Abstract
Additive manufacturing (AM) allows the fabrication of customized bone scaffolds in terms of shape, pore size, material type and mechanical properties. Combined with the possibility to obtain a precise 3D image of the bone defects using computed tomography or magnetic resonance imaging, it is now possible to manufacture implants for patient-specific bone regeneration. This paper reviews the state-of-the-art of the different materials and AM techniques used for the fabrication of 3D-printed scaffolds in the field of bone tissue engineering. Their advantages and drawbacks are highlighted. For materials, specific criteria, were extracted from a literature study: biomimetism to native bone, mechanical properties, biodegradability, ability to be imaged (implantation and follow-up period), histological performances and sterilization process. AM techniques can be classified in three major categories: extrusion-based, powder-based and liquid-base. Their price, ease of use and space requirement are analyzed. Different combinations of materials/AM techniques appear to be the most relevant depending on the targeted clinical applications (implantation site, presence of mechanical constraints, temporary or permanent implant). Finally, some barriers impeding the translation to human clinics are identified, notably the sterilization process.
Keywords: additive manufacturing, bone regeneration, 3D printing, scaffolds, tissue engineering
1. Introduction
For the last four decades, additive manufacturing (AM), also called rapid prototyping or solid free form fabrication or 3D printing, has emerged as a promising set of fabrication techniques in different fields including in biomedical research.[1] Indeed, the market of 3D-printed medical devices is predicted to reach $26 billion by 2022.[2] AM is especially used in bone tissue engineering,[3] where the aim is to regenerate bones as close as possible to native bones, by providing a 3D template, named hereafter scaffold, with macropores in which cells and blood can penetrate.[3] Indeed, AM has changed the design and fabrication of scaffolds since it allows, at a very reasonable cost, a complete control over the scaffold architecture that can be easily adapted to the needs. Thus, it is more and more used by clinicians and engineers to treat bone defects as shown by Ricles et al.[2] Moreover, AM allows to replicate bone anisotropy, which is an important property but usually difficult to replicate with more conventional methods such as freeze-drying or sol-gel manufacturing methods.[4] Nowadays, 5-10% of bone fractures result in critical-size bone defects, meaning that they are not able to self-heal.[5] Currently, the gold standard to repair this kind of defects is the autologous graft.[6] However, this solution is far from being ideal since it is painful and requires several surgeries, the quantity of bone is limited, and there is morbidity at the donor site.[7] Thus, researchers are focusing their efforts on the development of synthetic bone graft substitutes, called synthetic scaffolds. Initially, the aim of using scaffolds was simply to replace bones and to act as “space filler”. Progressively, the research has evolved and the idea is now that these scaffolds would actively guide bone regeneration in order to reconstruct a new bone similar to the native bone.[8] These scaffolds should ideally have a physico-chemical composition and mechanical properties close to that of native bone. For example, bone has an elastic modulus ranging 3-20 GPa,[9] so similar properties are expected from the scaffolds if they are not fixed with osteosynthesis plates. Else, they should have sufficient mechanical properties to support bone growth and to ensure that the newly-formed bone will have similar mechanical properties compared with native bone. Moreover, natural bone is composed of an organic phase and approximately 50% of calcium phosphates (inorganic phase) and has a specific internal architecture.[10] As a result, the scaffold needs to be macroporous and have interconnected pores to allow cells and fluids to invade the scaffold and to activate the bone formation process.[11] The porous structure will also favor cell migration, adhesion and proliferation. This will allow the newly-formed bone to mimic natural bone composition and architecture.[1] Before AM, conventional techniques such as sol-gel method, gas foaming or freeze-drying were used to manufacture bone scaffolds.[11] However, these techniques did not allow a thorough control over the architecture of the 3D scaffold.[12] On the other hand, AM offers the possibility to customize both the global shape and internal structure of the scaffold at high reproducibility and reliability. This provides a unique possibility to fabricate patient-specific implants, which enable to reduce the surgery time, since the surgeon does not need to adjust the implant to the patient anymore.[13–15] Thus, AM is used in personalized medicine and particularly well adapted for bone defects resulting from trauma or tumor resection where the defects shapes can be very complex.
AM workflow consists in different steps that have already been described.[11,16] First, the 3D scaffold is designed using a computer aided design (CAD) software and the design is exported as a standard triangulation language file (stl file), which is a standard file format for 3D printing. Then, the stl file is imported in a software called a slicer, which is dividing the 3D structure into several 2D cross-layers and compute the path that the AM machine needs to take to fabricate the 3D scaffold. Finally, the scaffold can be manufactured using an extrusion-based, powder-based or vat photopolymerization machine depending on the raw material. After the fabrication, some post-treatment may be needed depending on the machine and material used. This post-treatment may be a material removal or sintering of the piece to render it more mechanically resistant. The development of AM is tightly related to that of imaging techniques, which provide the precise parameters of the bone defects. Among the existing imaging techniques, computed tomography (CT) and magnetic resonance imaging (MRI) are the most used to image bones since their strong contrast allows to distinguish easily bone from soft tissue. CT is by far more used than MRI since it is cheaper and faster, but the advantage of MRI is to avoid X-rays scans, which may damage the human body.[17] The CT-scans data or MRI files are used to produce a 3D CAD model of the scaffold that is perfectly adapted to the shape of the defect.[18–20] As a result of this precise 3D defect imaging followed by AM fabrication techniques, it is possible to manufacture a scaffold that perfectly fits into a particular defect. This customization is particularly interesting for clinical use in the context of personalized medicine.
Several groups have already reviewed the different types of AM machines and materials that can be used for bone regeneration.[11,21–29] Extrusion-based, powder-based and vat photopolymerization AM techniques allow to print polymers, ceramics and metals that are biocompatible and sometimes biodegradable. However, although AM has already been widely used in research in the biomedical field, still very few products are available on the market to date. Yet, many pre-clinical and some clinical studies are currently conducted to prove the biosafety and efficiency of these products.
The objectives of the present progress report are to provide a view on materials and additive manufacturing techniques that are specifically used to repair bone defects. We will more particularly focus on the materials and processes that can be translated to the clinics. Thus, we will highlight the specificities and criteria that are required for an effective clinical translation. We will survey the state-of-the art in the development of the different techniques and materials with a particular focus on pre-clinical experiments in large animals and clinical experiments in humans.
2. Synthetic bone graft materials
In bone tissue engineering, six criteria are important to consider when choosing the material to be used (Table 1): (i) biomimetism, i.e its ability to mimic the properties of native bone and to interact well with the tissue that will be regenerated; the biocompatibility is included in this criterion; (ii) mechanical properties, namely the elastic modulus and compression strength, that should be sufficient to support bone growth and not too high to avoid stress shielding; (iii) long-term biodegradability since ideally, the scaffold should be progressively replaced by newly-formed bone until it completely disappears from the body, so the biodegradation rate and safety of the biodegradation products are included in this criterion;[30] (iv) the ease of imaging without altering the information in order to follow its positioning during the post-operative period and bone growth without interference with native bone, which is determined by the contrast between the host bone and the scaffold material;[30] (v) the ability to easily cut histological slices without spoiling it and (vi) its compatibility with the sterilization processes that are clinically approved. Biomimetism also includes the osteoconductive and osteoinductive properties of the materials. Osteoconductivity is the ability of a material to guide bone regeneration while osteoinductivity is its ability to activate the bone progenitor cells and to trigger bone repair.[31] The fifth criterion is important mainly for the pre-clinical studies, where there is a need to assess the efficiency and quality of newly-formed bone, eventual signs of foreign body reaction, the maturity and type of newly-formed bone, neo-vascularization, as well as its interactions with the scaffold (that is, the contact with the scaffold surface or not, penetration in the pores, etc.).
Table 1.
Performances of the materials analyzed based on six criteria: biomimetism, mechanical properties, biodegradability, ease of imaging, ability to perform histological sections for pre-clinical studies and sterilization process Materials are qualitatively ranked from -- (very bad properties) to +++ (excellent properties) based on the literature analysis.
| Material | Examples | Biomimetism | Mechanical properties | Biodegradability | Imaging (µCT) | Histology | Sterilization process |
|---|---|---|---|---|---|---|---|
| Natural polymers | Collagen, HA, gelatin | +++ | -- | +++ | +++ | +++ | Ethylene oxide[43,44] |
| Synthetic biodegradable polymers | PCL, PLGA, PLA | + | + | +++ | +++ | +++ | UV,[56] γ-irradiation,[57] β-irradiation[59] |
| Synthetic non degradable polymers | PEEK, PEKK | + | +++ | -- | +++ | +++ | γ-irradiation[70] |
| Ceramics | HAP, TCP | +++ | +/- | + | - | + | γ-irradiation[81] |
| Metals | Ti | ++ | ++ | -- | + | - | Autoclaving[111,112] |
The sterilization step is important to prevent infection and is needed to obtain a regulatory approval.[32] The different sterilization processes that can be used are ethylene oxide sterilization, steam sterilization (also called autoclaving), hydrogen peroxide plasma sterilization (simply called plasma sterilization afterwards), γ-irradiation, electron-beam irradiation (also called β-irradiation) and UV sterilization.[32] The technical details of each process have been reviewed elsewhere.[32] Each material can react differently to the different processes, so it is important to determine which type of sterilization process is suitable for a given material (see Table 1). Indeed, the sterilization process may induce adverse reactions such as changes in physico-chemical properties or morphology and might produce toxic byproducts by degrading the material.[32] Yet, the sterilization step is too often neglected and considered as secondary as can be seen in Table 3, where the information is often missing. Table 3 reviews pre-clinical and clinical studies on 3D-printed scaffolds for bone regeneration and for each study, the sterilization process used was reported. In fact, the question of the sterilization process is crucial and needs to be raised at the very beginning of the implant development.[32] The availability and cost of some types of sterilization processes may influence the choice of the implant material. Indeed, the adverse effects of sterilization of a given material should be known in order to make the best choice for the targeted application. If not carefully thought at the beginning of the study design, the question of sterilization may quickly become a blocking point.
Table 3.
Translational potential for each type of material associated to a given fabrication technique Only pre-clinical studies on large animals (rabbit or larger) and clinical studies were included except one on rats (in italic). For each study, the scaffold modifications, sterilization process, animal model and site of implantation are given. The translational potential was evaluated regarding the bone regeneration performances in vivo, the criteria of Figure 3, the post-processing steps, the sterilization process used and the dimensional resolution and accuracy of the techniques.
