Abstract
Background
Maternal obesity is associated with offspring cardiometabolic risk. UPBEAT was a randomised controlled trial of an antenatal diet and physical activity intervention in 1,555 women with obesity. The intervention was associated with lower gestational weight gain, healthier diet and metabolic profile in pregnancy, and reduced infant adiposity at six months.
Objective
We have investigated whether the UPBEAT intervention influenced childhood cardiometabolic outcomes or was associated with sustained improvements in maternal lifestyle 3-years after delivery.
Methods
In UPBEAT mother-child dyads at the 3-year follow-up, we assessed childhood blood pressure, resting pulse rate, and adiposity (body mass index, skinfold thicknesses, body fat, waist and arm circumferences) and maternal diet, physical activity, and anthropometry.
Results
514 three-year-old children attended the appointment (49% intervention, 51% standard care). There was no difference in the main outcome of interest, subscapular skinfold thickness, between the trial arms (-0.30mm, 95% confidence interval: -0.92, 0.31). However, the intervention was associated with a lower resting pulse rate (-5bpm (-8.41, -1.07)). There was also a non-significant lower odds of overweight/obesity (OR 0.73; 0.50, 1.08). Maternal dietary improvements observed in the UPBEAT trial, including glycaemic load and saturated fat were maintained 3-years postpartum.
Conclusion
This study has demonstrated that an antenatal dietary and physical activity intervention in women with obesity is associated with lower offspring pulse rate and sustained improvement in maternal diet. Whilst larger than previous cohorts, there remains potential for bias from attrition and these findings require validation in future cohorts.
Keywords: Maternal obesity, randomised controlled trial, childhood obesity, developmental origins, cardiovascular function
Introduction
The World Health Organization (WHO) estimates that the global prevalence of childhood overweight and obesity will reach 70 million by 2025.1 The causal pathways, widely explored in observational studies2,3 suggest that maternal obesity may contribute to the development of childhood obesity through exposures during in utero development,4,5 which remain following adjustment for confounders.6,7 These relationships have been observed in animal studies, in which environmental and genetic contributions can be tightly controlled.8 In contrast, observational studies using Mendelian randomisation, in which maternal genetic variants are used as instrumental variables to test the effect of maternal obesity on offspring adiposity, have not supported a causal intrauterine effect of greater maternal BMI on offspring adiposity,9,10 inferring that the relationship is explained by shared genetic traits.
Epidemiological observations from mother-child cohorts have also reported associations between maternal obesity and cardiovascular morbidity and mortality rates in their children.11,12 The inference of in utero effects of maternal obesity on offspring cardiovascular function is supported by animal models; numerous studies in experimental animals, reported by ourselves13,14 and others,15 have described a relationship between pre-pregnancy maternal obesity and offspring cardiovascular dysfunction, including heart rate variability, enhanced cardiovascular response to stress, hypertension and higher circulating atherogenic lipids,16–18 observations which have been reported consistently across species.
Many antenatal randomised controlled trials (RCTs) have attempted to reduce gestational weight gain (GWG) or improve obesity related pregnancy outcomes, especially gestational diabetes through antenatal diet and/or physical activity interventions.19,20 Whilst improvement in gestational diabetes and other antenatal clinical outcomes has seldom been achieved, the majority of interventions have shown some benefit in limiting GWG and improving self-reported diet.20 These RCTs provide an important opportunity to explore the causal relationship between maternal obesity and subsequent obesity and cardiovascular risk in the offspring, by studying children born to women who participated in these trials. However, few studies have progressed to childhood follow-up and, in those, that have, the sample size has frequently been inadequate to detect any effects with certainty.21
The UK Pregnancies Better Eating and Activity Trial (UPBEAT), was a multi-centre RCT of a dietary and physical activity intervention in 1,555 pregnant women with obesity.22 Women were randomised to an intensive 8-week behavioural intervention or to standard antenatal care. The intervention had no effect on the primary outcomes, the incidence of gestational diabetes and large for gestational age (LGA) infants. However, there were improvements in several secondary maternal outcomes; specifically, lower total GWG, sum of skinfold thicknesses, dietary glycaemic load (GL) and saturated fat intake (SFA), and a modest increase in self-reported physical activity. The intervention also contributed to a healthier metabolic profile across pregnancy.23 At six months postpartum we found the maternal dietary benefits of the intervention were sustained and also observed a lower infant subscapular skinfold thicknesses in the offspring of women randomised to the intervention.24 The aim of the present study was to assess whether the UPBEAT intervention influenced childhood adiposity and cardiovascular function at three years of age and if improvements in maternal lifestyle behaviours were sustained three years after delivery.
Patients and Methods
Study design and setting
This was a secondary analysis of the UPBEAT RCT.22 We undertook a three-year post-partum follow-up study in eight trial centres. In the original trial, 1,555 women with obesity (≥16 years of age; pre-pregnancy BMI≥30kg/m2) were recruited in early pregnancy; exclusion criteria included pre-existing disease and multiple pregnancy. The participants were randomised to the intervention or standard antenatal care at 15+0–18+6 weeks’ gestation as reported previously. 25 In brief, the intention of the intervention was to prevent GDM through the promotion of healthy dietary intake and incremental increases in daily physical activity, over the 8-week intervention period. The dietary recommendations focused on reducing GL and SFA intake and were tailored to the woman’s habitual diet and cultural preferences. With respect to daily exercise, all women were provided with a pedometer and a DVD of suitable exercises. Further details are available in the protocol. 25
Participants and consent
Consent to the trial included agreement to contact the participants at a later date (UK Integrated Research Application System, IRAS, ref 09/H0802/5). The follow-up study design and protocol were approved by the NHS Research Ethics Committee (UK IRAS ref 13/LO/1108). Between August 2014 and October 2017, all participants in the trial were invited to attend a three year post-delivery visit with their child.22 Research midwives/research assistants completed the data collection. Continued training and regular contact between the sites was sustained throughout the data collection period. Women were excluded from the analysis if they were pregnant or had given birth in the previous four months at the time of follow-up. Children were excluded if they were suffering from severe illness (n=4) (chronic lung disease, developmental delay, down’s syndrome and Spina bifida) as these could affect growth or development or if they were born before 34 weeks’ gestation (n=5).
