Abstract
Mimicking the various facets of human psychiatric and neurodevelopmental disorders in animal models is a challenging task. Nevertheless, mice have emerged as a widely used model system to study pathophysiology and treatment strategies for these diseases. However, the corresponding behavioral tests are often elaborate and require extensive experience in behavioral testing. Here, we present protocols for two simple assays, nest building and nestlet shredding, that can serve as a starting point for the behavioral phenotyping of mouse models with (potential) features of psychiatric disorders. Both tests have been reported previously and we extend prior descriptions by including adaptations and refinements derived from our practical experience, like the use of the home cage instead of a fresh cage for nestlet shredding. Summarized, we provide ready-to-use protocols for two behavioral assays that allow the generation of robust data with minimal time and cost expenditure and enable an initial assessment of features of psychiatric or neurodevelopmental disorders in mouse models of these diseases.
Keywords: Behavior, Autism, Neurodevelopmental disorder, Repetitive behavior, Plasmalogen, Stereotypy, Stress, Neurological disease, Mouse model
Background
Due to the unmatched complexity of the human brain, it is difficult to model features of psychiatric and neurodevelopmental diseases using animal models. However, in spite of some limitations, in mice an impressive battery of tests targeting numerous individual aspects of these disorders has been developed in recent years ( Seong et al., 2002 ; Ricceri et al., 2007 ; Sukoff Rizzo and Crawley, 2017). Many of these assays are now widely accepted as valid tools to study disease mechanisms or therapeutic approaches. Here, we demonstrate two simple tests, nestlet shredding and nest building, widely used to study potential features of psychiatric and, particularly, neurodevelopmental disorders that we have – among others – used recently to characterize the behavioral phenotype of mice with a genetically caused deficiency to synthesize ether lipids ( Dorninger et al., 2019a ) (cf. Figure 1). The latter are compounds performing a wide range of biological activities, ranging from structural maintenance of cellular membranes to signal transduction ( Dorninger et al., 2017a ; Dorninger et al., 2020 ), and are particularly important for correct functioning of the brain ( Berger et al., 2016 ). Accordingly, ether lipid-deficient mice present with complex behavioral abnormalities including motor disturbances, hyperactivity and memory deficits as well as restricted social interaction and interest in novel objects ( Dorninger et al., 2017b ; Dorninger et al., 2019a ; Dorninger et al., 2019b ).
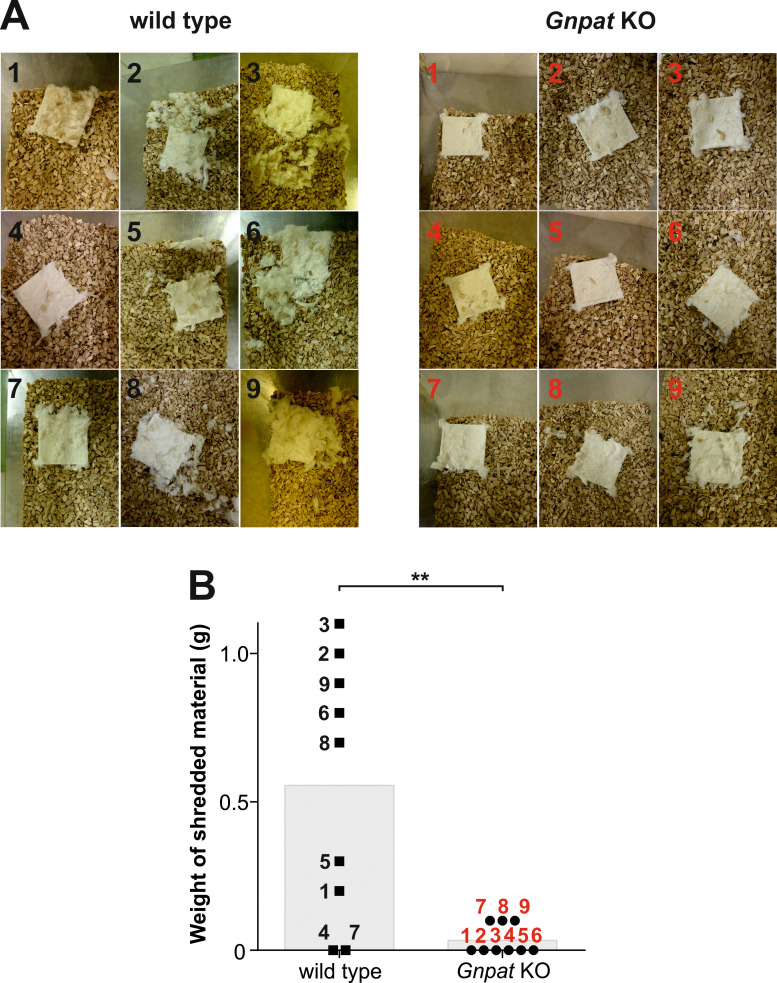
Figure 1. Nestlet shredding in a cohort of wild type and ether lipid-deficient (“Gnpat KO”) mice.
