Summary
Genes embedded in H3 lysine 9 methylation (H3K9me)–dependent heterochromatin are transcriptionally silenced1–3. In fission yeast, Schizosaccharomyces pombe, H3K9me-mediated heterochromatin can be transmitted through cell division provided the counteracting demethylase Epe1 is absent4,5. Under certain conditions wild-type cells might utilize heterochromatin heritability to form epimutations, phenotypes mediated by unstable silencing rather than DNA changes6,7. Here we show that resistant heterochromatin-dependent epimutants arise in threshold levels of caffeine. Unstable resistant isolates exhibit distinct heterochromatin islands, which reduce expression of underlying genes, some of which confer resistance when mutated. Targeting synthetic heterochromatin to implicated loci confirms that resistance results from heterochromatin-mediated silencing. Our analyses reveal that epigenetic processes promote phenotypic plasticity, allowing wild-type cells to adapt to non-favorable environments without altering their genotype. In some isolates, subsequent or co-occurring gene amplification events augment resistance. Caffeine impacts two anti-silencing factors: Epe1 levels are downregulated, reducing its chromatin association; and Mst2 histone acetyltransferase expression switches to a shortened isoform. Thus, heterochromatin-dependent epimutant formation provides a bet-hedging strategy that allows cells to remain genetically wild-type but adapt transiently to external insults. Unstable caffeine-resistant isolates show cross-resistance to antifungal agents, suggesting that related heterochromatin-dependent processes may contribute to antifungal resistance in plant and human pathogenic fungi.
H3K9me-heterochromatin can be copied by a read-write mechanism4,5,8 and has been observed to arise stochastically at various loci, albeit only in the absence of key anti-silencing factors9–13 or specific growth conditions14. We reasoned that if heterochromatin can redistribute in wild-type S. pombe cells, epimutations could be generated, allowing adaption to external insults. Unlike genetic mutants we predicted that such epimutants would be unstable, resulting in gradual loss of resistance following growth without the insult. We chose to use caffeine because deletion of genes with a variety of cellular roles confers caffeine resistance15, thereby increasing the chance of obtaining epimutations. We also reasoned that unstable epimutants would occur more frequently at moderate caffeine concentrations that prevent most cells from growing (16 mM) rather than the higher stringency (20 mM) used in screens for genetic caffeine-resistant mutants15.
As secondary events might occur upon prolonged growth on caffeine, we froze an aliquot of each isolate upon resistant colony formation and also froze consecutive aliquots of each isolate after continued growth on caffeine (Fig. 1a). This ‘time series’ permitted detection and separation of potential initiating and subsequent events. Colonies that grew after plating wild-type fission yeast (972 h -) cells in 16 mM caffeine (+CAF) were picked. Following freezing, isolates were then successively propagated without caffeine (-CAF). Re-challenging isolates with caffeine revealed that 23% lost caffeine resistance after 14 days of non-selective growth (‘unstable resistant’, UR) whereas 13% remained caffeine resistant (‘stable resistant’, SR). 64% of isolates did not display a clear phenotype (‘unclear’) (Fig. 1b and Extended Data Fig. 1a-c). Deletion of clr4 + (the sole S. pombe H3K9 methyltransferase16,17), but not a control locus, from resistant isolates resulted in loss of caffeine resistance in unstable, but not stable isolates (Fig. 1c and Extended Data Fig. 1d). Thus, caffeine resistance in unstable isolates requires heterochromatin.
Figure 1. Identification of heterochromatin-dependent epimutants resistant to caffeine.
a, Screening strategy. S. pombe wild-type (wt) cells were plated on caffeine-containing (+CAF) media. Caffeine-resistant isolates were picked and grown on +CAF for 4 days. Isolates were then grown on +CAF for a total of 7 or 20 days or on non-selective (-CAF) media for 2 and 14 days.
b, Unstable (UR) and stable (SR) caffeine-resistant isolates were identified. After non-selective growth for 2 and 14 days, caffeine-resistant isolates were serially diluted and spotted on -CAF and +CAF plates to assess resistance to caffeine.
c, Caffeine resistance in UR isolates depends on the Clr4 H3K9 methyltransferase. clr4 + (clr4Δ) or an unlinked intergenic region (controlΔ) were deleted in unstable (UR-1) and stable (SR-1) caffeine-resistant isolates.
Experiments in (b) and (c) were independently repeated at least twice with similar results.
Whole genome sequencing (WGS) of stable isolate SR-1 uncovered a mutation in pap1 + responsible for the caffeine-resistant phenotype (Extended Data Fig. 1e)18. ChIP-seq for H3K9me2 on SR-1 revealed no changes in heterochromatin distribution. WGS of unstable isolates revealed no genetic changes in any sequence involved in either caffeine resistance or H3K9me2-mediated silencing, and 8 of 30 analyzed unstable isolates had no detectable genetic change compared to wild-type (Extended Data Fig. 2a-e and Supplementary Table 1).
H3K9me2 ChIP-seq on unstable isolates revealed altered heterochromatin distributions. UR-1 exhibited a new H3K9me2 island over the hba1 locus, whereas UR-2-to-UR-6 exhibited H3K9me2 islands over the ncRNA.394, ppr4, grt1, fio1 and mbx2 loci, respectively (Fig. 2 and Supplementary Table 1). Deletion of hba1 + confers caffeine resistance19, suggesting that caffeine-induced heterochromatin islands may drive resistance by silencing underlying genes. Accordingly, RT-qPCR analysis revealed reduced expression of genes underlying the observed hba1 heterochromatin island (Extended Data Fig. 2f).
Figure 2. Ectopic islands of heterochromatin are detected in unstable (UR) caffeine-resistant isolates.
a-b, Genome-wide (a) and locus-specific (b) H3K9me2 ChIP-seq enrichment in wild-type (wt) cells and UR isolates. Data are represented as relative fold enrichment over input. Sequencing was performed once, and results were confirmed by qChIP. Red arrows in (b) indicate essential genes.
The ncRNA.394, ppr4, grt1, fio1 and mbx2 loci have not previously been implicated in caffeine resistance. Interestingly, 24/30 unstable isolates exhibited a heterochromatin island over the ncRNA.394 locus (Extended Data Fig. 3a, b and Supplementary Table 1), and reduced underlying transcript levels (Extended Data Fig. 2f and 3c), suggesting that transcriptional silencing within these loci mediates caffeine resistance.
ncRNA.394 was previously identified as a heterochromatin island that gains H3K9me2 in the absence of counteracting Epe1 demethylase9,20. We detected no H3K9me2 over ncRNA.394 in untreated wild-type cells (Fig. 2b and Extended Data Fig. 3a, b). Deletion of ncRNA.394 did not result in caffeine resistance (Extended Data Fig. 3d). Prolonged growth without caffeine of cells exhibiting the ncRNA.394 heterochromatin island resulted in H3K9me2 loss over this region, whereas growth with caffeine extended the H3K9me2 domain over the SPBC17G9.13c + and SPBC17G9.12c + genes (Extended Data Fig. 3e). Deletion of SPBC17G9.12c + or eno101 + did not result in caffeine resistance (Extended Data Fig. 3d). SPBC17G9.13c + is essential for viability, precluding testing its deletion for resistance.
