Abstract
Monoclonal antibodies targeting IgE or the type-2 cytokines IL-4, IL-5 and IL-13 are proving highly effective in reducing exacerbations and symptoms in people with severe allergic and eosinophilic asthma respectively. However, these therapies are not appropriate for 30-50% of patients in severe asthma clinics who present with non-allergic, non-eosinophilic, ‘type-2 low’ asthma. These patients constitute an important and common clinical asthma phenotype, driven by distinct, though poorly understood pathobiological mechanisms. In this review we describe the heterogeneity and clinical characteristics of type-2 low asthma and summarise current knowledge on the underlying pathobiological mechanisms, which includes neutrophilic airway inflammation often associated with smoking, obesity, occupational exposures and may be driven by persistent bacterial infections and by activation of a recently-described IL-6 pathway. We review the evidence base underlying existing treatment options for specific treatable traits which can be identified and addressed. We particularly focus on severe asthma as opposed to difficult-to-treat asthma, on emerging data on the identification of airway bacterial infection, on the increasing evidence base for the use of long-term low-dose macrolides, a critical appraisal of bronchial thermoplasty, and evidence for the use of biologics in type-2 low disease. Finally we review ongoing research into other pathways including TNF, IL-17, resolvins, apolipoproteins, type I interferons, IL-6 and mast cells. We suggest that type-2 low disease frequently presents opportunities for identification and treatment of tractable clinical problems and is currently a rapidly evolving field with potential for the development of novel targeted therapeutics.
Introduction
Asthma is a complex, chronic disease characterised by heterogeneous airway inflammation. 70-80% of corticosteroid-naïve and 50% of corticosteroid-treated asthma patients have a raised sputum eosinophil count[1], which is generally associated with enhanced expression of the type-2 cytokines interleukin (IL)-4, IL-5 and IL-13[2], increased fractional exhaled nitric oxide (FeNO), peripheral blood eosinophilia and a reproducible type-2 inflammatory epithelial gene signature[3]. This ‘type-2 high’ phenotype is characteristically responsive to treatment with inhaled corticosteroids (ICS), and, in severe disease, to biologic agents targeting these type-2 cytokines[4].
Whilst these treatments are proving highly-effective, there remain a significant proportion of people with ‘type-2 low’ disease characterised by normal sputum and peripheral blood eosinophil counts, and low FeNO, yet with persistent symptoms and airflow limitation and a poor response to corticosteroids (Table 1). Due to a relative paucity of research and limited therapeutic options these patients often present a clinical challenge. However several recent and ongoing studies provide a stimulus to optimism in this rapidly-evolving field. Here we review the heterogeneity and clinical characteristics of type-2 low asthma in adults and adolescents, summarise current knowledge on the underlying pathobiological mechanisms and review the evidence base underlying existing treatment options for specific ‘treatable traits’ within type-2 low asthma. Finally, we review ongoing research into other pathways constituting potential novel therapeutic targets.
Table 1. Challenges in developing therapeutics for type-2 low neutrophilic asthma, compared with successes in eosinophilic asthma.
| Characteristic | Eosinophilic asthma | Neutrophilic asthma |
|---|---|---|
| Biology of granulocytes | Eosinophils: | Neutrophils: |
| - long-lived haematopoietic cells. | - short-lived haematopoietic cells. | |
| - reside predominantly in mucosal tissues | - predominantly circulating in blood. | |
| - absent in sputum and airways in health. (e.g. airways). | - present in sputum and airways in health. | |
|
| ||
| Role in pathogenesis | Inflammatory eosinophils in the airways of patients with asthma are pathogenic and associate with exacerbations. | Role of neutrophils in the airways of patients with asthma is unknown; neutrophils are beneficial in airway infection. |
|
| ||
| Non-invasive biomarkers | Elevated FeNO | None. Non-invasive biomarkers (e.g. VOC) are not available in clinical practice. |
| Blood eosinophils correlate with sputum eosinophils in asthma. | Blood neutrophil levels do not correlate with sputum neutrophil levels in asthma. | |
|
| ||
| Heterogeneity of phenotype | Moderate heterogeneity within eosinophilic asthma: allergic versus nonallergic; early-onset versus late-onset. | Huge heterogeneity within neutrophilic asthma; multiple associated factors e.g. smoking, air pollution, obesity, infection. |
|
| ||
| Differential diagnosis | Limited: eosinophilic COPD; eosinophilic pneumonia; ABPA; EGPA. | Very broad: e.g. COPD; bronchiectasis; cystic fibrosis; diffuse panbronchiolitis; bacterial and fungal infections; tuberculosis; NTM infection. |
|
| ||
| Therapeutic targets | Clearly delineated: | Less well defined: [see Table 3] |
| - corticosteroids. | - pro-inflammatory cytokines such as IL-1β, IL-6, TNF, IL-17, IL-17R, IL-23 | |
| - type-2 cytokines and their receptors: IL-5, IL-5R and IL-4R. | - CXC chemokines or their receptors | |
| - IgE in allergic eosinophilic severe asthma. | - β-tryptase, G-CSF, GM-CSF | |
| - epithelial alarmins (e.g. TSLP, IL-33). | - epithelial alarmins (e.g. TSLP; IL-33) | |
ABPA, allergic bronchopulmonary aspergillosis; COPD, chronic obstructive pulmonary disease; CXC, C-X-C motif chemokine ligand; EGPA, eosinophilic granulomatosis with polyangiitis; FeNO, fractional exhaled nitric oxide; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte/monocyte colony-stimulating factor; IgE, immunoglobulin E; IL, interleukin; NTM, non-tuberculous mycobacteria; TNF, tumour necrosis factor; TSLP, thymic stromal lymphopoietin; VOC, volatile organic compounds.
How common is type-2 low asthma? A normal sputum eosinophil count is seen in 25% of patients with untreated symptomatic asthma[5] and 40-50% of patients with asthma treated with high doses of ICS[6–8]. Type-2 low asthma may be more common in mild-to-moderate disease, with estimates of 64-73%[9, 10] with a single sputum sample, and even with repeated sampling this may be approximately half of asthmatics[9]. Non-eosinophilic inflammation is also common in irritant-induced occupational asthma[6] and during virus-induced asthma exacerbations[11] and is increasingly seen in biologic-treated type-2 high patients experiencing infective exacerbations.
Type-2 low asthma encompasses both neutrophilic asthma and pauci-granulocytic asthma. Sputum neutrophilia is often defined as ≥61%[9, 12–14] or ≥73%[15–17] neutrophils on a cytospin. The optimal cut-off might differ according to local air pollution levels. For sputum eosinophilia several definitions have been used including cut-offs of 1%[12], 2%[18, 19], 3%[20–22]. Sputum eosinophilia >3% identifies individuals with corticosteroid responsive asthma[20, 22], and a definition of sputum eosinophils ≥3%, blood eosinophil ≥0.3×109 or FeNO ≥ 50 ppb identified patients responsive to anti-IL-5 therapy[23]. However for the purposes of identifying non-eosinophilic asthma a lower cut off for sputum eosinophils of <2%[9] may be more specific, and has been adopted in recent clinical trials[10] and GINA guidelines[24]. Whilst some patients may vary around these thresholds over time, in one study 96% of patients without sputum eosinophilia remained non-eosinophilic at 5-year follow-up[19], consistent with a previous report[12]. In a large study with 324 paired sputum samples 47% of participants with mild or moderate asthma had persistently non-eosinophilic samples on multiple occasions over one year, suggesting persistent non-eosinophilic asthma is a common finding[9]. Nonetheless, even in non-eosinophilic asthma, intermittent occurrence of airway eosinophilia is frequently observed[25], with an intraclass correlation coefficient for sputum neutrophils of 0.78[26]. The reasons for this variability are not known, but may be related to changes in treatment, environmental factors such as allergen exposures, seasonal changes and airway microbiology, and repeated measures may be required to obtain accurate phenotyping[26]. Inhaled and oral corticosteroids being used effectively to treat eosinophilic inflammation may lead to a non-eosinophilic phenotype in some individuals. In a cohort of 26 subjects with stable, persistent asthma and non-eosinophilic sputum at baseline, after 12 weeks withdrawal of maintenance inhaled corticosteroids 80% developed eosinophilic sputum, although it should be noted that the initial phenotype in all but one individual was paucigranulocytic, rather than neutrophilic disease[25]. It is important to recognize that a subset of eosinophilic asthmatics have eosinophil granules, rather than intact eosinophils, present in sputum which may result in misclassifying these patients as pauci-granulocytic asthmatics[27]. Occult eosinophilic inflammation can be identified in these individuals by demonstrating eosinophil granule components, such as eosinophil peroxidase (EPO), in sputum supernatants, or intracellular eosinophil proteins, such as eosinophil cationic protein and EPO, within sputum macrophages that have phagocytosed apoptotic eosinophils [27–29].
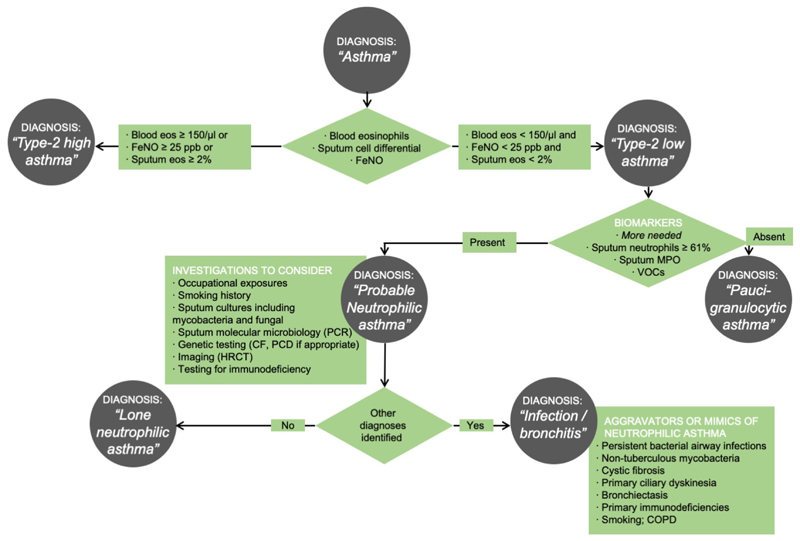
What are the clinical characteristics of type-2 low asthma, beyond an absence of type-2 biomarkers? A characteristic feature is a lack of response to systemic corticosteroids. By contrast patients with significant eosinophilic disease typically report symptomatic improvement within 1-2 days of starting oral corticosteroids. Non-eosinophilic asthma is associated with female sex, obesity[30], non-atopic status and adult onset symptoms[5]. It is also associated with smoking, occupational exposures to low-molecular weight compounds[31], and elite athletics[32, 33]. It is useful to enquire about a chronic mucopurulent cough, often a sign of chronic bacterial bronchitis or bronchiectasis, implying the need for additional diagnostic testing such as sputum microbiology (including Mycobacterium tuberculosis and non-tuberculous mycobacteria) and a high-resolution CT thorax. A suggested algorithm for diagnosis of neutrophilic asthma in clinical practice in shown in Figure 1.
Figure 1. Suggested algorithm for defining type-2 low asthma in clinical practice.
CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; Eos, eosinophils; FeNO, fractional exhaled nitric oxide; HRCT, high-resolution computed tomograph; PCD, primary ciliary dyskinesia; PCR, polymerase chain reaction.
Mechanisms
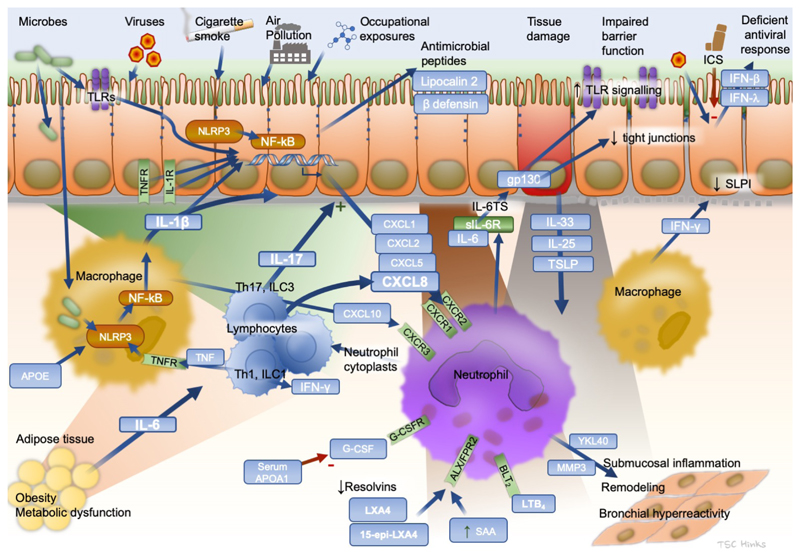
In contrast to type-2 high asthma, less is known about the mechanisms in type-2 low disease. Neutrophilic inflammation is likely due to innate and adaptive cell mediated immune responses (Figure 2). Airway neutrophilia is common during some viral infections and also during stable chronic asthma[2, 6, 8]. A transcriptomic analysis of airway samples in severe neutrophilic asthma found a strong upregulation of mucins, IL-17-inducible chemokines (CXCL1, CXCL2, CXCL3, and CSF-3) and the neutrophil chemoattractants CXCL8 (IL-8), CCL3 and LGALS3 [34]. Signatures of antibacterial responses including CD14, JUN and TLR2, implicate airway bacteria in driving the neutrophilia[34]. Neutrophilic asthma is associated with airway colonisation by bacteria including Haemophilus influenzae and Moraxella catarrhalis[35], which might induce Th17 responses[36]. Other microbiome studies have also linked neutrophilic asthma with the presence of H. influenzae[37] and of a reduced microbial diversity, suggesting dominance of a single airway pathogen[38]. Larger microbiome studies are needed to determine the exact role these bacteria play.
Figure 2. Key pathways mediating neutrophilic airway inflammation in type 2-low asthma.
APO, apolipoprotein; CXCL, C-X-C motif chemokine ligand; CXCR, C-X-C motif chemokine receptor; FPR, N-formyl peptide receptor; G-CSF, granulocyte colony-stimulating factor; G-CSFR, granulocyte colony-stimulating factor receptor; GM-CSF, granulocyte/monocyte colony-stimulating factor; GM-CSFR, granulocyte/monocyte colony-stimulating factor receptor; gp130, glycoprotein 130; ICS, inhaled corticosteroid; IFN interferon; IL, interleukin; ILC, innate lymphoid cell; IL-6TS, interleukin-6 trans-signaling; LXA, lipoxin; NLRP, nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain containing; sIL-6R, soluble interleukin-6 receptorm; SAA, serum amyloid A; SLPI, secretory leukocyte protease inhibitor; Th, T helper CD4+ lymphocyte; TNF, tumor necrosis factor; TSLP, thymic stromal lymphopoietin.
