Abstract
Objective
Despite a substantial consistency in recommendations for the management of children with acute gastroenteritis (AGE), a high variability in clinical practice and a high rate of inappropriate medical interventions persist in both developing and developed countries.
The aim of this study was to develop a set of clinical recommendations for the management of nonseverely malnourished children with AGE to be applied worldwide.
Methods
The Federation of International Societies of Pediatric Gastroenterology, Hepatology, and Nutrition (FISPGHAN) Working Group (WG) selected care protocols on the management of acute diarrhea in infants and children aged between 1 month and 18 years. The WG used a 3-step approach consisting of: systematic review and comparison of published guidelines, agreement on draft recommendations using Delphi methodology, and external peer-review and validation of recommendations.
Results
A core of recommendations including definition, diagnosis, nutritional management, and active treatment of AGE was developed with an overall agreement of 91% (range 80%–96%). A total of 28 world experts in pediatric gastroenterology and emergency medicine successively validated the set of 23 recommendations with an agreement of 87% (range 83%–95%). Recommendations on the use of antidiarrheal drugs and antiemetics received the lowest level of agreement and need to be tailored at local level. Oral rehydration and probiotics were the only treatments recommended.
Conclusions
Universal recommendations to assist health care practitioners in managing children with AGE may improve practitioners’ compliance with guidelines, reduce inappropriate interventions, and significantly impact clinical outcome and health care-associated costs.
Keywords: children, diarrhea, gastroenteritis, guidelines, recommendation
What Is Known
Comparing documents published in different countries, there is substantial consistency in guideline recommendations for children with acute gastroenteritis.
High variability exists in clinical practice.
Inappropriate medical interventions and a suboptimal adherence to recommendations persist both in developing and more developed areas.
What Is New
The development of universal recommendations, supported by evidence, public health authorities, and pediatric societies (general pediatrics, pediatric gastroenterology, emergency care and infectious diseases) would be of value to health care providers.
These guidelines would fill an unmet need in improving the outcomes of children with acute diarrhea, increasing the likelihood of meeting global targets.
Under-5-year children’s mortality declined by more than half from 1990 to 2015. However, the Millennium Development Goal 4 target of a two-thirds reduction was not met and the majority of deaths in children worldwide still derive from preventable infectious diseases (1). Among those, acute gastroenteritis (AGE) is the second leading cause of child mortality and morbidity, particularly in low-income countries. Although 30% reduction in mortality for diarrhea has been achieved in the last years, AGE still accounts for 550,000 deaths per year in infants and children younger than 5 years (2).
In high-income areas, mortality is a rare outcome, but AGE is a major cause of medical consultation and hospitalization resulting in a high health care and economic burden (3,4).
Although there is substantial consistency in guideline recommendations for children with AGE, a high variability in clinical practice, a substantial number of inappropriate medical interventions, and a suboptimal adherence to recommendations persist worldwide (5–10). There is evidence in different settings that providing a limited number of consistent recommendations may increase physicians’ compliance with standard of practice (11,12).
In this scenario, the Federation of International Societies of Pediatric Gastroenterology, Hepatology, and Nutrition (FISP-GHAN) Working Group (WG) for AGE identified the reduction of inappropriate medical interventions and the large-scale implementation of effective recommendations as 2 of the 3 highest priorities to reduce the burden of AGE in children worldwide, the third intervention was the use of e-learning as a tool to increase dissemination (13).
The WG considered that developing universal recommendations, supported by evidence, public health authorities, and pediatric societies (general pediatrics, pediatric gastroenterology, emergency care and infectious diseases), would fill an unmet need in improving the outcomes of children with AGE, increasing the likelihood of meeting global targets. The aim of this work was to develop a core of consensus clinical recommendations for the management of nonmalnourished children affected by AGE worldwide.
