Abstract
Context
Perturbed inositol physiology in insulin resistant conditions has led to proposals of inositol supplementation for gestational diabetes (GDM) prevention, but placental inositol biology is poorly understood.
Objective
Investigate associations of maternal glycemia with placental inositol content, determine glucose effects on placental expression of inositol enzymes and transporters, and examine relations with birthweight.
Design and Participants
Case-control study of placentae from term singleton pregnancies (GDM n=24, non-GDM n=26), and culture of another 9 placentae in different concentrations of glucose and myo-inositol for 48h.
Main Outcome Measures
Placental inositol was quantified by the Megazyme® assay. Relative expression of enzymes involved in myo-inositol metabolism and plasma membrane inositol transport was determined by quantitative RT-PCR and immunoblotting. Linear regression analyses adjusted for maternal age, BMI, ethnicity, gestational age and sex.
Results
Placental inositol content was 17% lower in GDM compared with non-GDM. Higher maternal mid-gestation glycemia were associated with lower placental inositol. Increasing fasting glycemia was associated with lower protein levels of the myo-inositol synthesis enzyme, IMPA1, and the inositol transporters, SLC5A11 and SLC2A13, the expression of which also correlated with placental inositol content. In vitro, higher glucose concentrations reduced IMPA1 and SLC5A11 mRNA expression. Increasing fasting glycemia positively associated with customized birthweight percentile as expected in cases with low placental inositol, but this association was attenuated with high placental inositol.
Conclusion
Glycemia-induced dysregulation of placental inositol synthesis and transport may be implicated in reduced placental inositol content in GDM, and this may in turn be permissive to accelerated fetal growth.
Keywords: Birthweight, Glycaemia, Myo-inositol, Inositol, Placenta, Pregnancy
Introduction
Inositols are 6-carbon polyols ubiquitously present in cells, of which myo-inositol is the most abundant naturally-occurring isomer (1). Inositol is enriched in fruits, grains and nuts in our diet, and is endogenously synthesized mainly by the kidneys (2). Tissue inositol and its derivatives can be found in water-soluble forms [e.g. free inositol, inositol phosphoglycans (IPG), inositol phosphates (IP)] and lipid-conjugated forms [e.g. phosphoinositide lipids, phosphatidylinositol (PI), phosphoinositide phosphates (PIP)], and both forms are critical in many cellular functions. Inositols are components of several important signaling molecules (e.g. PIP, IP) and secondary messenger pathways (e.g. PI3K) including that of insulin and IGF factors (3, 4), while some inositol derivatives (e.g. IPG) are themselves insulin mimics. Inositol derivatives also participate in regulation of membrane fluidity, protein trafficking and transport processes, cytoskeleton assembly, intracellular calcium, lipid metabolism and gene expression (3, 5).
Alterations in circulating and urinary inositol have been reported in non-pregnant adults with type 1 and type 2 diabetes mellitus (6–9), with studies in general reporting increased urinary myo-inositol excretion. In animal studies, diabetes is associated with decreased myo-inositol in neural tissue (10). Such tissue inositol depletion has been implicated in reduced insulin sensitivity and functional deficits with restoration of tissue function achieved through myo-inositol supplementation (11–13). It has been postulated that inositol depletion in diabetes could be secondary to increased urinary excretion due to competitive inhibition of renal tubular reabsorption by glucose, impaired tissue uptake, reduced synthesis, increased catabolism and enhanced cellular efflux secondary to sorbitol accumulation (14–16). Whether tissue inositol is similarly depleted in gestational diabetes mellitus (GDM) is unclear.
Pregnancy is a physiologically-induced insulin resistant state to promote nutrient mobilization to the fetus, but an exaggerated state of insulin resistance, relative to pancreatic beta cell insulin secretory capability, in some women can lead to GDM (17). Interestingly, maternal urinary inositol excretion was higher in the first trimester of pregnancy in women who subsequently developed GDM (18). In a separate study, an increased concentration of inositol was found in amniotic fluid at mid-gestation pre-dating the diagnosis of GDM (19). Both these studies suggest that altered inositol biology across the maternal-placental-fetal axis may contribute to the pathophysiology of GDM. With clinical trials of antenatal inositol supplementation now being conducted to investigate its efficacy in the prevention and treatment of GDM (20–23), it is important to understand the implications of inositol interventions on placental function.
The placenta is rich in inositol transporters (24), however, little is known of the role of inositol in the fetoplacental unit. The placenta plays a major part in GDM pathophysiology, with evidence of altered maternal-fetal crosstalk, and changed placental transport and metabolism (25), leading to adversity including placental hyperplasia, fetal hyperinsulinemia, fetal deficiency in long chain polyunsaturated fatty acid, fetal macrosomia and intrauterine demise. It is thus plausible that inositol has an important physiological role in the placenta and that an altered placental inositol status could contribute to GDM pathophysiology with consequences on the fetus, which in turn may also have implications on long term offspring cardiometabolic health.
We hypothesized that placentae from GDM pregnancies are depleted in inositol, due to a combination of systemic inositol insufficiency and to local glycemia-induced tissue disruption in inositol plasma membrane transport and metabolism. We also hypothesized that a reduction in placental inositol permits accelerated fetal growth, and hence the risk of fetal macrosomia. The objectives of this study were to describe the difference in the tissue content of inositol in placentae obtained from GDM pregnancies compared with those from uncomplicated pregnancies and examine whether placental inositol content relates to maternal glycemia. We also aimed to elucidate if increasing maternal glycemia in vivo and increasing glucose and myo-inositol exposure in vitro were associated with alterations in the placental expression of enzymes that metabolize inositol and of inositol transporters, and whether this in turn associated with differences in placental inositol content. Finally, we sought to determine whether placental inositol could modulate the maternal glycemic promotion of birthweight.
Materials and Methods
Participant Recruitment
Ethical approval was granted by the National Healthcare Group Domain Specific Research Board (Reference 2016/00183) and informed written consent was obtained from all participants.
In this case-control study, placentae were collected at elective cesarean sections of term singleton pregnancies at the National University Hospital, Singapore, from 26 non-GDM and 24 GDM cases (total n=50) of Asian ethnicity (Chinese, Malay or Indian), without any other complications such as hypertensive disorders or growth restriction (Table 1A). Indications for cesarean delivery were previous cesarean section (82%), breech presentation (8%), suspected macrosomia (6%) and maternal choice (4%). A further 9 placentae were collected for in vitro experiments from Chinese women with uncomplicated pregnancies (Table 1B).
Table 1. Clinical characteristics of participants in the study.
| A (n=50) Ex vivo biopsies |
B (n=9) In vitro culture |
|||
|---|---|---|---|---|
| Maternal | Non-GDM (n=26) | GDM (n=24) | Non-GDM | |
| Age (years) | 32.7 (4.1) | 33.0 (3.5) | 33 (2.8) | |
| BMI (kg/m2) | 23.5 (3.1) | 28.0 (5.8) | 20.9 (1.7) | |
| Ethnicity n (%) |
Chinese | 17 (65.4%) | 10 (42%) | 9 (100%) |
| Non-Chinese Asiana | 9 (34.6%) | 14 (58.3%) | --- | |
| Parity n (%) |
0 | 3 (11.5%) | 4 (16.6%) | 4 (44%) |
| 1 | 17 (65.4%) | 16 (66.7%) | 5 (56%) | |
| 2 or more | 6 (23.1%) | 4 (16.7%) | --- | |
| Glycemiab (mmol/L) | Fasting | 4.3 (0.3) | 4.9 (0.3) | 4.4 (0.3) |
| One-hour | 7.5 (0.8) | 10.7 (1.4) | 8.2 (1.1) | |
| Two-hour | 5.8 (0.9) | 8.8 (1.5) | 6.9 (1.1) | |
| Neonatal | … | … | … | |
| Gestation at Delivery (Days) | 270.1 (3.7) | 269.3 (3.9) | 269 (3.5) | |
| Sex – Male, n (%) | 19 (73%) | 11 (46%) | 4 (44%) | |
| Birthweight, grams | 3238.2 (297.2) | 3389.5 (282.6) | 3118.3 (313.2) | |
| Customized Birthweight Percentilec | 53.5 (23.6) | 67.4 (21.3) | 54.4 (25.7) | |
Values represent Mean (SD) or Number (%).
Indian or Malay
75g three-time point oral glucose tolerance test conducted at mid-gestation
Birthweight percentile customized for maternal BMI, parity, ethnicity, gestational age and neonatal sex (27)
Abbreviations: BMI, Body Mass Index
All participants conceived spontaneously, were self-declared non-smokers and, as part of universal screening, had undergone a 75g oral glucose tolerance test (OGTT) after an overnight fast at mid-gestation. GDM was diagnosed by a fasting glucose ≥5.1 mmol/L and/or 1h glucose ≥10.0 mmol/L and/or 2h glucose ≥8.5 mmol/L, according to World Health Organization (WHO) 2013 criteria (26). Those with pre-existing diabetes were excluded. Details of maternal and neonatal characteristics were obtained from medical records. Customized birthweight percentile for maternal ethnicity, BMI, parity, fetal sex and gestational age was derived (27).