Neutrophilic asthma is also associated with upregulation of oxidative stress responses – potentially driven by smoking or by inflammatory cell-derived reactive oxygen species – and matrix metalloproteases, including MMP-9, a type IV collagenase involved with CXCL8-induced neutrophilia[34]. MMPs are increased in airway samples from asthmatic smokers[39] and in severe neutrophilic asthma[8], and are implicated in tissue remodelling[8, 40, 41].
Tissue damage driven by stimuli such as viruses, bacteria, smoking or low-molecular-weight agents induces airway epithelium to release the alarmins thymic stromal lymphopoietin (TSLP), IL-25 and IL-33. Whilst these can drive type-2 pathology, they are likely to be released too by drivers of type-2 low pathology, which may explain the observed efficacy of anti-TSLP blockade in non-eosinophilic asthma[42].
Type-2 low asthma is associated with heightened capsaicin cough reflex sensitivity suggesting airway neuronal dysfunction, particularly of transient receptor potential vanilloid-1 (TRPV-1)[43–45]. A range of other immune mechanisms are implicated, including dysregulation of IL-6, IL-17, TNF and type 1 IFN signalling[2], each of which are discussed below.
Current therapeutic options
The role of corticosteroids
Since the 1950s it has been known that systemic corticosteroids are less effective in patients without sputum eosinophilia[20, 22, 46]. Lack of sputum eosinophilia predicts a lack of response to ICS[30, 47–50]. Indeed corticosteroid treatment can be safely reduced in non-eosinophilic asthma without increasing exacerbation frequency[20].
Mild asthma
In mild asthma a recent randomized controlled trial (RCT) found ICS effective in those with sputum eosinophilia, but not in those with <2% sputum eosinophils[10]. In mild-to-moderate asthma ICS didn’t improve lung function in those with type-2 low baseline gene expression profiles[3].
Given the poor steroid efficacy in type-2 low asthma an approach using as-required combination formoterol/corticosteroid inhalers as first line treatment in mild asthma has the dual benefits of increasing ICS delivery in those with type-2 high asthma, whilst reducing unnecessary steroid exposure by 50-80% in those least likely to benefit[51–53].
Severe asthma
In difficult-to-treat and severe asthma systemic corticosteroid prescribing is common, with up to 60% of treatment refractory asthmatics in a UK series receiving oral corticosteroids[54]. In symptom-predominant asthma phenotypes[55], or those with fixed airflow limitation[56], overtreatment with systemic corticosteroids can occur with inappropriate escalation of oral corticosteroids[57–59]. Biomarker-directed adjustment of dosing could reduce systemic corticosteroid prescribing. Adjusting ICS doses based on sputum eosinophil or FeNO effectively decreases exacerbations[60, 61]. Whilst induced sputum analysis availability is limited, protocolised reduction of inhaled and systemic steroids based on blood eosinophils, FeNO and serum periostin shows promise and is being evaluated prospectively[62].
Omalizumab less effective in type-2 low severe allergic asthma
Omalizumab[63], an injectable monoclonal antibody (mAb) targeting IgE reduces exacerbations in children and adults with severe allergic asthma. Patient selection is typically based on elevated serum IgE, although in severe persistent allergic asthma an omalizumab RCT found no significant benefit in type-2 low disease, stratified either by FeNO<20 ppb, or blood eosinophils <0.26×109 or periostin <50 ng/mL[64]. In contrast, in a retrospective observational study[65], the response rate was similar in high and low blood eosinophil subgroups. Since this discrepancy might arise from differences in study design, more research is required to delineate the role of type-2 biomarkers in predicting response to omalizumab.
Macrolides
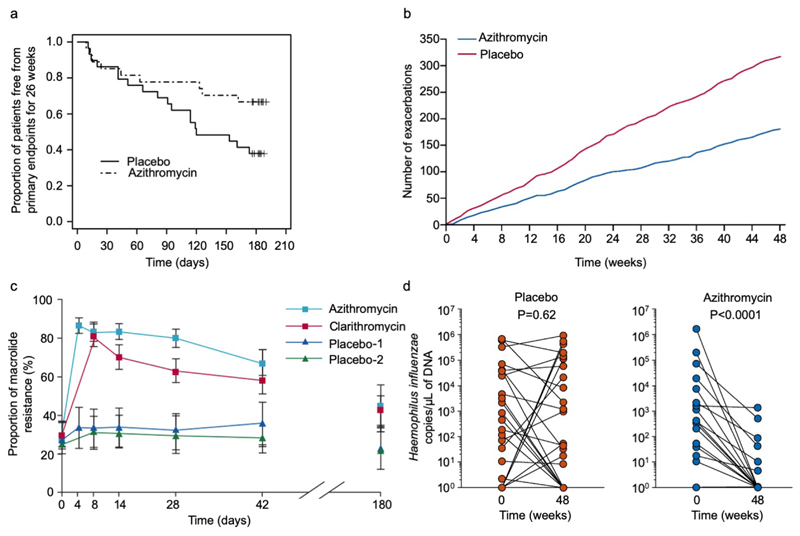
Several studies suggested that long-term use of macrolide antibiotics may have steroid-sparing or anti-inflammatory[66] effects in asthma. Macrolides reduce exacerbations in other neutrophilic chronic airways diseases including cystic fibrosis[67], non-CF bronchiectasis[68–70] and COPD[71]. Efficacy in asthma had not been shown in previous small studies[72, 73], however long-term low dose azithromycin has now been shown to be effective in two RCTs. The AZIZAST study randomised 109 adults with exacerbation-prone severe asthma to thrice-weekly azithromycin 250 mg or placebo for 26 weeks. Whilst the primary outcome was not reached in the overall population, in a pre-defined subgroup analysis azithromycin reduced the rate of severe exacerbations in the non-eosinophilic subjects (rate ratio 0.42 versus placebo in those with blood eosinophils <0.2×109/L)(Figure 3a)[74]. The larger AMAZES study randomised 420 adults with moderate-to-severe asthma to thrice-weekly azithromycin 500 mg or placebo for 48 weeks[75], finding a striking reduction in asthma exacerbations, with the proportion of patients experiencing an exacerbation reducing from 61% to 44%, an incidence rate ratio of 0.59, and with significant improvements in asthma-related quality of life (Figure 3b). This effect was also seen in those without a frequent exacerbation history. Unexpectedly a subgroup analysis found azithromycin effective in both eosinophilic and non-eosinophilic phenotypes. Subsequent meta-analysis of AZISAST and AMAZES confirmed efficacy of maintenance azithromycin as add-on therapy to ICS+LABA[76].
Figure 3. Clinical trial data for azithromycin data in severe asthma and for induction of resistance in healthy volunteers.
(a) Proportion of subjects with non-eosinophilic severe asthma (FeNO<upper limit of normal and a blood eosinophilia ≤200/ml) free from primary endpoints (severe exacerbations) for 26 weeks, according to study group (azithromycin or placebo) in AZISAST[74]. Azithromycin significantly decreased the number of patients with at least one primary endpoint from 9/27 (33%) azithromycin-treated subjects vs 18/29 (62%) placebo-treated subjects; relative risk 0.54, 95% CI 0.29 to 0.98, p=0.037). Reproduced from Brusselle GG and colleagues[74] with permission from BMJ Publishing Group Ltd. (b) Cumulative severe and moderate asthma exacerbations during 48 weeks of treatment with azithromycin 500 mg, three times per week, or placebo in AMAZES[75]. Reproduced with permission from Elsevier. (c) Temporal changes in the proportion of macrolide-resistant streptococci after azithromycin and clarithromycin use in healthy volunteers. Mean data are shown for 204 volunteers (of 224 recruited) assessed to day 42, and for 99 volunteers assessed to day 180. Error bars are 95% CIs. Reproduced from Malhotra-Kumar and colleagues[230] with permission from Elsevier. (d) Haemophilus influenzae copy number before and after either placebo (left, red) or azithromycin (right, blue) in AMAZES. Reproduced from Taylor SL[91] with permission from American Thoracic Society.
The mechanism of azithromycin efficacy is not understood. Azithromycin has antibacterial, antiviral[77] and anti-inflammatory[66] effects. These include inhibition of cytokines[78], chemokines[79], cytotoxicity[80], biofilms[77], and various immunomodulatory actions on neutrophils and T cells[78], including inhibiting calcineurin[81] and mTOR[82], besides reducing mucus production and stimulating phagocytosis[83]. It is unclear if the clinical efficacy is specific to azithromycin as macrolides differ in these properties[84]. Although short term clarithromycin reduced sputum IL-8[85] studies of other macrolides have been of short duration and small size[72], with a strong evidence base only for azithromycin.
Whilst macrolides are effective and recommended in current American Thoracic Society (ATS) / European Respiratory Society (ERS) and Global Initiative for Asthma (GINA) guidelines for selected persistently symptomatic adults with severe asthma[24, 86], there are several concerns about widespread use. They can cause diarrhoea, usually mild[75]. They have the potential for QT prolongation, although ECG screening excludes this effect[74, 75]. In a COPD trial a slight excess of hearing loss was observed using stringent sequential audiometry data, but this incidence was likely over estimated, was largely reversible[71] and was not observed in the two asthma studies[74, 75] perhaps because the patients were younger, and AMAZES excluded those with hearing loss. The greatest concern is inducing antimicrobial resistance. Macrolides may predispose to acquisition of mycobacteria by impairing autophagy, and inadvertent monotherapy could induce drug resistance in undiagnosed mycobacterial infection making subsequent treatment difficult, particularly of non-tuberculous mycobacteria[87]. Therefore sputa should be screened for acid fast bacilli prior to therapy. Global macrolide resistance is increasing rapidly amongst other bacteria with 90-100% resistance to Streptococcus pneumoniae[88] and Mycoplasma pneumoniae in China[89]. Due to its long, 3-day half-life azithromycin poses a particular ecological risk (Figure 3c). Resistance develops rapidly in oropharyngeal flora. Moreover resistance on mobile genetic elements is associated with resistance to other drug classes including chloramphenicol, tetracyclines and penicillins[87].
Azithromycin may act primarily as an antibacterial. Colonisation with Haemophilus, Streptococci and Moraxella is common in severe asthma [35] and Haemophilus may evade penicillins by paracellular and intracellular invasion[90]. In AMAZES the main change in sputum microbiome was a marked reduction in Haemophilus influenzae (Figure 3d)[91], perhaps related to high intracellular activity. Furthermore a post hoc analysis of baseline H. influenzae abundance by qPCR suggested those with highest H. influenzae abundance derived most benefit from the azithromycin therapy in terms of exacerbation reduction[92]. It is unknown whether other intracellular antibiotics like doxycycline are effective. Biomarker-directed antibiotic trials are required to determine which patients respond to antibiotics and the dose and duration of therapy. A greater understanding of their mechanisms could lead to novel macrolides or non-antibiotic macrolide compounds, with less potential for drug resistance[84]. However, we hypothesise their efficacy might be intermediate between placebo and azithromycin.
Long-acting muscarinic antagonists (LAMAs)
Several trials investigated addition of long acting anti-muscarinics (LAMAs), predominantly in severe asthma, leading to their inclusion as a step 4 or 5 option in current GINA guidelines[24, 86]. Meta-analyses suggest tiotropium provides significant improvements in lung function including peak expiratory flow and FEV1, though not in FVC[93, 94]. However, these effects are small, and despite a possible reduction in severe exacerbations LAMAs have not shown a meaningful improvement in quality of life or reduction in hospital admission[93, 94].
Bronchial Thermoplasty
Bronchial thermoplasty involves localised radiofrequency ablation of bronchial mucosa applied during three bronchoscopic treatment sessions. The mechanism is poorly understood but may involve reduced smooth muscle mass[95] or inhibition of airway neurons[96] or inflammatory cells[97]. Independence from type-2 pathways makes this theoretically attractive, but efficacy data are limited. Studies were confounded by large placebo responses for subjective patient-reported outcomes, compared with absence of objective effects on lung function[98, 99]. Data are lacking in those with very poor lung function (FEV1<60%), and treatment is associated with a temporary increase in exacerbations[99]. Long term-follow up data are limited. Current ATS/ERS and GINA guidelines recommend thermoplasty be considered as an add-on therapy for selected adult patients with medically-optimised severe disease, performed within an independent systematic registry or clinical study[24, 100].
Given the lack of many highly-effective options in type-2 low asthma, and especially in paucigranulocytic severe asthma, where airway remodelling may be a key driver of airway hyper-responsiveness (AHR) and symptoms, bronchial thermoplasty research should focus on type-2 low patients and developing predictors of response. Patient selection may be facilitated by emerging bronchoscopic imaging techniques, but must then be validated by long-term prospective registries.
Other treatable traits
Symptoms in asthma arise from a complex interplay of inflammation, airway-hyperreactivity and additional factors[101], including co-morbidities and psychological factors, many of which are not associated with airways inflammation[8, 30] (Table 2). Identifying and managing these ‘treatable traits’ may provide significant symptomatic benefit for individuals[56, 102]. Twenty-three treatable traits were identified within the U-BIOPRED cohort, being more common in severe asthma[103]. The most prevalent extra-pulmonary traits were atopy, rhinosinusitis, obesity, reflux and obstructive sleep apnoea. Poor adherence, anxiety and depression were the most common behavioural/ psychosocial treatable traits.
Table 2. Current therapeutic options in type-2 low asthma.
| Treatable trait | Phenotype | Potential Biomarkers | Investigations | Therapeutic option | Comments |
|---|---|---|---|---|---|
|
| |||||
| Fixed airflow obstruction | Persistent airflow obstruction despite ICS+LABA use. | Spirometry with reduced post-bronchodilator FEV1/FVC ratio. | Long acting antimuscarinics. | Effect small and may worsen cough so assess response and discontinue if no benefit. | |
|
| |||||
| Chronic bacterial airway colonisation | Persistent mucopurulent cough, frequent infective exacerbations. | Typical organisms on sputum culture. | Sputum culture. | Long term, low dose azithromycin. | Research needed into optimal patient selection, duration of therapy, potential use of other macrolides. |
| Bacterial colonisation with potentially pathogenic bacteria (e.g. Haemophilus influenzae). | Pathogenic specific quantitative PCR. | Exclude mycobacteria with sputum culture. | |||
| Consider CT to exclude bronchiectasis. | |||||
|
| |||||
| Cough reflex hypersensitivity | Female predominant, adult onset. | Capsaicin hypersensitivity. | Discontinue ACEi, treat GORD. | Research needed into cough suppressants including P2X3 inhibitors. | |
|
| |||||
| Airway hyperreactivity | Marked airway hyperreactivity and inadequate response to other therapies. | Paucigranulocytic. | Reversibility / bronchial hyperresponsiveness testing, CT to exclude bronchiectasis and tracheobronchomalacia. | Consider bronchial thermoplasty in highly-selected patients. | Optimal phenotype, long term outcomes and efficacy of retreatment remain to be defined. |
|
| |||||
| Steroid over use | Non-eosinophilic, patient reports symptoms are slow to improve after initiation of systemic steroids. | Peripheral blood eosinophil count. | Consider a steroid holiday: cautiously stopping systemic steroids. | Care to avoid iatrogenic adrenal insufficiency. | |
|
| |||||
| Vocal cord dysfunction (ILO) | Episodic, symptoms predominantly inspiratory, inspiratory stridor, minimal response to pharmacotherapy. | Flattened inspiratory flow loop, normal expiratory spirometry. | Laryngoscopy during provocation. | Specialist speech and language therapy. | Often coexists with asthma, triggers include inhalational irritants, exercise, and psychosocial disorders. |
|
| |||||
ACEi, angiotensin converting enzyme inhibitor; CT, computed tomography; GORD, gastro-oesophageal reflux; ILO, inducible laryngeal obstruction; LABA, long-acting beta-2 agonist; PCR, polymerase chain reaction.