Methods
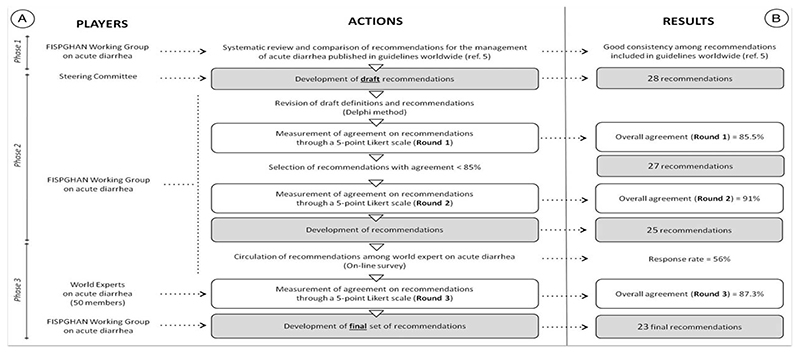
To identify common definitions and recommendations for the management of acute diarrhea in children worldwide, FISP-GHAN WG used a 3-step approach: systematic comparative review of published guidelines, agreement on draft recommendations, and external validation of universal recommendations (Fig. 1A).
Figure 1.
(A) Methods of the study according to the 3-phase approach. (B) Overall results reported according to any step of the applied methodology. FISPGHAN = Federation of the International Societies of Gastroenterology, Hepatology and Nutrition (5).
Phase 1
Phase I includes a review of guidelines and consensus statements on AGE management in subjects between 1 month and 18 years of age published in 1 of the 3 most spoken languages (English, Chinese, and Spanish) in the last 10 years, using previously described methodology (5). According to results of phase 1, there is consensus on the main pillars for the management of AGE: definition of diarrhea, assessment of dehydration, oral rehydration, age-appropriate diet, and possible use of selected products that could reduce the severity and duration of diarrhea (5).
Phase 2
The steering committee proposed definitions and recommendations aimed at managing children with AGE worldwide. This list was circulated to members of FISPGHAN WG to obtain their comments and corrections. Not essential recommendations were eventually removed. The Delphi method was used to reach an agreement (Fig. 1A) (14).
Subsequently, all members expressed their agreement by a 5-point Likert Scale (Round 1), a valuable tool used to compare and rank ordinal data (15). A specific target category of the scale was identified as referral category ("strongly agree" category) and the target consensus measure was calculated and expressed as a value between "0" and "1."
The overall agreement and the agreement for each recommendation were finally expressed as percentage. Those recommendations that did not reach at least 80% agreement in the first round, were re-discussed and tailored, and the entire list of recommendations was again scored with the same methodology (Round 2). Once all recommendations reached ≥80% agreement, a new list of recommendations was developed (Fig. 1A).
Since malnutrition may significantly change the approach to children with AGE, the list of recommendations developed by the WG applies to well-nourished to nonseverely malnourished children. This point was specifically highlighted in the final list of recommendations.
Phase 3
In Phase 3, an electronic questionnaire reporting definition and recommendations was circulated among 50 experts around the world (including at least 5 from each continent). We invited clinicians and/or researchers, members of the World Societies for Pediatric Gastroenterology, Hepatology, and Nutrition, of WHO and of European Society of Pediatric Infectious Diseases and Societies of Pediatric Emergency Medicine to participate.
Each participant was asked to express his/her agreement on each recommendation; the calculation of overall agreement was performed by the methodology previously reported.
A recommendation was finally accepted only if at least 75% external experts provided their agreement. The recommendations not reaching this threshold were discussed in the text but not included in the final set of recommendations.
Results
Figure 1B shows the results obtained in each phase. The results of Phase 1 were reported in detail in a previous publication (5). However, starting from the evidence obtained in Phase 1, the steering committee developed a list of 28 definitions/recommendations. After circulation of this list among the WG members, 1 recommendation was removed, 2 recommendations on the use of antiemetics were combined together, and a new recommendation on treatment was added, resulting in a final list of 27 recommendations.
In Round 1, the raters expressed an overall agreement of 86% (range 70%–98%) with 6 recommendations rating <80%. In Round 2, the overall agreement increased to 91% (range 81%–98%), with 2 recommendations on the pharmacological treatment with Smectite and Racecadotril showing a grade of agreement <80% (76% and 78%, respectively) (Fig. 1B).
