Abstract
Type 2 diabetes (T2D) and obesity are complex disorders that constitute major public health problems. The evidence for familial aggregation of both T2D and obesity is substantial. To date, more than 150 genetic loci are associated with the development of monogenic, syndromic, or multifactorial forms of T2D or obesity. However, the proportion of overall trait variance explained by these associated loci is modest (~5-10% for T2D, ~2% for body mass index (BMI)). Some of the familial aggregation not attributable to known genetic variation, as well as many of the effects of environmental exposures, may reflect epigenetic processes. In this review, we discuss the evidence concerning the genetic contribution to individual risk of T2D and obesity, and explore the potential role of epigenetic mechanisms. We also explain how genetics, epigenetics, and environment are likely to interact to define the individual risk of disease.
The worldwide rise in type 2 diabetes (T2D) presents a major public health problem. In 2011, there were 366 million people with T2D, a number projected to rise to 552 million by 2030.1 This corresponds to an annual growth of 2.7%, outstripping by a factor of 1.7 that of the global population.1 The increase in prevalence will disproportionately affect developing countries least able to cope with the medical and economic consequences—for example, 48% of the increase in prevalence is projected to occur in India and China.1 Even now, the proportion of global excess deaths attributable to T2D worldwide is estimated to be ~7%.2
T2D is characterized by sustained elevations of plasma glucose levels. In contrast to type 1 diabetes, an autoimmune disease that results in complete loss of the insulin-producing β-cells in the pancreatic islets, T2D typically results when insulin secretion from the islets fails to keep pace with increasing insensitivity to the action of circulating insulin on its target tissues (particularly muscle, liver, and fat) the latter is typically the consequence of a combination of age, obesity, and lifestyle.
Obesity, one of the major risk factors for T2D, has shown a parallel increase in global prevalence. The estimated 205 million men and 297 million women worldwide (as of 2008) to be obese (BMI ≥30 kg/m2)3 are at increased risk of T2D and a variety of diseases, including osteoarthritis and many cancers. These obesity-related diseases are projected to add $48-66 billion a year to health-care costs in the United States alone.4 The economic burden through lost productivity could be even greater—estimates for the United States run as high as $390-580 billion annually4. These estimates underline the need for a better understanding of the processes involved in T2D and obesity pathogenesis if we are to achieve more efficient disease prevention and treatment.
The evidence for the familial aggregation of both T2D and BMI/overall obesity is substantial and comes from a variety of population, family, and twin-based studies.5–7 Although the subclinical nature and late onset of T2D, and the variation of BMI phenotypes over time, complicate such assessments, parental T2D and obesity have been shown—in northern European populations, at least—to give rise to approximately three- and twofold increases in disease risk in the offspring, respectively.5,8 The increase in risk associated with having a sibling with T2D is of a similar degree.9
It is often simplistically assumed that familial aggregation equates to genetic influences, but the situation is almost certainly more complex. First, for T2D and obesity—diseases that have clear associations with lifestyle factors—familial aggregation may reflect nothing more than the effects of shared family environment. The recent explosion in prevalence rates of both T2D and obesity clearly reflects the impact of dramatic secular changes in lifestyle and environment that may tend to cluster within families (obvious examples include socioeconomic status, dietary preferences, food availability, and gut microbiome content). However, this is unlikely to be the whole story, given the results of studies that seek to control for the effects of genetics and/or environment. For instance, studies of twins reared apart10 or of twins undergoing overfeeding in controlled environments11 consistently suggest a strong influence of nonenvironmental factors (in other words, high heritability).
Alternatively, familial aggregation can reflect epigenetic processes that are able to generate patterns of enhanced intrafamilial and/or transgenerational phenotypic resemblance. One example is the increase in sibling recurrence that is widely thought to result from the long-term effects of early life events on adult risk of T2D and obesity.9 This could be viewed as a special case of shared family environment. The increased risk of offspring T2D associated with gestation in a pregnancy affected by maternal diabetes (see below) has the potential to lead to the propagation of T2D risk into successive generations through nongenetic means. More generally, the fetal origins (or Barker-Hales) hypothesis12 links events in early life (particularly an adverse uterine environment) with an increased risk of T2D in later life. This “metabolic programming” is very likely to involve epigenetic processes.
In this review, we first consider the evidence in favor of a genetic basis for T2D and obesity, focusing specifically on the specific DNA sequence variants that have been implicated in risk predisposition. Second, we critically review the evidence supporting a role for epigenetic mechanisms. Finally, we return to the theme of the interplay of genetics, epigenetics, and environment and highlight the ways in which these are likely to interact to define the individual risk of disease.