Childhood outcomes
Since we had previously reported lower subscapular skinfold thickness in six month old children in the intervention, compared with the control arm,24 subscapular skinfold thickness was the pre-specified childhood outcome of interest for the present study. Additional offspring outcomes included triceps, bicep, suprailiac, and abdomen skinfold thicknesses, and sum of skinfold thicknesses (calculated by addition of the five measures). All skinfold thicknesses were evaluated in triplicate using Holtain children skinfold callipers. Mid-upper arm and waist circumferences, estimated total body fat percentage (by bioelectrical impedance analysis; BIA, ImpediMed SFB7), weight (using a calibrated scale), WHO growth standard BMI z-score,26 and age adjusted International Obesity Task Force (IOTF) BMI centiles were also determined.27 The WHO reference standards are adjusted for age and sex and applicable irrespective of ethnicity and mode of early infant feeding. Childhood overweight and obesity were defined by IOTF sex-specific centiles (90.5th and 98.8th centiles for boys and 89.3th and 98.6th centiles for girls).27
For BIA estimation of body fat percentage, the child was asked to lie on a couch for five minutes during data collection, after which blood pressure was measured in duplicate when feasible and a single resting pulse rate measurement was recorded by a WelchAllyn 53S00-E4 device, with an appropriately sized arm cuff. This order ensured measurement of resting pulse rate. Blood pressure was converted to age and height appropriate centiles.28
Maternal outcomes
Maternal diet and physical activity were assessed with the same questionnaires used in the original UPBEAT study 25. These included a semi-quantitative food frequency questionnaire to estimate dietary GL, macronutrient, and energy intake. Women were excluded from this analysis if calorie intake was calculated to be under-reported at the baseline visit (15+0-18+6 weeks’ gestation). Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ) and summarised as metabolic equivalents (METs) of energy expenditure.29 Maternal anthropometric measurements at the three year follow-up included mid-upper arm, waist and thigh circumferences, subscapular, triceps, bicep, and suprailiac skinfold thicknesses (measured in triplicate using skinfold callipers). BMI was calculated from weight and height data using standardised methods.
Statistical analyses
For summary statistics, binary and categorical variables are presented using counts and percentages. The distribution of continuous variables was assessed using coefficients of skewness and then summarised by mean and standard deviation or median and interquartile range, where appropriate. Comparison of demographic details were made between the intervention and control groups; if the outcome of interest was binary, an odds ratio was calculated, when categorical, chi-squared test was used. Mann-Whitney U tests or t-tests were used for continuous data, depending on the distribution of the data.
Effect of the intervention on maternal and offspring outcomes three years postpartum
To analyse the effect of the intervention a complete case analysis (including only those with complete data on all variables used in any analyses) was undertaken for all participating mothers and children. Treatment effects for continuous outcomes were expressed as differences in means obtained from multivariable linear or quantile regression. Linear regression was used for most outcomes, with quantile regression employed for sum of skinfolds and maternal physical activity as the data were positively skewed. Binary endpoints were expressed as odds ratios with 95% confidence interval using logistic regression. Analyses were adjusted for minimisation variables (maternal BMI at trial enrolment, parity and ethnicity) and child sex and age at follow-up.
Sensitivity analyses to explore selection bias due to attrition
Although attrition was similar in each trial arm, we explored potential selection bias due to loss to follow-up by comparing maternal baseline characteristics and neonatal outcomes by randomisation arm for those included in this analysis (n=514) with those lost to follow-up (n=1,006).
We undertook additional analyses, imputing missing childhood outcome data due to loss to follow-up. For the offspring outcomes we used multivariate imputation chained equations to impute missing data for childhood adiposity and cardiovascular outcomes, to provide a total sample size of n=1,520. Data were imputed to create 50 datasets using 10 burn-in iterations for live-born infants using the multivariate imputation model including: maternal early pregnancy BMI, age, ethnicity, parity, early pregnancy smoking status, randomisation arm, measures of maternal anthropometry including GWG, maternal diet (glycaemic load, saturated fat, carbohydrate, protein, energy intake) and physical activity at 27+0-28+6, 34+0-36+0 weeks’ gestation, gestation at delivery, birthweight, mode of feeding on hospital discharge and 3-year maternal diet (glycaemic load, saturated fat, carbohydrate, protein, energy intake) and physical activity, and child sex and age at follow up. Analyses were performed using Stata version 15.0 (StataCorp, College Station, TX, USA).
Results
Participants
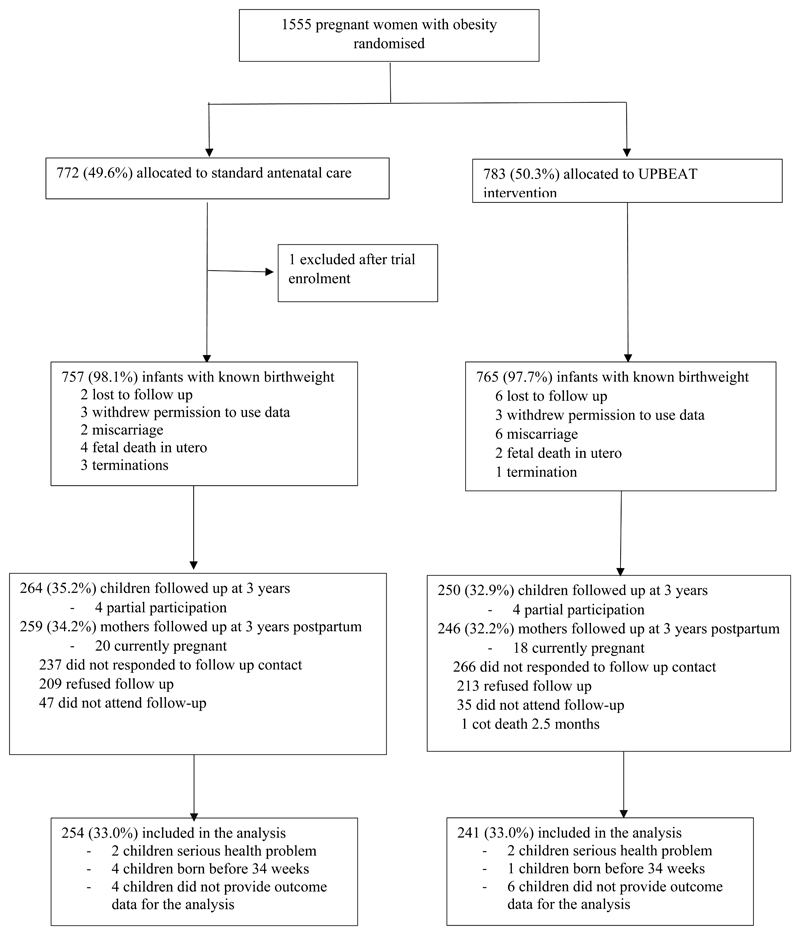
One thousand, five hundred and fifty-five participants were randomised to the UPBEAT trial and 1,233 were approached between three to four years after delivery with 1,018 of these responding to contact. The predominant reason for the reduction in numbers contacted compared to the original study population was the child being outside the prescribed age range. Of the women originally randomised, n=514 (33%) mother-child dyads took part (n= 250 intervention; 264 standard antenatal care), Figure 1. For the 514 mothers and children, 495 had complete outcome data, with 10 providing only questionnaire data completed at home. Nine children were excluded on the basis of severe illness or delivery <34 weeks’ gestation. For those who completed the follow-up there was no difference in the majority of maternal baseline (trial entry) characteristics (Table 1) or neonatal characteristics (Table 2) between the intervention and standard care arm, except for a significantly higher odds ratio of LGA and a higher birthweight for infants in the intervention arm. Mothers who attended the three-year follow-up were on average, compared to those who did not attend, older (1.1 years), had a lower early pregnancy BMI, more likely to be White European and nulliparous, and less likely to smoke (Supplementary T1). There was a higher proportion of breastfeeding on hospital discharge amongst infants who completed the three-year follow-up (Supplementary T2).
