Abstract
Glycerol-based ether lipids including ether phospholipids form a specialized branch of lipids that in mammals require peroxisomes for their biosynthesis. They are major components of biological membranes and one particular subgroup, the plasmalogens, is widely regarded as a cellular antioxidant. Their vast potential to influence signal transduction pathways is less well known. Here, we summarize the literature showing associations with essential signaling cascades for a wide variety of ether lipids, including platelet-activating factor, alkylglycerols, ether-linked lysophosphatidic acid and plasmalogen-derived polyunsaturated fatty acids. The available experimental evidence demonstrates links to several common players like protein kinase C, peroxisome proliferator-activated receptors or mitogen-activated protein kinases. Furthermore, ether lipid levels have repeatedly been connected to some of the most abundant neurological diseases, particularly Alzheimer’s disease and more recently also neurodevelopmental disorders like autism. Thus, we critically discuss the potential role of these compounds in the etiology and pathophysiology of these diseases with an emphasis on signaling processes. Finally, we review the emerging interest in plasmalogens as treatment target in neurological diseases, assessing available data and highlighting future perspectives. Although many aspects of ether lipid involvement in cellular signaling identified in vitro still have to be confirmed in vivo, the compiled data show many intriguing properties and contributions of these lipids to health and disease that will trigger further research.
Keywords: Alzheimer’s disease, Autism, Parkinson’s disease, Peroxisome, Plasmalogen, Phospholipid, Lysophosphatidic acid, Alkylglycerol, Platelet-activating factor, Signal transduction
1. Introduction
Lipids have manifold roles in physiology and pathophysiology, most prominently by ensuring the correct structure and function of biological membranes, but also by serving as important signaling messengers and in the homeostasis of reactive oxygen species. In the present review, we cover a particular lipid subgroup, ether (phospho)lipids, which are distinguished by the presence of an ether bond at the sn-1 position of the glycerol backbone. A broad variety of ether lipid species exist (cf. Section 2), of which the plasmalogens (“plasmenyl phospholipids”) are the most abundant and probably best studied subtype. Due to their high fraction of the total phospholipid mass (around 20% in humans), some authors even use the terms “plasmalogens” and “ether lipids” interchangeably, although that is clearly an oversimplification, as we attempt to highlight in the present review. In plasmalogens, the ether bond is desaturated constituting a cis vinyl ether bond and the head group is usually ethanolamine or choline, thus leading to their designation as plasmenylethanolamine (PlsEtn) or plasmenylcholine (PlsCho). Correspondingly, ether lipids without the vinyl ether bond are often termed plasmanyl phospholipids. Plasmalogens are abundant throughout the body, in humans with the highest levels in brain and heart and lower levels in the liver (Braverman and Moser, 2012). They were originally identified as compounds that are protective against oxidative stress (Zoeller et al., 1988; Hoefler et al., 1991), particularly for polyunsaturated fatty acids (PUFAs) in their proximity (Reiss et al., 1997). However, the relevance of these anti-oxidative properties in vivo have been debated more recently (Lessig and Fuchs, 2009). Over time, the unique properties of plasmalogens for the shape, organization and structure of biomembranes were discovered and are now probably seen as their most essential feature (Koivuniemi, 2017; Jimenez-Rojo and Riezman, 2019). Overall, many different biological tasks are ascribed to ether lipids (Dorninger et al., 2017a; Dean and Lodhi, 2018), including highly versatile roles in various signaling pathways.
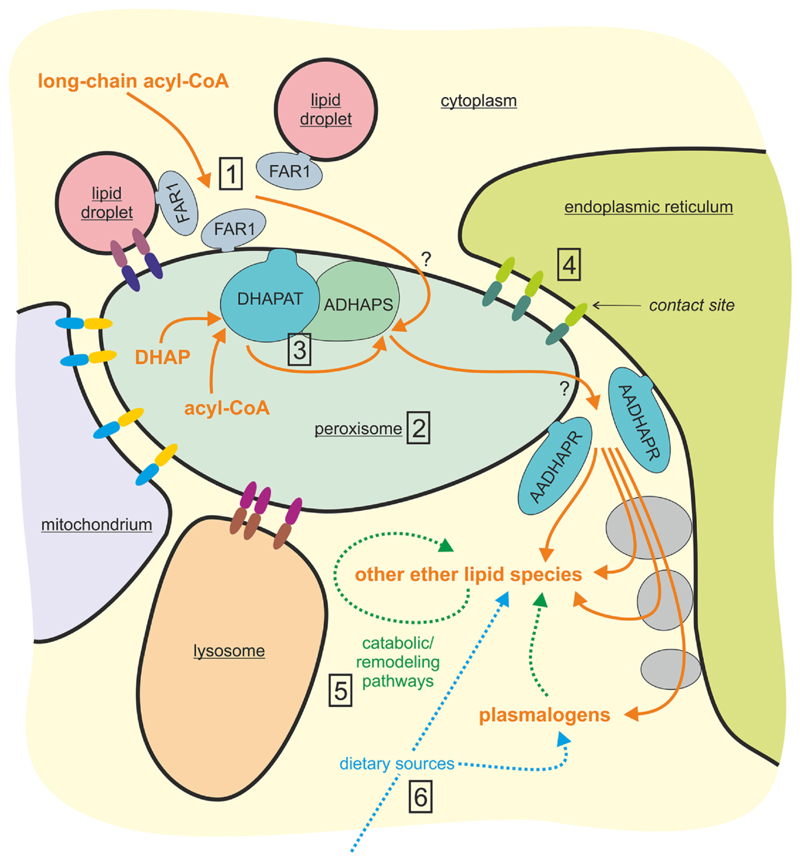
Similar to other lipid classes, the metabolism of ether lipids is complex (Fig. 1) and has been extensively reviewed previously (Watschinger and Werner, 2013). In mammals, de novo biosynthesis of these compounds originates in the peroxisome, a small organelle, which is in constant interaction with various other organelles via contact sites (Fig. 1) and which houses various anabolic as well as catabolic processes in lipid metabolism (Berger et al., 2016). Inside peroxisomes, a complex consisting of the sequentially acting enzymes dihydroxyacetone phosphate acyltransferase (DHAPAT; EC 2.3.1.42; gene name: glycerone phosphate acyltransferase, GNPAT) and alkyl-dihydroxyacetone phosphate synthase (ADHAPS; EC 2.5.1.26; gene name: alkylglycerone phosphate synthase, AGPS) generate the ether bond (Fig. 1). In fact, ADHAPS utilizes the 1-acyl-dihydroxyacetone phosphate (acyl-DHAP) intermediate produced by the DHAPAT reaction and a fatty alcohol to produce 1-alkyl-DHAP. The fatty alcohol for this reaction is provided by the peroxisomal tail-anchored protein fatty acyl-CoA reductase (FAR). Interestingly, a recent study demonstrated an alternative localization of FAR1 (EC 1.2.1.84), the major FAR subtype in ether lipid biosynthesis, to lipid droplets (Exner et al., 2019).
Fig. 1. Ether lipid levels are dynamically influenced by the cellular environment.
The crucial steps of ether lipid biosynthesis take place in peroxisomes, which are in close interaction with other organelles via various recently identified contact sites (reviewed in (Schrader et al., 2020)). Accordingly, multiple factors may influence the synthesis and levels of ether lipids in such a complex environment: The production of fatty alcohols from fatty acyl-CoA for generation of the ether bond has been proposed as rate-limiting step for ether lipid biosynthesis (Honsho et al., 2013). Accordingly, substrate availability and activity of FAR1 are essential regulators of the whole pathway (1). Owing to the localization of the enzymes carrying out the initial steps, ether lipid biosynthesis critically depends on peroxisome biogenesis and proliferation (2), processes which themselves are subject to regulation by multiple factors. Polymorphisms and mutations in biosynthetic enzymes can have a dramatic impact on ether lipid levels (3). Based on current knowledge, contact sites may be crucial for the exchange of ether lipid precursors between organelles (4). For example, tethering to the ER was shown to be required for maintenance of plasmalogen levels (Hua et al., 2017) and is influenced by cellular oxidative stress and other factors. After exiting the peroxisome by an unknown mechanism (indicated by a question mark), further enzymatic modification of the ether lipid precursor is accomplished at the ER or in the cytosol, thus producing the wide range of different ether lipid species. These are continuously exposed to remodeling (e.g. by phospholipases) or catabolic steps (5). Apart from endogenous biosynthesis, ether lipids can, to a lesser extent, also be derived from dietary sources (6).
AADHAPR, acyl/alkyl-DHAP reductase; ADHAPS, alkyl-DHAP synthase; DHAP, dihydroxyacetone phosphate; DHAPAT, DHAP acyltransferase; FAR, fatty acyl-CoA reductase.
Subsequently, the precursor compound is reduced by the enzyme acyl/alkyl-DHAP reductase (AADHAPR; EC 1.1.1.101; alternative name: peroxisomal reductase activating PPARγ, PexRAP; gene name: dehydrogenase/reductase SDR family member 7B, DHRS7B) at the outer face of the peroxisomal membrane or the ER membrane. The remaining biosynthesis steps are carried out in other subcellular compartments and differ between the different ether lipid species. For details, we may refer the reader to an excellent review (Watschinger and Werner, 2013). In the case of plasmalogens, synthesis is completed at the ER (Fig. 1). This includes the generation of the characteristic vinyl ether bond by desaturation, an enzymatic activity, which could recently be assigned to a transmembrane protein encoded by TMEM189 (Gallego-Garcia et al., 2019; Werner et al., 2020). Also the degradation of plasmalogens has been unraveled lately. It was known previously that after deacylation at the sn-2 position, the resulting lysoplasmalogen is cleaved by lysoplasmalogenase (Wu et al., 2011). However, in addition, a recent study proved that under certain conditions cytochrome c targets plasmalogens and acts as a plasmalogenase resulting in the production of a 2-acyl-lysolipid and a fatty aldehyde (Jenkins et al., 2018). The non-vinyl ether bond can be cleaved by the enzyme alkylglycerol monooxygenase (AGMO) generating glycerol and a fatty aldehyde, thus, paving the way for further metabolization (Taguchi and Armarego, 1998; Watschinger et al., 2010). Regulation of ether lipid biosynthesis and metabolism is multifaceted and can occur at many different levels (Fig. 1). Ether lipids are exposed to constant remodeling, not only within the group of ether lipids, but also serving as reservoir of fatty acids for other lipid classes, for example, cardiolipins (Kimura et al., 2018).
In humans, congenital deficiency in ether lipid biosynthesis evokes the rare, often fatal disease rhizomelic chondrodysplasia punctata (RCDP) (Berger et al., 2016). Genetically, most cases are caused by mutations in peroxin (PEX) 7 (RCDP type 1), coding for a receptor enabling the peroxisomal import of proteins, like ADHAPS, containing a peroxisome targeting signal 2 (PTS2) (Kunze, 2020). Other RCDP subtypes are assigned to mutations in GNPAT (RCDP type 2), AGPS (RCDP type 3), FAR1 (RCDP type 4) or PEX5 (RCDP type 5) affecting the long isoform of PEX5, a protein assisting in PEX7-mediated import. Clinically, the disease is characterized by skeletal dysplasia, a characteristic shortening of proximal long bones, developmental retardation, cataracts and structural abnormalities of the brain like cerebellar atrophy, enlargement of the ventricles and deficits in myelination. The disease course can be heterogeneous depending on the residual activity of the affected protein, but recent data document clearly reduced survival with about 25% of patients not reaching school age and about 50% dying prior to the age of 14 (Duker et al., 2020).
Several mouse models have been used to study the biological role of ether lipids in vivo, mostly the completely ether-lipid deficient Pex7 or Gnpat knockout (KO) mice (Brites et al., 2003; Rodemer et al., 2003) as well as hypomorphic Pex7 mice (Braverman et al., 2010). Although these models show a somewhat milder phenotype, the clinical features largely mimic those of human disease including impaired growth and survival, brain and ocular abnormalities, infertility and ossification defects (Brites et al., 2003; Rodemer et al., 2003; Dorninger et al., 2017b).
Apart from RCDP, ether lipids have been linked to an impressive number of different diseases, in which their biosynthesis is not directly affected, among them many neurological diseases (Dorninger et al., 2017a). In this review, we will highlight the multiple facets of ether lipids in signaling (Section 2), discuss their role in the etiology and pathology of neurodegenerative and neurodevelopmental disorders (Sections 3 and 4) and finally address some therapeutic approaches (Section 5).
2. Ether lipids in signaling
In the scientific literature, the discussion of ether lipids is frequently limited to plasmalogens. Undoubtedly, plasmalogens and in particular their role in membrane biology are important for the efficiency of signaling processes. However, in recent years, also a number of other, non-plasmalogen ether lipids have been associated with signaling. We therefore find it timely to highlight the multiple facets of different ether lipids, including but not restricted to plasmalogens, in this section. Apart from the major ether lipids discussed in the sections below, several novel subspecies with yet undetermined functions have recently been identified and partially characterized, like for example the first ether bond-containing bisretinoids in the human retina (Kim and Sparrow, 2018). With the current advances in lipidomic analysis techniques and given the low concentrations often needed for bioactive molecules to evoke important biological effects, it is conceivable that quite a few ether lipid species with significant bioactivity and involvement in signaling are still to be discovered.
Here, we will restrict our discussion to ether lipid species and classes that have been associated with signaling pathways in vertebrates, particularly in mammals. An even wider spectrum of lipid species and signaling functions is used by other kingdoms, especially prokaryotes and fungi, as exemplified by the occurrence of ether-linked phosphatidylglycerol in actinobacteria (Valero-Guillen et al., 2016) or the role of plasmanyl phospholipids with inositol head group as intracellular messengers in Dictyostelium (Clark et al., 2014), to name just a few. Also, we will focus on species, whose endogenous occurrence has been demonstrated. Many more ether lipids can be synthesized ex vivo and may have important biological or therapeutic properties, for example in battling cancer (Fromm et al., 1987; Jaffres et al., 2016), however, these are beyond the scope of the present review. Furthermore, we will attempt to dissect the physiological functions of the individual ether lipid (sub)classes independently. In some cases, it may appear more appropriate to treat ether lipids as a homogeneous group, for example, when considering their proposed global upregulation in cancer cells (Benjamin et al., 2013) or the activation of major signaling hubs, like peroxisome proliferator-activated receptor (PPAR) γ or protein kinase C (PKC), by several different types of ether lipids (see Sections 2.6, 2.7 and 2.9). However, on the whole, we find the species and their suggested activities so diverse (even differing between closely related ones) that a (sub)class-centered approach is justified. A simplified overview of the discussed ether lipid classes and subclasses as well as their links to key signaling pathways is provided in Table 1.
Table 1.
Ether lipids and derivatives with reported involvement in signaling and their association with key signaling components.
| Ether lipid subclass | Alternative names | Common representatives | Section | Common signaling components | ||||
|---|---|---|---|---|---|---|---|---|
| AKT/PKB | PKC | PPAR | GPCR | MAPK | ||||
| Plasmalogen (Plasmenyl phospholipids) | 1-(1Z-Alkenyl)-2-acyl-3-phosphocholine (plasmenylcholine) | 2.1 | ✔ | ✔ | ✔ | ✔ | ||
| 1-(1Z-Alkenyl)-2-acyl-3-phosphoethanolamine (plasmenylethanolamine) | 2.2 | |||||||
| Plasmalogen-derived PUFA* | AA DHA |
2.2 | ✔ | ✔ | ✔ | ✔ | ✔ | |
| 2-Halo fatty aldehydes | 2-Chloro fatty aldehyde 2-Bromo fatty aldehyde 2-Iodo fatty aldehyde |
2.2 | ✔ | ✔ | ||||
| Lysoplasmalogen | 1-(1Z-Alkenyl)-2-lyso-3-phosphocholine or –ethanolamine | 2.3 | ||||||
| pNAPE* | 1-(1Z-Alkenyl)-2-acyl-sn-glycero-3-phospho-(N-acyl)-ethanolamine | 2.4 | ✔ | ✔ | ||||
| PAF | 1-O-Hexadecyl-2-acetyl-sn-glyceryl-3-phosphocholine Acetyl-glyceryl-ether-phosphorylcholine PAF-acether |
2.5 | ✔ | ✔ | ✔ | |||
| Lyso-PAF | 1-O-Hexadecyl-2-lyso-sn-glyceryl-3-phosphocholine | 2.5 | ||||||
| Alkylglycerol | 1- O-Alkyl-sn-glycerol | Octadecylglycerol (batyl alcohol) Hexadecylglycerol (chimyl alcohol) |
2.6 | ✔ | ✔ | |||
| Alkyl-LPA | 1-O-Alkyl-sn-glycero-3-phosphate | 2.7 | ✔ | ✔ | ✔ | ✔ | ||
| Alkenyl-LPA | 1-(1Z-Alkenyl)-sn-glycero-3-phosphate | 2.7 | ✔ | ✔ | ✔ | |||
| Noladin ether | 2-O-Arachidonyl glyceryl ether 2-O-(5Z,8Z,11Z,14Z-Eicosatetraenyl)-sn-glycerol | 2.8 | ✔ | ✔ | ||||
| Ether-linked diglycerides | 1-O-Alkyl-2-acyl-sn-glycerol | 2.9 | ✔ | ✔ | ✔ | |||
| Plasmanyl phospholipids | 1-O-Alkyl-2-acyl-3-phosphocholine (plasmanylcholine) 1-O-Alkyl-2-acyl-3 phosphoethanolamine (plasmanylethanolamine) |
2.10 | ✔ | |||||
| Seminolipid | 1-O-Alkyl-2-acyl-3-[β-3’-sulfogalactosyl]-glycerol | 2.11 | ||||||
| Fecapentaene | 1-(1-Glycero)dodeca/ tetradeca-1,3,5,7,9-pentaene | Fecapentaene-12 Fecapentaene-14 |
2.12 | |||||
| GPI anchor | Glycosyl-phosphatidyl-inositol anchor | 2.13 | ✔ | ✔ | ✔ | |||
AA, arachidonic acid; AKT/PKB, protein kinase B; DHA, docosahexaenoic acid; GPCR, G protein-coupled receptor; LPA, lysophosphatidic acid; MAPK, mitogen- activated protein kinase; PAF, platelet-activating factor; PKC, protein kinase C; pNAPE, N-acyl ethanolamine plasmalogen; PPAR, peroxisome proliferator-activated receptor; PUFA, polyunsaturated fatty acid.
Involves PUFAs/pNAPEs themselves as well as their metabolites.
2.1. Signaling pathways affected by the contribution of plasmalogens to membrane composition
It is undisputed that plasmalogens (Fig. 2A) are important membrane constituents that play a crucial role in defining membrane characteristics of different subcellular compartments. The detailed biophysical properties of plasmalogens and their effect on membrane function have been the topic of excellent recent reviews (Koivuniemi, 2017; Jimenez-Rojo and Riezman, 2019) and are, therefore, not reiterated here. However, from all the available data, it is clear that changes in the availability or level of plasmalogens have fundamental effects on biomembranes, thus potentially modulating numerous cellular processes. For example, due to their importance for biophysical membrane properties affecting membrane curvature or the stabilization of non-bilayer structures, plasmalogens have long been speculated to play an important role in biological processes involving membrane fusion. We could recently show that the lack of ether lipids indeed has considerable effects on neurotransmission, which involves the fusion of synaptic vesicles in the axon terminal with the presynaptic membrane resulting in the release of neurotransmitters mediating interneuronal communication (Dorninger et al., 2019b). Also the neurotransmitter homeostasis is strikingly impaired in ether lipid-deficient mice, possibly due to altered kinetics of the synaptic vesicle cycle. Other evidence that intercellular communication deficits result from the lack of ether lipids comes from the mistargeting and/or downregulation of surface proteins required for cell-cell contact and formation of intercellular junctions. This was demonstrated in vitro to result in altered migration properties of cultured human breast cancer cells (Takahashi et al., 2019) and in vivo in arrested spermatogenesis in mice (Komljenovic et al., 2009).
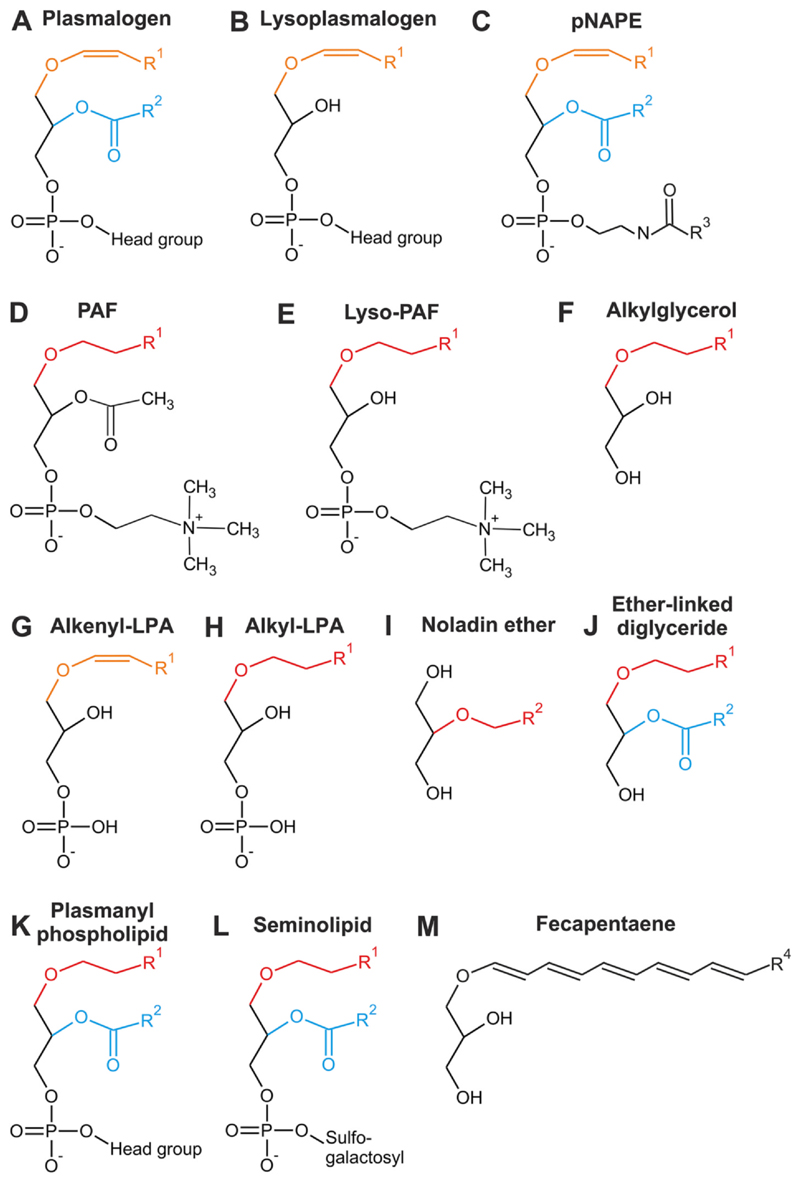
Fig. 2. Structure of ether lipids with reported involvement in signaling processes.
