Abstract
The majority of childhood deaths occur in low-income countries, with vaccine-preventable infections contributing greatly. Of the many possible environmental factors that could hamper a child’s immune response, mycotoxins rank among the least studied in spite of the high exposure in vulnerable populations. Aflatoxin crosses the placenta, is secreted in breast milk and is consumed widely in weaning diets by children with developing organ systems. This review describes the effects of mycotoxin exposure on immunity in children that may contribute to sub-optimal vaccine effectiveness. We searched electronic databases and references of identified articles for relevant studies on the effects of mycotoxins on the immune system in children. Geographical location, publication year, study design, sample selection, sample size, mean age, route of exposure were extracted on a standard template. Quality was assessed using Joanna Briggs Institute tool for appraisal of systematic reviews for prevalence studies. Our analyses and reporting were conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. Out of 806 articles screened, 5 observational studies met criteria for inclusion for review. The definition of exposures to mycotoxins and outcomes varied across the studies. Exposure to mycotoxins was positively associated with low birth weight and concentration of antibodies to asexual malaria parasites and hepatitis B surface antigen, and negatively associated with death and sIgA, antibodies to pneumococcal antigen 23. Despite the far-reaching clinical and public health effects of mycotoxin exposure among children, studies on the effects of mycotoxin exposure on immunity in children were few, small and mostly of low quality. There is an urgent need for carefully designed prospective studies in this neglected field to inform policy interventions for child health in settings where exposure to mycotoxins is high.
Introduction
Of the 7.7 million deaths among children and adolescents globally in 2013, over 80% occurred in children under 5 years. The two leading causes of deaths were lower respiratory tract infections and diarrheal diseases.1 There is wide geographic variation in mortality and morbidity in children. Sub-Saharan Africa contributed half of all the under 5 global deaths while Southern Asia contributed almost a third.2 The majority of these deaths are preventable through the implementation of evidence-based effective interventions.3–5 Among the available public health interventions, immunization,6 in spite of its implementation and technical challenges, ranks among the most cost-effective tools in public health.7
Vaccine efficacy indicates direct protection of the individual under optimal conditions. Vaccine effectiveness on the other hand connotes protection conferred by immunization in a defined population in ordinary circumstances. Effectiveness is influenced by other factors that include differential protection at the individual level. This immunological heterogeneity is a threat to the realization of the full benefits of immunization in tackling the targeted diseases.
There are many possible environmental factors that could hamper a child’s immune response. For example, animal data indicate that exposure in early life to dioxin-like polychlorinated biphenyls (PCBs) and to tetrachlorodibenzo-p-dioxin depresses immune function. To explore the potential immunotoxicity of PCBs and perfluoroalkyl and polyfluoroalkyl substances (PFAS) in developing infants, studies of the immune response to standard childhood immunizations were conducted in a birth cohort in the Faroe Islands, where the investigators found that the antibody response to diphtheria toxoid was depressed at age 18 months by 24.4% for each doubling of the cumulative PCB exposure.8–12
An environmental factor that has rarely been studied is exposure to mycotoxins. There are over 400 mycotoxins (secondary metabolites of fungi)13 that exist in nature and contaminate a broad spectrum of foods that make up the staple diet of people. In parts of Africa, many people live in areas that lack centralized food systems that ensure the food is safe.
In addition to the known genotoxic and carcinogenic effects of mycotoxins, there is emerging evidence of direct effects of mycotoxins on the immune system.8,9 Aflatoxins, fumonisins, deoxynivalenol, zearalenone and ochratoxin are the most important in human health with the first three having significant impact in the paediatric population.13
In addition to the known genotoxic and carcinogenic effects of mycotoxins, there is emerging evidence of direct effects of mycotoxins on the immune system.8,9
Aflatoxin crosses the placenta to the fetus and is excreted in breast milk. Consequently, aflatoxin exposure occurs at various critical developmental stages affecting several systems that include the immune system.
“Vaccine preventable conditions continue to cause unacceptably high mortality rates compared to countries with similar programs in the northern hemisphere”
“Vaccine preventable conditions continue to cause unacceptably high mortality rates compared to countries with similar programs in the northern hemisphere”
Animal experiments have shown that aflatoxin not only reduces primary immune and humoral responses, the immunosuppressive effect also is transferred across the placenta.14,15 This may impact on vaccination uptake in breastfeeding babies.10 Data on the immunotoxic effects of aflatoxins in humans are limited. This review aims to describe the effects of mycotoxin exposure on the immunity in children to explore if any gaps exists that may be contributing to the sub-optimal vaccine effectiveness alluded to in the introduction.
