Abstract
Despite the large number of drugs available for the treatment of asthma, in 5-10% of the patients this disease is not well controlled. While most treatments palliate symptoms, those suffering from severe and uncontrolled asthma could benefit more from a therapeutic approach addressing the root problem. An siRNA-based therapy targeting the transcription factor GATA3 in activated T helper cells subtype 2 (TH2 cells), one of the key upstream factors involved in asthma, could therefore represent a promising strategy. However, the difficult-to-transfect cell type has not extensively been explored for nucleic acid therapeutics. In this regard, our group first identified a suitable pathway, i.e. transferrin receptor mediated uptake, to target efficiently and specifically activated TH2 cells with a transferrin- polyethyleneimine (PEI)conjugate which forms polyplexes with siRNA. This system, despite efficient uptake in activated T cells in vivo, suffered from poor endosomal release and was later improved by a combination with a melittin-PEI conjugate. The new formulation showed improved endosomal escape and gene silencing efficacy. Additionally, in order to develop a clinically relevant dosage form for pulmonary delivery of siRNA we have lately focused on a dry powder formulation by spray drying for the production of inhalable nano-in-microparticles. In proof-of-concept experiments, DNA/PEI polyplexes were used in order to implement analytics and engineer process parameters to pave the way for spray drying also siRNA containing polyplexes and more sophisticated systems in general. Ultimately, our efforts are devoted to the development of a novel treatment of asthma that can be translated from bench to bedside and are reviewed and discussed here in the context of the current literature.
Introduction
Amongst chronic inflammatory diseases of the airway, asthma is still considered a great medical and socioeconomic burden under which nearly 340 million people suffer worldwide ( The Global Asthma Report 2018, 2018). The disease hallmarks, besides persistent lung inflammation, are shortness of breath, mucus hypersecretion, broncho obstruction with enhanced reactivity to spasmogens (airway hyperactivity) and airway remodeling ( The Global Asthma Report 2018, 2018; Sel et al., 2008; Weckmann et al., 2007; Wegmann et al., 2005). The current asthma treatment algorithm is based on bronchodilating and anti-inflammatory agents (inhaled and/or systemic glucocorticoids) targeting symptoms only. However, in a small subset of patients these symptoms cannot be controlled even with high doses of the recommended drugs (de Groot, Ten Brinke, & Bel, 2015).
The origin of these symptoms, amongst others is the activation, infiltration and accumulation of T cells positive for the cluster of differentiation 4, also known as T helper cells (TH2 cells), of subtype 2 (CD4+TH2 cells) into major airways and mucosa of small airways, and the subsequent release of proinflammatory cytokines (IL-4, IL-5, IL-9 and IL-13)(Sel et al., 2008; Walker, Barlow, & McKenzie, 2013; Weckmann et al., 2007; Wegmann et al., 2005).After it was found that the release of these interleukins is triggered by an upregulation of the GATA-binding protein 3 (GATA3) in TH2 cells upon activation (Ray & Cohn, 1999), GATA3, a transcription factor regulating T cell differentiation into TH2 cells, emerged as a powerful therapeutic target (Krug et al., 2017). Considering that the effect of silencing only single cytokines can be overcompensated by others (Lively et al., 2008),therapeutic downregulation of GATA3, preventing downstream release of all TH2 cytokines and concomitant symptoms in parallel holds great promise.
A powerful tool for post-transcriptional gene silencing is RNA interference (RNAi): Fire and Mello who earned the Nobel Prize, discovered a nuclease complex known as RNA induced silencing complex (RISC) which recognizes and destroys target mRNAs. The target is specifically identified by small interfering RNAs (siRNA) which are RNA strands of 21-25 base pairs with base-complementarity to the target mRNA. Upon pairing of the activated RISC with single-stranded siRNA and the complementary mRNA site, cleavage of mRNA is initiated and the translation of the protein alongside the degraded mRNA is prevented (Hannon, 2002). Accordingly, targeting mRNA coding for GATA3 with siRNA could enable post-transcriptional gene silencing of the transcription factor which is overexpressed in activated TH2 cells. Subsequently, downregulating the overexpressed level towards a more physiologic one could therapeutically be exploited towards a new asthma treatment without general immunosuppressive side effects. Delivering siRNA exogenously into activated TH2 cells, however, is not a simple task.
Considering the macromolecular nature of siRNAs which are highly negatively charged and the lack of nucleic acid specific active transporters on cell membranes in combination with ubiquitously present nucleases which quickly degrade siRNA in the body, intracellular siRNA delivery requires formulation of the latter. Compared to viral vectors, non-viral vectors are more advantageous regarding safety, manufacturability and immunogenicity (Ruigrok, Frijlink, & Hinrichs, 2016). Positively charged polymers are one class of non-viral vectors where polyethyleneimine (PEI) and its derivatives are the most studied representatives. These polymers form so called polyplexes on the nano-scale by electrostatic interaction with siRNA protecting it on the one hand from nucleases and enabling internalization and release into cells on the other (Liu, Nguyen, Steele, Merkel, & Kissel, 2009). However, in case of intracellular delivery into T cells, additional barriers, such as endosomal release, need to be considered (Olden, Cheng, Yu, & Pun, 2018).
In addition to avoiding nucleases which are present in high concentrations in blood but in very low concentrations in lung lining fluids, pulmonary administration of nucleic acids also avoids the rapid distribution within the body upon systemic delivery which comes with possible side effects (Weber, Zimmer, & Pardeike, 2014). Local pulmonary delivery can be achieved by inhaling particles (liquid or solid) of an aerodynamic size between 1 and 5 µm. Greater particles are deposited in the throat and upper airways unable to reach the area of interest. Smaller particles can undergo insufficient sedimentation and are exhaled. For optimal lung deposition, dry powder formulations are favored for siRNA delivery despite more complex formulation and preparation as compared with aerosolization (Chow & Lam, 2015). The advantages can be easily explained by increased physical and chemical stability and the resulting prolonged shelf life due to the absence of water and nucleases (Keil & Merkel, 2019).Typical procedures to produce inhalable powders are spray drying or a combination of spray drying and lyophilization – spray freeze drying – of drug only or drug-excipient combinations (Okuda et al., 2018). For the latter, solutions or suspensions are sprayed into liquid nitrogen resulting in frozen particles which are lyophilized afterwards to remove residual water. However, this process requires high energy and time consumption due to long lyophilization cycles. A more straight forward process is spray drying where droplets are generated and dried by hot air (Schulze et al., 2018). This technique leads to microparticles where polyplexes or other nanoparticles are embedded in an excipient matrix resulting in nano-in-microparticles which ideally resuspend into nanoparticles upon impaction on lung fluid.