Twenty-eight of 50 experts took part in the third phase of the project (response rate 56%). After Round 3, 4 recommendations focusing on the dehydration scores (1 recommendation), the use of antiemetics (1 recommendation), and specific antidiarrheal drugs (2 recommendations) did not reach the threshold of 75% agreement and were excluded from the final set of recommendations. The overall agreement expressed by the external experts was 87%. The agreement received by single recommendations in each phase of the study is reported in Table 1.
Table 1. Agreement expressed by experts of the FISPGHAN working group (round 1 and 2) and by external experts (round 3) on each recommendation.
| Agreement* (%) | |||||
|---|---|---|---|---|---|
| Recommendation | Topic | Round 1 | Round 2 | Round 3 | Final set of recommendations |
| #1 | Definition | 90.33 | 96.50 | 84.48 | Approved |
| #2 | Assessment of dehydration | 87.47 | 91.24 | 86.25 | Approved |
| #3 | 92.99 | 96.50 | 83.94 | Approved | |
| #4 | 89.49 | 92.99 | 84.98 | Approved | |
| #5 | 84.24 | 87.20 | 74.35 | Not approved | |
| #6 | Diagnostic work-up | 77.53 | 94.75 | 88.99 | Approved |
| #7 | 85.72 | 87.47 | 91.06 | Approved | |
| #8 | 94.75 | 94.75 | 89.68 | Approved | |
| #9 | Rehydration | 98.25 | 96.50 | 93.78 | Approved |
| #10 | 90.97 | 90.97 | 87.59 | Approved | |
| #11 | 83.70 | 81.58 | 75.61 | Approved | |
| #12 | 92.72 | 96.50 | 83.02 | Approved | |
| #13 | 82.42 | 91.24 | 88.99 | Approved | |
| #14 | Nutritional interventions | 98.25 | 98.25 | 92.43 | Approved |
| #15 | 88.95 | 94.48 | 91.06 | Approved | |
| #16 | 83.97 | 91.24 | 84.07 | Approved | |
| #17 | 70.26 | 92.99 | 94.50 | Approved | |
| #18 | 91.24 | 89.22 | 85.13 | Approved | |
| #19 | Anti-diarrheal treatment | 81.04 | 92.99 | 83.94 | Approved |
| #20 | 70.73 | 80.67 | 79.34 | Approved | |
| #21 | 67.60 | 75.51 | 66.59 | Not approved | |
| #22 | 72.65 | 78.17 | 67.61 | Not approved | |
| #23 | 92.08 | 98.25 | 93.70 | Approved | |
| #24 | Anti-emetic treatment | 83.33 | 87.74 | 71.16 | Not approved |
| #25 | 76.05 | 86.83 | 86.13 | Approved | |
| #26 | Antibiotic treatment | 98.25 | 98.25 | 95.18 | Approved |
| #27 | 83.97 | 92.99 | 84.29 | Approved | |
FISPGHAN = Federation of the International Societies of Gastroenterology, Hepatology and Nutrition.
Below are the definitions and recommendations developed by the FISPGHAN WG and, subsequently, validated by external revision and voting:
Definitions
Several definitions have been used by researchers, scientific societies, and health care authorities to describe acute infectious diarrhea in childhood. Johnston et al, analyzing the clinical trials published in pediatric age, found 64 unique definitions of diarrhea and 69 definitions of diarrhea resolution (16). Starting from the definitions used by other guidelines collected in the Phase 1, the WG agreed upon the following definition.
Recommendation 1 (Agreement 85%).
AGE is characterized by the presence of diarrhea defined as a decrease in the consistency of stool leading to loose or liquid stools and/or an increase in the frequency of evacuations to three or more in 24 hours, with or without fever or vomiting.
-
-
Acute diarrhea lasting 7 days or less.
-
-
Prolonged diarrhea lasting 8 to 13 days.
-
-
Chronic or persistent diarrhea lasting 14 days or more.
Assessment of Dehydration
Dehydration is the consequence of diarrhea, vomiting, and fever. It is a major risk for child health and the main reason for medical visit, hospitalization, and cause of death. Assessing dehydration in a reliable and rapid way is essential to prevent severe dehydration and its consequences and promptly start treatment.