“Studies have not pushed the agenda that vaccines may not be working as well as they should for reasons that can be addressed”
“Studies have not pushed the agenda that vaccines may not be working as well as they should for reasons that can be addressed”
Methods
Search Strategy and Selection Criteria
The protocol of this systematic review was registered at the International Prospective Register of Systematic Reviews (PROSPERO) under number CRD42016032838. We searched PubMed, Embase, and Cumulative Index to Nursing and Allied Health Literature with the term “(mycotoxins [MeSH] OR “aflatoxin”) AND (“immunity” OR “immune system” OR “immunomodulation” OR “immune modulation” OR “immunosuppression”)” for studies on the effects of mycotoxins on the immune system in children. No restrictions were applied on study design, language or time. We also searched relevant reference lists and relevant journals by hand and wrote to authors when additional data were required. The final search across the databases was conducted on 12 March 2018. Our analyses and reporting were conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.
Studies conducted only in adult populations and those conducted in adults and children but with outcomes not reported separately for children were excluded.
Titles for studies identified in the search were screened for eligibility independently by 2 reviewers. Abstracts of potentially eligible articles were further screened following which full texts of shortlisted articles were retrieved and assessed for final inclusion. A third reviewer was consulted in the event of disagreements between the two primary reviewers on articles eligible for the review. The decision of the third reviewer was considered final.
Data Extraction
A standard template was used to extract information about geographical location, year of publication, study design, method of sample selection, sample size, mean age and description of the route of mycotoxin exposure. Disagreement was resolved by consensus between the 2 reviewers or through consultation with a third reviewer, when necessary. If needed, we sought further clarifications from the authors of relevant studies.
Quality Assessment
Studies were assessed for quality using the Joanna Briggs Institute tool for appraisal of systematic reviews for prevalence studies.16 This tool defines 9 criteria aimed at determining representativeness of the study sample, appropriateness of the recruitment process, adequacy of the sample size, appropriate description and reporting of study subjects and setting, adequacy of data coverage of the identified sample, reliability and objectivity of the exposure and outcome measures, appropriateness of the statistical methods used, and adequate identification and consideration of confounding factors/subgroups/differences in the analyses. We applied a quality rating system derived from the 9 domains of the Joanna Briggs Institute tool whereby the risk of bias was categorized by the authors as “high” when the “yes” fields accounted for <50% of the total possible score, “moderate” if “yes” responses represented 50% - 69% of the total score, and “low” when it was more than 70%.
Heterogeneity
We initially intended to statistically pool results across individual studies to enhance generalizability and precision. However, marked heterogeneity in the study procedures, methods for determining exposure to mycotoxins and outcome measures precluded quantitative synthesis of the findings. We therefore restricted our results to a narrative synthesis, noting individual study findings and highlighting possible relationships across the included studies.
Results
Study Selection
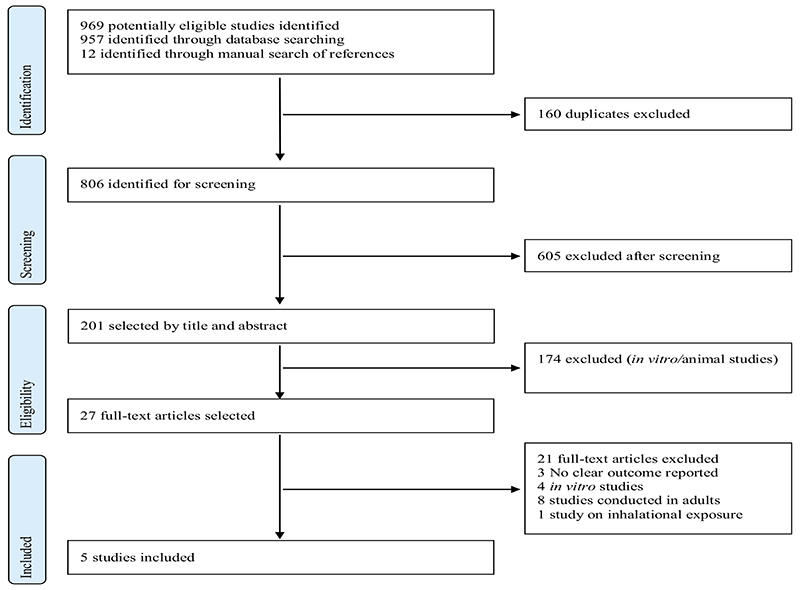
The search criteria identified 806 unduplicated articles for screening. We considered 27 eligible for full-text review, excluded a further 22 and determined that 5 met criteria for inclusion in the review (Fig. 1). One potentially eligible study conducted in the United States examined the immune response among 33 patients who presented with symptoms related to mold exposure (rhinitis, cough, other respiratory symptoms, ocular pruritus, gastrointestinal symptoms, headache, fatigue, and central nervous system symptoms) and 17 non-symptomatic controls.17 This study was excluded because of the small sample size and even smaller population of children (n=7), and because the conflicting results for associations observed in IgG and IgA responses to various molds among symptomatic and non-symptomatic patients are difficult to interpret.