Tobacco smoking is associated with neutrophilic airway inflammation, leads to worse symptoms[104], impaired steroid responsiveness[105], increased bronchial reactivity[106] and rapid lung function decline[104] so every effort should be made to encourage smoking cessation.
Vocal cord dysfunction, also called inducible laryngeal obstruction affects, an estimated 1 in 4 adults with asthma[107] often leading to over treatment. If identified early it responds to speech and language therapy[108]. Dysfunctional breathing is more common in type-2 low asthma[55] and responds to physiotherapy, leading to marked, sustained improvements in symptoms[109].
Type-2 low asthma is associated with obesity[55], perhaps related to increased systemic IL-6 inflammation[110]. Numerous studies have shown that weight loss, particularly when dietary changes are combined with increased exercise, lead to improved asthma control and lung function[111]. The magnitude of improvement is related to extent of weight loss, with at least 5% weight loss required to produce significant improvements[112]. Bariatric surgery is the most effective intervention for achieving sustained weight loss, and is consistently associated with improvements in asthma control, airway reactivity, and lung function[111].
There is less evidence for management of some other treatable traits. Whilst rhinitis, and gastro-oesophageal reflux cause asthma-like symptoms, there is little evidence their treatment improves asthma control[56, 113].
Anti-TSLP monoclonal antibody tezepelumab
Upstream targets for therapeutic intervention encompass the epithelial alarmin Thymic Stromal Lymphopoietin (TSLP), which is secreted by airway epithelial cells exposed to noxious stimuli such as cigarette smoke[114], diesel exhaust particles[115], proteases and microbes[116]. Airway TSLP expression is increased in severe asthma, and TSLP has been associated with steroid resistance of airway type 2 innate lymphoid cells in severe asthma[117, 118]. In a phase 2 RCT treatment with subcutaneous tezepelumab, a human anti-TSLP monoclonal antibody reduced asthma exacerbations by 60 to 70%, and improved lung function[42]. Efficacy occurred irrespective of blood eosinophil or FeNO levels, suggesting anti-TSLP might affect disease activity more broadly than inhibition of more downstream pathways. These promising results in type-2 and non-type-2 asthma need replication in larger phase 3 trials.
Potential Future treatment options
Development of new therapeutics for type-2 low disease will require a different approach from that taken in type-2 high disease, due to several important differences between these major phenotypes (Table 1). It is unlikely that a simple biologic blocking of a single interleukin pathway will replicate the successes of the anti-type-2 biologics. Neutrophils have a very distinct biology to eosinophils, are far more abundant and critical to many biological processes. Neutrophilia may even be a beneficial response in type-2 low airway inflammation. There is a lack of clinically-available non-invasive biomarkers, a much broader differential diagnosis to be considered and optimal therapeutic targets remain unclear. Nonetheless, several pathways in type 2-low asthma are potential therapeutic targets, which we review next (Table 3). Strategies targeting type-2 low pathways will need to maintain adequate host defence and immune surveillance functions to prevent infectious or neoplastic complications. Furthermore, the successful targeting of specific pathways mediating type-2 low asthma will require the identification of biomarkers to direct treatment in a precision medicine approach to those patients in whom the pathway is active and is mediating disease. Potential new biomarkers include serum and sputum levels of neutrophil products such as neutrophil lipocalin, neutrophil gelatinase-associated lipocalin[119, 120] and myeloperoxidase[8], although these require validation in large cohorts. Another potential approach is the measurement of volatile organic compounds such as in exhaled breath condensate. One study has reported the combination of nonanal, 1-propanol and hexane identifies neutrophilic asthma with 81% sensitivity, although the specificity was low at 43% and these approaches remain at the experimental stage[121].
Table 3. Potential future therapeutic targets in type-2 low asthma.
| Pathway | Pathobiological Mechanism | Potential Biomarkers | Potential Therapeutics |
|---|---|---|---|
| IL-1β | Activation of the NLRP3 inflammasome → NF-kB → cytokines including IL-1β and neutrophil chemokines | IL-1β | Anti-IL-1β (e.g. canakinumab) |
| IL-1R | IL-1β receptor antagonists (e.g. anakinra) | ||
| NLPR3 | NLRP3 small-molecule inhibitors IL-17A, IL-17F | ||
|
| |||
| IL-17A/F | Th17 / γδ T17 / ILC3 / MAIT cells → IL-17A & IL-17F → epithelial derived neutrophil chemoattractants and antimicrobial defence | IL-17A, IL-17F | Anti-IL-17RA (e.g. brodalumab) |
| IL-23A | Anti-IL-23 (e.g. risankizumab) | ||
| RORγt | DNAzymes | ||
| Small-molecule inhibitors | |||
|
| |||
| Alarmins | Epithelial tissue damage → release of alarmins TSLP / IL-33 / IL-25 | Anti-TSLP (e.g. tezepelumab) | |
|
| |||
| Resolvins | Lipoxin A4 promotes resolution of inflammation via ALX/FPR2 | Low LXA4 | LXA4 or analogues |
| Increased serum amyloid A inhibits resolvin signalling via ALX/FPR2 | High SAA | Specialized proresolving mediator precursors | |
|
| |||
| Colony stimulating factors | Apoliporoteins (e.g. APOA1) → ABCA1 inhibit G-CSF-induced neutrophilia | G-CSF | Neutralising antibodies |
| GM-CSF | APOA1 mimetic peptide | ||
|
| |||
| Type I interferons | Stable state: high ISG → type-2-independent inflammation | Blood ISG expression | |
| Acute viral infection: deficient type-I/III IFN → increased viral replication | Low IFN-α / -β / -λ | Inhaled IFN-β | |
|
| |||
| IL-6 | IL-6: obesity / granulocytes → IL-6 → steroid-resistant inflammation | IL-6 | Anti-IL-6 (e.g. clazakizumab) |
| Anti-IL-6R (e.g. tocilizumab) | |||
| IL-6 trans-signalling: bacteria → TLRs → granulocytes shed soluble IL-6R + IL-6 → local epithelial cell inflammation | sIL-6R | Antimicrobials | |
|
| |||
| Mast cells | IgE cross-linking → Mast cell degranulation → mediators including histamine, tryptase, chymase, carboxypeptidase | Tryptase | Anti-β-tryptase mAb |
| Chymase | KIT inhibitors (e.g. imatinib) | ||
|
| |||
| IFN-γ | Th1 / ILC1 / NK cells → IFN-γ → CXCL10 → neutrophilia & ↓ SLPI | TNF | Soluble TNFR (e.g. etanercept) IFN-γ, CXCL10, SLPI |
| IFN-γ, CXCL10, SLPI | Small-molecule inhibtors (JAK1) | ||
| Tbet | DNAzyme (Tbet) | ||
|
| |||
| CXCL8 (IL-8) | CXCL8 → CXCR2 → neutrophil recruitment | CXCL8 | Small-molecule inhibitors |
APOA1, apolipoprotein A1; BET, bromodomain and extraterminal; CXCL, C-X-C motif chemokine ligand; CXCR, C-X-C motif chemokine receptor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte/monocyte colony-stimulating factor; IFN, interferon; IL, interleukin; ILC, innate lymphoid cell; ISG, interferon-stimulated genes; JAK, Janus kinase; KIT, KIT proto-oncogene receptor tyrosine kinase; LXA, lipoxin A; mAb, monoclonal antibody; NLRP3, nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain containing; RORγt, retinoic acid-related orphan receptor γ thymus specific; SAA, serum amyloid A; SLPI, secretory leukocyte protease inhibitor; Tbet, T-box transcription factor TBX21; Th1, Th17, helper T-cell type 1 and type 17; TNF, tumour necrosis factor; TSLP, thymic stromal lymphopoietin.
Interleukin-1β(IL-1β)
IL-1β is a pro-inflammatory cytokine that promotes type 2-low neutrophilic asthma. IL-1β generation is mediated by the canonical NLRP3 inflammasome, which activates caspase-1 to process pro-IL-1β into its mature, secreted form[122]. Caspase-1 also cleaves gasdermin-D (GSDMD) into fragments that assemble into a plasma membrane pore releasing mature IL-1β from cells and inducing pyroptotic cell death. A non-canonical inflammasome comprised of caspase-4 and caspase-5 also cleaves GSDMD, with resultant activation of the NLRP3 inflammasome and caspase-1. Caspase-1 activation and GSDMD cleavage also generate neutrophil extracellular traps (NETs) and induce another pro-inflammatory form of lytic cell death, termed NETosis [123, 124].
Sputum levels of IL-1β, IL-1 receptors, NLRP3, caspase-1, caspase-4, and caspase-5 are increased in neutrophilic asthma[125–130]. Sputum IL-1β and NLRP3 correlate with neutrophilic airway inflammation and asthma severity[125, 126, 131]. Ozone exposure increases sputum neutrophils in atopic asthmatics, which correlate with increased sputum IL-1β and IL-8[132, 133]. In severe asthma, sputum neutrophils correlate with sputum extracellular DNA levels indicative of NET formation, while increased sputum extracellular DNA is associated with increased sputum IL-1β and asthma severity[131].
Murine and human studies support the concept of targeting IL-1β in type 2-low neutrophilic asthma. Administration of either a neutralizing anti-IL-1β antibody or a pharmacological NLRP3 inhibitor (MCC950) suppressed lung IL-1β production and neutrophilic airway inflammation in a murine model of severe, steroid-resistant asthma induced by concurrent allergic airways disease and Chlamydia respiratory infection[125]. Treatment of healthy subjects with the IL-1 receptor antagonist, anakinra, before inhalational endotoxin challenge suppressed sputum neutrophils, IL-1β, IL-6, and IL-8 levels, suggesting anakinra as a candidate treatment[134]. Canakinumab, a neutralizing anti-IL-1β humanized monoclonal antibody has proved safe in a Phase 1/2 clinical trial of mild asthma[135]. Clinical trials utilizing inhalational allergen challenge are underway to assess the effect of anakinra on inflammation and pulmonary function[136, 137]. The development of biomarkers to identify IL-1β-high asthmatics would allow these strategies to be administered in a precision medicine approach.
Inhibition of the IL-17 pathway
IL-17A and IL-17F are cytokines produced particularly by innate and adaptive lymphocytes (including Th17 cells, γδ T cells, group 3 ILCs, and mucosal associated invariant T (MAIT) cells) which induce epithelial cells to recruit neutrophils to sites of inflammation[36, 138, 139]. IL-17 cytokines have been implicated in asthma by human genetic studies[140–142], by murine models[143–145] and observations of increased IL-17 levels in human airway samples[146–152], particularly in neutrophilic asthma. However, such weak clinical associations from these small studies have not been replicated consistently. In a bronchoscopy study of 84 volunteers we found no evidence of increased IL-17A protein or Th17 frequencies in asthma in serum, sputum or bronchoalveolar lavage (BAL)[138]. Transcriptomic studies have suggested activation of steroid-resistant IL-17 pathways in severe asthma[34, 153] associated with neutrophilia, smoking, and frequent exacerbations.
It remains unclear whether IL-17 is driving pathology, or may be simply a consequence of epithelial damage or bacterial airway colonisation[36]. Indeed neutrophilic inflammation may promote IL-17 production[2]. Neutrophil cytoplasts (enucleated cell bodies generated when DNA-containing NETs are released) trigger IL-17 production by CD4+ T cells, which suggests neutrophilic inflammation may drive IL-17 expression[2, 154]. IL-17 may be protective in asthma, being important for antibacterial defence, promoting tissue repair, and in maintaining epithelial barrier function[36]. Indeed, the largest clinical trial of an anti-IL-17 receptor A monoclonal antibody (mAb), brodalumab, in >300 patients with moderate-to-severe asthma did not show improvement of symptoms or lung function [155], although patients weren’t selected for airway neutrophilia or IL-17 levels. Similarly, a clinical trial using an anti-IL-23 antibody, risankizumab, which blocks Th17 cell differentiation, worsened asthma control in severe asthmatics who were not selected by cytokine levels or airway neutrophils[156]. A phase 2 clinical trial is ongoing with an anti-IL-17A mAb in moderate to severe asthma[157]. Other strategies to inhibit this pathway include DNAzymes targeting the Th17 transcription factor, RORγt, and small molecule inhibitors[158].
Resolvins: Lipoxin 4 and Serum Amyloid A
Lipoxin A4 (LXA4) is a specialized proresolving mediator (SPM) that is enzymatically derived from arachidonic acid metabolism and promotes resolution of inflammation via interactions with the lipoxin A4/formyl peptide type 2 (ALX/FPR2) receptor[159, 160]. LXA4 levels are decreased in the blood, sputum, exhaled breath condensate, and BALF from severe adult and paediatric asthmatics, which suggests a causal relationship may exist between reductions in LXA4 levels and more severe asthma[159, 161–166]. Sputum cells from severe asthmatic children and peripheral blood granulocytes from adults with severe asthma have reduced ALX/FPR2 expression, which may further impair the effects of LXA4[164, 166]. BALF natural killer (NK) cells from severe asthmatics are skewed towards a cytotoxic CD56dim subset with reduced ALX/FPR2 expression, which might contribute to the impaired resolution of inflammation mediated by NK cell-dependent clearance of T cells and neutrophils[167].