Recommendation 2 (Agreement 86%).
The percentage of body weight lost is the best measure of dehydration. However, in most circumstances, preillness weight is frequently not available to estimate weight lost during an episode of diarrhea.
Recommendation 3 (Agreement 84%).
Dehydration reflects disease severity particularly in the community setting. In hospitalized children, although dehydration may also be considered, other parameters (such as respiratory distress, deep breathing, signs of shock, inability to drink, neurological signs, electrolyte abnormalities, or body weight loss) may also be used as markers of severity.
Recommendation 4 (Agreement 85%).
The best parameters to estimate the degree of dehydration are: skin turgor, sunken eyes, general appearance, capillary refill time, and mucous membranes. These parameters are frequently included in clinical scores that may be used to estimate the degree of dehydration in individual children. Reliability of those signs may be reduced in malnourished children.
Although none of the available scoring systems for dehydration reached the threshold of 75% agreement, the Clinical Dehydration Scale (17) is commonly considered a reliable tool to estimate the degree of dehydration in nonmalnourished children with AGE (agreement 74%). This 4-item score system, based on the evaluation of general appearance, mucous membranes, eyes, and presence of tears, has been mainly applied in high-income countries and has been recently demonstrated to be helpful in ruling in moderate-to-severe dehydration (≥6%) in this setting (18). However, its reliability needs to be better established in resource-poor settings.
Diagnostic Workup
Children otherwise healthy with AGE do not require a specific diagnostic work-up, as the results of laboratory and microbiological investigations are not likely to change their management. Diagnostic work-up may be required in selected cases.
Recommendation 5 (Agreement 89%).
In most cases, children with AGE do not require any diagnostic workup. In severe conditions and/or in the hospital setting, investigations may be appropriate in individual cases.
Recommendation 6 (Agreement 91%).
Children presenting with uncomplicated AGE do not require routine of microbiological investigation.
Recommendation 7 (Agreement 90%).
Microbiological investigations should be considered in the following circumstances:
Children with underlying chronic conditions (e.g., oncologic diseases, inflammatory bowel disease, immunodeficiency)
Extremely severe clinical conditions (e.g., sepsis)
Prolonged symptoms (>7 days)
During outbreaks (childcare, school, hospital)
Children with severe bloody diarrhea and high fever
History of travel to at-risk areas
Rehydration
Replacing fluid losses and avoiding dehydration are the primary aims of AGE management.
The oral route is effective in preventing and treating mild-to-moderate dehydration and should be started as soon as possible with appropriate solutions.
Different oral rehydration solutions (ORS) are available worldwide; however, an overall trend in recommending reduced osmolarity solutions (60–75 mmol Na+) has been observed in the last 10 years based on evidence of efficacy and better palatability than the WHO/UNICEF 90 mmol Na+ ORS. Different ORS compositions may show some different efficacy according to spread of Vibrio cholerae and the prevalence of malnutrition. In any case, the use of ORS is the key intervention to reduce AGE-related mortality, and it should be explained to mothers at well-being visits particularly in low-income areas as early as possible.
In children who do not tolerate oral rehydration, enteral or intravenous rehydration may be needed; however, a rapid switch back to oral route significantly reduces side effects and improves recovery.
Recommendation 8 (Agreement 94%).
ORS is the first-line treatment of AGE. Knowledge, attitude, and practice about oral rehydration by health service providers are essential and should be promoted.
Recommendation 9 (Agreement 88%).
Reduced osmolality ORS (60–75 mmol/L Na+) is recommended as first-line treatment of AGE. In case of cholera, 75 mmol/L Na+ is the standard rehydration regimen.
Recommendation 10 (Agreement 76%).
ReSoMal (Rehydration Solution for Malnutrition) containing 45 mmol/L Na+ and 40 mmol/L K+ may be indicated for malnourished children, although there are no conclusive data on its efficacy compared to standard reduced osmolality ORS.
Recommendation 11 (Agreement 83%).
In children who fail on oral rehydration, administration of rehydration fluids either by nasogastric tube or intravenously (IV) is effective and should be recommended. IV rehydration should be avoided where possible in severely malnourished children.