Fig. 1. PRISMA flow diagram.
Study Characteristics
All 5 included studies were observational in design; half prospective and half cross-sectional. Study participants ranged in age from 1 day to 12 years. The size of the individual studies ranged from 40 to 472. The study populations were from low- and middle-income countries (half of the studies were conducted in sub-Saharan Africa) (Table 1).
Table 1. Characteristics of included studies.
| Author (year) | Country | Study design | Age | Route of exposure | Outcomes | Length of follow-up | |
|---|---|---|---|---|---|---|---|
| Abdulrazzaq et al, (2005) | UAE | Cross sectional | 1 day | Transplacental | Low birth weight, Aflatoxin M1 levels | None | 250 |
| Allen et al, (1992) | Gambia | Prospective | 3-8 years | Diet | Antibodies to asexual malaria parasites and Hepatitis B surface antigen | 7 months | 391 |
| Denning et al, (1995) | Philippines | Prospective | 0-12 years | Diet | Duration of fever and mortality | Discharge, fever resolution or inpatient death | 115 |
| Oyelami et al, (2007) | Nigeria | Cross sectional | 4-168 months | Diet/Inhalation | AFB1 levels, malnutrition | None | 40 |
| Turner et al, (2003) | Gambia | Prospective | 6-9 years | Diet | sIGA levels; geometrical mean antibody titers for pneumococcal and rabies vaccine antigens; CMI response | 48 hrs, day 16, day 30 and day 60 | 472 |
Assessment of Exposures to Mycotoxins
In 315,18,19 of the 5 included studies, exposure to aflatoxins was determined based on aflatoxin-albumin adduct levels in serum analyzed using enzyme-linked immunosorbent assays (ELISA). One of these studies20 used a combination of ELISA and HPLC to determine aflatoxin levels in serum, while another21 studied levels of water-soluble AFB1 DNA and protein adducts in urine using ELISA. In one study that investigated the effects of transplacental exposure to aflatoxins in newborns,22 aflatoxin M1 concentration in cord blood samples was analyzed using HPLC. A post-mortem study23 analyzed aflatoxin levels in lung tissue specimens using HPLC. (Table 2)
Table 2. Definitions of measures of exposure and outcome.
| Study | Exposure(s) | Outcome(s) |
|---|---|---|
| Abdulrazzaq et al, (2005) |
|
|
| Allen et al, (1992) |
|
|
| Denning et al, (1995) |
|
|
| Oyelami et al, (2007) |
|
|
| Turner et al, (2003) |
|
|
Assessment of Outcomes
The outcomes varied across all the included studies. Clinically-derived measures were low birth weight (<2500 g), neonatal jaundice (not objectively defined), or infections (also not objectively defined)22 death (limit of time over which outcome was studied not defined), or duration of fever (temperature >38.5°C after study entry categorized as ≤7 vs. > 7 days)21 and clinically diagnosed kwashiokor.23
Two studies reported outcomes derived from a variety of immunological markers. One study investigated titers of antibodies against P. falciparum and hepatitis B core antigen, Hepatitis B surface antigen levels, and proliferative responses of PBMC following stimulation with purified protein derivative of Mycobacterium tuberculin (PPD), Candida albicans extract and surface antigens of the circumsporozoite protein and merozoite stages of P. falciparum 20 The other study15 reported a combination of mean secretory IgA (sIgA) in saliva measured by ELISA, cell-mediated immunity assessed by a CMI skin test for delayed type hypersensitivity reaction to tetanus, diphtheria, streptococcus, tuberculin, candida, tricophyton, and proteus antigens, titers of antibody to pneumococcal serotypes 1, 5, 14, and 23 antigens after administration of 23-valent pneumococcal capsular polysaccharide vaccine, and titers of antibody to rabies after two doses of rabies vaccine. (Table 2)
Risk of Bias for Included Studies
None of the included studies fulfilled all of the methodological quality criteria. Two studies ranked as having low risk of bias (quality score >70%). One study ranked as moderate quality (quality score 50-69%), while three were low quality (quality score <50%). Common reasons for downgrading the quality of individual studies were unclear or inappropriate approaches to sampling of participants, lack of justification for sample size used (particularly for the smaller studies), and inadequate measures to address potential confounding in statistical analyses. The results of the quality assessment are summarized in Table 3.