| Type of material | Material | AM technique | Scaffold modifications | Sterilization process | Animal | Site of implantation | Translation to clinics |
|---|---|---|---|---|---|---|---|
| Synthetic biodegradable polymers | PCL | SLS | rhPDGF-BB | Ethylene oxide | Human [51] | Periodontal | - depends on the application site |
| ∅ | NA | Pig[18] | Condylar ramus unit | ||||
| ∅ | NA | Rabbit[55] | Lateral epicondyle of the femur | ||||
| FDM | ∅ | UV[117] | Rabbit[117,118] | Calvaria | ++ | ||
| ∅ | NA | Goat[53] | Femur | ||||
| Nano-PCL coating,[119] autologous BMSCs,[119] BMP-2,[119] Bone marrow coating[121] | Ethanol soak[119] | Pig[119–121] | Calvaria,[119] Orbit[120,121] | ||||
| ∅ | Ethylene oxide [52] | Human [52] | Fresh extraction socket [52] | ||||
| MHDS | Deproteinized bovine bone grafting material | NA | Dog[86] | Alveolar | + | ||
| Dispense plotting | Fibrin, BMSC, BMP-2[12] | NA | Rabbit[12,54] | Tibia,[54] Ulna[12] | + depends on the application site | ||
| Filled with β-TCP powder | Dog[78] | Alveolar | |||||
| PLA | FDM | ∅ | Ethanol soak | Rat [122] | Subcutaneous | + | |
| PLGA | Inkjet printing | ∅ | NA | Rabbit[143] | Iliac crest | ++ | |
| Dispense plotting | ∅ | NA | Rabbit[54] | Tibia | + depends on the application site | ||
| Osteoblast-like cells derived from bone or periost | Plasma | Sheep[93] | Calvaria | ||||
| Synthetic non-degradable polymers | PEKK | SLS | Autologous ovine MSC | Autoclaving | Sheep[67] | Calvaria | + depends on the application site |
| ∅ | NA | Human [68] | Cervical | ||||
| PEEK | Inkjet printing | ∅ | NA | Human [61] | Scapula | ++ | |
| FDM | ∅ | NA | Human [69] | Rib | ++ | ||
| Ti-coated | Rabbit[63] | Tibia | |||||
| Ceramics | α-TCP | Inkjet printing | ∅ | Autoclaving [13,75] | Human [13,75,76] | Maxillofacial,[13,76] Facial [75] | +++ |
| NA | Dog[137] | Skull | |||||
| β-TCP | Robocasting | Bioglass or mesoporous bioglass coating[124] | NA | Rabbit[123–125] | Mandible,[123] Alveolar,[125] Skull[124] | ++ | |
| TCP | Dispense plotting | Collagen coating and osteoblast-like cells derived from bone or periost | Plasma | Sheep[93] | Calvaria | + | |
| DCP | Inkjet printing | ∅ | γ-irradiation | Goat[141] | Lumbar | ++ | |
| HA | SLA | ∅ | γ-irradiation | Human [77] | Skull | ++ | |
| Robocasting | ∅ | γ-irradiation | Rabbit[126] | Calvaria | +++ | ||
| Inkjet printing | ∅ | γ-irradiation | Rabbit[138,139] | Calvaria | + | ||
| OCP | Inkjet printing | ∅ | NA | Rabbit[140] | Cranial | + | |
| BCP | Robocasting | BMP-2 | Heating | Rabbit and pig[127] | Tibia (rabbit), maxillary (pig) | +++ | |
| Dispense plotting | Filled with autologous blood,[128] oAEC[129] | γ-irradiation[128] | Sheep[128–130] | Calvaria,[128] Sinus[129,130] | ++ | ||
| ∅ | NA | Dog[131] | Mandible | ||||
| DLP | ∅ | NA | Dog[146] | Mandible | - | ||
| Bioglass | Dispense plotting | BMP/CS and BMP/CS + rBMSCs | NA | Monkey[132] | Alveolar | +++ | |
| DLP | EPC/BMSC | NA | Rabbit[147] | Mandible | + | ||
| MgP | Dispense plotting | ∅ | NA | Rabbit[133] | Calvaria | -- | |
| Sr-HT-Gahnite | Robocasting | ∅ | NA | Rabbit[134] | Calvaria | ++ | |
| Composites | PCL/PLGA | MHDS | Filling with collagen containing rhBMP-2[85] | UV[85] | Rabbit[85,87] | Calvaria,[87] Radius[85] | ++ depends on the application site |
| PCL/PLGA/β-TCP | MHDS | Filling with collagen containing rhBMP-2[90] | NA | Rabbit[87,90] | Calvaria | ++ | |
| Filling with deproteinized bovine bone grafting material | Dog[88,89] | Dental | |||||
| PCL/β-TCP | MHDS | Deproteinized bovine bone grafting material | NA | Dog[86] | Alveolar | +++ | |
| Inkjet printing | pBMPC | Ethylene oxide | Pig[91] | Mandible | - | ||
| FDM | ∅ | NA | Rabbit[92] | Calvaria | +++ | ||
| rhBMP-7;[82,83] autologous BMSC;[82] MPC, tOB, mOB[84] | Sheep[82–84] | Tibia | |||||
| Lyophilisation with bovine collagen + rhBMP-2 | Pig[135] | Spine | |||||
| SLS | ∅ | NA | Sheep[74] | Tibia | -- | ||
| PLGA/β-TCP | FDM | HAP coating | Ethylene oxide | Rabbit[73] | Femur | + | |
| PCL/HA | SLS | ∅ | NA | Rabbit[55] | Lateral epicondyle of the femur | ++ | |
| FDM | ∅ | Ethanol soak | Goat[53] | Femur | +++ | ||
| BMSC | NA | Dog[94] | Sternal | ||||
| PLA/HA | FDM | ∅ | Ethanol soak | Rabbit[64] | Femur | +++ | |
| TCP/Chitosan/Collagen hydrogel | Dispense plotting | Osteoblast-like cells derived from bone or periost | Plasma | Sheep[93] | Calvaria | + | |
| Metals | Ti and Ti6Al4V | SLM | ∅ | NA | Human [99,100,107] | Maxillofacial,[99] Alveolar [100] , Orbital wall [107] | ++ |
| Pig[97] | Tibia | ||||||
| EBM | ∅ | NA | Human [101–103] | Skull,[101] Chest,[102] Sternocostal [103] | ++ | ||
| Sheep[104] | Spine | ||||||
| Rabbit[98] | Tibia | ||||||
| DMLS | Filling with bone graft and covering with PRGF[105] | NA | Human [17,20,105,106] | Cranial,[17] Dental,[105] Maxillofacial,[106] Orbital wall [20] | ++ |
rhPDGF-BB: recombinant human platelet-derived growth factor BB; BMSC: bone marrow-derived stem cell; BMP-2: bone morphogenetic protein 2; DCP: dicalcium phosphate; OCP: octacalcium phosphate; BCP: biphasic calcium phosphates (HA/TCP); oAEC: ovine amniotic epithelial cell; BMP/CS: BMP-2 gene loaded nanoparticles; EPC: endothelial progenitor cell; rBMSCs: rhesus bone marrow stem cells; MgP: magnesium phosphate; Sr-HT-Gahnite: strontium doped Hardystonite and Gahnite; pBMPC: porcine bone marrow progenitor cell; MPC: allogenic bone marrow-derived mesenchymal progenitor cells; tOB: allogenic osteoblasts isolated from the axial skeleton; mOB: allogenic osteoblasts isolated from the orofacial skeleton; Ti6Al4V: alloy of titanium widely used in the biomedical field; PRGF: platelet rich in growth factor
In terms of materials type, four types of materials are currently used as bone graft substitutes: polymers, ceramics, composites of polymers and ceramics, and metals. Table 1 gives our analysis of the six selected criteria, based on the literature study. Moreover, Figure 1 offers a global view of the major sites where bone graft substitutes are used in the human body and the kind of materials used to repair the different bone defects.
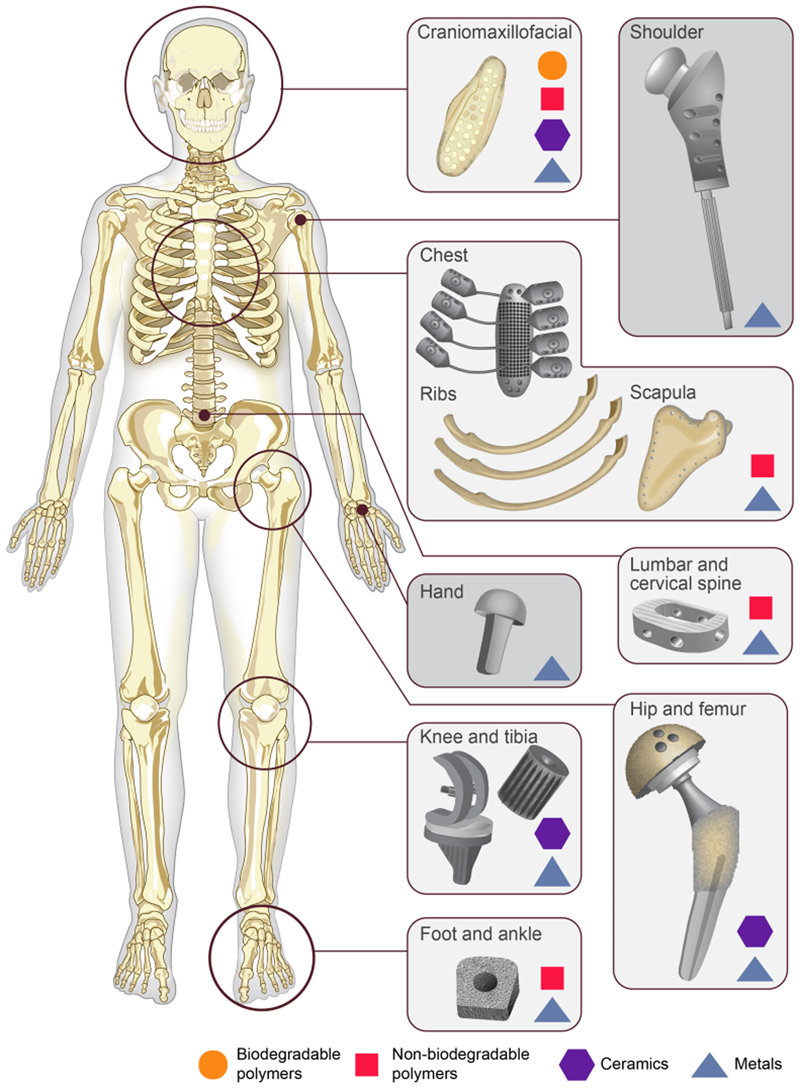
Figure 1.
Major implantation sites in the body where synthetic bone graft substitutes are used for bone repair For each implantation site, the different types of implantable materials already used in clinics (biodegradable polymers, non-biodegradable polymers, ceramics, metals) are presented.
2.1. Polymers
Polymers are divided in two groups: natural and synthetic polymers. Natural polymers, eg chitosan, collagen, hyaluronic acid, gelatin, silk fibroin … have excellent biocompatibility and biodegradation properties. They are usually degraded by natural enzymes within 2 to 24 weeks depending on the polymer crosslinking level, without producing any acidic byproducts.[26,33,34] However, they are mechanically weak and cannot sustain the forces exerted on bones (Table 1).[26] Indeed, their compressive modulus ranges from 0.005 MPa to 90 MPa whereas cancellous bone compressive modulus is higher than 100 MPa.[35–37] Thus, they are mainly used as additives or composites for their osteoinductive properties and their ability to enhance cell and protein adhesion as they benefit from excellent biomimetism (Table 1).[21] Some pre-clinical studies suggest that silk fibroin and chitosan could be used alone to heal craniofacial defects but these studies were only conducted on small animals (mice and rat) and have to be proved successful on larger animals.[38–42] Usually, ethylene oxide sterilization is used on natural polymers since it is a low temperature and gentle sterilization process (Table 1).[32,43,44] In contrary, both electron-beam irradiation and autoclaving are not recommended: electron-beam irradiation accelerates the degradation rate of the scaffolds and increases the chemical crosslinking of the material to use, while autoclaving reduces the viscosity and mechanical properties of the materials.[43,44]
Synthetic polymers are man-made. They can be biodegradable (poly-ε-caprolactone (PCL), poly(lactide-co-glycolide) (PLGA), poly(lactic acid) (PLA), poly(propylene fumarate) (PPF)…) or non-biodegradable, such as poly(ether ether ketone) (PEEK) and poly(ether ketone ketone) (PEKK). Biodegradable polymers are easily printable with good dimensional resolutions thanks to their low melting point (~60°C for PCL and ~175°C for PLA)[45] which makes it easy to heat the material without the need for a complex heating system.[22] Their degradation rate is tunable and is usually longer than one to two years. Their biodegradation induces the release of low acidic byproducts for PCL and non-toxic byproducts for PLA and PLGA.[23,46,47] They are also biocompatible and osteoconductive.[21] They have good mechanical properties with a compressive strength of the order of 2-39 MPa as compared to the native bone compressive strength (2-12 MPa) and compressive modulus (52-318 MPa), these values depending on the scaffold porosity)[22,48–50], are radiolucent, lightweight and histocompatible which is an important criterion for preclinical studies (Table 1).[23,24] However, they are not highly biomimetic when used alone since they are unable to induce bone formation by themselves (Table 1). These materials are often used in cranio-maxillofacial bone reconstruction (Figure 1),[51,52] and some pre-clinical studies look at their application in load-bearing sites such as femoral defects or tibiae defects.[12,53–55] Different processes are suitable to sterilize biodegradable polymers: UV exposure,[56] γ-irradiation and β-irradiation (Table 1).[57,58] However, autoclaving, ethylene oxide and plasma sterilization should be avoided because they induce a shrinkage of the materials,[58,59] toxicity and physical alterations respectively.[56] According to our literature study, biodegradable polymers appear to have the best overall score in Table 1. Their major drawback is the lack of osteoinductivity that can be addressed using different strategies listed in section 4.
Non-degradable polymers have mechanical properties that are closer to native bone with a compressive modulus ranging from 0.14 to 8.21 MPa and compressive strength ranging from 25 to 200 MPa depending on the scaffold porosity[60]. They are also biocompatible (Table 1).[61] Similarly to other polymers, they are radiolucent,[62] which makes them easy to image and compatible with histological imaging (Table 1). However, they are harder to print with extrusion machines because their melting point is very high (300-360°C as indicated in polymerdatabase.com) so the machines have to be thermally isolated and equipped with a high power heater.[63] Moreover, they are usually hydrophobic, resulting in a poor interaction with cells or growth factors and a low osseointegration (i.e. a poor integration of the scaffold with the host bone), altering their biomimetism (Table 1).[62,64] As a result, they often need a surface treatment such as gas plasma or coating with thin layers of other materials such as titanium or hydroxyapatite,[65,66] which improves the osseointegration.[63] These polymers are FDA-approved for craniomaxillofacial and spine fusion surgery (Figure 1).[67,68] They are also often used to repair ribs or sternal defects (Figure 1) and their potential to regenerate tibiae defects is studied on animals.[61,63,69] Some commercial products made of non-degradable polymers exist, including screws for foot and ankle bone healing (Figure 1). The main sterilization technique used for non-degradable polymers is γ-irradiation (Table 1). Autoclaving should be avoided since it induces changes in the material physicochemical properties.[70,71] The score of non-degradable polymers in Table 1 is lower than that of biodegradable polymers since the non-degradability of the material can be problematic and may require a surgery to remove the material, which is invasive and risky for the patient.
