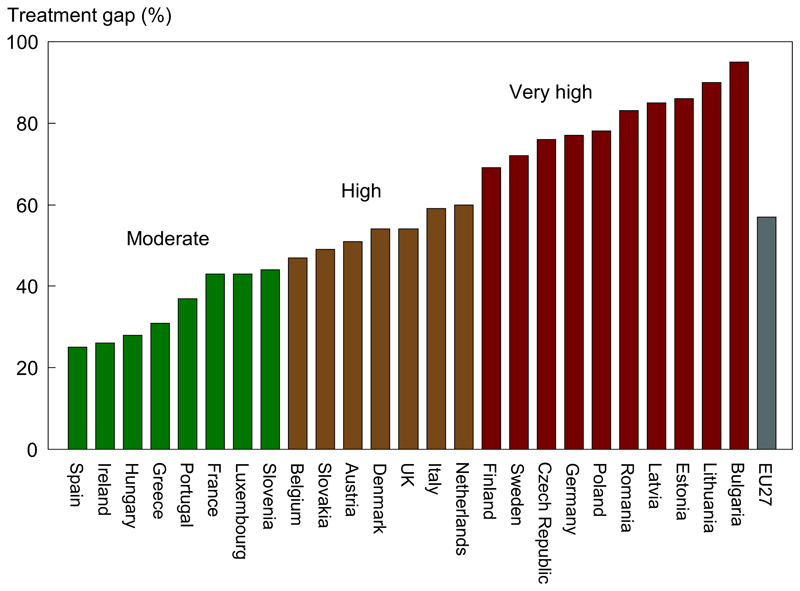
Figure 1.
Proportion of postmenopausal European women at moderate, high and very high risk of osteoporotic fracture who are un-treated – the “treatment gap” according to EU27 country with the EU27 mean in grey Ref 3.
2. Pharmacological treatment of osteoporosis
The effectiveness of a broad range of currently available osteoporosis treatments has been comprehensively reviewed elsewhere 4. The most commonly used agents in Europe are the bisphosphonates alendronate, risedronate, ibandronate and zoledronate. Additionally, raloxifene, agents derived from parathyroid hormone and denosumab. These have all been shown to reduce the risk of vertebral fracture. Some have also been shown to reduce the risk of non-vertebral fractures and, in some cases, agents have been shown specifically to decrease fracture risk at the hip (Table 1) 1.
Table 1.
Antifracture efficacy of the most frequently used treatments for postmenopausal osteoporosis when given with calcium and vitamin D, as derived from randomized controlled trials1. In this table ‘established osteoporosis’ is defined as osteoporosis in the presence of one or more fragility fractures.
| Effect on vertebral fracture risk | Effect on non-vertebral fracture risk | |||
|---|---|---|---|---|
| Osteoporosis | Established osteoporosisa | Osteoporosis | Established osteoporosisa | |
| Alendronate | + | + | NA | + (including hip) |
| Risedronate | + | + | NA | + (including hip) |
| Ibandronate | NA | + | NA | + b |
| Zoledronate | + | + | NA | + c |
| HRT | + | + | + | + (including hip) |
| Raloxifene | + | + | NA | NA |
| Teriparatide | NA | + | NA | + |
| Denosumab | + | + c | + (including hip) | + c |
NA, no evidence available;
effective drug;
women with a prior vertebral fracture;
in subsets of patients only (post-hoc analysis);
mixed group of patients with or without prevalent vertebral fractures
2.1. Bisphosphonates
2.1.1. Efficacy
Bisphosphonates are stable analogues of pyrophosphate characterized by a P-C-P bond. A variety of bisphosphonates have been synthesized, the potency of which depends on the length and structure of the side chain 5. Bisphosphonates have a strong affinity for bone apatite, both in vitro and in vivo, which is the basis for their clinical use. They are potent inhibitors of bone resorption and produce their effect by reducing the recruitment and activity of osteoclasts and increasing their apoptosis. The potency and chemical affinity to bone of bisphosphonates determines their effect to inhibit bone resorption and varies greatly from compound to compound. Potency differences can range 10,000-fold in vitro, so that the doses used clinically also vary. The primary action of nitrogen-containing bisphosphonates is to inhibit the farnesyl pyrophosphate synthase enzyme step in the mevalonate pathway, thereby modifying the isoprenylation of multiple guanosine triphosphate binding proteins involved in intracellular signalling and osteoclast function6–8.
Oral bioavailability of bisphosphonates is low, around 1% of the dose ingested, and is impaired by food, calcium, iron, coffee, tea and orange juice. Bisphosphonates are quickly cleared from plasma, about 50% being deposited in bone and the remainder excreted in urine. Their half-life in bone is very prolonged 9.
Alendronate 70 mg once weekly and risedronate 35 mg once weekly are the most commonly used bisphosphonates worldwide. In the Fracture Intervention (FIT) study, alendronate was shown to reduce the incidence of vertebral, wrist and hip fractures by approximately half in women with prevalent vertebral fractures 10. In women without prevalent vertebral fractures, there was no significant decrease in clinical fractures in the overall population, but the reduction was significant in the one-third of patients that had a baseline hip BMD T-score lower than -2.5 11. In a population of more than 90,000 men and women aged 80 years and older, with a prior fracture, a case-control analysis revealed that alendronate use was associated with a 34% decrease in hip fracture risk, and a 12% lower mortality risk. However there was a 58% increase in the risk of mild upper gastro-intestinal symptoms 12.