Table 3. Quality assessment for included studies.
| Author (year) | Quality rating |
|---|---|
| Abdulrazzaq et al, (2005) | |
| Allen et al, (1992) | |
| Denning et al, (1995) | |
| Oyelami et al, (2007) | |
| Turner et al, (2003) |
Synthesis of Results
The direction of association between aflatoxin exposure and immunity in children in the included studies is provided in Table 4. Mycotoxin exposure was associated with low birth weight, titres of antibodies to asexual malaria parasites, hepatitis B surface antigen, IgG to A. ochraceus, A. terreus, and proliferative responses of PBMC to A. alternate, C. herbarum, S. chartarum, A. versicolor and A. flavus. Undetectable serum aflatoxin concentrations were associated with death while IgG to C. herbarum and Trichoderma and IgA to S. chartarum was negatively associated with mycotoxin exposure. No association was observed between mycotoxin exposure and neonatal jaundice, neonatal infections and duration of fever.
Table 4. Direction of association between mycotoxin exposure and outcomes representing immune function.
| Author (year) | Direction of association between mycotoxin exposure and outcomes representing immune function | ||
|---|---|---|---|
| Positive | Negative | No association found | |
| Abdulrazzaq et al, (2005) | Low birth weight | Jaundice, infection | |
| Allen et al, (1992) | Antibodies to asexual malaria parasites and Hepatitis B surface antigen | Purified protein derivative of Mycobacterium tuberculin (PPD), Candida albicans extract and surface antigens of the circumsporozoite protein and merozoite stages of P. falciparum. | |
| Denning et al, (1995) | Death | Duration of fever | |
| Oyelami et al, (2007) | Kwashiokor | ||
| Turner et al, (2003) | sIgA, antibodies to pneumococcal antigen 23 | CMI, antibodies to pneumococcal antigens 1, 5, 14, Rabies | |
Effects of aflatoxin exposure on clinical outcomes
One study that described morbidity among neonates of mothers who had ingested aflatoxins demonstrated a strong correlation between maternal exposure to aflatoxins and levels of aflatoxin M1 in cord blood (r=0.797, p<0.0001).22 The same study demonstrated a negative correlation between aflatoxin level and birth weight (r=- 0.565, p<0.001). However, the study showed no association between aflatoxin M1 concentration in maternal or cord blood and rates of jaundice or infection. It is not clear whether the null finding for the latter clinical outcomes was due to the relatively small sample size as there was no description of the assumptions that informed the study sample size. The analyses also did not account for potential confounding variables.
Contrasting results were reported in a prospective study of 115 Philippino children with acute respiratory tract infections.21 Undetectable serum aflatoxin concentrations were strongly associated with death (p = 0.004). The authors attribute this unexpected finding to anorexia as a marker of severe illness, and further comment on the high levels of exposure to aflatoxin within the study population. Detectable levels of serum aflatoxin levels were not associated with duration of fever (≤ 7 days versus > 7 days) (p=0.89). Limitations of this study include the small sample size, unclear description of the process used for sampling of potential participants and the failure to account for confounding in the analyses for the primary outcomes.
A post-mortem study of 40 children who died of kwashiorkor (n=20) or other illnesses (pneumonia, gastroenteritis, measles, anaemia, xerophthalmia, meningitis, tuberculosis, pericarditis, nephrotic syndrome, Hirschprungs disease, sickle cell disease, chronic renal failure) conducted in Nigeria sought to determine whether patients with kwashiorkor or other diseases retain AFB1 in the lungs.23 Aflatoxins were detected in 18 children who died from kwashiorkor and in 13 of those who died from other diseases (95% confidence interval -1,019 to 1.459). Limitations of this study included the small sample size, inadequate description of the procedure used to sample the control population, and the failure to account for potential confounders in the analyses presented.