2.2. Ceramics
Ceramics are widely studied as bone graft substitute materials since they have similar composition with the inorganic phase of natural bone so they benefit from excellent biomimetism (Table 1).[35] Indeed, they are osteoconductive as well as sometimes osteoinductive.[21] The most widely used ceramics in bone tissue engineering are calcium phosphates, namely hydroxyapatite (HA) and tricalcium phosphate (TCP). Ceramics are mainly biodegradable but at different rates (Table 1).[72] However, ceramics are very brittle, and therefore not appropriate for load-bearing applications despite their high compressive strength which ranges from 3 to 18 MPa (Table 1).[22,23,73] Moreover, they are not easy to image and to distinguish from the native bone, due to their similar composition and density to native bones, which also complicates the histological analysis (Table 1).[74] Usually, ceramics are used to repair all kinds of defects especially in the craniofacial region (Figure 1).[13,75–77] They can also be used in composite materials, as scaffolds fillers and as coatings to enhance the bioactivity of a scaffold.[78,79] γ-irradiation is the best sterilization process for ceramics (Table 1), while autoclaving and ethylene oxide have been proven to degrade some calcium phosphates phases.[80,81]
2.3. Composites
In order to optimize the mechanical and biological properties of the implants, composites of ceramics and polymers can be manufactured. The most widely used composite is poly(ε-caprolactone) (PCL)/β-tricalcium phosphate (β-TCP). PCL degradation rate is very slow, so the addition of β-TCP allows for a higher degradation rate and better mechanical properties in compression as well as enhanced osteoinductivity.[82] PCL/β-TCP is often used in pre-clinical studies to treat femoral or tibiae bone defects,[64,73,74,82–85] but also craniomaxillofacial bone defects.[86–93] Other biodegradable polymers and ceramics are used in composites such as PLA, PLGA and HA (Table 3).[53,55,64,73,94] There are also composites made of ceramics and natural polymers which are used in the craniomaxillofacial region (Table 3).[93]
2.4. Metals
The most widely used metals in bone tissue engineering are titanium and its alloys as well as stainless steel, which are all non-degradable metals (Table 1). They have very good biocompatibility properties and their mechanical properties are close to natural bone, even a bit higher (compression modulus between 5 and 35 GPa and compression strength between 50 and 325 MPa) (Table 1).[21,95] The biocompatibility of titanium is particularly high thanks to the titanium oxide film that is formed upon corrosion. This film is known to be antibacterial and thus enhances the biocompatibility and biomimetism of the material (Table 1).[96] In view of their high mechanical properties, they are often used in load-bearing sites such as tibiae defects,[97,98] but also in craniofacial and spine fusion surgery and in chest reconstruction (Figure 1).[17,99–107] Metals are also used to manufacture hip prosthesis,[108] shoulder prosthesis and commercial screws used in hand, foot and ankle bone repair.[109] Nevertheless, they have important drawbacks which limit their use in vivo. Indeed, they may release metallic ions through corrosion or wear which may lead to tissue loss and because of their high elastic modulus, they can generate stress shielding.[9,23,110] Moreover, they are difficult to image because of their high density (Table 1).[62,110] Autoclaving is the main sterilization technique used on metals since it gives better histologic results than gamma- or UV-sterilized scaffolds (Table 1).[111,112] However, cutting metals slides for histology is challenging because of the hardness of the material (Table 1).
3. Additive manufacturing for bone regeneration
AM has revolutionized the fabrication of scaffolds for bone tissue engineering. The ability to customize both the global shape and internal architectures, as well as the possibility to perfectly fit the defect shape regardless of its complexity have driven the development of AM in bone tissue engineering.[11]
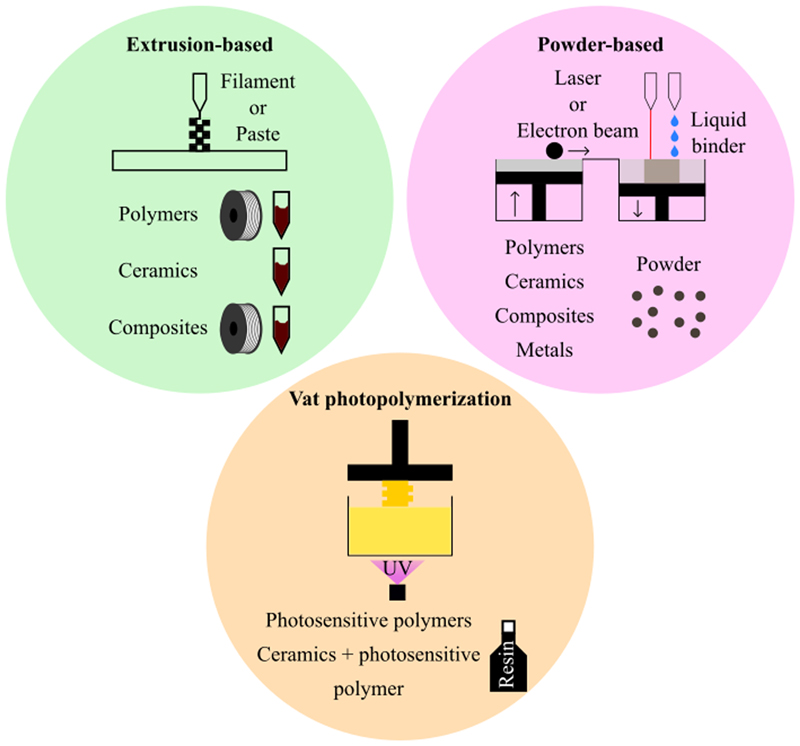
The different AM techniques used in bone tissue engineering can be divided in three major groups:[113] i) extrusion-based techniques: the material is deposited using a nozzle fixed on a robotic arm, ii) powder-based techniques: the material is in the form of particles that constitute a powder, which is then sintered or chemically bounded and iii) vat photopolymerization techniques: a liquid resin is photopolymerized using UV light. Each technique has its own characteristics, which are reported in Table 2. Table 2 compares the different AM techniques regarding seven criteria: i) the dimensional resolution, i.e. the size of the minimum printable feature ; ii) the dimensional accuracy, which quantifies the match between the actual and theoretical dimensions of the printed piece ; iii) the machine cost; iv) the material cost which depends on the technique used; v) the machine size; vi) the ease of use of the machine and vii) the translational potential of the technique taking into account the six previous criteria. Figure 2 offers a representation of each group of techniques associated to the materials that it can process. The materials forms are also represented.
Table 2.
Comparison of the different AM techniques based on seven criteria: i) the dimensional resolution reachable; ii) the dimensional accuracy of the technique; iii) the machine cost; iv) the material cost which depends on the technique used; v) the machine size; vi) the ease of use of the machine and vii) the translational potential of the technique taking into account the six previous criteria The two last criteria are qualitatively ranked from -- (very bad) to +++ (excellent).
| Dimensional resolution | Dimensional accuracy | Machine cost | Material cost | Machine size | Ease of use | Translational potential | ||
|---|---|---|---|---|---|---|---|---|
| Extrusion-based | FDM | 100 μm | ± 0.5 mm | 3 k$ | 30 $/kg | 0.15 m3 | +++ | +++ |
| Robocasting | 100 μm | Particle size dependent | 10 k$ | 3-10 $/g | 1 m3 | ++ | ++ | |
| Dispense plotting | 100-250 μm | NA | 100 k$ | 10-75 $/mL | 1 m3 | ++ | ++ | |
| MHDS | 100 μm | NA | NA | 20 $/g | NA | ++ | ++ | |
| Powder-based | Inkjet printing | 100 μm | ± 0.3 mm | 5-200 k$ | 3-10 $/g | 1 m3 | ++ | +++ |
| SLS | Particle size dependent | ± 0.3 mm | 40 k$ | 3-10 $/g | 8 m3 | ++ | ++ | |
| SLM | 100 μm | ± 0.1 mm | 300 k$ | 100 $/kg | 8 m3 | + | + | |
| DMLS | 100 μm | ± 0.1 mm | 400 k$ | 100 $/kg | 4 m3 | + | + | |
| EBM | 50 μm | ± 0.1 mm | 500 k$ | 100 $/kg | 4 m3 | -- | - | |
| VPP | SLA | 25 μm | 0.01-0.1 mm | 10 k$ | 150 $/L | 0.15-6 m3 | ++ | - |
| DLP | 35 μm | ± 0.1 mm | 10 k$ | 150 $/L | 0.5 m3 | ++ | - |
Figure 2.
The three major types of AM techniques: extrusion-based techniques, powder-based techniques and vat photopolymerization techniques For each one, the types of materials that can be used and their form (filament, paste, powder…) are indicated.
3.1. Extrusion-based techniques
Fused Deposition Modeling (FDM), robocasting (also called direct-write assembly or direct ink writing), dispense plotting (also called material extrusion or bioplotting) and Multi Head Deposition System (MHDS) are all AM techniques based on the extrusion of one or several materials. The materials are processed in the form of a filament as in the case of FDM or as a paste (dispense plotting, MHDS and robocasting) (Figure 2). They are loaded into a nozzle or a syringe that is fixed on a robotic arm, which will execute the path defined by the slicer. The material is dispensed from the nozzle using heat (FDM) or pressure (robocasting, dispense plotting and MHDS). The precise technical explanations of each technique will not be detailed here since it has already been reviewed by several groups.[11,22,25,29,114–116]
Extrusion printing, notably FDM, is the most widely used AM technique since it allows to print a large variety of materials, including polymers,[12,52–54,63,69,78,86,93,117–122] ceramics,[93,123–134] and composites (Figure 2)[53,64,73,82–90,92–94,135] at a low cost and with good accuracy (± 0.5 mm according to 3D Hubs, see Table 2). Indeed, polymers such as PCL, PLA, PLGA or PEEK are easily fabricated in the form of filaments, or as powder that can be mixed to a solution to form a paste. Ceramics are largely available as powders and can form a paste when mixed with polymers such as polyvinyl alcohol (PVA). Finally, composites of polymers and ceramics can be fabricated in filament form by embedding ceramics particles into the polymer matrix, or in paste form. Metals cannot be printed via extrusion-based machines because their melting point is too high and is not reachable using this type of techniques. Moreover, the machines are relatively small compared to other techniques, which makes their transport and use in hospital easier (Table 2). Also, a short training is sufficient to learn how to use the machines as they are quite intuitive (Table 2).[136] Thus, they may potentially be easily implemented in the context of a hospital (Table 2).
The major drawback of these techniques is the dimensional resolution. Indeed, it is impossible to print features of size below 100 µm (Table 2).[116] However, this resolution is sufficient to print synthetic bone graft substitutes since the ideal pore diameter for bone growth should be higher than about 400 µm.[134] The dimensional accuracy of extrusion-based techniques is technique-dependent but known to be lower for dispense plotting (Table 2). For robocasting, it will depend on the ceramics particles sizes that are put in suspension into the polymer matrix (Table 2). Also, dispense plotting often requires a post-processing to improve the mechanical properties of the printed parts, which is not the case for the other techniques.