Risedronate has been shown to reduce the incidence of vertebral and non-vertebral fractures by 40-50% and 30-36%, respectively in women with prevalent vertebral fractures 13, 14. In a large population of elderly women, risedronate significantly decreased the risk of hip fractures by 30%, an effect that was greater in osteoporotic women age 70-79 years (-40%), and not significant in women over the age of 80 years without evidence of osteoporosis. A delayed-release formulation of 35mg risedronate weekly, given before or immediately following breakfast showed a similar or greater effect on spine and hip BMD than traditional immediate-release 5mg risedronate in a daily dose. This formulation allows osteoporotic patients to take their weekly risedronate dose immediately after breakfast, hence offering a potential for improved adherence and persistence to treatment 15.
Ibandronate given daily (2.5 mg) reduces the risk of vertebral fractures by 50-60%. An effect on non-vertebral fractures was only demonstrated in a post hoc analysis of women with a baseline of BMD T-score below -3.0 17–19. Bridging studies have shown that oral ibandronate 150 mg once monthly is equivalent or superior to daily ibandronate in increasing BMD and decreasing biochemical markers of bone turnover, giving rise to its approval for the prevention of vertebral fracture in postmenopausal osteoporosis 20. The efficacy and safety of oral monthly ibandronate was confirmed for up to 5 years in women with post-menopausal osteoporosis 20, 21. Similarly, bridging studies comparing intermittent intravenous ibandronate to daily oral treatment has led to the approval of intravenous ibandronate 3 mg every 3 months for the same indication 22. A post-hoc analysis of pooled individual patient-data from the studies assessing the long-term (5 years) efficacy of oral20 and intravenous23 ibandronate concluded that for ibandronate regimens with annual cumulative exposure ≥ 10.8mg, time-to-fracture was significantly longer for all clinical fractures, non-vertebral and clinical vertebral fractures versus placebo and that for all fracture types, the rate of fracture appeared stable up to 5 years 24.
Based on the result of a phase II study 25, a large phase III trial in over 7700 postmenopausal osteoporotic patients, assessed the efficacy of yearly infusion of zoledronate 5 mg over 3 years. As compared to the placebo group, zoledronate was found to reduce the incidence of vertebral fractures by 70% and that of hip fractures by 40% 26, and is now available for the treatment of postmenopausal osteoporosis. Intravenous zoledronate has also been shown to decrease the risk of fracture and mortality when given shortly after a first hip fracture 27. The phase III trial was extended to 6 28 and 9 29 years. The overall conclusion was that pursuing treatment beyond 3 years only provided marginal benefits 29. Some authors even argue that an annual administration of 5mg zoledronate might represent over treatment 30. A single dose of 5mg zoledronate given to frail elderly women improved spine and total hip BMD over 2 years, compared to placebo but the treated group had an increase in fractures, multiple falls and mortality 31 suggesting that zoledronate may not be an appropriate treatment for such patients. Of course, the intravenous delivery of zoledronate has the potential to improve adherence to bisphosphonates provided that infusions are not missed.
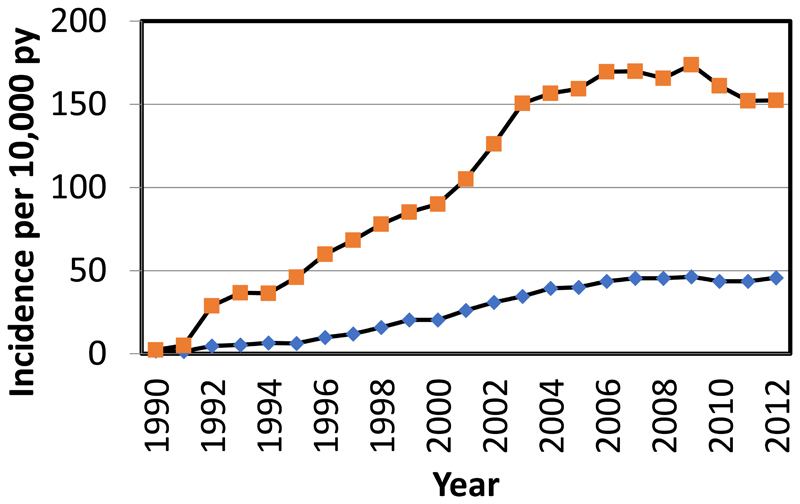
Despite widespread evidence of the efficacy of these agents, there has been a plateau in their use in Europe and North America over recent years (Figure 2).
Figure 2.
Frequency of use of anti-osteoporotic medication in England and Wales, 1990-2012. Orange squares denote incidence for women and blue diamonds the incidence for men.16
2.1.2. Safety of bisphosphonates
The overall safety profile of bisphosphonates is favourable. Oral bisphosphonates are associated with mild gastrointestinal disturbances, and some nitrogen-containing bisphosphonates (alendronate and pamidronate) can rarely cause oesophagitis. A network meta-analysis compared the gastro-intestinal safety of all oral and injectable bisphosphonates given to osteoporotic patients. It concluded that zoledronate has the highest probability of causing gastro-intestinal adverse events, possibly related to nausea 32. Intravenous nitrogen-containing bisphosphonates can induce a transient acute phase reaction with fever, bone and muscle pain that ameliorates or disappears after subsequent courses 33. Concerns have been raised about a possible association between bisphosphonate therapy and atrial fibrillation. Subsequent studies have produced conflicting results, but have not excluded the possibility of such an association in people at increased risk of fracture 34. Patients to whom zoledronate was administered for up to 9 years had a higher risk of cardiac arrhythmias compared to those who discontinued the treatment after 6 years 34. The possibility that bisphosphonate therapy is associated with increased risk of oesophageal cancer has been raised. Two studies from the General Practice Research Database in the UK have produced conflicting results, one failing to show any association, but another concluding that there was an increased risk with extended use over 5 years 35, 36. Other database studies have found no excessive risk of oesophageal cancer deaths or incidence37. However, the two major adverse effects of bisphosphonate therapy contributing to the treatment gap are atypical femoral fracture and osteonecrosis of the jaw.