This Focus Article highlights our previous and ongoing research regarding polyplexes designed for pulmonary delivery for the treatment of lung diseases in general, and of asthma specifically in the context of the current literature.
TH2-cell targeting
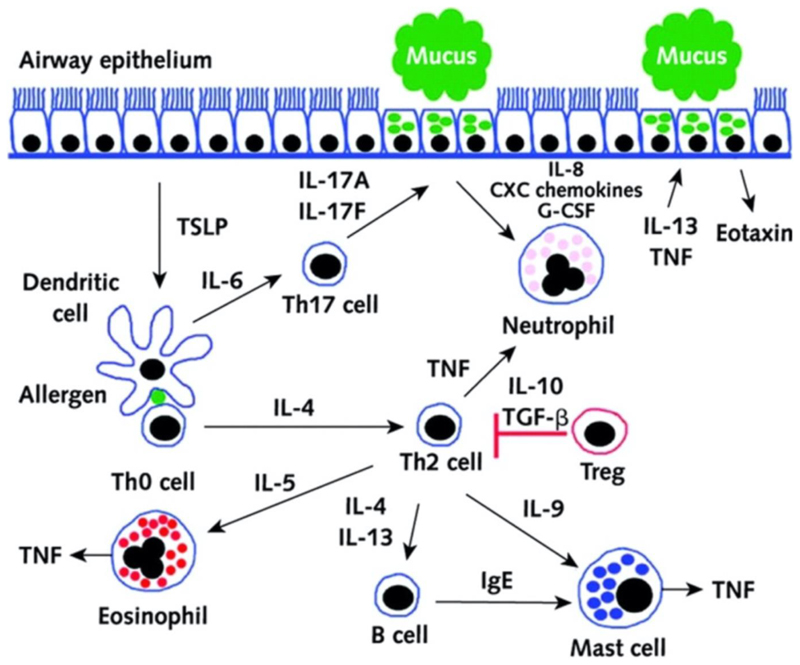
In the pathogenesis of asthma, TH2 cells play a central role in orchestrating the allergic reaction. Upon activation and concomitant upregulation of GATA3, TH2 cells secrete IL-4, IL-5, IL-9, IL-13 and tumor necrosis factor alpha (TNFα) (Barnes, 2002; Ishmael, 2011). As shown in Figure 1, these cytokines stimulate different cell types resulting in further downstream effects and symptoms known for asthma. Although symptoms were shown to be reduced by i.v. application of antibodies against single interleukins in mice, their use is limited due to whole body distribution after systemic administration resulting in various side effects (Wegmann, 2009) and due to lack of compliance if administered clinically.
Figure 1.
T helper-2 (TH2) cells in asthma pathogenesis. Inhaled allergens are thought to be processed by two mechanisms in asthmatic airways. Allergens either: (1) activate mast cells through cross-linking with IgE on their cell surfaces through the high-affinity type 1 IgE receptor (FcεR1) to release mediators that induce bronchoconstriction, such as histamine, cysteinyl leukotrienes, and prostaglandin D2 (PGD2) or (2) are processed by dendritic cells, which are induced to secrete the CC chemokine ligand (CCL) 17 and CCL22 by thymic stromal lymphopoietin (TSLP). Dendritic cells then attract and activate TH2 cells by the binding of CCL17 and CCL22 with CC chemokine receptor 4 (CCR4) on the TH2 cell surface. IL-33 is produced by airway epithelial cells and activates dendritic cells and TH2 by inducing the release of TNF-α from mast cells. TH2 secretes cytokines, including IL-4 and IL-13, which switch B cells to produce IgE, IL-5, which promotes the development and survival of eosinophils, and IL-9, which activates mast cells. Once IL-13 is produced, it can increase the survival and migration of eosinophils, and it promotes activation of macrophages to create an M2, or an allergic cell phenotype. Airway epithelial cells are stimulated, and through mediators such as periostin and transforming growth factor β1 (TGF-β1), they can increase airway inflammation and lead to the increased permeability of airway epithelial cells and mucous hypersecretion. IL-13 also has direct effects on airway smooth muscle, leading to increased contraction to agonists such as acetylcholine and decreased relaxation with beta-agonists. (Reproduced with permission from Thomson, Patel, and Smith (2012). Copyright 2012 Dove Medical Press Ltd.)
Rather than blocking or downregulating single cytokines, post-transcriptional interference with GATA3 expression has been reported to be a promising approach using intratracheal instillation with a bolus of GATA-3 shRNA lentiviral vector (Lee, Huang, & Chiang, 2007)or intranasal treatment with GATA-3 DNazyme (Dicke, Wegmann, Sel, Renz, & Garn, 2007). In those experiments, however, the therapeutic nucleic acids were administered as unstable, free DNA and were not specifically targeted towards TH2 cells.
The downregulation of GATA3 in activated TH2 cells via pulmonary administration is therefore preferred as the secretion of TH2 cytokines is ideally only downregulated in the activated T cells in the lung, preventing side effects of general immune suppression. It is furthermore expected that gene silencing via siRNA will not mediate a full knock out of GATA 3 but rather downregulation of a pathologically upregulated gene to a physiologic level. However, the transfection of T cells is challenging since they do not express caveolin and are devoid of caveolae, preventing them from active endocytosis of nanoparticles. Primary T cells are thus known to be resistant to common non-viral delivery vectors (Montixi et al., 1998).