Children with shock should be managed according to guidelines for hypovolemic shock. In non-shocked children with severe dehydration, moderate quality of evidence supports the use of rapid IV rehydration with 20 mL/kg/h of 0.9% saline solution for 2 to 4 hours. These rehydration regimens have been proposed in high-income settings and need to be tested in other settings before providing recommendations.
Recommendation 12 (Agreement 89%).
Enteral administration of ORS through a nasogastric tube is effective in rehydrating children with AGE and it is associated with fewer side effects than IV rehydration, especially in malnourished children. Its knowledge, attitude, and practice should be promoted among health workers as well as families and local workers.
Nutritional management
Several nutritional approaches are currently used worldwide according to local habits and beliefs to reduce severity and duration of diarrhea, but only few have been appropriately tested for efficacy. All international guidelines agree on continuation of breast-feeding throughout the episode, and an age-appropriate scheme should be started during or after initial rehydration (4–6 hours). Diet modification is usually unnecessary. However, several data support the addition of zinc to malnourished children.
Recommendation 13 (Agreement 92%).
Infants younger than 6 months should neither interrupt breast-feeding nor introduce diluted or modified formula. Where there is not the possibility to breast-feed, routine dilution of milk and routine use of lactose-free milk formula are not usually necessary.
Recommendation 14 (Agreement 91%).
Children should be re-fed early during the course of AGE. Regular oral feeding should be reintroduced no later than 4 to 6 hours after the onset of rehydration.
Recommendation 15 (Agreement 84%).
Lactose-free formula is generally not necessary in AGE episodes. However, lactose-restricted diets may be considered in hospitalized children and in children with prolonged diarrhea (>7 days). Lactose-free formula should be recommended in children with chronic diarrhea (>14 days).
Recommendation 16 (Agreement 94%).
Elimination diet is usually not indicated for children with AGE and it may further impair the child’s nutritional status.
Recommendation 17 (Agreement 85%).
Zinc is recommended as an adjunct to oral rehydration therapy in children older than 6 months living in low-income countries or in settings with medium or high risk of zinc deficiency. Its efficacy is not supported by solid evidence in well-nourished children living in high-income countries. In infants younger than 6 months zinc is not effective regardless of the nutritional status.
Active Treatment of Diarrhea
Many pharmacological interventions have been proposed in adjunct to ORS to reduce the severity and duration of symptoms. Only some have been specifically and accurately tested in clinical trials, whereas many products are currently available on the market with no proof of efficacy and safety. Children with diarrhea should receive only tested products. Physicians should discuss their use with families and caregivers explaining the modality of administration and expected effects, providing the important message that administration of any product should not replace oral rehydration therapy.
Recommendation 18 (Agreement 84%).
Active treatment of diarrhea with the administration of probiotics and/or drugs may be considered where there is solid proof of efficacy in reducing the intensity and duration of symptoms. To maximize efficacy, active treatment should be administered early in the course of the disease.
However, administration of any product should not replace oral rehydration therapy and should be always used as an adjunct to ORS treatment.
Because investigation of active therapies is rapidly evolving, the choice of best treatment should be always made along recommendations of evidence-based guidelines and in compliance with well-done randomized controlled trials.
Recommendation 19 (Agreement 79%).
Probiotics are effective in reducing the duration and intensity of symptoms of AGE. If available and in agreement with caregivers, selected probiotic strains (including Lactobacillus rhamnosus GG, Saccharomyces boulardii, and also L reuteri DSM 17938) can be considered in children with AGE, as an adjunct to ORS.
Antidiarrheal drugs recommended by several guidelines as adjunct to ORS treatment in children with AGE were considered for inclusion in the set of recommendations, but did not reach sufficient agreement among experts. Specifically, smectite (66.6%) and race-cadotril (67.6%), that are recommended in guidelines produced in Europe, South America, Malaysia, and China, were not endorsed based on our stringent criteria.
Recommendation 20 (Agreement 94%).
Loperamide and other antimotility drugs are not recommended in the treatment of AGE.