Placental Tissue Collection
Multiple random biopsies of villous placental tissue (excluding chorionic plate and decidua) were collected within 10min of delivery, snap-frozen in liquid nitrogen and stored at -80ºC. Five frozen biopsies from each placenta were later pooled for crushing in liquid nitrogen for further use.
Extraction and Measurement of Inositol from Placental Tissue
Inositol was extracted by three methods to isolate different inositol fractions. Method 1: To extract water-soluble inositol (including naturally-free inositols and heat-labile water-soluble inositol derivatives), 250mg of crushed placenta was homogenized in 1ml water for 2 min, then boiled (100ºC) for 2 min and centrifuged (12,000 rpm, 10 min). Method 2: A larger fraction of water-soluble inositols (including free inositols and inositols released from within organelles and intracellular vesicle pools by detergent action, or from inositol derivatives sensitive to base-catalyzed hydrolysis) was extracted by homogenizing in 1ml RIPA buffer (Sigma, R0278, MO) for 2 min before centrifugation, as previously described (28). Method 3: For total inositol extraction (comprising all water-soluble and lipid-conjugated forms) (29), 500mg of crushed placenta was hydrolyzed in 3ml of 6M Hydrochloric Acid (VWR International, 20252420, Solon, OH) at 100ºC for 48h. After hydrolysis, 3ml chloroform–methanol (2:1; Merck, 102445, 106009, Darmstadt, Germany) was added, then vortexed and centrifuged (2500 x g, 20 min). For the inositol assay, 25µl of supernatant from water-soluble extracts (water-only and RIPA) and 50μl of the aqueous phase from the Acid-H extract was used.
The Megazyme® kit (Dublin, Ireland), a colorimetric enzymatic assay for inositol detection, was used following manufacturer’s guidelines with minor modifications, as described previously (28). Briefly, the resultant formazan from the oxidation of free inositol was spectrophotometrically measured at λ492nm. Assays were done in duplicates. Standard curves were generated by spiking pooled placental samples with myo-inositol (0, 62.5µg, 125µg; >99% purity; Sigma-Aldrich, I7508, St Louis, MO) to calculate absolute amounts and for standardization across runs, and expressed as µg inositol per gram of wet placental tissue weight (µg/g).
Placental Explant Culture
Tiny biopsies (3x3x3mm) randomly collected from across the villous placenta (n=9) were cultured in serum-free CMRL media (containing 5mM glucose and 0.3μM myo-inositol; Thermo Fisher Scientific, #11530037, Carlsbad, CA) with addition of (i) increasing concentrations of myo-inositol (Sigma-Aldrich, I7508, MO) of 0, 10, 30, 100 μM, corresponding to almost none, deficient and normal maternal plasma concentrations, and normal fetal plasma concentration, respectively, and (ii) increasing concentrations of glucose (Biowest, P5030, France) of final concentrations of 5, 10, 17 mM, corresponding to normal, high and very high maternal plasma concentrations. Experiments were conducted in triplicates in 6-well plates. After 48h of treatment, explants from triplicate wells were combined for storage at -80ºC after washing in phosphate buffered saline and centrifugation (12,000 rpm, 15 min).
Quantitative Taqman RT-PCR (qRT-PCR)
To quantify mRNA expression, total RNA was extracted by homogenization in TRIzol® Reagent (Gibco, Thermo Fisher Scientific, CA) following manufacturer’s guidelines. Total RNA was treated with DNase (Qiagen RNeasy® Mini kit, 74106, RNase-Free DNase set QIAGEN, 79254, Hilden, Germany) and then 1µg was reversed transcribed in a 20µl reaction (Superscript ®III First-Strand Synthesis System, 18080051 Invitrogen™, Life Technologies, CA). Primers and probes (Applied Biosystems™ TaqMan® Assays, Pleasanton, CA) for three enzymes IMPA1 [Inositol Monophosphatase-1; Hs04188597], ISYNA1 [Inositol-3-Phosphate Synthase-1; Hs01126940], MIOX [Myo-inositol oxygenase; Hs00367743], three inositol solute carrier transporters SLC5A3 [Hs00272857], SLC5A11 [Hs01086559], SLC2A13 [Hs00369423] and three control genes SDHA [Succinate dehydrogenase complex, subunit A; Hs00188166], TBP [TATA-Box binding protein; Hs00427620], CYC1 [Cytochrome C1; Hs00357718] were used in singleplex qRT-PCR reactions (2µl cDNA per reaction) using the Applied Biosystems®7500 Fast Real-Time PCR System (Thermo Fisher Scientific). Relative expression of each gene was calculated by the formula 2(-ΔCT) and normalized to the geometric mean of expression of the three control genes (30).
Western Immunoblotting
Protein assessments were made in a representative subset of frozen ex vivo biopsies selected from across the fasting glycemic range (n=12), and in a subset of experiments of cultured explants (n=4). Total protein was prepared by homogenizing the tissue in RIPA buffer (Sigma, R0728, St. Loius, MO) with Halt™ Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific, 1861281, Rockford, IL). Protein concentration was quantified using Pierce™ bicinchoninic acid kit (Pierce Biotechnology, 23227, IL). Protein samples (30µg) were resolved using 10% SDS-PAGE and transferred to a 0.2µm PVDF membrane (Amersham, 10600021, Germany). Blocking was done in 5% non-fat dry milk in 1X Tris Buffered Saline with 0.05% Tween®20 at room temperature. The membranes were incubated overnight at 4ºC in primary antibodies to IMPA1 (rabbit monoclonal, 1:20,000 for ex vivo biopsies, 1:40,000 for explants, Abcam, ab184165), ISYNA1 (rabbit polyclonal, 1:2000, Abcam ab118241), SLC5A3 (rabbit polyclonal, 1:250, Thermo Fisher Scientific, PA5-51062), SLC5A11 (rabbit polyclonal, 1:1000, Proteintech, 14089-1-AP) and SLC2A13 (rabbit polyclonal, 1:750, Thermo Fisher Scientific, PA5-76466), followed by horseradish peroxidase; conjugated secondary antibody (goat anti-rabbit IgG,1:3000, Thermo Fisher Scientific, 31460) for 1h at room temperature. As a negative control, non-immune rabbit IgG (Santa Cruz, sc2027) in place of primary antibodies was used (data not shown). Blots were processed using Enhanced Chemiluminescence substrate (Bio-Rad, Clarity Max™ Western ECL substrate, 1705062, Italy) and developed on film. As loading control, β-actin (mouse monoclonal, 1:1000, Santa Cruz, sc47778 or 1:10,000 Sigma Aldrich A5441) immunoblotting was used for the ex vivo biopsies, and total protein quantified by Ponceau S (Sigma, P3504) was used for the explants. Relative densitometry was performed using Image J software (NIH).
Statistical Analysis
A sample size of fifty has 90% power (at alpha 5%) to detect a 10% difference in placental inositol content between 2 groups assuming 15% SD in mean levels. Comparisons of GDM versus non-GDM groups used t-test (parametric data) or Mann-Whitney U (non-parametric data) for continuous variables with Graph Pad Prism (v7.0). Spearman’s correlation was used to compare results from the three different inositol extractions. Multiple linear regression using Stata (v15.0) was performed to assess the associations between parameters with adjustments for important covariates and factors found to differ between the GDM and non-GDM groups. Maternal age, BMI, glycemia and gestational age at delivery were used as continuous variables, and ethnicity categorized as Chinese or non-Chinese Asian. Expression data from in vitro experiments was logarithmically transformed before statistical analysis using a two-way ANOVA and Tukey’s multiple comparison post-hoc test with Graph Pad Prism (v7.0). Statistical significance was taken at P<0.05.
Results
Participant Characteristics
GDM cases showed significantly higher fasting, 1h and 2h glycemia (all P<0.0001) in a mid-gestation OGTT compared with the non-GDM cases (Table 1A). Maternal age, parity and gestational age at delivery were similar between groups. However, those with GDM had higher BMI (P=0.0017), were predominantly non-Chinese Asian (P=0.001), delivered fewer male neonates (P=0.0001), and showed trends of a slightly higher mean birthweight (P=0.072) and customized birthweight percentile (P=0.051). Among GDM cases, 29% were treated with insulin while the rest were diet-controlled. All the samples used for in vitro culture were from Chinese participants with normal BMI and normoglycemia (Table 1B).