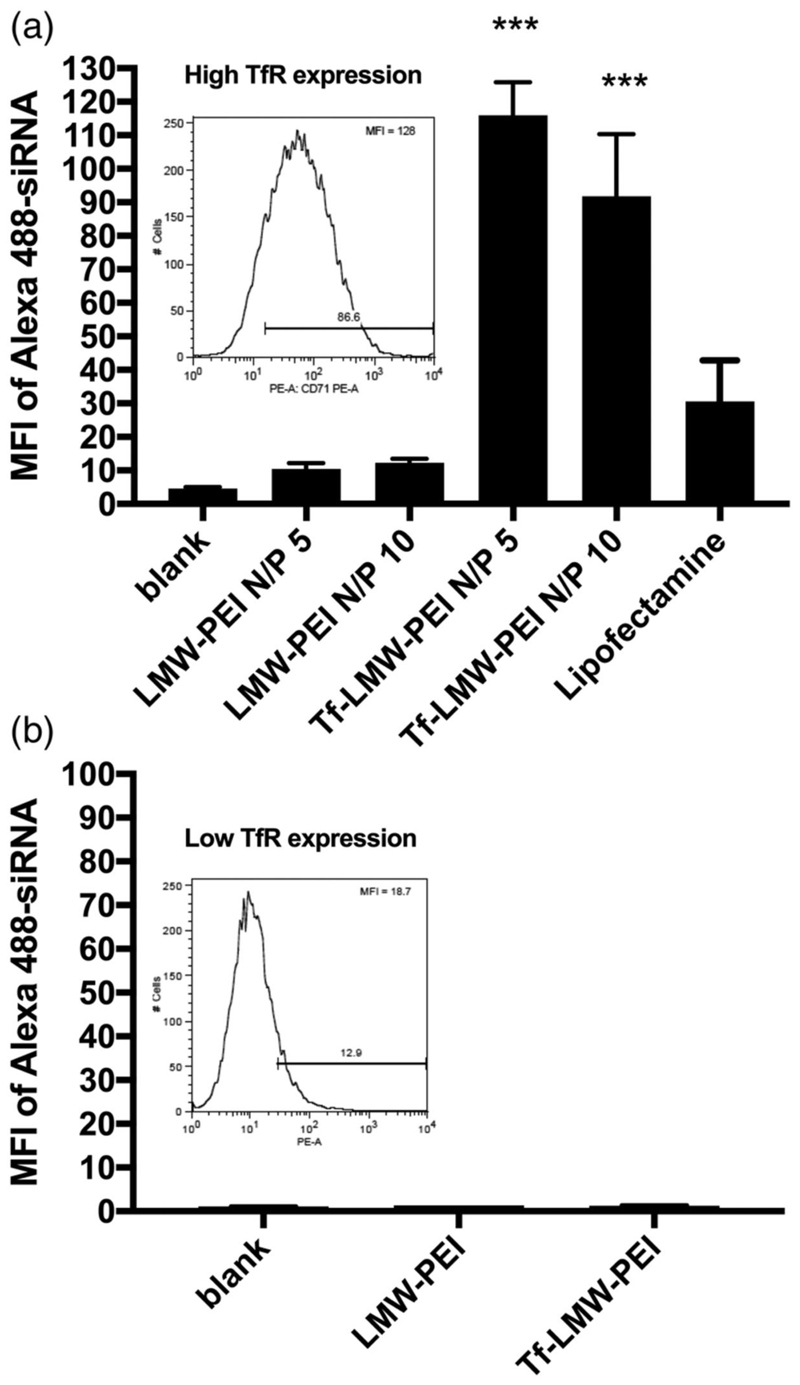
Since viruses efficiently transduce T cells, we sought to find a virus-like tool to target activated TH2 cells specifically and efficiently in a receptor-mediated manner. While in the 1980s, an overexpression of transferrin receptor (CD71) in activated T cells was found whereas naïve T cells lacked the expression of CD71 (Galbraith, Werner, Arnaud, & Galbraith, 1980; Neckers & Cossman, 1984), this finding had so far not been exploited for nucleic acid delivery to T cells. This idea was picked up by our group in the early 2010s. First experiments were conducted to confirm differential receptor expression in naïve vs. activated T cells and to test whether receptor mediated uptake was possible ex vivo in primary T cells exploiting transferrin (Tf) as a targeting ligand (Kim, Nadithe, Elsayed, & Merkel, 2013). Therefore, in proof-of-concept experiments,low molecular weight (LMW) PEI was conjugated to Tf (Tf-PEI) and complexed with fluorescently labelled siRNA into polyplexes at different ratios. These formulations were initially only tested regarding intracellular delivery in primary T cells ex vivo. As seen in Figure 2a, Tf-PEI polyplexes were significantly more efficiently taken up by activated T cells (ATCs) compared to blank, compared to unmodified PEI and even compared to the positive lipofectamine control. Also, no efficient uptake was observed in naïve T cells for either formulation (Figure 2b),which was expected based on the lack of caveolae in T cells (Montixi et al., 1998).The activation dependent expression of CD71 in both activated and naïve primary T cells was confirmed by anti-CD71 antibody binding assays (see Figure 2 insets). These results confirmed our hypothesis that specific targeting of activated T cells via transferrin is possible and further research was conducted.
Figure 2.
Specific Uptake of siRNA in activated T cells with a Tf-PEI conjugate. Uptake of Alexa488-labaled siRNA a) at different N/P ratios into fully activated T cells with high TfR expression (inset 2a), and b) lack of uptake into T cells with low TfR expression (inset 2b). The expression of TfR in the T cells was confirmed by anti-CD71 antibody binding assay. The siRNA taken up into T cells was analyzed by flow cytometry. Lipofectamine was used as a positive control. Reproduced with permission from (Kim et al., 2013). Copyright 2013 Elsevier B.V.
While successful targeting of T cells was also shown by Ramishetti et al. after systemic administration of lipid nanoparticles which were surface functionalized with an CD4+ antibody (Ramishetti et al., 2015), no differentiation between resting and activated T cells was made as the intention was to target and downregulate T cell specific genes systemically. In contrast, CD71 targeting results in the benefit of addressing only activated T cells which are strongly involved in asthma.
To test specificity of Tf-PEI polyplexes toward activated T cells in the complex environment of the lung, an in vivo biodistribution study was performed in a murine asthma model in comparison to healthy control groups:Mice were intratracheally administrated on four consecutive days with Tf-PEI or PEI polyplexes.After euthanizing animals, bronchoalveolar lavage (BAL) cells were collected to investigate the distribution of siRNA in different cells types (macrophages, eosinophils, type II pneumocytes, B and T cells). We observed a specific uptake in T cells in comparison to the other investigated cell types. In line with the previous ex vivo study, the uptake in TH2 cells was also significantly higher in asthmatic than in healthy mice (Xie et al., 2016). In healthy animals, only macrophages took up siRNA independent of the formulation, and non-targeted PEI mediated uptake in type II epithelial cells and macrophages in both inflamed and healthy animals.However it has been described that macrophages do not express GATA3 (Saraiva & O'Garra, 2010), which leads us to hypothesize a lack of severe side effects due to nanoparticle delivery to macrophages. Type II pneumocytes, however, do express GATA3 and are involved in TH2 cytokine production. Therefore, non-specific delivery to lung epithelial cells is expected to have a positive, anti-inflammatory effect. Since GATA3 does however have a protective effect, for example in mammary luminal cells (Chu et al., 2012), we will investigate biodistribution of siRNA after pulmonary delivery to assess potential risks and side effects of the treatment.