Figure 1. Consort diagram of participants enrolled in the UPBEAT trial at 3 years after delivery.
Table 1. UPBEAT 3-year follow-up: Comparison of maternal characteristics of those who attended the 3-year follow-up, by randomisation arm.
| Maternal | Intervention | Control | Difference in means/ OR (95%CI) | |||
|---|---|---|---|---|---|---|
| Mean (SD)/ Median (IQR) N (%) | ||||||
| Age (years) at baseline | 250 | 31.2 (5.0) | 264 | 31.3 (5.5) | -0.09 (-1.01 to 0.82) | |
| BMI (kg/m2) at baseline | 250 | 34.5 (32.5-38.0) | 264 | 34.9 (32.6-37.8) | -0.14 (-0.96 to 0.68) | |
| ethnicity | Asian | 250 | 13 (5) | 264 | 9 (3) | 1.47 (0.61 to 3.52) |
| Black | 55 (22) | 64 (24) | 0.87 (0.57 to 1.32) | |||
| White | 173 (69) | 176 (67) | ref | |||
| Other | 9 (4) | 15 (6) | 0.61 (0.26 to 1.43) | |||
| Multiparous | 250 | 124 (50) | 264 | 138 (52) | 0.90 (0.63 to 1.27) | |
| Smoking status at baseline | 250 | 5 (2) | 264 | 14 (5) | 0.88 (0.56 to 1.38) | |
| IMD quintiles* | 1 (least deprived) | 247 | 14 (6) | 264 | 16 (6) | 0.78 (0.36 to 1.68) |
| 2 | 21 (8) | 15 (6) | 1.24 (0.61 to 2.55) | |||
| 3 | 28 (11) | 30 (11) | 0.83 (0.46 to 1.50) | |||
| 4 | 75 (31) | 106 (40) | 0.63 (0.42 to 0.94) | |||
| 5 (mot deprived) | 109 (44) | 97 (37) | ref | |||
| Sum of skinfolds (cm) at baseline | 246 | 121.6 (29.5) | 263 | 122.7 (25.7) | -1.15 (-5.97 to 3.66) | |
| Antenatal characteristics | GDM† | 234 | 56 (24) | 250 | 69 (27) | 0.82 (0.55 to 1.24) |
| PE‡ | 249 | 6 (2) | 260 | 10 (4) | 0.62 (0.22 to 1.72) | |
| Total GWG from 15-18 weeks§ | 222 | 7.3 (4.5) | 230 | 7.7 (4.2) | -0.38 (-1.17 to 0.42) | |
Abbreviations: BMI, body mass index; CI, confidence intervals; GDM, gestational diabetes; GWG, gestational weight gain; IMD, indices of multiple deprivation; IQR, interquartile range; PE, pre-eclampsia; SD, standard deviation.
IMD quintiles are calculated for the region of residence, by fifths of the population. UK wide scores were developed by reconciling Scottish data to English norms.
Gestational diabetes (GDM) diagnosis by International Association of Diabetes in Pregnancy Study Group criteria at 27+0 to 28+6 weeks’ gestation.
Pre-eclampsia defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or both, on at least two occasions 4 hours apart, with proteinuria ≥300 mg/ 24 hours.
Total gestational weight gain calculated using estimated weight before pregnancy and weight at 36 weeks’.
Table 2. UPBEAT 3-year follow-up: Comparison of neonatal characteristics, of those who attended the 3-year follow-up, by randomisation arm.
| Intervention | Control | Difference in means/ OR (95% CI) | ||||
|---|---|---|---|---|---|---|
| Mean (SD)/ N (%) | ||||||
| Birth characteristics | Gestation at birth (weeks) | 250 | 39.8 (1.5) | 264 | 39.5 (2.2) | 0.25 (-0.07 to 0.58) |
| Anthropometry | Birthweight (grams) | 250 | 3523 (526) | 264 | 3426 (578) | 97.2 (1.3 to 193.0) |
| Birthweight >4kg | 250 | 40 (16) | 264 | 28 (11) | 1.6 (0.95 to 2.69) | |
| LGA >90th Centile * | 250 | 39 (15) | 264 | 25 (9) | 1.76 (1.03 to 3.01) | |
| Subscapular skinfold thickness (mm) | 113 | 5.7 (1.4) | 113 | 5.5 (1.3) | 0.24 (-0.13 to 0.61) | |
| Triceps skinfold thickness (mm) | 115 | 5.3 (1.3) | 119 | 5.2 (1.6) | 0.08 (-0.30 to 0.47) | |
| Neonatal feeding history at 72 hrs | Formula feeding | 249 | 47 (19) | 264 | 53 (20) | 0.91 (0.58 to 1.43) |
| Breast feeding | 158 (63) | 163 (62) | Ref | |||
| Partially breastfeeding | 44 (18) | 48 (18) | 0.94 (0.59 to 1.50) | |||
Abbreviations: CI, confidence intervals; LGA, large for gestational age; OR, odds ratio; SD, standard deviation.
Customised birthweight centile adjusting for maternal height and weight, ethnicity, parity and sex of the infant.