A prototypic plasmalogen (A), lysoplasmalogen (B), N-acyl ethanolamine plasmalogen (pNAPE; C), platelet-activating factor (PAF; D), lyso-PAF (E), alkyl- glycerol (F), alkenyl-lysophosphatidic acid (alkenyl- LPA; G), alkyl-LPA (H), noladin ether (I), ether- linked diglyceride (J), plasmanyl phospholipid (K), seminolipid (L) and fecapentaene (M) are shown with alkyl groups (ether-bonded) colored red, al-kenyl groups (vinyl ether-bonded) orange and acyl groups (ester-bonded) blue. Head groups in (A), (B) and (J) are predominantly ethanolamine or choline. R1 represents alkyl residues originating from primary alcohols synthesized by FAR (mainly C16:0, C18:1 or C18:0 but also other, less common species have been reported). R2 and R3 designate a wider range of saturated and unsaturated fatty acyl chains. In plasmalogens (A), R2 is usually a PUFA residue; in the case of noladin ether (H), R2 indicates the ether- bonded arachidonyl moiety. R4 constitutes C2H5 (fecapentaene-12) or C4H9 (fecapentaene-14).
Furthermore, PlsEtn are prominent constituents of exosomes, representing around 3% of total lipids in exosomes from human prostate cancer cells (an enrichment factor of 1.2 compared with the cells themselves) (Llorente et al., 2013) or around 5% in those isolated from human urine (Skotland et al., 2017). Exosomes are extracellular vesicles, which among other functions are proposed to mediate intercellular communication (Pegtel and Gould, 2019). Whether the presence of plasmalogens in these structures serves any particular function or mainly reflects the cellular lipid composition, has yet to be shown but it has been speculated that plasmalogens regulate fusion processes and contribute to the extracellular stability of exosomes (Skotland et al., 2019).
Moreover, plasmalogens are evidently enriched in the small membrane domains termed “membrane rafts” or “lipid rafts” (Pike et al., 2002), which by definition “compartmentalize cellular processes” (Pike, 2006) like signal transduction. It is not yet clear how a change in plasmalogen levels manifests in terms of lipid raft functionality, but an impact on signaling pathways is well conceivable. In accordance with this concept, decreased phagocytosis capacity of plasmalogen-deficient macrophages was recently described as a physiological consequence of lipid raft perturbation and its resulting effects on signal transduction (Rubio et al., 2018).
Receptors for extracellular signaling molecules are usually associated with the plasma membrane, making them susceptible to functional impairment upon any type of membrane perturbation, including alterations in plasmalogen composition. Already in the 1990s, Han and Gross pointed out that hydrolysis of plasmalogens by phospholipases necessarily causes changes in membrane properties that are bound to impact membrane-associated signaling pathways (Han and Gross, 1991). Indeed, a number of recent studies have demonstrated a dysfunction of essential signaling cascades as consequence of the lack of ether lipids. In the peripheral nervous system of Gnpat –/– mice, a model of complete ether lipid deficiency, the recruitment of AKT (protein kinase B, PKB) to the plasma membrane of Schwann cells was found to be impaired (Fig. 3A) and was proposed to elicit a series of pathological changes in downstream signaling processes culminating in a severe defect in myelination (da Silva et al., 2014). An analogous phenotype in the CNS myelin of these mice suggests a similar disturbance of AKT signaling also in oligodendrocytes (Malheiro et al., 2019). Furthermore, the neuromuscular junction of Gnpat -–/– mice shows abnormal sprouting and ramification of the phrenic nerve innervating the diaphragm, likely due to impaired signaling at the presynaptic (neuronal) or the postsynaptic (muscular) side of the junction (Dorninger et al., 2017b). Another key signal transduction pathway directly affected by decreased plasmalogen levels involves the mitogen-activated protein (MAP) kinase ERK. Downregulation of plasmalogen synthesis by injection of lentiviral shRNAs targeting the Gnpat mRNA causes reduced phosphorylation of ERK in the cerebral cortex of mice, an observation that was accompanied by a proinflammatory state of microglia in the manipulated brain region (Hossain et al., 2017). Vice versa, complementary experiments in vitro indicate that plasmalogen supplementation can modulate several essential signaling pathways including those associated with AKT, ERK, PKCδ and brain-derived neurotrophic factor (BDNF), while shifting microglia cell lines to a less proinflammatory state (Hossain et al., 2016; Ali et al., 2019; Youssef et al., 2019) and protecting neurons from apoptosis (Yamashita et al., 2015a; Che et al., 2020). However, although the number of signaling pathways reported to be altered by supplementation of plasmalogens in cell culture is impressive, the relevance of these findings in vivo largely still remains to be demonstrated.
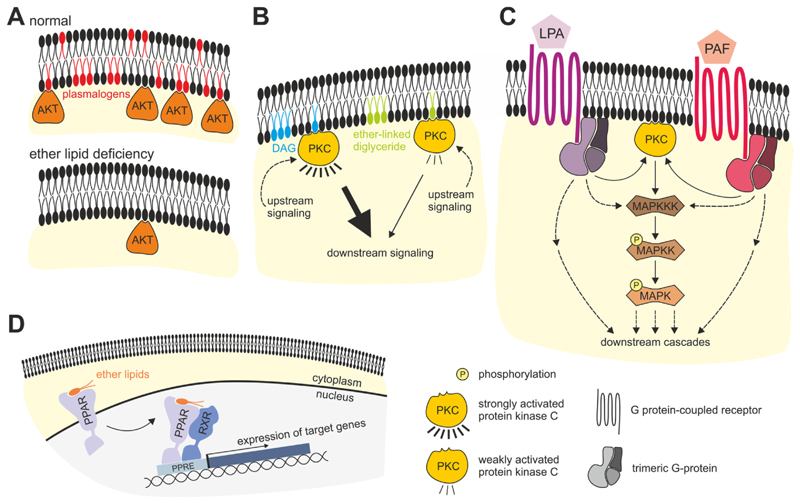
Fig. 3. Schematic overview of the versatile impact of ether lipids on signaling pathways.
(A) Plasmalogens are essential membrane constituents that modulate membrane properties. Their lack can disturb the dynamics of membrane-associated signaling processes, as demonstrated for the impaired membrane recruitment and functioning of AKT/PKB. Other pathways may be affected similarly. (B) Ether-linked diglycerides have been proposed to compete with diacylglycerol (DAG) for binding of PKC but exert a weaker (or no) activation, thus dampening downstream responses. (C) Secreted ether-linked species like alkyl-LPA, alkenyl-LPA and PAF bind G protein-coupled receptors on the cell surface; receptor binding triggers a cascade involving activation and dissociation of G proteins followed by induction of downstream pathways including, but not limited to, MAP kinase pathways. Note that LPA and PAF receptors are not necessarily expressed by the same cell type, as shown here for simplicity. (D) Ether lipids serve as ligands for nuclear receptors like PPARα or PPARγ. Upon ligand binding in the nucleus, or in the cytoplasm triggering nuclear translocation, the PPARs bind to response elements in the DNA. As heterodimers with the retinoid X receptor (RXR), PPARs associate with coactivators (not shown), thus inducing the expression of target genes. Several ether lipid ligands have been suggested, including chlorinated plasmalogens and noladin ether for PPARα as well as alkyl-LPA and plasmanyl phospholipids for PPARγ.
For image clarity, the signaling pathways are drawn highly simplified and several intermediate steps are omitted for easier understanding. MAPKK, MAP kinase kinase; MAPKKK, MAP kinase kinase kinase.
The plasmalogen status can also have more indirect effects on signaling pathways. For example, a recent study indicated that plasmalogen deficiency perturbs cholesterol homeostasis resulting in altered levels of ligands for the nuclear receptor liver X receptor (LXR) and, consequently, changes in the activation pattern of this transcription factor (Honsho et al., 2019).
To sum up this section, there is ample evidence that plasmalogens as integral membrane constituents have profound influence on a wide variety of signaling cascades, whether by affecting the membrane association of involved proteins or by influencing the properties of membranous organelles like vesicles. Nevertheless, future scientific work on this issue will still have the responsibility to translate interesting observations and possible coincidences into causal relationships. Furthermore, many results have been gained under extreme conditions like complete deficiency or strong overexposure of plasmalogens, leaving room for speculation on how smaller, more physiological alterations in plasmalogen levels would influence signaling pathways in vivo.
2.2. Plasmalogens as source of signaling mediators
Apart from accommodating signal transduction processes as a structural component of cellular membranes, plasmalogens themselves can be a source of signaling mediators released by cleavage. For instance, after oxidation of the sn-1 vinyl ether bond of plasmalogens by hypochloric acid (HOCl) produced by myeloperoxidase, the hallmark enzyme of neutrophils, plasmalogens release chlorinated lipids (Thukkani et al., 2003). These 2-chloro fatty aldehydes and fatty acids modulate inflammatory and immune processes, for example, by participating in the formation of “neutrophil extracellular traps”, thus assisting in the defense against bacterial intruders (Palladino et al., 2018b). Furthermore, they increase the permeability of endothelial cells and promote neutrophil migration by so far not elucidated molecular mechanisms (Thukkani et al., 2002; Ullen et al., 2010). The identified involvement of these oxidized lipids as ligands in signaling pathways include the activation of the nuclear receptor PPARα (Fig. 3D) (Palladino et al., 2017) and the inhibition of endothelial nitric oxide synthase (Marsche et al., 2004) and, thus, vasodilatation (Ford et al., 2016). For further information on this unusual type of lipid as well as other 2-halo fatty aldehydes derived from plasmalogens (e.g. 2-bromo fatty aldehydes or 2-iodo fatty aldehydes), we refer the reader to excellent recent reviews (Palladino et al., 2018a; Ebenezer et al., 2020).
Although the fatty acid composition of plasmalogens varies across cell types and tissues, the sn-2 position is mostly occupied by PUFAs (fatty acids with more than one double bond). This has led to the prevalent assumption that plasmalogens act as a depot for these bio-logically essential fatty acids (Horrocks, 1972; Thomas et al., 1990). However, considering that also other phospholipid classes readily store PUFAs and that PUFAs are shifted to phosphatidylethanolamines upon deficiency in plasmalogens (Dorninger et al., 2015b), lack of plasmalogens does not necessarily cause a deficit in PUFAs. This is also underlined by recent data from human macrophages demonstrating that the levels of plasmalogens do not determine the total cellular levels of arachidonic acid (AA) (Lebrero et al., 2019). Nevertheless, our own studies in both human fibroblasts and murine gray matter have indicated that plasmalogen deficiency perturbs the balance between ω-3 and ω-6 fatty acids, the two major PUFA subgroups in phospholipids, which by itself may have physiological consequences (Dorninger et al., 2015b).
Fatty acids can be cleaved off the glycerol backbone of phospholipids at the sn-2 position by phospholipases of the A2 subtype (PLA2). Remarkably, a calcium-independent and cytosolic enzyme variant, which is selective for plasmalogens, was purified and characterized from bovine brain (Hirashima et al., 1992). At that time, it was hypothesized that plasmalogen-selective PLA2 is stimulated by external signals and contributes to the generation of lipid mediators like eicosanoids, thus propagating inflammatory reactions (Yang et al., 1996). Indeed, recent data suggest that AA release from plasmalogens is an essential contributor to priming of cultured macrophages in response to the bacterial endotoxin lipopolysaccharide (Gil-de-Gomez et al., 2017). However, other studies state that rather docosahexaenoic acid (DHA), an ω-3 PUFA with mainly anti-inflammatory and anti-apoptotic properties, is preferentially released by plasmalogen-selective PLA2 (Ong et al., 2010) leaving the particular biological role of this specific PLA2 variant still unresolved. Adding a further piece to the puzzle, a recent study using KO mice (iPLA2γ –/–) implied that PlsEtn, together with phosphatidylglycerol, are the preferred substrates of phospholipases of the calcium-independent VIB group mainly releasing AA for further metabolization (Yoda et al., 2014).
DHA and AA are the main representatives of the groups of ω-3 and ω-6 fatty acids, respectively. These PUFAs are aggressively promoted as nutritional supplements but also widely studied in numerous scientific contexts covering essential biological processes like brain development, memory, (chronic) inflammation or apoptosis as well as the pathogenesis of countless diseases. AA, being a precursor of the mostly pro-inflammatory prostaglandins, leukotrienes, lipoxins, epoxyeicosatrienoic acids or thromboxanes, is commonly depicted as the “evil” counterpart to DHA, which is converted to the anti-inflammatory and (neuro)protective resolvins, maresins and protectins. In reality, an adequate balance between the two types of fatty acids may be desirable. In view of the wealth of literature on the topic, we may refer the reader to recent reviews for further details on the physiological roles and interplay of different PUFAs (Bazinet and Laye, 2014; Calder, 2015; Serhan et al., 2015; Innes and Calder, 2018; Serhan and Levy, 2018). It should also be noted that the presence of PUFAs at the sn-2 position of plasmalogens (and also other phospholipids) is not only essential for the production of signaling mediators, but PUFAs also contribute significantly to the fine-tuning of membrane composition and properties (for DHA reviewed in (Hishikawa et al., 2017)).
In summary, it is undisputed that PUFAs are vital compounds with a broad spectrum of crucial functions and that they are frequently encountered at the sn-2 position of plasmalogens. However, with the current knowledge, it cannot be assessed if, apart from shaping membrane characteristics, the storage of PUFAs in plasmalogens per se is important and has any advantage over the storage of PUFAs in other phospholipids like phosphatidylethanolamine. In this context, the discovery of a plasmalogen-specific PLA2 is interesting and could hint at facilitated mobilization of PUFAs as a benefit from their accumulation in plasmalogens. However, no additional data on this topic have emerged in the last decade and further information is required to really elucidate the role of plasmalogens in PUFA metabolism.
2.3. Lysoplasmalogens
Lysoplasmalogens (1-Alkenyl-2-lyso-3-phosphocholines or –ethanolamines; Fig. 2B) serve an important role as both precursor and metabolite of plasmalogens, and frequently they are regarded just as degradation product of plasmalogens or as acceptor for transacylation in the synthesis or remodeling of plasmalogens. However, also the lysoplasmalogens themselves have bioactivity. They are presented to semi-invariant natural killer T (iNKT) cells as self-antigens, assisting in the maturation and stimulation of these immune cells in the thymus (Facciotti et al., 2012). The use of lysoplasmalogens has, among others, enabled the identification of T cell receptor sequences essential for auto-stimulation of iNKT cells (Chamoto et al., 2016). Furthermore, choline lysoplasmalogens have been suggested to activate cAMP-dependent protein kinase (PKA) (Williams and Ford, 1997) and, thus, directly contribute to signal transduction, which may be the mechanism behind their reported effect on neutrophil adherence to human endothelial cells (White et al., 2007).
Lysoplasmalogens can also be indirectly involved in signaling, as an auxiliary molecule in lipid remodeling generating PAF by accepting fatty acids released from alkyl-acyl-glycerophosphocholine, thus supporting the formation of lyso-PAF and, subsequently, PAF (Uemura et al., 1991). Finally, studies in rabbit renal cells have indicated an inhibitory effect of lysoplasmalogens, similar to other lysolipids, on Na+-K+-ATPase (Schonefeld et al., 1996). Such a mechanism could underlie the observation that choline lysoplasmalogen induces a strong depolarization of myocytes thus potentially disturbing cardiac rhythmicity (Caldwell and Baumgarten, 1998).
2.4. N-acylated ethanolamine plasmalogens
N-acylated ethanolamine phospholipids are formed by transfer of an acyl group to the free amine group of phosphatidylethanolamine and PlsEtn, thus generating N-acyl phosphatidylethanolamine (NAPE) and N-acyl ethanolamine plasmalogen (pNAPE; Fig. 2C), respectively. They are found in prokaryotes as well as in diverse eukaryotic kingdoms, however, in mammalian tissues levels are usually very low in the range of a few nmol/g tissue, with the highest levels in the brain (Schmid et al., 1990; Wellner et al., 2013). The major role of N-acylated ethanolamine phospholipids lies in serving as a precursor for the group of N-acylethanolamine species, which are produced via the action of a specific phospholipase D (NAPE-phospholipase D) (Schmid et al., 1983; Okamoto et al., 2004). Among these biologically active compounds are for example palmitoylethanolamide, a PPARα agonist with anti-inflammatory activity; oleoylethanolamide, a proposed regulator of energy metabolism (Bowen et al., 2017), which binds to receptors of the G protein-coupled receptor and transient receptor potential families as well as PPARα; and arachidonoyl-ethanolamide, better known as anandamide, one of the main endocannabinoids in the mammalian nervous system. More recent research suggests that also the N-acylated phosphatidylethanolamines themselves have biological functions like the stabilization of membranes or the regulation of food intake, thus serving as a kind of lipid hormone (Wellner et al., 2013).
At the moment, it is still enigmatic if pNAPEs serve any specific functions different from those of NAPEs or if their generation is simply a consequence of the availability of precursor phospholipids (i.e. PlsEtn). It has, however, been specifically shown that pNAPE is a substrate for NAPE-phospholipase D leading to the generation of N-acylethanolamines like anandamide (Schmid et al., 1983; Tsuboi et al., 2011). Also, an alternative pathway exists, involving deacylation to lyso-pNAPE by the serine hydrolase ABHD4 and subsequent action of a phospholipase D (Lee et al., 2015). Remarkably, adding complexity to the relation between ether lipids and N-acylated phospholipids, it was found that overexpression of phospholipase A/acyltransferase (PLAAT)-3 (also termed H-Ras-like suppressor, HRASLS, 3), one of the enzymes generating N-acyl ethanolamine phospholipids, causes impairment of peroxisomal functions, including ether lipid biosynthesis, in HEK293 cells (Uyama et al., 2012). The physiological relevance of this observation and the underlying molecular mechanism are not yet unraveled, but appear to involve a general downregulation of the peroxisome number via binding of PEX19 (Uyama et al., 2015).
2.5. Platelet-activating factor (PAF) and its metabolites
Platelet-activating factor (PAF; 1-O-Alkyl-2-acetyl-sn-glycero-3-phosphocholine; Fig. 2D) is a potent and short-lived signaling molecule that is able to act in intercellular communication at very low concentrations (10–14 M). Structurally, it contains an ether-bonded sn-1 alkyl chain, an acetyl group at sn-2 and a choline head group. PAF has gained major attention as a versatile inflammatory mediator produced by different immune cell types, in particular neutrophils, eosinophils and macrophages but also endothelial cells and platelets (Triggiani et al., 1991). Biosynthesis of PAF is often induced by an exogenous trigger, for example oxidative stress, and can occur either by substitution of the acyl residue of an alkylacyl phospholipid for an acetyl residue in the “remodeling pathway” or de novo by transfer of a phosphocholine group to alkylacetylglycerol. While synthesis via remodeling is of major importance in the response to inflammatory or allergic stimuli and requires activation of the PAF-synthesizing cell, the de novo pathway is mainly responsible for constitutive generation of basal PAF levels (Venable et al., 1993). Of note, also alkylacetylglycerol has been implicated in signaling processes; specifically, it was shown to promote differentiation of cultured leukemia cells towards a macrophage-like phenotype (McNamara et al., 1984) and, presumably after being phosphorylated, to inhibit PKC, thus limiting platelet aggregation and granule secretion in models for thrombosis (Holly et al., 2019).
From a physiological point of view, PAF has been ascribed roles in a plethora of important processes, like wound healing, angiogenesis, apoptosis, where ambivalent effects have been described (Southall et al., 2001; Hostettler et al., 2002), and inflammation. PAF signaling is initiated by binding to the PAF receptor, a G protein-coupled seven transmembrane receptor found on the surface of several key cell types of the hemostatic and the immune system (Honda et al., 1991; Stafforini et al., 2003). The binding of PAF to its receptor can activate various types of G proteins, thereby triggering classical downstream signal transduction cascades involving MAP kinase pathways, PKA activation, GTPase activity or intracellular calcium mobilization (Fig. 3C) (Brown et al., 2006). Depending on the cell type, PAF receptor activation finally results in – among others – facilitation of leukocyte binding by upregulation of surface molecules, the release of proinflammatory mediators like cytokines, or migration and proliferation of endothelial cells (Stafforini et al., 2003; Yost et al., 2010). Just recently, a study in alkylglycerol-fed mice demonstrated that PAF secreted by adipose tissue macrophages acts in an autocrine manner to stimulate the production of interleukin-6, which in turn induces the differentiation of adipocytes into beige fat via the JAK/STAT3 pathway (Yu et al., 2019).
By the action of particular PLA2 enzymes named PAF-acetylhydrolases, of which several subtypes exist (McIntyre et al., 2009; Kono and Arai, 2019), PAF is hydrolyzed to lyso-PAF (Fig. 2E), which is thought to be biologically inactive (Marathe et al., 2001) and quickly metabolized (Snyder, 1994) either by further degradation or by reacetylation back to PAF. Interestingly, AGMO, an enzyme mainly associated with the degradation of alkylglycerols (cf. Section 2.6), has been suggested to cleave the O-alkyl bond of lyso-PAF, producing an aldehyde and glycerophosphocholine, and thus be an important player in the regulation of PAF levels (Tokuoka et al., 2013). The importance of PAF for the mammalian immune system is emphasized by the fact that mice with a genetic deficiency in the PAF receptor exhibit im-munological hyporesponsiveness to a variety of stimuli, like allergens or viral infections (Ishii et al., 1998; Souza et al., 2009). On the other hand, PAF receptor antagonists might help to dampen acute inflammatory responses or assist in the treatment of neuropathic pain (Tsuda et al., 2011).
The action of PAF is not restricted to inflammatory reactions. Instead, PAF activity has also been linked to the accurate structure and function of the CNS. More precisely, PAF has been ascribed a role in long-term potentiation (LTP), a process involving synaptic plasticity, which is essential for memory formation. In hippocampal slices, PAF secreted from postsynaptic neurons acts in a retrograde fashion to increase presynaptic neurotransmitter release, thus inducing or strengthening LTP (Wieraszko et al., 1993; Kato et al., 1994; Bazan, 2003). These findings are supported by in vivo experiments in rats evidencing that memory-related behavior is improved by the infusion of a PAF analog into the hippocampus, the amygdala or the entorhinal cortex (Izquierdo et al., 1995). This phenomenon appears to be restricted to a concentration window, as higher (unphysiological) concentrations of PAF have been associated with an inhibitory effect on LTP (Reiner et al., 2016). In addition to a role in LTP, PAF has been implicated in neuronal migration (Bix and Clark, 1998) and, consequently, in brain development. This is supported by the observation of neuronal layering abnormalities in some brain regions of mice with defects in PAF-acetylhydrolase or the PAF receptor (Hirotsune et al., 1998; Tokuoka et al., 2003). Collectively, these findings emphasize the broad spectrum of physiological functions engaging PAF in different tissues.
2.6. Alkylglycerols and the role of alkylglycerol monooxygenase (AGMO)
Alkylglycerols (1-O-Alkylglycerols) are compounds of simple bio-chemical structure (Fig. 2F), which can be enzymatically converted into a wide range of different ether lipid species. In particular, they are frequently used in supplementation strategies to provide plasmalogens to biological systems in vitro and in vivo. Like other ether lipids, alkylglycerols have prominent roles in the regulation of the immune system. Treatment of healthy obese humans has been reported to downregulate inflammatory markers in the blood (Parri et al., 2016), but the mechanism behind this observation remains elusive. On the other hand, alkylglycerols have been linked to immune activation and stimulation in several studies. They have the potential to promote proliferation and maturation of T and B lymphocytes in vitro (Qian et al., 2014), possibly via conversion to PAF. A recent paper showed convincingly that alkylglycerols, being present in the micromolar range, are prominent constituents of mammalian breast milk and are converted to PAF by adipose tissue macrophages in the beige fat of the pups to prevent overproduction of white adipose tissue (Yu et al., 2019). Similar me-chanisms may also be responsible for the observation that feeding rat dams alkylglycerol induces the production of granulocytes and immunoglobulins in the pups (Oh and Jadhav, 1994). Based on these findings, it is tempting to speculate that alkylglycerols represent an inactive precursor, which can be transported at higher concentrations to allow the generation of sufficient PAF locally, at sites where it is actually required. In human breast milk, alkylglycerols are present in an excess of several orders of magnitude compared with PAF (Akisu et al., 1998; Yu et al., 2019), presumably due to the presence of plasma PAFacetylhydrolases (group VII PLA2) secreted by macrophages (Furukawa et al., 1993).