2.1.3. Atypical femoral fracture
Schilcher and colleagues reviewed radiographs of Swedish women who sustained a hip fracture in 2008, identifying 59 atypical femoral fractures 38. Data on medications and coexisting conditions were obtained from national registries. The relative and absolute risk of atypical fractures associated with bisphosphonate use was estimated by means of a nationwide cohort analysis. The 59 case patients were also compared with 263 control patients who had ordinary subtrochanteric or shaft fractures. In this study, the age-adjusted relative risk of atypical fracture was 47.3 (95% confidence interval [CI], 25.6 to 87.3) in the cohort analysis. The increase in absolute risk was 5 cases per 10,000 patient-years (95% CI, 4 to 7). A total of 78% of the case patients and 10% of the controls had received bisphosphonates, corresponding to a multivariable-adjusted odds ratio of 33.3 (95% CI, 14.3 to 77.8). The duration of use influenced the risk (odds ratio per 100 daily doses, 1.3; 95% CI, 1.1 to 1.6). Importantly after drug withdrawal, the risk diminished by 70% per year since the last use (odds ratio, 0.28; 95% CI, 0.21 to 0.38). In a follow-up paper of 172 patients with atypical femoral fractures the age-adjusted relative risk (RR) of atypical fracture associated with bisphosphonate use was 55 (95% CI: 39-79) in women and 54 (CI: 15-192) in men 39. In bisphosphonate users, women had a 3-fold higher risk than men (RR = 3.1, CI: 1.1-8.4). Alendronate users had higher risk than risedronate users (RR = 1.9, CI: 1.1-3.3). The RR after 4 years or more of use reached 126 (CI: 55-288), with a corresponding absolute risk of 11 (CI: 7-14) fractures per 10,000 person-years of use. The risk decreased by 70% per year since last use. These data are complementary to previous reported figures that suggest the incidence of atypical femoral fracture appears related to duration of exposure. This observation receives some support from preliminary reports from the Southern California Osteoporosis Cohort Study (SOCS) where the risk of radiology adjudicated AFFs declined by 44% in the first year after discontinuation compared to women who continued to use BP (HR 0.56, CI 0.38-0.82). After 4 years or more, the AFF risk was found to be reduced by 78% (HR 0.22, CI 0.08-0.59) compared to current users. The rate of AFFs among current users was reported as 4.6 per 10,000 patient years 40. A recent systematic review 41 included 23 studies on atypical femoral fractures: 14 on epidemiology and 11 on treatment outcomes (2 articles reported on both aspects). The review showed that the incidence of atypical femoral fractures is low (3.0-9.8 per 100,000 person-years), but relative risk increased with longer duration of bisphosphonates use, especially after more than 3 years.
It should be noted that some of the most recent data in this area come from a study of nearly 200,000 women (over 50 year of age) which modelled the risk-benefit profile from 1 to 10 years with regard to fragility fracture prevention (benefit) and atypical femoral fracture occurrence (risk). This found that the absolute risk was very low and certainly outweighed by the benefits in terms fracture prevention42.
2.1.4. Osteonecrosis of the jaw
Osteonecrosis of the jaw (ONJ) is a very rare clinical event that was first reported in connection with bisphosphonate use in 2003 43 and has been addressed in numerous reviews since44. The incidence of ONJ is rare and increases with exposure, suggesting an inflection point of 4 years. The American Society for Bone and Mineral Research (ASBMR) estimated ONJ incidence as between 1 in 10, 000-100,000 patient-treatment years. The AAOMS using data from Lo 45 estimated 210/100,000 patient years. Most of the reported cases have been in association with the use of zoledronate or pamidronate used intravenously to control metastatic bone disease 45, 46. The risk of ONJ in association with the use of oral bisphosphonates is very much less, and was reviewed by Masoodi in 2009, who concluded that the use of oral bisphosphonates did not increase the risk of ONJ in osteoporosis patients 47. Furthermore, no cases of ONJ were reported in over 3000 patients participating in clinical studies of effectiveness of alendronate and zoledronate 46. More recent studies have suggested that pre-existing dental disease and prior dental extraction are the strongest risk factors 26. Danish national health data also suggest a low incidence rate of surgically treated ONJ of 2.5 (95% confidence interval 2.1 to 3.1) per 10,000 patient years for users of oral bisphosphonates, albeit a higher risk in users with 5 years of exposure or more. The risk was higher in patient with rheumatoid disorders or diabetes 48. Denosumab therapy has also been associated with ONJ. The incidence of adverse and serious adverse events did not increase over time in the denosumab extension study. However, through extension year 5, there were 8 confirmed events of osteonecrosis of the jaw and 2 events of atypical femoral fracture. Very recently, 10-year data have been published for denosumab therapy. Serious adverse event rates were generally stable over time, varying between 11·5 and 14·4 per 100 participant-years. One atypical femoral fracture occurred in each group during the extension. Seven cases of osteonecrosis of the jaw were reported in the long-term group and 6 cases in the crossover group.
There is increasing interest in the potential, non-skeletal benefits of bisphosphonates on lifespan and in conditions including progeria (when used in conjunction with statins)49, cardiovascular disease50 neurodegenerative disorders, neoplasia and infections9 (particularly pneumonia risk reduction51).