In this sub-population (n=514), and in common with the original trial, sum of maternal skinfold thicknesses at 26+0-28+6 weeks’ gestation were lower in the intervention arm compared to the standard care arm, as were GL and reported SFA intake (Supplementary T3). In common with the main trial population, physical activity was higher in those in the intervention arm (Supplementary T3). In contrast to the main trial, there was no significant difference in total GWG between the intervention and control groups (difference in mean in main trial -0.55kg (95% CI: -1.08 to -0.02) vs -0.38kg (-1.17 to 0.42), or in the metabolic profile in pregnancy (Supplementary F1).22,23
Intervention effects on childhood adiposity outcomes
34% of all children with adiposity measurements were classified as having a BMI equivalent to the adult BMI classification of ≥25.0kg/m2, with 8% having obesity. The mean (standard deviation) BMI z-score was 0.88 (1.0). There were no differences in the adjusted coefficients for BMI z-score between the intervention and standard care arms. Despite a trend for lower odds of overweight/obesity in the intervention arm (OR 0.73; 95CI: 0.50, 1.08) and for lower adiposity as measured by skinfold thicknesses there was no statistical evidence for a difference between arms (Table 3): for the primary outcome of subscapular skinfold thickness the adjusted difference in mean was -0.30mm (95% CI: -0.92 to 0.31), for sum of skinfold thicknesses -2.00mm (95% CI -4.64 to 0.62) and for body fat percentage (by bioelectrical impedance analysis) -0.30% (95% CI -1.62 to 1.01). There were also no differences in the adjusted coefficients for waist circumference and mid-upper arm circumference between trial arms (Tables 3).
Table 3. UPBEAT 3-year follow-up: Child anthropometry at 3 years of age, by UPBEAT randomisation arm.
| Intervention | Control | Difference in means /Odds ratio (95% CI)* | p-value | |||
|---|---|---|---|---|---|---|
| Mean (SD)/ Median (IQR) N (%) | ||||||
| Child age at 3-year follow up (months) | 250 | 41.8 (3.4) | 264 | 41.8 (3.4) | 0.05 (-0.53 to 0.64) | 0.85 |
| Weight (kg) | 240 | 17.2 (2.7) | 254 | 17.1 (2.9) | 0.16 (-0.30 to 0.63) | 0.49 |
| height (cm) | 241 | 101.1 (4.9) | 252 | 100.8 (5.5) | 0.34 (-0.46 to 1.14) | 0.40 |
| Subscapular skinfold thickness (mm) | 204 | 7.8 (3.2) | 215 | 8.1 (3.4) | -0.30 (-0.92 to 0.31) | 0.33 |
| Triceps skinfold thickness (mm) | 216 | 12.3 (4.1) | 228 | 12.1 (3.7) | 0.23 (-0.49 to 0.97) | 0.52 |
| Biceps skinfold thickness (mm) | 212 | 8.3 (3.7) | 226 | 8.3 (3.3) | 0.01 (-0.65 to 0.67) | 0.97 |
| Super iliac skinfold thickness (mm) | 194 | 6.8 (3.4) | 202 | 7.2 (4.14) | -0.40 (-1.15 to 0.34) | 0.28 |
| Abdomen skinfold thickness (mm) | 196 | 9.4 (4.8) | 211 | 9.6 (4.2) | -0.20 (-1.06 to 0.66) | 0.64 |
| Sum of skinfolds (mm) | 185 | 39.8 (33.4 to 48.8) | 196 | 42 (34.5 to 51.0) | -2.00 (-4.64 to 0.62) | 0.13 |
| Waist Circumference (cm) | 238 | 53.0 (4.5) | 241 | 53.2 (4.2) | -0.16 (-0.92 to 0.60) | 0.67 |
| Mid upper arm circumference (cm) | 231 | 17.8 (1.6) | 239 | 17.7 (1.9) | 0.04 (-0.26 to 0.36) | 0.76 |
| BMI for age z-score †, ‡ | 236 | 0.88 (1.0) | 249 | 0.88 (1.0) | 0.004 (-0.18 to 0.19) | 0.96 |
| Percentage with obesity (IOTF definition) ‡ | 230 | 20 (8.8) | 243 | 20 (8.2) | 1.06 (0.55 to 2.04) | 0.86 |
| Percentage overweight/obesity (IOTF definition) ‡ | 230 | 73 (31.7) | 243 | 93 (38.3) | 0.73 (0.50 to 1.08) | 0.11 |
| Body fat percentage calculated from BIA | 186 | 22.3 (7.1) | 196 | 22.4 (6.1) | -0.30 (-1.62 to 1.01) | 0.65 |
| Pulse rate (bpm) | 199 | 91 (20.0) | 204 | 96 (17.4) | -4.8 (-8.41 to -1.07) | 0.01 |
| Systolic blood pressure percentile | 197 | 80 (63 to 91) | 207 | 78 (63 to 90) | 2.79 (-1.81 to 7.39) | 0.23 |
| Diastolic blood pressure percentile | 196 | 79 (57 to 91) | 205 | 82 (64 to 88) | -2.98 (-7.76 to 1.08) | 0.22 |
Abbreviations: BIA, bioelectrical impedance analysis; BMI, body mass index; BPM: beats per minute; CI: confidence interval; IOTF: international obesity task force; IQR: interquartile range; mm, millimetres; cm, centimetres; kg, kilograms, SD, standard deviation.
Treatment effect adjusted for minimisation variables of randomisation maternal BMI, parity & ethnicity, child age at 3 year follow up and sex.
Z-scores calculated using WHO Anthro (de Onis, 2006).
Not adjusted for child age or sex.