Use of Antiemetics in Children With Acute Gastroenteritis
Vomiting is a major cause of dehydration (additional fluid losses and impaired oral rehydration). The use of antiemetics has been proposed with the primary aim of reducing vomiting in the first hours after disease onset, allowing effective oral rehydration and avoiding hospital admission. Routine use of antiemetics is still debated, mainly in the outpatients setting. However, if prescribed, the potential side effects of antiemetics should be carefully taken into account by physicians.
Recommendation 21 (Agreement 86%).
Metoclopramide, although effective, has significant side effects and is therefore not recommended for children with vomiting owing to AGE. The efficacy of domperidone is not supported by randomized controlled trials.
Ondansetron administered either orally or intravenously is effective in reducing vomiting and may avoid hospital admission. A single dose at the dosages used in the available studies may be considered in young children presenting to an emergency department with vomiting to ensure oral rehydration and reduce hospital admission. However, the use of ondansetron has been associated with QT prolongation and severe cardiac arrhythmias and the drug carries a warning label by both the Food and Drug Administration and the European Medicines Agency that should be taken into account by health care providers. Current cost of the product is also an issue that must be considered in many settings.
Anti-infectious Treatment
AGE in otherwise healthy children is usually self-limited regardless of etiology. Even without specific antimicrobial therapy, clinical recovery generally occurs within a few days and the causative organism is cleared in a few days or weeks. Hence, an empiric anti-infectious treatment is not routinely indicated in children with AGE. An empiric antibiotic treatment may be considered in severe cases and in at-risk children with underlying chronic conditions or immunodeficiency. Overall, a pathogen-based approach within the local epidemiological pattern of antibiotic resistance should be considered only in particular circumstances.
Recommendation 22 (Agreement 95%).
Routine use of antibiotics is not recommended for the treatment of AGE.
Recommendation 23 (Agreement 84%).
The use of antibiotics should be started immediately and may be considered in specific situations, including:
infants younger than 3 months
children with underlying chronic conditions, including those with sickle cell anemia or immunodeficiency and those at risk for developing severe or extraintestinal dissemination
isolation of specific pathogens such as Shigella, enterotoxigenic (but not Shiga-like toxin-producing) Escherichia coli, V cholerae, Yersinia enterocolitica, and Entamoeba histolytica.
Campylobacter colitis can be treated with antibiotics, but treatment is effective only if administered within the first 2 days from the onset of symptoms.
Discussion
Despite guidelines produced in different settings sharing similar recommendations for the management of children with AGE worldwide (5), a low adherence to standard indications and a significant variability in clinical practice have been widely reported (6–10).
The management of AGE is essentially based on 5 steps: assessment of dehydration by simple, reproducible, and validated parameters and/or clinical score; prompt rehydration with reduced osmolality ORS; avoidance of elimination diets and continuing of breast-feeding in infants and regular diet in children; limiting laboratory investigations to selected circumstances and increased risk for bacterial infection; consider active treatment of diarrhea with products supported by compelling clinical evidence in children.
In 2012, the FISPGHAN WG identified the top priorities for the reduction of AGE burden worldwide (13) and now developed a set of clinical recommendations based on indications provided by guidelines produced in different countries and settings worldwide; the list has been validated by external experts in pediatric gastroenterology, emergency medicine, and infectious diseases. The rate of agreement slightly decreased when the core of recommendations was circulated among external experts from different disciplines as expected. Nevertheless, an overall agreement >85% was eventually achieved.
Some recommendations initially developed by the FISP-GHAN WG were not validated by external experts and hence were not included in the final set of recommendations.
Overall, the FISPGHAN WG recommendations for the management of AGE in children closely match those of the World Health Organization (19); however, relevant differences are present in treatment recommendations, including positive recommendation for selected antidiarrheal products (i.e., selected probiotic strains) or antiemetics (in particular circumstances), indications to antibiotic treatment and negative recommendations for the use of zinc in not malnourished or zinc-deficient children.
The use of the Clinical Dehydration Score was proposed to assess dehydration in children with AGE. Although the degree of agreement did not allow formal approval as final recommendation (agreement 74%), the evidence reporting efficacy, reliability, and ease use also by non-medical personnel suggest that Clinical Dehydration Scale could still be considered in clinical practice.