2.1.5. Treatment discontinuation
These observations have led to a number of position papers and guidelines 4, which generally reinforce the importance of continuing therapy among women who remain at high risk of fracture, as intermission of therapy, even for those with residual effects on bone turnover after intermission such as BPs, will be followed by an increase in fracture incidence in those subjects. Despite these recommendations, many patients discontinue therapy despite remaining at high risk. When considering long-term therapy though, one has to balance benefits and risks. With the exception of denosumab, the number of patients in RCTs carried through to 10 years or longer is very small. A particular concern of patients and physicians alike is the apparent association of osteoporosis treatment with atypical femoral fractures and ONJ. However, this risk remains less than 1 in 1000 subjects treated even for 10 years according to most long-term extensions of RCTs and observational studies. Although the long-term benefits remain difficult to exactly evaluate in absence of large placebo-controlled extension studies, assuming a long-term reduction of fracture risk in the order of 30% with anti-resorptive therapy, as suggested by the available evidence, particularly among high risk subjects, would result in a benefit: risk ratio (fractures prevented: adverse skeletal event) of at least 100:1. Observational data suggest that patients treated with oral bisphosphonates in excess of 10 dose years maintain a low incidence of both hip fractures and fractures of the subtrochanteric femur and femoral shaft. The available evidence from prospective and retrospective analyses indicates that treatment cessation is associated with an increase in fracture risk. The risk of new clinical fracture was about 20-40% higher in patients who stopped treatment and vertebral fracture risk was approximately doubled. These findings suggest that the concept of a ‘drug holiday’ as routine must be challenged. This is an urgent public health message that should be conveyed to health professionals, policy makers and patients.
3. Other Measures to Close the Treatment Gap
3.1. Secondary prevention: treating those who have already had a fracture
As described by the evidence detailed above, fragility fractures represent a huge burden on societies worldwide. Patient perception of fracture risk is often underestimated as osteoporosis is a silent condition until a fracture happens, so primary prevention initiation is usually reliant on health care practitioners who need to have the time and incentive to assess fracture risk and explain the purpose of treatment to their patients. Secondary prevention, in which patients are identified for treatment on the basis of a previous low trauma fracture, is therefore the approach usually taken.
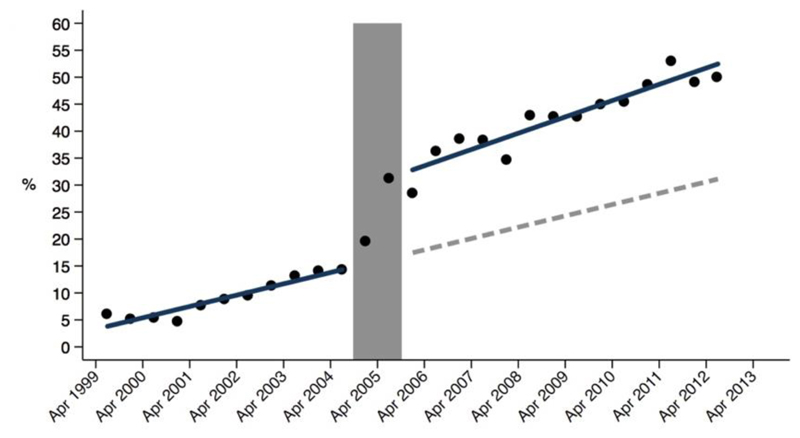
Several methods have been explored to enable fracture risk assessment and initiation of appropriate treatment – some based upon staff, others on IT and others upon a combination of the two. The multi-disciplinary Fracture Liaison Service (FLS) is one of the most successful of these systems 52, 53, incorporating rheumatologists, ortho-geriatricians, other physicians, clinical nurse specialists and allied health professionals. Members of the FLS multidisciplinary team, coordinated by a lead clinician, work together to optimise the medical management of patients admitted with fracture, both in hospital and for long term fracture prevention 54. This approach has been demonstrated to optimise osteoporosis treatment (Figure 3).
Figure 3.
Increase in frequency of prescribing anti-osteoporotic medication in UK following installment of secondary fracture prevention services nationwide. Percentage of anti-osteoporosis medication in the first year after index hip fracture among treatment naïve patients at baseline before and after the commencement of the secondary fracture prevention service (shown in grey). The blue line represents the actual (observed) increase in the proportion of patients receiving anti-osteoporosis medication before and after the instigation of the secondary fracture prevention service and the dotted line represents the modelled trajectory without the secondary fracture prevention service.62
“Capture the Fracture®”, an initiative instituted by the International Osteoporosis Foundation (http://www.capturethefracture.org/) is “a global campaign to facilitate the implementation of coordinated, multi-disciplinary models of care for secondary fracture prevention.” Capture the Fracture has provided secondary fracture prevention guidance, and also a global map of secondary fracture prevention services, with a quality grading scheme, graded by assessed application and description of the service 55, 56. This scheme has helped to document the huge variation in the quality, scope and availability of secondary prevention facilities, not only within, but also between countries. The Capture the Fracture initiative aims to raise the quality and coverage of these fracture liaison services. Vertebral fracture case finding is an additive approach to secondary fracture prevention as many such events go undetected. It has been shown that around 12% of postmenopausal women with osteoporosis have one or more vertebral deformities, but fewer than one in three of these individuals come to clinical attention 57. Primary care-based screening strategies 58 and history taking methods distinguishing “vertebral fracture-type back pain” from other types of back pain may assist their detection 59. Different methods for radiological assessment of vertebral fractures exist, including radiographs, CT scans and automatic detection using artificial intelligence modalities are moving nearer to clinical usage60, 61.
3.2. Primary prevention: starting treatment in individuals at high fracture risk
In osteoporosis, there is ongoing debate regarding the benefits of a widespread systematic screening approach, leading to higher treatment rates (with its associated cost and side-effect risk), and a case-finding approach focused on those at highest individual risk (with its associated issue of under-treatment). Whilst DXA-based osteoporosis screening is officially a standard policy in the US (at age 65 years in women, age 70 in men, and in individuals over 50 years who have suffered a fracture as an adult) 63, in the majority of countries population screening is not judged to be cost-effective. Primary prevention is therefore generally focused on case-finding strategies, reliant on the physician identifying clinical risk factors 64.