3.2. Powder-based techniques
In these techniques, materials are in a powder-form (Figure 2). The major powder-based techniques used in bone tissue engineering are inkjet printing, selective laser sintering (SLS), selective laser melting (SLM), direct metal laser sintering (DMLS) and electron beam melting (EBM). The technical details of the different techniques are explained elsewhere.[11,21,22,24–26,29] Briefly, the powder is set in a collector and then spread using a roller on the building plate. After that, a laser or an ink composed of a liquid binder follows the path calculated by the slicer to sinter or melt the powder particles to fuse them together. A new layer of powder is spread and the process is repeated until completion of the part. The dimensional resolution of this type of techniques is better than for the extrusion-based techniques. Indeed, it is possible to reach a resolution of about 50 µm but it is technique-dependent (Table 2).[1,116] However, the dimensional accuracy is not always very high, especially for the sintering techniques, the melting techniques being more precise (Table 2). The dimensional accuracy also depends on the particles sizes and on the technique used.[136] Usually, the printed parts will exhibit a rough surface (except with SLM) with porosities due to the partial fusion of the particles (sintering is at a lower temperature compared to melting).[116]
These techniques are used to print ceramics (inkjet printing,[13,75,76,137–141] SLS),[25,142] polymers in powder-form (SLS,[18,50,51,55,67,68] inkjet printing),[61,143] composites (SLS,[74] inkjet printing)[91] and metals (SLM,[97,99,100,107] DMLS,[17,20,105,106] EBM, Figure 2).[98,101–104] All types of materials can be printed using a laser since they can all be found in powder form and the laser has sufficient power to fuse all kinds of particles. However, metals cannot be printed by inkjet printing because the liquid binder can not induce the fusion of metal particles. Powder-based machines have excellent dimensional resolution and their dimensional accuracy depends on the technique but is sufficient for bone tissue engineering. Indeed, the minimum accuracy required for bone tissue engineering depends on the pore size that is printed but dimensional errors of less than 200 μm are considered as satisfying (Table 2).[144] For example, the dimensional accuracy of SLS and inkjet printing is ± 0.3 mm while it is ± 0.1 mm for DMLS, SLM and EBM (according to 3D Hubs, see Table 2).[145] However, these techniques tend to be very expensive and the machines, which are bulky, need to be installed in a large room (Table 2). Last but not least, they require an extensive training before using them safely and with sufficient know-how (Table 2).
3.3. Vat photopolymerization techniques
Stereolithography (SLA) is the main vat photopolymerization (VPP) technique. [11,22,26] A resin-based material is located in a collector. The building plate moves along the z direction to enable the spreading of the resin at its surface. Then, a UV laser or a UV light projection (for the technique called digital light processing, DLP) is drawing the path designed in the slicer, which will photopolymerize the resin. These steps are repeated layer by layer until the part is completed. At the end of the printing process, a post-processing step is needed to remove the resin from the material and to cure the piece. Two-photon polymerization is also a vat photopolymerization technique but is not used in bone tissue engineering. Indeed, its build volume is very limited, the building speed is low and the compatible materials limited.[116]
SLA and DLP can be used to print photosensitive polymers and ceramics scaffolds (Figure 2).[77,146,147] Without a photoinitiator, the materials cannot be printed: this requirement excludes ceramics and metals alone. It is versatile and has an excellent resolution at ∼25 µm and accuracy (± 0.1 mm according to 3D Hubs, see Table 2).[22] However, it is not commonly used in bone tissue engineering since the resin can be cytotoxic.[136,148] Besides, it is quite expensive,[14] even if the machines are small and their use intuitive (Table 2).
3.4. Toward clinical application
As already mentioned above, a great advantage of AM is that it allows a customization of the implant architecture. To have the best fit possible between the implant and the bone defect dimensions, a whole procedure is often followed in clinics.[18–20,69,106] After obtaining a 3D model of the defect from imaging data, the DICOM files resulting from these acquisitions are converted to stl files so that the 3D model can be sliced in 2D cross-layers and finally 3D-printed. This production of the defect model helps to plan the surgery, identify the difficulties, decide the shape of the implant and requirements for its fixation. Then, the scaffold is designed to fit the 3D model using a CAD software. A first print of the scaffold is usually made to assess its perfect fit with the 3D model. Then the final scaffold is manufactured. This procedure is being democratized for the production of patient-specific implants in personalized medicine. To note, CT-based models contained an average error of 0.15 mm while the MRI-based models contained an average error of 0.23 mm.[149] Therefore, the accuracy of CT and MRI does not limit the ability of AM to produce high precision parts since the error of CT-based and MRI-based model is lower than the error of fabrication via AM (usually ~ 0.3 mm, i.e. 300 µm).
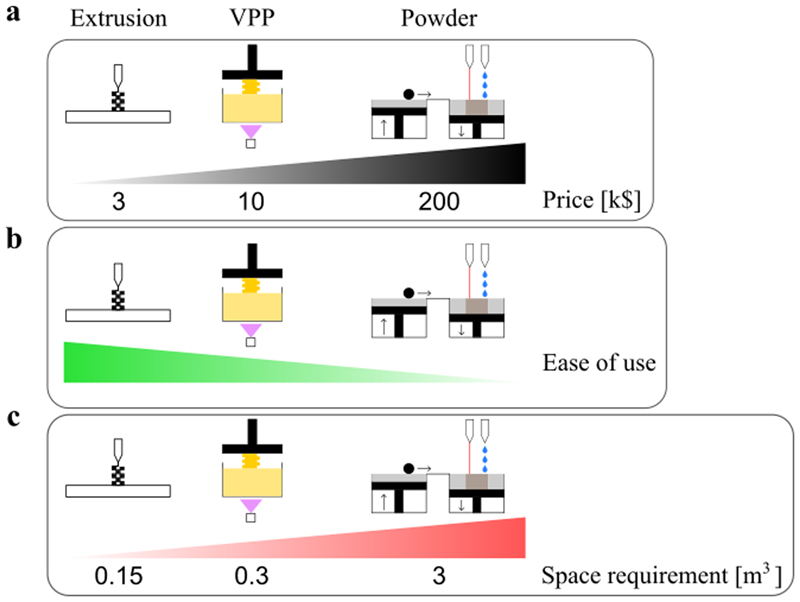
In view of clinical translation, AM techniques need to be relatively cheap, easy to use and ideally easy to install and use directly in a hospital. The different groups of fabrication techniques were compared regarding these criteria by summarizing the information from Table 2 (Figure 3). In Figure 3A, the price (in k$) of the AM techniques does not take into account the price of materials which is the lowest for the extrusion-based techniques (around 30$/kg, Makershop, see Table 2) and the highest for the vat photopolymerization techniques (around 150$/L, Formlabs). However, researchers should keep the materials prices in mind because facing two materials with the same bone regeneration potential, clinicians will prefer using the cheapest product. While extrusion-based machines are the cheapest (~3 k$ in average), vat photopolymerization ones are a bit more expensive (~10 k$ in average) and the powder-based machines are very expensive due to the use of complex technologies (~200 k$ in average). The ease of use of each technique (Figure 3B) is evaluated by considering the time needed for a beginner to be able to produce a part (training needed) and the complexity to print a part, i.e. how many operations are needed from the beginning of the print to the post-treatment. For example, vat photopolymerization machines are harder to use than extrusion-based ones because toxic products are used so the user has to be particularly focused and a post-treatment is required after each print. Another example, EBM requires the use of secondary vacuum, which makes its use more complex. The space requirement of each technique (Figure 3C) was measured as the volume needed to install a machine in a room. Extrusion-based machines do not require a lot of space since they just have to be installed on a bench. The average space needed to install an extrusion-based machine is 0.15 m3 (Figure 3C). Vat photopolymerization machines require only a small space but space is needed for the post-treatment processes, notably the removal of the resin, which may require to work under a hood. Thus the average space needed to install a vat photopolymerization machine in a room is 0.3 m3 (Figure 3C). Depending on the specific technique used (SLS, SLM, DMLS, EBM or inkjet printing), powder-based machines may require less space than what is indicated on Figure 3C but an average volume of 3 m3 was chosen. For example, if a SLS machine requires approximately the same space as a FDM one, an EBM machine requires much more space because of the pumps needed to reach a secondary vacuum.
Figure 3.
(a) Price of the AM machines indicated on a scale in k$ From left to right: extrusion-based machines, vat photopolymerization machines (VPP) and powder-based machines, these later ones being the most expensive ones.
(b) Ease of use of the different machines, assessed based on the training required and the complexity of the machine The scale corresponds to the difficulty level, from easy to difficult. From left to right: extrusion-based machines, VPP machines and powder-based machines.
(c) Space requirement of each technique estimated in m3. From left to right: extrusion-based machines, VPP machines and powder-based machines.
For each criterion, extrusion-based techniques appear to have a slight advantage over powder-based techniques which are more complex to implement. However, since there is a large number of techniques, and since each technique has different characteristics, different techniques are adapted for different clinical use. Some inkjet printing machines may be interesting for a clinical application. Vat photopolymerization could be more widely used in the future if the availability of biocompatible materials increases. The performances of extrusion-based techniques and especially FDM were also confirmed in a recent study comparing the dimensional accuracy of different AM techniques for the fabrication of an anatomical mandibular model.[150]
In order to use AM directly in hospitals, it is also important to be aware of the regulations. According to a recent study, it appears relatively easy to implement custom-made fabrication of bone implants directly in healthcare facilities since hospitals are not required to fully meet the requirements of the regulation that industrials need to meet.[151] However, a strict quality control of the scaffolds implanted should be implemented. For now, the fabrication of AM scaffolds directly in hospitals is still rare. As a result, there are no study on the quality control of the scaffolds printed and implanted. Each surgeon is responsible for the safety control of each product he/she implanted.
4. Translational studies on custom-made synthetic bone grafts
As mentioned earlier, although a large number of pre-clinical studies are currently being conducted on materials and AM techniques for bone tissue engineering, still very few products are commercially available to date. In this part, we review translational studies on custom-made synthetic bone grafts. From this analysis, we aim to understand the specific difficulties associated to the clinical translation.
In addition to the criteria already discussed and reported in Figure 3, the scaffolds produced have to perform well in animal models in view of clinical application. In Table 3, different couples of materials and fabrication techniques are reviewed for their clinical use. The materials are put in relation to the fabrication techniques since the materials or AM machines performances alone are not relevant for a clinical application. Instead, it is rather the performance of the couple material/machine that provides a more complete information about the potential for clinical translation. In Table 3, we reviewed pre-clinical and clinical studies on 3D-printed scaffolds and reported the eventual scaffold modifications, the sterilization process used as well as the animal model and site of implantation targeted in each study. Then, we rated the potential for clinical translation for the materials combined with fabrication techniques using a scale from very low (--) to promising (+++). Our rating is based on the following parameters: i) fabrication performances (dimensional accuracy and resolution), ii) need for post-processing, related to the time required to manufacture a scaffold, and iii) bone regeneration performances of the implant in vivo. Thus, it appears that several combinations of materials and AM techniques are promising for a clinical use, notably PCL/dispense plotting with bone marrow-derived stem cells (BMSC) seeding and bone morphogenetic proteins 2 (BMP-2) loading,[12] α-TCP/inkjet printing which has already been successfully tested on humans,[13,75,76,137] some ceramics printed via robocasting with or without BMP-2,[126,127] bioglass/dispense plotting with BMP and BMSC,[132] PCL/β-TCP printed via MHDS or FDM and with different scaffold modifications,[82–84,86] and PCL and PLA with HA printed via FDM with or without BMSC seeding.[53,64,94] Indeed, for these combinations, the dimensional accuracy was better than 100 μm, post-processing was needed only when using inkjet printing (immersion in a curing solution for 6h) or robocasting (heating and sintering) and bone regeneration performances were encouraging with bridging of the defects, new bone formation in good quantity and quality and patients satisfied with the implants in the case of clinical studies.[13,53,54,64,75,76,82–84,86,94,126,127,132,137] PLA combined with FDM as well as PLGA combined with inkjet printing have a high potential for clinical translation too but the scaffolds need to be modified since the materials alone are only osteoconductive but not osteoinductive.[122,143] Thus, their osteogenic performances cannot be high enough without any modification.
We limited this progress report to studies on large animals (rabbit, sheep, goat, dog, pig or monkey) and humans. Indeed, small animal models (mice, rats) are useful to assess the very first steps of the implant development, notably biocompatibility, osteoinductivity and bone repair but have too small bones to be replaced by 3D printed scaffolds. In fact, it is not possible to architecture a scaffold that is adapted to a too small bone defect.[84] In vitro studies are not included either since a lot of successful in vitro studies do not lead to good results in vivo because of a lack of similarity between the conditions.[134] To note, only one pre-clinical study on rats was included since the material used (PLA) combined with the FDM process appears to have a clinical potential (Table 3), although it has not been widely studied to date.[122]
4.1. Application site
The vast majority of pre-clinical studies on large animals are for applications in the cranio-maxillofacial region, which represent ∼64% of the pre-clinical studies reported in Table 3).[18,67,78,86–93,117–121,123–134,137–140,146,147] Around 32% of the pre-clinical studies reviewed in Table 3 are conducted in load-bearing sites.[12,53,54,63,64,73,74,82–85,97,98,104,127,135,141] This difference may be due to the specific requirement for bone healing in a load-bearing sites: good mechanical properties of the scaffolds are a prerequisite.
It is very important to identify good material/process couples to translate what has been achieved so far in cranio-maxillofacial to weight-bearing applications.