A. Nestlets after the 30 min-observation period are documented for all tested animals (n = 9 per genotype). Numbers inside the panels indicate the test ID (black: wild type; red: Gnpat KO). Note the markedly higher shredding activity of wild type compared with Gnpat KO mice. B. The difference in nestlet weight before and after the trial was calculated for each test animal and results are shown as individual data points. Grey bars indicate the mean value. Numbers next to the data points designate the mouse test ID, as defined in (A). Statistical testing was performed using Mann-Whitney U-test (**p < 0.01). The quantitative data shown here have also been presented in our previous publication ( Dorninger et al., 2019a ).
Both of the presented assays have several obvious advantages: They are low-cost experiments, by which a significant amount of data can be obtained in a relatively short time frame. Furthermore, they can be reliably performed by investigators with only modest experience in behavioral analysis. Both tests are hardly dependent on physical abilities of the animals, thus allowing the use of genetically modified mouse models with marked phenotypes. For example, we used mice with considerable developmental defects and visual impairments (the ether lipid-deficient Gnpat knockout (KO) mouse model) ( Rodemer et al., 2003 ), which precluded the application of many behavioral tests. However, as demonstrated by their performance in the nest building assay ( Dorninger et al., 2019a ), the animals are perfectly capable to utilize nestlets. Certainly, the simplicity of the assays also comes with some caveats: Both tests provide only an initial estimation on potential features of neurodevelopmental disorders and should not be used isolated. Also, they do not yield numeric data that can be directly related to the extent of human disease. Instead, they need to be complemented by additional behavioral tests assessing similar features ( Silverman et al., 2010 ) and, optimally, embedded in a battery of assays allowing a comprehensive description of the behavioral phenotype. Furthermore, results need to be interpreted with care: Excessive nestlet shredding is generally seen as an indicator of repetitive behavior, a symptom also seen in human autism spectrum disorders or obsessive-compulsive disorder (Angoa- Perez et al., 2013 ). On the contrary, reduced engagement in the task may be viewed as restricted interest in novel objects, another feature commonly associated with autism ( Bernard et al., 2015 ; Dorninger et al., 2019a ). We feel that both interpretations are valid but need to be considered in the context of other aspects of behavior of a certain mouse model. Nest building, in turn, can be indicative of general well-being, distress or pain (Jirkof, 2014). At the same time, impairments in this task could hint towards structural or functional deficits in the brain, as demonstrated by mouse models of brain injury or with genetic modifications ( Lijam et al., 1997 ; Deacon et al., 2002 ; Sager et al., 2010 ).
Our procedures for both tests are based on the previous protocols (Deacon, 2006; Angoa- Perez et al., 2013 ; Neely et al., 2019 ) that we have slightly modified. Furthermore, we provide more detailed descriptions of the testing protocols, including novel recommendations on the testing environment and extended illustration of the procedures and results, and share our experiences in test execution and analysis.