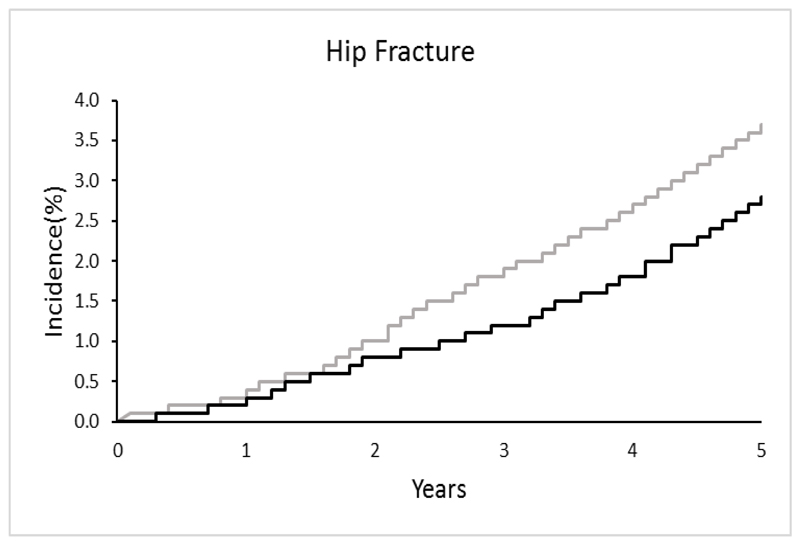
In the UK, a randomised controlled trial across 7 centres was recently undertaken (the UK SCOOP study), examining both the clinical and cost-effectiveness of screening older women in primary care for primary fracture prevention. Around 12,500 older women were randomised to either screening and subsequent treatment (stratified using FRAX hip fracture probability) or usual care. The screening intervention was shown to lead to reduction in hip fracture risk by 28% (Figure 4) 65, 66. Those at highest baseline fracture risk appeared to benefit most from screening (as would be expected, since these were the individuals targeted for treatment) 67, and importantly, was shown to be cost-effective (the cost per quality adjusted life year (QALY) gained was £2772 compared with the control arm) 68. The finding that women who were identified by FRAX as moderate or high risk of fracture benefited most from a screening programme was supported by the Danish Risk Stratified Osteoporosis Strategy Evaluation (ROSE) study, though this study found no overall effect on fracture incidence of a screening strategy 69. A recent evidence report and systematic review for the US Preventive Services Task Force, concluded that screening to prevent osteoporosis in women may reduce hip fractures.
Figure 4.
Results of MRC SCOOP Trial: Over 5 years, risk assessment using FRAX to target bisphosphonate therapy resulted in a 24% (p<0.01) reduction in the risk of hip fracture 65. The grey line shows the incidence of hip fracture in the non-screening (control) arm and the black line shows the lower incidence of hip fracture in the screening (experimental) arm.
Once a patient has been identified as requiring fracture risk assessment, the threshold at which treatment should be given will vary according to factors such as healthcare provision, willingness to pay and cost of medications. The majority of guidelines internationally use FRAX as the arbiter of fracture risk, but 38 of the 120 guidelines identified in a recent systematic review gave no direction on translating FRAX probabilities into a treatment decision. Threshold setting is as much a philosophical as scientific process, with decisions around whether a level should be fixed, or age dependent, and calibration to the specific country. Given the marked variation in fracture rates between countries, this latter consideration seems mandatory, and the benefits and caveats associated with fixed or age-dependent thresholds are presented in detail in. In the UK, FRAX is linked to the age-dependent (up to the age of 70 years) thresholds of the National Osteoporosis Guideline Group (NOGG), with the threshold predicated on the probability of future fracture conferred by a prior fracture. This approach has been shown to be cost effective in the UK, and contrasts markedly with that of the UK National Institute of Health and Care Excellence (NICE). In the 2017 technology appraisal of bisphosphonates, a pure health economic approach, in the context of a very common disease and extremely inexpensive therapies, led of a 1% risk of major osteoporotic fracture over 10 years as the threshold above which these medications were considered cost-effective. Unfortunately this was often interpreted by payers as an intervention threshold, a situation which if permitted to continue would have resulted in many adults at low risk of fracture being inappropriately treated, but which was later resolved by referral to NOGG guidance for clinical, rather than health economic thresholds.
4.0. Conclusion
Recent decades have seen a dramatic transformation in osteoporosis, from having been historically viewed as an inescapable result of ageing, to now being a well- characterised chronic non-communicable disease, with diagnostic criteria, well-established methods of risk assessment and an enviable range of therapeutic medications. Despite this backdrop, however, there is evidence from the UK, US and continental Europe that treatment rates have declined substantially in the last 5 years. With ageing populations and overstretched health services, osteoporosis may often fall off the bottom of the list of priorities for both clinicians and patients. Although many of the studies included in this review have focussed on women, it is vital to remember that osteoporosis is often more under-diagnosed and under-treated in men. The rare adverse effects of anti-resorptive therapies have become a disproportionately (and inappropriately) major concern, amplified by sensationalised media reports, which have usually been inadequately countered by the clinical academic community. These fears and the resulting reduced prescribing have been exacerbated by reductions in reimbursement in the US, mirrored in new guidance. It is apparent that many patients, doctors and dentists now appear more concerned by the rare but serious side effects of anti-resorptives than they are of the osteoporosis and fragility fractures.
The clear imperative to urgently tackle this issue has been recognised by key organisations such as the International Osteoporosis Foundation and the American Society for Bone and Mineral Research, leading to the publication of recommendations and roadmaps to address the critical care gap in osteoporosis treatment 1, 2. It is also important to acknowledge that with bisphosphonates now generic compounds, their cost has dropped substantially. This is good news for the accessibility of medications but the field will be reliant on the development of novel therapies to bring in revenue which can be used to educate patients, healthcare professionals and large-scale clinical trials.