However, other studies have claimed bioactivity of alkylglycerols themselves. For example, alkylglycerols were found to bind and inhibit purified PKC in vitro (McNeely et al., 1989; Warne et al., 1995). Correspondingly, reduced PKC activity in confluent cultured Madin-Darby canine kidney cells was accompanied by accumulation of alkylglycerols, particularly those with a C18:0 alkyl chain, and PKC inhibition was presumed to be causative for restricted growth in this cell population (Warne et al., 1995). Another group found that an alkylglycerol mix purified from shark liver oil and containing species of various chain lengths induces calcium influx in cultured human lymphocytes (Pedrono et al., 2004a) and prevents tumor propagation in mice, supposedly by inhibiting angiogenesis (Pedrono et al., 2004b). However, as alkylglycerols are readily remodeled to other ether lipid species in biological systems (Dorninger et al., 2015b), it is unclear, exactly which compound mediates these effects. Additional evidence for a direct involvement of alkylglycerols in signaling processes comes from studies in adipocytes, where these ether lipids accumulate upon differentiation and act as regulators of adipogenesis (Homan et al., 2011). As judged by the activation of downstream genes, this effect seems to operate via PPARγ but does not involve direct binding of this nuclear receptor. Interestingly, impaired PPARγ expression and adipogenesis in fibroblasts with defective peroxisome biogenesis could be rescued by supplementation with alkylglycerols (Hofer et al., 2017).
The metabolism of alkylglycerols is strongly influenced by the activity of AGMO, the only enzyme known to date capable of cleaving the ether bond of alkylglycerols (Taguchi and Armarego, 1998; Watschinger et al., 2010). Notably, based on studies in Xenopus tropicalis, a recent report claims a crucial role of AGMO in developmental biology. Specifically, genetic downregulation of AGMO leads to scrambled left-right patterning of embryos, presumably due to a perturbation of Wnt-dependent signaling cascades (Duncan et al., 2019). The importance of AGMO in this process evidently relies on its ability to cleave the ether bond. Accordingly, AGMO was also suggested as the gene causing congenital heterotaxy syndrome in a patient harboring a larger deletion on chromosome 7 (Fakhro et al., 2011; Duncan et al., 2019). Also in the context of diabetes, a genetic link was observed between a single nucleotide polymorphism close to the AGMO gene and high fasting glucose as well as type 2 diabetes (Dupuis et al., 2010). It remains to be seen, though, if indeed an involvement of ether lipids in these disorders holds true.
2.7. Alkyl-LPA and alkenyl-LPA
Lysophosphatidic acid (LPA; 1-acyl-sn-glycero-3-phosphate) is a widely studied, crucial phospholipid mediator with a small negatively charged head group. It is present in a broad variety of tissues and exerts its effect via binding to different G protein-coupled LPA receptors (LPA1–6). LPA has been implicated in numerous crucial processes including development of the mammalian brain (Yung et al., 2015), reproduction (Ye and Chun, 2010) and cancer cell metastasis (Willier et al., 2013) to name just a few. Notably, also bioactive ether lipid variants exist like alkenyl-LPA (1-alkenyl-sn-glycero-3-phosphate; Fig. 2G), which stimulates MAP kinases in vitro in mouse fibroblasts (Liliom et al., 1998). Complementing experiments showed that treatment of ovarian cancer cells with alkenyl-LPA leads to phosphorylation of several key signaling proteins including the MAP kinase ERK and AKT (PKB) (Lu et al., 2002). Originally, alkenyl-LPA was hypothesized to exhibit subtype selectivity (Liliom et al., 1998). Later, its binding was demonstrated for the LPA1, LPA2 and LPA4 receptors, albeit with lower affinity than its acyl counterpart (Bandoh et al., 2000; Noguchi et al., 2003).
More experimental data is available concerning the bioactivity of alkyl-LPA (1-alkyl-sn-glycero-3-phosphate; Fig. 2H), which appears to be present in substantial amounts in vivo, as suggested by studies in rat brain (Sugiura et al., 1999) and human atherosclerotic plaque tissue (Rother et al., 2003), where alkyl-LPA makes up approximately one tenth (≈ 0.4 nmol/g wet weight) and one fifth (≈ 5 nmol/g wet weight), respectively, of total LPA levels. Like lysoplasmalogen, alkyl-LPA can act as self-antigen to stimulate iNKT cells (Facciotti et al., 2012). Similar to alkenyl-LPA, also alkyl-LPA binds to several LPA receptors (Fig. 3C); however, the reported potency differs between studies or experimental systems. While one study using different human, murine and insect cell lines indicated that alkyl-LPA and acyl-LPA bind to the LPA receptors of the endothelial differentiation gene (EDG) family (LPA1–3) with similar potency (Xu et al., 2004), others principally confirm the binding ability, but found alkyl-LPA to be a consistently weaker ligand for the three receptors compared with the acyl analog, when the receptors were heterologously expressed in insect (Bandoh et al., 2000) or rat hepatoma cells (Khandoga et al., 2008). Among the more recently identified non-EDG family receptors (LPA4–6), which are phylogenetically related to the PAF receptor (Noguchi et al., 2003), alkyl-LPA is a particularly strong agonist for LPA5 (Khandoga et al., 2008).
Already in the 1980s, alkyl-LPA was reported to be much more potent than its acyl analog for the induction of platelet aggregation (Simon et al., 1982; Tokumura et al., 1987). More recently, this effect was credited to signaling activity via the LPA5 receptor, which is an especially efficient target of alkyl-LPA (Williams et al., 2009). Furthermore, a proinflammatory response and activation of human mast cells and murine microglia can be triggered via the same receptor by alkyl-LPA in vitro (Kozian et al., 2016). Similar to alkenyl-LPAs, also alkyl-LPA species have been shown to activate signaling pathways promoting proliferation and increasing migration of ovarian cancer cells (Lu et al., 2002). This is in good accordance with later findings showing that suppressing ether lipid synthesis considerably impedes invasiveness and migratory properties of human cancer cell lines and that alkyl-LPA is the major determinant for these observations (Benjamin et al., 2013).
Another line of experiments addressing the bioactivity of alkyl-LPA (C18:1) suggests that it exerts its effects both via cell surface receptors and as a ligand of the intracellular receptor and transcription factor PPARγ (McIntyre et al., 2003; Zhang et al., 2004; Tsukahara et al., 2006). In macrophages, PPARγ engagement induced CD36 gene expression resulting in lipid accumulation (McIntyre et al., 2003). Follow-up investigations showed that alkyl-LPA interaction with PPARγ can also stimulate glucose uptake in muscle cells and promote oxidative stress in microglial cells via upregulation of CD36 in vitro (Tsukahara et al., 2013; Tsukahara, 2020).
To sum up, ether-linked LPA species may not be the primary species responsible for the main physiological actions of LPA. However, based on their specific properties and binding kinetics, compared with acyl-LPA they could constitute a biochemically more stable option under certain conditions (Lu et al., 2002) allowing increased flexibility in the fine-tuning of physiological responses.
2.8. Noladin ether
Noladin ether (arachidonyl glyceryl ether; Fig. 2I) is another example of a non-plasmalogen ether lipid with postulated signaling function. Originally isolated from porcine brain as an endocannabinoid binding to the G protein-coupled CB1 receptor, it was later also detected in the rat brain (particularly hippocampus and thalamus) and shown to be taken up and metabolized by a glioma cell line (Fezza et al., 2002). Its specific binding to the CB1 receptor has also been confirmed in human neocortical tissue, and an agonistic effect was hypothesized due to its binding kinetics and downstream effects (Steffens et al., 2005). However, other authors have later refuted the existence of noladin ether in mammals in vivo (Oka et al., 2003; Richardson et al., 2007) and the question, whether noladin ether is indeed an endogenous compound or its detection in animal tissues was an experimental artifact, is still an open issue. Also, species differences in the endogenous presence of this compound cannot be ruled out. Furthermore, given that noladin ether carries the ether bond at the sn-2 and not at the sn-1 position, where DHAPAT and ADHAPS are known to act, it is also questionable whether the peroxisomal ether lipid biosynthesis pathway is responsible for the generation of noladin ether. On the other hand, no other route is known for de novo biosynthesis of ether lipids in vertebrates.
Irrespective of the discourse on the occurrence of noladin ether in mammals, its biological activity has been repeatedly demonstrated in vitro and in vivo. Physiological consequences of its application in vivo include increased food intake, hypothermia, sedation, reduced defecation after i.p. injection in mice and rats (Hanus et al., 2001; Avraham et al., 2005; Jones and Kirkham, 2012) and reduction of intraocular pressure after ocular administration in rabbits (Laine et al., 2002). All these actions have been ascribed to the binding of noladin ether to the CB1 receptor and downstream effects on neurotransmission. Additionally, noladin ether has been shown to bind to other receptors of the cannabinoid system, namely the CB2 receptor (Shoemaker et al., 2005), with impact on the opioid system as well (Paldyova et al., 2008), and GPR55 (Ryberg et al., 2007), a putative cannabinoid receptor that has also been shown to be targeted by lysophosphatidylinositol and lysophosphatidylglucoside (Oka et al., 2007; Guy et al., 2015).
Beyond the cannabinoid system, noladin ether has been reported to bind to the ligand-binding domain of murine PPARα and activate downstream transcriptional activity in vitro (Fig. 3D) (Sun et al., 2007). Furthermore, one report claims an antiproliferative effect on carcinoma cell lines by preventing nuclear translocation of NF-κB and reducing the protein levels of essential cyclins (Nithipatikom et al., 2011). However, the exact mechanism, which apparently is independent of the cannabinoid system and PPARγ, of such an effect remains unclear. Yet an-other investigation, using rat mesenteric arterial bed preparations ex vivo, suggested that exposure to noladin ether activates a non-specified signaling mechanism, not involving the cannabinoid system, to induce vasorelaxation via reduced sensory neurotransmission (Duncan et al., 2004).
Noladin ether, thus, represents an unconventional (due to its ether bond at the sn-2 position) ether lipid with considerable potency in various signaling pathways. However, whether it is indeed of relevance in vivo remains a riddle to be solved. Most data on noladin ether were gathered in the post-millennium decade just before the recent advances in lipid methodology, which may now be sensitive enough to detect the putative endogenous compound.
2.9. Ether-linked diglycerides
The ether-linked counterparts (Fig. 2J) of the widely studied second messenger diacylglycerol (DAG) are the ether-linked diglycerides, also termed alkylacylglycerols or alkyl-diglycerides. A major physiological function of DAG is the binding and activation of PKC, a key signaling regulator, of which several subclasses and isoforms exist: the classical (or conventional) isoforms requiring DAG and calcium for activation; the novel isoforms needing solely DAG but not calcium for activation; and the atypical isoforms depending neither on DAG nor on calcium (Webb et al., 2000). Also ether-linked diglycerides reportedly bind to PKC. However, their activation of classical, calcium-dependent PKC isoforms apparently requires particularly high concentrations of calcium (Ford et al., 1989), which may only be present in strongly stimulated cells in vivo. This is in line with earlier studies finding no PKC activation upon exposure to these compounds and concluding that the ester bond at sn-1 is a requirement for PKC activation under physiological conditions (Cabot and Jaken, 1984; Ganong et al., 1986; Heymans et al., 1987). Later, it was suggested that ether-linked diglycerides are specifically generated in response to activation of the interleukin-1 receptor and act as a competitive inhibitor of the DAG-binding site of PKC, most effectively the novel, calcium-independent isoforms δ and ε, thus preventing its activation and DAG downstream signaling (Fig. 3B) (Musial et al., 1995; Mandal et al., 1997). This view is also supported by in vitro experiments in human polymorphonuclear leukocytes, whose activation by DAG was inhibited by alkylacylglycerol (Bass et al., 1988). A more recent study using smooth muscle cells indicated that the inhibitory action of 1-alkyl-diglyceride prevents the downstream activation of the MAP kinase ERK resulting in stalled cell proliferation and migration (Houck et al., 2008). A similar mechanism but involving additional players has been proposed for the regulation of the PI3K/ AKT pathway by alkyl-containing diglycerides (Houck et al., 2008).
Ether-linked diglycerides, thus, like many other ether lipid species, appear to modulate signaling activities, in this case by dampening the DAG-induced PKC response, while also being able to activate PKC under specific circumstances like high calcium levels.
Similar properties as for alkyl-diglycerides have been proposed also for vinyl ether (alkenyl) diglycerides (Musial et al., 1995). However, it must be noted that both ether and vinyl ether-bonded diglyceride species are rare, at levels close to the detection limit of current meth-odology in murine brain and heart tissue (Yang et al., 2015).
Furthermore, ether bonds have been identified also in triacylglycerol. Such ether-linked neutral lipids are enriched in lipid droplets, where they can make up 10%–20% of all neutral lipids, and likely serve storage functions but, so far, without any identified role in signaling (Bartz et al., 2007). Yet, they possibly serve as a reservoir for etherlinked signaling molecules like diglycerides, which can easily be generated by a triacylglycerol lipase.
2.10. Plasmanyl phospholipids
Strictly speaking, the term “plasmanyl phospholipids” also includes PAF and alkyl-LPA. However, here, for the purpose of distinguishing between the different subclasses, we refer to plasmanyl phospholipids as those phospholipids, which contain an alkyl group at sn-1, an acyl chain at sn-2 and a polar head group like ethanolamine or choline (1-O-alkyl-2-acyl-3-phosphocholine or –ethanolamine; Fig. 2K). Plasmanyl phospholipids are similar in structure to plasmalogens, but contain a non-vinyl ether bond instead of the vinyl ether at the sn-1 position of the glycerol backbone. In an oxidatively modified state, they constitute potent ligands for the nuclear receptor PPARγ in vitro, and it was suggested that these oxidized ether lipids mediate some of the effects of oxidized LDL particles, like the maintenance of an inflammatory state during atherosclerotic plaque formation (Davies et al., 2001). Later studies indicated binding of (non-oxidized) plasmanylcholine lipids to PPARγ (Fig. 3D) and the agonistic action of one particular species was proposed to be crucial for adipogenesis (Lodhi et al., 2012).
Plasmanylcholine species are also enriched in some immune cell types, especially in neutrophils, where they account for almost half of all choline-containing phospholipids and are presumed to serve as precursors for the production of PAF (Mueller et al., 1982; Mueller et al., 1984). A more contemporary study suggested a crucial role of the plasmanylcholine lipids themselves in the maturation and development of neutrophils (Lodhi et al., 2015). However, the neutropenia hypothesized to result from the lack of these lipids, based on experiments in mice lacking AADHAPR (PexRAP), an enzyme involved in ether lipid biosynthesis (cf. Section 1; Fig. 1), is not reproduced in Gnpat KO mice with complete ether lipid deficiency (Dorninger et al., 2015a).
Plasmanyl phospholipids are present in various forms in different tissues – even species with inositol or serine head groups have been detected in trace amounts in human macrophage cell lines (Ivanova et al., 2010) – but their biological role is still largely unassigned.
2.11. Seminolipid and other ether-linked sulfoglycolipids
Seminolipid, a trivial name for 1-O-alkyl-2-acyl-3-[β-3′-sulfogalactosyl]-glycerol (Fig. 2L), represents the most prominent sulfoglycolipid in mammals (Ishizuka et al., 1973) accounting for up to 90% of total testicular glycolipids (i.e. 25–160 nmol/mg tissue in humans de-pending on age) (Ueno et al., 1977). As suggested by the name, it is almost exclusively found in the testes, where it is particularly enriched in the plasma membrane of spermatozoa (Ishizuka, 1997). In mice, seminolipid deficiency, caused by genetic disruption of the essential biosynthetic enzymes, leads to arrested spermatogenesis and, consequently, infertility in males (Fujimoto et al., 2000; Honke et al., 2002). The latter feature is shared by Gnpat KO mice (Rodemer et al., 2003) and it is tempting to assume that the lack of seminolipid causes the infertility in these animals, although here all ether lipid species are missing and also dysfunctional tight junctions of the blood-testis barrier may play a role (Komljenovic et al., 2009). The detailed molecular mechanisms underlying infertility in seminolipid-deficient mice and whether seminolipid is involved in signaling processes during spermatogenesis are not yet fully established; hypotheses range from a function of seminolipid in cell–cell-interaction and intercellular communication to the potential importance of this lipid for lactate transport and energy generation in male germ cells (Honke et al., 2002; Honke, 2013; Luddi et al., 2017).
Other sulfated glycoglycerolipids containing an ether bond have been discovered in the brain of adult rats and their levels decreased with age (Ishizuka and Inomata, 1979). A role of these lipids in myelination was hypothesized, but more detailed follow-up studies have never been reported.
2.12. Fecapentaenes
Fecapentaenes are glycerol-based (non-vinyl) ether lipids containing a polyunsaturated alkyl chain at the sn-1 position (Fig. 2M). They are mainly found in the digestive tract, where they have been detected in several mammalian species, but are not inherently produced. Instead, these compounds are generated by colonic bacteria (Lederman et al., 1980; Van Tassel et al., 1982) and were, accordingly, originally isolated from and characterized in human feces (Hirai et al., 1982; Gupta et al., 1983). Two species, namely fecapentaene-12 and fecapentaene-14, differing in the length of the alkyl chain, are the most prominent. In humans, fecal fecapentaene content varies considerably between individuals (Baptista et al., 1984), which is likely a result of diverse dietary habits (de Kok et al., 1992). Since their discovery, fecapentaenes have been connected to the development of colon cancer. Their genotoxic and mutagenic potential has been shown in vitro in bacterial and mammalian – including human – cells (Plummer et al., 1986; Curren et al., 1987) as well as in vivo in mice and rats, where they drive tumorigenesis when applied exogenously (Weisburger et al., 1990; Zarkovic et al., 1993). The mechanism underlying these properties likely involves oxidative damage to DNA by radicals produced from fecapentaenes (Szekely and Gates, 2006), a process, which may be mediated by the enzyme prostaglandin H synthase (Plummer et al., 1995). In spite of these remarkable findings, research progress on the in vivo actions of fecapentaenes has stalled in recent years and, thus, their actual role in cancer development in humans remains unresolved.
2.13. The ether lipid component in GPI anchors
A series of papers have shown that the glycosyl-phosphatidyl-inositol (GPI) anchor of many membrane-associated proteins contains an alkyl-acyl moiety, which is generated by the peroxisomal ether lipid biosynthesis pathway (Houjou et al., 2007; Kanzawa et al., 2009; Kanzawa et al., 2012). GPI-anchored proteins can make up a considerable portion (up to 0.5%) of the total protein content in eukaryotes. Many of these, like the ephrinA group of proteins specifically acting in the nervous system, are critically involved in signaling. However, whether the alkyl chain is of any significance or can be replaced by an acyl residue without a functional consequence, remains enigmatic to date. Ether lipid-deficient human fibroblasts derived from RCDP patients show strongly increased surface levels of the diacyl variant of the GPI-anchored protein urokinase-type plasminogen activator as compared with control fibroblasts (Kanzawa et al., 2012), but the biological impact of this observation is unclear. Accordingly, further studies in ether lipid-deficient cells and animal models will be required to assess the physiological consequences of a lack of ether-linked GPI anchors. As we have recently elaborated on this topic more extensively elsewhere and no related, additional data have been published since then, we may refer the reader to our previous work (Dorninger et al., 2017a).
3. Ether lipids in neurodegenerative and neurodevelopmental disorders – current evidence and reported alterations
Ether lipids, particularly the plasmalogens with reported amounts of up to 30 mol% of total phospholipids are abundant in the nervous system, where they contribute to the organization of neuronal membranes and the myelin sheath. Thus, it is not surprising that ether lipids have in various ways been implicated in a variety of neurologic diseases. We have previously given an overview over the current state of knowledge in this field (Dorninger et al., 2017a) and, here, want to exclusively focus on neurodegenerative and neurodevelopmental diseases. Abnormal levels of ether lipids, usually plasmalogens, have been described in several pathologic conditions involving neurodegeneration. For example, a recent publication revealed that mutations in the enzyme ethanolamine phosphotransferase (selenoprotein 1), required for the biosynthesis of ethanolamine phospholipids, causes a syndrome characterized among others by neurodegeneration, which was largely ascribed to the deficit in plasmalogens (Horibata et al., 2018). However, ether lipids have gained most attention based on their proposed involvement in the two frequent neurodegenerative disorders Alzheimer’s disease (AD) and Parkinson’s disease (PD). Here, we first review the available evidence of ether lipid involvement in these diseases and in neurodevelopmental disorders, which have more recently been linked to ether lipids (Sections 3.1–3.3). Subsequently we discuss the role ether lipids may play in the etiology of these disorders (Section 4), particularly in light of the signaling involvement of ether lipids as discussed in Section 2. Finally, we will address recent research developments addressing the potential use of ether lipids as therapeutic agents targeting neurodegeneration (Section 5).
3.1. Alzheimer’s disease and other types of dementia
AD constitutes the most common neurodegenerative disease worldwide and accounts for the major fraction of the 40–50 million individuals (Nichols et al., 2019) currently suffering from dementia. Because high age is a major risk factor and increased aging is a feature particularly of the Western societies, AD prevalence is predicted to continuously rise in the foreseeable future. Structurally, AD is characterized by neuronal damage and the histopathological hallmarks of β-amyloid (Aβ) deposition in extracellular plaques and intracellular ac-cumulation of abnormally phosphorylated tau protein.
Already more than twenty years ago, reports indicated a deficit of PlsEtn in the brain of patients with sporadic AD (Ginsberg et al., 1995; Guan et al., 1999). Han and collaborators were among the first to substantiate these observations by demonstrating that the deficiency is detectable at early stages of the disease in the white matter (Han et al., 2001). Further work showed that in gray matter, albeit less pronounced than in white matter, PlsEtn loss progresses in parallel to cognitive decline (Han, 2005). Concordantly, PlsEtn levels were found to be the lowest in brain tissue with the most pronounced neuropathology according to Braak staging (Kou et al., 2011). Depending on the brain region and exact plasmalogen species, the reported reductions mostly lie between 15% and 40%. Contrasting all these findings, a few reports have described increased amounts of PlsEtn in AD brains (Pettegrew et al., 2001) or of ether-linked ethanolamine phospholipid species in (a low number of) familial AD cases (Villamil-Ortiz et al., 2018). The origin of these discrepancies is unclear but the selection of AD cohorts and/or control samples may play a role. Despite the contrasting reports, the case for a plasmalogen deficit in AD is strong and further emphasized by studies showing that not only PlsEtn, but also PlsCho is affected (Grimm et al., 2011a; Igarashi et al., 2011) and by the analyses of brain lipids in various mouse models of AD (Han et al., 2001; Fabelo et al., 2012; Tajima et al., 2013; Uruno et al., 2020). A detailed overview of the extent of plasmalogen alterations at the species level in published studies involving human AD patients was presented in a recent review (Fontaine et al., 2020).