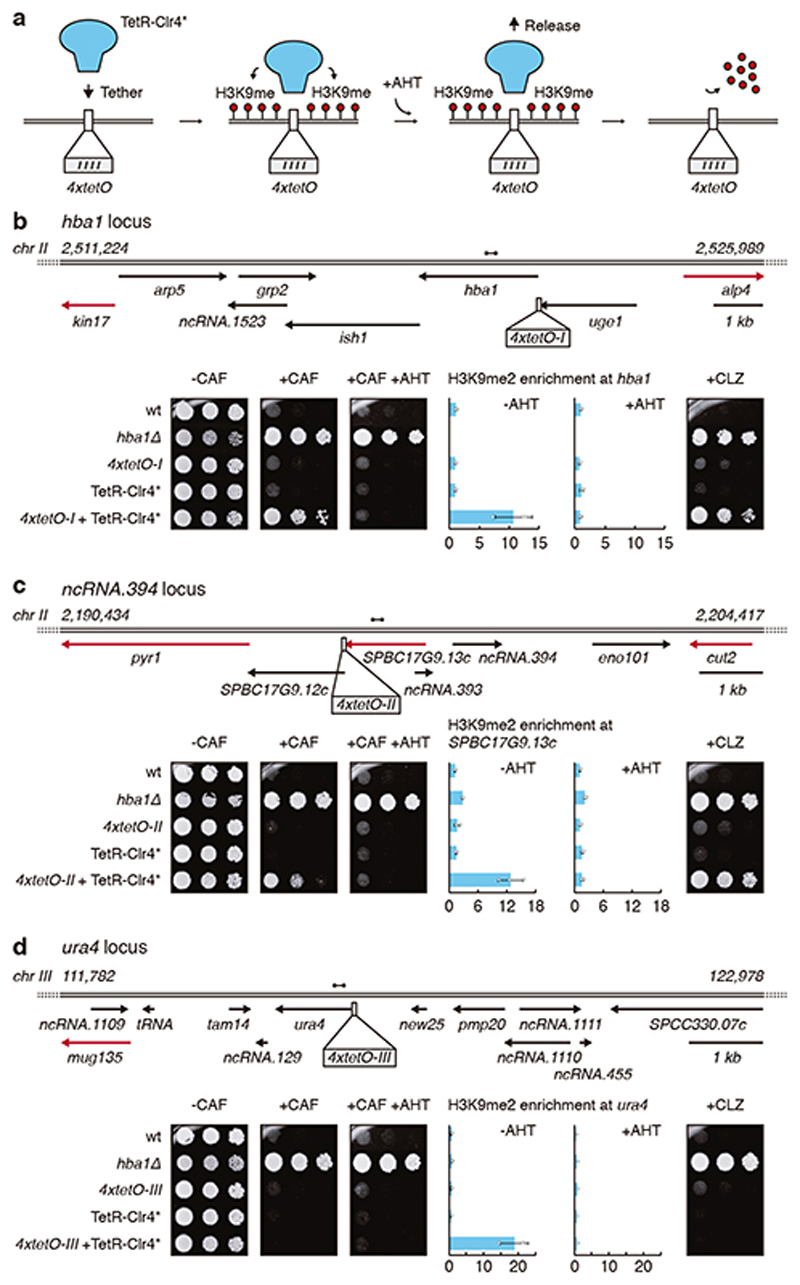
To test if heterochromatin formation at these specific loci alone results in caffeine resistance, tetO binding sites were inserted at hba1, ncRNA.394 and mbx2 loci to force synthetic heterochromatin assembly upon recruitment of TetR-Clr4* fusion protein4,5. Combining tetO with TetR-Clr4* without anhydrotetracycline (-AHT) resulted in novel H3K9me2 domains and growth on caffeine (Fig. 3 and Extended Data Fig. 4a-d). Thus, heterochromatin-mediated silencing at hba1, ncRNA.394 or mbx2 loci results in caffeine resistance.
Figure 3. Forced synthetic heterochromatin targeting to the identified loci is sufficient to drive caffeine resistance in wild-type cells.
a, TetR-Clr4* mediates H3K9me deposition at 4xtetO binding sites. Addition of anhydrotetracycline (+AHT) releases TetR-Clr4* from 4xtetO sites, resulting in removal of H3K9me.
b-d, Wild-type (wt) cells harbouring 4xtetO binding sites at the hba1 or ncRNA.394 loci (or ura4 as control) and expressing TetR-Clr4* were assessed for caffeine (+CAF) or clotrimazole (+CLZ) resistance in the absence or presence of AHT. qChIP of H3K9me2 levels on hba1 (b), SPBC17G9.13c (c) and ura4 (d) loci. Data are mean ± s.d. from three biological replicates. Dumbbells indicate primer pairs used. Red arrows indicate essential genes. Note hba1 is not present in hba1Δ.
Remarkably, strains with forced synthetic heterochromatin at either hba1 or ncRNA.394 loci displayed resistance to the widely-used antifungals clotrimazole, tebuconazole and fluconazole (Fig. 3 and Extended Data Fig. 4e). Unstable caffeine-resistant isolates with heterochromatin islands at hba1 (UR-1) or ncRNA.394 (UR-2) loci also displayed resistance to antifungals and produced small interfering RNAs (siRNAs) homologous to surrounding genes (Extended Data Fig. 5a-c). Consistent with RNAi pathway involvement, caffeine resistance was abolished upon removal of RNAi components (dcr1Δ, ago1Δ; Extended Data Fig. 5d).
TetR-Clr4* tethering close to SPBC17G9.13c +, upstream of ncRNA.394, resulted in caffeine resistance (Fig. 3c), suggesting that reduced SPBC17G9.13c + expression may mediate resistance. We therefore reduced expression of SPBC17G9.13c + (named cup1+, caffeine unstable phenotype 1) by increasing degradation of its mRNA (LocusPX:cup1-3xDSR) or attenuating its transcription (cup1-TT; see Methods). Both approaches resulted in reduced cup1 + transcript levels and caffeine resistance (Extended Data Fig. 6a, b). Cup1 contains a LYR domain often found in mitochondrial proteins21 and Cup1-GFP exhibited mitochondrial localisation (Extended Data Fig. 6c). LYR-domain mutation led to caffeine resistance (Extended Data Fig. 6d). Thus, reduced expression or mutation of mitochondrial protein Cup1 (SPBC17G9.13c) renders cells caffeine resistant. We conclude that cup1 + silencing by heterochromatin island formation mediates caffeine resistance.
In addition to the ncRNA.394/cup1 heterochromatin island, analysis of ChIP-seq input DNA indicated that many independent unstable caffeine-resistant isolates also contained increased copy number of a chromosome III region (Extended Data Fig. 7a). The minimal region of overlap in 11/12 isolates contained cds1 +, whose overexpression confers caffeine resistance22. To determine if cds1 + amplification occurred before or after ncRNA.394/cup1 heterochromatin island formation, we analyzed UR-2 samples frozen at earlier and later time points. The ncRNA.394/cup1 H3K9me2 island was detected in the initial caffeine-resistant isolate (4day/+CAF), whereas cds1 locus amplification arose later (7day/+CAF) (Extended Data Fig. 7b). Thus, development of resistance appears to be a multistep process where combinatorial events facilitate adaption to the insult.
In agreement with this hypothesis, deletion of clr4 + from the initial UR-2 isolate (4day/+CAF) resulted in caffeine resistance loss in all transformants (6/6). However, only half of the transformants (3/6; transformants 1, 4 and 5) lost caffeine resistance upon clr4 + deletion from the later UR-2 isolate with cds1 locus amplification (7day/+CAF). Transformants that retained resistance after clr4 + removal (3/6; transformants 2, 3 and 6) exhibited higher cds1 + copy numbers compared to clr4Δ transformants that lost resistance or to wild-type cells (Extended Data Fig. 7c). We conclude that once cds1 locus amplification occurs heterochromatin is no longer required for caffeine resistance. In UR-2 the new ncRNA.394/cup1 heterochromatin island arose before cds1 + amplification, but it is likely that these events are stochastic and occur in no fixed order. Interestingly, both adaptations – island formation and locus amplification – are unstable and lost following growth without caffeine (Extended Data Fig. 7d).
Instability of the amplified region suggested it resulted from excision and extrachromosomal circular DNA (eccDNA) formation which can be rapidly accumulated and lost23–26. CNV plots revealed repetitive elements at junctions of putative eccDNA (5S rRNA.24/26 for UR-2 (7day/+CAF) and LTR3/27 for UR-4). PCR specific for putative circle junctions and Southern analysis confirmed the presence of chromosome-III-derived eccDNA (Extended Data Fig. 8). Therefore, repeat-mediated eccDNA generation provides an alternative, or supplementary, mechanism for the evolution of caffeine, and perhaps other, resistances in fission yeast. Accumulation of additional changes may allow further adaption to insults through other pathways or by bolstering silencing at particular loci27.