Optimization of Endosomal Release: Tf-Mel-PEI
After confirming effective targeting and uptake of Tf-PEI polyplexes in TH2 cells in the lung, their therapeutic efficacy was tested in vivo by evaluating the knockdown of GATA3 and subsequent downstream effects. Despite significantly higher gene silencing rates of Tf-PEI compared to PEI ex vivo in primary TH2 cells (Xie et al., 2016), a single treatment with Tf-PEI polyplexes did not result in significant gene silencing of GATA3 in lung tissue or of IL13 in pulmonary T cells, as determined by qRT-PCR and intracellular cytokine staining, respectively (Kandil, Feldmann, Xie, & Merkel, 2019). The reason for the lack of significant gene silencing was hypothesized to depend on the single administration and/or on insufficient endosomal escape of the nanocarrier after endocytosis.
Endosomal escape represents acrucial factor and is considered the rate-limiting step in cytoplasmatic delivery of nanoparticle-based therapies. In fact, a failure of escape would result in a probable degradation of the cargo in the lysosome which merges with the late endosome, leading to a loss of therapeutic activity (Smith, Selby, Johnston, & Such, 2019). Several strategies have been proposed to overcome this problem, such as including positively charged or pH-sensitive moieties able to disrupt the endosomal membrane (Selby, Cortez-Jugo, Such, & Johnston, 2017). In this respect, it is to be noted that endosomal acidification, leading to the so-called proton sponge effect and osmotic rupture of polyamine-loaded endosomes, is slower and less robust in T cells as compared to epithelial cell lines (Olden, Cheng, Cheng, & Pun, 2019). In this view, the use of melittin, a pore-forming peptide, was hypothesized to yield more efficient endosomal escape. Melittin is a peptide derived from bee venom and consisting of 26 amino acids that has an inherent capacity to disrupt cell membranes also at acidic pH. Moreover, this peptide was shown to be effective as a delivery system for siRNA itself, being able to reach significant transfection efficiencies (Hou, Pan, Lanza, & Wickline, 2013). Additionally, a virus-inspired polymer for efficient in vitro and in vivo gene delivery, called VIPER (Cheng, Yumul, & Pun, 2016), containing melittin, had been shown to efficiently mediate gene silencing in the lung of healthy mice and had been tolerated very well after pulmonary delivery (Feldmann et al., 2018). Based on these observations, we decided to include melittin in our Tf-PEI conjugate to improve the endosomal escape. However, melittin was modified by 2,3-Dimethyl-maleic anhydride (DMMAn) to reduce side effects by directing its activity to acidic pH and hence to the endosomal membrane only. Thus, general membrane activity was reduced, and this melittin derivative was conjugated to PEI and blended with different ratios of Tf-PEI. As a result,we obtained a delivery system which retained its selectivity toward activated T cells due to the presence of transferrin at improved endosomolytic activity (Kandil et al., 2019). The new blend composed of Tf-PEI and Mel-PEI (Tf-Mel-PEI, 50:50) displayed optimal characterization parameters, showing particle sizes below 200 nm, low polydispersity indices and stability in lung lining fluids. In line with the results obtained with the Tf-PEI conjugate, we achieved significant cellular uptake results in both Jurkat and human primary activated T cells in comparison to both free siRNA and siRNA/lipofectamine. Moreover, after determining binding kinetics of the different formulations with CD71 using Surface Plasmon Resonance technique, we observed that Tf-Mel-PEI stably bound the transferrin receptor and also showed superior affinity over Tf-PEI (Kandil et al., 2019).
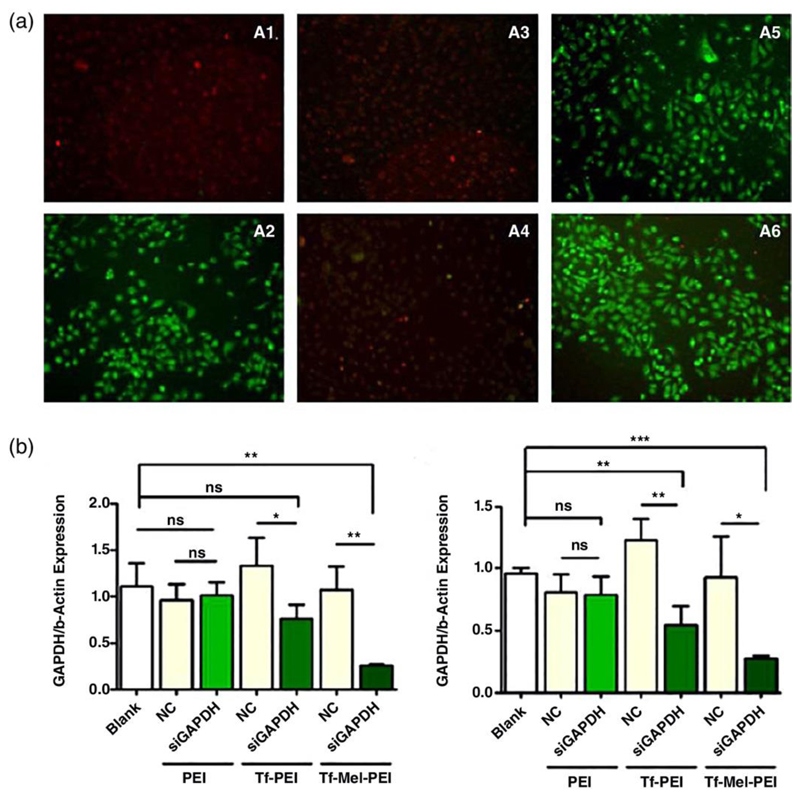
In the next step, the effect of melittin on the endosomal membrane was investigated via acridine orange staining of living cells. This technique is based on a differential fluorescence emission of the cell-permeable nucleic acid binding dye which emits red light if trapped in the endosome and green light if located within cytoplasmic pH. As shown in Figure 3A, chloroquine, as positive control for successful endosomal release, as well as transfection with melittin-containing conjugates resulted in efficient endosomal escape, reflected in a color change of the dye from red to green. Positive and negative controls in fluorescence based assays with chloroquine treatment are especially important as chloroquine is a mild base used to neutralize endosomal and lysosomal pH, and pH dependent dyes used to visualize these compartments can potentially render a negative result in the presence of this drug. After transfection with the Tf-PEI conjugate, only few green dots were detected while in PEI transfected cells, only red dye was found, supporting the findings by Olden et al. describing a lack of endosomal acidification in T cells (Olden et al., 2018) resulting in endosomal entrapment of PEI. Regarding endosomal escape, Tf-Mel-PEI clearly showed superior characteristics over the previously used Tf-PEI formulations (Kandil et al., 2019).