In the wake of the hunt for peripheral biomarkers for the early detection or prognosis of AD, plasmalogen deficiency was also identified in the circulation of AD patients (Goodenowe et al., 2007; Yamashita et al., 2015b). Such findings were utilized to argue for plasmalogens, particularly PlsEtn species containing PUFAs (Song et al., 2018) as diagnostic or prognostic biomarkers, either individually or as part of a panel consisting of several lipid species (Mapstone et al., 2014), with the latter appearing to be the more promising strategy. In addition, recent studies applying multivariate regression models pin-pointed serum levels of PlsEtn and ether-linked choline phospholipids as factors associated with impaired cognitive function (Toledo et al., 2017; Goodenowe and Senanayake, 2019). Remarkably, whereas low PlsEtn levels were linked to dementia (Goodenowe and Senanayake, 2019), pathologically high serum levels of Aβ peptides were found to go along with increased ether-containing choline species (Toledo et al., 2017). The latter results are in good agreement with our recent longitudinal study on choline phospholipid levels, which indicated that PlsCho levels in plasma increase with age in healthy controls and more strikingly in individuals converting to AD (Dorninger et al., 2018).
Next to plasmalogens, also PAF, due to its proposed role in neuroinflammation (Stafforini et al., 2003), has been the subject of several studies in the context of AD. One of the first studies on this issue found increased binding of PAF to platelets derived from patients with AD or multi-infarct dementia, and PAF binding was associated with the extent of cognitive impairment (Hershkowitz and Adunsky, 1996). In a more recent investigation, a two-and threefold accumulation of C16:0 PAF and lyso-PAF, respectively, was detected in brain specimens of patients with AD and in a transgenic AD mouse model compared with non-transgenic controls (Ryan et al., 2009). Of note, also lyso-PAF levels in plasma were found to increase strongly (1.5–2-fold compared with baseline) upon development of AD (Dorninger et al., 2018). These findings may be explained by the increased activity of PAF-acetylhydrolase in the plasma of AD patients that was shown to correlate with cognitive decline (Bacchetti et al., 2015).
Apart from AD, ether lipids have also been analyzed in the context of other types of dementia. For example, in post mortem brain tissue from patients diagnosed with Lewy body dementia, reduced levels of plasmalogens, particularly the C18:0 subtype (-50% compared with controls), were detected in lipid rafts isolated from frontal cortex (Marin et al., 2017). Similarly, a change in the species composition of plasmalogens – with higher levels of several PlsCho species and decreases in polyunsaturated PlsEtn species in favor of more highly saturated ones – were found in gray matter from temporal cortex of patients with mixed (AD and vascular) dementia (Lam et al., 2014).
3.2. Parkinson’s disease
Lipid abnormalities may be one of several factors participating in the pathophysiological process of PD (Hallett et al., 2019), a disease characterized by the degeneration of dopaminergic neurons and aggregations of the protein a-synuclein (Lewy bodies) that manifests in motor impairments like tremor, stiffness and imbalance. Although not as excessively as in AD, also in PD research have alterations in plasmalogen levels been a topic in recent years. Upon investigation of lipid raft fractions from cortical gray matter, the levels of C18:0 and C18:1 plasmalogens, as indicated by dimethylacetals after acidic hydrolysis, as well as PUFAs were found reduced in samples derived from classic (particularly C18:0 subspecies, approximately -50%) or incidental (particularly C18:1 subspecies, approximately -60%) PD (Fabelo et al., 2011). Of note, however, an earlier study could not detect altered amounts of PlsEtn in patient samples from the substantia nigra, the major site of neurodegeneration in PD (Ginsberg et al., 1995).
Also less marked than in AD, decreased plasmalogen-derived C16:0 dimethylacetal levels (approximately -20% compared with controls; no other subspecies were measured) were identified in the plasma of PD patients (Dragonas et al., 2009). Adding further evidence, a just published report found the levels of ether lipids with ethanolamine head group to be reduced by about 30% in plasma and erythrocytes of PD patients, although with a relatively low number of samples (Mawatari et al., 2020). A series of papers have also investigated plasmalogens in the context of mouse models of PD. Treatment of mice with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which is widely used to provoke symptoms mimicking human PD, goes along with lower plasmalogen levels in the serum and plasma (Miville-Godbout et al., 2016; Miville-Godbout et al., 2017; Nadeau et al., 2019). Strongly reduced levels of some choline ether lipids were described in a lipidomic study using another PD mouse model, however, this observation was not specific for ether lipids but appears to generally affect phospholipids with a choline head group (Farmer et al., 2015).
3.3. Neurodevelopmental disorders
In several recent publications, we have speculated about an association between neurodevelopmental disorders, particularly autism spectrum disorders (ASD) and attention-deficit hyperactivity disorder (ADHD), and ether lipids (Dorninger et al., 2017a; Dorninger et al., 2019a; Dorninger et al., 2019b). Indeed, there is growing evidence for an involvement of ether lipids in the pathophysiology of these diseases. First, several cases of RCDP, the disease caused by inherited ether lipid deficiency, have been reported to show features of ASD or ADHD (Moser, 1999; Yu et al., 2013). Conversely, plasmalogen levels were found to be statistically significantly reduced by about 15% in the blood of autistic patients (Bell et al., 2004; Wiest et al., 2009). Furthermore, a genetic association between ether lipid biosynthesis and neurodevelopmental disorders has been revealed by two independent studies applying whole-exome sequencing (Yu et al., 2013) and single nucleotide polymorphism analysis (Ro et al., 2012). Recently, we could additionally strengthen the putative link by a series of behavioral analyses of a mouse model of ether lipid deficiency, indicating marked hyperactivity and signs of autistic behavior like stereotypy, impaired social interaction and highly abnormal marble burying behavior in Gnpat KO mice (Dorninger et al., 2019a; Dorninger et al., 2019b).
The relationship between ether lipids and neurodevelopmental disorders appears to be quite complex. Not only has the deficiency of ether lipids been linked to these diseases; a recent study in rats suggests that also elevated levels of PlsEtn in the prefrontal cortex, resulting from maternal food restriction in early pregnancy, leads to hyper-activity in the young adult offspring compared with pups from dams fed a control diet (Hino et al., 2019). Furthermore, there is increasing experimental support for the hypothesis that disruption of AGMO can cause neurodevelopmental disorders. AGMO has been listed as one of the genes, in which de novo mutations were identified in autistic patients (Sebat et al., 2007; Awadalla et al., 2010). Two subsequent reports have corroborated this association by describing cases of complex human neurodevelopmental disorders, which are presumably caused by null mutations in AGMO (Alrayes et al., 2016; Okur et al., 2019).
Interestingly, apart from conventional ether lipids, a relationship to neurodevelopmental disorders has also been established for noladin ether (cf. Section 2.8). In mice, intraperitoneal application of noladin ether leads to increased locomotor activity, which could be reversed by concomitant application of a CB1 cannabinoid receptor antagonist (Avraham et al., 2005).
4. Ether lipid alterations in disease – causative, modulatory or bystander damage?
Due to the large compensatory potential and continuous lipid remodeling, we have previously expressed some skepticism about the biological relevance of changes in individual lipid species in human diseases, for which no connection to the disease process can be estab-lished (Dorninger et al., 2017a). However, the pronounced deficit in plasmalogen levels in AD has now been confirmed by many groups and is clearly a characteristic of AD. Whether it is a general feature of neurodegenerative diseases, though, may be debated. Whereas some authors explicitly claim that depletion of plasmalogens is specific for AD and not a feature of other neurodegenerative disease like PD or Huntington’s disease (Ginsberg et al., 1995), others have recently postulated a general association between reduced plasmalogen levels and neurodegeneration (Senanayake and Goodenowe, 2019). The observations of reduced plasmalogen levels sporadically also in PD and in the “normal” brain as a consequence of aging (Rouser and Yamamoto, 1968), another process involving neurodegeneration (Rossini et al., 2007), may indeed favor the latter hypothesis. However, of all neuro-degenerative diseases, the involvement of plasmalogens is most prominent and consistently detected in AD.
Often the discussion on various aspects of ether lipids in AD pathophysiology and neurodegeneration, as well as a potential involvement of ether lipid signaling, is centered around one question: are ether lipids in general affected or is this phenomenon specific for plasmalogens? Several hypotheses on the contribution of ether lipids to AD pathophysiology have been expressed: A generalized dysfunction of peroxisomes was hypothesized based on biochemical and histological findings in post mortem brain tissue from AD patients in a study involving our own group (Kou et al., 2011). In that study, next to reduced plasmalogen levels, also other metabolic changes, for example the accumulation of very long-chain fatty acids, which are degraded in peroxisomes, indicated a compromised peroxisomal function in AD patients. Remarkably, biochemical changes as well as peroxisomal volume density in neuronal somata showed a stronger association with neurofibrillary tangles than with neuritic plaques (Kou et al., 2011).
Others postulated that decreased expression of AGPS, due to disturbed processing of the amyloid precursor protein (Grimm et al., 2011b), or of GNPAT, as a consequence of inflammatory signaling via NF-κB and c-Myc (Hossain et al., 2017), accounts for the decrease in plasmalogen levels in AD. All these theories build on the assumption that ether lipid biosynthesis is disturbed, thus affecting all ether lipid species similarly. For ether lipids other than plasmalogens, though, information on the levels in neurodegenerative diseases is sparse, presumably due to their low abundance. Interestingly, in aging mice, reduced plasmalogen levels in various tissues were accompanied by an increase in the amounts of ether-linked diglycerides (Ando et al., 2019), suggesting that there is no general ether lipid deficiency in these animals. In fact, several concepts have been proposed to explain a selective degradation of plasmalogens upon neurodegeneration. Plasmalogens are preferentially degraded under conditions of oxidative stress (Felde and Spiteller, 1995; Zoeller et al., 1999), which is considered as a key component of AD etiology (Butterfield and Halliwell, 2019). Another hypothesis proposes that plasmalogen-selective phospholipase A2 is stimulated by Aβ (Farooqui, 2010) or by accumulating ceramide (Latorre et al., 2003), thus leading to enhanced enzymatic cleavage of plasmalogens. Many researchers have simply attributed plasmalogen loss to membrane breakdown in the course of neurodegeneration, referring to altered levels in the blood to support this idea. Furthermore, a recent study interpreted increased serum choline ether lipid levels in subjects with AD and pathologically high Aβ levels in the CSF as sign of early neurodegeneration (Toledo et al., 2017), thus adding further facets to the puzzling findings involving plasmalogens in neurodegenerative diseases.
Because the quest for the origin of the plasmalogen deficit in AD (and neurodegeneration in general) is tightly connected to a potential role in the pathophysiology, also considerations concerning the consequences of the proposed lipid alterations heavily rely on whether all ether lipids or only plasmalogens are affected. Actually, there is a striking variety of ways, in which plasmalogen deficiency could modulate or propagate disease pathology in neurodegenerative disease. Suggestions in previous literature range from an amplification of oxidative reactions (Ullen et al., 2010), an altered critical temperature for membrane breakdown increasing susceptibility for neurodegeneration (Ginsberg et al., 1998) or a dysfunction of cellular membranes including impaired function of lipid rafts (Diaz et al., 2018) to the increased generation of lysoplasmalogens finally resulting in synaptic failure (Bennett et al., 2013). However, here, we mainly want to focus on the potential modulation or alteration of signaling processes resulting from plasmalogen deficiency. Dysregulation of fundamental signaling cascades, as described for the ERK and AKT pathways upon ether lipid deficiency (da Silva et al., 2014; Hossain et al., 2017), can have multiple devastating consequences and, thus, may also aggravate pathology in neurodegenerative diseases. Increased activity of the kinase GSK-3β has been implicated as a downstream effect of impaired AKT activity in Schwann cells surrounding peripheral nerves of ether lipid-deficient mice (da Silva et al., 2014). Based on the similarity of the phenotype, i.e. demyelination and hampered myelin formation (Malheiro et al., 2019), a comparable defect also prevails in oligodendrocytes of the CNS. In the context of AD, this could be particularly intriguing, as both GSK-3β and ERK are linked to phosphorylation of the tau protein (Pei et al., 2003; Rankin et al., 2007). Given that hyperphosphorylation of tau causes formation of neurofibrillary tangles, one of the pathological hallmarks of AD brains, these kinases could represent a plausible link between plasmalogen deficiency and AD etiology. Also the second main feature of AD, extracellular Aβ plaques, has been associated with plasmalogens. In membranes isolated from neuronal cells, plasmalogens stimulated the activity of a-secretase (Rothhaar et al., 2012), which counteracts the formation of toxic Aβ peptides by cleaving the precursor protein at a non-pathogenic site. Conversely, a deficit in plasmalogens may, therefore, lead to reduced a-secretase activity, thus enabling the excessive generation of Aβ. Furthermore, several recent reports suggest that plasmalogens have the capacity to enhance BDNF signaling and restrict neuroinflammation (Ali et al., 2019; Youssef et al., 2019; Che et al., 2020). This provides yet another potential mechanism by which a deficit in plasmalogens may amplify disease burden, as both loss of neuroprotective BDNF and neuroinflammation have been connected to neurodegenerative disease (Lu et al., 2013; Heneka et al., 2015). Lastly, also PUFAs have often been discussed in the context of plasmalogens and AD, due to the presumed function of plasmalogens as a storage reservoir for PUFAs and the potential role of a DHA deficit in AD (Bazinet and Laye, 2014). Indeed, there is a subtle reduction of DHA levels in the brain of ether lipid-deficient (Gnpat –/–) mice (Rodemer et al., 2003; Dorninger et al., 2015b). However, given that the DHA deficit is only minor upon complete plasmalogen deficiency and that the reduction of plasmalogens in AD is only partial, we consider a major impact of the alteration in DHA levels contributed by plasmalogen deficiency on the disease state unlikely.
The notion, that also other ether lipid species than plasmalogens are affected in neurodegeneration, leaves even more room for speculation on the contribution of ether lipid signaling in disease mechanisms. In Section 2, we documented that a number of low-abundant ether lipid species have the potential to modulate several crucial signaling pathways that have been associated with neurodegenerative diseases. For instance, the activation of PPARγ has been suggested to alleviate neuroinflammatory processes, thus counteracting Aβ deposition (Heneka et al., 2011). Considering that several ether lipid species, including alkylglycerols and plasmanyl phospholipids, have been proposed as PPARγ ligands, their deficit may lead to an amplification of disease burden.
Likewise, a modulation of PKC activity, as for example reported for alkylgylcerols or ether-linked diglycerides, could contribute to the augmentation of neurodegenerative diseases like AD. However, in view of the facts that ether lipids are regarded as PKC antagonists and that AD is associated with decreased rather than increased PKC activity (Masliah et al., 1991; Wang et al., 1994), it seems questionable that reduced ether lipid levels would have a substantial impact on disease development via PKC. Also LPA signaling has been mentioned in the context of AD, particularly in mediating boosted Aβ generation (Shi et al., 2013). Consequently, it could be speculated that a reduced contribution of ether-linked LPA species to LPA downstream signaling might induce disease progression. Interestingly, cannabinoid signaling involving the CB1 receptor, like that evoked by noladin ether, has been shown to mitigate Aβ-mediated toxicity in cultured cells via the activation of MAP kinase pathways (Milton, 2002). Also in PD, signaling through CB1 is extensively discussed due to the key role of this pathway in movement control (Brotchie, 2003). However, considering the still open debate on the in vivo occurrence of noladin ether, an involvement in the etiology of neurodegenerative disease currently seems far-fetched.
Apart from plasmalogens, most evidence for a role of ether lipids in the pathophysiology of neurodegenerative diseases has been gathered for PAF. In contrast to plasmalogens, the mechanism, by which PAF may influence disease burden, appears clearer at a first glance. PAF is a mediator of inflammatory responses also in the nervous system, and immune mechanisms including neuroinflammation have been implicated as important disease drivers in AD (Heneka et al., 2015). Furthermore, PAF can promote apoptosis of neurons (Stafforini et al., 2003; Ryan et al., 2007). Accordingly, interference with PAF-associated signaling restricts neuronal damage induced by Aβ treatment in cultured cells (Bate et al., 2007; Ryan et al., 2009; Simmons et al., 2014). Thus, based on current knowledge, PAF presumably does not serve as a trigger in neurodegenerative disorders but may well be one of many driving factors in disease progression and propagation.
Much less than for neurodegenerative diseases is known about the mechanisms linking ether lipids to neurodevelopmental disorders like autism and ADHD. In a recent study, we could prove that hyperactivity and restricted social interaction of ether lipid-deficient mice are ac-companied by systematically altered neurotransmitter levels in the brain (Dorninger et al., 2019b). Based on previous literature (Oades et al., 2005; Swanson et al., 2007), we hypothesized that a general dysregulation of neurotransmission, particularly in the monoaminergic systems, evokes the behavioral phenotype of these animals. According to the current state of knowledge, neurodevelopmental disorders are the product of complex interactions of various genetic and environmental factors (Thapar et al., 2005; Rossignol et al., 2014; Bourgeron, 2015). Thus, it is considerable that also other factors than impaired neurotransmission contribute to the abnormal behavioral features upon ether lipid deficiency. An interesting candidate in this respect is (etherlinked) LPA. LPA signaling has been associated with many of the molecular and neurotransmitter pathways implicated in neuropsychiatric diseases (Yung et al., 2015). Moreover, in mice with a deletion in the Lpar1 gene, encoding one of the receptors (LPA1) cited as binding partner for alkenyl-LPA and alkyl-LPA, behavioral abnormalities are paired with dysregulated neurotransmitter levels in different brain regions (Harrison et al., 2003). It is, thus, conceivable that also a lack of alkenyl-and alkyl-LPA impacts downstream pathways that could influence disease development. Also AKT and MAP kinase signaling are indispensable for nervous system development (Samuels et al., 2009; Wang et al., 2017), and both signaling cascades have been linked to the etiology of autism (Kitagishi et al., 2015; Vithayathil et al., 2018), representing another mechanism by which ether lipid could act as disease modulators (Fig. 3). Finally, as has to be expected based on the overall physiological importance of this pathway, the endocannabinoid system has been assigned a key regulatory role in pathways involved in autism spectrum disorders (Brigida et al., 2017). Yet, whether noladin ether can indeed be classified as endocannabinoid and, thus, potentially has an impact on these diseases remains to be established. Furthermore, even though pNAPE species have been identified as some of the precursors of anandamide in mouse brain and cultured cells (cf. Section 2.4) (Tsuboi et al., 2011; Uyama et al., 2013), there is currently no evidence showing that the levels of endocannabinoids like anandamide are affected by a deficiency in ether lipids.
We are certainly aware that the role of most non-plasmalogen ether lipids in signaling in the context of neurodegeneration remains speculative, until the exact involvement of these species in the various signaling pathways has been better elucidated (cf. Section 2). Never-theless, one should bear in mind that a dysfunction of ether lipid biosynthesis, as proposed repeatedly in the context of neurodegenerative diseases, does not only impact plasmalogens, but also many other phospholipid mediators with the potential to influence disease pathology. Therefore, for all the discussed pathways, extensive future studies will be first required to clarify, how individual signaling cascades are impacted by ether lipid levels. Only then can the postulated relevance for disease-specific pathways and a role of ether lipids in pathophysiology be reasonably evaluated. In spite of these considerations, the manifold involvement of ether lipids in crucial signaling events provides interesting research perspectives and may reveal some surprising connections in the future.
5. Ether lipids as treatment target in neurodegenerative disease
To date, there is no cure for individuals affected by ether lipid deficiency. In particular, no strategy has been developed to successfully tackle deficits in the brain, probably due to an inability to overcome the blood-brain barrier and/or to postnatally correct the lipid composition of certain brain cell types like myelin-forming oligodendrocytes, which presumably contribute the largest fraction of ether lipids in the brain. With the emerging hypotheses about a potential role of plasmalogen loss in neurodegenerative diseases, and despite the current lack of evidence for the causative involvement of plasmalogen deficiency in any of these diseases, therapeutic options targeting brain ether lipids have gained remarkable interest (Paul et al., 2019). However, when discussing treatment strategies involving oral intake of ether lipids to increase their brain levels, three major questions need consideration: (1) How can ether lipids or their precursors be delivered to the brain? (2) Which amounts and subspecies of ether lipids must be replenished to obtain functional effects in nervous tissue? (3) If ether lipid replacement indeed has an appreciable impact on cognitive abnormalities like those seen in AD, what could be the underlying mechanism?
None of these questions have yet been satisfactorily answered. Particularly, to our knowledge, no replacement of plasmalogens in the brain has been documented by peripheral application so far. Nevertheless, several recent publications claim an improvement of nervous system-related readout parameters in patients with neurodegenerative diseases or animal models thereof. Most strikingly, a trial with orally administered plasmalogens purified from scallops reported cognitive improvements in a subgroup of AD patients after multiple stratification of the trial cohort (Fujino et al., 2017). Similarly, a recent publication describes improvements of clinical symptoms in a small group of PD patients after oral intake of an ether lipid mixture (Mawatari et al., 2020). In rats infused with Aβ into the ventricles, oral supplementation with plasmalogens led to enhanced performance in behavioral tests assessing cognition together with correction of different markers of oxidative stress, neuroinflammation and neuropathology (Yamashita et al., 2017; Che et al., 2018b), which was ascribed to the increased availability of, in particular, DHA-containing species (Yamashita et al., 2017). Also in AD mouse models, several reports indicate improvements in memory-related tests after oral plasmalogen ingestion accompanied by reduced neuroinflammation (Ifuku et al., 2012; Che et al., 2018a; Hossain et al., 2018). In the context of animal models of PD, application of a plasmalogen precursor compound (“PPI-1011”, a glycerol-based lipid containing a C16:0 alkyl group at sn-1, DHA at sn-2 and a lipoic acyl group at sn-3) was described to ameliorate side effects of L-DOPA treatment in MPTP-injected monkeys (Gregoire et al., 2015). In addition, the same substance as well as a related one (“PPI-1025”, containing a C18:0 alkyl group at sn-1 and oleic acid at sn-2) reportedly has positive effects on markers of dopaminergic neurons in MPTP-treated mice (Miville-Godbout et al., 2016; Miville-Godbout et al., 2017). Remarkably, also the hyperactive behavior of an ether lipid-deficient mouse model was just recently demonstrated to be reversed by a novel plasmalogen-like compound (Fallatah et al., 2020).
On the other hand, all of the mentioned studies fail to demonstrate a meaningful increase in the levels of brain plasmalogens, which leaves some uncertainty as to the mechanism behind the clinical findings. This is in line with earlier systematic investigations of the tissue distribution upon food supplementation with alkylglycerols showing that these are readily converted to plasmalogens in the periphery, while the levels of plasmalogens in the nervous system of ether lipid-deficient mice are only minimally increased (Brites et al., 2011).
Certainly, the lack of an established mechanism of action does not preclude the use of therapeutic compounds with a proven clinical benefit in patients. However, particularly in a disease causing as large a burden as posed by AD, the growing commercial interest in purified plasmalogens cannot be entirely ignored when evaluating the scientific literature, as these compounds are now in some countries marketed as dietary supplements promising a prevention or amelioration of AD. In this context, we feel some concern warranted that in some of the related publications the authors might not have adequately declared all conflicts of interest, thus potentially obscuring a bias. Furthermore, the list of compounds suggested as food supplements to protect from cognitive impairment is lengthy, but as demonstrated by a recent meta-analysis, scientific evidence for a clinically relevant effect is largely insufficient (Butler et al., 2018).