Materials and Reagents
Cotton fiber nestlets (Ancare; product no. NES3600; 1 per mouse and behavioral test)
Labeled plastic bags for storage of used nestlets
-
Animals
There is no restriction concerning the mouse strain to be used for the current protocols. The presented data were generated using animals on an outbred C57BL/6 x CD1 background carrying a targeted inactivation of the Gnpat gene (Gnpattm1Just, MGI:2670462; Rodemer et al., 2003 ) and their wild type littermates.
Equipment
Common laboratory balance (minimum accuracy: 0.1 g)
Clean mouse cages with fresh bedding (as conventionally used by your animal facility)
Standard camera
Timer
Forceps
Glass or plexiglass plate; dimensions depending on the size of the cage used for testing (the plate should be large enough to replace the cage lid)
Software
Standard statistics software: Prism (GraphPad; version 6 or higher), Sigma Plot (Systat; version 11.0 or higher) or similar
Optional: Video tracking software (e.g., VideoTrack, ViewPoint or similar)
Procedure
-
Nestlet shredding test
House mice individually for at least 7 days prior to the test. Make sure that housing conditions fulfill common standards like controlled lighting (in our case 12:12 h light-dark), temperature and humidity as well as low noise level, enriched cage environment and provision of chow and water ad libitum.
24 h prior to the test, remove any material for nesting and environmental enrichment from the cage of test mice.
On the testing day, relocate testing animals to the test area. Make sure the animals are accustomed to their environment to avoid distraction, for example by unfamiliar smells. This can be achieved by performing the test in a separated space of the room, in which the mice are housed. Alternatively, habituate mice to the test area on the days prior to testing for a minimum of 1 h per day.
Remove food and water from the test animal’s cage and replace the lid by a transparent object with a smooth surface, such as a (glass or plexiglass) plate, to prevent mice from climbing during the test time.
Take a nestlet using a forceps, weigh it and place it in the test animal’s cage.
Ensure documentation by taking a photo at the beginning of the trial. Alternatively, an automated video system (e.g., VideoTrack, ViewPoint or similar) may be used. Make sure to document identifying information of the tested animal (e.g., by picturing the cage label including the mouse ID prior to picturing the nestlet).
Start the timer. During the observation period of 30 min, keep a distance of at least 1 m from the test area.
After 30 min, stop the trial and gently remove the test animal from the cage. Make sure all nestlet material remains in the cage.
Document the state after the end of the trial using the camera (Figure 1).
Remove the nestlet and weigh it. If the nestlet is torn into several pieces, use the largest one. Do not include pieces that fall off during removal of the nestlet, but do not deliberately try to shake off loose material.
Store the nestlet in a plastic bag for documentation. Do not forget to label the bag with the mouse ID.
When testing animals sequentially, alternate between treatment groups to avoid bias in your analysis.
-
Nest building test
House mice individually for at least 7 days prior to the test. Make sure that housing conditions fulfill common standards like controlled lighting (in our case 12:12 h light-dark), temperature and humidity as well as low noise level, enriched cage environment and provision of chow and water ad libitum. Testing can be performed in the animals’ standard housing room, which ensures maximal familiarization of mice with their environment.
24 h prior to the test, remove any nesting material from the cage of test mice.
Avoid any setting that could provoke a bias in your test results (e.g., spatially separating different test groups).
On the test day (preferably in the evening), supply every test mouse with a nestlet. Distribute nestlets using a clean forceps.
Document the state at the beginning of the trial by taking a photo.
After 20 h, document the status quo (“t20”). Take care not to disorganize the nest – thus, avoid any agitation of the animals. Therefore, to take a photo, wait till the mouse either sits steadily in its nest (make sure that the nest is clearly visible) or leaves its nest voluntarily. Do not dislocate mice forcibly from the nest. Make sure to document identifying information of the tested animal (e.g., by picturing the cage label including the mouse ID prior to picturing the nestlet).