Routine use of antiemetics is mostly discussed in developed areas and emergency settings, where stopping vomiting to enhance oral rehydration can avoid hospital admission. Recent data support a single oral dose of ondansetron in the emergency department (20,21). However, the reports of potentially severe, but extremely rare, side effects hamper its use on a large scale (22,23), at least, until the warning will be cleaned by post-marketing analysis.
Recommendations for active treatment included effective probiotic strains but no drugs, including racecadotril and smectite.
This study has some limitations. First the WG sets arbitrary thresholds to rule out or accept recommendations. A high threshold of 80% was set in the first 2 rounds to exclude unnecessary and ambiguous recommendations and put the basis for a core set of recommendations. A slightly lower threshold (75%) was used in the third round to include recommendations strongly agreed upon by experts from different pediatrics specialties.
Second, the article did not discuss prevention of AGE in terms of public health and contacts measures or active immunization. The FISPGHAN WG identified rotavirus immunization as a public health priority and recently reported an unacceptably low vaccination rate in many countries also identifying costs and limited perception of rotavirus severity by public health authorities, families, and physicians as major barriers to local implementation (24).
Finally, the recommendations provided are not applicable to severely malnourished children. Although malnourished children are at higher risk for developing recurrent and complicated AGE episodes, only a few interventions have been tested in this population with variable results. For example, probiotics are considered a standard of practice in otherwise healthy children, but obtained inconsistent results in a malnourished population (25). We strongly believe that malnutrition, and not geographical location, is the major determinant for considering changes in the management of AGE. Malnourished children need specific recommendations supported by evidence. However, irrespective of geographical setting, all other children may benefit from similar interventions and may be safely managed in the same way based on “universal" recommendations.
This article is expected to increase adherence to effective management of AGE according to evidence-based indications.
Acknowledgments
P.M.S. has received honoraria for CME presentations from Nestle Nutrition, Abbott Nutrition, and Mead Johnson Nutrition, and collaborates on research grants related to probiotics awarded from the Canadian Institutes of Health Research, Lallemand Human Nutrition, and BloKPlus.
Footnotes
The authors report no conflicts of interest.
References
- 1.United Nation Millenum Goals. [Accessed on July 20, 2016]; Available at http://www.un.org/millenniumgoals/childhealth.shtml.
- 2.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–40. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 3.Ogilvie I, Khoury H, Goetghebeur MM, et al. Burden of community-acquired and nosocomial rotavirus gastroenteritis in the pediatric population of Western Europe: a scoping review. BMC Infect Dis. 2012;12:62. doi: 10.1186/1471-2334-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiegering V, Kaiser J, Tappe D, et al. Gastroenteritis in childhood: a retrospective study of 650 hospitalized pediatric patients. Int J Infect Dis. 2011;15 doi: 10.1016/j.ijid.2011.02.006. e401–7. [DOI] [PubMed] [Google Scholar]
- 5.Lo Vecchio A, Dias JA, Berkley JA, et al. Comparison of recommendations in Clinical Practice Guidelines for acute gastroenteritis in children. J Pediatr Gastroenterol Nutr. 2016;63:226–35. doi: 10.1097/MPG.0000000000001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo Vecchio A, Liguoro I, Bruzzese D, et al. Accreditation and Quality Improvement Working Group of Italian Society of Pediatrics. Adherence to guidelines for management of children hospitalized for acute diarrhea. Pediatr Infect Dis J. 2014;33:1103–8. doi: 10.1097/INF.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 7.Powell EC, Hampers LC. Physician variation in test ordering in the management of gastroenteritis in children. Arch PediatrAdolesc Med. 2003;157:978–83. doi: 10.1001/archpedi.157.10.978. [DOI] [PubMed] [Google Scholar]