Alternatively, there may be plasmalogen-independent ways, through which ether lipid supplementation could beneficially impact neurodegeneration. However, this consideration applies only to studies using precursor compounds with a simple ether, not a vinyl ether bond, as no enzyme is known to convert vinyl ether lipids into alkyl ether lipids. Non-plasmalogen ether lipids, particularly such with ethanolamine head groups, have been detected in the brains of several species including mouse, rat and human (Horrocks, 1972). Given that many of the pathways alluded to in Section 2 require only minimal amounts of (ether) lipids for activation, the delivery of traces of these compounds to the brain could be sufficient for a therapeutic effect. Yet, until proven that the CNS is reached after peripheral administration, for example, in experiments using radioactively labeled precursors or ether lipid-deficient animals, also this hypothesis remains speculative. Finally, several papers have shown a positive impact of plasmalogens on CNS cells like neurons and microglia in vitro, particularly by reducing the expression and activation of proteins and pathways propagating inflammation (Sejimo et al., 2018; Ali et al., 2019; Youssef et al., 2019). However, to exert such anti-inflammatory action in vivo, plasmalogens have to reach the brain in the first place.
Overall, there is still insufficient evidence to judge if indeed plasmalogens, or ether lipids in general, provide a worthwhile treatment target in neurodegenerative or neurodevelopmental diseases. Many questions remain concerning delivery of these molecules to the brain and their beneficial effect in disease development or pathology.
6. Concluding remarks
So far, in the discussion of ether lipids, their involvement in various signaling pathways has hardly gained more than a passing remark in the scientific literature compared with their more extensively studied functions in the defense against oxidative stress and the organization of membrane biology. When signaling is mentioned in the context of ether lipids, the debate is usually restricted to DHA and AA and the pro- and anti-inflammatory mediators derived from these PUFAs. While these physiological tasks are certainly important, we have demonstrated in Section 2 that ether lipids have a tremendous potential to modulate signaling activities in a variety of ways. Specifically, this overview reveals that: (i) a large number of different ether lipid species have been associated with functions in signaling; (ii) diverse pathways have been implicated in signaling involving ether lipids; (iii) the mode of action of ether lipids in signal transduction is highly versatile (Fig. 3). For example, ether lipids are able to bind to intracellular receptors and proteins like PPARγ and PKC or may be released and target extracellular receptors like the PAF or LPA receptors. Furthermore, they can alter membrane properties thus potentially affecting numerous membrane-associated signaling activities. Some pathways like those involving PPARs, PKC or MAP kinases have been linked to several different ether lipids, whereas others may be unique for individual species like the activation of PAF receptor by PAF or of cannabinoid receptors by noladin ether.
Still, many – or even most – aspects of the signaling involvement of ether lipids remain unclear. For instance, can ether lipid deficiency be compensated by other lipid species or do certain functions rely exclusively on ether lipids? Are the ether lipid species with documented bioactivity merely by-products of other reactions, or are they generated in a targeted manner? If the latter holds true, the triggers for the production and action of ether lipids in signaling pathways as well as the cell types in which ether lipids play a major role, still need to be clarified in most cases. Often, the in vivo significance of ether lipid involvement has not yet been elucidated. For some species, the initial reports of their activity in certain pathways date back decades, without any follow-up surfacing, thus prompting questions about the physiological relevance.
The data available to date indicate that in many of the signaling pathways alluded to here, ether lipids play a modulatory rather than a major regulatory role. The relatively low potency of many species suggests a function in fine-tuning of responses possibly preventing ex-cessive activation by other, more potent ligands. Interestingly, the converse situation is true for PAF, itself a highly powerful mediator, with a much less potent acyl-analogue ascribed modulatory activity (Chaithra et al., 2018). Future analyses with state-of-the-art methodology will undoubtedly allow the generation of additional quantitative data on the levels of less abundant ether lipid classes, thus facilitating interpretation and evaluation of their role in vivo.
The above considerations make evident that much is still in the dark, when it comes to the versatile biological functions of ether lipids. Obviously, it is even more difficult to evaluate the consequences of deficits in these compounds in complex disorders, as discussed for plasmalogens in neurodegenerative or neurodevelopmental disorders. Many observations are still anecdotal, indeed making it difficult to put the puzzle together from the pieces gathered so far and untangle the potential contributions of ether lipids to neurodegeneration. Currently, the most robust fact is the deficit of PlsEtn in the brain and blood of cognitively impaired patients. More advanced analytic technologies should soon enable us to determine, whether and to which extent also the levels of other ether lipids are affected, which would constitute a major step forward in understanding their role in the disease process. As elaborated in Section 4, a general reduction of ether lipid levels, including less abundant species involved in different signaling pathways, may well aggravate the damage in neurodegeneration. In such a pathological scenario, given the complex web of interactions of different signaling pathways, also minor deficits could evoke chain reactions, whose consequences are currently unpredictable.
With regard to the proposed use of plasmalogens as therapeutic approach in neurodegenerative diseases, we are, presently, still not convinced of the rationale, as neither a pathogenic role of reduced plasmalogen levels in AD nor the delivery of exogenously supplied plasmalogens to the brain has been demonstrated. It is undoubted that strategies enabling the transport of ether lipids to the brain are urgently required, not only for neurodegenerative disorders but even more for individuals affected by inborn ether lipid deficiency. We are aware that substantial increases in plasmalogens in nervous tissue may be masked by low turnover in myelin although reaching other cell types like neurons. It is, however, desirable for a therapeutic strategy targeting plasmalogens to achieve a noticeable increase in the level of brain plasmalogens. In this respect, the strategies applied so far, do not appear particularly promising. A newly suggested approach involves the supplementation with shorter-chain alkylglycerols (myristyl alcohol or tetradecanol), which appear to be more readily integrated into the membranes of myelinating cells (Malheiro et al., 2019), resulting in remarkable phenotypical improvements in myelination in an ether lipid-deficient mouse model. Hence, it will be of interest to learn whether diminished plasmalogen levels can be raised efficiently with these substances.
Overall, ether lipids still bear many mysteries ranging from their various biological tasks, as demonstrated here by their involvement in multiple signaling pathways, to their role in different diseases. Time will tell, whether their use in successful therapeutic strategies against neurodegenerative disorders will emerge as another revelation in the research on this intriguing group of phospholipids.
Acknowledgements
The authors thank Markus Kunze and Christoph Wiesinger for helpful discussions.
Funding
This work was supported by the Austrian Science Fund (FWF, P24843-B24, P31082-B21 and I2738-B26), the ERA-Net for Research Programmes on Rare Diseases (E-Rare; project acronym “PERescue”) and RhizoKids International. The funders were not involved in writing the article or the decision to submit for publication.
Abbreviations
- Aβ
β-amyloid
- AA
arachidonic acid
- AD
Alzheimer’s disease
- ADHAPS/AGPS
alkyl-dihydroxyacetone phosphate/alkylglycerone phosphate synthase
- ADHD
attention-deficit hyperactivity disorder
- AGMO
alkylglycerol monooxygenase
- ASD
autism spectrum disorders
- BDNF
brain-derived neurotrophic factor
- DAG
diacylglycerol
- DHA
docosahexaenoic acid
- FAR
fatty acyl-CoA reductase
- GNPAT/DHAPAT
glycerone phosphate/dihydroxyacetone phosphate O- acyltransferase
- KO
knockout
- LPA
lysophosphatidic acid
- LTP
long-term potentiation
- MAP
mitogen-activated protein
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetra- hydropyridine
- NAPE
N-acyl phosphatidylethanolamine
- PD
Parkinon’s disease
- PEX
peroxin
- PKC
protein kinase C
- PLA
phospholipase A
- PlsEtn
plasmenylethanolamine
- PlsCho
plasmenylcholine
- pNAPE
N-acyl ethanolamine plasmalogen
- PPAR
peroxisome proliferator-activated receptor
- PexRAP
peroxisomal reductase activating PPARγ
- PD
Parkinson’s disease
- RCDP
rhizomelic chondrodysplasia punctata
Footnotes
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- Akisu M, Kultursay N, Ozkayin N, Coker I, Huseyinov A. Platelet-activating factor levels in term and preterm human milk. Biol Neonate. 1998;74(4):289–293. doi: 10.1159/000014036. [DOI] [PubMed] [Google Scholar]
- Ali F, Hossain MS, Sejimo S, Akashi K. Plasmalogens inhibit endocytosis of toll-like receptor 4 to attenuate the inflammatory signal in microglial cells. Mol Neurobiol. 2019;56(5):3404–3419. doi: 10.1007/s12035-018-1307-2. [DOI] [PubMed] [Google Scholar]
- Alrayes N, Mohamoud HS, Ahmed S, Almramhi MM, Shuaib TM, Wang J, Al-Aama JY, Everett K, Nasir J, Jelani M. The alkylglycerol monooxygenase (AGMO) gene previously involved in autism also causes a novel syndromic form of primary microcephaly in a consanguineous Saudi family. J Neurol Sci. 2016;363:240–244. doi: 10.1016/j.jns.2016.02.063. [DOI] [PubMed] [Google Scholar]
- Ando A, Oka M, Satomi Y. Deoxysphingolipids and ether-linked diacylglycerols accumulate in the tissues of aged mice. Cell Biosci. 2019;9:61. doi: 10.1186/s13578-019-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham Y, Menachem AB, Okun A, Zlotarav O, Abel N, Mechoulam R, Berry EM. Effects of the endocannabinoid noladin ether on body weight, food consumption, locomotor activity, and cognitive index in mice. Brain Res Bull. 2005;65(2):117–123. doi: 10.1016/j.brainresbull.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Awadalla P, et al. Direct measure of the de novo mutation rate in autism and schizophrenia cohorts. Am J Hum Genet. 2010;87(3):316–324. doi: 10.1016/j.ajhg.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti T, Vignini A, Giulietti A, Nanetti L, Provinciali L, Luzzi S, Mazzanti L, Ferretti G. Higher levels of oxidized low density lipoproteins in Alzheimer’s disease patients: roles for platelet activating factor acetyl hydrolase and paraoxonase-1. J Alzheimers Dis. 2015;46(1):179–186. doi: 10.3233/JAD-143096. [DOI] [PubMed] [Google Scholar]
- Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species Structure-activity relationship of cloned LPA receptors. FEBS Lett. 2000;478(1–2):159–165. doi: 10.1016/s0014-5793(00)01827-5. [DOI] [PubMed] [Google Scholar]
- Baptista J, Bruce WR, Gupta I, Krepinsky JJ, van Tassell RL, Wilkins TD. On distribution of different fecapentaenes, the fecal mutagens, in the human population. Cancer Lett. 1984;22(3):299–303. doi: 10.1016/0304-3835(84)90166-6. [DOI] [PubMed] [Google Scholar]
- Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, Welti R, Anderson RG, Liu P, Chapman KD. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res. 2007;48(4):837–847. doi: 10.1194/jlr.M600413-JLR200. [DOI] [PubMed] [Google Scholar]
- Bass DA, McPhail LC, Schmitt JD, Morris-Natschke S, McCall CE, Wykle RL. Selective priming of rate and duration of the respiratory burst of neutrophils by 1,2-diacyl and 1-O-alkyl-2-acyl diglycerides. Possible relation to effects on protein kinase C J Biol Chem. 1988;263(36):19610–19617. [PubMed] [Google Scholar]
- Bate C, Rumbold L, Williams A. Cholesterol synthesis inhibitors protect against platelet-activating factor-induced neuronal damage. J Neuroinflammation. 2007;4:5. doi: 10.1186/1742-2094-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44(12):2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- Bazinet RP, Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15(12):771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- Bell JG, MacKinlay EE, Dick JR, MacDonald DJ, Boyle RM, Glen AC. Essential fatty acids and phospholipase A2 in autistic spectrum disorders. Prostaglandins Leukot Essent Fatty Acids. 2004;71(4):201–204. doi: 10.1016/j.plefa.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Benjamin DI, Cozzo A, Ji X, Roberts LS, Louie SM, Mulvihill MM, Luo K, Nomura DK. Ether lipid generating enzyme AGPS alters the balance of structural and signaling lipids to fuel cancer pathogenicity. Proc Natl Acad Sci; U S A. 2013. pp. 14912–14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SA, Valenzuela N, Xu H, Franko B, Fai S, Figeys D. Using neurolipidomics to identify phospholipid mediators of synaptic (dys)function in Alzheimer’s disease. Front Physiol. 2013;4:168. doi: 10.3389/fphys.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Dorninger F, Forss-Petter S, Kunze M. Peroxisomes in brain development and function. Biochim Biophys Acta. 2016;1863(5):934–955. doi: 10.1016/j.bbamcr.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix GJ, Clark GD. Platelet-activating factor receptor stimulation disrupts neuronal migration in vitro. J Neurosci. 1998;18(1):307–318. doi: 10.1523/JNEUROSCI.18-01-00307.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci. 2015;16(9):551–563. doi: 10.1038/nrn3992. [DOI] [PubMed] [Google Scholar]
- Bowen KJ, Kris-Etherton PM, Shearer GC, West SG, Reddivari L, Jones PJH. Oleic acid-derived oleoylethanolamide: a nutritional science perspective. Prog Lipid Res. 2017;67:1–15. doi: 10.1016/j.plipres.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012;1822(9):1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Braverman N, Zhang R, Chen L, Nimmo G, Scheper S, Tran T, Chaudhury R, Moser A, Steinberg S. A Pex7 hypomorphic mouse model for plasmalogen deficiency affecting the lens and skeleton. Mol Genet Metab. 2010;99(4):408–416. doi: 10.1016/j.ymgme.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigida AL, Schultz S, Cascone M, Antonucci N, Siniscalco D. Endocannabinod signal dysregulation in autism spectrum disorders: a correlation link between inflammatory state and neuro-immune alterations. Int J Mol Sci. 2017;18(7) doi: 10.3390/ijms18071425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brites P, Motley AM, Gressens P, Mooyer PA, Ploegaert I, Everts V, Evrard P, Carmeliet P, Dewerchin M, Schoonjans L, Duran M, Waterham HR, Wanders RJ, Baes M. Impaired neuronal migration and endochondral ossification in Pex7 knockout mice: a model for rhizomelic chondrodysplasia punctata. Hum Mol Genet. 2003;12(18):2255–2267. doi: 10.1093/hmg/ddg236. [DOI] [PubMed] [Google Scholar]
- Brites P, Ferreira AS, Ferreira da Silva T, Sousa VF, Malheiro AR, Duran M, Waterham HR, Baes M, Wanders RJ. Alkyl-glycerol rescues plasmalogen levels and pathology of ether-phospholipid deficient mice. PLoS One. 2011;6(12):e28539. doi: 10.1371/journal.pone.0028539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotchie J. CB cannabinoid receptor signalling in Parkinson’s disease. Curr Opin Pharmacol. 2003;3(1):54–61. doi: 10.1016/s1471-4892(02)00011-5. [DOI] [PubMed] [Google Scholar]
- Brown SL, Jala VR, Raghuwanshi SK, Nasser MW, Haribabu B, Richardson RM. Activation and regulation of platelet-activating factor receptor: role of G(i) and G(q) in receptor-mediated chemotactic, cytotoxic, and cross-regulatory signals. J Immunol. 2006;177(5):3242–3249. doi: 10.4049/jimmunol.177.5.3242. [DOI] [PubMed] [Google Scholar]
- Butler M, Nelson VA, Davila H, Ratner E, Fink HA, Hemmy LS, McCarten JR, Barclay TR, Brasure M, Kane RL. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168(1):52–62. doi: 10.7326/M17-1530. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20(3):148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabot MC, Jaken S. Structural and chemical specificity of diacylglycerols for protein kinase C activation. Biochem Biophys Res Commun. 1984;125(1):163–169. doi: 10.1016/s0006-291x(84)80349-6. [DOI] [PubMed] [Google Scholar]
- Calder PC. Functional roles of fatty acids and their effects on human health. JPEN J Parenter Enteral Nutr. 2015;39(1):18S–32S. doi: 10.1177/0148607115595980. [DOI] [PubMed] [Google Scholar]
- Caldwell RA, Baumgarten CM. Plasmalogen-derived lysolipid induces a depolarizing cation current in rabbit ventricular myocytes. Circ Res. 1998;83(5):533–540. doi: 10.1161/01.res.83.5.533. [DOI] [PubMed] [Google Scholar]
- Chaithra VH, Jacob SP, Lakshmikanth CL, Sumanth MS, Abhilasha KV, Chen CH, Thyagarajan A, Sahu RP, Travers JB, McIntyre TM, Kemparaju K, Marathe GK. Modulation of inflammatory platelet-activating factor (PAF) receptor by the acyl analogue of PAF. J Lipid Res. 2018;59(11):2063–2074. doi: 10.1194/jlr.M085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoto K, Guo T, Imataki O, Tanaka M, Nakatsugawa M, Ochi T, Yamashita Y, Saito AM, Saito TI, Butler MO, Hirano N. CDR3beta sequence motifs regulate autoreactivity of human invariant NKT cell receptors. J Autoimmun. 2016;68:39–51. doi: 10.1016/j.jaut.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che H, Zhou M, Zhang T, Zhang L, Ding L, Yanagita T, Xu J, Xue C, Wang Y. EPA enriched ethanolamine plasmalogens significantly improve cognition of Alzheimer’s disease mouse model by suppressing β-amyloid generation. J Funct Foods. 2018a;41:9–18. [Google Scholar]
- Che H, Li Q, Zhang T, Ding L, Zhang L, Shi H, Yanagita T, Xue C, Chang Y, Wang Y. A comparative study of EPA-enriched ethanolamine plasmalogen and EPA-enriched phosphatidylethanolamine on Abeta42 induced cognitive deficiency in a rat model of Alzheimer’s disease. Food Funct. 2018b;9(5):3008–3017. doi: 10.1039/c8fo00643a. [DOI] [PubMed] [Google Scholar]
- Che H, Zhang L, Ding L, Xie W, Jiang X, Xue C, Zhang T, Wang Y. EPA-enriched ethanolamine plasmalogen and EPA-enriched phosphatidylethanolamine enhance BDNF/TrkB/CREB signaling and inhibit neuronal apoptosis in vitro and in vivo. Food Funct. 2020;11(2):1729–1739. doi: 10.1039/c9fo02323b. [DOI] [PubMed] [Google Scholar]
- Clark J, Kay RR, Kielkowska A, Niewczas I, Fets L, Oxley D, Stephens LR, Hawkins PT. Dictyostelium uses ether-linked inositol phospholipids for intracellular signalling. EMBO J. 2014;33(19):2188–2200. doi: 10.15252/embj.201488677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curren RD, Putman DL, Yang LL, Haworth SR, Lawlor TE, Plummer SM, Harris CC. Genotoxicity of fecapentaene-12 in bacterial and mammalian cell assay systems. Carcinogenesis. 1987;8(2):349–352. doi: 10.1093/carcin/8.2.349. [DOI] [PubMed] [Google Scholar]
- Davies SS, Pontsler AV, Marathe GK, Harrison KA, Murphy RC, Hinshaw JC, Prestwich GD, Hilaire AS, Prescott SM, Zimmerman GA, McIntyre TM. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. J Biol Chem. 2001;276(19):16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- Dean JM, Lodhi IJ. Structural and functional roles of ether lipids. Protein Cell. 2018;9(2):196–206. doi: 10.1007/s13238-017-0423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M, Fabelo N, Ferrer I, Marin R. “Lipid raft aging” in the human frontal cortex during nonpathological aging: gender influences and potential implications in Alzheimer’s disease. Neurobiol Aging. 2018;67:42–52. doi: 10.1016/j.neurobiolaging.2018.02.022. [DOI] [PubMed] [Google Scholar]
- Dorninger F, Wiesinger C, Braverman NE, Forss-Petter S, Berger J. Ether lipid deficiency does not cause neutropenia or leukopenia in mice and men. Cell Metab. 2015a;21(5):650–651. doi: 10.1016/j.cmet.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorninger F, Brodde A, Braverman NE, Moser AB, Just WW, Forss-Petter S, Brugger B, Berger J. Homeostasis of phospholipids - the level of phosphatidylethanolamine tightly adapts to changes in ethanolamine plasmalogens. Biochim Biophys Acta. 2015b;1851(2):117–128. doi: 10.1016/j.bbalip.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorninger F, Forss-Petter S, Berger J. From peroxisomal disorders to common neurodegenerative diseases - the role of ether phospholipids in the nervous system. FEBS Lett. 2017a;591(18):2761–2788. doi: 10.1002/1873-3468.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorninger F, Herbst R, Kravic B, Camurdanoglu BZ, Macinkovic I, Zeitler G, Forss-Petter S, Strack S, Khan MM, Waterham HR, Rudolf R, Hashemolhosseini S, Berger J. Reduced muscle strength in ether lipid-deficient mice is accompanied by altered development and function of the neuromuscular junction. J Neurochem. 2017b;143(5):569–583. doi: 10.1111/jnc.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorninger F, Moser AB, Kou J, Wiesinger C, Forss-Petter S, Gleiss A, Hinterberger M, Jungwirth S, Fischer P, Berger J. Alterations in the plasma levels of specific choline phospholipids in Alzheimer’s disease mimic accelerated aging. J Alzheimers Dis. 2018;62(2):841–854. doi: 10.3233/JAD-171036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorninger F, Gundacker A, Zeitler G, Pollak DD, Berger J. Ether lipid deficiency in mice produces a complex behavioral phenotype mimicking aspects of human psychiatric disorders. Int J Mol Sci. 2019a;20(16):3929. doi: 10.3390/ijms20163929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorninger F, Konig T, Scholze P, Berger ML, Zeitler G, Wiesinger C, Gundacker A, Pollak DD, Huck S, Just WW, Forss-Petter S, Pifl C, Berger J. Disturbed neurotransmitter homeostasis in ether lipid deficiency. Hum Mol Genet. 2019b;28(12):2046–2061. doi: 10.1093/hmg/ddz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragonas C, Bertsch T, Sieber CC, Brosche T. Plasmalogens as a marker of elevated systemic oxidative stress in Parkinson’s disease. Clin Chem Lab Med. 2009;47(7):894–897. doi: 10.1515/CCLM.2009.205. [DOI] [PubMed] [Google Scholar]