After 48 h, end the trial by moving test mice to a clean cage. Document the final nest and avoid disorganizing it.
Data analysis
-
Nestlet shredding test
For every test animal, calculate the difference between the nestlet weight before and after the trial (Figure 1).
Do not exclude animals, which did not engage in nestlet shredding. In general, we recommend to make use of exclusion sparingly (if a statistical exclusion criterion is chosen, use > 5-7 x SD; Barbato et al., 2011 ). We usually take the position that uncommon behavior in a certain behavioral task is insufficient to exclude animals, as this may create a bias in your analysis. Thus, only exclude animals that are obviously impaired (e.g., hurt) or physically unable to perform the task. However, this should be done prior to performing the trial, in compliance with current ARRIVE guidelines (Percie du Sert et al., 2020 ).
Compare your test groups using the statistics software of your choice. In the case of normally distributed values, parametric tests may be used (e.g., Student’s t-test for comparison of two groups, one-way ANOVA for comparison of more than two groups). However, with the mouse strain that we used, a considerable number of values equaled to 0, thus precluding the use of parametric tests. In that case, use non-parametric tests like the Mann-Whitney U-test (for comparison of two groups) or the Kruskal-Wallis test (for comparison of more than two groups).
-
Nest building test
For the assessment of nests, engage 2-3 independent investigators that were not involved in the testing procedure or in handling the mice.
Do not exclude animals, which did not engage in nest building. If any other exclusion criteria are applied, make sure to follow the ARRIVE guidelines (Percie du Sert et al., 2020 ).
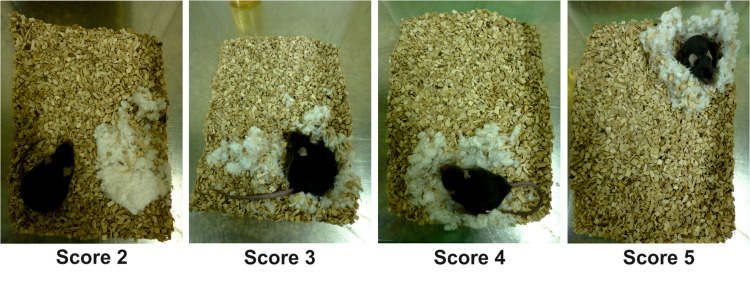
Grade nests using scores ranging from 1 (very poor/no nest building) to 5 (optimal nest building) according to a previously published scoring scheme (Deacon, 2006) (Table 1 and Figure 2). Assessors must be blinded to the genotype or treatment group of the test animals.
Use non-parametric tests for comparison of your test groups at each time point. Correction for multiple testing at the two time points should be considered (in particular, if analysis at t20 and at t48 do not target different research questions).
Table 1. Scoring scheme for assessment of nests, as described by Deacon, 2006.
Note that grading steps of 0.5 can be assigned for more exact evaluation.
| Score | Description |
| 1 | Nestlet (mostly) untouched |
| 2 | Parts of the nestlet shredded; 50% or more remain intact |
| 3 | Nestlet mostly shredded but material often dispersed with no delimited nest site |
| 4 | Identifiable but flat nest with most of the nestlet shredded |
| 5 | Nestlet fully shredded to form a crater-like, delimited nest |
Figure 2. Scoring in the nest building test.
Scoring was performed according to a previously published assessment scheme (Table 1; Deacon, 2006) using grades ranging from 1 (very poor) to 5 (optimal). Examples of nests reaching scores of 2 (A), 3 (B), 4 (C) and 5 (D) are shown. For all of these nests, the vote of two independent evaluators was unanimous. Note that grade 1 would correspond to an untouched nestlet. However, no animal graded lower than a score of 2 in our test cohort.
Notes
Like for any behavioral assay, make sure you test all animals at approximately the same time of the day, preferably in the evening, which marks the beginning of the active phase of mice when housed at a conventional light-dark cycle.