A biochemical endotype of severe asthma has been defined with decreased BALF LXA4 levels and high BALF levels of the acute-phase protein, serum amyloid A (LXA4loSAAhi)[168]. Since SAA also signals via the ALX/FPR2 receptor, the increased BALF SAA can compete with and overwhelm the ability of LXA4 to interact with ALX/FPR2. LXA4loSAAhi asthmatics have increased BALF neutrophils, more severe asthma, comorbidities, and pruned pulmonary vasculature[168, 169]. BALF from LXA4loSAAhi asthmatics induced IL-8 production by A549 cells that express ALX/FPR2, which was inhibited by another SPM, 15-epi-LXA4. This suggests a potential therapeutic role for SPMs in severe, neutrophilic-predominant asthma. In support of this concept, administration of a stable LXA4 analogue inhibited allergic airway inflammation and AHR in an experimental murine model of OVA-induced airways disease[170], while inhalation of LXA4 by 5 mild asthmatics antagonized the bronchoconstrictive effects of leukotriene C4[171]. Inhalation of a stable LXA4 analogue (5(S),6(R)-LXA4 methyl ester) was also safe in a small cohort of asthmatic children[172]. Thus, LXA4, stable LXA4 analogues, or other SPMs might be utilized in trials assessing efficacy in type 2-low, neutrophilic-predominant asthma[159, 160]. SPM precursors might represent another therapeutic modality based upon a recent study that utilized diet supplementation with n-3 long-chain polyunsaturated fatty acids during the third trimester of pregnancy to reduce the risk of asthma during early childhood[173].
Apolipoproteins: an inhaled formulation of an APOA1 mimetic peptide
Studies in murine models of allergen-induced airways diseases identified that APOA1, the major protein component of high-density lipoprotein particles, interacts with ABCA1 transporters on alveolar macrophages and pulmonary vascular endothelial cells, to suppress neutrophilic airway inflammation via a G-CSF-dependent mechanism[174, 175]. In addition, higher serum APOA1 levels are associated with less severe airflow obstruction in allergic asthmatics[176]. These results suggest APOA1 might be beneficial for type 2-low neutrophilic asthma and have supported efforts to develop an inhaled APOA1 mimetic peptide, such as the 5A APOA1 mimetic peptide, that re-capitulates the biological activity of the endogenous APOA1 molecule[158, 177].
Type I interferons
People with asthma are predisposed to lower respiratory symptoms during an upper respiratory tract viral infection[178], which has been linked to deficiency of type I/III interferons (IFN) IFN-β and IFN-λ [179–183]. This may favour rhinovirus replication, mucin production and impair antimicrobial peptide responses[184]. Whilst these responses may be deficient in an acute exacerbation, in stable state interferon stimulated genes are upregulated in airway epithelial cells in mild asthma, and in peripheral blood in stable severe asthma[185]. Upregulation is not associated with type-2 inflammation. Potentially elevated baseline IFN responses during stable state may lead to densitisation of type I IFN responses during acute infection[185]. A trial of inhaled IFN-β showed some efficacy in reducing viral-exacerbation induced asthma symptoms in frequently-exacerbating severe asthma, although the trial did not reach its primary endpoint across all asthma[186] and a subsequent trial was negative[187, 188]. It remains unknown if IFN deficiency is a feature of asthma or a consequence of corticosteroid treatment[184], which can suppress IFN-stimulated genes[185].
Inhibition of the IL-6 pathway
IL-6 is a biomarker of systemic inflammation, metabolic dysfunction, and obesity. IL-6 is increased in serum and airways in asthma[189–191] and has recently been found to be elevated in plasma in severe asthma associated with obesity, metabolic dysfunction and blood neutrophilia[110]. This may reflect increased IL-6 production by inflammatory macrophages in adipose tissue of obese individuals. This increases asthma severity via an “outside-in” mechanism of lung dysfunction due to systemic inflammation[110]. Furthermore, systemic IL-6 inflammation and obesity are associated with a deficiency of airway cytotoxic CD8+ T cells in type 2-low asthmatics, which may reflect T-cell exhaustion as a mechanism of increased exacerbations due to impaired anti-viral immune responses[192]. The importance of systemic IL-6 in asthma will need confirmation by trials inhibiting IL-6 signalling, such as an ongoing trial of clazakizumab in severe asthma[157]. As anti-IL-6 monoclonals are already in clinical use and high serum IL-6 levels have been identified as a biomarker[110], such clinical translation could follow swiftly.
IL-6 may also play a mechanistic role in severe asthmatics with a SNP in the IL-4 receptor α chain that converts a glutamine to arginine at residue 576 [193]. Experiments in mice showed that Il4ra R576 promotes conversion of induced regulatory T cells to Th17-like cells by a pathway involving growth-factor-receptor-bound protein 2 (GRB2) adaptor protein, mitogen-activated protein kinase (MAPK) kinase signalling, IL-6, and STAT3, which can be inhibited by a neutralizing anti-IL-6 antibody. The anti-IL-6 antibody also suppressed mixed neutrophilic/eosinophilic airway inflammation and mucous cell metaplasia in a murine model of house dust mite-induced airways disease, while a humanized anti-IL-6 receptor monoclonal antibody, tocilizumab, has been used to successfully treat two paediatric severe asthmatics with the IL4R R576 allele and evidence of mixed Th2/Th17 inflammation [194].
In addition to acting on neutrophils and macrophages, during inflammation IL-6 can also bind to soluble IL-6R shed by inflammatory neutrophils and cause IL-6 trans-signalling on epithelial cells. sIL-6R is increased in serum[195], BAL[195] and sputum[196] in asthma. Activation of IL-6TS reduces epithelial integrity and induces gene signatures associated with airway remodelling. These signatures are expressed in epithelial brushings from frequently-exacerbating, type-2 low asthmatics, associated with submucosal macrophage and T cell infiltration, evidence of impaired epithelial barrier function and induction of the alarmin IL-33[197]. IL-6ST high patients had low expression of the epithelial type-2 gene signature, although did have elevated eosinophils, implying eosinophilia in these individuals is driven by type-2 independent mechanisms. IL-6TS occurred in the absence of a systemic IL-6 signal, and was associated with TLR signalling and inflammasome activation, suggesting this phenotype was driven by local, likely bacterially-driven inflammation, with pathogens, such as H. influenzae. Experiments using human airway smooth muscle cells have also shown that IL-6TS induced proliferative responses, as well as the expression of genes regulating immune responses, airway remodelling, glucose metabolism, and hypoxia[198]. Single nucleotide polymorphisms in the IL6R gene are associated with increased serum sIL-6R that may promote IL-6TS[199, 200]. The IL6R rs4129267 SNP is associated with both higher serum sIL-6R levels and an increased risk of asthma[199], while the rs2228145 SNP has been associated with worse lung function and severe asthma[201]. Furthermore, elevated serum IL-6R levels were associated with more severe airflow obstruction, which suggests a role for IL-6 trans-signalling in severe asthma.
Mast cells
Mast cells contribute to the pathobiology of severe asthma by mediating airflow obstruction and AHR[202]. A role for mast cells in severe asthma was shown with imatinib, which blocks stem cell factor signalling by inhibiting the mast/stem cell growth factor receptor KIT with reductions in airway methacholine reactivity, serum tryptase levels, and airway mast cell counts[203]. Recent studies identified that mast cell-derived tryptase, the dominant secretory granule protein in mast cells, is elevated in BALF and blood from severe asthmatics with either type 2-low or type 2-high disease[138, 202]. Moreover, due to common polymorphisms in the two genes producing β-tryptase, it is possible to have 2,3 or 4 active β-tryptase alleles, and serum tryptase levels correlate with the number of active β-tryptase alleles[202]. A neutralizing antibody directed against human β-tryptase has been developed that inhibits airway tryptase in non-human primates and can potentially be developed into a new treatment option for type 2-low severe asthmatics. An approach that administers anti-tryptase antibodies to type 2-low asthmatics with increased numbers of active β-tryptase alleles or elevated tryptase levels can potentially target treatment to individuals with a mast cell asthma endotype.
TH1/ILC1 Cytokines
Interferon-γ, produced by Th1 CD4+ T cells, type 1 ILCs and NK cells is important in innate immunity[158, 167]. CD4+ T cells from severe asthmatics produce high levels of IFN-γ, which induces increases in CXCL10 (C-X-X motif chemokine ligand 10) that recruits Th1 CD4+ T cells and neutrophils[204, 205]. Murine experiments showed that IFN-γ decreases airway epithelial cell production of secretory leukocyte protease inhibitor (SLPI), which causes increased AHR and steroid resistance[204–206]. Furthermore, SLPI inhibits mast cell tryptase[206]. Therefore, strategies that target the IFN-γ/SLPI pathway might be developed for severe asthmatics with this endotype, however the consequences of impaired IFN-γ activity on airway host defence will need to be considered.
Tumour necrosis factor (TNF), another pro-inflammatory cytokine which recruits pulmonary neutrophils, is increased in BALF from severe asthmatics and causes AHR[158, 167, 207]. Although in a preliminary trial a soluble TNF receptor, etanercept, reduced AHR and improved bronchodilator responsiveness, in a subsequent larger study of severe asthma golimumab, an anti-TNF neutralizing antibody failed to improve asthma control and lung function, but was associated with serious infections and malignancies[207, 208]. If future anti-TNF trials are considered, strategies to target TNF-high asthma could potentially identify a responsive subset[158].
TL1A (TNFSF15A) is a TNF superfamily member that functions as a ligand for Death Receptor 3 (DR3, TNFRSF25) on T cells and promotes type 2-high allergic lung inflammation in mice[209–213]. TL1A also amplifies Th1 and Th17 responses, which suggests that TL1A inhibition might be considered for the treatment of type 2-low asthma[213–215]. PF-06480605, a monoclonal antibody that neutralizes TL1A, has entered phase 2b clinical trials for inflammatory bowel disease[216, 217] and could be potentially be utilized in studies of type 2-low asthmatics.
CD6/ALCAM Axis
CD6 is a T cell co-stimulatory receptor for ALCAM (Activated Leukocyte Cell Adhesion Molecule, CD166) that enhances the activation and differentiation of Th1 and Th17 cells to promote autoimmunity[218]. The CD6/ALCAM pathway also promotes type-2 high, allergic asthma in mice, while serum and sputum ALCAM levels are increased in asthmatic children, especially those with severe disease[219]. Furthermore, a genome-wide association study has identified single nucleotide polymorphisms in the region of the ALCAM gene that were associated with an adult-onset, non-allergic asthmatic phenotype[220]. Collectively, these studies suggest that targeting the CD6/ALCAM pathway might be investigated for the treatment of type 2-low asthma[213]. A phase Ib clinical trial is currently in progress to evaluate the safety of an anti-CD6 antibody, itolizumab, in patients with moderate-to-severe uncontrolled asthma, however, additional studies will be required to assess its efficacy in type 2-low asthmatics[221].
IL-8(CXCL8)/CXCR2 Axis
Given the key role that IL-8 (CXCL8) plays in neutrophil recruitment and activation, treatments that block binding to its high-affinity C-X-C motif chemokine receptor, CXCR2, have been considered. Although a selective CXCR2 antagonist reduced sputum neutrophils and mild exacerbations in a small trial of severe asthmatics with increased sputum neutrophils, a larger study utilizing a different CXCR2 antagonist did not reduce the frequency of severe exacerbations in severe asthmatics with low peripheral blood eosinophil counts[222, 223]. Although this result called into question the strategy of targeting CXCR2 and neutrophils in uncontrolled asthma, the future development of a biomarker that identifies IL-8-high, neutrophilic asthmatics that can easily be utilized in clinical practice may allow this approach to be re-visited.
Leukotriene B4 (LTB4)
5-lipoxygenase-activating protein (FLAP) is a key component of the leukotriene synthetic pathway that generates leukotriene B4 (LTB4), which is both a pro-inflammatory neutrophil product, as well as a potent neutrophil chemoattractant[27, 224, 225]. Administration of the FLAP inhibitor, GSK2190915, to a small cohort of neutrophilic asthmatics for 14 days, suppressed sputum LTB4 levels, but not sputum neutrophils, which suggests that targeting FLAP may not be an effective strategy for treating neutrophilic asthma. Targeting the interaction between LTB4 and its high affinity receptor, BLT1, may represent an alternative strategy to attenuate neutrophilic airway inflammation in asthma[226, 227].
Mitochondrial Reactive Oxygen Species (ROS)
LC28-0126 is a novel mitochondria-targeted scavenger of ROS and reactive nitrogen species that attenuates ischemic-reperfusion injury in cardiomyocytes[27, 228]. LC28-0126 has also been shown to ameliorate neutrophilic airway inflammation and airway hyperresponsiveness in mice. Although LC28-0126 has been administered to healthy volunteers, it has not yet been investigated in clinical trials of type-2 low asthmatics.
Conclusion
Currently the limited treatment options in type-2 low asthma contrast with the dramatic efficacy of novel drugs for type-2 high disease. However, an approach which focuses on identifying specific treatable traits is effective in selected patients with severe type-2 low disease. More trials of biomarker-directed macrolide therapy are required. The need to develop novel biomarkers for specific type-2 low pathways, such as molecular microbiology and exhaled volatile organic compounds[121], and to understand the underlying pathological mechanisms are recognised research priorities[229], and the present refocussing of research on type-2 low disease holds genuine promise for novel therapies on the near horizon.
Take home message.
1/3 of severe asthma is type-2 low, presenting a challenge to clinicians. Here we review currently available treatment options including macrolides, bronchodilators, thermoplasty and other treatable traits, and review a range of therapies in development.
Funding
This work was supported by grants from the Wellcome Trust (104553/z/14/z, 211050/Z/18/z) and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC) (to TSCH), the Division of Intramural Research of the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (to SJL), and the BOF19/GOA/008 Concerted Research Action of Ghent University.
Footnotes
Author contributions: TSCH, SJL, GB jointly conceived the article, conducted the literature review and drafted the manuscript. All authors approved the final manuscript.