- Duker AL, Niiler T, Kinderman D, Schouten M, Poll-The BT, Braverman N, Bober MB. Rhizomelic chondrodysplasia punctata morbidity and mortality, an update. Am J Med Genet A. 2020;182(3):579–583. doi: 10.1002/ajmg.a.61413. [DOI] [PubMed] [Google Scholar]
- Duncan M, Millns P, Smart D, Wright JE, Kendall DA, Ralevic V. Noladin ether, a putative endocannabinoid, attenuates sensory neurotransmission in the rat isolated mesenteric arterial bed via a non-CB1/CB2 G(i/o) linked receptor. Br J Pharmacol. 2004;142(3):509–518. doi: 10.1038/sj.bjp.0705789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AR, Gonzalez DP, Del Viso F, Robson A, Khokha MK, Griffin JN. Alkylglycerol monooxygenase, a heterotaxy candidate gene, regulates left-right patterning via Wnt signaling. Dev Biol. 2019;456(1):1–7. doi: 10.1016/j.ydbio.2019.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenezer DL, Fu P, Ramchandran R, Ha AW, Putherickal V, Sudhadevi T, Harijith A, Schumacher F, Kleuser B, Natarajan V. S1P and plasmalogen derived fatty aldehydes in cellular signaling and functions. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865(7) doi: 10.1016/j.bbalip.2020.158681. 158681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner T, Romero-Brey I, Yifrach E, Rivera-Monroy J, Schrul B, Zouboulis CC, Stremmel W, Honsho M, Bartenschlager R, Zalckvar E, Poppelreuther M, Fullekrug J. An alternative membrane topology permits lipid droplet localization of peroxisomal fatty acyl-CoA reductase 1. J Cell Sci. 2019;132(6) doi: 10.1242/jcs.223016. jcs223016. [DOI] [PubMed] [Google Scholar]
- Fabelo N, Martin V, Santpere G, Marin R, Torrent L, Ferrer I, Diaz M. Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol Med. 2011;17(9–10):1107–1118. doi: 10.2119/molmed.2011.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabelo N, Martin V, Marin R, Santpere G, Aso E, Ferrer I, Diaz M. Evidence for premature lipid raft aging in APP/PS1 double-transgenic mice, a model of familial Alzheimer disease. J Neuropathol Exp Neurol. 2012;71(10):868–881. doi: 10.1097/NEN.0b013e31826be03c. [DOI] [PubMed] [Google Scholar]
- Facciotti F, Ramanjaneyulu GS, Lepore M, Sansano S, Cavallari M, Kistowska M, Forss-Petter S, Ni G, Colone A, Singhal A, Berger J, Xia C, Mori L, De Libero G. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat Immunol. 2012;13(5):474–480. doi: 10.1038/ni.2245. [DOI] [PubMed] [Google Scholar]
- Fakhro KA, Choi M, Ware SM, Belmont JW, Towbin JA, Lifton RP, Khokha MK, Brueckner M. Rare copy number variations in congenital heart disease patients identify unique genes in left-right patterning. Proc Natl Acad Sci; U S A. 2011. pp. 2915–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallatah W, Smith T, Cui W, Jayasinghe D, Di Pietro E, Ritchie SA, Braverman N. Oral administration of a synthetic vinyl-ether plasmalogen normalizes open field activity in a mouse model of rhizomelic chondrodysplasia punctata. Dis Model Mech. 2020;13(1) doi: 10.1242/dmm.042499. dmm042499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer K, Smith CA, Hayley S, Smith J. Major alterations of phosphatidylcholine and lysophosphotidylcholine lipids in the substantia Nigra using an early stage model of Parkinson’s disease. Int J Mol Sci. 2015;16(8):18865–18877. doi: 10.3390/ijms160818865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui AA. Studies on plasmalogen-selective phospholipase A2 in brain. Mol Neurobiol. 2010;41(2–3):267–273. doi: 10.1007/s12035-009-8091-y. [DOI] [PubMed] [Google Scholar]
- Felde R, Spiteller G. Plasmalogen oxidation in human serum lipoproteins. Chem Phys Lipids. 1995;76(2):259–267. doi: 10.1016/0009-3084(94)02448-e. [DOI] [PubMed] [Google Scholar]
- Fezza F, Bisogno T, Minassi A, Appendino G, Mechoulam R, Di Marzo V. Noladin ether, a putative novel endocannabinoid: inactivation mechanisms and a sensitive method for its quantification in rat tissues. FEBS Lett. 2002;513(2–3):294–298. doi: 10.1016/s0014-5793(02)02341-4. [DOI] [PubMed] [Google Scholar]
- Fontaine D, Figiel S, Felix R, Kouba S, Fromont G, Maheo K, Potier-Cartereau M, Chantome A, Vandier C. Roles of endogenous ether lipids and associated PUFAs in the regulation of ion channels and their relevance for disease. J Lipid Res. 2020;61(6):840–858. doi: 10.1194/jlr.RA120000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DA, Miyake R, Glaser PE, Gross RW. Activation of protein kinase C by naturally occurring ether-linked diglycerides. J Biol Chem. 1989;264(23):13818–13824. [PubMed] [Google Scholar]
- Ford DA, Honavar J, Albert CJ, Duerr MA, Oh JY, Doran S, Matalon S, Patel RP. Formation of chlorinated lipids post-chlorine gas exposure. J Lipid Res. 2016;57(8):1529–1540. doi: 10.1194/jlr.M069005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M, Berdel WE, Schick HD, Fink U, Pahlke W, Bicker U, Reichert A, Rastetter J. Antineoplastic activity of the thioether lysophospholipid derivative BM 41.440 in vitro. Lipids. 1987;22(11):916–918. doi: 10.1007/BF02535554. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Tadano-Aritomi K, Tokumasu A, Ito K, Hikita T, Suzuki K, Ishizuka I. Requirement of seminolipid in spermatogenesis revealed by UDP-galactose: ceramide galactosyltransferase-deficient mice. J Biol Chem. 2000;275(30):22623–22626. doi: 10.1074/jbc.C000200200. [DOI] [PubMed] [Google Scholar]
- Fujino T, Yamada T, Asada T, Tsuboi Y, Wakana C, Mawatari S, Kono S. Efficacy and blood plasmalogen changes by oral administration of plasmalogen in patients with mild Alzheimer’s disease and mild cognitive impairment: a multicenter, randomized, double-blind, placebo-controlled trial. EBioMedicine. 2017;17:199–205. doi: 10.1016/j.ebiom.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa M, Narahara H, Yasuda K, Johnston JM. Presence of platelet-activating factor-acetylhydrolase in milk. J Lipid Res. 1993;34(9):1603–1609. [PubMed] [Google Scholar]
- Gallego-Garcia A, Monera-Girona AJ, Pajares-Martinez E, Bastida-Martinez E, Perez-Castano R, Iniesta AA, Fontes M, Padmanabhan S, Elias-Arnanz M. A bacterial light response reveals an orphan desaturase for human plasmalogen synthesis. Science. 2019;366(6461):128–132. doi: 10.1126/science.aay1436. [DOI] [PubMed] [Google Scholar]
- Ganong BR, Loomis CR, Hannun YA, Bell RM. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc Natl Acad Sci; U S A. 1986. pp. 1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-de-Gomez L, Astudillo AM, Lebrero P, Balboa MA, Balsinde J. Essential role for ethanolamine plasmalogen hydrolysis in bacterial lipopolysaccharide priming of macrophages for enhanced arachidonic acid release. Front Immunol. 2017;8:1251. doi: 10.3389/fimmu.2017.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg L, Rafique S, Xuereb JH, Rapoport SI, Gershfeld NL. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer’s disease brain. Brain Res. 1995;698(1–2):223–226. doi: 10.1016/0006-8993(95)00931-f. [DOI] [PubMed] [Google Scholar]
- Ginsberg L, Xuereb JH, Gershfeld NL. Membrane instability, plasmalogen content, and Alzheimer’s disease. J Neurochem. 1998;70(6):2533–2538. doi: 10.1046/j.1471-4159.1998.70062533.x. [DOI] [PubMed] [Google Scholar]
- Goodenowe DB, Senanayake V. Relation of serum plasmalogens and APOE genotype to cognition and dementia in older persons in a cross-sectional study. Brain Sci. 2019;9(4):92. doi: 10.3390/brainsci9040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenowe DB, Cook LL, Liu J, Lu Y, Jayasinghe DA, Ahiahonu PW, Heath D, Yamazaki Y, Flax J, Krenitsky KF, Sparks DL, Lerner A, Friedland RP, Kudo T, Kamino K, Morihara T, Takeda M, Wood PL. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer’s disease and dementia. J Lipid Res. 2007;48(11):2485–2498. doi: 10.1194/jlr.P700023-JLR200. [DOI] [PubMed] [Google Scholar]
- Gregoire L, Smith T, Senanayake V, Mochizuki A, Miville-Godbout E, Goodenowe D, Di Paolo T. Plasmalogen precursor analog treatment reduces levodopa-induced dyskinesias in parkinsonian monkeys. Behav Brain Res. 2015;286:328–337. doi: 10.1016/j.bbr.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grosgen S, Riemenschneider M, Tanila H, Grimm HS, Hartmann T. From brain to food: analysis of phosphatidylcholins, lyso-phosphatidylcholins and phosphatidylcholin-plasmalogens derivates in Alzheimer’s disease human post mortem brains and mice model via mass spectrometry. J Chromatogr A. 2011a;1218(42):7713–7722. doi: 10.1016/j.chroma.2011.07.073. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Kuchenbecker J, Rothhaar TL, Grosgen S, Hundsdorfer B, Burg VK, Friess P, Muller U, Grimm HS, Riemenschneider M, Hartmann T. Plasmalogen synthesis is regulated via alkyl-dihydroxyacetonephosphate-synthase by amyloid precursor protein processing and is affected in Alzheimer’s disease. J Neurochem. 2011b;116(5):916–925. doi: 10.1111/j.1471-4159.2010.07070.x. [DOI] [PubMed] [Google Scholar]
- Guan ZZ, Wang YA, Cairns NJ, Lantos PL, Dallner G, Sindelar PJ. Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J Neuropathol Exp Neurol. 1999;58(7):740–747. doi: 10.1097/00005072-199907000-00008. [DOI] [PubMed] [Google Scholar]
- Gupta I, Baptista J, Bruce WR, Che CT, Furrer R, Gingerich JS, Grey AA, Marai L, Yates P, Krepinsky JJ. Structures of fecapentaenes, the mutagens of bacterial origin isolated from human feces. Biochemistry. 1983;22(2):241–245. doi: 10.1021/bi00271a001. [DOI] [PubMed] [Google Scholar]
- Guy AT, Nagatsuka Y, Ooashi N, Inoue M, Nakata A, Greimel P, Inoue A, Nabetani T, Murayama A, Ohta K, Ito Y, Aoki J, Hirabayashi Y, Kamiguchi H. Glycerophospholipid regulation of modality-specific sensory axon guidance in the spinal cord. Science. 2015;349(6251):974–977. doi: 10.1126/science.aab3516. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Engelender S, Isacson O. Lipid and immune abnormalities causing age-dependent neurodegeneration and Parkinson’s disease. J Neuroinflammation. 2019;16(1):153. doi: 10.1186/s12974-019-1532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X. Lipid alterations in the earliest clinically recognizable stage of Alzheimer’s disease: implication of the role of lipids in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2005;2(1):65–77. doi: 10.2174/1567205052772786. [DOI] [PubMed] [Google Scholar]
- Han XL, Gross RW. Alterations in membrane dynamics elicited by amphiphilic compounds are augmented in plasmenylcholine bilayers. Biochim Biophys Acta. 1991;1069(1):37–45. doi: 10.1016/0005-2736(91)90101-d. [DOI] [PubMed] [Google Scholar]
- Han X, Holtzman DM, McKeel DW., Jr Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J Neurochem. 2001;77(4):1168–1180. doi: 10.1046/j.1471-4159.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci; U S A. 2001. pp. 3662–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, et al. LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Mol Cell Neurosci. 2003;24(4):1170–1179. doi: 10.1016/j.mcn.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Reyes-Irisarri E, Hull M, Kummer MP. Impact and therapeutic potential of PPARs in Alzheimer’s disease. Curr Neuropharmacol. 2011;9(4):643–650. doi: 10.2174/157015911798376325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, et al. Neuroinflammation in Alzheimer’s disease. The Lancet Neurology. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershkowitz M, Adunsky A. Binding of platelet-activating factor to platelets of Alzheimer’s disease and multiinfarct dementia patients. Neurobiol Aging. 1996;17(6):865–868. doi: 10.1016/s0197-4580(96)00073-5. [DOI] [PubMed] [Google Scholar]
- Heymans F, Da Silva C, Marrec N, Godfroid JJ, Castagna M. Alkyl analogs of diacylglycerol as activators of protein kinase C. FEBS Lett. 1987;218(1):35–40. doi: 10.1016/0014-5793(87)81013-x. [DOI] [PubMed] [Google Scholar]
- Hino K, Kaneko S, Harasawa T, Kimura T, Takei S, Shinohara M, Yamazaki F, Morita SY, Sato S, Kubo Y, Kono T, Setou M, Yoshioka M, Fujino J, Sugihara H, Kojima H, Yamada N, Udagawa J. Change in brain plasmalogen composition by exposure to prenatal undernutrition leads to behavioral impairment of rats. J Neurosci. 2019;39(39):7689–7702. doi: 10.1523/JNEUROSCI.2721-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai N, Kingston DGI, Van Tassel RL, Wilkins TD. Structure elucidation of a potent mutagen from human feces. J Am Chem Soc. 1982;104:6149–6150. [Google Scholar]
- Hirashima Y, Farooqui AA, Mills JS, Horrocks LA. Identification and purification of calcium-independent phospholipase-A2 from bovine brain cytosol. J Neurochem. 1992;59(2):708–714. doi: 10.1111/j.1471-4159.1992.tb09426.x. [DOI] [PubMed] [Google Scholar]
- Hirotsune S, Fleck MW, Gambello MJ, Bix GJ, Chen A, Clark GD, Ledbetter DH, McBain CJ, Wynshaw-Boris A. Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nat Genet. 1998;19(4):333–339. doi: 10.1038/1221. [DOI] [PubMed] [Google Scholar]
- Hishikawa D, Valentine WJ, Iizuka-Hishikawa Y, Shindou H, Shimizu T. Metabolism and functions of docosahexaenoic acid-containing membrane glycer-ophospholipids. FEBS Lett. 2017;591(18):2730–2744. doi: 10.1002/1873-3468.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefler G, Paschke E, Hoefler S, Moser AB, Moser HW. Photosensitized killing of cultured fibroblasts from patients with peroxisomal disorders due to pyrene fatty acid-mediated ultraviolet damage. J Clin Invest. 1991;88(6):1873–1879. doi: 10.1172/JCI115509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer DC, Pessentheiner AR, Pelzmann HJ, Schlager S, Madreiter-Sokolowski CT, Kolb D, TO, Eichmann, Rechberger G, Bilban M, Graier WF, Kratky D, Bogner-Strauss JG. Critical role of the peroxisomal protein PEX16 in white adipocyte development and lipid homeostasis. Biochim Biophys Acta. 2017;1862(3):358–368. doi: 10.1016/j.bbalip.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly SP, Gera N, Wang P, Wilson A, Guan Z, Lin L, Cooley B, Alfar HR, Patil RG, Piatt R, Leisner TM, Bergmeier W, Majumder R, Parise LV. Ether lipid metabolism by AADACL1 regulates platelet function and thrombosis. Blood Adv. 2019;3(22):3818–3828. doi: 10.1182/bloodadvances.2018030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan EA, Kim YG, Cardia JP, Saghatelian A. Monoalkylglycerol ether lipids promote adipogenesis. J Am Chem Soc. 2011;133(14):5178–5181. doi: 10.1021/ja111173c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Z, Nakamura M, Miki I, Minami M, Watanabe T, Seyama Y, Okado H, Toh H, Ito K, Miyamoto T, et al. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature. 1991;349(6307):342–346. doi: 10.1038/349342a0. [DOI] [PubMed] [Google Scholar]
- Honke K. Biosynthesis and biological function of sulfoglycolipids. Proc Jpn Acad Ser B Phys Biol Sci. 2013;89(4):129–138. doi: 10.2183/pjab.89.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honke K, Hirahara Y, Dupree J, Suzuki K, Popko B, Fukushima K, Fukushima J, Nagasawa T, Yoshida N, Wada Y, Taniguchi N. Paranodal junction formation and spermatogenesis require sulfoglycolipids. Proc Natl Acad Sci; U S A. 2002. pp. 4227–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honsho M, Asaoku S, Fukumoto K, Fujiki Y. Topogenesis and homeostasis of fatty acyl-CoA reductase 1. J Biol Chem. 2013;288(48):34588–34598. doi: 10.1074/jbc.M113.498345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honsho M, Dorninger F, Abe Y, Setoyama D, Ohgi R, Uchiumi T, Kang D, Berger J, Fujiki Y. Impaired plasmalogen synthesis dysregulates liver X receptor-dependent transcription in cerebellum. J Biochem. 2019;166(4):353–361. doi: 10.1093/jb/mvz043. [DOI] [PubMed] [Google Scholar]
- Horibata Y, Elpeleg O, Eran A, Hirabayashi Y, Savitzki D, Tal G, Mandel H, Sugimoto H. EPT1 (selenoprotein I) is critical for the neural development and maintenance of plasmalogen in humans. J Lipid Res. 2018;59(6):1015–1026. doi: 10.1194/jlr.P081620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrocks LA. Content, composition, and metabolism of mammalian and avian lipids that contain ether groups. In: Snyder F, editor. Ether Lipids: Chemistry and Biology. Academic Press; New York: 1972. pp. 177–272. [Google Scholar]
- Hossain MS, Mineno K, Katafuchi T. Neuronal orphan G-protein coupled receptor proteins mediate plasmalogens-induced activation of ERK and Akt signaling. PLoS One. 2016;11(3):e0150846. doi: 10.1371/journal.pone.0150846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MS, Abe Y, Ali F, Youssef M, Honsho M, Fujiki Y, Katafuchi T. Reduction of ether-type glycerophospholipids, plasmalogens, by NF-kappaB signal leading to microglial activation. J Neurosci. 2017;37(15):4074–4092. doi: 10.1523/JNEUROSCI.3941-15.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MS, Tajima A, Kotoura S, Katafuchi T. Oral ingestion of plasmalogens can attenuate the LPS-induced memory loss and microglial activation. Biochem Biophys Res Commun. 2018;496(4):1033–1039. doi: 10.1016/j.bbrc.2018.01.078. [DOI] [PubMed] [Google Scholar]
- Hostettler ME, Knapp PE, Carlson SL. Platelet-activating factor induces cell death in cultured astrocytes and oligodendrocytes: involvement of caspase-3. Glia. 2002;38(3):228–239. doi: 10.1002/glia.10065. [DOI] [PubMed] [Google Scholar]
- Houck KL, Fox TE, Sandirasegarane L, Kester M. Ether-linked diglycerides inhibit vascular smooth muscle cell growth via decreased MAPK and PI3K/Akt signaling. Am J Physiol Heart Circ Physiol. 2008;295(4):H1657–H1668. doi: 10.1152/ajpheart.00141.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houjou T, Hayakawa J, Watanabe R, Tashima Y, Maeda Y, Kinoshita T, Taguchi R. Changes in molecular species profiles of glycosylphosphatidylinositol anchor precursors in early stages of biosynthesis. J Lipid Res. 2007;48(7):1599–1606. doi: 10.1194/jlr.M700095-JLR200. [DOI] [PubMed] [Google Scholar]
- Hua R, Cheng D, Coyaud E, Freeman S, Di Pietro E, Wang Y, Vissa A, Yip CM, Fairn GD, Braverman N, Brumell JH, Trimble WS, Raught B, Kim PK. VAPs and ACBD5 tether peroxisomes to the ER for peroxisome maintenance and lipid homeostasis. J Cell Biol. 2017;216(2):367–377. doi: 10.1083/jcb.201608128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifuku M, Katafuchi T, Mawatari S, Noda M, Miake K, Sugiyama M, Fujino T. Anti-inflammatory/anti-amyloidogenic effects of plasmalogens in lipopoly-saccharide-induced neuroinflammation in adult mice. J Neuroinflammation. 2012;9:197. doi: 10.1186/1742-2094-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Gao F, Kim HW, Rapoport SI, Rao JS. Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer’s disease prefrontal cortex. J Alzheimers Dis. 2011;24(3):507–517. doi: 10.3233/JAD-2011-101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–48. doi: 10.1016/j.plefa.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, Cao WH, Kume K, Fukuchi Y, Ikuta K, Miyazaki J, Kumada M, Shimizu T. Impaired ana-phylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187(11):1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka I. Chemistry and functional distribution of sulfoglycolipids. Prog Lipid Res. 1997;36(4):245–319. doi: 10.1016/s0163-7827(97)00011-8. [DOI] [PubMed] [Google Scholar]
- Ishizuka I, Inomata M. Sulphated glycoglycerolipids in rat brain: decrease and disappearance after developmental age. J Neurochem. 1979;33(1):387–388. doi: 10.1111/j.1471-4159.1979.tb11749.x. [DOI] [PubMed] [Google Scholar]
- Ishizuka I, Suzuki M, Yamakawa T. Isolation and characterization of a novel sulfoglycolipid, ‘seminolipid’, from boar testis and spermatozoa. J Biochem. 1973;73(1):77–87. [PubMed] [Google Scholar]
- Ivanova PT, Milne SB, Brown HA. Identification of atypical ether-linked glycerophospholipid species in macrophages by mass spectrometry. J Lipid Res. 2010;51(6):1581–1590. doi: 10.1194/jlr.D003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Fin C, Schmitz PK, Da Silva RC, Jerusalinsky D, Quillfeldt JA, Ferreira MB, Medina JH, Bazan NG. Memory enhancement by intrahippocampal, intraamygdala, or intraentorhinal infusion of platelet-activating factor measured in an inhibitory avoidance task. Proc Natl Acad Sci; U S A. 1972. pp. 5047–5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffres PA, Gajate C, Bouchet AM, Couthon-Gourves H, Chantome A, Potier-Cartereau M, Besson P, Bougnoux P, Mollinedo F, Vandier C. Alkyl ether lipids, ion channels and lipid raft reorganization in cancer therapy. Pharmacol Ther. 2016;165:114–131. doi: 10.1016/j.pharmthera.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Jenkins CM, Yang K, Liu G, Moon SH, Dilthey BG, Gross RW. Cytochrome c is an oxidative stress-activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1 vinyl ether linkage. J Biol Chem. 2018;293(22):8693–8709. doi: 10.1074/jbc.RA117.001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Rojo N, Riezman H. On the road to unraveling the molecular functions of ether lipids. FEBS Lett. 2019;593(17):2378–2389. doi: 10.1002/1873-3468.13465. [DOI] [PubMed] [Google Scholar]
- Jones EK, Kirkham TC. Noladin ether, a putative endocannabinoid, enhances motivation to eat after acute systemic administration in rats. Br J Pharmacol. 2012;166(6):1815–1821. doi: 10.1111/j.1476-5381.2012.01888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzawa N, Maeda Y, Ogiso H, Murakami Y, Taguchi R, Kinoshita T. Peroxisome dependency of alkyl-containing GPI-anchor biosynthesis in the endoplasmic reticulum. Proc Natl Acad Sci; U S A. 2009. pp. 17711–17716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzawa N, Shimozawa N, Wanders RJ, Ikeda K, Murakami Y, Waterham HR, Mukai S, Fujita M, Maeda Y, Taguchi R, Fujiki Y, Kinoshita T. Defective lipid remodeling of GPI anchors in peroxisomal disorders, Zellweger syndrome, and rhizomelic chondrodysplasia punctata. J Lipid Res. 2012;53(4):653–663. doi: 10.1194/jlr.M021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Clark GD, Bazan NG, Zorumski CF. Platelet-activating factor as a potential retrograde messenger in CA1 hippocampal long-term potentiation. Nature. 1994;367(6459):175–179. doi: 10.1038/367175a0. [DOI] [PubMed] [Google Scholar]
