Abstract
The Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO) is a preconception, longitudinal cohort study that aims to study the effects of nutrition, lifestyle, and maternal mood prior to and during pregnancy on the epigenome of the offspring and clinically important outcomes including duration of gestation, fetal growth, metabolic and neural phenotypes in the offspring. Between February 2015 and October 2017, the S-PRESTO study recruited 1039 Chinese, Malay or Indian (or any combinations thereof) women aged 18 to 45 years and who intended to get pregnant and deliver in Singapore, resulting in 1032 unique participants and 373 children born in the cohort.
The participants were followed up for 3 visits during the preconception phase and censored at 12 months of follow up if pregnancy was not achieved (N=557 censored). Women who successfully conceived (N=475) were characterised at gestational weeks 6-8, 11-13, 18-21, 24-26, 27-28 and 34-36. Follow up of their index offspring (N=373 singletons) is on-going at birth, 1, 3 and 6 weeks, 3, 6, 12, 18, 24 and 36 months and beyond. Women are also being followed up post-delivery. Data is collected via interviewer-administered questionnaires, metabolic imaging (magnetic resonance imaging), standardized anthropometric measurements and collection of diverse specimens, i.e. blood, urine, buccal smear, stool, skin tapes, epithelial swabs at numerous timepoints.
S-PRESTO has extensive repeated phenotypic data collected which include genetic and epigenetic sampling from preconception which is unique in mother-offspring epidemiological cohorts This enables prospective assessment of a wide array of potential determinants of future health outcomes in women from preconception to post-delivery and in their offspring across the earliest development from embryonic stages into early childhood. In addition the S-PRESTO study, draws from the three major Asian ethnic groups that represent 50% of the global population, increasing the relevance of its findings to global efforts to address non-communicable diseases.
Introduction
Epidemiological studies strongly suggest that maternal health and nutrition during preconception and over the course of embryonic, fetal and postnatal development influence the risk for major disease outcomes in the offspring, including obesity, type 2 diabetes (T2D), cardiovascular disease and mental disorders. The influence of the early environment on health over the lifespan forms the basis for the “Developmental Origins of Health and Disease (DOHaD)” paradigm [1, 2]. The DOHaD paradigm proposes that environmental influences in early life affect the development of the newborn, allowed by the occurrence of normal developmental plasticity, partly mediated through epigenetic processes, thus influencing later health and vulnerability for disease. There is now strong evidence in humans supporting the importance of prenatal and early pregnancy influences on childhood development and health profiles in later life [1, 3, 4]. For example, maternal gestational diabetes during pregnancy has been linked to increased risk for cardiovascular disease, obesity, elevated blood pressure, T2D and neurodevelopmental outcomes in the offspring [5–7]. The importance of early life influences has been demonstrated in a number of studies; e.g. reduced infant growth was found to be associated with an increased risk for ischaemic heart disease in adulthood [8], and rapid weight gain in infants with a low birth weight has been associated with later increased risk of death from coronary heart disease [9]. Besides early life influences, the preconceptional period has been highlighted to play a pivotal role; maternal weight trajectories in preconception have been associated with increase in offspring’s BMI [10] and maternal stress during preconception has been linked to child’s eczema development [11].
Besides human studies, the DOHaD paradigm has received support from findings in animal studies showing that conditions around conception have a major impact on offspring adiposity, cardiovascular, metabolic and growth outcomes [12–15]. Maternal obesity during preconception and in early life has been reported to result in alterations in development of the pancreas, leading to pancreatic dysfunction and subsequent diabetes in the murine offspring. Besides this, findings from murine models also showed that exposure to fine particulate matter during preconception result in cardiac dysfunction in adult offspring, emphasizing the importance of the preconception period [16]. Further, data from in vitro manipulations [17] suggest that the periconceptual conditions may be associated with epigenetic changes in the offspring [18].
Scope of research
We sought to address the hypothesis that conditions before and in early pregnancy affect offspring epigenetics and phenotype, which in turn influences vulnerabilities to non-communicable diseases in later life. Understanding the evolution of the biological pathways involved will have major implications for preventative interventions during preconception, pregnancy and after delivery as well as impact public health and formation of government policies.
The Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO) aims at prospectively defining the relationships between preconceptional maternal characteristics including metabolism and nutritional status, genetic and epigenetic markers and mood to demonstrate more robust and a wider range of evidence to support the notion that periconceptional factors strongly impact offspring health. In addition, S-PRESTO provides a critical platform for replication of novel epigenetic findings from our other ongoing mother-offspring cohort study GUSTO (Growing Up in Singapore Towards healthy Outcomes) which started recruitment during early pregnancy [19]. Aside from the validation of candidate markers which are predictive of child metabolic and neural phenotypes using a prospective study design [19], the S-PRESTO study also seeks to identify other novel biomarkers and modifiable risk factors, particularly in the preconception period where intervention could potentially have the most significant impact.
The S-PRESTO cohort study aims to examine the following hypotheses:
-
1
That nutrition, lifestyle, and maternal mood prior to or in early pregnancy alter the epigenome of the offspring and that these changes are associated with variation in clinically important outcomes including duration of gestation, fetal growth, and metabolic and neurobehavioral phenotypes in the offspring;
-
2
That there are changes in offspring body composition and epigenetic state detected already at lesser degrees of maternal glucose intolerance than that defined by gestational diabetes mellitus (GDM);
-
3
That there is a high incidence of previously unanticipated micronutrient deficiencies in Singaporean women that have clinical significance for the offspring and are reflected in their epigenotype, growth trajectories and developmental patterns;
-
4
That maternal nutrition prior to and during pregnancy affects human breast milk composition, with effects on infant growth and development.
Additionally, the study design incorporates assessment of reproductive age women before and after pregnancy which allows a number of other hypotheses to be tested including:
-
5
That insulin sensitivity and body composition differ by ethnicity in the Singaporean female population;
-
6
That the metabolic state of women prior to conception will identify those at greater risk for developing gestational diabetes mellitus (GDM) and other pregnancy complications, permitting earlier interventions.