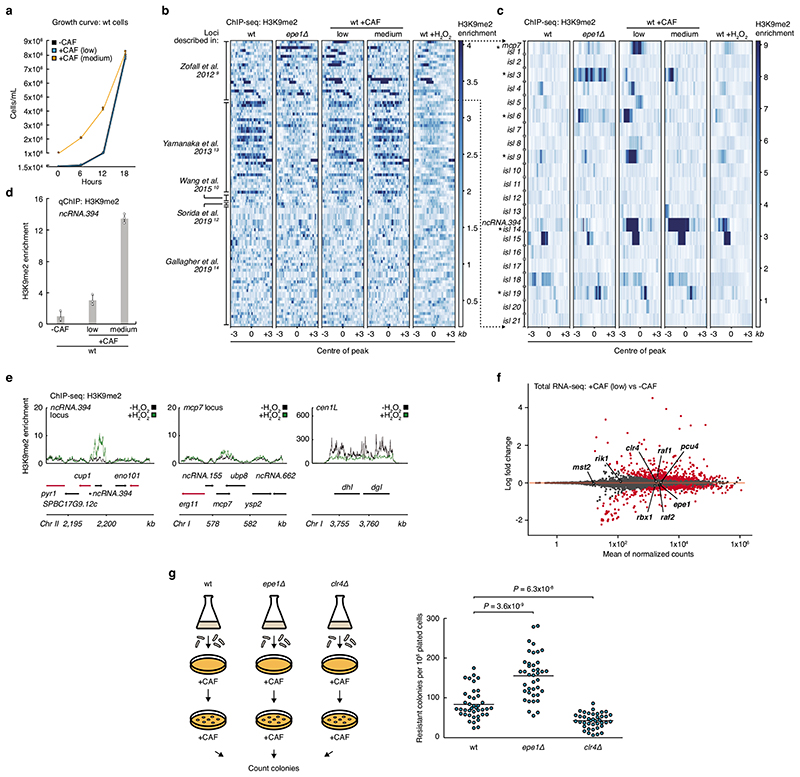
To investigate the dynamics of heterochromatin island formation in response to caffeine we exposed wild-type cells to low (7 mM) or medium (14 mM) doses of caffeine. Cells in low or medium caffeine doubled ~8 or ~3 times, respectively, in 18 hours (Extended Data Fig. 9a). Several H3K9me2 heterochromatin islands were detected following exposure to low caffeine (Fig. 4a top and Extended Data Fig. 9b, c). These low-caffeine-induced islands represent a subgroup of those that accumulate H3K9me2 in the absence of Epe19,10,12, including ncRNA.394/cup1, but did not overlap with H3K9me2-heterochromatin domains that accumulate without nuclear exosome function13 or at 18°C14. Remarkably, ectopic heterochromatin was restricted to ncRNA.394/cup1 following medium caffeine treatment and H3K9me2 levels at this locus were ~4-fold greater after medium compared to low caffeine exposure (Fig. 4a and Extended Data Fig. 9d). These data indicate that exposure to near-lethal doses of caffeine (14 mM) allows wild-type cells to develop resistance rapidly by forming heterochromatin over a locus (ncRNA.394/cup1) that confers resistance when silenced.
Figure 4. Dynamic heterochromatin redistribution following short exposure to caffeine in wild-type cells.
a, H3K9me2 ChIP-seq enrichment at ncRNA.394/cup1 and mcp7 loci (or at pericentromeric dgI/dhI repeats of chromosome I as control) in wild-type (wt) cells following 18 hr exposure to low (7 mM, top) or medium (14 mM, bottom) concentrations of caffeine. Data are represented as relative fold enrichment over input. Red arrows indicate essential genes.
b, Effect of caffeine treatment on retention of synthetic heterochromatin upon release of tethered Clr4 methyltransferase. qChIP of H3K9me2 levels on 4xtetO-ura4 + before and after TetR-Clr4* release in wt cells untreated or treated with low caffeine. epe1Δ cells were used as positive control. Dumbbells indicate primer pairs used. H3K9me2 levels were normalized to spike-in control. Data are mean ± s.d. from three biological replicates.
c, Top: Western analysis of 3xFLAG-Epe1 (endogenous gene tagged) levels before and after low caffeine treatment. Loading control: α-tubulin. For gel source data, see Supplementary Figure 1a.
Bottom: Quantification of 3xFLAG-Epe1 protein levels normalized to α-tubulin. Data are mean ± s.d. from four biological replicates. P value: two-tailed Student’s t-test.
d, Effect of caffeine treatment on association of Epe1 with chromatin. qChIP analysis of Epe1-GFP levels at sub-telomeric tlh2 locus and centromere 1 (dg repeats: cendg; outer boundary: cen-IRC) in wt cells treated with no, low or medium caffeine. Epe1-GFP levels were normalized to spike-in control. Data are mean ± s.d. from three biological replicates.
e, Model. Resistant isolates arise following exposure to a lethal insult. Resistance could be mediated by permanent, DNA-based changes (resistant mutants) or reversible, heterochromatin-based epimutations (resistant epimutants). Upon insult removal, resistant epimutants can revert to wild-type (sensitive phenotype) by disassembling ectopic heterochromatin islands, whereas resistant mutants continue displaying the mutant phenotype due to the genetic nature of DNA mutations.
To determine if other insults also induce heterochromatin islands, we exposed wild-type cells to oxidative stress (1 mM hydrogen peroxide). Heterochromatin islands were detected at similar locations to those observed in low caffeine, albeit H3K9me2 levels were lower (Extended Data Fig. 9b, c and e).
The heterochromatin profile of wild-type cells treated with low caffeine resembles that of untreated epe1Δ cells (Extended Data Fig. 9c). We hypothesized that caffeine might negatively regulate Epe1, thereby allowing adaptive ectopic heterochromatin islands to form in wild-type cells. TetR-Clr4*-mediated synthetic heterochromatin can be transmitted through cell division upon release of TetR-Clr4* from tetO sites only in cells lacking Epe14,5. To further test if caffeine imparts an epe1Δ-like phenotype, we treated wild-type cells with low caffeine and released TetR-Clr4* from 4xtetO sites inserted at ura4 + (Fig. 4b). Caffeine treatment, like epe1Δ, allowed heterochromatin retention at the tethering site for longer compared to untreated cells. epe1 + RNA levels were not significantly altered by caffeine, suggesting post-transcriptional regulation (Extended Data Fig. 9f). 3xFLAG-Epe1 levels decreased by 33% and Epe1 association with various heterochromatic locations was reduced following exposure to caffeine (Fig. 4c, d). These data suggest that down-regulation of Epe1 putative H3K9 demethylase levels plays a critical role in the response to external insults by allowing formation of adaptive ectopic H3K9me-heterochromatin islands that, in turn, reduce expression of underlying genes to confer resistance. Consistent with this scenario, epe1Δ cells form more, and clr4Δ cells fewer, caffeine resistant colonies than wild-type cells (Extended Data Fig. 9g).
Although caffeine down-regulates Epe1 protein levels, higher levels of H3K9me2 accumulate at heterochromatin islands following caffeine exposure than in untreated epe1Δ cells (Extended Data Fig. 9c). Therefore, reduced Epe1 levels alone cannot account for the high levels of H3K9me2 observed at islands upon caffeine treatment. Mst2 histone acetyltransferase acts synergistically with Epe1 to prevent heterochromatin island formation10. Interestingly, caffeine exposure results in production of a shorter Mst2 protein by wild-type cells (52 kDa versus 62 kDa; Extended Data Fig. 10a). RNA-seq suggests this shorter isoform arises through use of an alternative transcriptional start site in caffeine, such as that detected in other stresses28 (Extended Data Fig. 10b). We suggest that this caffeine-induced shortened Mst2 isoform, lacking the MYST-Zinc finger domain29, may be inactive and unable to prevent heterochromatin island formation. Thus, caffeine, by both lowering Epe1 levels and likely disabling Mst2, allows greater accumulation of H3K9me2 at islands than in epe1Δ cells. These findings reveal an adaptive epigenetic response to external insults that stimulates phenotypic plasticity, and suggest that stress-response pathways may regulate heterochromatin modulation activities, thereby ensuring cell survival in fluctuating environmental conditions (Fig. 4e).
DNA methylation-dependent epimutations frequently arise in plants and are propagated by maintenance methyltransferases30,31. RNAi-mediated epimutations occur in the fungus Mucor circinelloides 32, but their DNA methylation or heterochromatin dependence is unknown. As fission yeast lacks DNA methylation33,34 this epigenetic mark cannot be responsible for the epimutations described here. Instead our analyses indicate that these adaptive epimutations are transmitted in wild-type cells by the Clr4/H3K9me read-write mechanism4,5,8.
Why have epimutants not been detected previously in mutant screens? Stringent phenotypic screens mean strong mutants are investigated further and eccentric mutants discarded. Here we selected for weak mutants by applying sublethal doses of drug at the threshold of growth prevention. Selection was time-limited to maximize identification of isolates exhibiting unstable phenotypes prior to development of genetic alterations.
Fungal infections are on the rise, especially in immunocompromised humans. Few effective antifungal agents exist and resistance is rendering them increasingly ineffective35. Widespread use of related azole compounds to control fungus-mediated crop deterioration may leave residual antifungals in the soil, possibly allowing unwitting selection of resistant epimutants in fungi, ultimately driving increasing cases of azole-resistant Aspergillosis and Cryptococcosis in the clinic. Monitoring resistance in clinical isolates involves mutation identification by genome sequencing, but resistance due to epimutations – similar to those described here – would be missed, leading to inaccurate diagnoses. Re-engineering existing so-called ‘epigenetic drugs’ – compounds that inhibit histone-modifying enzymes – or development of novel agents, may identify molecules that specifically block fungal, not host, heterochromatin formation, hence reducing the emergence of antifungal resistance in clinical and agricultural settings.
Methods
Yeast strains and manipulations
Standard methods were used for fission yeast growth, genetics and manipulation36. S. pombe strains used in this study are described in Supplementary Table 2. Oligonucleotide sequences are listed in Supplementary Table 3. For pDUAL-adh21-TetR-2xFLAG-Clr4-CDΔ (abbreviated as TetR-Clr4*), the nmt81 promoter of pDUAL-nmt81-TetR-2xFLAG-Clr4-CDΔ4, was replaced by the adh21 promoter (pRAD21, gift from Y. Watanabe). NotI-digested plasmid was integrated at leu1 +.