The Genetic Basis of T2D and Obesity
As of 2012, more than 150 genetic loci have been conclusively implicated in the development of monogenic, syndromic, or multifactorial forms of obesity or (nonautoimmune) diabetes.13 In principle, each of these loci provides an opportunity to define the genetic architecture and pathophysiology of these traits.
The earliest successes for genetic discovery in diabetes and obesity arose from the study of monogenic and syndromic forms of disease, for which the segregation of rare, but highly penetrant, alleles could be tracked using family-based linkage approaches that are well suited to that setting. Maturity-onset diabetes of the young, for example, accounts for ~1-2% of cases of nonautoimmune diabetes presenting in early adulthood.14 Most cases of maturity-onset diabetes of the young are now known to result from rare coding mutations in either the hepatocyte nuclear factor-1A (HNF1A) or glucokinase (GCK) genes. In patients with these conditions, a precise molecular diagnosis brings important benefits in terms of individual prognostication and treatment optimization.14 These discoveries have also generated valuable insights into the cellular and molecular processes—operating in the pancreatic islet and other tissues—that control glucose homeostasis.15 To give a further example, identification of the mutations underlying syndromic forms of obesity, including Bardet-Biedl, has uncovered a whole class of diseases, the ciliopathies, that result from defects in the genetic control of ciliary development and function.16
Early attempts to apply family-based linkage approaches to more common forms of diabetes and obesity proved to be unrewarding. In their seminal paper in 1996, Risch and Merikangas17 highlighted the merits of association, as opposed to linkage, analysis for the detection of the low-penetrance alleles most likely to be relevant to common disease. It would take a decade before the density of available markers would allow genomewide screens for association to be implemented.18 In the interim, association analyses that focused attention on genetic variation within presumed biological candidates resulted in some successes in risk variant detection. For T2D, these included associations with variants in the genes encoding key therapeutic targets such as the peroxisome proliferator-activated receptor-y (PPARG) and the islet KATP channel (KCNJ11);19,20 an equivalent example for obesity would relate to variants in the melanocortin 4 receptor (MC4R) gene.21 More often than not, however, these candidate gene studies were plagued by inadequate sample size and overly liberal significance thresholds, a lethal combination that led to a profusion of unreliable reports of association.22
Over the past 5 years, the ability to conduct genome-wide studies of association has led to a massive acceleration in the number of loci now known to be associated with common forms of T2D and obesity, as well as with closely related continuous traits such as fasting glucose, BMI, and fat distribution.18,23–26 Given the content of the genotyping arrays employed, these studies have focused on the detection of signals attributable to common variants (typically of a minor allele frequency above 5%). Increasingly, these efforts are characterized by the aggregation of data from multiple samples24–27—the largest ongoing studies for BMI include data from more than 330,000 individuals —and, although early studies typically involved samples of European descent, the genetic basis of trait variability is now being explored across a broader range of ethnic groups.28–31 In the case of T2D, the current count of risk loci, each confirmed to genome-wide significance, is around 65;27–29 for BMI and obesity, the count is about half that number.25 Looking across these loci, several important features emerge.
First, these common variant signals have at most a modest effect. The largest signal for T2D, at the TCF7L2 (transcription factor 7-like 2) locus, is associated with a difference in T2D risk of approximately twofold between the two homozygote classes,32 whereas the strongest association for BMI (at the fat mass and obesity-associated (FTO) locus) is responsible for a ~2.5-kg difference in weight between homozygote groups.33 The other loci have substantially smaller effects.25,27
Second, most of these signals map to noncoding sequence. For only a minority, such as the T2D associations mapping to the genes encoding the glucokinase regulatory protein (GCKR) and ZnT-8 zinc transporter (SLC30A8), are alterations in transcript coding sequence thought to be responsible for the association signal.34–36
Third, it is clear at relatively few of the loci which of the genes in the region is mechanistically responsible for the association effects observed. This reflects both our inadequate understanding of the regulatory sequences controlling the expression of each gene37,38 and the correlation structure of human variation (“linkage disequilibrium”), which frustrate efforts to distinguish the specific causal variant from its highly correlated neighbors.39 However, it also illustrates the poverty of our current understanding of the processes involved in the regulation of body mass and metabolic homeostasis; surprisingly few of these genomewide association signals map near strong biological candidates. Notable exceptions include, for T2D, the melatonin receptor 1B (MTNR1B) and insulin receptor substrate-1 (IRS1)40–42 and, for BMI and obesity, MC4R and proopiomelanocortin (POMC).25,43 Considerable effort, using fine-mapping and functional approaches, is now being made to characterize pathophysiological mechanisms at the other loci.