Our overall aim is to facilitate the translation of “Developmental Origins of Health and Disease (DOHaD)” science into advances in prevention and intervention strategies designed to target the most relevant windows of development for optimal promotion of healthy childhood development, and to reduce the risk of metabolic and mental health disorders both in the mother and her offspring.
Study design
Eligibility and enrollment
The S-PRESTO study recruited 1039 Chinese, Malay or Indian (or any combinations of these 3 ethnic groups) women aged 18 to 45 years between February 2015 and October 2017. An upper age limit of 35 years was introduced from mid July 2016 onwards to increase the likelihood that recruited women conceive within the time frame of the study. A pre-conception clinic was established in KK Women's and Children's Hospital, the main public maternity hospital in Singapore, to facilitate recruitment. Public recruitment outside the hospital occurred through a variety of approaches, including distributed brochures, publicly displayed posters at out-patient clinics in general practices, community/religious centers, advertisements on social media platforms and the radio. Participants who were recruited were planning to conceive within 1 year of recruitment and had the intention to reside in Singapore for the next 5 years. Women who had been actively trying to conceive for more than 18 months before recruitment, who were currently pregnant, were using oral or implanted contraception, or with an intrauterine contraceptive device (IUCD) in situ in the past 1 month or who were undergoing fertility treatment (apart from those taking clomiphene or letrozole alone in the past 1 month) were excluded from the study. Women with health conditions including established type 1 or type 2 diabetes, on systemic steroids, anticonvulsants, HIV or Hepatitis B or C medication in the past 1 month were also excluded. The reasons for pre-screening failure are indicated in supplementary table 1.
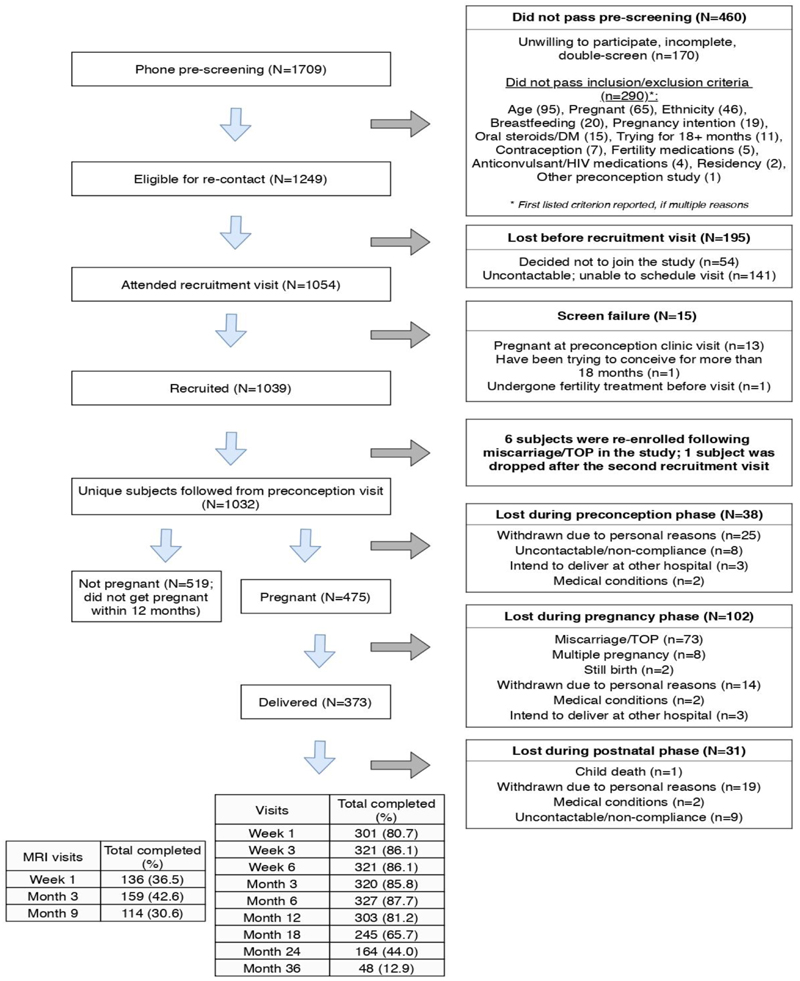
Of the 1054 who attended the recruitment visit, 1039 were recruited. Majority of the screen failures (13/15=86.7%) was already pregnant at the recruitment visit. Nine women participated in two recruitment visits: 6 reenrolled after first miscarriage / termination of pregnancy in the study; 1 was dropped after failing the second screen; and 2 only enrolled after the second visit, resulting in follow-up for N = 1032 uniquely enrolled women (Figure 1). Participants who did not get pregnant within a year of enrolment (N = 519) or withdrew for other reasons were censored from the study (N= 38). Of 475 confirmed pregnancies, 373 (79%) gave live birth to singletons. The majority of the remainder were lost to miscarriage or termination of pregnancy (73/102 = 72%). To date, 48 (12.9%) mothers and index children have been followed through 36 months with 48 month visits in progress (Figure 1). Written informed consent was provided by participants. Ethical approval was obtained from the SingHealth Centralised Institutional Review Board (reference 2014/692/D). This study has been registered at ClinicalTrials.gov (NCT 03531658).
Figure 1. Progress of the S-PRESTO study.
*postnatal follow up numbers are of subjects that have completed visits as of 11 February 2020. There are 342 active subjects still in the study.
Figure 1 shows the flow chart of the progress of participants through the study.
Overview
In the preconception period, 3 visits were conducted. During pregnancy, 6 visits were conducted at 6-8, 11-13, 18-21, 24-26, 27-28 and 34-36 weeks’ gestation for detailed assessments, and for ultrasound scans to assess gestational age and fetal growth. Birth tissues (e.g. cord, placenta) were obtained at delivery and anthropometric measurements of the newborn were performed within 24 h of birth. During early childhood, the offspring are being examined at ages 1, 3, 6 weeks, 3, 6, 9, 12, 18, 24 and 36months.