Improved public awareness and public health strategies to improve bone health from a young age will also contribute to prevention of osteoporosis in future generations. Given the rapid ageing of the global population and the importance of good musculoskeletal health in old age, we must come together to ensure that during the coming decade, 2020-2030, hailed by the WHO and others as the “Decade of Healthy Ageing”, bone health and fracture prevention become the priority they so urgently need to be.
Highlights.
Bisphosphonates are effective at reducing the risk of fragility fractures and have an important role to play in closing the osteoporosis treatment gap
They are generally safe and the rates of osteonecrosis of the jaw and atypical femoral fractures are low
Secondary fracture prevention services are an effective and cost-efficient method of closing the treatment gap
The SCOOP trial demonstrated that population screening for primary prevention was clinically effective for hip fractures and cost-effective
Acknowledgements
This manuscript was prepared for presentation at the 50th Anniversary Meeting for Bisphosphonates, Sheffield, July 2019. It relies upon material published by the principal author in the IOF-ESCEO European Guidelines for the Management and Treatment of Osteoporosis (1); the IOF Report on Closing the Osteoporosis Treatment Gap (2); the IOF Working Group Report on Discontinuation of Osteoporosis Therapy (3); and a recently published review on optimising osteoporosis therapy (4).
References
- 1.Kanis JA, Cooper C, Rizzoli R, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30:3–44. doi: 10.1007/s00198-018-4704-5. 2018/10/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey NC, McCloskey EV, Mitchell PJ, et al. Mind the (treatment) gap: a global perspective on current and future strategies for prevention of fragility fractures. Osteoporos Int. 2017;28:1507–1529. doi: 10.1007/s00198-016-3894-y. 2017/02/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernlund E, Svedbom A, Ivergard M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. 2013/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennison EM, Cooper C, Kanis JA, et al. Fracture risk following intermission of osteoporosis therapy. Osteoporos Int. 2019;30:1733–1743. doi: 10.1007/s00198-019-05002-w. 2019/06/09. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson M, Jones ML, De Nigris E, et al. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess. 2005;9:1–160. doi: 10.3310/hta9220. 2005/06/03. [DOI] [PubMed] [Google Scholar]
- 6.Rogers MJ, Mönkkönen J, Munoz MA. Molecular mechanisms of action of bisphosphonates and new insights into their effects outside the skeleton. Bone. 2020;139 doi: 10.1016/j.bone.2020.115493. 115493. [DOI] [PubMed] [Google Scholar]
- 7.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. 2011/05/11. [DOI] [PubMed] [Google Scholar]
- 8.Ebetino FH, Hogan AM, Sun S, et al. The relationship between the chemistry and biological activity of the bisphosphonates. Bone. 2011;49:20–33. doi: 10.1016/j.bone.2011.03.774. 2011/04/19. [DOI] [PubMed] [Google Scholar]
- 9.Cremers S, Ebetino F, Phipps R. On the pharmacological evaluation of bisphosphonates in humans. Bone. 2020;139 doi: 10.1016/j.bone.2020.115501. 115501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85:4118–4124. doi: 10.1210/jcem.85.11.6953. 2000/11/30. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. Jama. 1998;280:2077–2082. doi: 10.1001/jama.280.24.2077. 1999/01/06. [DOI] [PubMed] [Google Scholar]
- 12.Axelsson KF, Wallander M, Johansson H, et al. Hip fracture risk and safety with alendronate treatment in the oldest-old. J Intern Med. 2017;282:546–559. doi: 10.1111/joim.12678. 2017/09/01. [DOI] [PubMed] [Google Scholar]
- 13.Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. 2000/02/09. [DOI] [PubMed] [Google Scholar]
- 14.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. Jama. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. 1999/10/20. [DOI] [PubMed] [Google Scholar]
- 15.McClung MR, Balske A, Burgio DE, et al. Treatment of postmenopausal osteoporosis with delayed-release risedronate 35 mg weekly for 2 years. Osteoporos Int. 2013;24:301–310. doi: 10.1007/s00198-012-2175-7. 2012/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Velde RY, Wyers CE, Curtis EM, et al. Secular trends in fracture incidence in the UK between 1990 and 2012. Osteoporos Int. 2016;27:3197–3206. doi: 10.1007/s00198-016-3650-3. 2016/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris ST, Blumentals WA, Miller PD. Ibandronate and the risk of non-vertebral and clinical fractures in women with postmenopausal osteoporosis: results of a meta-analysis of phase III studies. Curr Med Res Opin. 2008;24:237–245. doi: 10.1185/030079908x253717. 2007/12/01. [DOI] [PubMed] [Google Scholar]
- 18.Chesnut CH, 3rd, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249. doi: 10.1359/jbmr.040325. 2004/07/03. [DOI] [PubMed] [Google Scholar]
- 19.Delmas PD, Recker RR, Chesnut CH, 3rd, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15:792–798. doi: 10.1007/s00198-004-1602-9. 2004/04/09. [DOI] [PubMed] [Google Scholar]
- 20.Reginster JY, Adami S, Lakatos P, et al. Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis. 2006;65:654–661. doi: 10.1136/ard.2005.044958. 2005/12/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller PD, Recker RR, Reginster JY, et al. Efficacy of monthly oral ibandronate is sustained over 5 years: the MOBILE long-term extension study. Osteoporos Int. 2012;23:1747–1756. doi: 10.1007/s00198-011-1773-0. 2011/09/29. [DOI] [PubMed] [Google Scholar]