Fourth, even though it has not been possible to identify the “causal” allele and transcript at each locus, these discoveries represent powerful tools for exploring the etiological relationships between related traits, such as those between T2D on the one hand and continuous traits including fasting glucose, insulin secretion, BMI, and birth weight on the other.24,27,44,45 Such analyses have shown, for example, that the variants altering T2D risk, as well as those influencing fasting glucose in healthy nondiabetic individuals, only partially overlap.24,27 The inference is that the processes involved in the physiological regulation of glucose levels are not identical to those responsible for the development of T2D, and that not all individuals with raised fasting glucose levels are at equal genetic risk of developing T2D. It has also been possible, by exploring the effects of T2D-risk alleles on indexes of insulin secretion and action in large numbers of nondiabetic individuals,27 to demonstrate that most of the known T2D-risk loci exert their primary effects on the former, placing the pancreatic islet at center stage in terms of functional investigation.38 The subset of T2D-risk loci that act primarily through insulin resistance includes the locus near FTO (fat mass and obesity-associated gene), which influences insulin sensitivity through its primary effect on BMI,46 and others, including variants near the gene encoding the Kruppel-like factor 14 (KLF14), that generate insulin resistance through obesity-independent disruption of adipocyte function.27,47 Similar approaches applied to genome-wide association studies for overall obesity (BMI) and fat distribution (BMI-adjusted waist-hip ratio) have suggested distinct biological processes involved in each—the former implicating transcripts involved in hypothalamic function and the latter implicating transcripts influencing adipocyte development and function.25,26
Fifth, the variants implicated in disease development have relatively weak effects on individual response to therapeutic and preventative interventions. Certainly, T2D-risk variants at TCF7L2 have been shown to influence sulfonylurea response, but the effect is too modest to be of direct clinical utility.48 Progress in applying genome-wide association approaches to identify novel signals associated with treatment response has been frustrated by the limited sample size of available cohorts. However, there have been recent successes— for example, the revelation that variants near ATM (ataxia telangiectasia mutated) influence individual response to metformin may provide valuable clues to the mechanism of action of this drug, which remains the leading first-line treatment for T2D.49
Finally, the small effect sizes of the established risk variants mean that, even in combination, the proportion of overall trait variance (and therefore the proportion of the observed familial aggregation) explained is modest. The ~65 T2D-risk variants account for no more than 5-10% of overall trait variance (and therefore, perhaps, 10-20% of overall heritability).27 The variance in BMI attributable to the known common variant signals (~2%)25 lies in stark contrast to the overall genetic variance estimated from twin and family studies (see above). For neither disease do these known variants provide a platform for clinically useful disease prediction.50
These estimates have led to the conclusion that much of the genetic variance is unexplained and have engendered much debate over the nature of this so-called “missing heritability”51 From the perspective of genetics, these results have motivated efforts to extend discovery beyond the common single-nucleotide polymorphisms that have been the focus of previous waves of genome-wide association studies to embrace variants of other types and frequencies (in particular, low-frequency and rare alleles). Variation at both common (e.g., at neuronal growth regulator 1 (NEGR1))52 and rare (e.g., on chromosome 16p11.2)53 copy-number polymorphisms has been shown to be associated with BMI, but these findings do not add substantially to the overall variance explained. Advances in next-generation sequencing are now being deployed—in the form of whole-exome and whole-genome sequencing—to provide ever more complete surveys of DNA sequence variation and its relationship to these and other traits. Over the next few years, such efforts can be expected to quantify the contribution of low-frequency and rare alleles of all types to T2D and obesity predisposition.
Some argue, based largely on population-genetics grounds, that much of the unexplained familial aggregation will turn out to be attributable to very rare alleles of relatively recent origin.54 However, there are alternative explanations. Recent analyses using the full set of genome-wide association data (rather than just the signals reaching genome-wide significance) have suggested that the accumulated effect of many hundreds of weakly associated loci may be sufficient to explain much more of the variance—as much as 50% of overall trait variance for T2D (and therefore most of the inherited component)55 and ~20% for BMI.56 Others have argued that gene-gene interaction effects will provide a substantial component.57 As we describe below, the indistinct boundaries between genetic and epigenetic effects may also help to explain why genetic effects cannot be expected to fully account for familial aggregation, or even measured heritability.
The Epigenetic Basis of T2D and Obesity
Changes in gene expression and cellular phenotypes that are mitotically stable but that occur without accompanying changes in primary DNA sequence are collectively referred to as epigenetic58 and, at the molecular level, are encoded by DNA methylation and histone modifications.59 Several lines of evidence point to a substantial epigenetic component to the development of T2D and obesity.