References
- 1.Gibson PG, Fujimura M, Niimi A. Eosinophilic bronchitis: clinical manifestations and implications for treatment. Thorax. 2002;57(2):178–182. doi: 10.1136/thorax.57.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambrecht BN, Hammad H, Fahy JV. The Cytokines of Asthma. Immunity. 2019;50(4):975–991. doi: 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavord I, Bahmer T, Braido F, Cosio BG, Humbert M, Idzko M, Adamek L. Severe T2-high asthma in the biologics era: European experts' opinion. Eur Respir Rev. 2019;28(152) doi: 10.1183/16000617.0054-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57(10):875–879. doi: 10.1136/thorax.57.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119(5):1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 7.Kuo CS, Pavlidis S, Loza M, Baribaud F, Rowe A, Pandis I, Sousa A, Corfield J, Djukanovic R, Lutter R, Sterk PJ, et al. T-helper cell type 2 (Th2) and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in U-BIOPRED. Eur Respir J. 2017;49(2) doi: 10.1183/13993003.02135-2016. [DOI] [PubMed] [Google Scholar]
- 8.Hinks TS, Brown T, Lau LC, Rupani H, Barber C, Elliott S, Ward JA, Ono J, Ohta S, Izuhara K, Djukanovic R, et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol. 2016;138(1):61–75. doi: 10.1016/j.jaci.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, Fahy JV. Asthma Clinical Research Network of the National Heart L Blood I. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012;185(6):612–619. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazarus SC, Krishnan JA, King TS, Lang JE, Blake KV, Covar R, Lugogo N, Wenzel S, Chinchilli VM, Mauger DT, Dyer AM, et al. Blood Institute A. Mometasone or Tiotropium in Mild Asthma with a Low Sputum Eosinophil Level. N Engl J Med. 2019;380(21):2009–2019. doi: 10.1056/NEJMoa1814917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95(4):843–852. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 12.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11(1):54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 13.Simpson JL, Grissell TV, Douwes J, Scott RJ, Boyle MJ, Gibson PG. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax. 2007;62(3):211–218. doi: 10.1136/thx.2006.061358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax. 2010;65(5):384–390. doi: 10.1136/thx.2009.126722. [DOI] [PubMed] [Google Scholar]
- 15.da Silva J, Hilzendeger C, Moermans C, Schleich F, Henket M, Kebadze T, Mallia P, Edwards MR, Johnston SL, Louis R. Raised interferon-beta, type 3 interferon and interferon-stimulated genes - evidence of innate immune activation in neutrophilic asthma. Clin Exp Allergy. 2017;47(3):313–323. doi: 10.1111/cea.12809. [DOI] [PubMed] [Google Scholar]
- 16.Schleich F, Brusselle G, Louis R, Vandenplas O, Michils A, Pilette C, Peche R, Manise M, Joos G. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR) Respir Med. 2014;108(12):1723–1732. doi: 10.1016/j.rmed.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Schleich FN, Manise M, Sele J, Henket M, Seidel L, Louis R. Distribution of sputum cellular phenotype in a large asthma cohort: predicting factors for eosinophilic vs neutrophilic inflammation. BMC pulmonary medicine. 2013;13:11. doi: 10.1186/1471-2466-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayaram L, Pizzichini MM, Cook RJ, Boulet LP, Lemiere C, Pizzichini E, Cartier A, Hussack P, Goldsmith CH, Laviolette M, Parameswaran K, Hargreave FE. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006;27(3):483–494. doi: 10.1183/09031936.06.00137704. [DOI] [PubMed] [Google Scholar]
- 19.van Veen IH, Ten Brinke A, Gauw SA, Sterk PJ, Rabe KF, Bel EH. Consistency of sputum eosinophilia in difficult-to-treat asthma: a 5-year follow-up study. J Allergy Clin Immunol. 2009;124(3):615–617. doi: 10.1016/j.jaci.2009.06.029. 617 e611-612. [DOI] [PubMed] [Google Scholar]
- 20.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 21.Green RH, Pavord I. Stability of inflammatory phenotypes in asthma. Thorax. 2012;67(8):665–667. doi: 10.1136/thoraxjnl-2012-201657. [DOI] [PubMed] [Google Scholar]
- 22.Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet. 1999;353(9171):2213–2214. doi: 10.1016/S0140-6736(99)01813-9. [DOI] [PubMed] [Google Scholar]
- 23.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 24.Asthma. GIf. Global strategy for asthma management and prevention. 2019 [Google Scholar]
- 25.Hancox RJ, Cowan DC, Aldridge RE, Cowan JO, Palmay R, Williamson A, Town GI, Taylor DR. Asthma phenotypes: consistency of classification using induced sputum. Respirology. 2012;17(3):461–466. doi: 10.1111/j.1440-1843.2011.02113.x. [DOI] [PubMed] [Google Scholar]
- 26.Kupczyk M, Dahlen B, Sterk PJ, Nizankowska-Mogilnicka E, Papi A, Bel EH, Chanez P, Howarth PH, Holgate ST, Brusselle G, Siafakas NM, et al. investigators B. Stability of phenotypes defined by physiological variables and biomarkers in adults with asthma. Allergy. 2014;69(9):1198–1204. doi: 10.1111/all.12445. [DOI] [PubMed] [Google Scholar]
- 27.Sze E, Bhalla A, Nair P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy. 2020;75(2):311–325. doi: 10.1111/all.13985. [DOI] [PubMed] [Google Scholar]
- 28.Nair P, Ochkur SI, Protheroe C, Radford K, Efthimiadis A, Lee NA, Lee JJ. Eosinophil peroxidase in sputum represents a unique biomarker of airway eosinophilia. Allergy. 2013;68(9):1177–1184. doi: 10.1111/all.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni NS, Hollins F, Sutcliffe A, Saunders R, Shah S, Siddiqui S, Gupta S, Haldar P, Green R, Pavord I, Wardlaw A, et al. Eosinophil protein in airway macrophages: a novel biomarker of eosinophilic inflammation in patients with asthma. J Allergy Clin Immunol. 2010;126(1):61–69 e63. doi: 10.1016/j.jaci.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haldar P, Pavord ID. Noneosinophilic asthma: a distinct clinical and pathologic phenotype. J Allergy Clin Immunol. 2007;119(5):1043–1052. doi: 10.1016/j.jaci.2007.02.042. quiz 1053-1044. [DOI] [PubMed] [Google Scholar]
- 31.Anees W, Huggins V, Pavord ID, Robertson AS, Burge PS. Occupational asthma due to low molecular weight agents: eosinophilic and non-eosinophilic variants. Thorax. 2002;57(3):231–236. doi: 10.1136/thorax.57.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helenius I, Lumme A, Haahtela T. Asthma, airway inflammation and treatment in elite athletes. Sports Med. 2005;35(7):565–574. doi: 10.2165/00007256-200535070-00002. [DOI] [PubMed] [Google Scholar]
- 33.Selge C, Thomas S, Nowak D, Radon K, Wolfarth B. Asthma prevalence in German Olympic athletes: A comparison of winter and summer sport disciplines. Respir Med. 2016;118:15–21. doi: 10.1016/j.rmed.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Singhania A, Wallington JC, Smith CG, Horowitz D, Staples KJ, Howarth PH, Gadola SD, Djukanovic R, Woelk CH, Hinks TSC. Multitissue Transcriptomics Delineates the Diversity of Airway T Cell Functions in Asthma. Am J Respir Cell Mol Biol. 2018;58(2):261–270. doi: 10.1165/rcmb.2017-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L, Carroll MP, Bruce KD, Howarth PH. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One. 2014;9(6):e100645. doi: 10.1371/journal.pone.0100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hynes GM, Hinks TSC. The Role of Interleukin-17 in Airways Disease. ERJ Open. 2020 doi: 10.1183/23120541.00364-2019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson JL, Daly J, Baines KJ, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Hugenholtz P, Willner D, Gibson PG. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur Respir J. 2016;47(3):792–800. doi: 10.1183/13993003.00405-2015. [DOI] [PubMed] [Google Scholar]
- 38.Taylor SL, Leong LEX, Choo JM, Wesselingh S, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C, Peters MJ, et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J Allergy Clin Immunol. 2018;141(1):94–103 e115. doi: 10.1016/j.jaci.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhuri R, McSharry C, Brady J, Donnelly I, Grierson C, McGuinness S, Jolly L, Weir CJ, Messow CM, Spears M, Miele G, et al. Sputum matrix metalloproteinase-12 in patients with chronic obstructive pulmonary disease and asthma: relationship to disease severity. J Allergy Clin Immunol. 2012;129(3):655–663 e658. doi: 10.1016/j.jaci.2011.12.996. [DOI] [PubMed] [Google Scholar]
- 40.Mukhopadhyay S, Sypek J, Tavendale R, Gartner U, Winter J, Li W, Page K, Fleming M, Brady J, O'Toole M, Macgregor DF, et al. Matrix metalloproteinase-12 is a therapeutic target for asthma in children and young adults. J Allergy Clin Immunol. 2010;126(1):70–76 e16. doi: 10.1016/j.jaci.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Hunninghake GM, Cho MH, Tesfaigzi Y, Soto-Quiros ME, Avila L, Lasky-Su J, Stidley C, Melen E, Soderhall C, Hallberg J, Kull I, et al. MMP12, lung function, and COPD in high-risk populations. N Engl J Med. 2009;361(27):2599–2608. doi: 10.1056/NEJMoa0904006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, van der Merwe R. Tezepelumab in Adults with Uncontrolled Asthma. N Engl J Med. 2017;377(10):936–946. doi: 10.1056/NEJMoa1704064. [DOI] [PubMed] [Google Scholar]
- 43.Kanemitsu Y, Fukumitsu K, Kurokawa R, Takeda N, Suzuki M, Yap J, Nishiyama H, Tajiri T, Fukuda S, Uemura T, Ohkubo H, et al. Increased Capsaicin Sensitivity in Severe Asthmatics Associated with Worse Clinical Outcome. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.201911-2263OC. [DOI] [PubMed] [Google Scholar]
- 44.Satia I, Tsamandouras N, Holt K, Badri H, Woodhead M, Ogungbenro K, Felton TW, O'Byrne PM, Fowler SJ, Smith JA. Capsaicin-evoked cough responses in asthmatic patients: Evidence for airway neuronal dysfunction. J Allergy Clin Immunol. 2017;139(3):771–779 e710. doi: 10.1016/j.jaci.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 45.Satia I, Watson R, Scime T, Dockry RJ, Sen S, Ford JW, Mitchell PD, Fowler SJ, Gauvreau GM, O'Byrne PM, Smith JA. Allergen challenge increases capsaicin-evoked cough responses in patients with allergic asthma. J Allergy Clin Immunol. 2019;144(3):788–795 e781. doi: 10.1016/j.jaci.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 46.Brown HM. Treatment of chronic asthma with prednisolone; significance of eosinophils in the sputum. Lancet. 1958;2(7059):1245–1247. doi: 10.1016/s0140-6736(58)91385-0. [DOI] [PubMed] [Google Scholar]
- 47.Bacci E, Cianchetti S, Bartoli M, Dente FL, Di Franco A, Vagaggini B, Paggiaro P. Low sputum eosinophils predict the lack of response to beclomethasone in symptomatic asthmatic patients. Chest. 2006;129(3):565–572. doi: 10.1378/chest.129.3.565. [DOI] [PubMed] [Google Scholar]
- 48.Little SA, Chalmers GW, MacLeod KJ, McSharry C, Thomson NC. Non-invasive markers of airway inflammation as predictors of oral steroid responsiveness in asthma. Thorax. 2000;55(3):232–234. doi: 10.1136/thorax.55.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meijer RJ, Postma DS, Kauffman HF, Arends LR, Koeter GH, Kerstjens HA. Accuracy of eosinophils and eosinophil cationic protein to predict steroid improvement in asthma. Clin Exp Allergy. 2002;32(7):1096–1103. doi: 10.1046/j.1365-2222.2002.01412.x. [DOI] [PubMed] [Google Scholar]
- 50.Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, Bradding P, Wardlaw AJ, Pavord ID. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62(12):1043–1049. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beasley R, Holliday M, Reddel HK, Braithwaite I, Ebmeier S, Hancox RJ, Harrison T, Houghton C, Oldfield K, Papi A, Pavord ID, et al. Controlled Trial of Budesonide-Formoterol as Needed for Mild Asthma. N Engl J Med. 2019;380(21):2020–2030. doi: 10.1056/NEJMoa1901963. [DOI] [PubMed] [Google Scholar]
- 52.O'Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, Jorup C, Lamarca R, Ivanov S, Reddel HK. Inhaled Combined Budesonide-Formoterol as Needed in Mild Asthma. N Engl J Med. 2018;378(20):1865–1876. doi: 10.1056/NEJMoa1715274. [DOI] [PubMed] [Google Scholar]
- 53.Bateman ED, Reddel HK, O'Byrne PM, Barnes PJ, Zhong N, Keen C, Jorup C, Lamarca R, Siwek-Posluszna A, FitzGerald JM. As-Needed Budesonide-Formoterol versus Maintenance Budesonide in Mild Asthma. N Engl J Med. 2018;378(20):1877–1887. doi: 10.1056/NEJMoa1715275. [DOI] [PubMed] [Google Scholar]
- 54.Sweeney J, Brightling CE, Menzies-Gow A, Niven R, Patterson CC, Heaney LG. British Thoracic Society Difficult Asthma N. Clinical management and outcome of refractory asthma in the UK from the British Thoracic Society Difficult Asthma Registry. Thorax. 2012;67(8):754–756. doi: 10.1136/thoraxjnl-2012-201869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, Cullinan P, Custovic A, Ducharme FM, Fahy JV, Frey U, et al. After asthma: redefining airways diseases. Lancet. 2017 doi: 10.1016/S0140-6736(17)30879-6. [DOI] [PubMed] [Google Scholar]
- 57.Heaney LG, Conway E, Kelly C, Johnston BT, English C, Stevenson M, Gamble J. Predictors of therapy resistant asthma: outcome of a systematic evaluation protocol. Thorax. 2003;58(7):561–566. doi: 10.1136/thorax.58.7.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heaney LG, Robinson DS. Severe asthma treatment: need for characterising patients. Lancet. 2005;365(9463):974–976. doi: 10.1016/S0140-6736(05)71087-4. [DOI] [PubMed] [Google Scholar]
- 59.Robinson DS, Campbell DA, Durham SR, Pfeffer J, Barnes PJ, Chung KF. Systematic assessment of difficult-to-treat asthma. Eur Respir J. 2003;22(3):478–483. doi: 10.1183/09031936.03.00017003. [DOI] [PubMed] [Google Scholar]
- 60.Petsky HL, Cates CJ, Lasserson TJ, Li AM, Turner C, Kynaston JA, Chang AB. A systematic review and meta-analysis: tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils) Thorax. 2012;67(3):199–208. doi: 10.1136/thx.2010.135574. [DOI] [PubMed] [Google Scholar]
- 61.Petsky HL, Cates CJ, Kew KM, Chang AB. Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils): a systematic review and meta-analysis. Thorax. 2018;73(12):1110–1119. doi: 10.1136/thoraxjnl-2018-211540. [DOI] [PubMed] [Google Scholar]
- 62.Hanratty CE, Matthews JG, Arron JR, Choy DF, Pavord ID, Bradding P, Brightling CE, Chaudhuri R, Cowan DC, Djukanovic R, Gallagher N, et al. A randomised pragmatic trial of corticosteroid optimization in severe asthma using a composite biomarker algorithm to adjust corticosteroid dose versus standard care: study protocol for a randomised trial. Trials. 2018;19(1):5. doi: 10.1186/s13063-017-2384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, Beeh KM, Ramos S, Canonica GW, Hedgecock S, Fox H, Blogg M, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316. doi: 10.1111/j.1398-9995.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 64.Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, Lal P, Arron JR, Harris JM, Busse W. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–811. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 65.Humbert M, Taille C, Mala L, Le Gros V, Just J, Molimard M, investigators S. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur Respir J. 2018;51(5) doi: 10.1183/13993003.02523-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amayasu H, Yoshida S, Ebana S, Yamamoto Y, Nishikawa T, Shoji T, Nakagawa H, Hasegawa H, Nakabayashi M, Ishizaki Y. Clarithromycin suppresses bronchial hyperresponsiveness associated with eosinophilic inflammation in patients with asthma. Ann Allergy Asthma Immunol. 2000;84(6):594–598. doi: 10.1016/S1081-1206(10)62409-X. [DOI] [PubMed] [Google Scholar]
- 67.Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev. 2012;11:CD002203. doi: 10.1002/14651858.CD002203.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, Milne D, Fergusson W, Tuffery C, Sexton P, Storey L, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):660–667. doi: 10.1016/S0140-6736(12)60953-2. [DOI] [PubMed] [Google Scholar]
- 69.Altenburg J, de Graaff CS, Stienstra Y, Sloos JH, van Haren EH, Koppers RJ, van der Werf TS, Boersma WG. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013;309(12):1251–1259. doi: 10.1001/jama.2013.1937. [DOI] [PubMed] [Google Scholar]
- 70.Serisier DJ, Martin ML, McGuckin MA, Lourie R, Chen AC, Brain B, Biga S, Schlebusch S, Dash P, Bowler SD. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA. 2013;309(12):1260–1267. doi: 10.1001/jama.2013.2290. [DOI] [PubMed] [Google Scholar]
- 71.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, Make B, Marchetti N, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kew KM, Undela K, Kotortsi I, Ferrara G. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9) doi: 10.1002/14651858.CD002997.pub4. CD002997. [DOI] [PubMed] [Google Scholar]