Data collected in the preconception period
The 3 visits during the pre-conception phase consisted of an initial visit for eligibility screening, consenting and baseline assessments (sociodemographic/metabolic/nutrition). A second visit encompassed cognitive and mood assessment, and a third comprised cardiovascular assessment and retinal photography. All participants were screened for pregnancy using a urinary pregnancy test kit at each visit. Pregnancy test kits were also provided to the participants to screen for pregnancy in between visits and they were asked to inform the research coordinators when a positive pregnancy test was obtained. An appointment for viability ultrasound scan would be arranged to confirm the pregnancy. In addition, research coordinators contacted the participants to find out if they were pregnant when there were no updates by the participants. Interviewer-administered questionnaires ascertained detailed medical history, dietary habits, lifestyle and physical activity, and several self-administered mood and sleep questionnaires were completed. This was followed by a physical examination that included anthropometric measurements (height, weight, mid arm circumference, waist and hip circumference, skinfold thickness). Body fat percentage was measured by bioelectrical impedance analysis and air displacement plethysmography which is the criterion method of measuring body composition. The air displacement plethysmography uses whole-body densitometry to determine body composition (fat and fat-free mass).
All participants were approached to undergo magnetic resonance imaging (MRI) and dual energy x-ray absorptiometry (DXA) scans, however only a subset consented due to concern related to electromagnetic field and radiation exposure, respectively. MRI was carried out for assessment of ectopic fat deposits and body composition related assessments. DXA-scans were done to assess bone mineral density of hip and lumbar spine.
An accelerometer (Actigraph wGT3X-BT) was used to measure activity-rest pattern over 7 consecutive days following the first clinic visit. Cardiovascular assessment was carried out by examining arterial stiffness using applanation tonometry and flow-mediated dilation of the brachial artery by ultrasonography. The extent of changes in maternal cardiovascular functions from preconception to the postpartum period and its correlation to infant outcomes will be investigated. Ocular health was assessed and retinal photographs have been taken together with autorefraction to capture the fundus and evaluate its blood vessels and the optic nerve. We will evaluate if such retinal assessments could be reflective of differences within the microvasculature of the utero-placental circulation and be associated with fetal growth and health outcomes.
A 2-hour 75g 3-timepoint oral glucose tolerance test (OGTT) was conducted at 0, 30 and 120 min during the first preconception visit. On top of fasting glucose, fasting blood samples will be used to test for insulin (to derive insulin resistant index), nutritional and endocrine biomarkers. Blood and buccal swabs were taken for genomic and epigenomic analysis. Hair was collected to determine hair metabolite profile and assess environmental exposures. Participants were given stool collection kits for collecting fecal samples at the preconception visit after the OGTT testing. This will be used for metagenomic and other microbiome analyses. Mid-stream urine and saliva were sampled for metabolite profiling. Household dust samples were collected by trained research coordinators from 3 sites: the bed, the sofa in the living room and the area furthest away from the main door to analyse levels of allergens, environmental microbiome and pollutants at home to correlate with allergic outcomes. Dust samples are collected using vacuum cleaners (Dyson DC63; Dyson, UK) with 40 μm nylon mesh DUSTREAM® filters (Indoor Biotechnologies, India). Each area was vacuumed for 4 minutes. Socio-emotional assessment was carried out by interviews and questionnaires.
Table 1 specifies the information collected in the preconception period.
Table 1. Data collected at the preconception period of the S-PRESTO study.
| PRECONCEPTION | Clinic visit 1 | Clinic visit 2 | Neurodevelopment visit 1 | MRI visit |
|---|---|---|---|---|
| Consent taking | ✓ | |||
| Pregnancy test | ✓ | ✓ | ||
| Interviews & self-administered questionnaires | ✓ | ✓ | ||
| Measurements | ||||
| Height/weight/skinfolds | ✓ | |||
| BODPOD/BIA (Bioelectrical Impedance Analysis) | ✓ | |||
| Blood pressure | ✓ | |||
| Cardiovascular assessment | ✓ | |||
| Retinal Photography | ✓ | |||
| Accelerometry | ✓ | |||
| Metabolic imaging (MRI/MRS) | ✓ | |||
| Biosample collection | ||||
| Blood (fasting) | ✓ | |||
| Urine (fasting) | ✓ | ✓ | ||
| Buccal smear | ✓ | ✓ | ✓ | |
| Hair | ✓ | ✓ | ||
| Stool | ✓ | ✓ | ||
| Dust | ✓ | |||
| Socio-emotional assessment | ||||
| Online or hard copy self-administered questionnaires1 | ✓ | |||
| Tasks: SCID, Flanker - Emotional and Neutral, DCCS - Emotional and Neutral, Relational Binding - Emotional and Neutral, Emotional Perception, CANTAB (Spatial Working Memory), Shipley Language Test, Heart Rate | ✓ |
Interviews & online self-administered questionnaires include: EPDS, BDI, STAI, Big 5, Perceived stress scale, Life Experiences Survey, Multidimensional Scale of Perceived Support, Sufficiency of practical/emotional support, Other Sources of Social Support, Need to Belong, SES + income, Belief in a Dangerous World, Interpersonal Orientation Measure, Pittsburgh Sleep Questionnaire, Morningness Eveningness Questionnaire, Epworth Sleepiness Scale Questionnaire, GHQ-12, Body Image Expectations and Dissatisfaction, Childhood Trauma Questionnaire, Parental Bonding Instrument, SF12 and Edinburgh Handedness Inventory
Data collected in the pregnancy period
The women were asked to contact the study team as soon as they had a positive urinary pregnancy test. They were examined at gestational weeks 6-8, 11-13, 18-21, 24-26, 27-28 and 34-36. Lifestyle and behavioural factors such as dietary intake, smoking and alcohol consumption, physical activity and mental health were recorded. Maternal anthropometry (height, weight, mid arm circumference and skinfold thickness) was measured. Obstetric ultrasound scans were conducted at 7 weeks to confirm likely conception date, viability of the pregnancy, singleton or multiple pregnancy; at 12 weeks to estimate date of delivery and to measure nuchal thickness; at 12, 20, 28 and 34 weeks to determine fetal size, i.e.head circumference (HC), biparietal diameter (BPD), abdominal circumference (AC), femur length. An anomaly scan was done at 20 weeks. Doppler ultrasound measures of uterine artery, umbilical cord and fetal regional blood flow were undertaken at 20, 28 and 34 weeks. Scans were conducted in a standardized manner by trained ultrasonographers. Socio-emotional assessment at 26 weeks was carried out by interviews and questionnaires.