Figure 3.
A) Acridine orange staining of untreated A549 cells (A1) and of A549 cells after incubation of polyplexes with Chloroquine (A2), PEI (A3), Tf-PEI (A4), Mel-PEI (A5) and Tf-Mel-PEI (A6). B) GAPDH knockdown in Jurkat cells (B1) and human primary activated T cells (B2) after treatment with GAPDH-siRNA or scrambled siRNA as negative control. Data points indicate mean ±SD, n = 3; One-way ANOVA, *P<0.05, **P < 0.01, *** P < 0.001. Reproduced with permission from (Kandil et al., 2019). Copyright 2019 Wiley.
To determine whether the effect of improved endosomal escape was also reflected on higher mRNA downregulation, the knockdown of GAPDH was evaluated via qRT-PCR (Figure 3B). Indeed, in human primary activated T cells the Tf-Mel-PEI blend achieved higher gene silencing levels than Tf-PEI with 76% gene knockdown compared to 43%, respectively (Kandil et al., 2019). Most importantly, however, the increased endosomal escape was not paralleled by increased cytotoxicity or membrane destabilization (Kandil et al., 2019).
Our published efforts so far describe a formulation of Tf-Mel-PEI polyplexes with optimal particle characteristics able to selectively target activated T cells and improved endosomal escape and gene silencing efficacy. Tf-Mel-PEI was therefore considered a suitable tool for follow up in vivo studies concerning in vivo specificity for activated T cells, biodistribution and therapeutic gene silencing efficiencies in a murine asthma model. Despite the fact that LMW-PEI and its Tf-PEI conjugate have been tolerated well in healthy and asthmatic mice (Xie et al., 2016), we are currently developing oligospermine derivatives to replace the PEI block in our approach (Elsayed et al., 2014). It is expected that in the inflamed lung, even Tf-shielded LMW-PEI is not well tolerated, especially intracellulary where the disulfide bond between Tf and PEI will be reduced. Therefore, non-biodegradable PEI is not a promising approach for the treatment of a chronic disease, and biodegradable alternatives are currently developed by us and others. By mimicking PEI with a polyamine that endogenously acts as nucleic acid condenser, we believe that similar condensation efficacy of siRNA will be obtained at reduced toxicity and immunogenicity but at increased biodegradability (Elsayed et al., 2014). In parallel, inhalable dry powder formulations are being developed to pave the way for translation from bench to bed side.
Pulmonary delivery of nucleic acids
Therapeutic approaches of delivering nucleic acids to the lung via inhalation benefit from direct accessibility and the ease of its administration route. However, several barriers, such as the architecture of the lung, the presence of mucus and surfactant, mucociliary clearance and phagocytosis by cells of the immune system, need to be overcome in the lung for successful delivery of nucleic acids to their target cells and sites of action (O. M. Merkel & Kissel, 2012; Olivia M. Merkel, Zheng, Debus, & Kissel, 2012). After carefully optimizing our formulations for gene silencing in T cells over the past years, we are currently assessing their efficacy in 3D cell culture models (Zscheppang et al., 2018) and in mucus mobility assays based on Fluorescence Correlation Spectroscopy (FCS) (O. M. Merkel et al., 2009). Current clinical trials for inhalable gene-based therapies have been focused mainly on the use of viral and liposome-based vectors, such as in the case of cystic fibrosis, to deliver cDNA for replacing the defective CFTR gene (Caplen et al., 1995; Griesenbach, Geddes, & Alton, 2004). However, transfection efficiency in the lung in these clinical trials was disappointing. The study and development of new formulation strategies for inhalable gene therapies is therefore of paramount importance.