- 73.Reiter J, Demirel N, Mendy A, Gasana J, Vieira ER, Colin AA, Quizon A, Forno E. Macrolides for the long-term management of asthma--a meta-analysis of randomized clinical trials. Allergy. 2013;68(8):1040–1049. doi: 10.1111/all.12199. [DOI] [PubMed] [Google Scholar]
- 74.Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, Verleden G, Demedts IK, Verhamme K, Delporte A, Demeyere B, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68(4):322–329. doi: 10.1136/thoraxjnl-2012-202698. [DOI] [PubMed] [Google Scholar]
- 75.Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C, Peters MJ, Marks GB, Baraket M, Powell H, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10095):659–668. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 76.Hiles SA, McDonald VM, Guilhermino M, Brusselle GG, Gibson PG. Does maintenance azithromycin reduce asthma exacerbations? An individual participant data meta-analysis. Eur Respir J. 2019;54(5) doi: 10.1183/13993003.01381-2019. [DOI] [PubMed] [Google Scholar]
- 77.Altenburg J, de Graaff CS, van der Werf TS, Boersma WG. Immunomodulatory effects of macrolide antibiotics - part 1: biological mechanisms. Respiration. 2011;81(1):67–74. doi: 10.1159/000320319. [DOI] [PubMed] [Google Scholar]
- 78.Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacology & therapeutics. 2014;143(2):225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 79.Marjanovic N, Bosnar M, Michielin F, Wille DR, Anic-Milic T, Culic O, Popovic-Grle S, Bogdan M, Parnham MJ, Erakovic Haber V. Macrolide antibiotics broadly and distinctively inhibit cytokine and chemokine production by COPD sputum cells in vitro. Pharmacol Res. 2011;63(5):389–397. doi: 10.1016/j.phrs.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 80.Lin SJ, Yan DC, Lee WI, Kuo ML, Hsiao HS, Lee PY. Effect of azithromycin on natural killer cell function. Int Immunopharmacol. 2012;13(1):8–14. doi: 10.1016/j.intimp.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Rodriguez-Cerdeira C, Sanchez-Blanco E, Molares-Vila A. Clinical application of development of nonantibiotic macrolides that correct inflammation-driven immune dysfunction in inflammatory skin diseases. Mediators of inflammation. 2012;2012:563709. doi: 10.1155/2012/563709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ratzinger F, Haslacher H, Poeppl W, Hoermann G, Kovarik JJ, Jutz S, Steinberger P, Burgmann H, Pickl WF, Schmetterer KG. Azithromycin suppresses CD4(+) T-cell activation by direct modulation of mTOR activity. Scientific reports. 2014;4 doi: 10.1038/srep07438. 7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129(6):1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 84.Porter JD, Watson J, Roberts LR, Gill SK, Groves H, Dhariwal J, Almond MH, Wong E, Walton RP, Jones LH, Tregoning J, et al. Identification of novel macrolides with antibacterial, anti-inflammatory and type I and III IFN-augmenting activity in airway epithelium. The Journal of antimicrobial chemotherapy. 2016;71(10):2767–2781. doi: 10.1093/jac/dkw222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008;177(2):148–155. doi: 10.1164/rccm.200707-1134OC. [DOI] [PubMed] [Google Scholar]
- 86.Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, Gaga M, Kellermeyer L, Khurana S, Knight S, McDonald VM, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55(1) doi: 10.1183/13993003.00588-2019. [DOI] [PubMed] [Google Scholar]
- 87.Serisier DJ. Risks of population antimicrobial resistance associated with chronic macrolide use for inflammatory airway diseases. Lancet Respir Med. 2013;1(3):262–274. doi: 10.1016/S2213-2600(13)70038-9. [DOI] [PubMed] [Google Scholar]
- 88.Xiao YH, Giske CG, Wei ZQ, Shen P, Heddini A, Li LJ. Epidemiology and characteristics of antimicrobial resistance in China. Drug Resist Updat. 2011;14(4-5):236–250. doi: 10.1016/j.drup.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Xin D, Mi Z, Han X, Qin L, Li J, Wei T, Chen X, Ma S, Hou A, Li G, Shi D. Molecular mechanisms of macrolide resistance in clinical isolates of Mycoplasma pneumoniae from China. Antimicrob Agents Chemother. 2009;53(5):2158–2159. doi: 10.1128/AAC.01563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sriram KB, Cox AJ, Clancy RL, Slack MPE, Cripps AW. Nontypeable Haemophilus influenzae and chronic obstructive pulmonary disease: a review for clinicians. Crit Rev Microbiol. 2018;44(2):125–142. doi: 10.1080/1040841X.2017.1329274. [DOI] [PubMed] [Google Scholar]
- 91.Taylor SL, Leong LEX, Mobegi FM, Choo JM, Wesselingh S, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C, et al. Long-Term Azithromycin Reduces Haemophilus influenzae and Increases Antibiotic Resistance in Severe Asthma. Am J Respir Crit Care Med. 2019;200(3):309–317. doi: 10.1164/rccm.201809-1739OC. [DOI] [PubMed] [Google Scholar]
- 92.Taylor SL, Ivey KL, Gibson PG, Simpson JL, Rogers GB. Group ASR. Airway abundance of Haemophilus influenzae predicts response to azithromycin in adults with persistent uncontrolled asthma. Eur Respir J. 2020 doi: 10.1183/13993003.00194-2020. [DOI] [PubMed] [Google Scholar]
- 93.Meng JF, Li H, Luo MJ, Li HB. Efficacy of tiotropium in treating patients with moderate-to-severe asthma: A meta-analysis and systematic review based on 14 randomized controlled trials. Medicine (Baltimore) 2019;98(33):e16637. doi: 10.1097/MD.0000000000016637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kew KM, Dahri K. Long-acting muscarinic antagonists (LAMA) added to combination long-acting beta2-agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ICS for adults with asthma. Cochrane Database Syst Rev. 2016;(1):CD011721. doi: 10.1002/14651858.CD011721.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown RH, Wizeman W, Danek C, Mitzner W. In vivo evaluation of the effectiveness of bronchial thermoplasty with computed tomography. J Appl Physiol (1985) 2005;98(5):1603–1606. doi: 10.1152/japplphysiol.01210.2004. [DOI] [PubMed] [Google Scholar]
- 96.Facciolongo N, Di Stefano A, Pietrini V, Galeone C, Bellanova F, Menzella F, Scichilone N, Piro R, Bajocchi GL, Balbi B, Agostini L, et al. Nerve ablation after bronchial thermoplasty and sustained improvement in severe asthma. BMC pulmonary medicine. 2018;18(1):29. doi: 10.1186/s12890-017-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Niven R, Aubier M, Bonta P, Puente-Maestu L, Facciolongo N, Ryan D. European consensus meeting/statement on Bronchial Thermoplasty Who? Where? How? Respir Med. 2019;150:161–164. doi: 10.1016/j.rmed.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 98.Cox G, Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, Olivenstein R, Pavord ID, McCormack D, Chaudhuri R, Miller JD, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327–1337. doi: 10.1056/NEJMoa064707. [DOI] [PubMed] [Google Scholar]
- 99.Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, Fiss E, Olivenstein R, Thomson NC, Niven RM, Pavord ID, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116–124. doi: 10.1164/rccm.200903-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet LP, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 101.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372(9643):1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 102.Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, Humbert M, Jones P, Gibson PG, Vestbo J, Beasley R, Pavord ID. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. doi: 10.1183/13993003.01359-2015. [DOI] [PubMed] [Google Scholar]
- 103.Simpson AJ, Hekking PP, Shaw DE, Fleming LJ, Roberts G, Riley JH, Bates S, Sousa AR, Bansal AT, Pandis I, Sun K, et al. Treatable traits in the European U-BIOPRED adult asthma cohorts. Allergy. 2019;74(2):406–411. doi: 10.1111/all.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thomson NC, Chaudhuri R, Livingston E. Asthma and cigarette smoking. Eur Respir J. 2004;24(5):822–833. doi: 10.1183/09031936.04.00039004. [DOI] [PubMed] [Google Scholar]
- 105.Lazarus SC, Chinchilli VM, Rollings NJ, Boushey HA, Cherniack R, Craig TJ, Deykin A, DiMango E, Fish JE, Ford JG, Israel E, et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med. 2007;175(8):783–790. doi: 10.1164/rccm.200511-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chinn S, Jarvis D, Luczynska CM, Ackermann-Liebrich U, Anto JM, Cerveri I, de Marco R, Gislason T, Heinrich J, Janson C, Kunzli N, et al. An increase in bronchial responsiveness is associated with continuing or restarting smoking. Am J Respir Crit Care Med. 2005;172(8):956–961. doi: 10.1164/rccm.200503-323OC. [DOI] [PubMed] [Google Scholar]
- 107.Lee JH, An J, Won HK, Kang Y, Kwon HS, Kim TB, Cho YS, Moon HB, Song WJ, Hull JH. Prevalence and impact of comorbid laryngeal dysfunction in asthma: A systematic review and meta-analysis. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2019.12.906. [DOI] [PubMed] [Google Scholar]
- 108.Baxter M, Ruane L, Phyland D, Leahy E, Heke E, Lau KK, Low K, Hamza K, MacDonald M, Bardin PG. Multidisciplinary team clinic for vocal cord dysfunction directs therapy and significantly reduces healthcare utilization. Respirology. 2019;24(8):758–764. doi: 10.1111/resp.13520. [DOI] [PubMed] [Google Scholar]
- 109.Holloway EA, West RJ. Integrated breathing and relaxation training (the Papworth method) for adults with asthma in primary care: a randomised controlled trial. Thorax. 2007;62(12):1039–1042. doi: 10.1136/thx.2006.076430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, Phillips BR, Mauger DT, Comhair SA, Erzurum SC, Johansson MW, et al. Blood Institute Severe Asthma Research P. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4(7):574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169–1179. doi: 10.1016/j.jaci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ma J, Strub P, Xiao L, Lavori PW, Camargo CA, Jr, Wilson SR, Gardner CD, Buist AS, Haskell WL, Lv N. Behavioral weight loss and physical activity intervention in obese adults with asthma. A randomized trial. Ann Am Thorac Soc. 2015;12(1):1–11. doi: 10.1513/AnnalsATS.201406-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Network BTSSIG. British guideline on the management of asthma. London: 2019. Jul, 2019. [Google Scholar]
- 114.Nakamura Y, Miyata M, Ohba T, Ando T, Hatsushika K, Suenaga F, Shimokawa N, Ohnuma Y, Katoh R, Ogawa H, Nakao A. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol. 2008;122(6):1208–1214. doi: 10.1016/j.jaci.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 115.Bleck B, Tse DB, Curotto de Lafaille MA, Zhang F, Reibman J. Diesel exhaust particle-exposed human bronchial epithelial cells induce dendritic cell maturation and polarization via thymic stromal lymphopoietin. J Clin Immunol. 2008;28(2):147–156. doi: 10.1007/s10875-007-9149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183(2):1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, Shelley M, Abbas AR, Austin CD, Jackman J, Wu LC, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129(1):104-111–e101-109. doi: 10.1016/j.jaci.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 118.Liu S, Verma M, Michalec L, Liu W, Sripada A, Rollins D, Good J, Ito Y, Chu H, Gorska MM, Martin RJ, Alam R. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: The role of thymic stromal lymphopoietin. J Allergy Clin Immunol. 2018;141(1):257–268 e256. doi: 10.1016/j.jaci.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eagan TM, Damas JK, Ueland T, Voll-Aanerud M, Mollnes TE, Hardie JA, Bakke PS, Aukrust P. Neutrophil gelatinase-associated lipocalin: a biomarker in COPD. Chest. 2010;138(4):888–895. doi: 10.1378/chest.09-2718. [DOI] [PubMed] [Google Scholar]
- 120.Wang J, Lv H, Luo Z, Mou S, Liu J, Liu C, Deng S, Jiang Y, Lin J, Wu C, Liu X, He J, Jiang D. Plasma YKL-40 and NGAL are useful in distinguishing ACO from asthma and COPD. Respir Res. 2018;19(1):47. doi: 10.1186/s12931-018-0755-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schleich FN, Zanella D, Stefanuto PH, Bessonov K, Smolinska A, Dallinga JW, Henket M, Paulus V, Guissard F, Graff S, Moermans C, et al. Exhaled Volatile Organic Compounds Are Able to Discriminate between Neutrophilic and Eosinophilic Asthma. Am J Respir Crit Care Med. 2019;200(4):444–453. doi: 10.1164/rccm.201811-2210OC. [DOI] [PubMed] [Google Scholar]
- 122.Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity. 2019;50(4):778–795. doi: 10.1016/j.immuni.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B, Kruger R, et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol. 2018;3(26) doi: 10.1126/sciimmunol.aar6689. [DOI] [PubMed] [Google Scholar]
- 124.Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, Schroder K. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol. 2018;3(26) doi: 10.1126/sciimmunol.aar6676. [DOI] [PubMed] [Google Scholar]
- 125.Kim RY, Pinkerton JW, Essilfie AT, Robertson AAB, Baines KJ, Brown AC, Mayall JR, Ali MK, Starkey MR, Hansbro NG, Hirota JA, et al. Role for NLRP3 Inflammasome-mediated, IL-1beta-Dependent Responses in Severe, Steroid-Resistant Asthma. Am J Respir Crit Care Med. 2017;196(3):283–297. doi: 10.1164/rccm.201609-1830OC. [DOI] [PubMed] [Google Scholar]
- 126.Simpson JL, Phipps S, Baines KJ, Oreo KM, Gunawardhana L, Gibson PG. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J. 2014;43(4):1067–1076. doi: 10.1183/09031936.00105013. [DOI] [PubMed] [Google Scholar]
- 127.Baines KJ, Simpson JL, Wood LG, Scott RJ, Gibson PG. Transcriptional phenotypes of asthma defined by gene expression profiling of induced sputum samples. J Allergy Clin Immunol. 2011;127(1):153-160–160 e151-159. doi: 10.1016/j.jaci.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 128.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER, National Heart, Lung, and Blood Institute Severe Asthma Research Program Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125(5):1028–1036 e1013. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Evans MD, Esnault S, Denlinger LC, Jarjour NN. Sputum cell IL-1 receptor expression level is a marker of airway neutrophilia and airflow obstruction in asthmatic patients. J Allergy Clin Immunol. 2018;142(2):415–423. doi: 10.1016/j.jaci.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rossios C, Pavlidis S, Hoda U, Kuo CH, Wiegman C, Russell K, Sun K, Loza MJ, Baribaud F, Durham AL, Ojo O, et al. Sputum transcriptomics reveal upregulation of IL-1 receptor family members in patients with severe asthma. J Allergy Clin Immunol. 2018;141(2):560–570. doi: 10.1016/j.jaci.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 131.Lachowicz-Scroggins ME, Dunican EM, Charbit AR, Raymond W, Looney MR, Peters MC, Gordon ED, Woodruff PG, Lefrancais E, Phillips BR, Mauger DT, et al. Extracellular DNA: Neutrophil Extracellular Traps, and Inflammasome Activation in Severe Asthma. Am J Respir Crit Care Med. 2019;199(9):1076–1085. doi: 10.1164/rccm.201810-1869OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hernandez ML, Lay JC, Harris B, Esther CR, Jr, Brickey WJ, Bromberg PA, Diaz-Sanchez D, Devlin RB, Kleeberger SR, Alexis NE, Peden DB. Atopic asthmatic subjects but not atopic subjects without asthma have enhanced inflammatory response to ozone. J Allergy Clin Immunol. 2010;126(3):537–544 e531. doi: 10.1016/j.jaci.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aleman MM, Kesic MJ, Mills KH, Peden DB, Hernandez ML. The IL-1 axis is associated with airway inflammation after O3 exposure in allergic asthmatic patients. J Allergy Clin Immunol. 2015;136(4):1099–1101 e1092. doi: 10.1016/j.jaci.2015.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hernandez ML, Mills K, Almond M, Todoric K, Aleman MM, Zhang H, Zhou H, Peden DB. IL-1 receptor antagonist reduces endotoxin-induced airway inflammation in healthy volunteers. J Allergy Clin Immunol. 2015;135(2):379–385. doi: 10.1016/j.jaci.2014.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pascoe S, Kanniess F, Bonner J. A monoclonal antibody to IL-1lll attenuates the late asthmatic response to antigen challenge in patients with mild asthma. Oral presentation 752; Annual ERS International Congress; London, UK. 2016. [Google Scholar]
- 136.University of North Carolina CH. Early Phase Administration of Anakinra as a Rescue Treatment for Inhaled Allergen Challenge-Induced Airway Inflammation. [cited 2020 4/01/2020];2018 Available from: http://clinicaltrials.gov/ct2/show/NCT03513471.