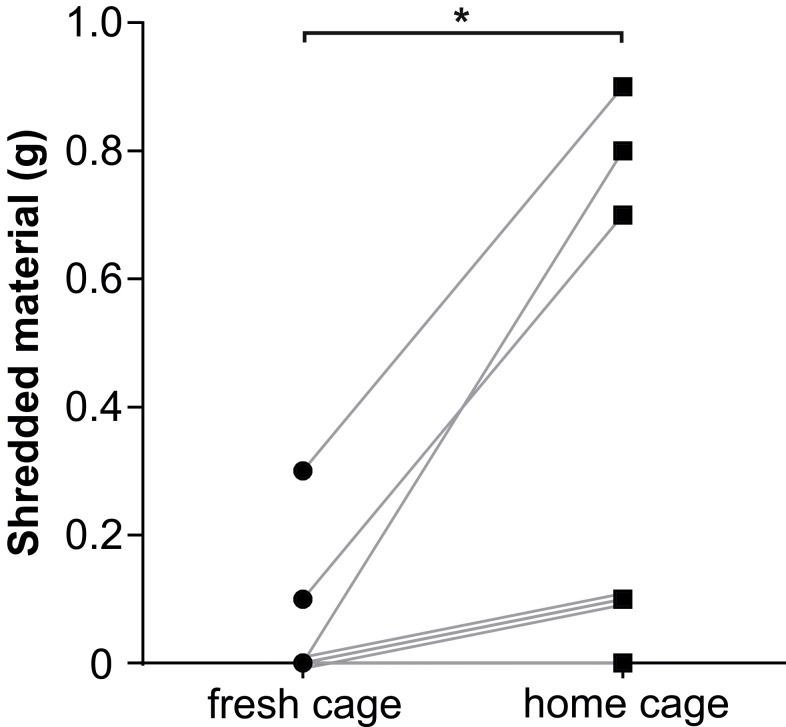
In previous protocols, nestlet shredding was described in clean cages with fresh bedding (Angoa- Perez et al., 2013 ). However, in the mouse strain we used, this led to increased interest of the animals in the unfamiliar environment and less engagement in nestlet shredding. To avoid this kind of distraction, we performed the assay in the animals’ home cage, which clearly increased shredding activity and generated more discriminative results (Figure 3).
Additional information can be gathered by repeating the nestlet shredding assay on consecutive days. However, for our purposes, we decided against repeated measurements to avoid habituation to the task.
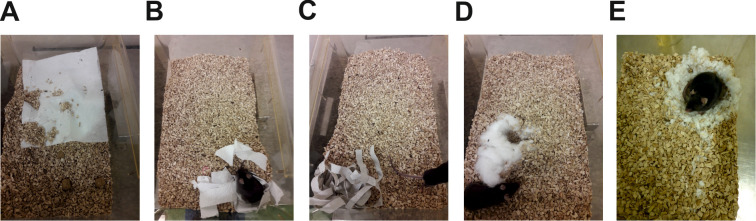
We tested several types of nesting material for use in the nest building test. In our hands, nestlets proved to produce most reproducible results and led to a more discriminative performance compared with paper towels, customary kitchen roll (cut in stripes or uncut) or cotton wool (Figure 4). Please note that other authors suggested to conduct four consecutive trials with different types of material ( Neely et al., 2019 ), which may be considered as an alternative option.
The time points, at which nests are analyzed in the nest building test, can be varied at the experimenter’s discretion. However, make sure to cover at least one overnight period, as this constitutes the animals’ active phase, i.e., we recommend a minimum of 12 h until the first analysis.
Mice of both sexes can be used for the presented tests. To minimize variability, male and female mice should be analyzed separately. However, keep in mind that in this case statistical correction for multiple comparisons may have to be performed.
Similarly, there are only limited constraints concerning the age of the test animals. Basically, adult animals of any age can be used. Yet, we generally recommend to keep the age range as narrow as possible for low variability. Also, the phenotype of genetically modified models may change with age, thus affecting performance in behavioral assays.