Dry powder formulation
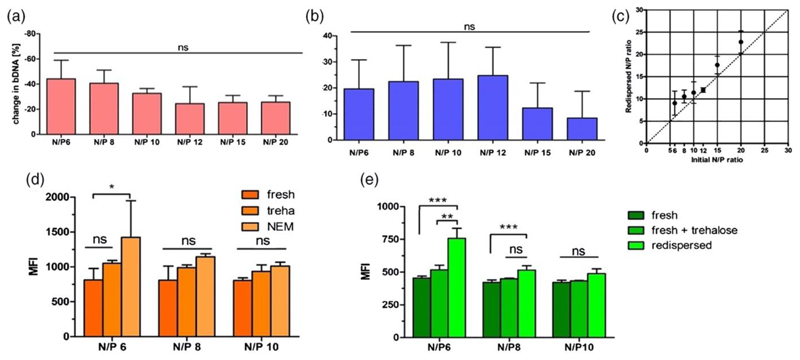
Spray drying (SD) is the most straightforward technique to produce inhalable particles. Although several groups have applied SD to obtain dry powder formulations of their nucleic acid formulations, analytics for characterizing nanoparticles before and after spray drying have been very scarce. However, an understanding of changes between freshly prepared and redispersed nanoparticles is needed to fully understand effects of tubing material, pump stress, shear forces and heat stress which are applied to the particles during production (Jensen et al., 2010; Schulze et al., 2018). Loss of nucleic acid and or polymer could ultimately lead to decreased in vitro and in vivo performance. Hence, we successfully developed and set up protocols for nucleic acid as well as amine based polymer quantification to easily quantify the components of dried formulations and redispersed suspensions (Keil et al., 2019). Additionally, the effect of mannitol and trehalose on the redispersability of polyplexes consisting of 25k PEI and bulk DNA (bDNA) after SD was investigated. Both materials were chosen as they are generally recognized as safe (GRAS) substances, known for their lyo-and desicco-protection and commonly applied in SD (Vehring, 2008). We found that a distinct concentration of excipient was needed to preserve PEI-bDNA polyplex size and particle distribution, independent of the excipient’s nature. Initial changes in zeta potentials of the formulations after SD and redispersion could also be eliminated. Furthermore, it was confirmed by cascade impaction analysis that particles were prepared with an aerodynamic diameter between 1 and 5 µm, which is a conducive size range for pulmonary administration. SEM revealed round smooth microparticles for mannitol based formulations and also round but partly fused trehalose based microparticles. These findings were explained by the state of mannitol and trehalose via x-ray powder diffraction and differential scanning calorimetry revealing that mannitol crystallized upon spray drying while trehalose formulations dried without forming a crystalline but an amorphous state. This most probably led to the high residual moisture content of 3.2% of trehalose formulations compared to 0.4% of mannitol formulations and hence the fusion of trehalose microparticles. However, crystallinity and water content did not affect aerodynamic properties on short term. After establishing and ultimately applying the new set of analytical methods to SD powders, important changes to the initial formulations were detected: trehalose formulations showed ~32% nucleic acid loss at low N/P ratios reaching a plateau of ~20% at higher N/P ratios of the polymer/nucleic acid polyplexes, suggesting a stabilizing effect of excess polymer (Figure 4A). Considering the measured polymer loss of the different formulations after SD (Figure 4B), N/P ratios of the redispersed formulations overall increased (Figure 4C). It was therefore hypothesized that the N/P increase at least partially explained the improved uptake and transfection efficiency of redispersed formulations in vitro compared to their freshly prepared counterparts with and without excipient as control (Figure 4D and 4E) (Keil et al., 2019).
Figure 4.
Figure 4. Polymer and nucleic acid quantification. Quantification of A) bulk DNA and B) PEI in the 10% trehalose nano-in-microparticle (NIM) formulations following spray drying; C) Comparing the redispersed N/P ratio with the initial N/P ratio of the PEI polyplexes loaded with bulk DNA; D) Uptake and E) Transfection efficiency of redispersed nano-in-microparticle formulations in A549 cells. Median fluorescence intensity (MFI) was determined by flow cytometry to evaluate efficiency of D) fluorescently labeled bDNA or E) pEGFP in a human non-small cell lung carcinoma cell line (A549) of fresh or redispersed polyplexes from 10% trehalose NIM formulations at N/P ratios of 6, 8 and 10 with D) 0.5µg of bDNA or E) 0.75 µg of GFP plasmid in comparison to freshly prepared formulations in presence of trehalose. Blank samples consisted of A549 cells treated with 5% glucose only. Data points indicate mean ± SD (n=3). Two-way ANOVA, Bonferroni post-test, *P<0.05, **P < 0.01, *** P < 0.001, ns = non-significant. Reproduced with permission from (Keil et al., 2019). Copyright 2019 Elsevier B. V.
Even if both DNA and RNA are nucleic acids, we have more than once shown that circular plasmid DNA, for example, behaves very differently from rigid, short double-stranded siRNA when condensed with cationic polymers (Zheng et al., 2012). Therefore, our current research on dry powder formulations focuses on the preparation of polyplexes consisting of siRNA and different PEI based delivery vectors with the goal of developing a platform technology for inhalable nucleic acid formulations.
Conclusion
siRNA-based therapies offer the chance to potentially targetany single mRNA specifically and efficiently and mediate its downregulation. In the treatment of asthma, this technique could be exploited to target GATA3 in activated TH2 cells, one of the main factors involved in the pathogenesis of asthma. Almost 10 years ago, our group started a journey aimed to find a suitable delivery system able to downregulate GATA3 and to produce a final spray dried powder formulation to be administered to patients via inhalation. After identifying transferrin as a targeting ligand for activated T cells, we developed the Tf-PEI conjugate, a delivery system that displayed high cellular selectivity and intracellular uptake with high transfection efficiencies ex vivo. Due to insufficient in vivo gene silencing, however, this formulation was then improved in terms of endosomal escape by blending it with the Mel-PEI conjugate. The new formulation showed optimal particle characteristics and ex vivo parameters. In parallel, we have also focused on the development of a dry powder formulation, an essential step to produce a final formulation that could be transferred from bench to bedside. We successfully produced nano-in-microparticles with ideal characteristics while retaining high transfection efficacies after redispersion.
Current research is therefore focusing on the in vivo testing of Tf-Mel-PEI in a murine asthma model and the dry powder formulation of siRNA based polyplexes. Ultimately, both research fields will be combined and hopefully result in a new therapy for the treatment of severe, uncontrolled asthma.
Acknowledgments
The authors like to thank all former and current lab members both in Detroit and Munich who made this research progress possible.
Funding Information
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant agreement ERC-2014-StG637830)
Contributor Information
Tobias WM Keil, Email: tobias.keil@lmu.de, Pharmaceutical Technology and Biopharmaceutics, LMU Munich, Butenandtstr.5-13, 81377 München.
Domizia Baldassi, Email: domizia.baldassi@cup.uni-muenchen.de, Pharmaceutical Technology and Biopharmaceutics, LMU Munich, Butenandtstr.5-13, 81377 München.
Olivia M Merkel, Email: olivia.merkel@lmu.de, Pharmaceutical Technology and Biopharmaceutics, LMU Munich, Butenandtstr. 5-13, 81377 München.