Similar to the preconception stage, blood pressure measurements, cardiovascular assessments as well as retinal photography were carried out during pregnancy. Accelerometers were used to measure activity-rest patterns over 7 consecutive days following the fourth pregnancy clinic visit at 24-28 weeks. A 2-hour 75g 5-timepoint OGTT (0, 30, 60, 90, 120 min) was conducted at 27-28 weeks’ gestation. Maternal micronutrient status, metabolic (including assessment of trace elements, pollutants, metals) and epigenetic profiles will be assessed using urine, blood, hair and buccal swabs (for DNA) during the antenatal visits. Fecal samples and swabs from the vaginal, vulval, rectal and breast epithelia were collected to examine maternal microbiota in relation to pregnancy and infant outcomes. Dust sample collection was repeated at 34-36 weeks. Small skin tapes were applied on the mothers’ forehead and antecubital fossa during pregnancy visit to collect superficial skin bacterial samples and understand the evolution of the skin microbiome during pregnancy. Table 2 specifies the information collected in the pregnancy period.
Table 2. Data collected at the pregnancy period of the S-PRESTO study.
| PREGNANCY VISIT | Visit 1 (6-8wk) | Visit 2 (11-13 wk) | Visit 3 (18-21 wk) | Neurodevelopment visit 2 (24-26wk) | Visit 4 (27-28 wk) | Visit 5 (34-36 wk) |
|---|---|---|---|---|---|---|
| Ultrasound scan of pregnancy | Dating | Nuchal | Anomaly | - | Growth | Growth |
| Interviews & self-administered questionnaires | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Measurements | ||||||
| Height/weight/skinfolds | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Blood pressure | ✓ | ✓ | ✓ | |||
| Cardiovascular assessment | ✓ | |||||
| Retinal Photography | ✓ | ✓ | ✓ | |||
| Accelerometry | ✓ | |||||
| Biosample collection | ||||||
| Blood | ✓ | ✓ | ✓ | (Fasting) | ✓ | |
| OGTT (0, 30, 60, 90, 120 min) | ✓ | |||||
| Urine | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Hair | ✓ | ✓ | ✓ | ✓ | ||
| Buccal smear | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Stool | ✓ | ✓ | ||||
| Dust | ✓ | |||||
| Skin microbiome (Forehead, antecubital fossa) | ✓ | |||||
| Epithelial swabs (Vaginal, vulva, rectal and breast) | ✓ | |||||
| Socio-emotional assessment | ||||||
| Online or hard-copy self-administered questionnaires 2 * | ✓ | ✓ | ✓ | |||
| Tasks: Flanker - Emotional and Neutral, DCCS - Emotional and Neutral, Relational Binding – Neutral, Emotional Perception, CANTAB Spatial Working Memory, Adult Attachment Interview, Heart Rate | ✓ |
EPDS, BDI, STAI, Perceived Stress Scale, Multidimensional Scale of Perceived Support, Sufficiency of practical/emotional support, Other Sources of Social Support, Need to Belong, SES + income, Belief in a Dangerous World one item, Interpersonal Orientation Measure, Pittsburgh Sleep Questionnaire, Epworth Sleepiness Scale Questionnaire, Body Image Expectations and Dissatisfaction, Parental Bonding Instrument, Pregnancy Anxiety Scale (from Buss), Pregnancy Experiences Scale - Short version, Sleep Diary & Actiwatch, SF12, Loss Questionnaire
selected questionnaires are administered at each timepoint
Data collected at delivery
At birth, maternal blood, cord blood, cord tissue, placental villous biopsies, as well as neonatal buccal swabs, hair and heel prick blood spots were collected to determine maternal and offspring micronutrient status, metabolic and epigenetic profiles for testing study hypotheses. During labour or at the start of the caesarean section, maternal swabs from vaginal, vulval, rectal and breast epithelia were collected for metagenomic analysis of the human microbiome Meconium or first fecal stools from the baby were collected. Nurses helped to collect the meconium from the babies in the hospital while collection kits were passed to the participants for collection of first fecal stools for the babies. For a sub-sample of participants who delivered by caesarean section, maternal subcutaneous fat sample/biopsies were obtained for metabolic and molecular studies. Clinical and laboratory data (e.g. weight, blood pressure, full blood count, urine dipstick) was obtained. Antenatal and neonatal hospital medical records were retrieved. Details of antenatal, intrapartum and post-partum events during the study period were captured from hospital medical records. All routinely recorded birth data including sex, birth weight, head circumference and body length etc. were extracted from case-notes. The infants had extensive body composition measurements following delivery including skinfold thicknesses, anthropometry and air displacement plethysmography [20–23].
Follow up visits
Offspring follow-up postnatally (0-3 years)
The offspring are being followed up at birth, 1, 3 and 6 weeks, 3, 6, 12, 18, 24 and 36 months. At selected timepoints, measurements including longitudinal anthropometry, body composition measures using PEA POD® air displacement plethysmography (for infants up to 8 kg) and EchoMRI™ body composition analysis device. Buccal smears, hair and fecal samples for microbiome measurement along with nutritional and health records are taken at multiple timepoints. Infant dietary assessment methods include a weaning food journal at age 3 months, 3-day food diaries at age 12 months, food frequency questionnaires at ages 6, 12 and 18 months and questions related to intakes of dietary supplements, mothers’ feeding practices and infant/child eating behavior questionnaires at ages 6, 12 and 18 months.
Anogenital distance, a biomarker of prenatal androgen exposure, is measured. Allergic status is determined through questionnaires, clinical evaluation (e.g. SCORAD for eczema) at 6,18 and 36 months, skin examination at 3 and 12 months, and skin prick testing to common allergens at 6,18 and 36 months. Infants who were diagnosed with eczema by clinicians at age 6 months will undergo skin prick testing to a number of allergens(cow’s milk, whole egg, soy, wheat and peanut). At 18 and 36 months, the allergens tested are cow’s milk, whole egg, soy, wheat, peanut, shrimp, crab, dust mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae and Blomia tropicalis).