- Khandoga AL, Fujiwara Y, Goyal P, Pandey D, Tsukahara R, Bolen A, Guo H, Wilke N, Liu J, Valentine WJ, Durgam GG, Miller DD, Jiang G, Prestwich GD, Tigyi G, Siess W. Lysophosphatidic acid-induced platelet shape change revealed through LPA(1-5) receptor-selective probes and albumin. Platelets. 2008;19(6):415–427. doi: 10.1080/09537100802220468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Sparrow JR. Novel bisretinoids of human retina are lyso alkyl ether glycerophosphoethanolamine-bearing A2PE species. J Lipid Res. 2018;59(9):1620–1629. doi: 10.1194/jlr.M084459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Kimura AK, Ren M, Berno B, Xu Y, Schlame M, Epand RM. Substantial decrease in plasmalogen in the heart associated with tafazzin deficiency. Biochemistry. 2018;57(14):2162–2175. doi: 10.1021/acs.biochem.8b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagishi Y, Minami A, Nakanishi A, Ogura Y, Matsuda S. Neuron membrane trafficking and protein kinases involved in autism and ADHD. Int J Mol Sci. 2015;16(2):3095–3115. doi: 10.3390/ijms16023095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivuniemi A. The biophysical properties of plasmalogens originating from their unique molecular architecture. FEBS Lett. 2017;591(18):2700–2713. doi: 10.1002/1873-3468.12754. [DOI] [PubMed] [Google Scholar]
- de Kok TM, van Faassen A, Bausch-Goldbohm RA, ten Hoor F, Kleinjans JC. Fecapentaene excretion and fecal mutagenicity in relation to nutrient intake and fecal parameters in humans on omnivorous and vegetarian diets. Cancer Lett. 1992;62(1):11–21. doi: 10.1016/0304-3835(92)90193-y. [DOI] [PubMed] [Google Scholar]
- Komljenovic D, Sandhoff R, Teigler A, Heid H, Just WW, Gorgas K. Disruption of blood-testis barrier dynamics in ether-lipid-deficient mice. Cell Tissue Res. 2009;337(2):281–299. doi: 10.1007/s00441-009-0809-7. [DOI] [PubMed] [Google Scholar]
- Kono N, Arai H. Platelet-activating factor acetylhydrolases: an overview and update. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(6):922–931. doi: 10.1016/j.bbalip.2018.07.006. [DOI] [PubMed] [Google Scholar]
- Kou J, Kovacs GG, Hoftberger R, Kulik W, Brodde A, Forss-Petter S, Honigschnabl S, Gleiss A, Brugger B, Wanders R, Just W, Budka H, Jungwirth S, Fischer P, Berger J. Peroxisomal alterations in Alzheimer’s disease. Acta Neuropathol. 2011;122(3):271–283. doi: 10.1007/s00401-011-0836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozian DH, von Haeften E, Joho S, Czechtizky W, Anumala UR, Roux P, Dudda A, Evers A, Nazare M. Modulation of hexadecyl-LPA-mediated activation of mast cells and microglia by a chemical probe for LPA5. ChemBioChem. 2016;17(9):861–865. doi: 10.1002/cbic.201500559. [DOI] [PubMed] [Google Scholar]
- Kunze M. The type-2 peroxisomal targeting signal. Biochim Biophys Acta Mol Cell Res. 2020;1867(2) doi: 10.1016/j.bbamcr.2019.118609. 118609. [DOI] [PubMed] [Google Scholar]
- Laine K, Jarvinen K, Mechoulam R, Breuer A, Jarvinen T. Comparison of the enzymatic stability and intraocular pressure effects of 2-arachidonylglycerol and noladin ether, a novel putative endocannabinoid. Invest Ophthalmol Vis Sci. 2002;43(10):3216–3222. [PubMed] [Google Scholar]
- Lam SM, Wang Y, Duan X, Wenk MR, Kalaria RN, Chen CP, Lai MK, Shui G. Brain lipidomes of subcortical ischemic vascular dementia and mixed dementia. Neurobiol Aging. 2014;35(10):2369–2381. doi: 10.1016/j.neurobiolaging.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre E, Collado MP, Fernandez I, Aragones MD, Catalan RE. Signaling events mediating activation of brain ethanolamine plasmalogen hydrolysis by ceramide. Eur J Biochem. 2003;270(1):36–46. doi: 10.1046/j.1432-1033.2003.03356.x. [DOI] [PubMed] [Google Scholar]
- Lebrero P, Astudillo AM, Rubio JM, Fernandez-Caballero L, Kokotos G, Balboa MA, Balsinde J. Cellular plasmalogen content does not influence arachidonic acid levels or distribution in macrophages: a role for cytosolic phospholipase A2gamma in phospholipid remodeling. Cells. 2019;8(8):799. doi: 10.3390/cells8080799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman M, van Tassell R, West SE, Ehrich MF, Wilkins TD. In vitro production of human fecal mutagen. Mutat Res. 1980;79(2):115–124. doi: 10.1016/0165-1218(80)90079-8. [DOI] [PubMed] [Google Scholar]
- Lee HC, Simon GM, Cravatt BF. ABHD4 regulates multiple classes of N-acyl phospholipids in the mammalian central nervous system. Biochemistry. 2015;54(15):2539–2549. doi: 10.1021/acs.biochem.5b00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessig J, Fuchs B. Plasmalogens in biological systems: their role in oxidative processes in biological membranes, their contribution to pathological processes and aging and plasmalogen analysis. Curr Med Chem. 2009;16(16):2021–2041. doi: 10.2174/092986709788682164. [DOI] [PubMed] [Google Scholar]
- Liliom K, Fischer DJ, Virag T, Sun G, Miller DD, Tseng JL, Desiderio DM, Seidel MC, Erickson JR, Tigyi G. Identification of a novel growth factorlike lipid, 1-O-cis-alk-1′-enyl-2-lyso-sn-glycero-3-phosphate (alkenyl-GP) that is present in commercial sphingolipid preparations. J Biol Chem. 1998;273(22):13461–13468. doi: 10.1074/jbc.273.22.13461. [DOI] [PubMed] [Google Scholar]
- Llorente A, Skotland T, Sylvanne T, Kauhanen D, Rog T, Orlowski A, Vattulainen I, Ekroos K, Sandvig K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta. 2013;1831(7):1302–1309. doi: 10.1016/j.bbalip.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Lodhi IJ, Yin L, Jensen-Urstad AP, Funai K, Coleman T, Baird JH, El Ramahi MK, Razani B, Song H, Fu-Hsu F, Turk J, Semenkovich CF. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARgamma activation to decrease diet-induced obesity. Cell Metab. 2012;16(2):189–201. doi: 10.1016/j.cmet.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi IJ, Wei X, Yin L, Feng C, Adak S, Abou-Ezzi G, Hsu F, Link DC, Semenkovich CF. Peroxisomal lipid synthesis regulates inflammation by sustaining neutrophil membrane phospholipid composition and viability. Cell Metab. 2015;21(1):51–64. doi: 10.1016/j.cmet.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Xiao Yj YJ, Baudhuin LM, Hong G, Xu Y. Role of ether-linked lysophosphatidic acids in ovarian cancer cells. J Lipid Res. 2002;43(3):463–476. [PubMed] [Google Scholar]
- Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14(6):401–416. [Google Scholar]
- Luddi A, Gori M, Crifasi L, Marrocco C, Belmonte G, Costantino-Ceccarini E, Piomboni P. Impaired spermatogenesis in the twitcher mouse: a morphological evaluation from the seminiferous tubules to epididymal transit. Syst Biol Reprod Med. 2017;63(2):77–85. doi: 10.1080/19396368.2016.1271918. [DOI] [PubMed] [Google Scholar]
- Malheiro AR, Correia B, Ferreira da Silva T, Bessa-Neto D, Van Veldhoven PP, Brites P. Leukodystrophy caused by plasmalogen deficiency rescued by glyceryl 1-myristyl ether treatment. Brain Pathol. 2019;29(5):622–639. doi: 10.1111/bpa.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A, Wang Y, Ernsberger P, Kester M. Interleukin-1-induced ether-linked diglycerides inhibit calcium-insensitive protein kinase C isotypes Implications for growth senescence. J Biol Chem. 1997;272(32):20306–20311. doi: 10.1074/jbc.272.32.20306. [DOI] [PubMed] [Google Scholar]
- Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, Hall WJ, Fisher SG, Peterson DR, Haley JM, Nazar MD, Rich SA, Berlau DJ, Peltz CB, Tan MT, Kawas CH, Federoff HJ. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20(4):415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marathe GK, Silva AR, de Castro Faria Neto HC, Tjoelker LW, Prescott SM, Zimmerman GA, McIntyre TM. Lysophosphatidylcholine and lyso-PAF display PAF-like activity derived from contaminating phospholipids. J Lipid Res. 2001;42(9):1430–1437. [PubMed] [Google Scholar]
- Marin R, Fabelo N, Martin V, Garcia-Esparcia P, Ferrer I, Quinto-Alemany D, Diaz M. Anomalies occurring in lipid profiles and protein distribution in frontal cortex lipid rafts in dementia with Lewy bodies disclose neurochemical traits partially shared by Alzheimer’s and Parkinson’s diseases. Neurobiol Aging. 2017;49:52–59. doi: 10.1016/j.neurobiolaging.2016.08.027. [DOI] [PubMed] [Google Scholar]
- Marsche G, Heller R, Fauler G, Kovacevic A, Nuszkowski A, Graier W, Sattler W, Malle E. 2-chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis. Arterioscler Thromb Vasc Biol. 2004;24(12):2302–2306. doi: 10.1161/01.ATV.0000148703.43429.25. [DOI] [PubMed] [Google Scholar]
- Masliah E, Cole GM, Hansen LA, Mallory M, Albright T, Terry RD, Saitoh T. Protein kinase C alteration is an early biochemical marker in Alzheimer’s disease. J Neurosci. 1991;11(9):2759–2767. doi: 10.1523/JNEUROSCI.11-09-02759.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawatari S, Ohara S, Taniwaki Y, Tsuboi Y, Maruyama T, Fujino T. Improvement of blood plasmalogens and clinical symptoms in Parkinson’s disease by oral administration of ether phospholipids: a preliminary report. Parkinsons Dis. 2020;2020 doi: 10.1155/2020/2671070. 2671070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, Hinshaw JC, Zimmerman GA, Hama K, Aoki J, Arai H, Prestwich GD. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci; U S A. 2003. pp. 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre TM, Prescott SM, Stafforini DM. The emerging roles of PAF acetyl-hydrolase. J Lipid Res. 2009;50:S255–S259. doi: 10.1194/jlr.R800024-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara MJ, Schmitt JD, Wykle RL, Daniel LW. 1-O-Hexadecyl-2-acetyl-sn-glycerol stimulates differentiation of HL-60 human promyelocytic leukemia cells to macrophage-like cells. Biochem Biophys Res Commun. 1984;122(2):824–830. doi: 10.1016/s0006-291x(84)80108-4. [DOI] [PubMed] [Google Scholar]
- McNeely TB, Rosen G, Londner MV, Turco SJ. Inhibitory effects on protein kinase C activity by lipophosphoglycan fragments and glycosylphosphatidylinositol antigens of the protozoan parasite Leishmania. Biochem J. 1989;259(2):601–604. doi: 10.1042/bj2590601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton NG. Anandamide and noladin ether prevent neurotoxicity of the human amyloid-beta peptide. Neurosci Lett. 2002;332(2):127–130. doi: 10.1016/s0304-3940(02)00936-9. [DOI] [PubMed] [Google Scholar]
- Miville-Godbout E, Bourque M, Morissette M, Al-Sweidi S, Smith T, Mochizuki A, Senanayake V, Jayasinghe D, Wang L, Goodenowe D, Di Paolo T. Plasmalogen augmentation reverses striatal dopamine loss in MPTP mice. PLoS One. 2016;11(3):e0151020. doi: 10.1371/journal.pone.0151020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miville-Godbout E, Bourque M, Morissette M, Al-Sweidi S, Smith T, Jayasinghe D, Ritchie S, Di Paolo T. Plasmalogen precursor mitigates striatal dopamine loss in MPTP mice. Brain Res. 2017;1674:70–76. doi: 10.1016/j.brainres.2017.08.020. [DOI] [PubMed] [Google Scholar]
- Moser HW. Genotype-phenotype correlations in disorders of peroxisome biogenesis. Mol Genet Metab. 1999;68(2):316–327. doi: 10.1006/mgme.1999.2926. [DOI] [PubMed] [Google Scholar]
- Mueller HW, O’Flaherty JT, Wykle RL. Ether lipid content and fatty acid distribution in rabbit polymorphonuclear neutrophil phospholipids. Lipids. 1982;17(2):72–77. doi: 10.1007/BF02535178. [DOI] [PubMed] [Google Scholar]
- Mueller HW, O’Flaherty JT, Greene DG, Samuel MP, Wykle RL. 1-O-alkyl-linked glycerophospholipids of human neutrophils: distribution of arachidonate and other acyl residues in the ether-linked and diacyl species. J Lipid Res. 1984;25(4):383–388. [PubMed] [Google Scholar]
- Musial A, Mandal A, Coroneos E, Kester M. Interleukin-1 and endothelin stimulate distinct species of diglycerides that differentially regulate protein kinase C in mesangial cells. J Biol Chem. 1995;270(37):21632–21638. doi: 10.1074/jbc.270.37.21632. [DOI] [PubMed] [Google Scholar]
- Nadeau J, Smith T, Lamontagne-Proulx J, Bourque M, Al Sweidi S, Jayasinghe D, Ritchie S, Di Paolo T, Soulet D. Neuroprotection and immunomodulation in the gut of parkinsonian mice with a plasmalogen precursor. Brain Res. 2019;1725 doi: 10.1016/j.brainres.2019.146460. 146460. [DOI] [PubMed] [Google Scholar]
- Nichols E, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithipatikom K, Isbell MA, Endsley MP, Woodliff JE, Campbell WB. Antiproliferative effect of a putative endocannabinoid, 2-arachidonylglyceryl ether in prostate carcinoma cells. Prostaglandins Other Lipid Mediat. 2011;94(1–2):34–43. doi: 10.1016/j.prostaglandins.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278(28):25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- Oades RD, Sadile AG, Sagvolden T, Viggiano D, Zuddas A, Devoto P, Aase H, Johansen EB, Ruocco LA, Russell VA. The control of responsiveness in ADHD by catecholamines: evidence for dopaminergic, noradrenergic and interactive roles. Dev Sci. 2005;8(2):122–131. doi: 10.1111/j.1467-7687.2005.00399.x. [DOI] [PubMed] [Google Scholar]
- Oh SY, Jadhav LS. Effects of dietary alkylglycerols in lactating rats on immune responses in pups. Pediatr Res. 1994;36(3):300–305. doi: 10.1203/00006450-199409000-00006. [DOI] [PubMed] [Google Scholar]
- Oka S, Tsuchie A, Tokumura A, Muramatsu M, Suhara Y, Takayama H, Waku K, Sugiura T. Ether-linked analogue of 2-arachidonoylglycerol (noladin ether) was not detected in the brains of various mammalian species. J Neurochem. 2003;85(6):1374–1381. doi: 10.1046/j.1471-4159.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun. 2007;362(4):928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279(7):5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Okur V, Watschinger K, Niyazov D, McCarrier J, Basel D, Hermann M, Werner ER, Chung WK. Biallelic variants in AGMO with diminished enzyme activity are associated with a neurodevelopmental disorder. Hum Genet. 2019;138(11–12):1259–1266. doi: 10.1007/s00439-019-02065-x. [DOI] [PubMed] [Google Scholar]
- Ong WY, Farooqui T, Farooqui AA. Involvement of cytosolic phospholipase A(2), calcium independent phospholipase A(2) and plasmalogen selective phospholipase A(2) in neurodegenerative and neuropsychiatric conditions. Curr Med Chem. 2010;17(25):2746–2763. doi: 10.2174/092986710791859289. [DOI] [PubMed] [Google Scholar]
- Paldyova E, Bereczki E, Santha M, Wenger T, Borsodi A, Benyhe S. Noladin ether, a putative endocannabinoid, inhibits mu-opioid receptor activation via CB2 cannabinoid receptors. Neurochem Int. 2008;52(1–2):321–328. doi: 10.1016/j.neuint.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Palladino EN, Wang WY, Albert CJ, Langhi C, Baldan A, Ford DA. Peroxisome proliferator-activated receptor-alpha accelerates alpha-chlorofatty acid catabolism. J Lipid Res. 2017;58(2):317–324. doi: 10.1194/jlr.M069740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino END, Hartman CL, Albert CJ, Ford DA. The chlorinated lipidome originating from myeloperoxidase-derived HOCl targeting plasmalogens: Metabolism, clearance, and biological properties. Arch Biochem Biophys. 2018a;641:31–38. doi: 10.1016/j.abb.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino END, Katunga LA, Kolar GR, Ford DA. 2-Chlorofatty acids: lipid mediators of neutrophil extracellular trap formation. J Lipid Res. 2018b;59(8):1424–1432. doi: 10.1194/jlr.M084731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri A, Fito M, Torres CF, Munoz-Aguayo D, Schroder H, Cano JF, Vazquez L, Reglero G, Covas MI. Alkylglycerols reduce serum complement and plasma vascular endothelial growth factor in obese individuals. Inflammopharmacology. 2016;24(2–3):127–131. doi: 10.1007/s10787-016-0265-4. [DOI] [PubMed] [Google Scholar]
- Paul S, Lancaster GI, Meikle PJ. Plasmalogens: a potential therapeutic target for neurodegenerative and cardiometabolic disease. Prog Lipid Res. 2019;74:186–195. doi: 10.1016/j.plipres.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Pedrono F, Khan NA, Legrand AB. Regulation of calcium signalling by 1-O-alkylglycerols in human Jurkat T lymphocytes. Life Sci. 2004a;74(22):2793–2801. doi: 10.1016/j.lfs.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Pedrono F, Martin B, Leduc C, Le Lan J, Saiag B, Legrand P, Moulinoux JP, Legrand AB. Natural alkylglycerols restrain growth and metastasis of grafted tumors in mice. Nutr Cancer. 2004b;48(1):64–69. doi: 10.1207/s15327914nc4801_9. [DOI] [PubMed] [Google Scholar]
- Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Gong CX, An WL, Winblad B, Cowburn RF, Grundke-Iqbal I, Iqbal K. Okadaic-acid-induced inhibition of protein phosphatase 2A produces activation of mitogen-activated protein kinases ERK1/2, MEK1/2, and p70 S6, similar to that in Alzheimer’s disease. Am J Pathol. 2003;163(3):845–858. doi: 10.1016/S0002-9440(10)63445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettegrew JW, Panchalingam K, Hamilton RL, McClure RJ. Brain membrane phospholipid alterations in Alzheimer’s disease. Neurochem Res. 2001;26(7):771–782. doi: 10.1023/a:1011603916962. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Rafts defined: a report on the keystone symposium on lipid rafts and cell function. J Lipid Res. 2006;47(7):1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41(6):2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- Plummer SM, Grafstrom RC, Yang LL, Curren RD, Linnainmaa K, Harris CC. Fecapentaene-12 causes DNA damage and mutations in human cells. Carcinogenesis. 1986;7(9):1607–1609. doi: 10.1093/carcin/7.9.1607. [DOI] [PubMed] [Google Scholar]
- Plummer SM, Hall M, Faux SP. Oxidation and genotoxicity of fecapentaene-12 are potentiated by prostaglandin H synthase. Carcinogenesis. 1995;16(5):1023–1028. doi: 10.1093/carcin/16.5.1023. [DOI] [PubMed] [Google Scholar]
- Qian L, Zhang M, Wu S, Zhong Y, Van Tol E, Cai W. Alkylglycerols modulate the proliferation and differentiation of non-specific agonist and specific antigen-stimulated splenic lymphocytes. PLoS One. 2014;9(4):e96207. doi: 10.1371/journal.pone.0096207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CA, Sun Q, Gamblin TC. Tau phosphorylation by GSK-3beta promotes tangle-like filament morphology. Mol Neurodegener. 2007;2:12. doi: 10.1186/1750-1326-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner B, Wang W, Liu J, Xiong H. Platelet-activating factor attenuation of long-term potentiation in rat hippocampal slices via protein tyrosine kinase signaling. Neurosci Lett. 2016;615:83–87. doi: 10.1016/j.neulet.2016.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, Beyer K, Engelmann B. Delayed oxidative degradation of polyunsaturated diacyl phospholipids in the presence of plasmalogen phospholipids in vitro. Biochem J. 1997;323(3):807–814. doi: 10.1042/bj3230807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D, Ortori CA, Chapman V, Kendall DA, Barrett DA. Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry. Anal Biochem. 2007;360(2):216–226. doi: 10.1016/j.ab.2006.10.039. [DOI] [PubMed] [Google Scholar]
- Ro M, Park J, Nam M, Bang HJ, Yang J, Choi KS, Kim SK, Chung JH, Kwack K. Association between peroxisomal biogenesis factor 7 and autism spectrum disorders in a Korean population. J Child Neurol. 2012;27(10):1270–1275. doi: 10.1177/0883073811435507. [DOI] [PubMed] [Google Scholar]
- Rodemer C, Thai TP, Brugger B, Kaercher T, Werner H, Nave KA, Wieland F, Gorgas K, Just WW. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum Mol Genet. 2003;12(15):1881–1895. doi: 10.1093/hmg/ddg191. [DOI] [PubMed] [Google Scholar]
- Rossignol DA, Genuis SJ, Frye RE. Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry. 2014;4:e360. doi: 10.1038/tp.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Babiloni C, Polich J. Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Prog Neurobiol. 2007;83(6):375–400. doi: 10.1016/j.pneurobio.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Rother E, Brandl R, Baker DL, Goyal P, Gebhard H, Tigyi G, Siess W. Subtype-selective antagonists of lysophosphatidic acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation. 2003;108(6):741–747. doi: 10.1161/01.CIR.0000083715.37658.C4. [DOI] [PubMed] [Google Scholar]
- Rothhaar TL, Grosgen S, Haupenthal VJ, Burg VK, Hundsdorfer B, Mett J, Riemenschneider M, Grimm HS, Hartmann T, Grimm MO. Plasmalogens Inhibit AβP Processing by directly affecting gamma-secretase activity in Alzheimer’s disease. Scientific World Journal. 2012;2012 doi: 10.1100/2012/141240. 141240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G, Yamamoto A. Curvilinear regression course of human brain lipid composition changes with age. Lipids. 1968;3(3):284–287. doi: 10.1007/BF02531202. [DOI] [PubMed] [Google Scholar]
- Rubio JM, Astudillo AM, Casas J, Balboa MA, Balsinde J. Regulation of phagocytosis in macrophages by membrane ethanolamine plasmalogens. Front Immunol. 2018;9:1723. doi: 10.3389/fimmu.2018.01723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SD, Harris CS, Mo F, Lee H, Hou ST, Bazan NG, Haddad PS, Arnason JT, Bennett SA. Platelet activating factor-induced neuronal apoptosis is initiated independently of its G-protein coupled PAF receptor and is inhibited by the benzoate orsellinic acid. J Neurochem. 2007;103(1):88–97. doi: 10.1111/j.1471-4159.2007.04740.x. [DOI] [PubMed] [Google Scholar]
- Ryan SD, Whitehead SN, Swayne LA, Moffat TC, Hou W, Ethier M, Bourgeois AJ, Rashidian J, Blanchard AP, Fraser PE, Park DS, Figeys D, Bennett SA. Amyloid-beta42 signals tau hyperphosphorylation and compromises neuronal viability by disrupting alkylacylglycerophosphocholine metabolism. Proc Natl Acad Sci; U S A. 2009. pp. 20936–20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152(7):1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels IS, Saitta SC, Landreth GE. MAP’ing CNS development and cognition: an ERKsome process. Neuron. 2009;61(2):160–167. doi: 10.1016/j.neuron.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid PC, Reddy PV, Natarajan V, Schmid HH. Metabolism of N-acy-lethanolamine phospholipids by a mammalian phosphodiesterase of the phospholipase D type. J Biol Chem. 1983;258(15):9302–9306. [PubMed] [Google Scholar]