Placental Inositol Content
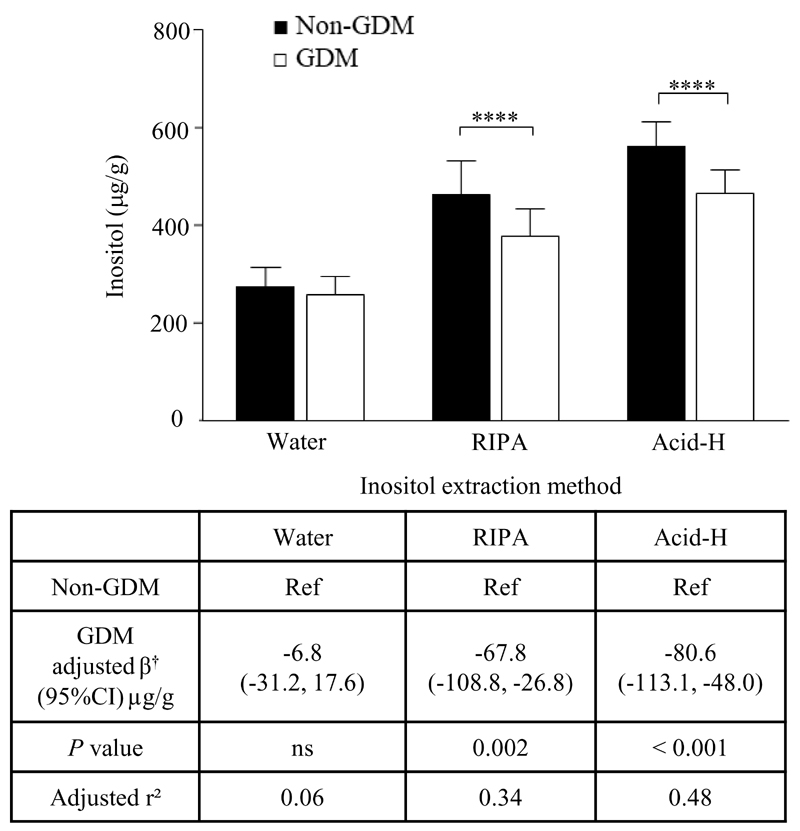
Placental inositol content was measured in three different types of inositol extracts. Overall, results showed good correlation between the inositol extracts (correlation co-efficient Rho: water-only vs RIPA 0.40, P=0.005; water-only vs Acid-H 0.48, P<0.0001; RIPA vs Acid-H 0.61, P<0.0001). In general, water-soluble inositols extracted from the water-only and RIPA methods constituted about 52% and 82%, respectively, of total inositols (water-soluble and lipid-conjugated) extracted by the Acid-H method.
When comparing GDM with non-GDM placentae, there was a significant reduction in placental inositol content in GDM, with differences in inositol partitioning. There were no differences in placental inositol content between the insulin-treated and diet-controlled GDM cases. Total inositol content was [mean (SD)] 464.9μg/g (47.8) in GDM placentae compared with 559.5μg/g (52.1) in non-GDM placentae, representing a 17% reduction in GDM (Figure 1). After adjustment for covariates, GDM placenta still showed significantly lower inositol content in RIPA (15% reduction) and Acid-H (14% reduction) extracts. The inositol fraction that accounted for most of this reduction was the additional water-soluble pool extracted by RIPA but not by water-only methods. This additional water-soluble pool (by RIPA) amounted to 118.8μg/g (47.3) in GDM compared with 187.2μg/g (75.0) in non-GDM placentae (P=0.0003), representing a 37% reduction in this fraction. Meanwhile, GDM placenta maintained a similar absolute amount of inositol in the water-only-extracted fraction, such that this fraction constituted a disproportionately higher percentage of the total placental inositol in GDM [55.6% (7.6)] compared with non-GDM [49.3% (6.2); P=0.003] cases.
Fig. 1. Placental inositol content.
Comparisons of inositol content in human term placental tissue between non-GDM (n=26; black bars) and GDM (n=24; white bars) pregnancies, quantified following extractions by three different methods: water-only, RIPA, acid hydrolysis (Acid-H). Graph data shows mean (SD), unadjusted, **** p≤0.0001 by Mann Whitney U. Table shows multiple linear regression †adjusted for maternal age, body mass index, ethnicity (Chinese/Non-Chinese), gestational age at delivery and neonatal sex. Abbreviations: GDM, gestational diabetes mellitus; Ref, reference; ns, not significant.
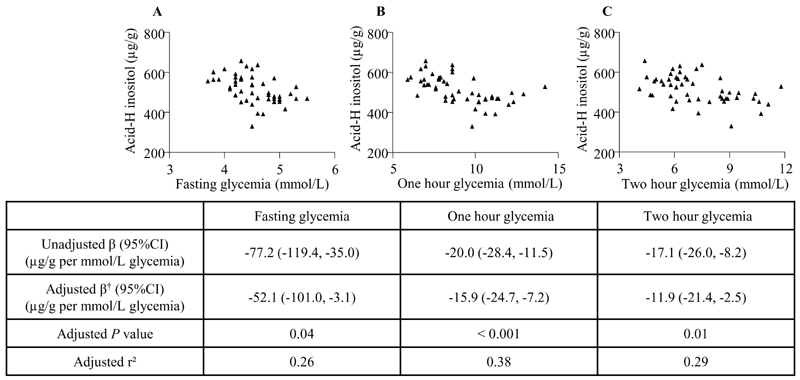
As a continuum across the glycemic range, total placental inositol was negatively associated with mid-gestation fasting, 1h and 2h glycemia assessed by OGTT, with and without covariate adjustment (Figure 2). The strongest association was observed for fasting glycemia. Adjusted r-squared values suggest that 26% of the variance in placental inositol content may be attributed to differences in fasting glycemia. Inositols in RIPA extracts also showed negative associations with fasting [adjusted β -86.1 (95%CI -137.6, -34.5)μg/g inositol per mmol/L glucose increment; P=0.002], 1h [-12.8 (-23.4, -2.2) μg/g/mmol/L; P=0.019] and 2h [-13.8 (-24.4, -3.3) μg/g/mmol/L; P=0.011] glycemia.
Fig. 2. Association of glycemia with total placental inositol content.
Associations of maternal fasting glycemia (A), one hour glycemia (B), and two hour glycemia (C) as assessed in a mid-gestation oral glucose tolerance test (n=50) with total placental inositol extracted by acid hydrolysis (Acid-H). Graphs show unadjusted data. Table shows multiple linear regression †adjusted for maternal age, body mass index, ethnicity (Chinese/Non- Chinese), gestational age at delivery and neonatal sex.
In unadjusted analysis, placental inositol was also negatively correlated with maternal BMI [unadjusted β -5.6 (-9.2, -2.0)μg/g per kg/m2; P=0.003], but after adjustment for covariates no significant association was observed, with maternal fasting glycemia being the main factor accounting for the BMI-associated change in placental inositol.
Association of Glycemia with expression of Inositol Enzymes and Transporters
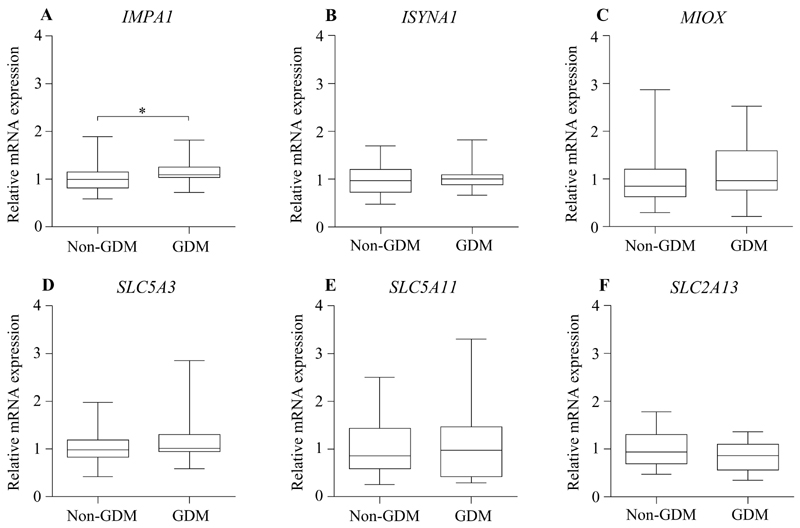
We explored if the decline in placental inositol content with maternal glycemia could be associated with alterations in the local placental expression of enzymes that synthesize (IMPA1, ISYNA1) or catabolize (MIOX) inositol, and of plasma membrane inositol transporters (SLC5A3, SLC5A11, SLC2A13). In frozen ex vivo biopsies, IMPA1 mRNA was higher in GDM placenta compared with non–GDM placenta in unadjusted analysis (Figure 3), but this difference was abolished following adjustment for maternal age, BMI, ethnicity, gestational age and neonatal sex.
Fig. 3. Expression of enzymes in inositol metabolism and inositol plasma membrane transporters in ex vivo biopsies.