Intervention effects on child cardiovascular outcomes
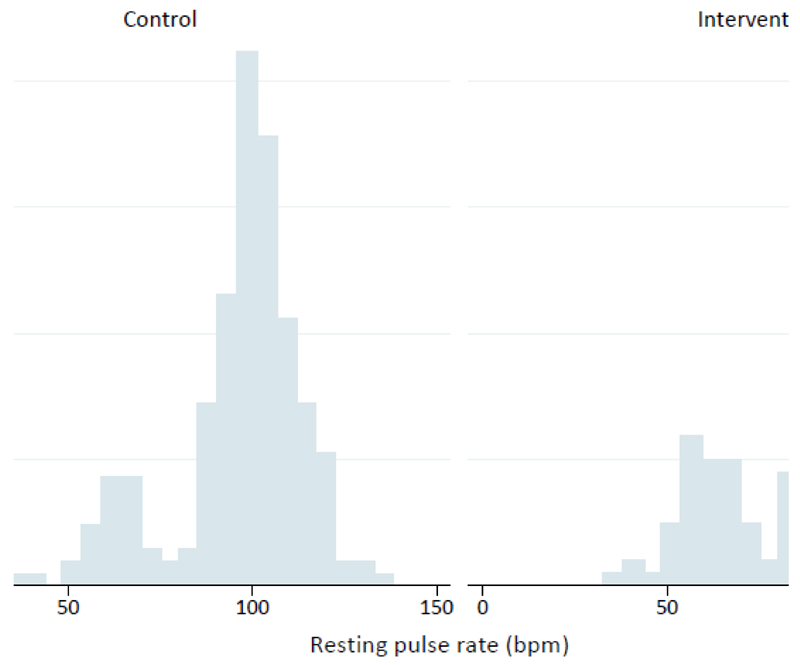
Resting pulse rate was -5 beats per minute (bpm) (-8.6 to -1.07) lower in the intervention arm (P<0.01), compared with standard care (Table 3). Further analysis identified a bimodal distribution of pulse rate in children in both trial arms (Figure 2). Bimodality was not associated with maternal dietary intake and resting pulse rate in pregnancy, child dietary intake, child’s BMI z-score, physical activity, sedentary time and time of day, season or trial centre. Logistic regression identified a shift from the higher (76-135bpm) to the lower (45-75bpm) modality as a result of the intervention; odds ratio 0.54 (0.32, 0.90). A trend towards lower diastolic blood pressure percentiles for children born to mothers from the intervention arm (-2.98; -7.76, 1.08) did not reach statistical significance (Table 3). Sensitivity analyses using multiple imputation for the whole trial population demonstrated a consistent reduction of resting pulse rate in the intervention arm (-4.8 bpm (-8.37 to -1.23)) and similar results for other offspring outcomes (Supplementary T4).
Figure 2. Resting pulse rate at 3-years of age, by randomisation arm.
Effect of the intervention on maternal diet and body composition three years postpartum
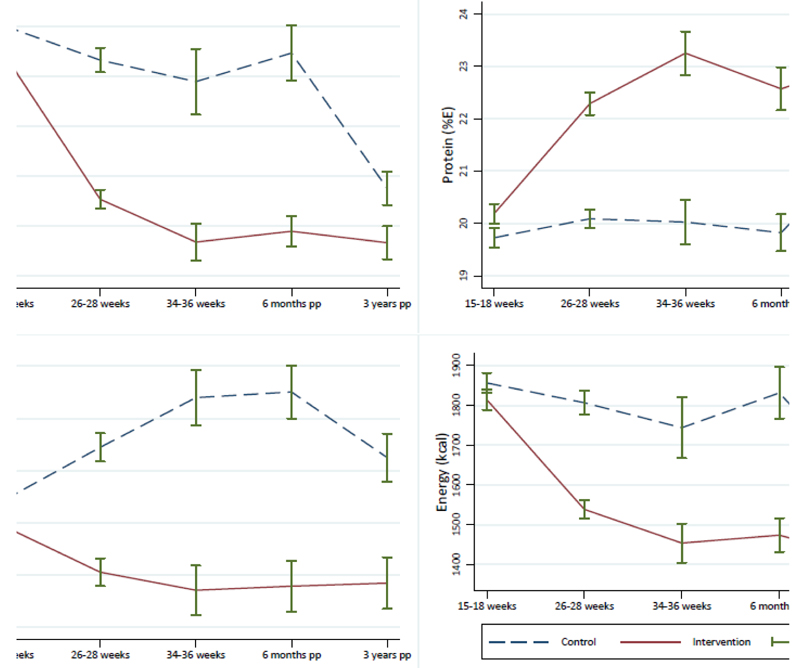
Compared to women who received standard antenatal care, women in the intervention arm who provided complete dietary data reported lower glycaemic load, maternal energy and SFA intake, and higher protein intake three years after delivery (Supplementary T5). Figure 3 illustrates these data with previous measurements throughout the index pregnancy and at six months postpartum, showing a sustained effect of the intervention from pregnancy to three years post-delivery. There were no differences in self-reported physical activity (Supplementary T5) or in measures in body composition between the two trial arms (Supplementary T6).
Figure 3. Maternal dietary intake across pregnancy and to 3-years postpartum, by randomisation arm.
Discussion
To our knowledge this investigation of 514 pre-school children born to mothers with obesity randomised to a lifestyle intervention in pregnancy is the most comprehensive reported to date. In a previous study in UPBEAT infants at six months of age (n=698), we reported a reduction in subscapular skinfold thickness, a measure which, in adults, is associated with risk of metabolic disease.30 At three years of age this effect was not sustained, despite trends towards lower adiposity, a lower incidence of overweight/obesity and diastolic blood pressure in the intervention group. The reduction in resting pulse rate in three-year-old children of mothers randomised to a lifestyle intervention, is an entirely novel observation. Notably, we found that the improvement in maternal diet during pregnancy in response to the UPBEAT intervention is still evident three years after delivery.
The reduction in the resting pulse rate of the three-year-old children could imply reduced cardiovascular risk. In adult populations, increased resting pulse rate is associated with hypertension and cardiovascular dysfunction.31 Of the few reports in children, a higher resting pulse rate has, as might be anticipated, been related to higher blood pressure.32 Resting pulse rate in children has also been reported to be inversely related to physical activity,33 but this is an unlikely explanation for the difference in pulse rates observed between intervention arms in this study, as there was no association with parent-reported child activity and sedentary time and resting pulse rate. An association between maternal obesity and offspring cardiometabolic dysfunction is widely reported in experimental animals.13–16,34 Rodent maternal obesity has been related to a sustained increase in offspring central sympathetic activity at the level of the hypothalamic neuronal pathways involved in peripheral autonomic regulation. In turn, this central pathway has been implicated in the sustained increase in blood pressure and altered heart rate variability observed.16 Mechanistically this may occur through permanently changed hypothalamic function through epigenetic processes.35,36 Our data could support a similar pathway to sympathetic activation in the children of women with obesity, and prompts more detailed investigation of sympathetic pathways and the epigenome in UPBEAT children at an older age. Assessment of the heart rate variability using ECG recordings, for example, would provide a read out of efferent parasympathetic and sympathetic autonomic activity37 and the peripheral blood epigenome may provide insight into sustained epigenetic signals originating in utero. A recently published study of 184 women provides added support for in utero origins of altered autonomic nervous system activity.38 Using the method of magnetoencephalography, the authors reported that maternal overweight and obesity was associated with lower fetal heart rate variability and a higher heart rate, in comparison to normal weight mothers. Furthermore, in a preliminary analysis of a study focusing on MRI assessment of newborn cardiovascular function, we have recently shown a significantly higher heart rate associated with abnormal cardiac structure and function in neonates born to mothers with obesity compared to those born to lean mothers (A. Groves, personal communication).