Effects of aflatoxin exposure on immunological markers
In a prospective study of 391 Gambian children, exposure to aflatoxin AFB1 was associated with an increased risk of infection with malaria (p=0.01) and hepatitis B (p=0.04) quantified by levels of IFAT for antibodies to asexual malaria parasites and HBsAg respectively.20 Limitations of this study included the poor description of the sampling procedure.
A study of 472 children recruited in rural Gambia, aimed to determine the effect of dietary aflatoxin exposure (serum aflatoxin-albumin (AF-alb) adducts) on sIgA, CMI, antibody response to rabies and pneumococcal vaccine in Gambian children. Secretory IgA was quantified in microgram per mg protein of secretory IGA; CMI response was assessed by measuring the diameter of skin induration in mm, antibody response to the pneumococcal and rabies vaccines was based on the geometrical mean antibody titer for 4 pneumococcal antigens contained in 23 valent vaccine and the rabies vaccine respectively. Levels of sIgA were markedly lower in children with detectable AF-alb compared with those with non-detectable levels p < 0.0001. Antibody response to one of 4 pneumococcal serotypes (serotype 23) (p=0.05), was weakly associated with higher levels of AF-alb adducts. No response was observed for antibodies to serotypes 1, 5, 14 or rabies. No association was found between CMI responses to test antigens and AF-alb adducts. The authors acknowledge the unexpected direction of the findings, but caution against ruling out the role of chance in explaining the results given the weak association observed. This was a well-conducted observational study nested within a large intervention study. The authors used regression analyses to examine independent associations between predictors and outcomes. However, the investigators failed to describe the appropriateness (power) of sample size to detect the associations of interest.
Conclusions
This systematic analysis encountered difficulties in finding common outcomes in the very diverse studies. For that reason, this review was difficult to conduct.
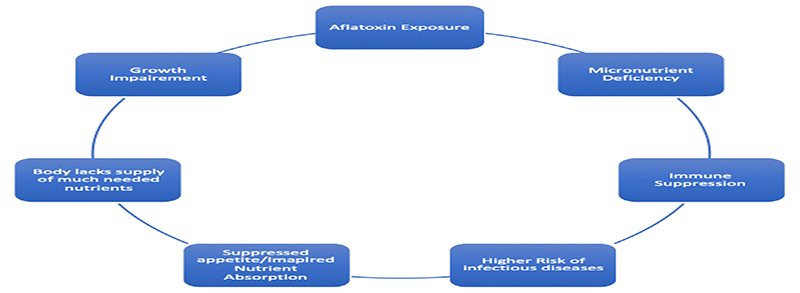
There is a complex interrelationship between mycotoxins, macro- and micro- nutrient deficiencies, immune suppression, growth impairment and infective conditions (Fig. 2).
Fig. 2. Interrelationships between mycotoxins and health.
Mycotoxins and in particular, aflatoxins, impact immunity through the route of malnutrition as well as directly as shown by laboratory animals, in vitro studies and a handful of human studies.
Funding
AA is supported by funds from a Wellcome Trust Strategic Award, the Initiative to Develop African Research Leaders (IDeAL) Wellcome Trust award, and a Wellcome Trust core grant awarded to the KEM-RI–Wellcome Trust Research Programme. The funders had no role in study design, data collection, data analysis, data interpretation, or writing of this report.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Global Burden of Disease Pediatrics Collaboration. Kyu HH, Pinho C, Wagner JA, Brown JC, Bertozzi-Villa A, et al. Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013: Findings From the Global Burden of Disease 2013 Study. JAMA Pediatr. 2016;98121(3):1–21. doi: 10.1001/jamapediatrics.2015.4276. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Inter-agency Group for Child Mortality Estimation (UN IGME) Levels & Trends in Child Mortality: Report 2017, Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation. New York: United Nations Children’s Fund; 2017. [Accessed April 15, 2018]. http://www.childmortality.org/files_v21/download/IGME%20report%202017%20child%20mortality%20final.pdf. [Google Scholar]
- 3.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giuglian E, et al. What works? Intervention for maternal and child undernutrition and survival. Lancet. 2008 doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 4.Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, De Bernis L. Evidence-based, cost-effective interventions: How many newborn babies can we save? Lancet. 2005;365(9463):977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 5.Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS. How many child deaths can we prevent this year? Lancet. 2003;362:65–71. doi: 10.1016/S0140-6736(03)13811-1. [DOI] [PubMed] [Google Scholar]