Relative mRNA expression of enzymes involved in inositol metabolism (A: IMPA1, B: ISYNA1, C: MIOX) and inositol plasma membrane transporters (D: SLC5A3, E: SLC5A11, F: SLC2A13) in human placental tissue obtained from non-GDM (n=26) and GDM (n=24) pregnancies, quantified by Taqman RT-PCR without adjustments for covariates. Mean expression in non-GDM placenta has been assigned a value of 1. Figures show median, interquartile range (box) and 95% confidence interval (whiskers). *P<0.05. Abbreviations: GDM, gestational diabetes mellitus.
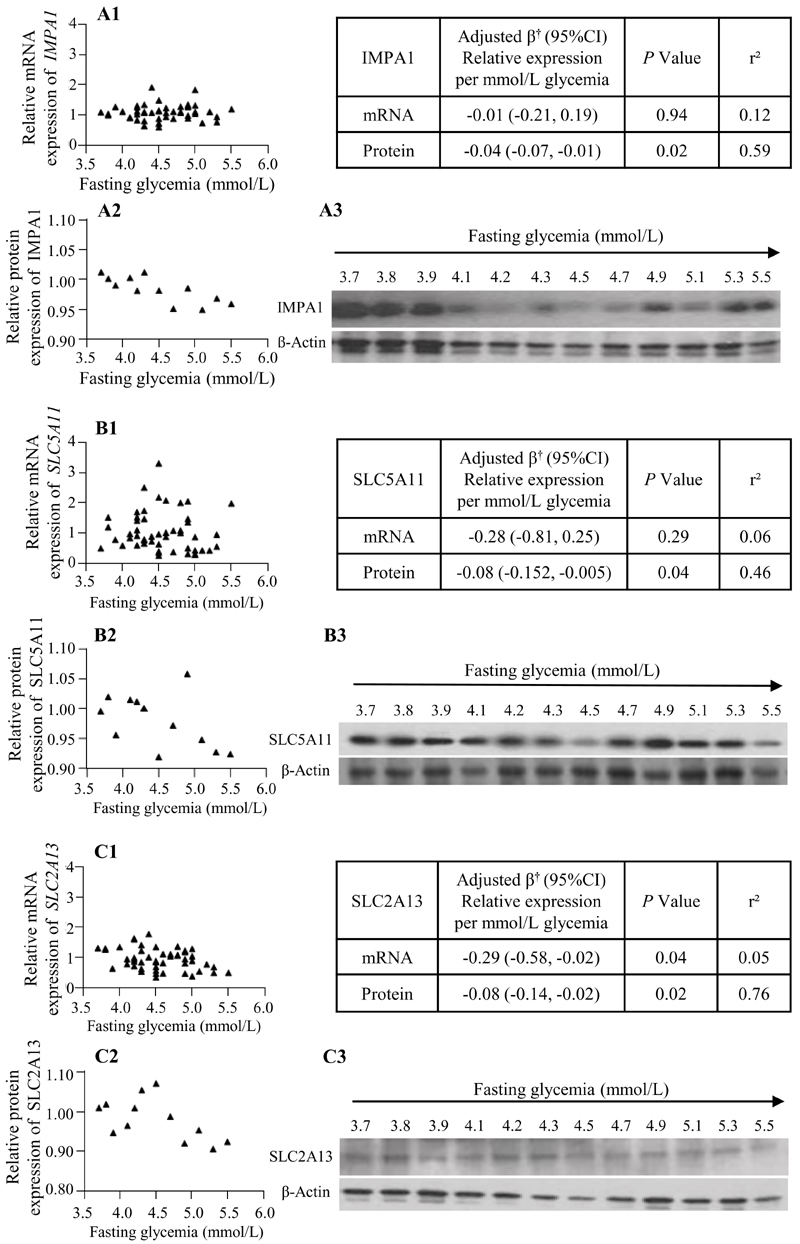
When considering maternal fasting glycemia and relative mRNA expression as continuous variables, we found a negative association between glycemia and SLC2A13 mRNA expression (Figure 4,C1). This relationship was replicated in a representative subset (n=12) at the protein level (Figure 4,C2-C3). Additionally, the protein, but not the mRNA, expression of IMPA1 and SLC5A11 showed a negative association with maternal fasting glycemia (Figure 4, A2, B2). For the other genes, no significant associations with fasting glycemia was observed [Supplementary Figure 1 (31)]. There was also no association between maternal 1h and 2h glycemia with the expression of any of the genes tested.
Fig. 4. Association of glycemia with expression of inositol enzymes and transporters.
Associations between maternal fasting glycemia and relative mRNA expression (A1, B1, C1; n=50) or protein expression (A2, B2, C2; n=12) of IMPA1 (A), SLC5A11 (B) and SLC2A13 (C). Graphs show unadjusted data; mean mRNA and protein expression in non-GDM placenta has been assigned a value of 1. Tables show multiple linear regression †adjusted for maternal age, body mass index, ethnicity (Chinese/Non-Chinese), gestational age at delivery and neonatal sex; β represents fold-change relative to mean expression in non-GDM placenta for each mmol/L increase in fasting glycemia. Western immunoblots demonstrate protein expression relative to β-actin (A3, B3, C3) across the fasting glycemia range (mmol/L).
Association of Inositol Enzymes and Transporters with Placental Inositol Content
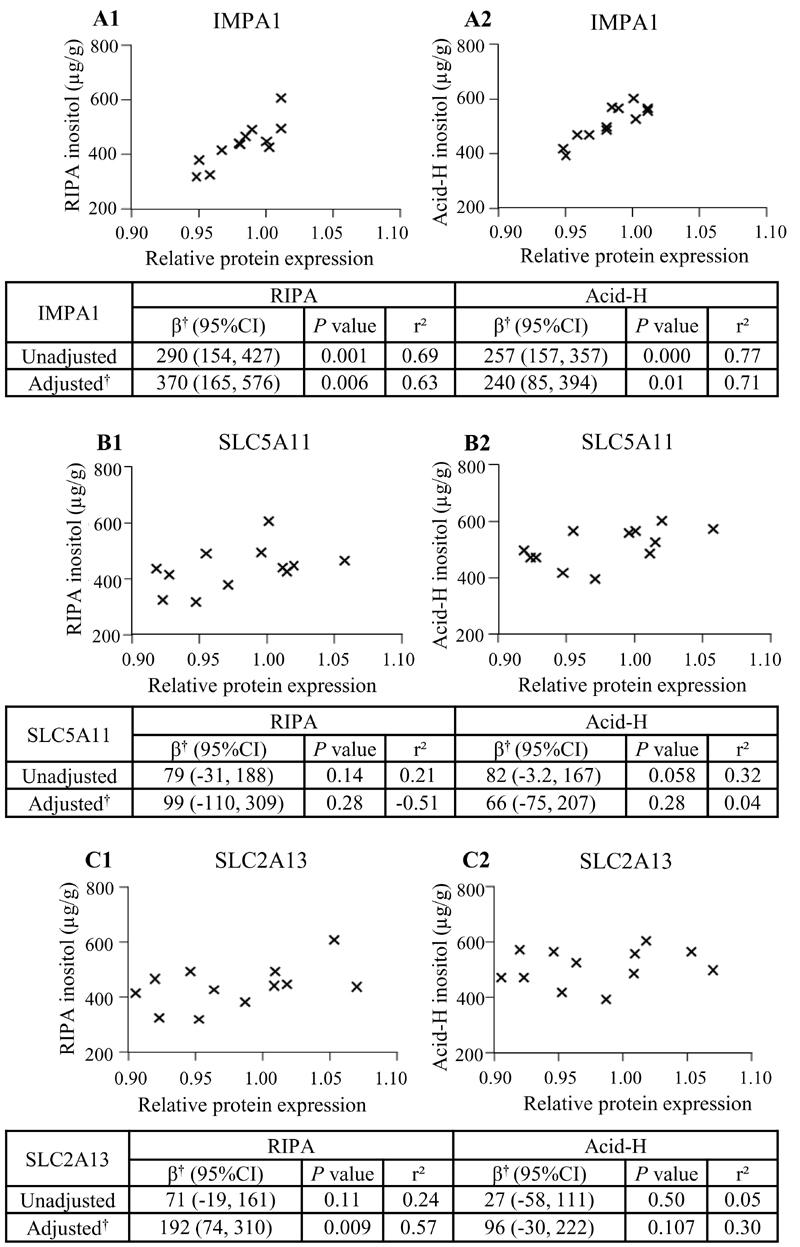
There was a strong association between IMPA1 protein expression and placental inositol content measured in RIPA and Acid-H extracts, in both unadjusted and adjusted analyses (Figure 5A). Meanwhile SLC2A13 protein expression was associated with RIPA-extracted placental inositol after adjustment for covariates (Figure 5C) with maternal BMI having the most influence in the model. There was also a trend of an association between SLC5A11 protein expression and placental inositol in both RIPA and Acid-H extracts, which became less statistically significant following adjustment for covariates (Figure 5B). However, there were no significant associations seen between inositol transporter and enzyme protein expression with placental inositol quantified in water-only extracts (data not shown).