We could find no obvious explanation for the bimodal distribution of heart rate observed in the children as it was not influenced by maternal dietary intake or resting pulse rate, child’s diet, weekly activity and sedentary time, time of day, seasonality or centre of measurement (heart rate monitoring device). To our knowledge this clear bimodal distribution has not been previously reported and the origin remains unknown.
The observation that the effect of the intervention on maternal diet was maintained to three years, having previously been demonstrated at six months24 is important and has potential implications for longer term maternal and family health. It supports the theory that pregnancy is a ‘teachable’ moment for initiating longer-term improvements in dietary intake.39 In a planned follow up of the older children, we shall explore long term effects of the intervention on the mothers’ behaviours and her health outcomes including obesity, Type 2 diabetes, hypertension and cardiovascular disease. Healthier behaviours in mothers may also impact upon the health of her offspring as they age, and of other family members, although we have previously reported no differences between dietary patterns in the 3year old children from mothers in the standard care and intervention arms 40. As the children grow older, we shall nonetheless assess relationships between in utero and contemporary family exposures with child health outcomes. As persistently healthier maternal behaviours may also impact on the next pregnancy, maternal and infant health in subsequent pregnancies will also be of interest.
Although there are many reports of the consequences of maternal obesity on offspring health from animal15 and cohort studies7 very few have addressed the influence of an antenatal intervention beyond infancy.21 The only study of an antenatal diet and physical activity intervention in women with obesity, which was effective at reducing GWG, reported no effect on offspring body composition at 2.8 years of age (n=254).41 In conjunction with our six month24 outcome data, this report and the present study suggest that these effects may diminish in the children over time. Alternatively, the lack of any significant reduction in overweight or obesity may reflect a different timing of the adiposity rebound, typically occurring in this age group. However, as the two arms of this RCT were well matched the distribution of age at adiposity rebound would be expected to similar between groups. Alternatively, the higher proportion of LGA infants in the intervention arm, not observed in the original study population,22 may have obscured a difference, as infants born LGA may retain a higher BMI throughout childhood and adolescence.42
Whilst UPBEAT and other similar RCTs have found positive effects of lifestyle interventions on maternal lifestyle behaviours and GWG, the magnitude of change is often modest. For example, in a recent individual participant meta-analysis of 36 antenatal lifestyle RCTs (n=12,526) in pregnant women of heterogenous BMI, the reduction in total GWG was a modest -0.7kg (-0.92, -0.48). A lower caesarean section rate was the clinical outcome to show improvement.20 The robust association between maternal obesity and childhood obesity across many observational studies6,7 could result from shared obesogenic genes, or shared family environment, or a persistent influence of in utero exposures on the developing fetus or a combination of all of these. Although we have found, as reported here, an effect of a lifestyle intervention which improved maternal metabolic health in women with obesity (and presumably fetal exposures) on the cardiovascular system of the children, interventions which substantially affect the maternal phenotype and metabolic health are required before firm conclusions can be made as to the contributions of pre and postnatal determinants of the child’s health. Future strategies may include, for example, targeted behavioural interventions in women with obesity stratified as being at higher risk of adverse outcomes, especially GDM, which may have a lasting independent effect on offspring health.43 The repeatedly demonstrated modest effect of lifestyle interventions on pregnancy outcomes, has contributed to a new focus on optimising BMI before pregnancy. Pre-conception interventions, not limited to the narrow gestational window of nine months, are now seen by many as a as a potentially more effective strategy for improving pregnancy and longer-term outcomes for mother and child.44 Ultimately, effective interventions which together improve health behaviours in the preconception period and achieve substantive improvements in pregnancy outcomes, will inevitably have greater reach and benefit.
Strengths and Limitations
Strengths of the study include the prospective collection of in-depth data of pregnancy demographic, health, metabolic and lifestyle variables and individual determinants of childhood body composition and health outcomes, allowing for adjustment of potential confounders. The principal limitation is the follow-up of 33% of those eligible from the original RCT,22 and some minor differences in baseline characteristics between the two trial arms, may have resulted in selection bias. The main outcomes were however consistent when comparing complete case analyses with those from analyses using multivariable regression to impute data from those lost to follow-up. The consistency between the two methods suggests that selection bias may not have importantly influenced our findings.45
The lack of a significant reduction in GWG and pregnancy metabolic function between women in the trial arms who attended the follow up study, contrasts with the main trial and may have influenced the childhood outcomes, although the intervention mothers in this study sample demonstrated a similar reduction in measures of adiposity as reported in the main trial. The higher resting pulse rate in the standard care arm versus the intervention arm was an additional outcome and has not been explored previously. This may be due to chance given the number of multiple tests performed. However, as described above, observations of a higher fetal and neonatal heart rate in offspring of mothers with obesity compared to those of women with a normal BMI add validity to this observation. Replication, ideally in another large RCT with minimal loss to follow-up is, nonetheless required for validation. Additionally, pulse rate should be assessed in duplicate or triplicate in future studies.
Skinfold thicknesses and BIA are indirect methods for assessing body fat mass and distribution and future studies should consider dual-energy x-ray absorptiometry (DXA) which is practically more feasible in older children. A further limitation is the use of self-reported FFQ for the dietary intake in the mothers, although, during the pilot for UPBEAT the food frequency questionnaire performed favourably against a more rigorous method for dietary assessment.46
In conclusion, this study provides some evidence to suggest that improving health behaviours in women with obesity may have a positive effect on cardiovascular health of the offspring but is not associated with a reduction in childhood adiposity at the age of three years. The intervention in pregnancy, in common with many other studies, had only modest effects on maternal diet, weight gain, and adiposity. It remains important therefore to develop better interventions in pregnant women with obesity before or during pregnancy which substantially improve maternal health, and to determine if these are associated with greater health benefits for the child. Importantly, we found that the improved dietary behaviours arising from the UPBEAT intervention were maintained three years after delivery. Further follow-up of the UPBEAT participants will be valuable in ascertaining the longer-term effects of the UPBEAT intervention and the mother’s diet on her and her offspring’s health, including detailed assessment of cardiovascular function and adiposity.
Supplementary Material
Acknowledgements
We thank all staff in the UPBEAT consortium and we are most grateful to all the women and their children who took part in the UPBEAT study.