Superficial skin bacterial samples are collected by applying small skin tapes on the child’s skin (forehead, antecubital fossa and lesional samples for those with eczema) at age 1 week, as well as at ages 3, 6 and 18 months. In addition, dust samples are again collected at 3 months from 3 sites (the bed that the infant sleeps on, sofa and the area furthest away from the main door) to correlate allergic outcomes with household allergen and microbiota levels.
Postpartum women
After delivery, the mothers are being nutritionally assessed to determine maternal diet during breastfeeding, the Asian confinement period (first month after delivery) and postnatally. Postnatal dietary intake is assessed using self-administered confinement food frequency questionnaires one month after delivery. In Asia, the period 21 to 40 days after parturition is believed to be a period of convalescence and also known as “the confinement period”. During this period, mothers follow specific dietary and behavioral restrictions and prescriptions, which are aimed at promoting restoration of maternal health and to protect mothers from future illnesses. Other behavioral prescriptions include not leaving the house, not washing one’s hair or shower, having traditional massages and abdominal binding throughout the confinement period [24].
At the visits, anthropometry and body composition measurements are being conducted. In line with the measures at the preconception and antenatal visits, MRI was conducted for women who underwent MRI at preconception and blood pressure is being measured together with cardiovascular assessment. Skin prick testing to common allergens are being conducted to determine allergic sensitization. The allergens tested are dust mites (Dermatophagoides pteronyssinus, Dermatophagoides farina, Blomia tropicalis), cockcroach (Blattella germanica, Periplanata Americana), cat and dog allergens. Superficial skin bacterial samples are being collected by applying small skin tapes on the mothers’ foreheads and antecubital fossa. An accelerometer is used to measure activity-rest patterns over 7 consecutive days following the month 12 visit.
Mother’s breast milk is being collected according to standard protocols at week 1, 3 and 6 as well as 3 and 6 months, to determine macronutrient, micronutrient, hormonal, adipocytokine and immune transcriptomic and metagenomic parameters. Mother’s blood, buccal smear, hair, urine and fecal samples are being collected at various time points, and the OGTT is repeated about 3 months post-delivery. Table 3 specifies the information collected in the post-delivery period.
Table 3. Data collected at the post-delivery period of the S-PRESTO study.
| Delivery | Post delivery | W1 | W3 | W6 | M3 | M6 | M9 | M12 | M18 | M24 | M36 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOTHER | ||||||||||||
| Interviews and self-administered questionnaires | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Measurements | ||||||||||||
| Accelerometry | ✓ | |||||||||||
| Cardiovascular assessment | ✓ | |||||||||||
| Blood pressure | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Anthropometry | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| BIA (Bioelectrical Impedance Analysis) | ✓ | ✓ | ✓ | |||||||||
| BODPOD | ✓ | ✓ | ||||||||||
| DXA (Dual-energy X-ray Absorptiometry) | ✓ | |||||||||||
| Metabolic imaging (MRI/MRS) Brain imaging | ✓ | |||||||||||
| Skin prick test | ✓ | ✓ | ||||||||||
| Biosamples collection | ||||||||||||
| Subcutaneous fat (from mothers who delivered by cesarean section) | ✓ | |||||||||||
| Skin microbiome sampling (Forehead, antecubital fossa) | ✓ | |||||||||||
| OGTT (0, 30, 120 min) | ✓ | ✓ | ✓ | |||||||||
| Maternal blood (fasting) | ✓ | ✓ | ✓ | |||||||||
| Buccal smear | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Hair | ✓ | |||||||||||
| Stools | ✓ | |||||||||||
| Urine | ✓ | ✓ | ✓ | |||||||||
| Dust collection | ✓ | |||||||||||
| Breast milk collection | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Epithelial swabs | ✓ | |||||||||||
| CHILD | ||||||||||||
| Interviews and questionnaires | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Measurements | ||||||||||||
| Anthropometry | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| QMR | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Peapod | ✓ | ✓ | ✓ | |||||||||
| Metabolic Imaging (MRI/MRS) | ✓ | ✓ | ✓ | |||||||||
| Anogenital distance | ✓ | ✓ | ✓ | ✓ | ||||||||
| Skin prick test and SCORAD | ✓ | ✓ | ✓ | |||||||||
| Skin examination | ✓ | ✓ | ||||||||||
| Biosamples collected | ||||||||||||
| Cord tissue | ✓ | |||||||||||
| Umbilical Cord Tissue Derived stem cells, Wharton-jelly-MSC and HUAVECs, Cord blood & cord blood derived stem cells (CBMCs) | ✓ | |||||||||||
| Placental villous biopsies | ✓ | |||||||||||
| Heel Prick | ✓ | |||||||||||
| Skin microbiome sampling (Forehead, antecubital fossa and lesional samples for those with eczema) | ✓ | ✓ | ✓ | ✓ | ||||||||
| Hair | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Buccal smear | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Stools | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Delivery | Post delivery | W1 | W3 | W6 | M3 | M6 | M9 | M12 | M18 | M24 | M36 | |
| Cognitive and socio-emotional assessment | ||||||||||||
| Tasks - Dimension Change Card Sort, Flanker, Relational Binding, Family Interview for Genetic Studies, Info on Lifestyle Practices, Baby photo taking (for M6 EEG experiment), Associative Memory, Parent Child Interaction, Emotion Regulation (EEG), Visual Oddball (EEG) Dotprobe (EEG), Strange Situation (Parent-Child interaction) | ✓ | ✓ | ✓ | |||||||||
| Online or hard-copy self-administered questionnaires3 * | ✓ | ✓ | ✓ |
STAI, EPDS, BDI, Belief in a Dangerous World, Body Image, Brief Infant Sleep Questionnaire, Daily Hassles and Uplifts of Parenting, Epsworth Sleepiness Scale, Experiences in Close Relationships, Interpersonal Orientation Scale, Interpersonal Reactivity Index, Life Experience Survey, Multidimensional Scale of Perceived Social Support, Need to Belong, Other Sources of Social Support, Parental Cognition about Sleep, Parental Expectations for Child's Social Development, Parent's Experience of Loss, Perceived Stress Scale, Pittsburg Sleep Quality Index, Primary Caregiver Questionnaire, Sufficiency of Practical and Emotional Support, The Parental Interactive Bedtime Behaviour Scale (PIBBS)
selected questionnaires are administered at each timepoint
Fathers
Fathers were introduced to the paternal neurobehavioral assessments which include tasks such as flanker, dimensional card sort, relational binding, emotion perception as well as self-administered questionnaires during the preconception phase. Once their partner conceived, fathers were invited to provide more detailed information including sociodemographics, dietary intake, physical activity, undergo measures of anthropometry, blood pressure and have blood, urine, hair and buccal smear samples collected when they were present, such as during pregnancy visits (preferably 7, 12 and 20 weeks), to examine paternal influences on offspring health outcomes.