Fig. 5. Placental inositol content in association with protein expression of inositol enzymes and transporters.
Associations between relative protein expression of IMPA1 (A), SLC5A11 (B) and SLC2A13 (C) with placental inositol extracted using RIPA buffer (A1, B1, C1) and by acid hydrolysis (Acid-H) (A2, B2, C2) (n=12). Graphs show unadjusted data; mean protein expression in non-GDM placentae has been assigned a value of 1. Tables show unadjusted regression analyses and multiple linear regression; *represents μg/g increase in placental inositol with 0.1-fold change increase in relative protein expression. † Adjusted for maternal age, body mass index, ethnicity (Chinese/Non-Chinese), gestational age at delivery and neonatal sex.
Changes in Expression of Inositol Enzymes and Transporters with in vitro treatment of placental explants with glucose and myo-inositol
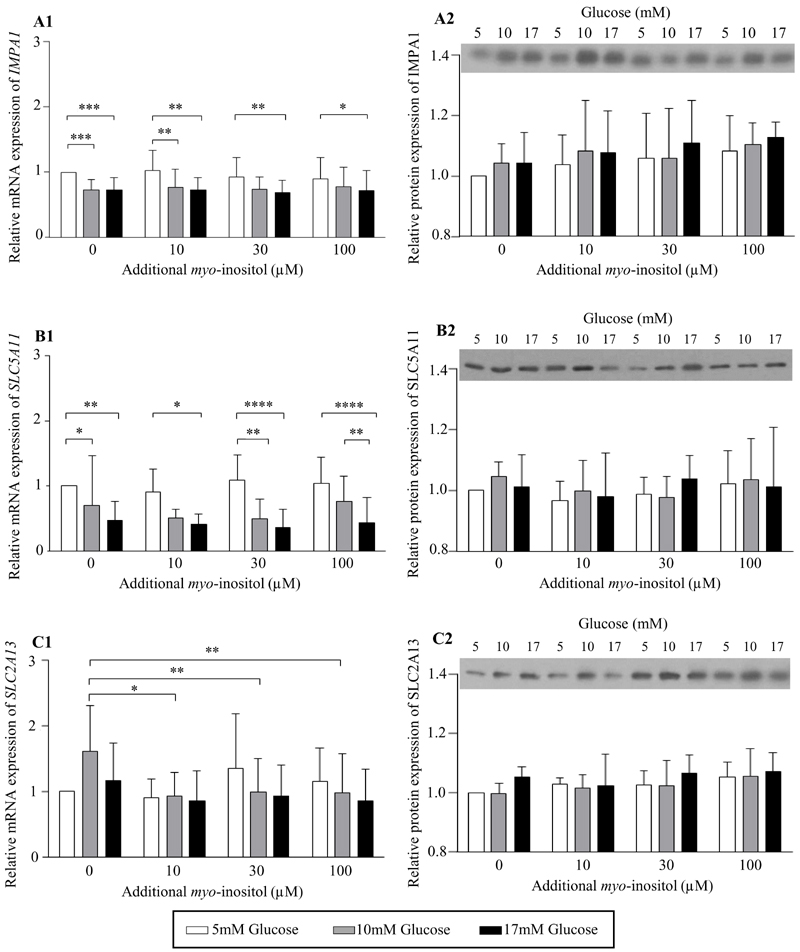
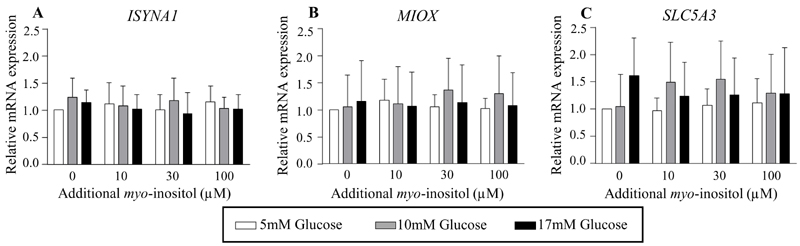
To assess if glucose plays a direct causal role in regulating placental expression of inositol enzymes and transporters, and to assess if this could be modulated by the level of myo-inositol exposure, placental explants were cultured in increasing concentrations of glucose and myo-inositol in vitro. Treatment with glucose suppressed the mRNA expression of the myo-inositol synthesis enzyme, IMPA1 (ANOVA effect of glucose P<0.0001; Figure 6A1), and the transporter, SLC5A11 (ANOVA effect of glucose P=0.0011; Figure 6B1). Compared with 5mM glucose, treatment with 10mM glucose reduced IMPA1 mRNA expression by 17-20%, with no further reductions observed with 17mM glucose. While a consistent decrease in IMPA1 mRNA expression was observed with 10mM glucose especially at lower myo-inositol exposures, the decrease with 17mM glucose was observed across the whole range of concentrations of myo-inositol. Expression of SLC5A11 mRNA demonstrated a clearer dose-dependent decrease with increasing glucose concentrations from 5mM to 17mM, particularly with exposure to higher myo-inositol concentrations (Figure 6B1). The most marked decreases in SLC5A11 mRNA expression were seen at the maternal physiological concentration of myo-inositol (additional 30μM) with a reduction of 51% with 10mM glucose and 64% with 17mM glucose compared with 5mM glucose. At the highest concentration of 100μM additional myo-inositol, a reduction of 43% was observed between 10mM and 17mM glucose treatment. In contrast, the mRNA expression of SLC2A13 showed no significant differences with increasing glucose treatment but instead demonstrated a significant reduction with additional myo-inositol treatment (ANOVA effect of myo-inositol P=0.022; Figure 6C1). Compared with no additional myo-inositol, exposure to 10, 30 and 100 μM additional myo-inositol reduced SLC2A13 mRNA expression by 39-42% when administered in conjunction with 10mM glucose treatment. There were no differences observed in the mRNA expression of the other genes (ISYNA1, MIOX, SLC5A3) with glucose and myo-inositol treatment (Figure 7). There were no sex differences in the expression of all these genes.
Fig. 6. Expression of inositol enzymes and transporters with glucose and myo-inositol treatment of placental explant cultures in vitro.
Relative mRNA expression of the enzyme involved in inositol synthesis, IMPA1 (A1), and the inositol plasma membrane transporters, SLC5A11 (B1), SLC2A13 (C1), quantified by Taqman RT-PCR in human placental explants (n=9) cultured in increasing concentrations of glucose of 5mM (white bars), 10mM (grey bars), 17mM (black bars), and of additional myo-inositol (0, 10, 30, 100µM) for 48h, without adjustment for covariates. The mean expression of explants treated with 5mM glucose and 0μM additional myo-inositol were assigned the arbitrary value of 1. Data was log transformed for statistical analyses. *P<0.05, **P<0.01, ***P<0.001. Representative western immunoblots demonstrating protein expression of IMPA1 (A2), SLC5A11 (B2) and SLC2A13 (C2) at 48h. Relative densitometry showing mean protein expression (relative to the total protein loading quantified by Ponceau S; Figure 8), normalized to the result of the corresponding explant treated with 5mM glucose and 0μM myo-inositol within each explant experiment (n=4; A2, B2, C2). Bars and error bars represent mean and standard deviations.
Fig. 7. Relative mRNA expression of other enzymes in inositol metabolism and inositol plasma membrane transporters in cultured placental explants treated with glucose and myo-inositol.
Relative mRNA expression of enzymes involved in inositol synthesis, ISYNA1 (A) (n=9), inositol catabolism, MIOX (B) (n=8), and inositol plasma membrane transport, SLC5A3 (C) (n=8), quantified by Taqman RT-PCR in human placental explants cultured in increasing concentrations of glucose of 5mM (white bars), 10mM (grey bars), 17mM (black bars), and of additional myo-inositol (0, 10, 30, 100µM) for 48h, without adjustment for covariates. The mean expression of explants treated with 5mM glucose and 0μM additional myo-inositol were assigned the arbitrary value of 1.
To examine if the observed changes in mRNA expression were also seen at the protein level, immunoblotting for IMPA1, SLC5A11 and SLC2A13 was done in a random subset of experiments but no significant changes were observed with either glucose or myo-inositol treatment [Figure 6; representative images of protein loading assessed by Ponceau S is shown in Supplementary Figure 2 (32)].
Influence on Birthweight
Given the wide-ranging role that inositol and its derivatives play in multiple cellular functions, including second messenger signaling of hormones such as insulin and IGF, which are known to be important in regulating fetal growth, we explored the potential implications of differences in placental inositol content in relation to birthweight. Placental inositol quantified in RIPA-extracts from ex vivo biopsies demonstrated a weak negative association with customized birthweight percentile (β -0.09% per µg/g inositol, P=0.03, r2=0.09) but no such association was observed for total placental inositol and water-only inositol extracts.