- 6.John TJ, Plotkin SA, Orenstein WA. Building on the success of the Expanded Programme on Immunization: Enhancing the focus on disease prevention and control. Vaccine. 2011;29:8835–7. doi: 10.1016/j.vaccine.2011.08.100. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Ten Great Public Health Achievements in the 20th Century, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999 [PubMed] [Google Scholar]
- 8.Heilmann C, Grandjean P, Weihe P, Nielsen F, Budtz-Jørgensen E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006;3:e311. doi: 10.1371/journal.pmed.0030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gascon M, Morales E, Sunyer J, Vrijheid M. Effects of persistent organic pollutants on the developing respiratory and immune systems: a systematic review. Environ Int. 2013;52:51–65. doi: 10.1016/j.envint.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Timmermann A, Budtz-Jørgensen E. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J Immunotoxicol. 2017;14(1):188–95. doi: 10.1080/1547691X.2017.1360968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Budtz-Jørgensen E. Serum vaccine antibody concentrations in adolescents exposed to perfluorinated compounds. Environ Health Perspect. 2017;125(7) doi: 10.1289/EHP275. 077018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandjean P, Andersen EW, Budtz-Jørgensen E, Nielsen F, Mølbak K, Weihe P, Heilmann C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307(4):391–7. doi: 10.1001/jama.2011.2034. Erratum in: JAMA. 2012; 307(11):1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Environmental Mycology in Public Health. doi: 10.1016/B978-0-12-411471-5.00001-6. [DOI] [Google Scholar]
- 14.Tang L, Xu L, Afriyie-Gyawu E, Liu W, Wang P, Tang Y, et al. Aflatoxin–albumin adducts and correlation with decreased serum levels of vitamins A and E in an adult Ghanaian population. Food Addit Contam - Part A Chem Anal Control Expo Risk Assess. 2009 doi: 10.1080/02652030802308472. [DOI] [PubMed] [Google Scholar]
- 15.Turner PC, Moore SE, Hall AJ, Prentice AM, Wild CP. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ Health Perspect. 2003 doi: 10.1289/ehp.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Joanna Briggs Institute. The Joanna Briggs Institute Reviewers’ Manual 2015: Methodology for JBI scoping reviews. Joanne Briggs Inst. 2015:1–24. [Internet]. Available from: http://joannabriggs.org/assets/docs/sumari/Reviewers-Manual_Mixed-Methods-Review-Methods-2014-ch1.pdf.
- 17.Edmonson DA, Barrios CS, Brasel TL, Staus DC, Kurup VP, Fink JN. Immune Response among Patients Exposed to Molds. Int J Mol Sci. 2009;10 doi: 10.3390/ijms10125471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marin DE, Taranu I, Bunaciu RP, Pascale F, Tudor DS, Avram N, et al. Changes in performance, blood parameters, humoral and cellular immune responses in weanling piglets exposed to low doses of aflatoxin. J Anim Sci. 2002 doi: 10.2527/2002.8051250x. [DOI] [PubMed] [Google Scholar]
- 19.Azam AH, Gabal MA. Aflatoxin and immunity in layer hens. Avian Pathol. 1998 doi: 10.1080/03079459808419386. [DOI] [PubMed] [Google Scholar]
- 20.Allen SJ, Wild CP, Wheeler JG, Riley EM, Montesano R, Bennett S, Whittle HC, Hall AJ, Greenwood BM. Aflatoxin exposure, malaria and hepatitis B infection in rural Gambian children. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1992;86:426–30. doi: 10.1016/0035-9203(92)90253-9. [DOI] [PubMed] [Google Scholar]
- 21.Denning DW, Quiepo SC, Altman DG, Makarananda KG, Neal GE, Camallere EL, Morgan MRA, Tupasi TE. Aflatoxin and outcome from acute lower respiratory infection in children in The Philippines. Annals of Tropical Paediatrics. 1995;15(3):209–16. doi: 10.1080/02724936.1995.11747774. [DOI] [PubMed] [Google Scholar]
- 22.Abdulrazzaq YM, Osman N, Yousif ZM, Trad O. Morbidity in neonates of mothers who have ingested aflatoxins. Annals of Tropical Paediatrics. 2004;24:145–51. doi: 10.1179/027249304225013420. [DOI] [PubMed] [Google Scholar]
- 23.Oyelami OA, Maxwell SM, Adelusola KA, Aladekoma TA, Oyelese AO. Aflatoxins in the lungs of children with kwashiorkor and children with miscellaneous diseases in Nigeria. Journal of Toxicology and Environmental Health. 1997;51(6):623–8. doi: 10.1080/00984109708984048. [DOI] [PubMed] [Google Scholar]