Key findings and publications
Out of the 1039 recruited women, 9 women participated in two recruitment visits: 6 reenrolled after first miscarriage / termination of pregnancy in the study; 1 was dropped after failing the second screen; and 2 only enrolled after the second visit, resulting in follow-up for 1032 uniquely enrolled women and 373 children born in the study. The study design and progress of the study is illustrated in Figure 1. The baseline demographic characteristics of the participants are listed in Table 4. Of the 1032 women, 743 (72.0%) were Chinese, 159 (15.4%) were Malay, 95 (9.2%) were Indian and 35 (3.4%) were of mixed ethnicity. The mean age of women at recruitment was 30.8 years (range 19-45 years) and 61.5% of them had completed tertiary education (Table 4).
Table 4. Baseline characteristics of participants in the S-PRESTO cohort.
| Characteristics | N | N(%) |
|---|---|---|
| Age (year ± SD) | 1032 | 30.8 ± 3.8 (range: 19-45) |
| Ethnicity * | ||
| Chinese | 743 (72.0) | |
| Malay | 159 (15.4) | |
| Indians | 95 (9.2) | |
| Mixed | 35 (3.4) | |
| Highest education level | ||
| Tertiary | 635 (61.5) | |
| Non-Tertiary | 384 (37.2) | |
| Unknown | 13 (1.3) | |
| Smoking status during preconception | ||
| Never smoke | 904(87.6) | |
| Smoker | 45 (4.4) | |
| Ex-smoker | 62 (6.0) | |
| Unknown | 21 (2.0) | |
| Height (cm, mean±SD) | 1004 | 159.82 ± 5.6, (range: 142.75 - 176.50) |
| Pre-pregnancy weight (kg, mean±SD) | 1005 | 60.84 ± 13.8, (range: 33.95 - 140.85) |
| Pre-pregnancy BMI (kg/m2, mean ±SD) | 1004 | 23.83 ± 5.3, (range: 15.14 - 50.11) |
Information on ethnicity are obtained from identification documents for 7 participants.
As this study is still in progress, limited initial findings are described here.
We examined if higher overall and central adiposity were associated with reduced fecundability in a subset of women [25]. BMI, sum of 4-site skinfold thickness (SFT) and total body fat percentages (TBF%, measured using air displacement plethysmography) were used to determine overall adiposity, while waist circumference, waist-to-hip ratio, waist-to-height ratio and A Body Shape Index were used to determine central adiposity. We found no associations between measures of central adiposity and fecundability, which is in contrast to previous studies among black women [26]. However, we observed that higher amounts of subcutaneous fat as determined by measurements of SFT and TBF% are associated with longer time-to-pregnancy, instead of central adiposity, in this Asian population. It is possible that abdominal adipocytes are not similarly hormonally active across populations which could affect fecundability.
Analysis of self-reported physical activity and screen time, a proxy for sedentary lifestyle, in the preconception period revealed that about 32% of women reported being active, and 36% watched television ≥2 h/day and 26% used electronic devices ≥3 h/day [27]. This study was carried out in a subset of women who had at least one glucose measurement. There was an inverse association between vigorous physical activity (VPA) and lower glucose concentrations, but no association with moderate physical activity (MPA). There were no associations between screen time and glucose concentrations. Engaging in VPA may, independently from MPA and screen time, could be a modifiable factor to improve glucose metabolism in women of reproductive age of Asian ethnicities.
Strengths and limitations
Interaction between human genome and the environment plays a significant role in determining an individual’s susceptibility to health adversities. Epigenetics identifies genomic loci modifiable by the environment. Hence one of the primary focus of S-PRESTO study is to map the genetic and epigenetic variability in the cohort and study its association with environmental variables and phenotypes of interest. The longitudinal S-PRESTO study has the advantage of prospectively assessing a wide array of potential determinants of future health outcomes, able to relate environmental exposures to epigenetic states and clinical phenotypes in women from preconception to post-delivery and in their offspring across the earliest development from embryonic stages into early childhood. This approach permits identification of the key differences in environmental conditions occurring during critical time windows that can impact on epigenetic marks and associated phenotypic outcomes. This includes the evaluation of pre-conception, early and later pregnancy, and postnatal environmental influences on infant development (as well as their potential interactions with each other and with genotype).
The current evidence from DOHaD-related studies supports the importance of suboptimal environmental exposures on the maternal and neonatal epigenome and subsequent disease. However, most of these studies have either focused on DNA methylation analysis at a specific time point in pregnancy, or at birth in infant cord blood, making it hard to study the mediation and causation effects of the alterations in the epigenome. S-PRESTO sample collection provides a unique opportunity to address this limitation as its longitudinal DNA methylation profiling from preconception to early childhood will help assess (1) assessment of baseline changes in the maternal blood epigenome from preconception through three trimesters and post-delivery, (2) examination of dynamics of epigenetic changes in response to environmental variables such as diet, lifestyle and exposure to pollutants, (3) study of the combined influences of genotype and environment on the variability in the DNA methylomes, and (4) Determination of epigenetic inheritance of environmental effects from mother to the child.
Importantly, our studies also permit assessment of the stability of biomarkers, including epigenetic states, as well as the analysis of dynamic variation in growth and other clinical phenotypes over the course of development. Finally, because we focus on early life, starting periconceptually, we can examine the earliest possible developmental influences independent of numerous confounders that emerge in traditional retrospective adult studies. This will enable the identification of early biological pathways to disease where interventions may be more effective. These strengths provide a solid platform for launching a translational research program that aims to advance current public health policy and clinical practices and is the basis for our strong partnerships with healthcare institutions, governmental organizations and ministries, public health services, translational applications, and academic clinical medicine.