- 22.Delmas PD, Adami S, Strugala C, et al. Intravenous ibandronate injections in postmenopausal women with osteoporosis: one-year results from the dosing intravenous administration study. Arthritis Rheum. 2006;54:1838–1846. doi: 10.1002/art.21918. 2006/05/27. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi G, Czerwinski E, Kenwright A, et al. Long-term administration of quarterly IV ibandronate is effective and well tolerated in postmenopausal osteoporosis: 5-year data from the DIVA study long-term extension. Osteoporos Int. 2012;23:1769–1778. doi: 10.1007/s00198-011-1793-9. 2011/10/07. [DOI] [PubMed] [Google Scholar]
- 24.Miller PD, Recker RR, Harris S, et al. Long-term fracture rates seen with continued ibandronate treatment: pooled analysis of DIVA and MOBILE long-term extension studies. Osteoporos Int. 2014;25:349–357. doi: 10.1007/s00198-013-2518-z. 2013/10/19. [DOI] [PubMed] [Google Scholar]
- 25.Reid IR, Brown JP, Burckhardt P, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346:653–661. doi: 10.1056/NEJMoa011807. 2002/03/01. [DOI] [PubMed] [Google Scholar]
- 26.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. The New England journal of medicine. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 27.Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. The New England journal of medicine. 2007;357:1799–1809. doi: 10.1056/NEJMoa074941. 09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT) J Bone Miner Res. 2012;27:243–254. doi: 10.1002/jbmr.1494. 2011/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Black DM, Reid IR, Cauley JA, et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT) J Bone Miner Res. 2015;30:934–944. doi: 10.1002/jbmr.2442. 2014/12/30. [DOI] [PubMed] [Google Scholar]
- 30.Grey A. Intravenous zoledronate for osteoporosis: less might be more. Ther Adv Musculoskelet Dis. 2016;8:119–123. doi: 10.1177/1759720x16650866. 2016/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenspan SL, Perera S, Ferchak MA, et al. Efficacy and safety of single-dose zoledronic acid for osteoporosis in frail elderly women: a randomized clinical trial. JAMA Intern Med. 2015;175:913–921. doi: 10.1001/jamainternmed.2015.0747. 2015/04/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tadrous M, Wong L, Mamdani MM, et al. Comparative gastrointestinal safety of bisphosphonates in primary osteoporosis: a network meta-analysis. Osteoporos Int. 2014;25:1225–1235. doi: 10.1007/s00198-013-2576-2. 2013/11/30. [DOI] [PubMed] [Google Scholar]
- 33.Rizzoli R, Reginster JY, Boonen S, et al. Adverse reactions and drug-drug interactions in the management of women with postmenopausal osteoporosis. Calcif Tissue Int. 2011;89:91–104. doi: 10.1007/s00223-011-9499-8. 2011/06/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pazianas M, Compston J, Huang CL. Atrial fibrillation and bisphosphonate therapy. J Bone Miner Res. 2010;25:2–10. doi: 10.1359/jbmr.091201. 2010/01/22. [DOI] [PubMed] [Google Scholar]
- 35.Green J, Czanner G, Reeves G, et al. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. Bmj. 2010;341 doi: 10.1136/bmj.c4444. c4444. 2010/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1–23. doi: 10.1002/jbmr.1998. 2013/05/29. [DOI] [PubMed] [Google Scholar]
- 37.Abrahamsen B, Pazianas M, Eiken P, et al. Esophageal and gastric cancer incidence and mortality in alendronate users. J Bone Miner Res. 2012;27:679–686. doi: 10.1002/jbmr.1481. 2011/11/25. [DOI] [PubMed] [Google Scholar]
- 38.Schilcher J, Michaëlsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med. 2011;364:1728–1737. doi: 10.1056/NEJMoa1010650. 2011/05/06. [DOI] [PubMed] [Google Scholar]
- 39.Adams AL. Fracture Risk During and After Bisphosphonate Drug Holidays: A Matter of Methods? Med Care. 2020;58:417–418. doi: 10.1097/mlr.0000000000001317. 2020/03/10. [DOI] [PubMed] [Google Scholar]
- 40.Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544–2550. doi: 10.1002/jbmr.1719. 2012/07/28. [DOI] [PubMed] [Google Scholar]
- 41.Khow KS, Shibu P, Yu SC, et al. Epidemiology and Postoperative Outcomes of Atypical Femoral Fractures in Older Adults: A Systematic Review. J Nutr Health Aging. 2017;21:83–91. doi: 10.1007/s12603-015-0652-3. 2016/12/22. [DOI] [PubMed] [Google Scholar]
- 42.Black DM, Geiger EJ, Eastell R, et al. Atypical Femur Fracture Risk versus Fragility Fracture Prevention with Bisphosphonates. N Engl J Med. 2020;383:743–753. doi: 10.1056/NEJMoa1916525. 2020/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. 2003/09/11. [DOI] [PubMed] [Google Scholar]
- 44.Cummings SR, Santora AC, Black DM, et al. History of alendronate. Bone. 2020;137 doi: 10.1016/j.bone.2020.115411. 115411. [DOI] [PubMed] [Google Scholar]
- 45.Lo JC, O'Ryan FS, Gordon NP, et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68:243–253. doi: 10.1016/j.joms.2009.03.050. 2009/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marx RE, Sawatari Y, Fortin M, et al. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–1575. doi: 10.1016/j.joms.2005.07.010. 2005/10/26. [DOI] [PubMed] [Google Scholar]
- 47.N. M. Oral bisphosphonates and the risk for osteonecrosis of the jaw. BJMP. 2009;2:11–15. [Google Scholar]