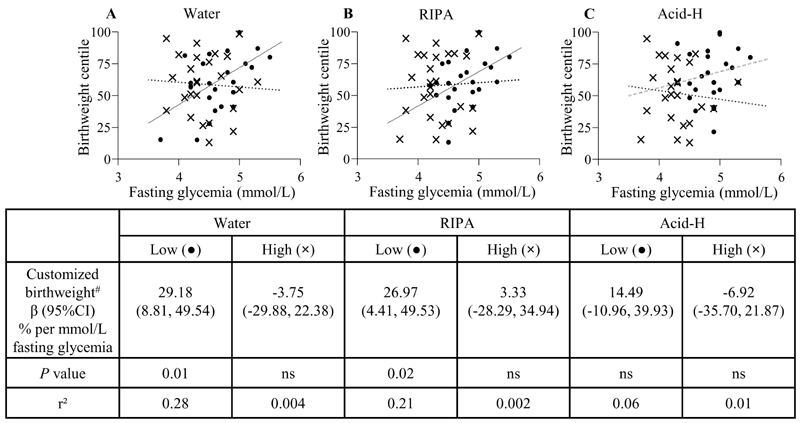
Since fetal size is known to closely correlate with maternal glycemia (33), we next examined the potential modulating influence of placental inositol on the fetal growth-promoting effects of maternal glycemia. In regression models associating fasting glycemia with birthweight, there were significant interactions observed between glycemia and placental inositol on the outcome of customized birthweight percentile for RIPA-extracts (P-for interaction=0.037) and water-only extracts (P-for interaction=0.022), but not for Acid-H. We therefore stratified cases by placental inositol content into two groups, above and below the median for each inositol extract (median values: water-only 265.1µg/g, RIPA 422.3µg/g, Acid-H 502.9µg/g). The expected positive association between maternal fasting glycemia and customized birthweight percentile was observed only among cases demonstrating lower placental inositol content by water-only and RIPA extraction (Figure 8). However, among cases with higher inositol content, classified by any of the three extracts, this positive association between glycemia and birthweight centile was attenuated.
Fig. 8. Glycemia-associated birthweight changes stratified by levels of placental inositol content.
Associations of maternal fasting glycemia and customized birthweight percentile stratified by low placental inositol (dots) and high placental inositol (crosses) groups (n=25 per group) determined by inositol quantification following three different extraction methods: (A) Water, (B) RIPA, (C) Acid-H. #Birthweight percentile already customized for maternal BMI, parity, ethnicity, gestational age and sex (27). Continuous trendlines (—) show statistically significant associations by linear regression while dotted (….) and dashed (---) trendlines indicate non-significant relationships. In (C), the grey dashed (---) trendline represents the low placental inositol group and the black dotted (….) trendline represents the high placental inositol group. Abbreviations: ns, not significant.
Discussion
This novel placental inositol study confirmed our hypothesis that there is a significant reduction in placental total inositol content in GDM, especially in the RIPA-extracted water-soluble inositol fraction. Similarly, there were negative associations between the continuum of maternal antenatal glycemia and these placental inositol pools. Decreased placental inositol in GDM may be partly mediated by glucose-associated downregulation of the local placental expression of IMPA1, that synthesizes myo-inositol, and the inositol plasma membrane transporters SLC5A11 and SLC2A13. Among cases of low placental inositol, increasing maternal glycemia was clearly associated with the promotion of birthweight, a phenomenon that was attenuated in cases of high placental inositol. This suggests that placental inositol may suppress the acceleration of fetal growth due to rising maternal glycemia.
Placental inositol comes from both maternal (34) and fetal circulations (19, 35, 36), as well as from endogenous placental synthesis, but their relative contributions are unknown. Ultimately, tissue levels of inositol would be determined by a balance of systemic inositol bioavailability, local transporter activity and local tissue inositol synthesis and metabolism, as well as the presence of other related saccharides, including glucose, which can either compete with inositol or alter osmolarity. It has been suggested that increased urinary excretion of inositol and possible systemic inositol depletion could lead to GDM (18), and if true, such reduced bioavailability may also partly explain the reduction in placental inositol. Findings of our study suggest that this could be exacerbated further by glycemia-associated downregulation of local placental inositol transport and synthesis. Since differences in placental inositol quantified at the time of delivery was preceded by and closely associated with maternal glucose concentrations assessed approximately 10 weeks prior to delivery, it is plausible that increasing glycemia could have played a role in reducing placental inositol.
We specifically focused on the expression of the three main inositol transporters that regulate placental inositol influx down a sodium or proton gradient, and the expression of enzymes involved in metabolism of myo-inositol, the main isomer in placenta (28). Although previous studies (37, 38) demonstrated upregulation of placental SLC5A3 [a sodium-inositol symporter, SMIT1] in GDM, we observed no such change, either with GDM/increasing glycemia in the frozen ex vivo biopsies, or in placental explants treated with glucose. Discrepancy in findings could be due to population differences. Novel findings from our study showed that the protein expression of SLC5A11 (SMIT2) and SLC2A13 (proton-inositol symporter, HMIT), and IMPA1 (myo-inositol synthesis), were downregulated in association with increasing maternal glycemia. A direct causal role of glucose in the regulation of expression of these genes is suggested by our findings in placental explants where a downregulation in IMPA1 and SLC5A11 mRNA expression was observed with increasing glucose treatment in vitro. Changes in IMPA1, SLC5A11 and SLC2A13 expression with glucose treatment or glycemia have never been reported before in placenta nor in any other tissue. However, the in vitro changes at transcript level were not reflected at the protein level possibly because of longer protein turnover such that 48h of culture might not provide sufficient length of time to allow potential changes in protein to be detected. Whereas in ex vivo biopsies maternal glycemia associated with changes in protein expression of these genes but not at the transcript level. This lack of concordance may be due to differences in responses to short-term and chronic exposure to glucose as well as in the presence of other potential co-regulators in vivo which might be absent in in vitro culture conditions. Indeed, opposing effects of acute and chronic glucose exposure on SMIT1 expression have been reported in cultured rat pancreatic islets (39). Additionally, the discrepancy between mRNA and protein expressions of IMPA1 and SLC5A11 in the ex vivo biopsies suggest that regulation by glucose may also occur at a post-transcriptional level in vivo.
Results of a reduction in SLC2A13 mRNA expression with myo-inositol treatment may suggest some autoregulation of placental myo-inositol content, however, this reduction was only observed in a high glucose environment of 10mM and also not observed at the protein level. Both glucose and myo-inositol thus appear to be involved in the regulation of local transporters that may affect placental inositol content. It should be noted that all the placental explants cultured were obtained from women with normal BMI and could not have been “programed” by factors associated with a high maternal BMI. Thus findings of glucose-induced changes in placental IMPA1 and SLC5A11 mRNA expression cannot be confounded by increased maternal BMI, further supporting our postulation that maternal glycemia is the main factor driving the reduction in placental inositol content.
Associations between IMPA1, SLC2A13 and SLC5A11 protein levels, with placental inositol content in ex vivo biopsies support the idea that local regulation of inositol metabolism and uptake are major determinants of placental inositol content. However, our sample size did not permit the performance of mediation analysis to determine whether the reduction in placental inositol with increasing maternal glycemia is actually mediated by a glucose-induced decline in IMPA1, SLC2A13 and SLC5A11 expression.
The biological implications of reduced placental inositol content in GDM are unclear. Our findings suggesting that a higher placental inositol content may attenuate the fetal growth-promoting effects of maternal glycemia are consistent with our previous report from the Singapore GUSTO mother-offspring cohort (40). This supports the prospect of using inositol supplementation during pregnancy to increase placental inositol and reduce the risk of fetal macrosomia. Indeed, a meta-analyses of three clinical trials of myo-inositol supplementation from early pregnancy showed a reduced incidence of GDM in high risk populations (22, 41, 42) as well as reduced odds of macrosomia (43). One presumed mechanism being a direct insulin-mimetic effect of the supplement on maternal tissues to reduce glycemia and hence transplacental glucose supply, and now our findings suggest another possible mechanism being a direct impact on placental function to suppress glycemia-induced fetal growth. The relative importance of these mechanisms to fetal growth regulation remains to be determined. It also remains unclear if maternal inositol supplementation can increase placental inositol content per se, nor how placental function may be altered. Possible mechanisms may include the inositol regulation of placental fatty acid uptake, metabolism and supply to the fetus, as suggested by in-vitro placental studies (44). However, we cannot exclude the possibility that increased placental inositol content is reflective of increased fetal inositol production, which may itself modulate fetal growth independently of placental function.