Both tests can be performed with the same cohort of animals. However, in order to avoid habituation to the nestlets, a resting interval of 7 days should be maintained between the tests.
In our experiments, we used mice of a mixed background strain (outbred C57BL/6 x CD1). These animals reliably showed nesting behavior. However, nesting has been reported to be highly strain-sensitive (Deacon, 2006 and 2012), thus baseline data should be obtained in a test cohort of your background strain. Average nestlet shredding activity in our strain was similar as described previously (Angoa- Perez et al., 2013 ). Yet, we experienced considerable variability ( Dorninger et al., 2019a ), thus emphasizing the need for statistical power calculation using baseline data before performing the assay in your test cohort.
In case you are working with a mouse strain/genotype/treatment condition that does not produce good nests, the percentage of nestlet material used for nest building can be taken as an alternative readout parameter. For this, weigh the nestlet prior to supplying it to the mice (step 4). After the trial, weigh the part of the nestlet that was not utilized for nest building and calculate the fraction of nestlet material that has been converted to the nest.
Figure 3. Different nestlet shredding activity in the home cage versus a fresh cage.
The same cohort of mice (n = 8) was exposed to the nestlet shredding test in a cleaned cage filled with fresh bedding (“fresh cage”) and, one week later to exclude habituation to the task, in their home cage. Connected data points derive from the same test animal. Note that the same shredding score (0 g in the fresh cage and 0.1 g in the home cage) was reached by three different mice. Statistical analysis was performed using paired Student’s t test. *p < 0.05
Figure 4. Comparison of different material types for use in the nest building test.
Test animals were supplied with a sheet of customary kitchen roll (either uncut, A, or cut to stripes, B), a paper towel (cut to stripes, C), cotton wool (D) or a nestlet (E) and the nests produced after a trial period of 24 h are shown. For the present protocol, the nestlet was selected as the most suitable material, as it produced the most discriminative results.
Acknowledgments
This work was supported by the Austrian Science Fund (FWF, P24843-B24, P31082-B21 and I2738-B26) and RhizoKids International. Data derived from the use of the protocols described here have been published recently ( Dorninger et al., 2019a ).
Competing interests
The authors declare no competing interests.
Ethics
All described protocols and experiments were approved by the Institutional Animal Care and Use Committee of the Medical University of Vienna and the Austrian Federal Ministry of Science and Research (BMWF-5.011/0003-II/10b/2009 and BMBWF-66.009/0174-V/3b/2019).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Angoa-Perez M., Kane M. J., Briggs D. I., Francescutti D. M. and Kuhn D. M.(2013). Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J Vis Exp(82): 50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barbato G., Barini E. M., Genta G. and Levi R.(2011). Features and performance of some outlier detection methods. J Appl Stat 38(10): 2133-2149. [Google Scholar]
- 3. Berger J., Dorninger F., Forss-Petter S. and Kunze M.(2016). Peroxisomes in brain development and function. Biochim Biophys Acta 1863(5): 934-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernard P. B., Castano A. M., Beitzel C. S., Carlson V. B. and Benke T. A.(2015). Behavioral changes following a single episode of early-life seizures support the latent development of an autistic phenotype. Epilepsy Behav 44: 78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deacon R.(2012). Assessing burrowing, nest construction, and hoarding in mice. J Vis Exp(59): e2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deacon R. M.(2006). Assessing nest building in mice. Nat Protoc 1(3): 1117-1119. [DOI] [PubMed] [Google Scholar]