- 137.University of North Carolina CH. Late Phase Administration Anakinra as a Rescue Treatment for Inhaled Allergen Challenge-Induced Airway Inflammation (LateAna) [cited 2020 04/01/2020];2018 Available from: http://clinicaltrials.gov/ct2/show/NCT03513458.
- 138.Hinks TS, Zhou X, Staples KJ, Dimitrov BD, Manta A, Petrossian T, Lum PY, Smith CG, Ward JA, Howarth PH, Walls AF, et al. Innate and adaptive T cells in asthmatic patients: Relationship to severity and disease mechanisms. J Allergy Clin Immunol. 2015;136(2):323–333. doi: 10.1016/j.jaci.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162(4):2347–2352. [PubMed] [Google Scholar]
- 140.Du J, Han J-C, Zhang Y-J, Qi G-B, Li H-B, Zhang Y-J, Cai S. Single-Nucleotide Polymorphisms of IL-17 Gene Are Associated with Asthma Susceptibility in an Asian Population. Medical science monitor: international medical journal of experimental and clinical research. 2016;22:780–787. doi: 10.12659/MSM.895494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Silva MJ, de Santana MBR, Tosta BR, Espinheira RP, Alcantara-Neves NM, Barreto ML, Figueiredo CA, Costa RDS. Variants in the IL17 pathway genes are associated with atopic asthma and atopy makers in a South American population. Allergy Asthma Clin Immunol. 2019;15:28. doi: 10.1186/s13223-019-0340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kawaguchi M, Takahashi D, Hizawa N, Suzuki S, Matsukura S, Kokubu F, Maeda Y, Fukui Y, Konno S, Huang SK, Nishimura M, et al. IL-17F sequence variant (His161Arg) is associated with protection against asthma and antagonizes wild-type IL-17F activity. J Allergy Clin Immunol. 2006;117(4):795–801. doi: 10.1016/j.jaci.2005.12.1346. [DOI] [PubMed] [Google Scholar]
- 143.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, Huang X, et al. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012;18(4):547–554. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Murdoch JR, Lloyd CM. Resolution of allergic airway inflammation and airway hyperreactivity is mediated by IL-17-producing {gamma}{delta}T cells. Am J Respir Crit Care Med. 2010;182(4):464–476. doi: 10.1164/rccm.200911-1775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scanlon KM, Hawksworth RJ, Lane SJ, Mahon BP. IL-17A induces CCL28, supporting the chemotaxis of IgE-secreting B cells. Int Arch Allergy Immunol. 2011;156(1):51–61. doi: 10.1159/000322178. [DOI] [PubMed] [Google Scholar]
- 146.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97(6):726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 148.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, Hamid Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123(5):1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 149.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, Martin RJ, Alam R. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134(5):1175–1186.e1177. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, Querci V, Angeli R, Matucci A, Fambrini M, Liotta F, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125(1):222–230 e221-224. doi: 10.1016/j.jaci.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 151.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, Ryan PH, Budelsky AL, Khurana Hershey GK. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132(5):1194–1204.e1192. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Siew LQC, Wu SY, Ying S, Corrigan CJ. Cigarette smoking increases bronchial mucosal IL-17A expression in asthmatics, which acts in concert with environmental aeroallergens to engender neutrophilic inflammation. Clin Exp Allergy. 2017;47(6):740–750. doi: 10.1111/cea.12907. [DOI] [PubMed] [Google Scholar]
- 153.Ostling J, van Geest M, Schofield JPR, Jevnikar Z, Wilson S, Ward J, Lutter R, Shaw DE, Bakke PS, Caruso M, Dahlen SE, et al. IL-17-high asthma with features of a psoriasis immunophenotype. J Allergy Clin Immunol. 2019 doi: 10.1016/j.jaci.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 154.Krishnamoorthy N, Douda DN, Bruggemann TR, Ricklefs I, Duvall MG, Abdulnour RE, Martinod K, Tavares L, Wang X, Cernadas M, Israel E, et al. Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci Immunol. 2018;3(26) doi: 10.1126/sciimmunol.aao4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, Lin SL. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188(11):1294–1302. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 156.National_Institutes_of_Health_Clinical_Center. Efficacy and safety of BI 655066/ABBV-066 (risankizumab) in patients with severe persistent asthma. [cited 2020 22/01/2020]];ClinicalTrials.gov. 2015 NCT02443298. Available from: http://clinicaltrials.gov/ct2/show/NCT02443298. [Google Scholar]
- 157.National_Institutes_of_Health_Clinical_Center. Study to assess the efficacy and safety of CJM112 in patients with inadequately controlled severe asthma. [cited 2020 22/01/2020];ClinicalTrialsgov. 2017 NCT03299686. Available from: http://clinicaltrials.gov/ct2/show/NCT03299686. [Google Scholar]
- 158.Kalchiem-Dekel O, Yao X, Levine SJ. Meeting the Challenge of Identifying New Treatments for Type 2-Low Neutrophilic Asthma. Chest. 2020;157(1):26–33. doi: 10.1016/j.chest.2019.08.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Barnig C, Frossard N, Levy BD. Towards targeting resolution pathways of airway inflammation in asthma. Pharmacology & therapeutics. 2018;186:98–113. doi: 10.1016/j.pharmthera.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 160.Krishnamoorthy N, Abdulnour RE, Walker KH, Engstrom BD, Levy BD. Specialized Proresolving Mediators in Innate and Adaptive Immune Responses in Airway Diseases. Physiol Rev. 2018;98(3):1335–1370. doi: 10.1152/physrev.00026.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E, Severe Asthma Research Program NHL. Blood I Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172(7):824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bonnans C, Vachier I, Chavis C, Godard P, Bousquet J, Chanez P. Lipoxins are potential endogenous antiinflammatory mediators in asthma. Am J Respir Crit Care Med. 2002;165(11):1531–1535. doi: 10.1164/rccm.200201-053OC. [DOI] [PubMed] [Google Scholar]
- 163.Vachier I, Bonnans C, Chavis C, Farce M, Godard P, Bousquet J, Chanez P. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. J Allergy Clin Immunol. 2005;115(1):55–60. doi: 10.1016/j.jaci.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 164.Gagliardo R, Gras D, La Grutta S, Chanez P, Di Sano C, Albano GD, Vachier I, Montalbano AM, Anzalone G, Bonanno A, Riccobono L, et al. Airway lipoxin A4/formyl peptide receptor 2-lipoxin receptor levels in pediatric patients with severe asthma. J Allergy Clin Immunol. 2016;137(6):1796–1806. doi: 10.1016/j.jaci.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 165.Kazani S, Planaguma A, Ono E, Bonini M, Zahid M, Marigowda G, Wechsler ME, Levy BD, Israel E. Exhaled breath condensate eicosanoid levels associate with asthma and its severity. J Allergy Clin Immunol. 2013;132(3):547–553. doi: 10.1016/j.jaci.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Planaguma A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, Bleecker ER, Curran-Everett D, Erzurum SC, Calhoun WJ, Castro M, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178(6):574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Duvall MG, Barnig C, Cernadas M, Ricklefs I, Krishnamoorthy N, Grossman NL, Bhakta NR, Fahy JV, Bleecker ER, Castro M, Erzurum SC, et al. Natural killer cell-mediated inflammation resolution is disabled in severe asthma. Sci Immunol. 2017;2(9) doi: 10.1126/sciimmunol.aam5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ricklefs I, Barkas I, Duvall MG, Cernadas M, Grossman NL, Israel E, Bleecker ER, Castro M, Erzurum SC, Fahy JV, Gaston BM, et al. ALX receptor ligands define a biochemical endotype for severe asthma. JCI Insight. 2017;2(14) doi: 10.1172/jci.insight.93534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ash SY, Rahaghi FN, Come CE, Ross JC, Colon AG, Cardet-Guisasola JC, Dunican EM, Bleecker ER, Castro M, Fahy JV, Fain SB, et al. Pruning of the Pulmonary Vasculature in Asthma. The Severe Asthma Research Program (SARP) Cohort. Am J Respir Crit Care Med. 2018;198(1):39–50. doi: 10.1164/rccm.201712-2426OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8(9):1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 171.Christie PE, Spur BW, Lee TH. The effects of lipoxin A4 on airway responses in asthmatic subjects. Am Rev Respir Dis. 1992;145(6):1281–1284. doi: 10.1164/ajrccm/145.6.1281. [DOI] [PubMed] [Google Scholar]
- 172.Kong X, Wu SH, Zhang L, Chen XQ. Pilot application of lipoxin A4 analog and lipoxin A4 receptor agonist in asthmatic children with acute episodes. Exp Ther Med. 2017;14(3):2284–2290. doi: 10.3892/etm.2017.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, Schoos AM, Wolsk HM, Pedersen TM, Vinding RK, Thorsteinsdottir S, Folsgaard NV, et al. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N Engl J Med. 2016;375(26):2530–2539. doi: 10.1056/NEJMoa1503734. [DOI] [PubMed] [Google Scholar]
- 174.Dai C, Yao X, Keeran KJ, Zywicke GJ, Qu X, Yu ZX, Dagur PK, McCoy JP, Remaley AT, Levine SJ. Apolipoprotein A-I attenuates ovalbumin-induced neutrophilic airway inflammation via a granulocyte colony-stimulating factor-dependent mechanism. Am J Respir Cell Mol Biol. 2012;47(2):186–195. doi: 10.1165/rcmb.2011-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dai C, Yao X, Vaisman B, Brenner T, Meyer KS, Gao M, Keeran KJ, Nugent GZ, Qu X, Yu ZX, Dagur PK, et al. ATP-binding cassette transporter 1 attenuates ovalbumin-induced neutrophilic airway inflammation. Am J Respir Cell Mol Biol. 2014;51(5):626–636. doi: 10.1165/rcmb.2013-0264OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Barochia AV, Kaler M, Cuento RA, Gordon EM, Weir NA, Sampson M, Fontana JR, MacDonald S, Moss J, Manganiello V, Remaley AT, et al. Serum apolipoprotein A-I and large high-density lipoprotein particles are positively correlated with FEV1 in atopic asthma. Am J Respir Crit Care Med. 2015;191(9):990–1000. doi: 10.1164/rccm.201411-1990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yao X, Dai C, Fredriksson K, Dagur PK, McCoy JP, Qu X, Yu ZX, Keeran KJ, Zywicke GJ, Amar MJ, Remaley AT, et al. 5A, an apolipoprotein A-I mimetic peptide, attenuates the induction of house dust mite-induced asthma. J Immunol. 2011;186(1):576–583. doi: 10.4049/jimmunol.1001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359(9309):831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 179.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201(6):937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12(9):1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 181.Edwards MR, Regamey N, Vareille M, Kieninger E, Gupta A, Shoemark A, Saglani S, Sykes A, Macintyre J, Davies J, Bossley C, et al. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal immunology. 2013;6(4):797–806. doi: 10.1038/mi.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sykes A, Edwards MR, Macintyre J, del Rosario A, Bakhsoliani E, Trujillo-Torralbo MB, Kon OM, Mallia P, McHale M, Johnston SL. Rhinovirus 16-induced IFN-alpha and IFN-beta are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol. 2012;129(6):1506–1514 e1506. doi: 10.1016/j.jaci.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 183.Bosco A, Ehteshami S, Stern DA, Martinez FD. Decreased activation of inflammatory networks during acute asthma exacerbations is associated with chronic airflow obstruction. Mucosal immunology. 2010;3(4):399–409. doi: 10.1038/mi.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Singanayagam A, Glanville N, Girkin JL, Ching YM, Marcellini A, Porter JD, Toussaint M, Walton RP, Finney LJ, Aniscenko J, Zhu J, et al. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nature communications. 2018;9(1) doi: 10.1038/s41467-018-04574-1. 2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bhakta NR, Christenson SA, Nerella S, Solberg OD, Nguyen CP, Choy DF, Jung KL, Garudadri S, Bonser LR, Pollack JL, Zlock LT, et al. IFN-stimulated Gene Expression, Type 2 Inflammation, and Endoplasmic Reticulum Stress in Asthma. Am J Respir Crit Care Med. 2018;197(3):313–324. doi: 10.1164/rccm.201706-1070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Djukanovic R, Harrison T, Johnston SL, Gabbay F, Wark P, Thomson NC, Niven R, Singh D, Reddel HK, Davies DE, Marsden R, et al. The effect of inhaled IFN-beta on worsening of asthma symptoms caused by viral infections. A randomized trial. Am J Respir Crit Care Med. 2014;190(2):145–154. doi: 10.1164/rccm.201312-2235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.AstraZeneca. A Study in Asthma Patients to Evaluate Efficacy, Safety and Tolerability of 14 Days Once Daily Inhaled Interferon Beta-1a After the Onset of Symptoms of an Upper Respiratory Tract Infection (INEXAS) [cited 2020 06/02/2020];2019 Available from: http://clinicaltrials.gov/ct2/show/results/NCT02491684.