- 48.Choi WS, Lee JI, Yoon HJ, et al. Medication-related osteonecrosis of the jaw: a preliminary retrospective study of 130 patients with multiple myeloma. Maxillofac Plast Reconstr Surg. 2017;39:1. doi: 10.1186/s40902-016-0099-4. 2017/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reid IR, Green JR, Lyles KW, et al. Zoledronate. Bone. 2020;137 doi: 10.1016/j.bone.2020.115390. 115390. [DOI] [PubMed] [Google Scholar]
- 50.Fuggle NR, Cooper C, Harvey NC, et al. Assessment of Cardiovascular Safety of Anti-Osteoporosis Drugs. Drugs. 2020;80:1537–1552. doi: 10.1007/s40265-020-01364-2. 2020/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Center JR, Lyles KW, Bliuc D. Bisphosphonates and lifespan. Bone. 2020;141 doi: 10.1016/j.bone.2020.115566. 115566. 2020/08/04. [DOI] [PubMed] [Google Scholar]
- 52.Eisman JA, Bogoch ER, Dell R, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012;27:2039–2046. doi: 10.1002/jbmr.1698. 2012/07/28. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell PJ. Best practices in secondary fracture prevention: fracture liaison services. Curr Osteoporos Rep. 2013;11:52–60. doi: 10.1007/s11914-012-0130-3. 2013/01/22. [DOI] [PubMed] [Google Scholar]
- 54.Drew S, Judge A, Cooper C, et al. Secondary prevention of fractures after hip fracture: a qualitative study of effective service delivery. Osteoporos Int. 2016;27:1719–1727. doi: 10.1007/s00198-015-3452-z. 2016/01/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akesson K, Marsh D, Mitchell PJ, et al. Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int. 2013;24:2135–2152. doi: 10.1007/s00198-013-2348-z. 2013/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Javaid MK, Kyer C, Mitchell PJ, et al. Effective secondary fracture prevention: implementation of a global benchmarking of clinical quality using the IOF Capture the Fracture® Best Practice Framework tool. Osteoporos Int. 2015;26:2573–2578. doi: 10.1007/s00198-015-3192-0. 2015/06/14. [DOI] [PubMed] [Google Scholar]
- 57.Cooper C, Atkinson EJ, O'Fallon WM, et al. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res. 1992;7:221–227. doi: 10.1002/jbmr.5650070214. 1992/02/01. [DOI] [PubMed] [Google Scholar]
- 58.Clark EM, Gould V, Morrison L, et al. Randomized controlled trial of a primary care-based screening program to identify older women with prevalent osteoporotic vertebral fractures: Cohort for Skeletal Health in Bristol and Avon (COSHIBA) J Bone Miner Res. 2012;27:664–671. doi: 10.1002/jbmr.1478. 2011/11/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clark EM, Gooberman-Hill R, Peters TJ. Using self-reports of pain and other variables to distinguish between older women with back pain due to vertebral fractures and those with back pain due to degenerative changes. Osteoporos Int. 2016;27:1459–1467. doi: 10.1007/s00198-015-3397-2. 2015/11/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Derkatch S, Kirby C, Kimelman D. Identification of Vertebral Fractures by Convolutional Neural Networks to Predict Nonvertebral and Hip Fractures: A Registry-based Cohort Study of Dual X-ray Absorptiometry. 2019;293:405–411. doi: 10.1148/radiol.2019190201. [DOI] [PubMed] [Google Scholar]
- 61.Tomita N, Cheung YY, Hassanpour S. Deep neural networks for automatic detection of osteoporotic vertebral fractures on CT scans. Comput Biol Med. 2018;98:8–15. doi: 10.1016/j.compbiomed.2018.05.011. 2018/05/15. [DOI] [PubMed] [Google Scholar]
- 62.Hawley S, Leal J, Delmestri A, et al. Anti-Osteoporosis Medication Prescriptions and Incidence of Subsequent Fracture Among Primary Hip Fracture Patients in England and Wales: An Interrupted Time-Series Analysis. J Bone Miner Res. 2016;31:2008–2015. doi: 10.1002/jbmr.2882. 2016/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi: 10.1007/s00198-014-2794-2. 2014/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lekamwasam S, Adachi JD, Agnusdei D, et al. An appendix to the 2012 IOF-ECTS guidelines for the management of glucocorticoid-induced osteoporosis. Arch Osteoporos. 2012;7:25–30. doi: 10.1007/s11657-012-0070-7. 2012/12/12. [DOI] [PubMed] [Google Scholar]
- 65.Shepstone L, Lenaghan E, Cooper C, et al. Screening in the community to reduce fractures in older women (SCOOP): a randomised controlled trial. Lancet. 2018;391:741–747. doi: 10.1016/s0140-6736(17)32640-5. 2017/12/20. [DOI] [PubMed] [Google Scholar]
- 66.Shepstone L, Fordham R, Lenaghan E, et al. A pragmatic randomised controlled trial of the effectiveness and cost-effectiveness of screening older women for the prevention of fractures: rationale, design and methods for the SCOOP study. Osteoporos Int. 2012;23:2507–2515. doi: 10.1007/s00198-011-1876-7. 2012/02/09. [DOI] [PubMed] [Google Scholar]
- 67.McCloskey E, Johansson H, Harvey NC, et al. Management of Patients With High Baseline Hip Fracture Risk by FRAX Reduces Hip Fractures-A Post Hoc Analysis of the SCOOP Study. J Bone Miner Res. 2018;33:1020–1026. doi: 10.1002/jbmr.3411. 2018/02/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner DA, Khioe RFS, Shepstone L, et al. The Cost-Effectiveness of Screening in the Community to Reduce Osteoporotic Fractures in Older Women in the UK: Economic Evaluation of the SCOOP Study. J Bone Miner Res. 2018;33:845–851. doi: 10.1002/jbmr.3381. 2018/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rubin KH, Rothmann MJ, Holmberg T, et al. Effectiveness of a two-step population-based osteoporosis screening program using FRAX: the randomized Risk-stratified Osteoporosis Strategy Evaluation (ROSE) study. Osteoporos Int. 2018;29:567–578. doi: 10.1007/s00198-017-4326-3. 2017/12/09. [DOI] [PubMed] [Google Scholar]