To complete the information provided by Table 3, clinical trials were searched on ClinicalTrials.gov. The research was done using these key words: “TCP”, “ankle”, “3D” and “3D printing”. The associated topics were “Bone diseases” for the first three key words and “bone” for the last one. There were respectively 7, 85, 201 and 14 studies found. Among them, only the studies focusing on bone regeneration using a 3D printed implant were included in this progress report.
75 % of the clinical studies in Table 3 and three clinical trials (NCT03735199, NCT03608280 and NCT03673865) have been conducted in the cranio-maxillofacial region.[13,17,20,51,52,75–77,99–101,105–107] Other clinical studies in Table 3 were on chest (ribs and sternocostal repair) and scapula,[61,69,102,103] which are not load-bearing sites. One clinical trial has focused on 3D printing for ankle bone repair lately (NCT03185286) but no results were published. The other clinical trials found in load-bearing sites are for applications in spine surgery (NCT02926391 (see Amelot et al. results)[68], NCT03018392, NCT03647501, NCT04086784 and NCT03761563).
Inkjet printed α-TCP implants are already brought to the clinic but only for craniofacial bone defects (Table 3).[13,75,76] These implants have a high potential to be translated and developed as commercial products. However, they are too brittle for load-bearing applications.
The clinical studies reported in Table 3 have brought some new information about the physico-chemical properties of the scaffolds required to obtain satisfying clinical results, depending on the targeted application site. Thus, it has been proven that a scaffold made of PCL is not ideal for dental applications since its degradation rate is too slow.[51,52] This degradation rate should be faster than one year and ideally between 5 and 6 months but PCL degrades in more than two years. Furthermore, dental implants should be porous (a porosity of about 70% was proven to lead to good results)[52] with interconnected pores, which would avoid wound dehiscence and exposure and therefore microbial contamination.[51] A pore size between 100 and 400 μm is considered as ideal for these applications.[105] Sumida et al. [100] showed that a 3D printed titanium dental implant with pores of 1mm in diameter led to less infection and a reduced operation time compared to available commercial products. Thus, titanium appears as a better solution than PCL for dental implants although its non-degradability induces other issues. For the treatment of facial bone deformities (maxillofacial deformities included), macroporous scaffolds made by α-TCP and printed via inkjet printing gave good results with bone bridging and satisfied patients.[13,75,76] Large pores of 1-2 mm in diameter lead to better results than smaller pores. SLM-printed macroporous Ti6Al4V also showed good results in terms of both reconstruction and patient satisfaction.[99] Macroporous scaffolds with pore sizes between 300 and 550 μm and strut sizes between 150 and 500 μm are the best candidates for the treatment of cranial defects.[77,101] Interestingly, they can be made of ceramics or Ti6Al4V. These pore sizes, along with a 50-70% porosity, do not induce any foreign body reaction but promote bone ingrowth. For orbital wall reconstruction, the use of titanium is encouraged compared to PEEK because titanium has better osseointegration, osteoinduction, stiffness and thermoconduction properties than PEEK.[107] Titanium scaffolds with pore sizes of 860 to 3000 μm and a porosity greater than 50% have shown good results and led to patients satisfaction.[20,107]
For applications in tibia, femur or ulna repair using titanium, polymer or composite scaffolds, the pre-clinical studies of Table 3 show that large pore sizes of at least 550 μm with a fully interconnected pore structure lead to results in terms of bone formation results.[12,53,54,82,83,97,98] Moreover, a porosity > 60% leads to better bone ingrowth than lower porosity.[73,74,82,83,98] Contrary to what is required for dental implants, a slower degradation rate is better for these application sites to provide a sustained mechanical support until full bone regeneration.[54] Thus, the use of PCL is preferred rather than PLGA for example. Furthermore, PLGA is more brittle and less ductile than PCL, which are undesirable properties for bone regeneration in load-bearing sites. For the fabrication of lumbar interbody fusion cages, titanium, ceramics or composite scaffolds are used but no consensus has been found regarding ideal pore size and porosity for an optimized bone repair although pore interconnectivity seems to be preferable.[104,135,141]
It thus appears that most in vivo studies are conducted in the cranio-maxillofacial region. Indeed, it has already been proven that AM is useful for clinical applications in cranio-maxillofacial surgery.[152] While the number of pre-clinical studies conducting in load-bearing sites is increasing, there are still few clinical studies resulting in these sites. It is now important to find ways to translate the knowledge gained from the cranio-maxillofacial to the load-bearing sites.
4.2. Additional modifications to the scaffolds
Since the majority of materials used in bone tissue engineering is osteoconductive but not osteoinductive, different strategies have been implemented to modify the scaffolds in order to make them osteoinductive.[5] The most widely used strategy is to seed cells in the scaffold prior to its implantation. In this case, stem cells are cultured in vitro and then seeded at the surface of the scaffold, before the cell-containing scaffold is implanted. The underlying idea is that cells should enable a faster bone regeneration in vivo thanks to their secretion of various growth factors and chemokines.[153] Around 15% of studies reviewed in Table 3 used this strategy.[12,67,82,84,91,93,94,119,129,132] The cells used were usually stem cells derived from the animal’s bone marrow. These cells improve the scaffold biocompatibility and osseointegration and are able to differentiate into osteogenic cells and to recruit host cells, which results in a higher quantity of newly-formed bone.[154] However, this technique is time consuming because it requires to culture the cells in vitro prior scaffold implantation. Moreover, this strategy is more complicated to implement from a regulatory point of view because the addition of cells to the implant makes it fall in the specific category called “advanced therapy medicinal products”.[155] Thus, the number of experiments before achieving a clinical application is increased as well as the time and cost associated to the implant development.
About 10% of the studies of Table 3 explored a second strategy, which consists in the loading of growth factors inside the scaffold or at its surface.[12,51,82,83,85,90,119,127,132,135] The most used growth factors to trigger stem cells differentiation in bone cells are the bone morphogenetic proteins (BMP), especially BMP-2 and BMP-7 that are FDA- and EMEA-approved.[156] The BMPs can either be loaded onto the scaffold surface after the scaffold fabrication or loaded into a hydrogel that fills the scaffold.[90,127] However, the affinity between the scaffold material and the growth factor has to be sufficient to allow a controlled release in vivo and avoid the use of supraphysiological doses.[157] In few examples, a combination of the two previous strategies is used to optimize the formation of new bone.[12,132]
Finally, the third major strategy used to improve the bioactivity of a scaffold is its coating or filling with bone graft, bone marrow, calcium phosphates or titanium.[63,73,86,88,89,121,124] About 8% of studies reviewed in Table 3 used this strategy. The coating (or filling) will make the scaffold more bioactive and it will interact more easily with its surrounding environment. It is also possible to use the two last strategies together, by coating the scaffold with a film or membrane that will allow a more efficient growth factors delivery.[90]
According to studies in Table 3, the use of BMPs may seem to be more efficient than the use of cells for bone regeneration. Indeed, Kang et al. [12] compared a PCL scaffold loaded with BMP-2 with a PCL scaffold loaded with BMSCs and showed that after the two first months of implantation, more bone was apparent on radiographs with BMP-2 than with BMSCs. This difference was less evident at three months but was more visible on histological sections. Similarly, Jensen et al. .[119] showed the same difference between a PCL scaffold loaded with mononuclear cells and a PCL scaffold loaded with BMP-2 and also showed that less material degradation happened with cells than with BMP-2. Reichert et al. proved that a PCL/β-TCP scaffold loaded with BMP-7 led to more newly-formed bone and better biomechanical properties than a PCL/β-TCP scaffold loaded with MSCs.[82] However, Kang et al. and Wang et al. both showed that a combination of BMPs and BMSCs was the most efficient strategy to induce bone formation.[12,132]
The coating of scaffolds with titanium or hydroxyapatite led to more bone formation but not significantly.[63,73] On the other hand, coating scaffolds with bone marrow or a nanolayer of bioactive glass induced significantly more bone formation but less in comparison with the loading of BMPs.[121,124]
Thus, based on this articles, the most efficient strategy to render a scaffold osteoinductive appears to be its loading with growth factors. The combination of growth factors and cells is promising but difficult to implement since regulations are tight. The dual use of growth factor andc cells leads to a longer preparation time of the scaffolds, which is an advantage in view of a clinical application.
4.3. Barriers to the clinical translation
Despite these progresses toward the application of 3D printed bone scaffolds in the clinics, several barriers impeding the translation remain. The first barrier is the lack of availability of medical grade materials for additive manufacturing. Indeed, although some materials such as PCL, PLGA, PLA, calcium phosphates and titanium exist in medical grade with sometimes only few provides, this is not the case for all the materials. Moreover, the medical grade materials are expensive. Another barrier to the translation of additive manufactured implants is the cost associated to the manufacturing. Indeed, as every piece is unique because it is patient-specific, the cost of each piece will be much higher than pieces from industrial batches. Furthermore, translating the AM technology to the clinics in hospital will require onsite training at hospital of clinicians or assistants, so that they can use the machines and associated software: this will take time and be costly. As mentioned earlier, the sterilization of the scaffolds is an important step. However, there are only few studies regarding the effects of sterilization on the materials. Moreover, the sterilization studies are often conducted on raw materials and very few are conducted on the materials processes via AM. Since the fabrication process may modify some of the materials properties, it would be useful to study the effect of different sterilization processes on 3D-printed scaffolds.
5. Conclusions and perspectives
The treatment of bone defects and especially critical-sized bone defects remains challenging since it requires the use of an implant that needs to match a large number of requirements. Indeed, it should i) be biocompatible and preferably biodegradable, ii) be sufficiently porous to let cells and fluids penetrate but mechanically strong enough to resist physiological loads, iii) fit the shape of the defect, (iv) be easily processed and v) be easily manipulated by surgeons. AM offers the possibility to fabricate customized scaffolds with an external shape perfectly adapted to a given defect and an internal architecture optimizing bone repair. Combining AM with the right materials and scaffolds modifications enables the control of the mechanical and biological properties of the implant. Nevertheless, clinical applications of these technologies still lack.
Here, we reviewed different associations of materials with AM techniques and evaluated their potential for clinical translation. We then identified promising combinations of materials and processes to help researchers in translating the technologies to the clinic. Thus, PCL associated with dispense plotting,[12] different ceramics printed via robocasting,[126,127] inkjet printing or dispense plotting,[13,75,76,132,137] and composites of biodegradable polymers and ceramics combined with MHDS or FDM were rated as promising because of their high bone regeneration performances in vivo.[53,64,82–84,86,94]
Barriers to the clinical translation were also identified. The availability of medical grade materials, the cost associated to the fabrication of patient-specific products, the training of clinicians or assistants and the sterilization of scaffolds have been identified as current obstacles to the application of 3D printed scaffolds in the clinics. Efforts from the research community and AM engineers should be concentrated on these points to facilitate the clinical translation easier.
It is difficult to adapt what has already been done in the cranio-maxillofacial region to load-bearing sites, in particular for the treatment of segmental long bone defects. Indeed, the bones to repair and their environments are different and require implants with different physico-chemical properties. As a result, more pre-clinical studies on load-bearing applications should be conducted and these studies need to be performed on large animals, ideally on the targeted implantation site, such as to be clinically relevant.
Acknowledgements
CP is a senior member of the Institut Universitaire de France whose support is greatly acknowledged. The work was supported by the “Association Gueules Cassées” (contract n° 21-2016 and 10-2018); by the European commission under the PF7 program (European Research Council grant BIOMIM GA259370 and Proof of Concept REGENERBONE 790435 to CP) and by the ANR (Grant OBOE, ANR-18-CE17-0016).
Biographies
Author biographies
 Charlotte GAROT received her Master degree from the Ecole Centrale de Lille (France) in 2019. She is currently a Ph.D. student in the Equipe de Recherche labelisée (ERL) “Biomimetism and regenerative medicine”(ERL 5000, CNRS/UGA/CEA) at the University of Grenoble Alpes under the supervision of Dr. Catherine Picart and Prof. Georges Bettega. Her research project is focused on the development of bioactive medical devices for bone regeneration.
Charlotte GAROT received her Master degree from the Ecole Centrale de Lille (France) in 2019. She is currently a Ph.D. student in the Equipe de Recherche labelisée (ERL) “Biomimetism and regenerative medicine”(ERL 5000, CNRS/UGA/CEA) at the University of Grenoble Alpes under the supervision of Dr. Catherine Picart and Prof. Georges Bettega. Her research project is focused on the development of bioactive medical devices for bone regeneration.
 Georges BETTEGA is Professor of maxillofacial surgery at Annecy Genevois hospital in France. He is member of the French society of stomatology and maxillofacial surgery and of the European Association of cranio-maxillo-facial Surgeons. His research focuses on reconstructive surgery specially bone reconstruction and on computer assisted surgery. He is a researcher at the Institute of Advanced Biosciences in Grenoble (INSERM-UGA U1209/CNRS UMR 5309), belonging to the “Cancer Targets and Experimental Therapeutics” team.