A majority of other cohort studies such as Avon Longitudinal Study of Parents and Children (ALSPAC) [28], ECHO-US [29], the Hokkaido Study on Environment and Children’s health in Japan [30], and the Taiwan birth panel study [31] recruits participants from pregnancy onwards and studies the effect of environmental factors on child’s health. There are other preconception studies such as the Shanghai birth cohort [32], PRESTO study [33], LifeCYcle project [34] and the Southampton Women’s Survey [35] that assessed lifestyle and environmental factors since preconception. To our knowledge, there is no existing pre-conception cohort with such extensive repeated phenotypic data collection including genetic and epigenetic sampling and only one (that of our collaborators in the UK, the Southampton Women’s Survey) with any at all. The strengths of S-PRESTO are the objective assessment of body composition by imaging as well as assessment of environmental factors via collection of household dust and skin samples since preconception, allowing the determination of environmental microbiome and its influence on host microbiome, dysbiosis of which leads to disease development. Future planned research includes evaluating the stability of maternal concentrations of environmental chemicals from preconception through pregnancy and their potentially varying effects on developing fetuses. Besides this, S-PRESTO also has extensive data and biosample collected, providing potential to harmonize the data with other cohorts.
The specific design and focus of this preconceptional study on metabolic phenotypes can offer new insights into the earliest precursors and risk factors for GDM and other cardiometabolic outcomes in an Asian population. This may provide unique insights to the Asian phenotypes and could lead to innovative preventative as well as approaches for intervention. The pre-conception phase of this study allows us to study ethnic differences in the biology of glucose-insulin metabolism in women. Our research will guide us in introducing better nutritional practices in mothers and children, improve evidence-based parental practice and better public health education. The genetic and epigenetic work will inform the evolution of individualized approaches to the prevention and management of chronic disorders. Environmental influences during early development appear especially profound in countries experiencing dramatic socioeconomic transitions, such as Singapore. We therefore, anticipate that the S-PRESTO study, which encompasses the three major Asian ethnic groups that constitute 50% of the global population, increasing the relevance of its findings to global efforts to address non-communicable diseases A limitation of this study is that we only have a subset of fathers who participated in this study.
Collaborations
The research team looks forward to potential collaborations and investigators interested in exploring the possibility of collaborations should contact lead principal investigator Professor Chong Yap Seng (obgcys@nus.edu.sg), principal investigators Professor Johan Gunnar Eriksson (obgjge@nus.edu.sg), Professor Lynette Shek Pei-Chi (paeshekl@nus.edu.sg) and Professor Jerry Chan (jerrychan@duke-nus.edu.sg). S-PRESTO has a website, mainly focused on information for the participants, at http://www.s-presto.sg/.
Supplementary Material
Acknowledgements
We thank the S-PRESTO study group and all clinical and home-visit staff involved. The voluntary participation of all participants is greatly appreciated.
Funding
This work is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore - NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science and Technology.
Footnotes
Conflict of interest
Chong YS has received reimbursement for speaking at conferences sponsored by Abbott Nutrition, Nestle, and Danone. Godfrey KM, Chan SY and Lee YS has received reimbursement for speaking at conferences sponsored by Nestle and Shek LP has received reimbursement for speaking at -conferences sponsored by Danone and Nestle and consulting for Mead Johnson and Nestle.
Godfrey KM, Chong YS, Chan SY and Karnani N are part of an academic consortium that has received research funding from Abbot Nutrition, Nestle and Danone. Shek LP has received research funding from Danone.
Contributor Information
The SPRESTO study group includes includes:
Airu Chia, Anna Magdalena Fogel, Anne Eng Neo Goh, Anne Hin Yee Chu, Anne Rifkin-Graboi, Anqi Qiu, Bee Wah Lee, Bobby Kyungbeom Cheon, Candida Vaz, Christiani Jeyakumar Henry, Ciaran Gerard Forde, Claudia Chi, Dawn Xin Ping Koh, Desiree Y. Phua, Doris Ngiuk Lan Loh, Elaine Phaik Ling Quah, Elizabeth Huiwen Tham, Evelyn Chung Ning Law, Faidon Magkos, Falk Mueller-Riemenschneider, George Seow Heong Yeo, Hannah Ee Juen Yong, Helen Yu Chen, Heng Hao Tan, Hong Pan, Hugo P S van Bever, Hui Min Tan, Izzuddin Bin Mohd Aris, Jeannie Tay, Jerry Kok Yen Chan, Jia Xu, Joanne Su-Yin Yoong, Johan Gunnar Eriksson, Jonathan Tze Liang Choo, Jonathan Y. Bernard, Jonathan Yinhao Huang, Jun Shi Lai, Karen Mei Ling Tan, Keith M. Godfrey, Kenneth Yung Chiang Kwek, Keri McCrickerd, Kothandaraman Narasimhan, Kok Wee Chong, Kuan Jin Lee, Li Chen, Lieng Hsi Ling, Ling-Wei Chen, Lourdes Mary Daniel, Lynette Pei-Chi Shek, Marielle V. Fortier, Mary Foong-Fong Chong, Mei Chien Chua, Melvin Khee-Shing Leow, Michelle Zhi Ling Kee, Min Gong, Mya Thway Tint, Navin Michael, Ngee Lek, Oon Hoe Teoh, Priti Mishra, Queenie Ling Jun Li, Sambasivam Sendhil Velan, Seng Bin Ang, Shirong Cai, Si Hui Goh, Sok Bee Lim, Stella Tsotsi, Stephen Chin-Ying Hsu, Sue-Anne Ee Shiow Toh, Suresh Anand Sadananthan, Teng Hong Tan, Tong Wei Yew, Varsha Gupta, Victor Samuel Rajadurai, Wee Meng Han, Wei Wei Pang, Wen Lun Yuan, Yanan Zhu, Yin Bun Cheung, Yiong Huak Chan, and Zai Ru Cheng
References