To reduce expression of SPBC17G9.13c +/cup1 + we used two independent strategies. First, we expressed an additional copy of cup1 + with three nuclear exosome RNA degradation motifs (DSR; Determinant of Selective Removal37,38) fused to its 3’ untranslated region from an intergenic locus (LocusPX:cup1-3xDSR). Following insertion of cup1-3xDSR at LocusPX, endogenous cup1 + was deleted and cells expressing only cup1-3xDSR were analysed. Second, the 144-bp transcriptional terminator site from ura4 + was inserted in place of part of the putative cup1 + promoter (cup1-TT) and cells were analysed.
pap1-N424STOP, clr5-Q264STOP meu27-S100Y, LocusPX:cup1-3xDSR, cup1-TT, cup1-L73G, cup1-F99G, cup1-GFP, 3xFLAG-epe1 and strains carrying 4xtetO insertions were constructed by CRISPR/Cas9-mediated genome editing using the SpEDIT system (Allshire Lab; available on request) with oligonucleotides listed in Supplementary Table 3. The mitochondrial protein Arg1139, Epe1 and Mst2 were C-terminally tagged with mCherry (Arg11), GFP (Epe1) or 13xMyc (Mst2) using the Bähler tagging method40.
Yeast extract plus supplements (YES) was used to grow all cultures. 16 mM caffeine (Sigma, C0750) was added to media for caffeine resistance screens and serial dilution assays. To screen for unstable caffeine-resistant isolates, caffeine-resistant colonies that formed seven days after plating wild-type cells on 16 mM caffeine YES (+CAF) plates were picked and patched to +CAF plates. After four days of growth, isolates were frozen (4day/+CAF). 4day/+CAF isolates were re-patched and grown for three days on +CAF plates and then frozen (7day/+CAF). Subsequently, 7day/+CAF isolates were re-patched every three days on +CAF plates up to twenty days of total growth on +CAF plates and then frozen (20day/+CAF).
0.29 μM clotrimazole (Sigma, C6019) was added to media for clotrimazole resistance serial dilution assays. 1.6 μM tebuconazole (Sigma, 32013) was added to media for tebuconazole resistance serial dilution assays. 0.6 mM fluconazole (Sigma, PHR1160) was added to media for fluconazole resistance serial dilution assays.
7 or 14 mM caffeine (Sigma, C0750), or 1 mM hydrogen peroxide (Sigma, H1009) were added to media for 18 hours for drug treatment experiments. To release TetR-Clr4*, 10 μM anhydrotetracycline (AHT) was added to the media.
Serial dilution assays
Equal amounts of starting cells were serially diluted five-fold and then spotted onto appropriate media. Cells were grown at 30-32°C for 3-5 days and then photographed.
Chromatin immunoprecipitation (ChIP)
ChIP experiments were performed as previously described41 using anti-H3K9me2 (5.1.1, gift from Takeshi Urano) or anti-GFP (Invitrogen, A11122). Immunoprecipitated DNA was recovered with Chelex-100 resin (BioRad) for ChIP-qPCR (qChIP) experiments or with QIAquick PCR Purification Kit (Qiagen) for ChIP-seq experiments.
Quantitative ChIP-qPCR (qChIP)
qChIPs were analysed by real-time PCR using Lightcycler 480 SYBR Green (Roche) with oligonucleotides listed in Supplementary Table 3. All ChIP enrichments were calculated as % DNA immunoprecipitated at the locus of interest relative to the corresponding input samples and normalized to % DNA immunoprecipitated at the act1 + locus. For spike-in qChIPs, an equal number (~20%) of Schizosaccharomyces octosporus cells (H3K9me2 spike-in qChIP)41 or Sgo1-GFP Saccharomyces cerevisiae cells (GFP spike-in qChIP)42 (gift from Adele Marston) were added to initial S. pombe pellets. Histograms represent data averaged over three biological replicates. Error bars represent standard deviations.
ChIP-seq library preparation and analysis
Illumina-compatible libraries were prepared as previously described41 using NEXTflex-96 barcode adapters (Bioo Scientific) and Ampure XP beads (Beckman Coulter). Libraries were then pooled to allow multiplexing and sequenced on an Illumina HiSeq2000, NextSeq or MiniSeq system (150-cycle high output kit) by 75 bp paired-end sequencing.
Approximately 6-10 million 75 bp paired-end reads were produced for each sample. Raw reads were then de-multiplexed and trimmed using Trimmomatic (v0.35)43 to remove adapter contamination and regions of poor sequencing quality. Trimmed reads were aligned to the S. pombe reference genome (972h -, ASM294v2.20) using Bowtie2 (v2.3.3)44. Resulting bam files were processed using Samtools (v1.3.1)45 and picard-tools (v2.1.0) (http://broadinstitute.github.io/picard) for sorting, removing duplicates and indexing. Coverage bigwig files were generated by BamCoverage (deepTools v2.0) and ratios IP/input were calculated using BamCompare (deepTools v2.0)46 in SES mode for normalisation47. Peaks were called using MACS248 in PE mode and broad peak calling (broad-cutoff = 0.05). Region-specific H3K9me2 enrichment plots were generated using the Sushi R package (v1.22)49. Heatmaps were generated using computeMatrix and plotHeatmap (deepTools v2.0)46 with genomic coordinates indicated in Supplementary Table 4.
SNP and indel calling
SNPs and indels were called as previously described50. Trimmed reads were mapped to the S. pombe reference genome (972h -, ASM294v2.20) using Bowtie2 (v2.3.3)44. GATK51,52 was used for base quality score recalibration. SNPs and indels were called with GATK HaplotypeCaller51,52 and filtered using custom parameters. Functional effect of variants was determined using Variant Effect Predictor53.
Copy number variation analysis
Copy number variation was determined using CNVkit54 in Whole-Genome Sequencing (-wgs) mode. Wild-type ChIP-seq input bam files were used as reference.
Extrachromosomal circular DNA diagnostic PCRs and Southern analysis
ChIP-input DNA samples were used as template for PCR with Taq polymerase (Roche, 4728858001) according to manufacturer’s instructions. Two types of PCR were performed: control PCR for loci present on endogenous chromosome III (expected to be present in wild-type, UR-2 (7day/+CAF) and UR-4) and circle-specific PCRs specific for putative extrachromosomal circles predicted to be present in UR-2 (7day/+CAF) or UR-4. For wild-type and UR-2 (7day/+CAF): control primers were located on either on side of 5S rRNA.24 (primers A (forward), B (reverse); see Supplementary Table 3) and 5S rRNA.26 (primers C, D); circle-specific primers were located on either side of a predicted junction between 5S rRNA.24 and 5S rRNA.26 (primers C and B). For wild-type and UR-4: control primers were located on either on side of LTR3 (primers E, F) and or LTR27 (primers G, H); circle-specific primers were located on either side of a predicted junction between LTR3 and LTR27 (primers G and F). For some locations, more than one forward and/or reverse primer was used, for instance: forward primers C1, C2 with reverse primers D1, D2. PCR products were electrophoresed on 2% agarose gels containing Ethidium Bromide.
For Southern analysis, genomic DNA was prepared from wild-type, UR-2 (7day/+CAF) and UR-4 cultures grown in YES. Briefly, cells were incubated with Zymolyase 100T (AMS Biotechnology) to digest the cell wall, pelleted, resuspended in TE and lysed with SDS, followed by addition of potassium acetate and precipitation with isopropanol. After treatment with RNase A and proteinase K, phenol chloroform and chloroform extractions were performed. DNA was precipitated in the presence of sodium acetate and ethanol, followed by centrifugation and washing of the pellet with 70% ethanol. After air drying the pellet was resuspended in TE. Approximately 8 μg of DNA was digested with the following restriction enzymes: wild-type and UR-2 (7day/+CAF): BsmBI, EcoRV, NdeI; wild-type and UR-4: EcoRI, BamHI + XbaI. Digested DNA was subjected to electrophoresis in a 0.9 % agarose gel containing ethidium bromide. Southern blotting was achieved by the alkali transfer method. Briefly, the gel was depurinated with 0.3 M HCl for 10 minutes, washed with distilled water, followed by two 15 min incubations in Denaturing Solution (0.5 M NaOH, 1.5 M NaCl). Overnight capillary transfer was used for transfer to Hybond XL membrane (Amersham), which was then washed with 50 mM Na2HPO4 pH7.2, followed by air drying. After drying at 80°C for 2 hours and UV-crosslinking, membranes were prehybridized in Church Buffer (0.5 M Na2HPO4 pH 7.2, 7% SDS, 1 mM EDTA, 1% BSA (Sigma, A0281) for 1 hour at 65°C. Probes were made using High Prime kit (Roche, 11585592001) and α-32P-dCTP (NEN), according to the manufacturer’s instructions. Heat denatured probes in Church Buffer were hybridized with relevant membranes at 65°C overnight with rotation. Following washes with Wash Buffer (40 mM Na2HPO4 pH 7.2, 1 mM EDTA, 1% SDS) blots were exposed to XAR-5 film (Kodak) at -80°C with an intensifying screen for several hours.