- 188.McCrae C, Olsson M, Aurell M, Lundin C, Paraskos J, Cavallin A, M K, Karlsson K, Marsden R, Malmgren A, Gustafson P, Harrison T. On-demand inhaled interferon-beta 1a for the prevention of severe asthma exacerbations: results of the INEXAS phase 2a study. Am J Respir Crit Care Med. 2018;197 [Google Scholar]
- 189.Yokoyama A, Kohno N, Fujino S, Hamada H, Inoue Y, Fujioka S, Ishida S, Hiwada K. Circulating interleukin-6 levels in patients with bronchial asthma. Am J Respir Crit Care Med. 1995;151(5):1354–1358. doi: 10.1164/ajrccm.151.5.7735584. [DOI] [PubMed] [Google Scholar]
- 190.Neveu WA, Allard JL, Raymond DM, Bourassa LM, Burns SM, Bunn JY, Irvin CG, Kaminsky DA, Rincon M. Elevation of IL-6 in the allergic asthmatic airway is independent of inflammation but associates with loss of central airway function. Respir Res. 2010;11:28. doi: 10.1186/1465-9921-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang JG, Chen XJ, Liu T, Jiang SJ. FOXP3(+) associated with the pro-inflammatory regulatory T and T helper 17 effector cells in asthma patients. Exp Ther Med. 2016;12(4):2753–2758. doi: 10.3892/etm.2016.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Peters MC, Ringel L, Dyjack N, Herrin R, Woodruff PG, Rios C, O'Connor B, Fahy JV, Seibold MA. A Transcriptomic Method to Determine Airway Immune Dysfunction in T2-High and T2-Low Asthma. Am J Respir Crit Care Med. 2019;199(4):465–477. doi: 10.1164/rccm.201807-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Massoud AH, Charbonnier LM, Lopez D, Pellegrini M, Phipatanakul W, Chatila TA. An asthma-associated IL4R variant exacerbates airway inflammation by promoting conversion of regulatory T cells to TH17-like cells. Nat Med. 2016;22(9):1013–1022. doi: 10.1038/nm.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Esty B, Harb H, Bartnikas LM, Charbonnier LM, Massoud AH, Leon-Astudillo C, Visner G, Subramaniam M, Phipatanakul W, Chatila TA. Treatment of severe persistent asthma with IL-6 receptor blockade. J Allergy Clin Immunol Pract. 2019;7(5):1639–1642 e1634. doi: 10.1016/j.jaip.2019.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yokoyama A, Kohno N, Sakai K, Kondo K, Hirasawa Y, Hiwada K. Circulating levels of soluble interleukin-6 receptor in patients with bronchial asthma. Am J Respir Crit Care Med. 1997;156(5):1688–1691. doi: 10.1164/ajrccm.156.5.9610070. [DOI] [PubMed] [Google Scholar]
- 196.Hinks TS, Brown T, Lau LCK, Rupani H, Barber C, Elliott S, Ward JA, Ono J, Ohra S, Izuhara K, Djukanovic R, et al. Multidimensional endotyping in severe asthma reveals inflammatory heterogeneity in MMPs and YKL-40. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.11.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jevnikar Z, Ostling J, Ax E, Calven J, Thorn K, Israelsson E, Oberg L, Singhania A, Lau LCK, Wilson SJ, Ward JA, et al. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J Allergy Clin Immunol. 2018 doi: 10.1016/j.jaci.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 198.Robinson MB, Deshpande DA, Chou J, Cui W, Smith S, Langefeld C, Hastie AT, Bleecker ER, Hawkins GA. IL-6 trans-signaling increases expression of airways disease genes in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2015;309(2):L129–138. doi: 10.1152/ajplung.00288.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ferreira MA, Matheson MC, Duffy DL, Marks GB, Hui J, Le Souef P, Danoy P, Baltic S, Nyholt DR, Jenkins M, Hayden C, et al. Identification of IL6R and chromosome 11q13.5 as risk loci for asthma. Lancet. 2011;378(9795):1006–1014. doi: 10.1016/S0140-6736(11)60874-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Galicia JC, Tai H, Komatsu Y, Shimada Y, Akazawa K, Yoshie H. Polymorphisms in the IL-6 receptor (IL-6R) gene: strong evidence that serum levels of soluble IL-6R are genetically influenced. Genes Immun. 2004;5(6):513–516. doi: 10.1038/sj.gene.6364120. [DOI] [PubMed] [Google Scholar]
- 201.Hawkins GA, Robinson MB, Hastie AT, Li X, Li H, Moore WC, Howard TD, Busse WW, Erzurum SC, Wenzel SE, Peters SP, et al. The IL6R variation Asp(358)Ala is a potential modifier of lung function in subjects with asthma. J Allergy Clin Immunol. 2012;130(2):510–515 e511. doi: 10.1016/j.jaci.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Maun HR, Jackman JK, Choy DF, Loyet KM, Staton TL, Jia G, Dressen A, Hackney JA, Bremer M, Walters BT, Vij R, et al. An Allosteric Anti-tryptase Antibody for the Treatment of Mast Cell-Mediated Severe Asthma. Cell. 2019;179(2):417–431 e419. doi: 10.1016/j.cell.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cahill KN, Katz HR, Cui J, Lai J, Kazani S, Crosby-Thompson A, Garofalo D, Castro M, Jarjour N, DiMango E, Erzurum S, et al. KIT Inhibition by Imatinib in Patients with Severe Refractory Asthma. N Engl J Med. 2017;376(20):1911–1920. doi: 10.1056/NEJMoa1613125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, Huff R, Pilewski J, Holguin F, Kolls J, Wenzel S, et al. High IFN-gamma and low SLPI mark severe asthma in mice and humans. J Clin Invest. 2015;125(8):3037–3050. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gauthier M, Chakraborty K, Oriss TB, Raundhal M, Das S, Chen J, Huff R, Sinha A, Fajt M, Ray P, Wenzel SE, et al. Severe asthma in humans and mouse model suggests a CXCL10 signature underlies corticosteroid-resistant Th1 bias. JCI Insight. 2017;2(13) doi: 10.1172/jci.insight.94580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Oriss TB, Raundhal M, Morse C, Huff RE, Das S, Hannum R, Gauthier MC, Scholl KL, Chakraborty K, Nouraie SM, Wenzel SE, et al. IRF5 distinguishes severe asthma in humans and drives Th1 phenotype and airway hyperreactivity in mice. JCI Insight. 2017;2(10) doi: 10.1172/jci.insight.91019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlen SE, Holgate ST, Meyers DA, Rabe KF, Antczak A, Baker J, et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am J Respir Crit Care Med. 2009;179(7):549–558. doi: 10.1164/rccm.200809-1512OC. [DOI] [PubMed] [Google Scholar]
- 208.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, Bradding P, Brightling CE, Wardlaw AJ, Pavord ID. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354(7):697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- 209.Fang L, Adkins B, Deyev V, Podack ER. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J Exp Med. 2008;205(5):1037–1048. doi: 10.1084/jem.20072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Meylan F, Hawley ET, Barron L, Barlow JL, Penumetcha P, Pelletier M, Sciume G, Richard AC, Hayes ET, Gomez-Rodriguez J, Chen X, et al. The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal immunology. 2014;7(4):958–968. doi: 10.1038/mi.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Khan SQ, Tsai MS, Schreiber TH, Wolf D, Deyev VV, Podack ER. Cloning, expression, and functional characterization of TL1A-Ig. J Immunol. 2013;190(4):1540–1550. doi: 10.4049/jimmunol.1201908. [DOI] [PubMed] [Google Scholar]
- 212.Collesano V, Brusotti C, Boieri G. [Preimplantation evaluation. Presentation of a clinical case] Dent Cadmos. 1979;47(5):21–29. [PubMed] [Google Scholar]
- 213.Corren J. New Targeted Therapies for Uncontrolled Asthma. J Allergy Clin Immunol Pract. 2019;7(5):1394–1403. doi: 10.1016/j.jaip.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 214.Bamias G, Martin C, Marini M, Hoang S, Mishina M, Ross WG, Sachedina MA, Friel CM, Mize J, Bickston SJ, Pizarro TT, Wei P, et al. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003;171(9):4868–4874. doi: 10.4049/jimmunol.171.9.4868. [DOI] [PubMed] [Google Scholar]
- 215.Papadakis KA, Prehn JL, Landers C, Han Q, Luo X, Cha SC, Wei P, Targan SR. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J Immunol. 2004;172(11):7002–7007. doi: 10.4049/jimmunol.172.11.7002. [DOI] [PubMed] [Google Scholar]
- 216.Banfield C, Rudin D, Bhattacharya I, Goteti K, Li G, Hassan-Zahraee M, Brown LS, Hung KE, Pawlak S, Lepsy C. First-in-human, randomized dose-escalation study of the safety, tolerability, pharmacokinetics, pharmacodynamics and immunogenicity of PF-06480605 in healthy subjects. Br J Clin Pharmacol. 2020;86(4):812–824. doi: 10.1111/bcp.14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pfizer. A Study to Evaluate the Efficacy and Safety of PF-06480605 in Adult Participants With Moderate to Severe Ulcerative Colitis. [cited 2020 19/05/2020];2020 Available from: https://clinicaltrials.gov/ct2/show/NCT04090411.
- 218.Consuegra-Fernandez M, Lin F, Fox DA, Lozano F. Clinical and experimental evidence for targeting CD6 in immune-based disorders. Autoimmun Rev. 2018;17(5):493–503. doi: 10.1016/j.autrev.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 219.Kim MN, Hong JY, Shim DH, Sol IS, Kim YS, Lee JH, Kim KW, Lee JM, Sohn MH. Activated Leukocyte Cell Adhesion Molecule Stimulates the T-Cell Response in Allergic Asthma. Am J Respir Crit Care Med. 2018;197(8):994–1008. doi: 10.1164/rccm.201703-0532OC. [DOI] [PubMed] [Google Scholar]
- 220.Siroux V, Gonzalez JR, Bouzigon E, Curjuric I, Boudier A, Imboden M, Anto JM, Gut I, Jarvis D, Lathrop M, Omenaas ER, et al. Genetic heterogeneity of asthma phenotypes identified by a clustering approach. Eur Respir J. 2014;43(2):439–452. doi: 10.1183/09031936.00032713. [DOI] [PubMed] [Google Scholar]
- 221.Equillium. A Study of Itolizumab (EQ001) to Evaluate the Safety, Tolerability, PK, PD, and Clinical Activity in Uncontrolled Asthma (EQUIP) [cited 2020 19/05/2020];2019 Available from: https://clinicaltrials.gov/ct2/show/NCT04007198.
- 222.Nair P, Gaga M, Zervas E, Alagha K, Hargreave FE, O'Byrne PM, Stryszak P, Gann L, Sadeh J, Chanez P. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2012;42(7):1097–1103. doi: 10.1111/j.1365-2222.2012.04014.x. [DOI] [PubMed] [Google Scholar]
- 223.O'Byrne PM, Metev H, Puu M, Richter K, Keen C, Uddin M, Larsson B, Cullberg M, Nair P. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4(10):797–806. doi: 10.1016/S2213-2600(16)30227-2. [DOI] [PubMed] [Google Scholar]
- 224.Chaudhuri R, Norris V, Kelly K, Zhu CQ, Ambery C, Lafferty J, Cameron E, Thomson NC. Effects of a FLAP inhibitor, GSK2190915, in asthmatics with high sputum neutrophils. Pulmonary pharmacology & therapeutics. 2014;27(1):62–69. doi: 10.1016/j.pupt.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 225.Sasaki F, Yokomizo T. The leukotriene receptors as therapeutic targets of inflammatory diseases. Int Immunol. 2019;31(9):607–615. doi: 10.1093/intimm/dxz044. [DOI] [PubMed] [Google Scholar]
- 226.Gelfand EW. Importance of the leukotriene B4-BLT1 and LTB4-BLT2 pathways in asthma. Semin Immunol. 2017;33:44–51. doi: 10.1016/j.smim.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hicks A, Goodnow R, Jr, Cavallo G, Tannu SA, Ventre JD, Lavelle D, Lora JM, Satjawatcharaphong J, Brovarney M, Dabbagh K, Tare NS, et al. Effects of LTB4 receptor antagonism on pulmonary inflammation in rodents and non-human primates. Prostaglandins Other Lipid Mediat. 2010;92(1-4):33–43. doi: 10.1016/j.prostaglandins.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 228.Shin E, Lee YC, Kim SR, Kim SH, Park J. Drug Signature-based Finding of Additional Clinical Use of LC28-0126 for Neutrophilic Bronchial Asthma. Scientific reports. 2015;5 doi: 10.1038/srep17784. 17784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Masefield S, Edwards J, Hansen K, Hamerlijnck D, Lisspers K, van der Schee M, Silva L, Matthews J, Gaga M, Adcock I, Holgate S, et al. The future of asthma research and development: a roadmap from the European Asthma Research and Innovation Partnership (EARIP) Eur Respir J. 2017;49(5) doi: 10.1183/13993003.02295-2016. [DOI] [PubMed] [Google Scholar]
- 230.Malhotra-Kumar S, Lammens C, Coenen S, Van Herck K, Goossens H. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: a randomised, double-blind, placebo-controlled study. Lancet. 2007;369(9560):482–490. doi: 10.1016/S0140-6736(07)60235-9. [DOI] [PubMed] [Google Scholar]