Georges BETTEGA is Professor of maxillofacial surgery at Annecy Genevois hospital in France. He is member of the French society of stomatology and maxillofacial surgery and of the European Association of cranio-maxillo-facial Surgeons. His research focuses on reconstructive surgery specially bone reconstruction and on computer assisted surgery. He is a researcher at the Institute of Advanced Biosciences in Grenoble (INSERM-UGA U1209/CNRS UMR 5309), belonging to the “Cancer Targets and Experimental Therapeutics” team.
 Catherine PICART is research director at CEA, direction of fundamental research (DRF), director of the Department of Health of “Interdisciplinary Research Institute of Grenoble” (IRIG). She also leads the “Biomimetism and Regenerative Medicine (BRM)” research team (ERL 5000, CNRS/CEA/UGA). She is senior member of the Institute Universitaire de France (IUF) (2016-2021). Her research is focused on tissue engineering, biomaterials, medical devices, drug delivery, molecular and cellular biophysics. Over the past 10 years, she was the PI of 4 ERC grants.
Catherine PICART is research director at CEA, direction of fundamental research (DRF), director of the Department of Health of “Interdisciplinary Research Institute of Grenoble” (IRIG). She also leads the “Biomimetism and Regenerative Medicine (BRM)” research team (ERL 5000, CNRS/CEA/UGA). She is senior member of the Institute Universitaire de France (IUF) (2016-2021). Her research is focused on tissue engineering, biomaterials, medical devices, drug delivery, molecular and cellular biophysics. Over the past 10 years, she was the PI of 4 ERC grants.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Javaid Mohd, Haleem A. J Clin Orthop Trauma. 2019;10:380. doi: 10.1016/j.jcot.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ricles LM, Coburn JC, Di Prima M, Oh SS. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan6521. eaan6521. [DOI] [PubMed] [Google Scholar]
- [3].Bagaria V, Deshpande S, Rasalkar DD, Kuthe A, Paunipagar BK. Eur J Radiol. 2011;80:814. doi: 10.1016/j.ejrad.2010.10.007. [DOI] [PubMed] [Google Scholar]
- [4].Rajasekharan AK, Lotsari A, Lutz-Bueno V, Liebi M, Andersson M. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201800466. 1800466. [DOI] [PubMed] [Google Scholar]
- [5].Ho-Shui-Ling A, Bolander J, Rustom LE, Johnson AW, Luyten FP, Picart C. Biomaterials. 2018;180:143. doi: 10.1016/j.biomaterials.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Laurencin C, Khan Y, El-Amin SF. Expert Rev Med Devices. 2006;3:49. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- [7].Pape HC, Evans A, Kobbe P. J Orthop Trauma. 2010;24:S36. doi: 10.1097/BOT.0b013e3181cec4a1. [DOI] [PubMed] [Google Scholar]
- [8].Holzapfel BM, Reichert JC, Schantz J-T, Gbureck U, Rackwitz L, Nöth U, Jakob F, Rudert M, Groll J, Hutmacher DW. Adv Drug Deliv Rev. 2013;65:581. doi: 10.1016/j.addr.2012.07.009. [DOI] [PubMed] [Google Scholar]
- [9].Liu C, Ren Z, Xu Y, Pang S, Zhao X, Zhao Y. Scanning. 2018;2018:1. doi: 10.1155/2018/9216314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Krut’ko VK, Kazbanov VV, Musskaya ON, Gaidash AA, Kulak AI, Chekan NM, Serdobintsev MS, Skrotskaya KV. Tech Phys. 2019;64:121. [Google Scholar]
- [11].Yang Y, Wang G, Liang H, Gao C, Peng S, Shen L, Shuai C. Int J Bioprinting. 2018;5:148. doi: 10.18063/IJB.v5i1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kang S-W, Bae J-H, Park S-A, Kim W-D, Park M-S, Ko Y-J, Jang H-S, Park J-H. Biotechnol Lett. 2012;34:1375. doi: 10.1007/s10529-012-0900-0. [DOI] [PubMed] [Google Scholar]
- [13].Saijo H, Igawa K, Kanno Y, Mori Y, Kondo K, Shimizu K, Suzuki S, Chikazu D, Iino M, Anzai M, Sasaki N, et al. J Artif Organs. 2009;12:200. doi: 10.1007/s10047-009-0462-7. [DOI] [PubMed] [Google Scholar]
- [14].Gul M, Arif A, Ghafoor R. J Indian Soc Periodontol. 2019;23:504. doi: 10.4103/jisp.jisp_46_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hikita A, Chung U, Hoshi K, Takato T. Tissue Eng Part A. 2017;23:515. doi: 10.1089/ten.TEA.2016.0543. [DOI] [PubMed] [Google Scholar]
- [16].Trevisan F, Calignano F, Aversa A, Marchese G, Lombardi M, Biamino S, Ugues D, Manfredi D. J Appl Biomater Funct Mater. 2018;16:57. doi: 10.5301/jabfm.5000371. [DOI] [PubMed] [Google Scholar]
- [17].Jardini AL, Larosa MA, de Carvalho C, Zavaglia A, Bernardes LF, Lambert CS, Kharmandayan P, Calderoni D, Maciel Filho R. Virtual Phys Prototyp. 2014;9:115. [Google Scholar]
- [18].Smith MH, Flanagan CL, Kemppainen JM, Sack JA, Chung H, Das S, Hollister SJ, Feinberg SE. Int J Med Robot. 2007;3:207. doi: 10.1002/rcs.143. [DOI] [PubMed] [Google Scholar]
- [19].van Eijnatten M, van Dijk R, Dobbe J, Streekstra G, Koivisto J, Wolff J. Med Eng Phys. 2018;51:6. doi: 10.1016/j.medengphy.2017.10.008. [DOI] [PubMed] [Google Scholar]
- [20].Salmi M, Tuomi J, Paloheimo K, Björkstrand R, Paloheimo M, Salo J, Kontio R, Mesimäki K, Mäkitie AA. Rapid Prototyp J. 2012;18:209. [Google Scholar]
- [21].Maroulakos M, Kamperos G, Tayebi L, Halazonetis D, Ren Y. J Dent. 2019;80:1. doi: 10.1016/j.jdent.2018.11.004. [DOI] [PubMed] [Google Scholar]
- [22].Rider P, Kačarević Ž, Alkildani S, Retnasingh S, Schnettler R, Barbeck M. Int J Mol Sci. 2018;19:3308. doi: 10.3390/ijms19113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang Y, Liu X, Zeng L, Zhang J, Zuo J, Zou J, Ding J, Chen X. Adv Funct Mater. 2019;29 1903279. [Google Scholar]
- [24].Zhang X-Y, Fang G, Zhou J. Materials. 2017;10:50. [Google Scholar]
- [25].Guzzi EA, Tibbitt MW. Adv Mater. 2019 doi: 10.1002/adma.201901994. 1901994. [DOI] [PubMed] [Google Scholar]
- [26].Wubneh A, Tsekoura EK, Ayranci C, Uludağ H. Acta Biomater. 2018;80:1. doi: 10.1016/j.actbio.2018.09.031. [DOI] [PubMed] [Google Scholar]
- [27].Trombetta R, Inzana JA, Schwarz EM, Kates SL, Awad HA. Ann Biomed Eng. 2017;45:23. doi: 10.1007/s10439-016-1678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bose S, Vahabzadeh S, Bandyopadhyay A. Mater Today. 2013;16:496. [Google Scholar]
- [29].Youssef A, Hollister SJ, Dalton PD. Biofabrication. 2017;9 doi: 10.1088/1758-5090/aa5766. 012002. [DOI] [PubMed] [Google Scholar]
- [30].Moreno Madrid AP, Vrech SM, Sanchez MA, Rodriguez AP. Mater Sci Eng C. 2019;100:631. doi: 10.1016/j.msec.2019.03.037. [DOI] [PubMed] [Google Scholar]
- [31].Bose S, Roy M, Bandyopadhyay A. Trends Biotechnol. 2012;30:546. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tipnis NP, Burgess DJ. Int J Pharm. 2018;544:455. doi: 10.1016/j.ijpharm.2017.12.003. [DOI] [PubMed] [Google Scholar]
- [33].Ratajska M, Strobin G, Wiśniewska-Wrona M, Ciechańska D, Struszczyk H, Boryniec S, Biniaś D, Biniaś W. FIBRES & TEXTILES in Eastern Europe. 2003;11:75. [Google Scholar]
- [34].Rothamel D, Schwarz F, Sager M, Herten M, Sculean A, Becker J. Clin Oral Implants Res. 2005;16:369. doi: 10.1111/j.1600-0501.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- [35].Lan Levengood SK, Polak SJ, Wheeler MB, Maki AJ, Clark SG, Jamison RD, Wagoner Johnson AJ. Biomaterials. 2010;31:3552. doi: 10.1016/j.biomaterials.2010.01.052. [DOI] [PubMed] [Google Scholar]
- [36].Wang S, Zhao Z, Yang Y, Mikos AG, Qiu Z, Song T, Cui F, Wang X, Zhang C. Regen Biomater. 2018;5:283. doi: 10.1093/rb/rby020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Luetchford KA, Chaudhuri JB, De Bank PA. Mater Sci Eng C. 2020;106 doi: 10.1016/j.msec.2019.110116. 110116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Karageorgiou V, Tomkins M, Fajardo R, Meinel L, Snyder B, Wade K, Chen J, Vunjak-Novakovic G, Kaplan DL. J Biomed Mater Res A. 2014;78:324. doi: 10.1002/jbm.a.30728. [DOI] [PubMed] [Google Scholar]
- [39].Riccio M, Maraldi T, Pisciotta A, Sala GBL, Ferrari A, Bruzzesi G, Motta A, Migliaresi C, Pol AD. Tissue Eng Part A. 2017;18:1006. doi: 10.1089/ten.TEA.2011.0542. [DOI] [PubMed] [Google Scholar]
- [40].Sahu N, Baligar P, Midha S, Kundu B, Bhattacharjee M, Mukherjee S, Mukherjee S, Maushart F, Das S, Loparic M, Kundu SC, Ghosh S, Mukhopadhyay A. Adv Healthc Mater. 2015;4:1709. doi: 10.1002/adhm.201500283. [DOI] [PubMed] [Google Scholar]
- [41].Li H, Ji Q, Chen X, Sun Y, Xu Q, Deng P, Hu F, Yang J. J Biomed Mater Res A. 2017;105:265. doi: 10.1002/jbm.a.35900. [DOI] [PubMed] [Google Scholar]
- [42].Issa JPM, Bel EAD, Iyomasa MM, Sebald W. Micron. 2008;39:373. doi: 10.1016/j.micron.2007.03.008. [DOI] [PubMed] [Google Scholar]
- [43].Monaco G, Cholas R, Salvatore L, Madaghiele M, Sannino A. Mater Sci Eng C. 2017;71:335. doi: 10.1016/j.msec.2016.10.030. [DOI] [PubMed] [Google Scholar]
- [44].O’Connell CD, Onofrillo C, Duchi S, Li X, Zhang Y, Tian P, Lu L, Trengove A, Quigley A, Gambhir S, Khansari A, et al. Biofabrication. 2019;11 doi: 10.1088/1758-5090/ab0b7c. 035003. [DOI] [PubMed] [Google Scholar]
- [45].Chen C-C, Chueh J-Y, Tseng H, Huang H-M, Lee S-Y. Biomaterials. 2003;24:1167. doi: 10.1016/s0142-9612(02)00466-0. [DOI] [PubMed] [Google Scholar]
- [46].Farah S, Anderson DG, Langer R. Adv Drug Deliv Rev. 2016;107:367. doi: 10.1016/j.addr.2016.06.012. [DOI] [PubMed] [Google Scholar]