- 8.Freedman SB, Gouin S, Bhatt M, et al. Prospective assessment of practice pattern variations in the treatment of pediatric gastroenteritis. Pediatrics. 2011;127 doi: 10.1542/peds.2010-2214. e287-95. [DOI] [PubMed] [Google Scholar]
- 9.Tieder JS, Robertson A, Garrison MM. Pediatric hospital adherence to the standard of care for acute gastroenteritis. Pediatrics. 2009;124 doi: 10.1542/peds.2009-0473. e1081-7. [DOI] [PubMed] [Google Scholar]
- 10.Mangione-Smith R, DeCristofaro AH, Setodji CM, et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357:1515–23. doi: 10.1056/NEJMsa064637. [DOI] [PubMed] [Google Scholar]
- 11.Marques AC, Calderaro D, Yu PC, et al. Impact of cardiology referral: clinical outcomes and factors associated with physicians’ adherence to recommendations. Clinics (Sao Paulo) 2014;69:666–71. doi: 10.6061/clinics/2014(10)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reuben DB, Maly RC, Hirsch SH, et al. Physician implementation of and patient adherence to recommendations from comprehensive geriatric assessment. Am J Med. 1996;100:444–51. doi: 10.1016/s0002-9343(97)89521-6. [DOI] [PubMed] [Google Scholar]
- 13.Guarino A, Winter H, Sandhu B, et al. Acute gastroenteritis disease: report of the FISPGHAN Working Group. J Pediatr Gastroenterol Nutr. 2012;55:621–6. doi: 10.1097/MPG.0b013e318272b5e2. [DOI] [PubMed] [Google Scholar]
- 14.Dalkey N. The Delphi method: an experimental study of group opinion. Rand; Santa Monica, CA: 1969. [Google Scholar]
- 15.Tastle WJ, Wierman MJ. Consensus and dissention: A measure of ordinal dispersion. Int J Approx Reason. 2007;45:531–45. [Google Scholar]
- 16.Johnston BC, Shamseer L, da Costa BR, et al. Measurement issues in trials of pediatric acute diarrheal diseases: a systematic review. Pediatrics. 2010;126:222–31. doi: 10.1542/peds.2009-3667. [DOI] [PubMed] [Google Scholar]
- 17.Friedman JN, Goldman RD, Srivastava R, et al. Development of a clinical dehydration scale for use in children between 1 and 36 months of age. J Pediatr. 2004;145:201–7. doi: 10.1016/j.jpeds.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 18.Falszewska A, Szajewska H, Dziechciarz P. Diagnostic accuracy of three clinical dehydration scales: a systematic review. Arch Dis Child. 2018;103:383–8. doi: 10.1136/archdischild-2017-313762. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Second. 2013. [Accessed on June 11, 2018]. Hospital Care for children. Guidelines for the management of common childhood illnesses; p. 125. Available at: http://apps.who.int/iris/bitstream/10665/81170/1/9789241548373_eng.pdf. [PubMed] [Google Scholar]
- 20.Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database of Systematic Reviews. (7) doi: 10.1002/14651858.CD005506.pub5. 2011CD005506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman SB, Steiner MJ, Chan KJ. Oral Ondansetron administered in emergency departments to children with gastroenteritis: an economic analysis. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000350. 1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKechnie K, Froese A. Ventricular tachycardia after ondansetron administration in a child with undiagnosed long QT syndrome. Can J Anaesth. 2010;57:453–7. doi: 10.1007/s12630-010-9288-2. [DOI] [PubMed] [Google Scholar]
- 23.FDA. Zofran (ondansetron): Drug Safety Communication-Risk of Abnormal Heart Rhythms. [Accessed on October 11,2011];2011 Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/Safety-AlertsforHumanMedicalProducts/ucm272041.htm Accessed December 1, 2017.
- 24.Lo Vecchio A, Liguoro I, Dias JA, et al. Rotavirus immunization: global coverage and local barriers for implementation. Vaccine. 2017;35:1637–44. doi: 10.1016/j.vaccine.2017.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grenov B, Namusoke H, Lanyero B, et al. Effect of probiotics on diarrhea in children with severe acute malnutrition: a randomized controlled study in Uganda. J Pediatr Gastroenterol Nutr. 2017;64:396–403. doi: 10.1097/MPG.0000000000001515. [DOI] [PubMed] [Google Scholar]