- Schmid HH, Schmid PC, Natarajan V. N-acylated glycerophospholipids and their derivatives. Prog Lipid Res. 1990;29(1):1–43. doi: 10.1016/0163-7827(90)90004-5. [DOI] [PubMed] [Google Scholar]
- Schonefeld M, Noble S, Bertorello AM, Mandel LJ, Creer MH, Portilla D. Hypoxia-induced amphiphiles inhibit renal Na+, K(+)-ATPase. Kidney Int. 1996;49(5):1289–1296. doi: 10.1038/ki.1996.184. [DOI] [PubMed] [Google Scholar]
- Schrader M, Kamoshita M, Islinger M. Organelle interplay-peroxisome interactions in health and disease. J Inherit Metab Dis. 2020;43(1):71–89. doi: 10.1002/jimd.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejimo S, Hossain MS, Akashi K. Scallop-derived plasmalogens attenuate the activation of PKCdelta associated with the brain inflammation. Biochem Biophys Res Commun. 2018;503(2):837–842. doi: 10.1016/j.bbrc.2018.06.084. [DOI] [PubMed] [Google Scholar]
- Senanayake V, Goodenowe DB. Plasmalogen deficiency and neuropathology in Alzheimer’s disease: causation or coincidence? Alzheimers Dement (N Y) 2019;5:524–532. doi: 10.1016/j.trci.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128(7):2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta. 2015;1851(4):397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Dong Y, Cui MZ, Xu X. Lysophosphatidic acid induces increased BACE1 expression and Abeta formation. Biochim Biophys Acta. 2013;1832(1):29–38. doi: 10.1016/j.bbadis.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JL, Joseph BK, Ruckle MB, Mayeux PR, Prather PL. The endocannabinoid noladin ether acts as a full agonist at human CB2 cannabinoid receptors. J Pharmacol Exp Ther. 2005;314(2):868–875. doi: 10.1124/jpet.105.085282. [DOI] [PubMed] [Google Scholar]
- da Silva TF, Eira J, Lopes AT, Malheiro AR, Sousa V, Luoma A, Avila RL, Wanders RJ, Just WW, Kirschner DA, Sousa MM, Brites P. Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. J Clin Invest. 2014;124(6):2560–2570. doi: 10.1172/JCI72063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C, Ingham V, Williams A, Bate C. Platelet-activating factor antagonists enhance intracellular degradation of amyloid-beta42 in neurons via regulation of cholesterol ester hydrolases. Alzheimers Res Ther. 2014;6(2):15. doi: 10.1186/alzrt245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MF, Chap H, Douste-Blazy L. Human platelet aggregation induced by 1-alkyl-lysophosphatidic acid and its analogs: a new group of phospholipid mediators? Biochem Biophys Res Commun. 1982;108(4):1743–1750. doi: 10.1016/s0006-291x(82)80113-7. [DOI] [PubMed] [Google Scholar]
- Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V, Sandvig K, Llorente A. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer. 2017;70:122–132. doi: 10.1016/j.ejca.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Skotland T, Hessvik NP, Sandvig K, Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res. 2019;60(1):9–18. doi: 10.1194/jlr.R084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder F. Metabolic processing of PAF. Clin Rev Allergy. 1994;12(4):309–327. doi: 10.1007/BF02802298. [DOI] [PubMed] [Google Scholar]
- Song S, Cheong LZ, Man QQ, Pang SJ, Li YQ, Ren B, Zhang J. Characterization of potential plasma biomarkers related to cognitive impairment by untargeted profiling of phospholipids using the HILIC-ESI-IT-TOF-MS system. Anal Bioanal Chem. 2018;410(12):2937–2948. doi: 10.1007/s00216-018-0975-0. [DOI] [PubMed] [Google Scholar]
- Southall MD, Isenberg JS, Nakshatri H, Yi Q, Pei Y, Spandau DF, Travers JB. The platelet-activating factor receptor protects epidermal cells from tumor necrosis factor (TNF) alpha and TNF-related apoptosis-inducing ligand-induced apoptosis through an NF-kappa B-dependent process. J Biol Chem. 2001;276(49):45548–45554. doi: 10.1074/jbc.M105978200. [DOI] [PubMed] [Google Scholar]
- Souza DG, Fagundes CT, Sousa LP, Amaral FA, Souza RS, Souza AL, Kroon EG, Sachs D, Cunha FQ, Bukin E, Atrasheuskaya A, Ignatyev G, Teixeira MM. Essential role of platelet-activating factor receptor in the pathogenesis of Dengue virus infection. Proc Natl Acad Sci; U S A. 2009. pp. 14138–14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet-activating factor, a pleiotrophic mediator of physiological and pathological processes. Crit Rev Clin Lab Sci. 2003;40(6):643–672. doi: 10.1080/714037693. [DOI] [PubMed] [Google Scholar]
- Steffens M, Zentner J, Honegger J, Feuerstein TJ. Binding affinity and agonist activity of putative endogenous cannabinoids at the human neocortical CB1 receptor. Biochem Pharmacol. 2005;69(1):169–178. doi: 10.1016/j.bcp.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Nakane S, Kishimoto S, Waku K, Yoshioka Y, Tokumura A, Hanahan DJ. Occurrence of lysophosphatidic acid and its alkyl ether-linked analog in rat brain and comparison of their biological activities toward cultured neural cells. Biochim Biophys Acta. 1999;1440(2–3):194–204. doi: 10.1016/s1388-1981(99)00127-4. [DOI] [PubMed] [Google Scholar]
- Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K, Murphy SP, Kendall DA, Bennett AJ. Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. Br J Pharmacol. 2007;152(5):734–743. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, Taylor E, Casey BJ, Castellanos FX, Wadhwa PD. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and en-vironmental factors and the dopamine hypothesis. Neuropsychol Rev. 2007;17(1):39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- Szekely J, Gates KS. Noncovalent DNA binding and the mechanism of oxidative DNA damage by fecapentaene-12. Chem Res Toxicol. 2006;19(1):117–121. doi: 10.1021/tx050197e. [DOI] [PubMed] [Google Scholar]
- Taguchi H, Armarego WL. Glyceryl-ether monooxygenase [EC 1.14.16.5] A microsomal enzyme of ether lipid metabolism. Med Res Rev. 1998;18(1):43–89. doi: 10.1002/(sici)1098-1128(199801)18:1<43::aid-med3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Tajima Y, Ishikawa M, Maekawa K, Murayama M, Senoo Y, Nishimaki-Mogami T, Nakanishi H, Ikeda K, Arita M, Taguchi R, Okuno A, Mikawa R, Niida S, Takikawa O, Saito Y. Lipidomic analysis of brain tissues and plasma in a mouse model expressing mutated human amyloid precursor protein/tau for Alzheimer’s disease. Lipids Health Dis. 2013;12:68. doi: 10.1186/1476-511X-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Honsho M, Abe Y, Fujiki Y. Plasmalogen mediates integration of adherens junction. J Biochem. 2019;166(5):423–432. doi: 10.1093/jb/mvz049. [DOI] [PubMed] [Google Scholar]
- Thapar A, O’Donovan M, Owen MJ. The genetics of attention deficit hyperactivity disorder. Hum Mol Genet. 2005;14:R275–R282. doi: 10.1093/hmg/ddi263. Spec No. 2. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Byers DM, Palmer FB, Spence MW, Cook HW. Incorporation of polyunsaturated fatty acids into plasmalogens, compared to other phospholipids of cultured glioma cells, is more dependent on chain length than on selectivity between (n - 3) and (n - 6) families. Biochim Biophys Acta. 1990;1044(3):349–356. doi: 10.1016/0005-2760(90)90079-d. [DOI] [PubMed] [Google Scholar]
- Thukkani AK, Hsu FF, Crowley JR, Wysolmerski RB, Albert CJ, Ford DA. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. J Biol Chem. 2002;277(6):3842–3849. doi: 10.1074/jbc.M109489200. [DOI] [PubMed] [Google Scholar]
- Thukkani AK, Albert CJ, Wildsmith KR, Messner MC, Martinson BD, Hsu FF, Ford DA. Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. J Biol Chem. 2003;278(38):36365–36372. doi: 10.1074/jbc.M305449200. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Yoshida J, Maruyama T, Fukuzawa K, Tsukatani H. Platelet aggregation induced by ether-linked phospholipids. 1. Inhibitory actions of bovine serum albumin and structural analogues of platelet activating factor. Thromb Res. 1987;46(1):51–63. doi: 10.1016/0049-3848(87)90206-4. [DOI] [PubMed] [Google Scholar]
- Tokuoka SM, Ishii S, Kawamura N, Satoh M, Shimada A, Sasaki S, Hirotsune S, Wynshaw-Boris A, Shimizu T. Involvement of platelet-activating factor and LIS1 in neuronal migration. Eur J Neurosci. 2003;18(3):563–570. doi: 10.1046/j.1460-9568.2003.02778.x. [DOI] [PubMed] [Google Scholar]
- Tokuoka SM, Kita Y, Shindou H, Shimizu T. Alkylglycerol monooxygenase as a potential modulator for PAF synthesis in macrophages. Biochem Biophys Res Commun. 2013;436(2):306–312. doi: 10.1016/j.bbrc.2013.05.099. [DOI] [PubMed] [Google Scholar]
- Toledo JB, et al. Metabolic network failures in Alzheimer’s disease: a bio-chemical road map. Alzheimers Dement. 2017;13(9):965–984. doi: 10.1016/j.jalz.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggiani M, Schleimer RP, Warner JA, Chilton FH. Differential synthesis of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine and platelet-activating factor by human inflammatory cells. J Immunol. 1991;147(2):660–666. [PubMed] [Google Scholar]
- Tsuboi K, Okamoto Y, Ikematsu N, Inoue M, Shimizu Y, Uyama T, Wang J, Deutsch DG, Burns MP, Ulloa NM, Tokumura A, Ueda N. Enzymatic formation of N-acylethanolamines from N-acylethanolamine plasmalogen through N-acylphosphatidylethanolamine-hydrolyzing phospholipase D-dependent and -independent pathways. Biochim Biophys Acta. 2011;1811(10):565–577. doi: 10.1016/j.bbalip.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Tozaki-Saitoh H, Inoue K. Platelet-activating factor and pain. Biol Pharm Bull. 2011;34(8):1159–1162. doi: 10.1248/bpb.34.1159. [DOI] [PubMed] [Google Scholar]
- Tsukahara T. 1-O-alkyl glycerophosphate-induced CD36 expression drives oxidative stress in microglial cells. Cell Signal. 2020;65 doi: 10.1016/j.cellsig.2019.109459. 109459. [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Tsukahara R, Yasuda S, Makarova N, Valentine WJ, Allison P, Yuan H, Baker DL, Li Z, Bittman R, Parrill A, Tigyi G. Different residues mediate recognition of 1-O-oleyllysophosphatidic acid and rosiglitazone in the ligand binding domain of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2006;281(6):3398–3407. doi: 10.1074/jbc.M510843200. [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Haniu H, Matsuda Y. Effect of alkyl glycerophosphate on the activation of peroxisome proliferator-activated receptor gamma and glucose uptake in C2C12 cells. Biochem Biophys Res Commun. 2013;433(3):281–285. doi: 10.1016/j.bbrc.2013.02.101. [DOI] [PubMed] [Google Scholar]
- Uemura Y, Lee TC, Snyder F. A coenzyme A-independent transacylase is linked to the formation of platelet-activating factor (PAF) by generating the lyso-PAF intermediate in the remodeling pathway. J Biol Chem. 1991;266(13):8268–8272. [PubMed] [Google Scholar]
- Ueno K, Ishizuka I, Yamakawa T. Glycolipid composition of human testis at different ages and the stereochemical configuration of seminolipid. Biochim Biophys Acta. 1977;487(1):61–73. [PubMed] [Google Scholar]
- Ullen A, Fauler G, Kofeler H, Waltl S, Nusshold C, Bernhart E, Reicher H, Leis HJ, Wintersperger A, Malle E, Sattler W. Mouse brain plasmalogens are targets for hypochlorous acid-mediated modification in vitro and in vivo. Free Radic Biol Med. 2010;49(11):1655–1665. doi: 10.1016/j.freeradbiomed.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uruno A, Matsumaru D, Ryoke R, Saito R, Kadoguchi S, Saigusa D, Saito T, Saido TC, Kawashima R, Yamamoto M. Nrf2 suppresses oxidative stress and inflammation in App knock-in Alzheimer’s disease model mice. Mol Cell Biol. 2020;40(6):e00467–00419. doi: 10.1128/MCB.00467-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama T, Ichi I, Kono N, Inoue A, Tsuboi K, Jin XH, Araki N, Aoki J, Arai H, Ueda N. Regulation of peroxisomal lipid metabolism by catalytic activity of tumor suppressor H-rev107. J Biol Chem. 2012;287(4):2706–2718. doi: 10.1074/jbc.M111.267575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama T, Inoue M, Okamoto Y, Shinohara N, Tai T, Tsuboi K, Inoue T, Tokumura A, Ueda N. Involvement of phospholipase A/acyltransferase-1 in N-acylphosphatidylethanolamine generation. Biochim Biophys Acta. 2013;1831(12):1690–1701. doi: 10.1016/j.bbalip.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Uyama T, Kawai K, Kono N, Watanabe M, Tsuboi K, Inoue T, Araki N, Arai H, Ueda N. Interaction of phospholipase A/Acyltransferase-3 with Pex19p: a possible involvement in the down-regulation of peroxisomes. J Biol Chem. 2015;290(28):17520–17534. doi: 10.1074/jbc.M114.635433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Guillen PL, Fernandez-Natal I, Marrodan-Ciordia T, Tauch A, Soriano F. Ether-linked lipids of Dermabacter hominis, a human skin actinobacterium. Chem Phys Lipids. 2016;196:24–32. doi: 10.1016/j.chemphyslip.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Van Tassel RL, MacDonald DK, Wilkins TD. Production of a fecal mutagen by Bacteroides spp. Infect Immun. 1982;37(3):975–980. doi: 10.1128/iai.37.3.975-980.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venable ME, Zimmerman GA, McIntyre TM, Prescott SM. Platelet-activating factor: a phospholipid autacoid with diverse actions. J Lipid Res. 1993;34(5):691–702. [PubMed] [Google Scholar]
- Villamil-Ortiz JG, Barrera-Ocampo A, Arias-Londono JD, Villegas A, Lopera F, Cardona-Gomez GP. Differential pattern of phospholipid profile in the temporal cortex from E280A-familiar and sporadic Alzheimer’s disease brains. J Alzheimers Dis. 2018;61(1):209–219. doi: 10.3233/JAD-170554. [DOI] [PubMed] [Google Scholar]
- Vithayathil J, Pucilowska J, Landreth GE. ERK/MAPK signaling and autism spectrum disorders. Prog Brain Res. 2018;241:63–112. doi: 10.1016/bs.pbr.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Wang HY, Pisano MR, Friedman E. Attenuated protein kinase C activity and translocation in Alzheimer’s disease brain. Neurobiol Aging. 1994;15(3):293–298. doi: 10.1016/0197-4580(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhou K, Fu Z, Yu D, Huang H, Zang X, Mo X. Brain development and Akt signaling: the crossroads of signaling pathway and neurodevelopmental diseases. J Mol Neurosci. 2017;61(3):379–384. doi: 10.1007/s12031-016-0872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne TR, Buchanan FG, Robinson M. Growth-dependent accumulation of monoalkylglycerol in Madin-Darby canine kidney cells Evidence for a role in the regulation of protein kinase C. J Biol Chem. 1995;270(19):11147–11154. doi: 10.1074/jbc.270.19.11147. [DOI] [PubMed] [Google Scholar]
- Watschinger K, Werner ER. Orphan enzymes in ether lipid metabolism. Biochimie. 2013;95(1):59–65. doi: 10.1016/j.biochi.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watschinger K, Keller MA, Golderer G, Hermann M, Maglione M, Sarg B, Lindner HH, Hermetter A, Werner-Felmayer G, Konrat R, Hulo N, Werner ER. Identification of the gene encoding alkylglycerol monooxygenase defines a third class of tetrahydrobiopterin-dependent enzymes. Proc Natl Acad Sci; U S A. 2010. pp. 13672–13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb BL, Hirst SJ, Giembycz MA. Protein kinase C isoenzymes: a review of their structure, regulation and role in regulating airways smooth muscle tone and mitogenesis. Br J Pharmacol. 2000;130(7):1433–1452. doi: 10.1038/sj.bjp.0703452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburger JH, Jones RC, Wang CX, Backlund JY, Williams GM, Kingston DG, Van Tassell RL, Keyes RF, Wilkins TD, de Wit PP, et al. Carcinogenicity tests of fecapentaene-12 in mice and rats. Cancer Lett. 1990;49(2):89–98. doi: 10.1016/0304-3835(90)90143-l. [DOI] [PubMed] [Google Scholar]
- Wellner N, Diep TA, Janfelt C, Hansen HS. N-acylation of phosphatidy-lethanolamine and its biological functions in mammals. Biochim Biophys Acta. 2013;1831(3):652–662. doi: 10.1016/j.bbalip.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Werner ER, Keller MA, Sailer S, Lackner K, Koch J, Hermann M, Coassin S, Golderer G, Werner-Felmayer G, Zoeller RA, Hulo N, Berger J, Watschinger K. The TMEM189 gene encodes plasmanylethanolamine desaturase which introduces the characteristic vinyl ether double bond into plasmalogens. Proc Natl Acad Sci; U S A. 2020. pp. 7792–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MC, Rastogi P, McHowat J. Lysoplasmenylcholine increases neutrophil adherence to human coronary artery endothelial cells. Am J Physiol Cell Physiol. 2007;293(5):C1467–C1471. doi: 10.1152/ajpcell.00290.2007. [DOI] [PubMed] [Google Scholar]
- Wieraszko A, Li G, Kornecki E, Hogan MV, Ehrlich YH. Long-term potentiation in the hippocampus induced by platelet-activating factor. Neuron. 1993;10(3):553–557. doi: 10.1016/0896-6273(93)90342-o. [DOI] [PubMed] [Google Scholar]
- Wiest MM, German JB, Harvey DJ, Watkins SM, Hertz-Picciotto I. Plasma fatty acid profiles in autism: a case-control study. Prostaglandins Leukot. Essent Fatty Acids. 2009;80(4):221–227. doi: 10.1016/j.plefa.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Williams SD, Ford DA. Activation of myocardial cAMP-dependent protein kinase by lysoplasmenylcholine. FEBS Lett. 1997;420(1):33–38. doi: 10.1016/s0014-5793(97)01482-8. [DOI] [PubMed] [Google Scholar]
- Williams JR, Khandoga AL, Goyal P, Fells JI, Perygin DH, Siess W, Parrill AL, Tigyi G, Fujiwara Y. Unique ligand selectivity of the GPR92/LPA5 lyso-phosphatidate receptor indicates role in human platelet activation. J Biol Chem. 2009;284(25):17304–17319. doi: 10.1074/jbc.M109.003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willier S, Butt E, Grunewald TG. Lysophosphatidic acid (LPA) signalling in cell migration and cancer invasion: a focussed review and analysis of LPA receptor gene expression on the basis of more than 1700 cancer microarrays. Biol Cell. 2013;105(8):317–333. doi: 10.1111/boc.201300011. [DOI] [PubMed] [Google Scholar]
- Wu LC, Pfeiffer DR, Calhoon EA, Madiai F, Marcucci G, Liu S, Jurkowitz MS. Purification, identification, and cloning of lysoplasmalogenase, the enzyme that catalyzes hydrolysis of the vinyl ether bond of lysoplasmalogen. J Biol Chem. 2011;286(28):24916–24930. doi: 10.1074/jbc.M111.247163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Tanaka M, Arai H, Aoki J, Prestwich GD. Alkyl lysophosphatidic acid and fluoromethylene phosphonate analogs as metabolically-stabilized agonists for LPA receptors. Bioorg Med Chem Lett. 2004;14(21):5323–5328. doi: 10.1016/j.bmcl.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Kanno S, Nakagawa K, Kinoshita M, Miyazawa T. Extrinsic plasmalogens suppress neuronal apoptosis in mouse neuroblastoma Neuro-2A cells: importance of plasmalogen molecular species. RSC Adv. 2015a;5(75):61012–61020. [Google Scholar]
- Yamashita S, Kiko T, Fujiwara H, Hashimoto M, Nakagawa K, Kinoshita M, Furukawa K, Arai H, Miyazawa T. Alterations in the levels of amyloidbeta, phospholipid hydroperoxide, and plasmalogen in the blood of patients with Alzheimer’s disease: possible interactions between amyloid-beta and these lipids. J Alzheimers Dis. 2015b;50(2):527–537. doi: 10.3233/JAD-150640. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Hashimoto M, Haque AM, Nakagawa K, Kinoshita M, Shido O, Miyazawa T. Oral administration of ethanolamine glycerophospholipid containing a high level of plasmalogen improves memory impairment in amyloid beta-infused rats. Lipids. 2017;52(7):575–585. doi: 10.1007/s11745-017-4260-3. [DOI] [PubMed] [Google Scholar]
- Yang HC, Farooqui AA, Horrocks LA. Plasmalogen-selective phospholipase A2 and its role in signal transduction. J Lipid Mediat Cell Signal. 1996;14(1–3):9–13. doi: 10.1016/0929-7855(96)01502-7. [DOI] [PubMed] [Google Scholar]
- Yang K, Jenkins CM, Dilthey B, Gross RW. Multidimensional mass spectrometry-based shotgun lipidomics analysis of vinyl ether diglycerides. Anal Bioanal Chem. 2015;407(17):5199–5210. doi: 10.1007/s00216-015-8640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Chun J. Lysophosphatidic acid (LPA) signaling in vertebrate reproduction. Trends Endocrinol Metab. 2010;21(1):17–24. doi: 10.1016/j.tem.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda E, Rai K, Ogawa M, Takakura Y, Kuwata H, Suzuki H, Nakatani Y, Murakami M, Hara S. Group VIB calcium-independent phospholipase A2 (iPLA2gamma) regulates platelet activation, hemostasis and thrombosis in mice. PLoS One. 2014;9(10):e109409. doi: 10.1371/journal.pone.0109409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost CC, Weyrich AS, Zimmerman GA. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie. 2010;92(6):692–697. doi: 10.1016/j.biochi.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef M, Ibrahim A, Akashi K, Hossain MS. PUFA-plasmalogens attenuate the LPS-induced nitric oxide production by inhibiting the NF-kB, p38 MAPK and JNK pathways in microglial cells. Neuroscience. 2019;397:18–30. doi: 10.1016/j.neuroscience.2018.11.030. [DOI] [PubMed] [Google Scholar]
- Yu TW, et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron. 2013;77(2):259–273. doi: 10.1016/j.neuron.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, et al. Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J Clin Invest. 2019;129(6):2485–2499. doi: 10.1172/JCI125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung YC, Stoddard NC, Mirendil H, Chun J. Lysophosphatidic acid signaling in the nervous system. Neuron. 2015;85(4):669–682. doi: 10.1016/j.neuron.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkovic M, Qin X, Nakatsuru Y, Oda H, Nakamura T, Shamsuddin AM, Ishikawa T. Tumor promotion by fecapentaene-12 in a rat colon carcinogenesis model. Carcinogenesis. 1993;14(7):1261–1264. doi: 10.1093/carcin/14.7.1261. [DOI] [PubMed] [Google Scholar]
- Zhang C, Baker DL, Yasuda S, Makarova N, Balazs L, Johnson LR, Marathe GK, McIntyre TM, Xu Y, Prestwich GD, Byun HS, Bittman R, Tigyi G. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J Exp Med. 2004;199(6):763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RA, Morand OH, Raetz CR. A possible role for plasmalogens in protecting animal cells against photosensitized killing. J Biol Chem. 1988;263(23):11590–11596. [PubMed] [Google Scholar]
- Zoeller RA, Lake AC, Nagan N, Gaposchkin DP, Legner MA, Lieberthal W. Plasmalogens as endogenous antioxidants: somatic cell mutants reveal the importance of the vinyl ether. Biochem J. 1999;338(3):769–776. [PMC free article] [PubMed] [Google Scholar]