Sources of Funding
Supported by the European Union’s 7th Framework Programme (FP7/2007–2013), project EarlyNutrition; grant agreement no. 289346 and the National Institute for Health Research (NIHR) (UK) Programme Grants for Applied Research Programme (RP-0407-10452). The contributions of HLM and DAL are supported by the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013) ERC grant agreement No 669545 (DevelopObese), the US National Institute of Health (R01 DK10324) and the NIHR Biomedical Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol. HLM and DAL work in a Unit that receives support from the UK Medical Research Council (MC_UU_00011/6). Support was also provided by the Chief Scientist Office Scotland, Guy’s and St Thomas’ Charity and Tommy’s Charity (Registered charity no. 1060508). KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the European Union (Erasmus+ Capacity-Building ENeASEA (573651-EPP-1-2016-1-DE-EPPKA2-CBHE-JP) and ImpENSA (598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP) projects), the US National Institute On Aging of the National Institutes of Health (Award No. U24AG047867) and the UK ESRC and BBSRC (Award No. ES/M00919X/1). LP, ACF, SLW an ALB are funded by Tommy’s Charity and KVD is supported by the British Heart Foundation FS/17/71/32953. PTS is partly funded by King’s Health Partners Institute of Women and Children’s Health (KHP), Tommy’s and by ARC South London (NIHR). DAL is a NIHR Senior Investigator (NF-0616-10102), KMG is a NIHR Senior Investigator (NF-SI-0515-10042) and LP is an NIHR Senior Investigator Emeritus (NI-SI-0512-10104). KMG is supported by the NIHR Southampton Biomedical Research Centre. This research was funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, KHP, the NIHR or the Department of Health. The funders had no role in study design, data collection, data analysis, data interpretation or writing of the final report. The corresponding author had access to all the data in the study and had final responsibility for the decision to submit for publication.
Abbreviations
- BMI
body mass index
- BPM
beats per minute
- ECG
Electrocardiography
- GL
glycaemic load
- GWG
gestational weight gain
- IOTF
International Obesity Task Force
- IPAQ
international physical activity questionnaire
- LGA
large for gestational age
- METs
metabolic equivalent task
- NMR
Nuclear Magnetic Resonance
- RCT
randomised controlled trial
- SFA
saturated fatty acids
- UPBEAT
The UK Pregnancy Better Eating and Activity Trial
- WHO
World Health Organization
Footnotes
Conflict of interest statement: The authors have no conflicts of interest to declare.
Contributors’ Statement
KVD, MOK, PTS, PDT, KMG and LP conceptualised and designed the study, drafted and carried out the initial analyses, critically reviewed the manuscript, and approved the final manuscript as submitted. LP, ALB, ACF, LH, SMN, SCR, NS, MKW and CS designed the data collection instruments, and coordinated and supervised data collection, critically reviewed the manuscript and approved the final manuscript as submitted. DAL, SLW and HLM contributed to the analysis plan for the metabolite data and completed this part of the analysis. PS contributed to the analysis of the pulse rate data. DAL and FAST completed additional statistical analyses and contributed to the final analysis plan reported in the paper. KVD wrote the first draft of the paper and coordinated updates following input from co-authors. All other authors critically reviewed the first and subsequent drafts. All authors approved the final version.
References
- 1.World Health Organisation. Report of the Commission on Ending Childhood Obesity. Geneva: 2016. [Accessed March 9, 2018]. http://www.who.int/end-childhood-obesity/en/ [Google Scholar]
- 2.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PloS One. 2013;8(4):e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godfrey KM, Reynolds RM, Prescott SL, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5(1):53–64. doi: 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksson JG, Sandboge S, Salonen MK, Kajantie E, Osmond C. Long-term consequences of maternal overweight in pregnancy on offspring later health: Findings from the Helsinki Birth Cohort Study. Ann Med. 2014;46(6):434–438. doi: 10.3109/07853890.2014.919728. [DOI] [PubMed] [Google Scholar]
- 5.Gaillard R, Steegers EA, Franco OH, Hofman A, Jaddoe VW. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int J Obes. 2015;39(4):677–685. doi: 10.1038/ijo.2014.175. [DOI] [PubMed] [Google Scholar]
- 6.Dalrymple KV, Thompson JMD, Begum S, et al. Relationships of maternal body mass index and plasma biomarkers with childhood body mass index and adiposity at 6 years: The Children of SCOPE study. Pediatr Obes. 2019 Jun;:e12537. doi: 10.1111/ijpo.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heslehurst N, Vieira R, Akhter Z, et al. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLOS Med. 2019;16(6):e1002817. doi: 10.1371/journal.pmed.1002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel N, Pasupathy D, Poston L. Determining the consequences of maternal obesity for offspring health. Exp Physiol. 2015;100(12):1421–1428. doi: 10.1113/EP085132. [DOI] [PubMed] [Google Scholar]
- 9.Fleten C, Nystad W, Stigum H, et al. Parent-offspring body mass index associations in the Norwegian Mother and Child Cohort Study: a family-based approach to studying the role of the intrauterine environment in childhood adiposity. Am J Epidemiol. 2012;176(2):83–92. doi: 10.1093/aje/kws134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richmond RC, Timpson NJ, Felix JF, et al. Using genetic variation to explore the causal effect of maternal pregnancy adiposity on future offspring adiposity: a Mendelian randomisation study. PLoS Med. 2017;14(1):e1002221. doi: 10.1371/journal.pmed.1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds RM, Allan KM, Raja E, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539. doi: 10.1136/bmj.f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard R, Steegers EA, Duijts L, et al. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension. 2014;63(4):683–691. doi: 10.1161/HYPERTENSIONAHA.113.02671. [DOI] [PubMed] [Google Scholar]
- 13.Samuelsson A-M, Matthews PA, Argenton M, et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance. Hypertension. 2008;51(2):383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 14.Samuelsson A-M, Clark J, Rudyk O, et al. Experimental Hyperleptinemia in Neonatal Rats Leads to Selective Leptin Responsiveness, Hypertension, and Altered Myocardial FunctionNovelty and Significance. Hypertension. 2013;62(3):627–633. doi: 10.1161/HYPERTENSIONAHA.111.00691. [DOI] [PubMed] [Google Scholar]
- 15.Menting MD, Mintjens S, Beek C, et al. Maternal obesity in pregnancy impacts offspring cardiometabolic health: Systematic review and meta‐analysis of animal studies. Obes Rev. 2019;20(5):675–685. doi: 10.1111/obr.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuelsson A-M, Morris A, Igosheva N, et al. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension. 2010;55(1):76–82. doi: 10.1161/HYPERTENSIONAHA.109.139402. [DOI] [PubMed] [Google Scholar]