- 1.Bateson P, et al. Developmental plasticity and human health. Nature. 2004;430(6998):419. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 2.Hanson M, et al. Developmental plasticity and developmental origins of non-communicable disease: theoretical considerations and epigenetic mechanisms. Progress in biophysics and molecular biology. 2011;106(1):272–280. doi: 10.1016/j.pbiomolbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, et al. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ, et al. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Y, et al. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ. 2019;367 doi: 10.1136/bmj.l6398. p. l6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Vargas L, et al. Gestational Diabetes and the Offspring: Implications in the Development of the Cardiorenal Metabolic Syndrome in Offspring. Cardiorenal Medicine. 2012;2(2):134–142. doi: 10.1159/000337734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Dam JM, et al. Reduced Cortical Excitability, Neuroplasticity, and Salivary Cortisol in 11–13-Year-Old Children Born to Women with Gestational Diabetes Mellitus. EBioMedicine. 2018;31:143–149. doi: 10.1016/j.ebiom.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker DJ, et al. Weight in infancy and death from ischaemic heart disease. The Lancet. 1989;334(8663):577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson JG, et al. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318(7181):427–431. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adane AA, et al. Maternal preconception weight trajectories are associated with offsprings' childhood obesity. Int J Obes (Lond) 2018;42(7):1265–1274. doi: 10.1038/s41366-018-0078-1. [DOI] [PubMed] [Google Scholar]
- 11.El-Heis S, et al. Maternal stress and psychological distress preconception: association with offspring atopic eczema at age 12 months. Clin Exp Allergy. 2017;47(6):760–769. doi: 10.1111/cea.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards LJ, McMillen IC. Periconceptional nutrition programs development of the cardiovascular system in the fetal sheep. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2002;283(3):R669–R679. doi: 10.1152/ajpregu.00736.2001. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair KD, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proceedings of the National Academy of Sciences Dec. 2007;104(49):19351–19356. doi: 10.1073/pnas.0707258104. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watkins AJ, et al. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. The Journal of Physiology. 2008;586(8):2231–2244. doi: 10.1113/jphysiol.2007.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortensen ELK, et al. Maternal preconceptional nutrition leads to variable fat deposition and gut dimensions of adult offspring mice (C57BL/6JBom) International Journal Of Obesity. 2010;34:1618. doi: 10.1038/ijo.2010.91. [DOI] [PubMed] [Google Scholar]
- 16.Tanwar V, et al. Preconception Exposure to Fine Particulate Matter Leads to Cardiac Dysfunction in Adult Male Offspring. J Am Heart Assoc. 2018;7(24):e010797. doi: 10.1161/JAHA.118.010797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bringhenti I, et al. Maternal obesity during the preconception and early life periods alters pancreatic development in early and adult life in male mouse offspring. PLoS One. 2013;8(1):e55711. doi: 10.1371/journal.pone.0055711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming TP, et al. Origins of lifetime health around the time of conception: causes and consequences. The Lancet. 2018;391(10132):1842–1852. doi: 10.1016/S0140-6736(18)30312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soh S-E, et al. Cohort Profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. International Journal of Epidemiology. 2013;43(5):1401–1409. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 20.Sainz RD, Urlando A. Evaluation of a new pediatric air-displacement plethysmograph for body-composition assessment by means of chemical analysis of bovine tissue phantoms. Am J Clin Nutr. 2003;77(2):364–70. doi: 10.1093/ajcn/77.2.364. [DOI] [PubMed] [Google Scholar]
- 21.Ellis KJ, et al. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr. 2007;85(1):90–5. doi: 10.1093/ajcn/85.1.90. [DOI] [PubMed] [Google Scholar]
- 22.Ma G, et al. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am J Clin Nutr. 2004;79(4):653–60. doi: 10.1093/ajcn/79.4.653. [DOI] [PubMed] [Google Scholar]
- 23.Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res. 2003;53(3):486–92. doi: 10.1203/01.PDR.0000049669.74793.E3. [DOI] [PubMed] [Google Scholar]
- 24.Pillsbury BLK. “Doing the month”: Confinement and convalescence of Chinese women after childbirth. Social Science & Medicine. Part B: Medical Anthropology. 1978;12:11–22. [PubMed] [Google Scholar]
- 25.Loy S, et al. Female adiposity and time-to-pregnancy: a multiethnic prospective cohort. Human Reproduction. 2018;33(11):2141–2149. doi: 10.1093/humrep/dey300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wise LA, Palmer JR, Rosenberg L. Body size and time-to-pregnancy in black women. Human reproduction (Oxford, England) 2013;28(10):2856–2864. doi: 10.1093/humrep/det333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernard JY, et al. Associations of physical activity levels and screen time with oral glucose tolerance test profiles in Singaporean women of reproductive age actively trying to conceive: the S‐PRESTO study. Diabetic Medicine. 2019 doi: 10.1111/dme.13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser A, et al. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tylavsky FA, et al. Understanding childhood obesity in the US: the NIH environmental influences on child health outcomes (ECHO) program. International Journal of Obesity. 2020;44(3):617–627. doi: 10.1038/s41366-019-0470-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kishi R, et al. Cohort profile: the Hokkaido study on environment and children's health in Japan. Int J Epidemiol. 2011;40(3):611–8. doi: 10.1093/ije/dyq071. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh C-J, et al. The Taiwan Birth Panel Study: a prospective cohort study for environmentally-related child health. BMC research notes. 2011;4:291–291. doi: 10.1186/1756-0500-4-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, et al. Cohort profile: the Shanghai Birth Cohort. Int J Epidemiol. 2019;48(1):21–21g. doi: 10.1093/ije/dyy277. [DOI] [PubMed] [Google Scholar]
- 33.Wise LA, et al. Design and Conduct of an Internet-Based Preconception Cohort Study in North America: Pregnancy Study Online. Paediatr Perinat Epidemiol. 2015;29(4):360–71. doi: 10.1111/ppe.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Contreras ZA, et al. Does early onset asthma increase childhood obesity risk? A pooled analysis of 16 European cohorts. The European respiratory journal. 2018;52(3) doi: 10.1183/13993003.00504-2018. p. 1800504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inskip HM, et al. Cohort profile: The Southampton Women's Survey. International Journal of Epidemiology. 2005;35(1):42–48. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.