We used different extraction methods in attempt to distinguish potentially different roles played by various pools of water-soluble and lipid-conjugated inositols and inositol derivatives in placenta. The final common step of inositol measurement using the Megazyme® assay would only quantify free inositols released during the extraction process. The water-only extracts would contain naturally-free inositols and inositols released from heat-labile water-soluble inositol derivatives. We postulate that treatment with RIPA-buffer would, in addition, liberate membrane-partitioned water-soluble inositols and inositol derivatives by detergent-disruption of organelles, vacuoles and vesicles, as well as the release of more inositols in free-form from the breakdown of water-soluble inositol derivatives sensitive to base-catalyzed hydrolysis. These additional water-soluble inositol derivatives may comprise key components of transmembrane signaling and second messenger pathways (e.g. IP, IPG) that regulate important cellular functions. Meanwhile the Acid-H extracts would capture all inositols released from both water-soluble inositol derivatives and lipid-conjugated inositols; the latter includes lipid-bound signaling compounds such as PIP and PI3K.
Our finding that it was the water-soluble fraction that was additionally extracted by the RIPA method (over and above the water-only method) that were most depleted in GDM placentae suggests that differences in this fraction may primarily mediate any observed glycemia-inositol related effects. Indeed, the strongest associations between the protein expression of IMPA1 and SLC2A13 with placental inositol was with the RIPA-extracted fraction followed by total inositol extracts. In contrast, we found that only extracts containing the water-soluble fractions (water-only and RIPA), and not total inositol (water-soluble and lipid-bound), were associated with attenuation of glycemia-associated fetal growth. Nonetheless, despite the absolute amounts of inositol in the water-only extracts being similar in GDM and non-GDM placentae, the overall mean customized birthweight percentile was still slightly higher in the GDM group, albeit not statistically significant. This, and the finding that only the RIPA-extracted inositols displayed an overall negative association with customized birthweight, suggest that the additional RIPA-extracted water-soluble inositol fraction is still likely to play the most important role in the modulation of fetal size.
Study strengths include robust placental collection protocols from elective cesarean sections only to optimize tissue preservation and the use of three different inositol extraction methods which has highlighted the importance of considering different inositol pools in the interpretation of results from tissue studies. A limitation is the use of the Megazyme® assay that is unable to resolve the different inositol isomers. This assay quantifies myo-, d-chiro-, epi-, allo- inositols but not scyllo-inositol. However, this is unlikely to compromise results since myo-inositol is by far the most predominant isomer in human placenta (28). We were also unable to characterize the inositol derivatives represented in the different inositol extracts. Analysis of mixed cell placental biopsies cannot determine cellular specificity with regard to inositol concentrations and localization of gene expression. Our sample size was relatively small, thus only adjustment for a limited number of covariates was possible. Maternal and fetal circulating levels of inositol were not measured so their relative contribution to placental inositol content cannot be ascertained. There is probably other unmeasured confounding, such as maternal dietary variations, involved in the regulation of placental inositol biology. Even though our findings showed no clear differences between the Chinese and non-Chinese Asians, results may not be generalizable to populations of other ethnicities.
In conclusion, placental inositol content is reduced in GDM and with increasing maternal glycemia. This may be partly mediated by glucose-induced downregulation in placental inositol synthesis and import. Further research is warranted to establish if reduced placental inositol is indeed permissive to fetal growth acceleration with maternal hyperglycemia, and if this could be addressed by inositol supplementation.
Supplementary Material
Acknowledgements
The authors acknowledge Celes Maria Catherine Dado, Samantha Grace Loon Magadia and Chen Zhenzhi in administration and recruitment, and staff of the National University Hospital who assisted with placental collection.
Funding
This research is supported by Singapore National Medical Research Council [(Clinician Scientist Award to SYC (NMRC/CSA-INV/0010/2016)], National University of Singapore, and Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research. KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042) and the NIHR Southampton Biomedical Research Centre) and the European Union (Erasmus+ Programme Early Nutrition eAcademy Southeast Asia-573651-EPP-1-2016-1-DE-EPPKA2-CBHE-JP).
Footnotes
Disclosure Statement
SYC and KMG are part of an academic consortium that has received research funding from Société Des Produits Nestlé S.A., Abbott Nutrition, Danone and Benevolent AI Bio Ltd outside the submitted work, and are co-inventors on patent filings by Nestlé S.A., which cover the use of inositol in human health applications. These patents do not draw on results reported in this manuscript. KMG and SYC have received reimbursement from Nestle for speaking at conferences. The other authors have no conflict of interest to declare.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1.Noventa M, Vitagliano A, Quaranta M, Borgato S, Abdulrahim B, Gizzo S. Preventive and Therapeutic Role of Dietary Inositol Supplementation in Periconceptional Period and During Pregnancy: A Summary of Evidences and Future Applications. Reprod Sci. 2016;23(3):278–88. doi: 10.1177/1933719115594018. [DOI] [PubMed] [Google Scholar]
- 2.Holub BJ. Metabolism and function of myo-inositol and inositol phospholipids. Annu Rev Nutr. 1986;6:563–97. doi: 10.1146/annurev.nu.06.070186.003023. [DOI] [PubMed] [Google Scholar]
- 3.Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93(3):1019–137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamazaki H, Zawalich KC, Zawalich WS. Physiologic implications of phosphoinositides and phospholipase C in the regulation of insulin secretion. J Nutr Sci Vitaminol (Tokyo) 2010;56(1):1–8. doi: 10.3177/jnsv.56.1. [DOI] [PubMed] [Google Scholar]
- 5.Croze ML, Soulage CO. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie. 2013;95(10):1811–27. doi: 10.1016/j.biochi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Daughaday WH, Larner J. The renal excretion of inositol in normal and diabetic human beings. J Clin Invest. 1954;33(3):326–32. doi: 10.1172/JCI102901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong JH, Jang HW, Kang YE, Lee JH, Kim KS, Kim HJ, et al. Urinary chiro- and myo-inositol levels as a biological marker for type 2 diabetes mellitus. Dis Markers. 2012;33(4):193–9. doi: 10.3233/DMA-2012-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung TS, Hahm JR, Kim JJ, Jung JH, Kang MY, Moon SW, et al. Determination of urinary Myo-/chiro-inositol ratios from Korean diabetes patients. Yonsei Med J. 2005;46(4):532–8. doi: 10.3349/ymj.2005.46.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki S, Kawasaki H, Satoh Y, Ohtomo M, Hirai M, Hirai A, et al. Urinary chiro-inositol excretion is an index marker of insulin sensitivity in Japanese type II diabetes. Diabetes Care. 1994;17(12):1465–8. doi: 10.2337/diacare.17.12.1465. [DOI] [PubMed] [Google Scholar]
- 10.Cogram P, Tesh S, Tesh J, Wade A, Allan G, Greene ND, et al. D-chiro-inositol is more effective than myo-inositol in preventing folate-resistant mouse neural tube defects. Hum Reprod. 2002;17(9):2451–8. doi: 10.1093/humrep/17.9.2451. [DOI] [PubMed] [Google Scholar]
- 11.Lubin V, Shojai R, Darmon P, Cosson E. A pilot study of gestational diabetes mellitus not controlled by diet alone: First-line medical treatment with myoinositol may limit the need for insulin. Diabetes Metab. 2016;42(3):192–5. doi: 10.1016/j.diabet.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Muscogiuri G, Palomba S, Lagana AS, Orio F. Inositols in the Treatment of Insulin-Mediated Diseases. Int J Endocrinol. 2016;2016:3058393. doi: 10.1155/2016/3058393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heimark D, McAllister J, Larner J. Decreased myo-inositol to chiro-inositol (M/C) ratios and increased M/C epimerase activity in PCOS theca cells demonstrate increased insulin sensitivity compared to controls. Endocr J. 2014;61(2):111–7. doi: 10.1507/endocrj.ej13-0423. [DOI] [PubMed] [Google Scholar]