References
- Barnes PJ. Cytokine modulators as novel therapies for asthma. Annual review of pharmacology and toxicology. 2002;42(1):81–98. doi: 10.1146/annurev.pharmtox.42.111901.111143. [DOI] [PubMed] [Google Scholar]
- Caplen NJ, Alton EW, Middleton PG, Dorin JR, Stevenson BJ, Gao X, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995;1(1):39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Yumul RC, Pun SH. Virus-Inspired Polymer for Efficient In Vitro and In Vivo Gene Delivery. Angewandte Chemie International Edition. 2016;55(39):12013–12017. doi: 10.1002/anie.201605958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow MY, Lam JK. Dry Powder Formulation of Plasmid DNA and siRNA for Inhalation. Current Pharmaceutical Design. 2015;21(27):3854–3866. doi: 10.2174/1381612821666150820105916. [DOI] [PubMed] [Google Scholar]
- Chu IM, Michalowski AM, Hoenerhoff M, Szauter KM, Luger D, Sato M, Green JE. GATA3 inhibits lysyl oxidase-mediated metastases of human basal triple-negative breast cancer cells. Oncogene. 2012;31(16):2017–2027. doi: 10.1038/onc.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JC, Ten Brinke A, Bel EH. Management of the patient with eosinophilic asthma: a new era begins. ERJ open research. 2015;1(1):00024–02015. doi: 10.1183/23120541.00024-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke T, Wegmann M, Sel S, Renz H, Garn H. Gata-3-specific dnazyme as an approach for asthma-therapy. Journal of allergy and clinical immunology. 2007;119(1):S1–S1. doi: 10.1016/j.jaci.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Elsayed M, Corrand V, Kolhatkar V, Xie Y, Kim NH, Kolhatkar R, Merkel OM. Influence of oligospermines architecture on their suitability for siRNA delivery. Biomacromolecules. 2014;15(4):1299–1310. doi: 10.1021/bm401849d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann DP, Cheng Y, Kandil R, Xie Y, Mohammadi M, Harz H, Merkel OM. In vitro and in vivo delivery of siRNA via VIPER polymer system to lung cells. Journal of Controlled Release. 2018;276:50–58. doi: 10.1016/j.jconrel.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith RM, Werner P, Arnaud P, Galbraith GM. Transferrin binding to peripheral blood lymphocytes activated by phytohemagglutinin involves a specific receptor. Ligand interaction. The Journal of clinical investigation. 1980;66(5):1135–1143. doi: 10.1172/JCI109943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Global Asthma Report 2018. Retrieved from Auckland. New Zealand: 2018. [Google Scholar]
- Griesenbach U, Geddes DM, Alton EW. Gene therapy for cystic fibrosis: an example for lung gene therapy. Gene Ther. 2004;11(1):S43–50. doi: 10.1038/sj.gt.3302368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon GJ. RNA interference. Nature. 2002;418(6894):244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- Hou KK, Pan H, Lanza GM, Wickline SA. Melittin derived peptides for nanoparticle based siRNA transfection. Biomaterials. 2013;34(12):3110–3119. doi: 10.1016/j.biomaterials.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishmael FT. The inflammatory response in the pathogenesis of asthma. The Journal of the American Osteopathic Association. 2011;111(11-7):S11–17. [PubMed] [Google Scholar]
- Jensen DMK, Cun D, Maltesen MJ, Frokjaer S, Nielsen HM, Foged C. Spray drying of siRNA-containing PLGA nanoparticles intended for inhalation. Journal of Controlled Release. 2010;142(1):138–145. doi: 10.1016/j.jconrel.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandil, Xie Y, Heermann R, Isert L, Jung K, Mehta A, Merkel OM. Coming in and Finding Out: Blending Receptor-Targeted Delivery and Efficient Endosomal Escape in a Novel Bio-Responsive siRNA Delivery System for Gene Knockdown in Pulmonary T Cells. Advanced therapeutics. 2019;2(7) doi: 10.1002/adtp.201900047. 1900047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandil R, Feldmann DP, Xie Y, Merkel OM. Evaluating the Regulation of Cytokine Levels After siRNA Treatment in Antigen-Specific Target Cell Populations via Intracellular Staining. Nanotechnology for Nucleic Acid Delivery. 2019:323–331. doi: 10.1007/978-1-4939-9092-4_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil TWM, Feldmann DP, Costabile G, Zhong Q, da Rocha S, Merkel OM. Characterization of spray dried powders with nucleic acid-containing PEI nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics. 2019 doi: 10.1016/j.ejpb.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil TWM, Merkel OM. Dry powder inhalation of siRNA. Therapeutic Delivery. 2019;10(5):265–267. doi: 10.4155/tde-2019-0018. [DOI] [PubMed] [Google Scholar]
- Kim NH, Nadithe V, Elsayed M, Merkel OM. Tracking and treating activated T cells. Journal of Drug Delivery Science and Technology. 2013;23(1):17–21. doi: 10.1016/s1773-2247(13)50002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug N, Hohlfeld JM, Buhl R, Renz J, Garn H, Renz H. Blood eosinophils predict therapeutic effects of a GATA3-specific DNAzyme in asthma patients. Journal of allergy and clinical immunology. 2017;140(2):625–628 e625. doi: 10.1016/j.jaci.2017.02.024. [DOI] [PubMed] [Google Scholar]
- Lee C-C, Huang H-Y, Chiang B-L. Lentiviral-mediated GATA-3 RNAi Decreases Allergic Airway Inflammation and Hyperresponsiveness. Mol Ther. 2007;16(1):60–65. doi: 10.1038/sj.mt.6300309. http://www.nature.com/mt/journal/v16/n1/suppinfo/6300309s1.html. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nguyen J, Steele T, Merkel O, Kissel T. A new synthesis method and degradation of hyper-branched polyethylenimine grafted polycaprolactone block mono-methoxyl poly (ethylene glycol) copolymers (hy-PEI-g-PCL-b-mPEG) as potential DNA delivery vectors. POLYMER. 2009;50(16):3895–3904. doi: 10.1016/j.polymer.2009.06.043. [DOI] [Google Scholar]
- Lively TN, Kossen K, Balhorn A, Koya T, Zinnen S, Takeda K, et al. Gelfand EW. Effect of chemically modified IL-13 short interfering RNA on development of airway hyperresponsiveness in mice. Journal of allergy and clinical immunology. 2008;121(1):88–94. doi: 10.1016/j.jaci.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel OM, Kissel T. Nonviral Pulmonary Delivery of siRNA. Acc Chem Res. 2012;45(7):961–970. doi: 10.1021/ar200110p. [DOI] [PubMed] [Google Scholar]