Cytology
Schizosaccharomyces pombe cultures were fixed before processing for immunofluorescence as described41. Briefly, cells in YES culture were fixed with 3.7% formaldehyde (Sigma, F8775) for 30 min, followed by cell wall digestion with Zymolyase-100T (AMS Biotechnology) in PEMS buffer (100 mM PIPES pH 7, 1 mM EDTA, 1 mM MgCl2, 1.2 M Sorbitol). After permeabilization with Triton-X100, cells were washed, blocked in PEMBAL (PEM containing 1% BSA, 0.1% sodium azide, 100 mM lysine hydrochloride). Rabbit anti-GFP (Invitrogen, A11122) was used in PEMBAL at 1:500 dilution, and Alexa 488-coupled chicken-anti-rabbit secondary antibody (Invitrogen, A21441) at 1:1000 dilution. Arg11-mCherry fluorescence survived fixation and no antibodies were used for localisation. Cells were stained with DAPI and mounted in Vectashield. Microscopy was performed with a Zeiss Imaging 2 microscope (Zeiss) using a 100x 1.4NA Plan-Apochromat objective, Prior filter wheel, illumination by HBO100 mercury bulb. Image acquisition with a Photometrics Prime sCMOS camera (Photometrics, https://www.photometrics.com) was controlled using Metamorph software (Version 7; Universal Imaging Corporation). Exposures were 3000 ms for FITC/Alexa-488 channel (Cup1-GFP/Alexa 488), 500 ms for TRITC channel (Arg11-mCherry) and 100 ms for DAPI. For display of images, maximum intensity was determined for e.g. Cup1-GFP staining in Cup1-GFP Arg11-mCherry strain (B4909) and this maximum was applied for scaling of all B4909 and B4912 (expresses only Arg11-mCherry) images. FITC and TRITC channels were scaled in this way; DAPI images were autoscaled.
qRT–PCR analysis
Total RNA was extracted using the Monarch Total RNA Miniprep Kit (New England Biolabs) according to the manufacturer’s instructions. Contaminating DNA was removed by treating with Turbo DNase (Invitrogen) and reverse transcription was performed using LunaScript RT Supermix Kit (New England Biolabs). Oligonucleotides used for qRT-PCR are listed in Supplementary Table 3. qRT-PCR histograms represent three biological replicates; error bars correspond to the standard deviation.
RNA-seq library preparation and analysis
Total RNA was extracted using the Monarch Total RNA Miniprep Kit (New England Biolabs) according to the manufacturer’s instructions. Contaminating DNA was removed by treating with Turbo DNase (Invitrogen). rRNA was removed using the Ribo-Zero Gold rRNA removal kit (Yeast) (Illumina) before library construction using NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (New England Biolabs). Libraries were pooled and sequenced on an Illumina NextSeq platform by 75 bp paired-end sequencing. Adapter-trimmed reads were aligned to the S. pombe reference genome (972h -, ASM294v2.20) using STAR (v2.2.1)55 and processed using Samtools (v1.3.1)45. Coverage bigwig files were generated by BamCoverage (deepTools v2.0)46.
Differential expression was analysed using the Bioconductor Rsamtools (v2.0.3), GenomicFeatures (v1.36.4)56 and DESeq2 (v.1.24)57 R libraries. Log2 fold changes were shrunk using the apeglm method58 and a MA-plot was generated using R. Genes with an adjusted p value below 0.01 are shown in red.
Small RNA-seq
50 mL of log-phase cells were collected and processed using the mirVana miRNA Isolation kit (Invitrogen). Resulting sRNA was treated with TURBO DNase (Invitrogen) and used for library construction using NEBNext Multiplex Small RNA Library Prep Set for Illumina (New England Biolabs) according to manufacturer’s instructions. Libraries were pooled and sequenced on an Illumina NextSeq platform by 50 bp single-end sequencing. Raw reads were then de-multiplexed and processed using Cutadapt (v1.17) to remove adapter contamination and discard reads shorter than 19 nucleotides or longer than 25 nucleotides. Coverage plots were generated using SCRAM59.
Protein extraction and western analysis
Protein samples were prepared as previously detailed60. Western blotting detection was performed using anti-FLAG-HRP (Sigma, A8591), anti-Myc (Cell Signalling, 9B11), anti-a-tubulin (gift from Keith Gull)61, goat anti-mouse (Sigma, A4416), anti-Bip162, goat anti-rabbit (Sigma, A6154), anti-Cdc11 (gift from Ken Sawin) and donkey anti-sheep (Abcam, ab6900). Gels were visualised using the ChemiDoc imaging system (BioRad) and analysed with ImageJ.
Extended Data
Extended Data Figure 1. Identification of heterochromatin-dependent epimutants resistant to caffeine.
a, Frequency of unstable (UR) and stable (SR) caffeine-resistant isolates obtained from 3 independent screens. 64% of isolates did not display a clear phenotype (unclear).
b, Unstable (UR) and stable (SR) caffeine-resistant isolates were identified using this screening strategy. After growth on non-selective media for 14 days caffeine resistance is lost in UR isolates but not in SR isolates.
c, Caffeine resistance is lost progressively in unstable (UR) isolates but maintained in stable (SR) isolates.
d, Caffeine resistance in UR isolates depends on the Clr4 H3K9 methyltransferase. clr4 + (clr4Δ) or an unlinked intergenic region (controlΔ) were deleted in unstable (UR-2) and stable (SR-2) caffeine-resistant isolates.
e, A mutation in pap1 + confers caffeine resistance in the stable isolate SR-1. Left: Whole genome sequencing of the stable isolate SR-1 revealed a 7-nucleotide insertion in pap1 +. The insertion results in a truncated Pap1 protein (Pap1-N424STOP) that lacks the Nuclear Export Signal (NES). CRD: Cysteine-rich domain. Right: Pap1-N424STOP is resistant to caffeine. The 7-nucleotide insertion identified in SR-1 was introduced into the pap1 + gene of wild-type cells (Pap1-N424STOP) and caffeine resistance assessed. hba1Δ and SR-1 cells were used as positive controls.
Experiments in (b-d) and (e, right) were independently repeated at least twice with similar results.
Extended Data Figure 2. Unstable (UR) caffeine-resistant isolates are bona fide epimutants.
a-e, Genetic changes (clr5-Q264STOP meu27-S100Y) found in 4 of 30 unstable isolates do not contribute to the caffeine-resistant phenotype nor cause the formation of ectopic heterochromatin.
a, Whole genome sequencing of unstable isolates UR-1/3/5/7 revealed a Single Nucleotide Polymorphism (SNP) in clr5 + (clr5-Q264STOP) and in meu27 + (meu27-S100Y).
b, Left: Schematic of experiment to determine whether clr5-Q264STOP meu27-S100Y cells form more caffeine-resistant colonies than wild-type cells. Wild-type (wt) and clr5-Q264STOP meu27-S100Y cells were plated on +CAF media (105 cells per plate, 20 plates per strain). Caffeine-resistant colonies were counted after 7 days. Right: clr5-Q264STOP meu27-S100Y form a similar number of caffeine-resistant colonies to wt cells. Data are mean from twenty technical replicates. P value from a two-tailed Student’s t-test is indicated.
c, clr5-Q264STOP meu27-S100Y cells are not resistant to caffeine. clr5-Q264STOP meu27-S100Y cells were serially diluted and spotted on -CAF and +CAF plates to assess caffeine resistance. hba1Δ cells served as a positive control. Experiment was independently repeated at least twice with similar results.
d, Genome-wide H3K9me2 ChIP-seq enrichment in wt and clr5-Q264STOP meu27-S100Y cells. Data are represented as relative fold enrichment over input.
e, H3K9me2 ChIP-seq enrichment at known heterochromatin islands detected in epe1Δ cells9 in wt and clr5-Q264STOP meu27-S100Y cells. Data are represented as relative fold enrichment over input.
f, Gene transcript levels within and flanking ectopic heterochromatin islands in individual isolates. See Figure 2b. Data are mean ± s.d. from three biological replicates. P values < 0.05 from a two-tailed Student’s t-test are indicated.