- 14.Baillargeon JP, Diamanti-Kandarakis E, Ostlund RE, Jr., Apridonidze T, Iuorno MJ, Nestler JE. Altered D-chiro-inositol urinary clearance in women with polycystic ovary syndrome. Diabetes Care. 2006;29(2):300–5. doi: 10.2337/diacare.29.02.06.dc05-1070. [DOI] [PubMed] [Google Scholar]
- 15.Ostlund RE, Jr, McGill JB, Herskowitz I, Kipnis DM, Santiago JV, Sherman WR. D-chiro-inositol metabolism in diabetes mellitus. Proc Natl Acad Sci U S A. 1993;90(21):9988–92. doi: 10.1073/pnas.90.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell WW, Ostlund RE, Jr, Joseph LJ, Farrell PA, Evans WJ. Relationships of plasma C-peptide and gender to the urinary excretion of inositols in older people. Horm Metab Res. 2001;33(1):44–51. doi: 10.1055/s-2001-12626. [DOI] [PubMed] [Google Scholar]
- 17.Catalano PM, Kirwan JP, Haugel-de Mouzon S, King J. Gestational diabetes and insulin resistance: role in short- and long-term implications for mother and fetus. J Nutr. 2003;133(5 Suppl 2):1674s–83s. doi: 10.1093/jn/133.5.1674S. [DOI] [PubMed] [Google Scholar]
- 18.Murphy A, Shamshirsaz A, Markovic D, Ostlund R, Koos B. Urinary Excretion of Myo-Inositol and D-Chiro-Inositol in Early Pregnancy Is Enhanced in Gravidas With Gestational Diabetes Mellitus. Reprod Sci. 2016;23(3):365–71. doi: 10.1177/1933719115602767. [DOI] [PubMed] [Google Scholar]
- 19.Santamaria A, Corrado F, Baviera G, Carlomagno G, Unfer V, D’Anna R. Second trimester amniotic fluid myo-inositol concentrations in women later developing gestational diabetes mellitus or pregnancy-induced hypertension. J Matern Fetal Neonatal Med. 2016;29(14):2245–7. doi: 10.3109/14767058.2015.1081886. [DOI] [PubMed] [Google Scholar]
- 20.Godfrey KM, Cutfield W, Chan SY, Baker PN, Chong YS, Ni PSG. Nutritional Intervention Preconception and During Pregnancy to Maintain Healthy Glucose Metabolism and Offspring Health (“NiPPeR”): study protocol for a randomised controlled trial. Trials. 2017;18(1):131. doi: 10.1186/s13063-017-1875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santamaria A, Di Benedetto A, Petrella E, Pintaudi B, Corrado F, D’Anna R, et al. Myo-inositol may prevent gestational diabetes onset in overweight women: a randomized, controlled trial. J Matern Fetal Neonatal Med. 2016;29(19):3234–7. doi: 10.3109/14767058.2015.1121478. [DOI] [PubMed] [Google Scholar]
- 22.D’Anna R, Scilipoti A, Giordano D, Caruso C, Cannata ML, Interdonato ML, et al. myo-Inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: a prospective, randomized, placebo-controlled study. Diabetes Care. 2013;36(4):854–7. doi: 10.2337/dc12-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes-Muñoz E, Guardo FD, Ciebiera M, Kahramanoglu I, Sathyapalan T, Lin LT, et al. Diet and Nutritional Interventions with the Special Role of Myo-Inositol in Gestational Diabetes Mellitus Management. An Evidence-Based Critical Appraisal. Curr Pharm Des. 2019;25(22):2467–73. doi: 10.2174/1381612825666190722155512. [DOI] [PubMed] [Google Scholar]
- 24.Toh N, Inoue T, Tanaka H, Kimoto E. Polyol accumulation in human placenta and umbilical cord. Biol Res Pregnancy Perinatol. 1987;8(1 1ST Half):13–5. [PubMed] [Google Scholar]
- 25.Gallo LA, Barrett HL, Dekker Nitert M. Review: Placental transport and metabolism of energy substrates in maternal obesity and diabetes. Placenta. 2017;54:59–67. doi: 10.1016/j.placenta.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. World Health Organization; 2013. [PubMed] [Google Scholar]
- 27.Gardosi J, Francis A, Turner S, Williams M. Customized growth charts: rationale, validation and clinical benefits. Am J Obstet Gynecol. 2018;218(2s):S609–s18. doi: 10.1016/j.ajog.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Islam MO, Selvam P, Appukuttan Pillai R, Watkins OC, Chan SY. An enzymatic assay for quantification of inositol in human term placental tissue. Anal Biochem. 2019;586:113409. doi: 10.1016/j.ab.2019.113409. [DOI] [PubMed] [Google Scholar]
- 29.Sun TH, Heimark DB, Nguygen T, Nadler JL, Larner J. Both myo-inositol to chiro-inositol epimerase activities and chiro-inositol to myo-inositol ratios are decreased in tissues of GK type 2 diabetic rats compared to Wistar controls. Biochem Biophys Res Commun. 2002;293(3):1092–8. doi: 10.1016/S0006-291X(02)00313-3. [DOI] [PubMed] [Google Scholar]
- 30.Loubiere LS, Vasilopoulou E, Bulmer JN, Taylor PM, Stieger B, Verrey F, et al. Expression of thyroid hormone transporters in the human placenta and changes associated with intrauterine growth restriction. Placenta. 2010;31(4):295–304. doi: 10.1016/j.placenta.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Pillai Reshma A, Islam Mohammed O, Selvam Preben, Sharma Neha, Chu Anne HY, Watkins Oliver C, et al. Supplementary Figure1, Data from: Placental Inositol Reduced in Gestational Diabetes as Glucose alters Inositol Transporters and IMPA1 enzyme expression. [Deposited 13 October 2020];Figshare data repository. doi: 10.1210/clinem/dgaa814. https://figshare.com/articles/figure/Association_of_Maternal_Glycemia_with_expression_of_Inositol_Enzymes_and_Transporters/13083647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pillai Reshma A, Islam Mohammed O, Selvam Preben, Sharma Neha, Chu Anne HY, Watkins Oliver C, et al. Supplementary Figure 2, Data from: Placental Inositol Reduced in Gestational Diabetes as Glucose alters Inositol Transporters and IMPA1 enzyme expression. [Deposited 13 October 2020];Figshare data repository. doi: 10.1210/clinem/dgaa814. https://figshare.com/articles/figure/Ponceau_S_staining/13083719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geurtsen ML, van Soest EEL, Voerman E, Steegers EAP, Jaddoe VWV, Gaillard R. High maternal early-pregnancy blood glucose levels are associated with altered fetal growth and increased risk of adverse birth outcomes. Diabetologia. 2019;62(10):1880–90. doi: 10.1007/s00125-019-4957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staat BC, Galan HL, Harwood JE, Lee G, Marconi AM, Paolini CL, et al. Transplacental supply of mannose and inositol in uncomplicated pregnancies using stable isotopes. J Clin Endocrinol Metab. 2012;97(7):2497–502. doi: 10.1210/jc.2011-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brusati V, Jozwik M, Jozwik M, Teng C, Paolini C, Marconi AM, et al. Fetal and maternal non-glucose carbohydrates and polyols concentrations in normal human pregnancies at term. Pediatr Res. 2005;58(4):700–4. doi: 10.1203/01.PDR.0000180549.86614.73. [DOI] [PubMed] [Google Scholar]
- 36.Campling JD, Nixon DA. The inositol content of foetal blood and foetal fluids. J Physiol. 1954;126(1):71–80. doi: 10.1113/jphysiol.1954.sp005192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mejia CA, Reynolds PR, Arroyo JA. Placental expression of NFAT5, SLC5A3, and aldose reductase in gestational diabetes mellitus. The FASEB Journal. 2016;30(1_supplement):851.5–5. [Google Scholar]
- 38.Mejia JF, Hirschi KM, Tsai KYF, Long MG, Tullis BC, Bitter EEK, et al. Differential placental ceramide levels during gestational diabetes mellitus (GDM) Reprod Biol Endocrinol. 2019;17(1):81. doi: 10.1186/s12958-019-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li SYT, Cheng STW, Zhang D, Leung PS. Identification and Functional Implications of Sodium/Myo-Inositol Cotransporter 1 in Pancreatic beta-Cells and Type 2 Diabetes. Diabetes. 2017;66(5):1258–71. doi: 10.2337/db16-0880. [DOI] [PubMed] [Google Scholar]
- 40.Chu AHY, Tint MT, Chang HF, Wong G, Yuan WL, Tull D, et al. High placental inositol content associated with suppressed pro-adipogenic effects of maternal glycaemia in offspring: the GUSTO cohort. Int J Obes (Lond) 2020 doi: 10.1038/s41366-020-0596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crawford TJ, Crowther CA, Alsweiler J, Brown J. Antenatal dietary supplementation with myo-inositol in women during pregnancy for preventing gestational diabetes. Cochrane Database Syst Rev. 2015;(12):Cd011507. doi: 10.1002/14651858.CD011507.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matarrelli B, Vitacolonna E, D’Angelo M, Pavone G, Mattei PA, Liberati M, et al. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial. J Matern Fetal Neonatal Med. 2013;26(10):967–72. doi: 10.3109/14767058.2013.766691. [DOI] [PubMed] [Google Scholar]
- 43.Santamaria A, Alibrandi A, Di Benedetto A, Pintaudi B, Corrado F, Facchinetti F, et al. Clinical and metabolic outcomes in pregnant women at risk for gestational diabetes mellitus supplemented with myo-inositol: a secondary analysis from 3 RCTs. Am J Obstet Gynecol. 2018;219(3):300.e1–.e6. doi: 10.1016/j.ajog.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 44.Watkins OC, Islam MO, Selvam P, Pillai RA, Cazenave-Gassiot A, Bendt AK, et al. Myo-inositol alters 13C-labeled fatty acid metabolism in human placental explants. J Endocrinol. 2019;234(1):73–84. doi: 10.1530/JOE-19-0267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.