- 17.Penfold NC, Ozanne SE. Developmental programming by maternal obesity in 2015: Outcomes, mechanisms, and potential interventions. Horm Behav. 2015;76:143–152. doi: 10.1016/j.yhbeh.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Roberts VHJ, Frias AE, Grove KL. Impact of Maternal Obesity on Fetal Programming of Cardiovascular Disease. Physiology. 2015;30(3):224–231. doi: 10.1152/physiol.00021.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flynn AC, Dalrymple K, Barr S, et al. Dietary interventions in overweight and obese pregnant women: a systematic review of the content, delivery, and outcomes of randomized controlled trials. Nutr Rev. 2016;74(5):312–328. doi: 10.1093/nutrit/nuw005. [DOI] [PubMed] [Google Scholar]
- 20.i-WIP Collaborative Group. i-WIP Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ. 2017;358:j3119. doi: 10.1136/bmj.j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalrymple KV, Martyni-Orenowicz J, Flynn AC, Poston L, O’Keeffe M. Can antenatal diet and lifestyle interventions influence childhood obesity? A systematic review. Matern Child Nutr. 2018;14(4):e12628. doi: 10.1111/mcn.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poston L, Bell R, Croker H, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(10):767–777. doi: 10.1016/S2213-8587(15)00227-2. [DOI] [PubMed] [Google Scholar]
- 23.Mills HL, Patel N, White SL, et al. The effect of a lifestyle intervention in obese pregnant women on gestational metabolic profiles: findings from the UK Pregnancies Better Eating and Activity Trial (UPBEAT) randomised controlled trial. BMC Med. 2019;17(1):15. doi: 10.1186/s12916-018-1248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel N, Godfrey K, Pasupathy D, et al. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy. Int J Obes. 2017;41(7):1018–1026. doi: 10.1038/ijo.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briley AL, Barr S, Badger S, et al. A complex intervention to improve pregnancy outcome in obese women; the UPBEAT randomised controlled trial. BMC Pregnancy Childbirth. 2014;14:74. doi: 10.1186/1471-2393-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Onis M. Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;95(S450):76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 27.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7(4):284–294. doi: 10.1111/j.2047-6310.2012.00064.x. [DOI] [PubMed] [Google Scholar]
- 28.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140(3):e20171904. doi: 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 29.The IPAQ Group. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) – Short and Long Forms Contents. 2005 [Google Scholar]
- 30.Srinivasan SR, Frontini MG, Berenson GS. Longitudinal changes in risk variables of insulin resistance syndrome from childhood to young adulthood in offspring of parents with type 2 diabetes: the Bogalusa Heart Study. Metabolism. 2003;52(4):443–450. doi: 10.1053/meta.2003.50065. [DOI] [PubMed] [Google Scholar]
- 31.Nanchen D, Locatelli I, Cornuz J, et al. Resting Heart Rate and the Risk of Heart Failure in Healthy Adults. Circ Heart Fail. 2013;6(3):403–410. doi: 10.1161/CIRCHEARTFAILURE.112.000171. [DOI] [PubMed] [Google Scholar]
- 32.Hu J, Shen H, Chu G-P, et al. Association of elevated resting pulse rate with increased risk of hypertension development in children. Medicine (Baltimore) 2017;96(32) doi: 10.1097/MD.0000000000007696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cordova A, Villa G, Sureda A, Rodriguez-Marroyo JA, Sánchez-Collado MP. Physical Activity and Cardiovascular Risk Factors in Spanish Children Aged 11-13 Years. Rev Esp Cardiol. 2012;65(7):620–626. doi: 10.1016/j.recesp.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 34.Kirk SL, Samuelsson A-M, Argenton M, et al. Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PloS One. 2009;4(6):e5870. doi: 10.1371/journal.pone.0005870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramamoorthy TG, Allen T-J, Davies A, et al. Maternal overnutrition programs epigenetic changes in the regulatory regions of hypothalamic Pomc in the offspring of rats. Int J Obes. 2018;42(8):1431–1444. doi: 10.1038/s41366-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor PD, Samuelsson A-M, Poston L. Maternal obesity and the developmental programming of hypertension: a role for leptin. Acta Physiol. 2014;210(3):508–523. doi: 10.1111/apha.12223. [DOI] [PubMed] [Google Scholar]
- 37.Ernst G. Heart-Rate Variability—More than Heart Beats? Front Public Health. 2017;5(11):240. doi: 10.3389/fpubh.2017.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Husin HM, Schleger F, Bauer I, et al. Maternal Weight, Weight Gain, and Metabolism are Associated with Changes in Fetal Heart Rate and Variability. Obesity. 2020;28(1):114–121. doi: 10.1002/oby.22664. [DOI] [PubMed] [Google Scholar]
- 39.Phelan S. Pregnancy: a “teachable moment” for weight control and obesity prevention. Am J Obstet Gynecol. 2010;202(2):135 e1–135 e8. doi: 10.1016/j.ajog.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalrymple KV, Flynn AC, Seed PT, et al. Associations between dietary patterns, eating behaviours, and body composition and adiposity in 3-year-old children of mothers with obesity. Pediatr Obes. 2019 Dec;:e12608. doi: 10.1111/ijpo.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanvig M, Vinter CA, Jorgensen JS, et al. Anthropometrics and body composition by dual energy x-ray in children of obese women: A follow-up of a randomized controlled trial (the Lifestyle in Pregnancy and Offspring [LiPO] study) PLoS ONE. 2014;9(2):e89590. doi: 10.1371/journal.pone.0089590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geserick M, Vogel M, Gausche R, et al. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N Engl J Med. 2018;379(14):1303–1312. doi: 10.1056/NEJMoa1803527. [DOI] [PubMed] [Google Scholar]
- 43.Lawlor DA, Lichtenstein P, Långström N. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation. 2011;123(3):258–265. doi: 10.1161/CIRCULATIONAHA.110.980169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephenson J, Heslehurst N, Hall J, et al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet Lond Engl. 2018;391(10132):1830–1841. doi: 10.1016/S0140-6736(18)30311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hughes RA, Heron J, Sterne JAC, Tilling K. Accounting for missing data in statistical analyses: multiple imputation is not always the answer. Int J Epidemiol. 2019;48(4):1294–1304. doi: 10.1093/ije/dyz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poston L, Briley AL, Barr S, et al. Developing a complex intervention for diet and activity behaviour change in obese pregnant women (the UPBEAT trial); assessment of behavioural change and process evaluation in a pilot randomised controlled trial. BMC Pregnancy Childbirth. 2013;13(1):148. doi: 10.1186/1471-2393-13-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.