Extended Data Figure 3. 24 of 30 unstable (UR) caffeine-resistant isolates display an ectopic heterochromatin island over the ncRNA.394 locus.
a, H3K9me2 ChIP-seq enrichment at the ncRNA.394 locus in individual isolates (left: coverage tracks; right: heatmaps). Data are represented as relative fold enrichment over input. Relevant genes within and flanking ectopic heterochromatin islands are indicated. Red arrows indicate essential genes. Dumbbells indicate primer pairs used in b, c and e.
b, Quantitative chromatin immunoprecipitation (qChIP) of H3K9me2 levels on ncRNA.394 in individual isolates. Data are mean ± s.d. from three biological replicates. Primer pairs used are indicated in a (ncRNA.394, primer pair 5).
c, SPBC17G9.13c + gene transcript levels in individual isolates. Data are mean ± s.d. from three biological replicates. P values from a two-tailed Student’s t-test are indicated. Primer pairs used are indicated in a (SPBC17G9.13c +, primer pair 3).
d, Deletion of ncRNA.394 or non-essential adjacent genes does not result in caffeine resistance. Experiment was independently repeated at least twice with similar results.
e, qChIP of H3K9me2 levels at the ncRNA.394 locus in UR-2 cells. UR-2 cells were grown in the absence (-CAF) or presence (+CAF) of caffeine overnight or in the absence of caffeine for 14 days (+14day/-CAF). Data are mean ± s.d. from three biological replicates. Primer pairs used are indicated in a.
Extended Data Figure 4. Forced synthetic heterochromatin targeting to the identified loci is sufficient to drive caffeine resistance in wild-type cells.
a-c, Quantitative chromatin immunoprecipitation (qChIP) of H3K9me2 levels in wild-type (wt) cells harbouring 4xtetO binding sites at the identified ectopic heterochromatin loci (or ura4 as control) and expressing TetR-Clr4* in the absence or presence of AHT. a, hba1 locus. b, ncRNA.394 locus. c, ura4 locus. Data are mean ± s.d. from three biological replicates. Dumbbells indicate primer pairs used. Red arrows indicate essential genes.
d, Forced synthetic heterochromatin targeting to the mbx2 locus is sufficient to drive caffeine resistance in wt cells. qChIP of H3K9me2 levels in wt cells harbouring 4xtetO binding sites at the mbx2 ectopic heterochromatin locus and expressing TetR-Clr4* in the absence or presence of AHT. Data are mean ± s.d. from three biological replicates. Dumbbells indicate primer pairs used.
e, Strains from a-c were assessed for resistance to the antifungal agents tebuconazole (+TEZ) and fluconazole (+FLZ). Experiments were independently repeated at least twice with similar results.
Extended Data Figure 5. Unstable (UR) caffeine-resistant isolates show cross-resistance to antifungals and siRNA generation at ectopic heterochromatin islands.
a, Unstable caffeine-resistant isolates UR-1 and UR-2 were serially diluted and spotted on non-selective (N/S), caffeine (+CAF), clotrimazole (+CLZ), tebuconazole (+TEZ) and fluconazole (+FLZ) media to assess resistance. Experiment was independently repeated at least twice with similar results.
b-c, Left: small RNA sequencing detects siRNAs (21-24 nucleotides) homologous to ectopic heterochromatin islands in UR-1 (b, hba1 locus) and UR-2 (c, ncRNA.394 locus) compared to wild-type (wt) cells. Right: siRNAs mapping to pericentromeric dgI/dhI repeats of chromosome I shown as control. Sequencing was performed once. *Transcripts mapping to the highly-expressed gene eno101 + in euchromatic wild-type conditions (note these are unidirectional RNAs and not siRNAs).
d, Caffeine resistance depends on RNAi. dcr1 + (dcr1Δ), ago1 + (ago1Δ) or an unlinked intergenic region (controlΔ) were deleted in UR-2 cells. Experiment was independently repeated at least twice with similar results.
Extended Data Figure 6. Decreased cup1 + transcript levels or Cup1 LYR-domain mutation results in caffeine resistance.
a, An additional copy of cup1 + with 3x Determinant of Selective Removal (DSR) motifs fused to its 3’ untranslated region was inserted at an intergenic region (LocusPX:cup1-3xDSR). Bottom left: After deletion of endogenous cup1 +, cells expressing only cup1-3xDSR were assessed for caffeine resistance. Bottom right: Transcript levels of cup1 + and SPBC17G9.12c + (as control) in cup1Δ locusPX:cup1-3xDSR cells compared to wild-type. Data are mean ± s.d. from three biological replicates. P value from a two-tailed Student’s t-test is indicated. Dumbbells indicate primer pairs used.
b, The 144-bp transcriptional terminator site from ura4 + was inserted in place of part of the putative cup1 + promoter (cup1-TT). Bottom left: Cells were assessed for caffeine resistance. Bottom right: Transcript levels of cup1 + and SPBC17G9.12c + (as control) in cup1-TT cells compared to wild type. Data are mean ± s.d. from three biological replicates. P value from a two-tailed Student’s t-test is indicated. Dumbbells indicate primer pairs used.
c, Cup1 localises to mitochondria. Cells expressing either untagged Cup1 (top row) or Cup1-GFP (bottom three rows) were fixed and processed for immunofluorescence with anti-GFP antibody and Alexa-488 secondary antibody and DNA was stained with DAPI. The mitochondrial protein Arg11-mCherry served as a positive control for mitochondrial localisation. All images in the green channel (Cup1-GFP) are scaled relative to each other, as are those in the red channel (Arg11-mCherry); DAPI images are autoscaled. Bar, 5 μm.
d, Point mutations (L73G and F99G) were introduced in the LYR domain of Cup1 and cells were assessed for caffeine resistance. Mutations were designed based on Phyre2 tool analysis. hba1Δ cells were used as positive control.
Experiments in (c) and (d) were independently repeated at least twice with similar results.
Extended Data Figure 7. Copy Number Variation (CNV) analysis reveals a partial duplication of chromosome III in 12 of 30 unstable (UR) caffeine-resistant isolates.
a, Chromosome III coverage plots with overlaid segments in UR isolates showing partial duplication of chromosome III. Location of cds1 + is highlighted. Wild-type ChIP-seq input data were used as the reference.
b-d, Epigenetic changes preceded genetic changes (CNV) in unstable caffeine-resistant isolate UR-2.
b, H3K9me2 ChIP-seq enrichment at the ncRNA.394/cup1 locus (left) and chromosome III coverage plots with overlaid segments (right) in UR-2 (4day/+CAF) cells and following their prolonged growth on +CAF for an additional 3 days (7day/+CAF). Wild-type ChIP-seq input data were used as the reference for CNV analysis.
c, clr4 + (clr4Δ) or an unlinked intergenic region (controlΔ) were deleted in UR-2 cells (4day/+CAF) and UR-2 (7day/+CAF). All (6/6) UR-2 (4day/+CAF) clr4Δ transformants lost resistance to caffeine whereas only 50% (3/6, transformants 1, 4 and 5) UR-2 (7day/+CAF) lost resistance to caffeine. Experiments were independently repeated at least twice with similar results. cds1 + DNA levels in extracted genomic DNA were assessed by qPCR. Data are mean ± s.d. from three biological replicates.
d, H3K9me2 ChIP-seq enrichment at the ncRNA.394/cup1 locus (left) and chromosome III coverage plots with overlaid segments (right) in UR-2 (7day/+CAF) cells and following their prolonged growth on non-selective media for 14 days (7day/+CAFà14day/-CAF). Wild-type ChIP-seq input data were used as the reference for CNV analysis.
Extended Data Figure 8. Copy Number Variation (CNV) of chromosome III corresponds to extrachromosomal circular DNA (eccDNA).
Junctions of putative extrachromosomal circles were identified at repetitive sequences by inspection of CNV plots for UR-2 (7day/+CAF) (a) and UR-4 (b). Maps and lower panels: Positions of 5S rRNA.24 and 5S rRNA.26 (pink arrows), LTR3 and LTR27 (green arrows) and flanking genes are indicated. PCR primers (half arrows) flanking 5S rRNA.24 (A (forward); B1,2 (reverse)) and 5S rRNA.26 (C1,2; D1,2) were used to amplify products from wild-type (wt) and UR-2 (7day/+CAF) ChIP input samples, along with primer combinations (C1,2; B1,2) specific for the putative circle junctions (vertical black lines). Primers flanking LTR3 (E; F1,2) and LTR27 (G1,2; H) were used to amplify products from wild-type and UR-4 ChIP input samples, along with primer combinations (G1,2; F1,2) specific for the putative circle junction. Shaded boxes indicate primer locations and predicted circle junctions (pink: 5S rRNA.24/26, green: LTR3/27). Right: Restriction enzyme-digested genomic DNA isolated from wild-type (wt), UR-2 (7day/+CAF) and UR-4 was separated on an Ethidium Bromide (EtBr)-containing gel followed by Southern analysis using the indicated probes (925: blue; 520: purple; 44: red). Relevant restriction enzyme sites are indicated. Predicted sizes of hybridising fragments and DNA size markers are indicated (kb). PCR experiments were independently repeated at least twice with similar results. For gel source data, see Supplementary Figure 1b.