- 7. Deacon R. M., Croucher A. and Rawlins J. N.(2002). Hippocampal cytotoxic lesion effects on species-typical behaviours in mice. Behav Brain Res 132(2): 203-213. [DOI] [PubMed] [Google Scholar]
- 8. Dorninger F., Forss-Petter S. and Berger J.(2017). From peroxisomal disorders to common neurodegenerative diseases- the role of ether phospholipids in the nervous system. FEBS Lett 591(18): 2761-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dorninger F., Forss-Petter S., Wimmer I. and Berger J.(2020). Plasmalogens, platelet-activating factor and beyond- Ether lipids in signaling and neurodegeneration. Neurobiol Dis 145: 105061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dorninger F., Gundacker A., Zeitler G., Pollak D. D. and Berger J.(2019). Ether lipid deficiency in mice produces a complex behavioral phenotype mimicking aspects of human psychiatric disorders. Int J Mol Sci 20(16): 3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dorninger F., Herbst R., Kravic B., Camurdanoglu B. Z., Macinkovic I., Zeitler G., Forss-Petter S., Strack S., Khan M. M., Waterham H. R., Rudolf R., Hashemolhosseini S. and Berger J.(2017). Reduced muscle strength in ether lipid-deficient mice is accompanied by altered development and function of the neuromuscular junction. J Neurochem 143(5): 569-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dorninger F., Konig T., Scholze P., Berger M. L., Zeitler G., Wiesinger C., Gundacker A., Pollak D. D., Huck S., Just W. W., Forss-Petter S., Pifl C. and Berger J.(2019). Disturbed neurotransmitter homeostasis in ether lipid deficiency. Hum Mol Genet 28(12): 2046-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jirkof P.(2014). Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods 234: 139-146. [DOI] [PubMed] [Google Scholar]
- 14. Lijam N., Paylor R., McDonald M. P., Crawley J. N., Deng C. X., Herrup K., Stevens K. E., Maccaferri G., McBain C. J., Sussman D. J. and Wynshaw-Boris A.(1997). Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell 90(5): 895-905. [DOI] [PubMed] [Google Scholar]
- 15. Neely C. L. C., Pedemonte K. A., Boggs K. N. and Flinn J. M.(2019). Nest Building Behavior as an Early Indicator of Behavioral Deficits in Mice. J Vis Exp (152). [DOI] [PubMed] [Google Scholar]
- 16. Percie du Sert N., Ahluwalia A., Alam S., Avey M. T., Baker M., Browne W. J., Clark A., Cuthill I. C., Dirnagl U., Emerson M., Garner P., Holgate S. T., Howells D. W., Hurst V., Karp N. A., Lazic S. E., Lidster K., MacCallum C. J., Macleod M., Pearl E. J., Petersen O. H., Rawle F., Reynolds P., Rooney K., Sena E. S., Silberberg S. D., Steckler T. and Wurbel H.(2020). Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol 18(7): e3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ricceri L., Moles A. and Crawley J.(2007). Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behav Brain Res 176(1): 40-52. [DOI] [PubMed] [Google Scholar]
- 18. Rodemer C., Thai T. P., Brugger B., Kaercher T., Werner H., Nave K. A., Wieland F., Gorgas K. and Just W. W.(2003). Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum Mol Genet 12(15): 1881-1895. [DOI] [PubMed] [Google Scholar]
- 19. Sager T. N., Kirchhoff J., Mork A., Van Beek J., Thirstrup K., Didriksen M. and Lauridsen J. B.(2010). Nest building performance following MPTP toxicity in mice. Behav Brain Res 208(2): 444-449. [DOI] [PubMed] [Google Scholar]
- 20. Seong E., Seasholtz A. F. and Burmeister M.(2002). Mouse models for psychiatric disorders. Trends Genet 18(12): 643-650. [DOI] [PubMed] [Google Scholar]
- 21. Silverman J. L., Yang M., Lord C. and Crawley J. N.(2010). Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 11(7): 490-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sukoff Rizzo S. J. and Crawley J. N.(2017). Behavioral phenotyping assays for genetic mouse models of neurodevelopmental, neurodegenerative, and psychiatric disorders. Annu Rev Anim Biosci 5: 371-389. [DOI] [PubMed] [Google Scholar]