- [47].Hutmacher DW. Biomaterials. 2000;21:2529. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- [48].Liu C-G, Zeng Y-T, Kankala R, Zhang S-S, Chen A-Z, Wang S-B. Materials. 2018;11:1832. doi: 10.3390/ma11101832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Eshraghi S, Das S. Acta Biomater. 2010;6:2467. doi: 10.1016/j.actbio.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, Hollister SJ, Das S. Biomaterials. 2005;26:4817. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- [51].Rasperini G, Pilipchuk SP, Flanagan CL, Park CH, Pagni G, Hollister SJ, Giannobile WV. J Dent Res. 2015;94:153S. doi: 10.1177/0022034515588303. [DOI] [PubMed] [Google Scholar]
- [52].Goh BT, Teh LY, Tan DBP, Zhang Z, Teoh SH. Clin Oral Implants Res. 2015;26:271. doi: 10.1111/clr.12486. [DOI] [PubMed] [Google Scholar]
- [53].Xu N, Ye X, Wei D, Zhong J, Chen Y, Xu G, He D. ACS Appl Mater Interfaces. 2014;6 doi: 10.1021/am502716t. 14952. [DOI] [PubMed] [Google Scholar]
- [54].Park SH, Park DS, Shin JW, Kang YG, Kim HK, Yoon TR, Shin J-W. J Mater Sci Mater Med. 2012;23:2671. doi: 10.1007/s10856-012-4738-8. [DOI] [PubMed] [Google Scholar]
- [55].Xia Y, Zhou P, Cheng X, Xie Y, Liang C, Li C, Xu S. Int J Nanomedicine. 2013;8:4197. doi: 10.2147/IJN.S50685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ghobeira R, Philips C, Declercq H, Cools P, De Geyter N, Cornelissen R, Morent R. Biomed Mater. 2017;12 doi: 10.1088/1748-605X/aa51d5. 015017. [DOI] [PubMed] [Google Scholar]
- [57].Turker NS, Özer AY, Çolak Ş, Kutlu B, Nohutçu R. Appl Radiat Isot. 2017;130:121. doi: 10.1016/j.apradiso.2017.09.026. [DOI] [PubMed] [Google Scholar]
- [58].Savaris M, dos Santos V, Brandalise RN. Mater Sci Eng C. 2016;69:661. doi: 10.1016/j.msec.2016.07.031. [DOI] [PubMed] [Google Scholar]
- [59].Zhao Y, Zhu B, Wang Y, Liu C, Shen C. Mater Sci Eng C. 2019;105 doi: 10.1016/j.msec.2019.110041. 110041. [DOI] [PubMed] [Google Scholar]
- [60].Senatov FS, Chubrik AV, Maksimkin AV, Kolesnikov EA, Salimon AI. Mater Lett. 2019;239:63. [Google Scholar]
- [61].Liu D, Fu J, Fan H, Li D, Dong E, Xiao X, Wang L, Guo Z. J Bone Oncol. 2018;12:78. doi: 10.1016/j.jbo.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].McGilvray KC, Easley J, Seim HB, Regan D, Berven SH, Hsu WK, Mroz TE, Puttlitz CM. Spine J. 2018;18:1250. doi: 10.1016/j.spinee.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jung H-D, Jang T-S, Lee JE, Park SJ, Son Y, Park S-H. Biofabrication. 2019;11 doi: 10.1088/1758-5090/ab376b. 045014. [DOI] [PubMed] [Google Scholar]
- [64].Chen X, Gao C, Jiang J, Wu Y, Zhu P, Chen G. Biomed Mater. 2019;14 doi: 10.1088/1748-605X/ab388d. 065003. [DOI] [PubMed] [Google Scholar]
- [65].Barkarmo S, Wennerberg A, Hoffman M, Kjellin P, Breding K, Handa P, Stenport V. J Biomed Mater Res A. 2013;101A:465. doi: 10.1002/jbm.a.34358. [DOI] [PubMed] [Google Scholar]
- [66].Johansson P, Barkarmo S, Hawthan M, Peruzzi N, Kjellin P, Wennerberg A. J Biomed Mater Res A. 2018;106:1440. doi: 10.1002/jbm.a.36345. [DOI] [PubMed] [Google Scholar]
- [67].Adamzyk C, Kachel P, Hoss M, Gremse F, Modabber A, Hölzle F, Tolba R, Neuss S, Lethaus B. J Cranio-Maxillofac Surg. 2016;44:985. doi: 10.1016/j.jcms.2016.04.012. [DOI] [PubMed] [Google Scholar]
- [68].Amelot A, Colman M, Loret J-E. Spine J. 2018;18:892. doi: 10.1016/j.spinee.2018.01.019. [DOI] [PubMed] [Google Scholar]
- [69].Kang J, Wang L, Yang C, Wang L, Yi C, He J, Li D. Biomech Model Mechanobiol. 2018;17:1083. doi: 10.1007/s10237-018-1015-x. [DOI] [PubMed] [Google Scholar]
- [70].Godara A, Raabe D, Green S. Acta Biomater. 2007;3:209. doi: 10.1016/j.actbio.2006.11.005. [DOI] [PubMed] [Google Scholar]
- [71].Kumar A, Yap WT, Foo SL, Lee TK. Bioengineering. 2018;5:18. doi: 10.3390/bioengineering5010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ebrahimi M, Botelho MG, Dorozhkin SV. Mater Sci Eng C. 2017;71:1293. doi: 10.1016/j.msec.2016.11.039. [DOI] [PubMed] [Google Scholar]
- [73].Kim J, McBride S, Tellis B, Alvarez-Urena P, Song Y-H, Dean DD, Sylvia VL, Elgendy H, Ong J, Hollinger JO. Biofabrication. 2012;4 doi: 10.1088/1758-5082/4/2/025003. 025003. [DOI] [PubMed] [Google Scholar]
- [74].Lohfeld S, Cahill S, Barron V, McHugh P, Dürselen L, Kreja L, Bausewein C, Ignatius A. Acta Biomater. 2012;8:3446. doi: 10.1016/j.actbio.2012.05.018. [DOI] [PubMed] [Google Scholar]
- [75].Kanno Y, Nakatsuka T, Saijo H, Fujihara Y, Atsuhiko H, Chung U, Takato T, Hoshi K. Regen Ther. 2016;5:1. doi: 10.1016/j.reth.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Saijo H, Fujihara Y, Kanno Y, Hoshi K, Hikita A, Chung U, Takato T. Regen Ther. 2016;5:72. doi: 10.1016/j.reth.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Brie J, Chartier T, Chaput C, Delage C, Pradeau B, Caire F, Boncoeur M-P, Moreau J-J. J Cranio-Maxillofac Surg. 2013;41:403. doi: 10.1016/j.jcms.2012.11.005. [DOI] [PubMed] [Google Scholar]
- [78].Park S, Lee H-J, Kim K-S, Lee S, Lee J-T, Kim S-Y, Chang N-H, Park S-Y. Materials. 2018;11:238. [Google Scholar]
- [79].Ratheesh G, Venugopal JR, Chinappan A, Ezhilarasu H, Sadiq A, Ramakrishna S. ACS Biomater Sci Eng. 2017;3:1175. doi: 10.1021/acsbiomaterials.6b00370. [DOI] [PubMed] [Google Scholar]
- [80].Wang I-C, Ju C-P, Lin J-HC. Mater Trans. 2005;46:1701. [Google Scholar]
- [81].Eliaz N, Metoki N. Materials. 2017;10:104. doi: 10.3390/ma10040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Reichert JC, Cipitria A, Epari DR, Saifzadeh S, Krishnakanth P, Berner A, Woodruff MA, Schell H, Mehta M, Schuetz MA, Duda GN, Hutmacher DW. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003720. 141ra93. [DOI] [PubMed] [Google Scholar]
- [83].Cipitria A, Wagermaier W, Zaslansky P, Schell H, Reichert JC, Fratzl P, Hutmacher DW, Duda GN. Acta Biomater. 2015;23:282. doi: 10.1016/j.actbio.2015.05.015. [DOI] [PubMed] [Google Scholar]
- [84].Berner A, Henkel J, Woodruff MA, Saifzadeh S, Kirby G, Zaiss S, Gohlke J, Reichert JC, Nerlich M, Schuetz MA, Hutmacher DW. J Tissue Eng Regen Med. 2017;11:2081. doi: 10.1002/term.2104. [DOI] [PubMed] [Google Scholar]
- [85].Shim J-H, Kim SE, Park JY, Kundu J, Kim SW, Kang SS, Cho D-W. Tissue Eng Part A. 2014;20:1980. doi: 10.1089/ten.tea.2013.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Shim J-H, Won J-Y, Park J-H, Bae J-H, Ahn G, Kim C-H, Lim D-H, Cho D-W, Yun W-S, Bae E-B, Jeong C-M, et al. Int J Mol Sci. 2017;18:899. doi: 10.3390/ijms18050899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shim J-H, Moon T-S, Yun M-J, Jeon Y-C, Jeong C-M, Cho D-W, Huh J-B. J Mater Sci Mater Med. 2012;23:2993. doi: 10.1007/s10856-012-4761-9. [DOI] [PubMed] [Google Scholar]
- [88].Shim J-H, Won J-Y, Sung S-J, Lim D-H, Yun W-S, Jeon Y-C, Huh J-B. Polymers. 2015;7:2061. [Google Scholar]
- [89].Won J-Y, Park C-Y, Bae J-H, Ahn G, Kim C, Lim D-H, Cho D-W, Yun W-S, Shim J-H, Huh J-B. Biomed Mater. 2016;11 doi: 10.1088/1748-6041/11/5/055013. 055013. [DOI] [PubMed] [Google Scholar]
- [90].Shim J-H, Yoon M-C, Jeong C-M, Jang J, Jeong S-I, Cho D-W, Huh J-B. Biomed Mater. 2014;9 doi: 10.1088/1748-6041/9/6/065006. 065006. [DOI] [PubMed] [Google Scholar]
- [91].Konopnicki S, Sharaf B, Resnick C, Patenaude A, Pogal-Sussman T, Hwang K-G, Abukawa H, Troulis MJ. J Oral Maxillofac Surg. 2015;73 doi: 10.1016/j.joms.2015.01.021. 1016.e1. [DOI] [PubMed] [Google Scholar]
- [92].Yeo A, Wong WJ, Teoh S-H. J Biomed Mater Res A. 2009;93A:1358. doi: 10.1002/jbm.a.32633. [DOI] [PubMed] [Google Scholar]
- [93].Haberstroh K, Ritter K, Kuschnierz J, Bormann K-H, Kaps C, Carvalho C, Mülhaupt R, Sittinger M, Gellrich N-C. J Biomed Mater Res B Appl Biomater. 2010;93B:520. doi: 10.1002/jbm.b.31611. [DOI] [PubMed] [Google Scholar]
- [94].Xuan Y, Tang H, Wu B, Ding X, Lu Z, Li W, Xu Z. J Biomed Mater Res A. 2014;102:3401. doi: 10.1002/jbm.a.35012. [DOI] [PubMed] [Google Scholar]
- [95].Ryan GE, Pandit AS, Apatsidis DP. Biomaterials. 2008;29:3625. doi: 10.1016/j.biomaterials.2008.05.032. [DOI] [PubMed] [Google Scholar]
- [96].Lozano D, Hernández-López JM, Esbrit P, Arenas MA, Gómez-Barrena E, de Damborenea J, Esteban J, Pérez-Jorge C, Pérez-Tanoira R, Conde A. J Biomed Mater Res A. 2015;103:1985. doi: 10.1002/jbm.a.35337. [DOI] [PubMed] [Google Scholar]
- [97].Li L, Shi J, Zhang K, Yang L, Yu F, Zhu L, Liang H, Wang X, Jiang Q. J Orthop Transl. 2019;19:94. doi: 10.1016/j.jot.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zhang T, Wei Q, Zhou H, Zhou W, Fan D, Lin X, Jing Z, Cai H, Cheng Y, Liu X, Li W, Song C, Tian Y, Xu N, Zheng Y, Liu Z. Biomater Sci. 2020;8:3106. doi: 10.1039/c9bm01968e. [DOI] [PubMed] [Google Scholar]
- [99].Ma J, Ma L, Wang Z, Zhu X, Wang W. Medicine. 2017;96:e7250. doi: 10.1097/MD.0000000000007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sumida T, Otawa N, Kamata Y, Kamakura S, Mtsushita T, Kitagaki H, Mori S, Sasaki K, Fujibayashi S, Takemoto M, Yamaguchi A, et al. J Cranio-Maxillofac Surg. 2015;43:2183. doi: 10.1016/j.jcms.2015.10.020. [DOI] [PubMed] [Google Scholar]
- [101].Park E-K, Lim J-Y, Yun I-S, Kim J-S, Woo S-H, Kim D-S, Shim K-W. J Craniofac Surg. 2016;27:943. doi: 10.1097/SCS.0000000000002656. [DOI] [PubMed] [Google Scholar]
- [102].Aragón J, Pérez Méndez I. J Thorac Dis. 2016;8:E385. doi: 10.21037/jtd.2016.03.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Aranda JL, Jiménez MF, Rodríguez M, Varela G. Eur J Cardiothorac Surg. 2015;48:e92. doi: 10.1093/ejcts/ezv265. [DOI] [PubMed] [Google Scholar]
- [104].Li P, Jiang W, Yan J, Hu K, Han Z, Wang B, Zhao Y, Cui G, Wang Z, Mao K, Wang Y, et al. J Biomed Mater Res A. 2019;107:1386. doi: 10.1002/jbm.a.36652. [DOI] [PubMed] [Google Scholar]