Extended Data Figure 9. The heterochromatin profile of low caffeine-treated wild-type cells resembles that of untreated epe1Δ cells.
a, Growth of cells in caffeine. Wild-type (wt) cells were grown in the presence of low (7 mM) or medium (14 mM) caffeine for 18 hours. Cell number was counted every 6 hours. Note: a larger inoculum was used for 14 mM caffeine culture to obtain an equivalent final number of cells. Data are mean ± s.d. from three biological replicates. Cells from the 18-hr time point were used for d.
b-c, H3K9me2 ChIP-seq enrichment at previously-detected facultative heterochromatin loci (described in Zofall et al., 20129 (b and c), Yamanaka et al., 201313 (b), Wang et al., 201510 (b), Sorida et al., 201912 (b) and Gallagher et al., 201914 (b)), in wt cells treated with low or medium dose of caffeine or low dose (1 mM) of H2O2, compared to untreated epe1Δ and wt cells. Data are represented as relative fold enrichment over input. A subset of facultative heterochromatin loci detected in untreated epe1Δ cells (Zofall et al., 20129, Wang et al., 201510 and Sorida et al., 201912) was detected in low caffeine-treated wt cells. Asterisks in c indicate loci with similar H3K9me2 patterns in low caffeine-treated wt cells and untreated epe1Δ cells, but not untreated wt cells. Facultative heterochromatin loci formed in the absence of the exosome (Yamanaka et al., 201313) or in wt cells grown at 18°C (Gallagher et al., 201914) were not detected in wt cells treated with low or medium caffeine or low H2O2.
d, Quantitative ChIP (qChIP) of H3K9me2 levels on ncRNA.394/cup1 in wt cells following 18 hr exposure to low or medium caffeine. H3K9me2 levels were normalized to S. octosporus spike-in control. Data are mean ± s.d. from three biological replicates.
e, H3K9me2 ChIP-seq enrichment at ncRNA.394/cup1 and mcp7 loci (or at pericentromeric dgI/dhI repeats of chromosome I as control) in wt cells following 18 hr exposure to low H2O2. Data are represented as relative fold enrichment over input. Red arrows indicate essential genes. Lower levels of H3K9me2 at pericentromeric repeats upon H2O2 treatment may be due to H2O2-specific regulation of limiting heterochromatin factors at this locus.
f, epe1 + RNA levels do not change upon caffeine treatment. Total RNA-seq of wt cells treated with low caffeine. Components of the Clr4 H3K9 methyltransferase CLRC complex (clr4+, rik1+, raf1+, raf2+, pcu4+ and rbx1+) and the antisilencing factors epe1 + and mst2 + are highlighted. Experiment was independently repeated twice with similar results.
g, epe1Δ cells display increased resistance to caffeine. Left: Schematic of experiment. Wild-type, epe1Δ and clr4Δ cells were plated on +CAF media (105 cells/plate, 40 plates/strain). Caffeine-resistant colonies were counted after 7 days. Right: Compared to wt cells, epe1Δ forms more, whereas clr4Δ forms fewer, caffeine-resistant colonies. Note that the total number of resistant colonies also includes genetic mutants. Data are mean from forty technical replicates. P values from a two-tailed Student’s t-test are indicated.
Extended Data Figure 10. A shortened version of the anti-silencing factor Mst2 is produced upon exposure to caffeine.
a, Western analysis of Mst2-13xMyc (left) and Gcn5-13xMyc (as HAT control, right) before and after caffeine treatment (medium concentration, 14 mM). Tagged proteins are expressed from their endogenous loci. Loading controls: left: Bip1; right: Cdc11. Experiments were independently repeated at least twice with similar results. For gel source data, see Supplementary Figure 1c.
b, Total RNA-seq for mst2 (left) and gcn5 (as HAT control, right) of untreated wild-type cells (top) or wild-type cells treated with medium caffeine concentration (bottom). Diagrams illustrate mst2 and gcn5 transcripts and predicted protein domains. Reads are normalized to RPKM. Red dashed lines indicate the region of full length mst2 transcript absent from the short isoform. The MYST zinc finger (ZnF) domain, required for S. cerevisiae Esa1 acetyltransferase activity29, is truncated in the short isoform of Mst2. The alternative mst2 TSS utilised in caffeine conditions was previously annotated28. Experiment was independently repeated twice with similar results.
Supplementary Material
Acknowledgments
We thank Lorenza Di Pompeo, Andreas Fellas and Rebecca Yeboah for laboratory support, Pin Tong, Marcel Lafos, Ryan Ard and Shaun Webb (Wellcome Centre for Cell Biology Bioinformatics Core) for sharing technical expertise, David Kelly (Wellcome Centre Optical Instrumentation Laboratory) for microscopy and instrumentation support, and members of the Allshire lab for valuable discussions. We are grateful to Adrian Bird, Wendy Bickmore and Lucia Massari for comments on the manuscript. We thank Takeshi Urano for the 5.1.1 (H3K9me) antibody, Yoshinori Watanabe for the pRAD21 plasmid, Keith Gull for the α-tubulin antibody, Adele Marston for the Sgo1-GFP S. cerevisiae strain, Ken Sawin for the Cdc11 antibody and both Edinburgh Genomics (NERC, R8/H10/56; MRC, MR/K001744/1; BBSRC, BB/J004243/1) and Genetics Core, Edinburgh Clinical Research Facility at the University of Edinburgh for sequencing. S.T-G. was supported by the Darwin Trust of Edinburgh. R.C.A. is a Wellcome Principal Research Fellow (095021, 200885); the Wellcome Centre for Cell Biology is supported by core funding from Wellcome (203149).
Footnotes
Author contributions
S.T-G., P.N.C.B.A. and R.C.A. conceived the project. S.T-G. and P.N.C.B.A. performed preliminary studies. S.T-G. performed experiments and bioinformatics. M.S. designed cup1-3xDSR experiments and contributed to ChIP-seq and qChIP experiments. A.L.P. performed cytology, cup1-TT and eccDNA experiments. I.Y. constructed Epe1 and Mst2 strains and performed western analysis. S.A.W. generated Cup1 point mutants, Cup1-GFP strain and contributed to Epe1 and Mst2 experiments. S.T-G., A.L.P. and R.C.A. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Supplementary Information is available for this paper.
Correspondence and requests for materials should be addressed to Robin Allshire.
Reprints and permissions information is available at www.nature.com/reprints
Data availability
Sequence data generated in this study have been submitted to GEO under accession number: GSE138436.
Code availability
The complete Workflow Description Language (WDL) pipeline script used for ChIP-seq and variation analyses is available at: https://github.com/SitoTorres/Torres-Garcia-et-al.-2019.