- Merkel OM, Librizzi D, Pfestroff A, Schurrat T, Buyens K, Sanders NN, Kissel T. Stability of siRNA polyplexes from poly (ethylenimine) and poly (ethylenimine)-g-poly (ethylene glycol) under in vivo conditions: effects on pharmacokinetics and biodistribution measured by Fluorescence Fluctuation Spectroscopy and Single Photon Emission Computed Tomography (SPECT) imaging. J Control Release. 2009;138(2):148–159. doi: 10.1016/j.jconrel.2009.05.016. S0168-3659.09.00305-8 [pii] [DOI] [PubMed] [Google Scholar]
- Merkel OM, Zheng M, Debus H, Kissel T. Pulmonary Gene Delivery Using Polymeric Nonviral Vectors. BIOCONJUGATE CHEMISTRY. 2012;23(1):3–20. doi: 10.1021/bc200296q. [DOI] [PubMed] [Google Scholar]
- Montixi C, Langlet C, Bernard AM, Thimonier J, Dubois C, Wurbel MA, He HT. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. The EMBO journal. 1998;17(18):5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers LM, Cossman J. Thymic Hormones and Lymphokines. Springer; 1984. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin-2 (TCGF) pp. 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Morishita M, Mizutani K, Shibayama A, Okazaki M, Okamoto H. Development of spray-freeze-dried siRNA/PEI powder for inhalation with high aerosol performance and strong pulmonary gene silencing activity. Journal of Controlled Release. 2018;279:99–113. doi: 10.1016/j.jconrel.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Olden BR, Cheng E, Cheng Y, Pun SH. Identifying key barriers in cationic polymer gene delivery to human T cells. Biomaterials science. 2019;7(3):789–797. doi: 10.1039/c8bm01262h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olden BR, Cheng Y, Yu JL, Pun SH. Cationic polymers for non-viral gene delivery to human T cells. Journal of Controlled Release. 2018;282:140–147. doi: 10.1016/j.jconrel.2018.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramishetti S, Kedmi R, Goldsmith M, Leonard F, Sprague AG, Godin B, et al. Peer D. Systemic Gene Silencing in Primary T Lymphocytes Using Targeted Lipid Nanoparticles. ACS Nano. 2015;9(7):6706–6716. doi: 10.1021/acsnano.5b02796. [DOI] [PubMed] [Google Scholar]
- Ray A, Cohn L. Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. Journal of Clinical Investigation. 1999;104(8):985–993. doi: 10.1172/Jci8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok MJR, Frijlink HW, Hinrichs WLJ. Pulmonary administration of small interfering RNA: The route to go? JOURNAL OF CONTROLLED RELEASE. 2016;235:14–23. doi: 10.1016/j.jconrel.2016.05.054. [DOI] [PubMed] [Google Scholar]
- Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Schulze J, Kuhn S, Hendrikx S, Schulz-Siegmund M, Polte T, Aigner A. Spray-Dried Nanoparticle-in-Microparticle Delivery Systems (NiMDS) for Gene Delivery, Comprising Polyethylenimine (PEI)-Based Nanoparticles in a Poly (Vinyl Alcohol) Matrix. Small. 2018;14(12):e1701810. doi: 10.1002/smll.201701810. [DOI] [PubMed] [Google Scholar]
- Sel S, Wegmann M, Dicke T, Sel S, Henke W, Yildirim AÖ, et al. Garn H. Effective prevention and therapy of experimental allergic asthma using a GATA-3–specific DNAzyme. Journal of Allergy and Clinical Immunology. 2008;121(4):910–916.e915. doi: 10.1016/j.jaci.2007.12.1175. [DOI] [PubMed] [Google Scholar]
- Selby LI, Cortez-Jugo CM, Such GK, Johnston APR. Nanoescapology: progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2017;9(5):e1452. doi: 10.1002/wnan.1452. [DOI] [PubMed] [Google Scholar]
- Smith SA, Selby LI, Johnston APR, Such GK. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjugate Chemistry. 2019;30(2):263–272. doi: 10.1021/acs.bioconjchem.8b00732. [DOI] [PubMed] [Google Scholar]
- Thomson NC, Patel M, Smith AD. Lebrikizumab in the personalized management of asthma. Biologics. 2012;6:329–335. doi: 10.2147/BTT.S28666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehring R. Pharmaceutical particle engineering via spray drying. Pharmaceutical research. 2008;25(5):999–1022. doi: 10.1007/s11095-007-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells—how did we miss them? Nature reviews immunology. 2013;13(2):75. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- Weber S, Zimmer A, Pardeike J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: a review of the state of the art. European Journal of Pharmaceutics and Biopharmaceutics. 2014;86(1):7–22. doi: 10.1016/j.ejpb.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Weckmann M, Collison A, Simpson JL, Kopp MV, Wark PA, Smyth MJ, et al. Whitehead B. Critical link between TRAIL and CCL20 for the activation of TH2 cells and the expression of allergic airway disease. Nature medicine. 2007;13(11):1308–1315. doi: 10.1038/nm1660. [DOI] [PubMed] [Google Scholar]
- Wegmann M. Th2 cells as targets for therapeutic intervention in allergic bronchial asthma. Expert review of molecular diagnostics. 2009;9(1):85–100. doi: 10.1586/14737159.9.1.85. [DOI] [PubMed] [Google Scholar]
- Wegmann M, Fehrenbach H, Fehrenbach A, Held T, Schramm C, Garn H, Renz H. Involvement of distal airways in a chronic model of experimental asthma. Clinical & Experimental Allergy. 2005;35(10):1263–1271. doi: 10.1111/j.1365-2222.2005.02306.x. [DOI] [PubMed] [Google Scholar]
- Xie YR, Kim NH, Nadithe V, Schalk D, Thakur A, Kilic A, et al. Merkel OM. Targeted delivery of siRNA to activated T cells via transferrin-polyethylenimine (Tf-PEI) as a potential therapy of asthma. Journal of Controlled Release. 2016;229:120–129. doi: 10.1016/j.jconrel.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Pavan GM, Neeb M, Schaper AK, Danani A, Klebe G, et al. Kissel T. Targeting the blind spot of polycationic nanocarrier-based siRNA delivery. ACS nano. 2012;6(11):9447–9454. doi: 10.1021/nn301966r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zscheppang K, Berg J, Hedtrich S, Verheyen L, Wagner DE, Suttorp N, et al. Hocke AC. Human Pulmonary 3D Models For Translational Research. Biotechnol J. 2018;13(1) doi: 10.1002/biot.201700341. [DOI] [PMC free article] [PubMed] [Google Scholar]