- [105].Tunchel S, Blay A, Kolerman R, Mijiritsky E, Shibli JA. Int J Dent. 2016;2016:1. doi: 10.1155/2016/8590971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Fernandes N, van den Heever J, Hoogendijk C, Botha S, Booysen G, Els J. J Prosthodont. 2016;25:589. doi: 10.1111/jopr.12487. [DOI] [PubMed] [Google Scholar]
- [107].Bachelet J-T, Cordier G, Porcheray M, Bourlet J, Gleizal A, Foletti J-M. J Oral Maxillofac Surg. 2018;76:2161. doi: 10.1016/j.joms.2018.04.006. [DOI] [PubMed] [Google Scholar]
- [108].Castagnini F, Bordini B, Stea S, Calderoni PP, Masetti C, Busanelli L. Int Orthop. 2019;43:1815. doi: 10.1007/s00264-018-4102-9. [DOI] [PubMed] [Google Scholar]
- [109].Zou Y, Yang Y, Han Q, Yang K, Zhang K, Wang J, Zou Y. Medicine. 2018;97 doi: 10.1097/MD.0000000000013282. e13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Cheng BC, Jaffee S, Averick S, Swink I, Horvath S, Zhukauskas R. Spine J. 2020;20:457. doi: 10.1016/j.spinee.2019.10.003. [DOI] [PubMed] [Google Scholar]
- [111].Manea A, Bran S, Baciut M, Armencea G, Pop D, Berce P, Vodnar D-C, Hedesiu M, Dinu C, Petrutiu A, Tomina D, et al. Med Pharm Rep. 2018;91:452. doi: 10.15386/cjmed-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].El-Wassefy N, El-Fallal A, Taha M. Angle Orthod. 2015;85:39. doi: 10.2319/012014-56.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Joshi SC, Sheikh AA. Virtual Phys Prototyp. 2015;10:175. [Google Scholar]
- [114].Kim JY, Cho D-W. Microelectron Eng. 2009;86:1447. [Google Scholar]
- [115].Saiz E, Gremillard L, Menendez G, Miranda P, Gryn K, Tomsia AP. Mater Sci Eng C. 2007;27:546. [Google Scholar]
- [116].Ligon SC, Liska R, Stampfl J, Gurr M, Mülhaupt R. Chem Rev. 2017;117 doi: 10.1021/acs.chemrev.7b00074. 10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Lee K-G, Lee K-S, Kang Y-J, Hwang J-H, Lee S-H, Park S-H, Park Y, Cho Y-S, Lee B-K. Tissue Eng Part C Methods. 2018;24:255. doi: 10.1089/ten.TEC.2017.0474. [DOI] [PubMed] [Google Scholar]
- [118].Lam CXF, Hutmacher DW, Schantz J-T, Woodruff MA, Teoh SH. J Biomed Mater Res A. 2009;90A:906. doi: 10.1002/jbm.a.32052. [DOI] [PubMed] [Google Scholar]
- [119].Jensen J, Rölfing JHD, Le Svend DQ, Kristiansen AA, Nygaard JV, Hokland LB, Bendtsen M, Kassem M, Lysdahl H, Bünger CE. J Biomed Mater Res A. 2014;102:2993. doi: 10.1002/jbm.a.34970. [DOI] [PubMed] [Google Scholar]
- [120].Jones AC, Milthorpe B, Averdunk H, Limaye A, Senden TJ, Sakellariou A, Sheppard AP, Sok RM, Knackstedt MA, Brandwood A, Rohner D, et al. Biomaterials. 2004;25:4947. doi: 10.1016/j.biomaterials.2004.01.047. [DOI] [PubMed] [Google Scholar]
- [121].Rohner D, Hutmacher DW, Cheng TK, Oberholzer M, Hammer B. J Biomed Mater Res. 2003;66B:574. doi: 10.1002/jbm.b.10037. [DOI] [PubMed] [Google Scholar]
- [122].Liang X, Gao J, Xu W, Wang X, Shen Y, Tang J, Cui S, Yang X, Liu Q, Yu L, Ding J. Biofabrication. 2019;11 doi: 10.1088/1758-5090/ab0f59. 035009. [DOI] [PubMed] [Google Scholar]
- [123].Lopez CD, Diaz-Siso JR, Witek L, Bekisz JM, Cronstein BN, Torroni A, Flores RL, Rodriguez ED, Coelho PG. J Surg Res. 2018;223:115. doi: 10.1016/j.jss.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zhang Y, Xia L, Zhai D, Shi M, Luo Y, Feng C, Fang B, Yin J, Chang J, Wu C. Nanoscale. 2015;7 doi: 10.1039/c5nr05421d. 19207. [DOI] [PubMed] [Google Scholar]
- [125].Shao H, Sun M, Zhang F, Liu A, He Y, Fu J, Yang X, Wang H, Gou Z. J Dent Res. 2018;97:68. doi: 10.1177/0022034517734846. [DOI] [PubMed] [Google Scholar]
- [126].Simon JL, Michna S, Lewis JA, Rekow ED, Thompson VP, Smay JE, Yampolsky A, Parsons JR, Ricci JL. J Biomed Mater Res A. 2007;83A:747. doi: 10.1002/jbm.a.31329. [DOI] [PubMed] [Google Scholar]
- [127].Abarrategi A, Moreno-Vicente C, Martínez-Vázquez FJ, Civantos A, Ramos V, Sanz-Casado JV, Martínez-Corriá R, Perera FH, Mulero F, Miranda P, López-Lacomba JL. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0034117. e34117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Carrel J-P, Wiskott A, Moussa M, Rieder P, Scherrer S, Durual S. Clin Oral Implants Res. 2016;27:55. doi: 10.1111/clr.12503. [DOI] [PubMed] [Google Scholar]
- [129].Barboni B, Mangano C, Valbonetti L, Marruchella G, Berardinelli P, Martelli A, Muttini A, Mauro A, Bedini R, Turriani M, Pecci R, et al. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0063256. e63256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Mangano C, Barboni B, Valbonetti L, Berardinelli P, Martelli A, Muttini A, Bedini R, Tetè S, Piattelli A, Mattioli M. J Oral Implantol. 2015;41:240. doi: 10.1563/AAID-JOI-D-13-00053. [DOI] [PubMed] [Google Scholar]
- [131].Carrel J-P, Wiskott A, Scherrer S, Durual S. Clin Implant Dent Relat Res. 2016;18:1183. doi: 10.1111/cid.12394. [DOI] [PubMed] [Google Scholar]
- [132].Wang L, Xu W, Chen Y, Wang J. Sci Rep. 2019;9 doi: 10.1038/s41598-019-54551-x. 18175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Kim J-A, Lim J, Naren R, Yun H, Park EK. Acta Biomater. 2016;44:155. doi: 10.1016/j.actbio.2016.08.039. [DOI] [PubMed] [Google Scholar]
- [134].Entezari A, Roohani I, Li G, Dunstan CR, Rognon P, Li Q, Jiang X, Zreiqat H. Adv Healthc Mater. 2018 doi: 10.1002/adhm.201801353. 1801353. [DOI] [PubMed] [Google Scholar]
- [135].Abbah SA, Lam CXL, Hutmacher DW, Goh JCH, Wong H-K. Biomaterials. 2009;30:5086. doi: 10.1016/j.biomaterials.2009.05.067. [DOI] [PubMed] [Google Scholar]
- [136].Babilotte J, Guduric V, Le Nihouannen D, Naveau A, Fricain J, Catros S. J Biomed Mater Res B Appl Biomater. 2019;107:2579. doi: 10.1002/jbm.b.34348. [DOI] [PubMed] [Google Scholar]
- [137].Igawa K, Mochizuki M, Sugimori O, Shimizu K, Yamazawa K, Kawaguchi H, Nakamura K, Takato T, Nishimura R, Suzuki S, Anzai M, et al. J Artif Organs. 2006;9:234. doi: 10.1007/s10047-006-0347-y. [DOI] [PubMed] [Google Scholar]
- [138].Simon JL, Rekow ED, Thompson VP, Beam H, Ricci JL, Parsons JR. J Biomed Mater Res A. 2008;85A:371. doi: 10.1002/jbm.a.31484. [DOI] [PubMed] [Google Scholar]
- [139].Dutta TRoy, Simon JL, Ricci JL, Rekow ED, Thompson VP, Parsons JR. J Biomed Mater Res. 2003;67A:1228. doi: 10.1002/jbm.a.20034. [DOI] [PubMed] [Google Scholar]
- [140].Komlev VS, Popov VK, Mironov AV, Fedotov AYu, Teterina AYu, Smirnov IV, Bozo IY, Rybko VA, Deev RV. Front Bioeng Biotechnol. 2015;3:81. doi: 10.3389/fbioe.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Habibovic P, Gbureck U, Doillon C, Bassett D, Vanblitterswijk C, Barralet J. Biomaterials. 2008;29:944. doi: 10.1016/j.biomaterials.2007.10.023. [DOI] [PubMed] [Google Scholar]
- [142].Nyberg EL, Farris AL, Hung BP, Dias M, Garcia JR, Dorafshar AH, Grayson WL. Ann Biomed Eng. 2017;45:45. doi: 10.1007/s10439-016-1668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Ge Z, Tian X, Heng BC, Fan V, Yeo JF, Cao T. Biomed Mater. 2009;4 doi: 10.1088/1748-6041/4/2/021001. 021001. [DOI] [PubMed] [Google Scholar]
- [144].Bartnikowski M, Vaquette C, Ivanovski S. Clin Oral Implants Res. 2020;31:431. doi: 10.1111/clr.13579. [DOI] [PubMed] [Google Scholar]
- [145].Franchitti S, Borrelli R, Pirozzi C, Carrino L, Polini W, Sorrentino L, Gazzerro A. Vacuum. 2018;157:340. [Google Scholar]
- [146].Kim J-W, Yang B-E, Hong S-J, Choi H-G, Byeon S-J, Lim H-K, Chung S-M, Lee J-H, Byun S-H. Int J Mol Sci. 2020;21:4837. doi: 10.3390/ijms21144837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Xu F, Ren H, Zheng M, Shao X, Dai T, Wu Y, Tian L, Liu Y, Liu B, Gunster J, Liu Y, et al. J Mech Behav Biomed Mater. 2020;103 doi: 10.1016/j.jmbbm.2019.103532. 103532. [DOI] [PubMed] [Google Scholar]
- [148].Kreß S, Schaller-Ammann R, Feiel J, Priedl J, Kasper C, Egger D. Materials. 2020;13:3011. doi: 10.3390/ma13133011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Rathnayaka K, Momot KI, Noser H, Volp A, Schuetz MA, Sahama T, Schmutz B. Med Eng Phys. 2012;34:357. doi: 10.1016/j.medengphy.2011.07.027. [DOI] [PubMed] [Google Scholar]
- [150].Msallem B, Sharma N, Cao S, Halbeisen FS, Zeilhofer H-F, Thieringer FM. J Clin Med. 2020;9:817. doi: 10.3390/jcm9030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Otero JJ, Vijverman A, Mommaerts MY. J Cranio-Maxillofac Surg. 2017;45:1542. doi: 10.1016/j.jcms.2017.06.018. [DOI] [PubMed] [Google Scholar]
- [152].Louvrier A, Marty P, Barrabé A, Euvrard E, Chatelain B, Weber E, Meyer C. J Stomatol Oral Maxillofac Surg. 2017;118:206. doi: 10.1016/j.jormas.2017.07.002. [DOI] [PubMed] [Google Scholar]
- [153].Brennan MÁ, Renaud A, Amiaud J, Rojewski MT, Schrezenmeier H, Heymann D, Trichet V, Layrolle P. Stem Cell Res Ther. 2014;5:114. doi: 10.1186/scrt504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Confalonieri D, Schwab A, Walles H, Ehlicke F. Tissue Eng Part B Rev. 2018;24:155. doi: 10.1089/ten.TEB.2017.0305. [DOI] [PubMed] [Google Scholar]
- [155].Blasimme A, Rial-Sebbag E. Stem Cells Dev. 2013;22:14. doi: 10.1089/scd.2013.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Santo VE, Gomes ME, Mano JF, Reis RL. Tissue Eng Part B Rev. 2013;19:308. doi: 10.1089/ten.teb.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Caballé-Serrano J, Abdeslam-Mohamed Y, Munar-Frau A, Fujioka-Kobayashi M, Hernández-Alfaro F, Miron R. Arch Oral Biol. 2019;100:57. doi: 10.1016/j.archoralbio.2019.02.006. [DOI] [PubMed] [Google Scholar]