References
- 1.Bannister AJ, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 2.Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 3.Allshire RC, Madhani HD. Ten principles of heterochromatin formation and function. Nat Rev Mol Cell Biol. 2017;45:153. doi: 10.1038/nrm.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Audergon PNCB, et al. Epigenetics. Restricted epigenetic inheritance of H3K9 methylation. Science. 2015;348:132–135. doi: 10.1126/science.1260638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ragunathan K, Jih G, Moazed D. Epigenetics. Epigenetic inheritance uncoupled from sequence-specific recruitment. Science. 2015;348 doi: 10.1126/science.1258699. 12586997#x2013;1258699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeggo PA, Holliday R. Azacytidine-induced reactivation of a DNA repair gene in Chinese hamster ovary cells. Mol Cell Biol. 1986;6:2944–2949. doi: 10.1128/mcb.6.8.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oey H, Whitelaw E. On the meaning of the word ‘epimutation’. Trends Genet. 2014;30:519–520. doi: 10.1016/j.tig.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Zhang K, Mosch K, Fischle W, Grewal SIS. Roles of the Clr4 methyltransferase complex in nucleation, spreading and maintenance of heterochromatin. Nat Struct Mol Biol. 2008;15:381–388. doi: 10.1038/nsmb.1406. [DOI] [PubMed] [Google Scholar]
- 9.Zofall M, et al. RNA elimination machinery targeting meiotic mRNAs promotes facultative heterochromatin formation. Science. 2012;335:96–100. doi: 10.1126/science.1211651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Reddy BD, Jia S. Rapid epigenetic adaptation to uncontrolled heterochromatin spreading. eLife. 2015;4:80. doi: 10.7554/eLife.06179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsa J-Y, Boudoukha S, Burke J, Homer C, Madhani HD. Polymerase pausing induced by sequence-specific RNA-binding protein drives heterochromatin assembly. Genes Dev. 2018;32:953–964. doi: 10.1101/gad.310136.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorida M, et al. Regulation of ectopic heterochromatin-mediated epigenetic diversification by the JmjC family protein Epe1. PLoS Genet. 2019;15:e1008129. doi: 10.1371/journal.pgen.1008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamanaka S, et al. RNAi triggered by specialized machinery silences developmental genes and retrotransposons. Nature. 2013;493:557–560. doi: 10.1038/nature11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher PS, et al. Iron homeostasis regulates facultative heterochromatin assembly in adaptive genome control. Nat Struct Mol Biol. 2018;25:372–383. doi: 10.1038/s41594-018-0056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvo IA, et al. Genome-wide screen of genes required for caffeine tolerance in fission yeast. PLoS ONE. 2009;4:e6619. doi: 10.1371/journal.pone.0006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanova AV, Bonaduce MJ, Ivanov SV, Klar AJ. The chromo and SET domains of the Clr4 protein are essential for silencing in fission yeast. Nat Genet. 1998;19:192–195. doi: 10.1038/566. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292:110–113. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- 18.Kudo N, Taoka H, Toda T, Yoshida M, Horinouchi S. A novel nuclear export signal sensitive to oxidative stress in the fission yeast transcription factor Pap1. J Biol Chem. 1999;274:15151–15158. doi: 10.1074/jbc.274.21.15151. [DOI] [PubMed] [Google Scholar]
- 19.Castillo EA, Vivancos AP, Jones N, Ayté J, Hidalgo E. Schizosaccharomyces pombe cells lacking the Ran-binding protein Hba1 show a multidrug resistance phenotype due to constitutive nuclear accumulation of Pap1. J Biol Chem. 2003;278:40565–40572. doi: 10.1074/jbc.M305859200. [DOI] [PubMed] [Google Scholar]
- 20.Zofall M, Smith DR, Mizuguchi T, Dhakshnamoorthy J, Grewal SIS. Taz1-Shelterin Promotes Facultative Heterochromatin Assembly at Chromosome-Internal Sites Containing Late Replication Origins. Mol Cell. 2016;62:862–874. doi: 10.1016/j.molcel.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angerer H. Eukaryotic LYR Proteins Interact with Mitochondrial Protein Complexes. Biology. 2015;4:133–150. doi: 10.3390/biology4010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang SW, Norbury C, Harris AL, Toda T. Caffeine can override the SM checkpoint in fission yeast. J Cell Sci. 1999;112:927–937. doi: 10.1242/jcs.112.6.927. [DOI] [PubMed] [Google Scholar]
- 23.Libuda DE, Winston F. Amplification of histone genes by circular chromosome formation in Saccharomyces cerevisiae. Nature. 2006;443:1003–1007. doi: 10.1038/nature05205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Møller HD, Parsons L, Jørgensen TS, Botstein D, Regenberg B. Extrachromosomal circular DNA is common in yeast. Proc Natl Acad Sci USA. 2015;112:E3114–22. doi: 10.1073/pnas.1508825112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hull RM, et al. Transcription-induced formation of extrachromosomal DNA during yeast ageing. PLoS Biol. 2019;17:e3000471. doi: 10.1371/journal.pbio.3000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S, et al. Circular ecDNA promotes accessible chromatin and high oncogene expression. Nature. 2019;575:699–703. doi: 10.1038/s41586-019-1763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stajic D, Perfeito L, Jansen LET. Epigenetic gene silencing alters the mechanisms and rate of evolutionary adaptation. Nat Ecol Evol. 2019;3:491–498. doi: 10.1038/s41559-018-0781-2. [DOI] [PubMed] [Google Scholar]
- 28.Thodberg M, et al. Comprehensive profiling of the fission yeast transcription start site activity during stress and media response. Nucleic Acids Res. 2019;47:1671–1691. doi: 10.1093/nar/gky1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Y, Barlev NA, Haley RH, Berger SL, Marmorstein R. Crystal structure of yeast Esa1 suggests a unified mechanism for catalysis and substrate binding by histone acetyltransferases. Mol Cell. 2000;6:1195–1205. doi: 10.1016/s1097-2765(00)00116-7. [DOI] [PubMed] [Google Scholar]
- 30.Cubas P, Vincent C, Coen E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature. 1999;401:157–161. doi: 10.1038/43657. [DOI] [PubMed] [Google Scholar]
- 31.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell. 2014;157:95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calo S, et al. Antifungal drug resistance evoked via RNAi-dependent epimutations. Nature. 2014;513:555–558. doi: 10.1038/nature13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antequera F, Tamame M, Villanueva JR, Santos T. DNA methylation in the fungi. J Biol Chem. 1984;259:8033–8036. [PubMed] [Google Scholar]
- 34.Wilkinson CR, Bartlett R, Nurse P, Bird AP. The fission yeast gene pmt1+ encodes a DNA methyltransferase homologue. Nucleic Acids Res. 1995;23:203–210. doi: 10.1093/nar/23.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science. 2018;360:739–742. doi: 10.1126/science.aap7999. [DOI] [PubMed] [Google Scholar]
- 36.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe . Meth Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 37.Harigaya Y, et al. Selective elimination of messenger RNA prevents an incidence of untimely meiosis. Nature. 2006;442:45–50. doi: 10.1038/nature04881. [DOI] [PubMed] [Google Scholar]
- 38.Watson AT, et al. Optimisation of the Schizosaccharomyces pombe urg1 expression system. PLoS ONE. 2013;8:e83800. doi: 10.1371/journal.pone.0083800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delerue T, et al. Loss of Msp1p in Schizosaccharomyces pombe induces a ROS-dependent nuclear mutator phenotype that affects mitochondrial fission genes. FEBS Lett. 2016;590:3544–3558. doi: 10.1002/1873-3468.12432. [DOI] [PubMed] [Google Scholar]
- 40.Bähler J, et al. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe . Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 41.Tong P, et al. Interspecies conservation of organisation and function between nonhomologous regional centromeres. Nat Commun. 2019;10 doi: 10.1038/s41467-019-09824-4. 2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nerusheva OO, Galander S, Fernius J, Kelly D, Marston AL. Tension-dependent removal of pericentromeric shugoshin is an indicator of sister chromosome biorientation. Genes Dev. 2014;28:1291–1309. doi: 10.1101/gad.240291.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramírez F, et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016;44:W160–5. doi: 10.1093/nar/gkw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz A, Park K, Lim DA, Song JS. Normalization, bias correction, and peak calling for ChIP-seq. Stat Appl Genet Mol Biol. 2012;11 doi: 10.1515/1544-6115.1750. Article 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phanstiel DH, Boyle AP, Araya CL, Snyder MP. Sushi.R: flexible, quantitative and integrative genomic visualizations for publication-quality multi-panel figures. Bioinformatics. 2014;30:2808–2810. doi: 10.1093/bioinformatics/btu379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeffares DC, et al. The genomic and phenotypic diversity of Schizosaccharomyces pombe . Nat Genet. 2015;47:235–241. doi: 10.1038/ng.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKenna A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van der Auwera GA, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43 doi: 10.1002/0471250953.bi1110s43. 11.10.1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLaren W, et al. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17:122. doi: 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talevich E, Shain AH, Botton T, Bastian BC. CNVkit: Genome-Wide Copy Number Detection and Visualization from Targeted DNA Sequencing. PLoS Comput Biol. 2016;12:e1004873. doi: 10.1371/journal.pcbi.1004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawrence M, et al. Software for computing and annotating genomic ranges. PLoS Comput Biol. 2013;9:e1003118. doi: 10.1371/journal.pcbi.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu A, Ibrahim JG, Love MI. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics. 2019;35:2084–2092. doi: 10.1093/bioinformatics/bty895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fletcher SJ, Boden M, Mitter N, Carroll BJ. SCRAM: a pipeline for fast index-free small RNA read alignment and visualization. Bioinformatics. 2018;34:2670–2672. doi: 10.1093/bioinformatics/bty161. [DOI] [PubMed] [Google Scholar]
- 60.Braun S, et al. The Cul4-Ddb1(Cdt)2 ubiquitin ligase inhibits invasion of a boundary-associated antisilencing factor into heterochromatin. Cell. 2011;144:41–54. doi: 10.1016/j.cell.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woods A, et al. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci. 1989;93(Pt 3):491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]
- 62.Pidoux AL, Armstrong J. The BiP protein and the endoplasmic reticulum of Schizosaccharomyces pombe: fate of the nuclear envelope during cell division. J Cell Sci. 1993;105(Pt 4):1115–1120. doi: 10.1242/jcs.105.4.1115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data generated in this study have been submitted to GEO under accession number: GSE138436.
The complete Workflow Description Language (WDL) pipeline script used for ChIP-seq and variation analyses is available at: https://github.com/SitoTorres/Torres-Garcia-et-al.-2019.