First, the fetal origins hypothesis established the notion of “metabolic programming” whereby nutritional and other exposures during early life generate long-term changes that later predispose to T2D and cardiovascular disease.12 This hypothesis builds on strong epidemiological data linking early life events to disease risk in late life, as seen, for example, in survivors of the Dutch “Hunger Winter”60 A growing body of data, from animal as well as human studies, has established that the molecular basis of programming involves altered DNA methylation.61
A second set of observations emerges from the longstanding follow-up of members of the Pima Native American community in Arizona, a population with an extremely high prevalence of T2D and obesity. The offspring of mothers who have T2D during pregnancy are at substantially higher risk of developing both T2D (45 vs. 1.4%) and obesity (58 vs. 17%) than are those born to women who are nondiabetic during pregnancy.61,62 Crucially, this difference is unlikely to completely reflect genetic transmission, as the distinction is preserved in children born to the same mother; that is, offspring born after the mother was diagnosed with T2D have higher rates of subsequent T2D and obesity than their siblings who arrived while their mother was nondiabetic.63 These findings suggest that the intrauterine environment is an important determinant of T2D and obesity predisposition, and they are broadly consistent with reports that the transmission of T2D and obesity is greater from mothers than from fathers.12,61 The increased risk of diabetes in female offspring of diabetic mothers clearly sets up the potential for an amplification of diabetes prevalence over successive generations.
Epigenetic processes are strong candidates for mediating the effects described above, and a growing body of data, largely, but not exclusively, derived from animal models provides experimental support for a causal link between early nutritional status and epigenetic changes that predispose to T2D and obesity risk in later life.64 Indeed, for some of these models there is evidence for epigenetic processes that can be transmitted through successive generations. Despite identical genetic makeup, the fur coat colors of agouti viable yellow (Avy/a) mice range from yellow to brown, and those colors are correlated with adult body weight.65 The variation in phenotypes is caused by an epigenetic modification of the Avy allele passed through the female germline that results in stable intergenerational transmission.65
To date, relatively few studies have explored the epigenetic characterization of humans at risk of T2D or obesity (Table 1). They have focused mostly on characterizing the methylation status of selected CpG sites in candidate genes, although the first epigenome-wide studies are now being performed (see below). Many of these studies have been poorly powered, and replication has rarely followed. Historically, research has been impeded by the relatively high cost and complexity of epigenetic analysis (certainly as compared with studies of the primary DNA sequence) as well as by tissue differences in methylation profile that mean that the findings obtained from accessible samples (e.g., peripheral blood) may not be representative of those happening in more disease-relevant, but largely inaccessible, tissues, such as the pancreatic islet or hypothalamus.
Table 1. DNA methylation candidate gene and epigenome-wide association studies for T2D and obesity.
| Candidate genes/EWAS | Phenotypes | Case control/quantitative trait | Tissue | Replication | Ref. |
|---|---|---|---|---|---|
| T2D CCL2 |
T2D | c/c: 32/15 | Peripheral blood mononuclear cells | Liu (2011) | |
| INS | T2D | c/c: 9/48 | Pancreatic islets | Yang (2011) | |
| PDX1 | T2D | c/c: 9/55 | Pancreatic islets | Yang (2012) | |
| PPARGC1A | T2D | c/c: 10/9 | Pancreatic islets | Ling (2008) | |
| EWAS | T2D | c/c: 17/17 | Skeletal muscle | Barres (2009) | |
| EWAS | T2D | c/c: 40/40 | Whole blood | Bell (2010) | |
| EWAS | T2D | c/c: 710a/459a | Whole blood | c/c: 198a/233a | Toperoff (2012) |
| EWAS | T2D | c/c: 11/5 | Pancreatic islets | Volkmar (2012) | |
| Obesity ALOX12, ALPL, BCL2A1, CASP10, CAV1, CCL3, CD9, CDKN1C, DSC2, EPHA1, EVI2A, HLA, IRF5, KRT1, LCN2, MLLT4, MMP9, MPL, NID1, NKX31, PMP22, S100A12, TAL1, VIM |
BMI, fat mass, and lean mass | qt: 178 | Umbilical cord blood | Relton (2012) | |
| KCNQ1OT1, H19, IGF2, GRB10, MEST, SNRPN, GNAS | BMI (discordance in twins) | c/c: 16/16 | Saliva | Souren (2011) | |
| MCHR1 | BMI | qt: 49 | Whole blood | Stepanow (2011) | |
| POMC | Obesity | c/c: 71/36 | Whole blood | c/c: 54/100 | Kuehnen (2012) |
| IL8, NOS3, PIK3CD, RXRA, SOD1 | Fat mass and %fat mass | qt: 78 | Umbilical cord tissue | qt: 239 | Godfrey (2011) |
| SLC6A4 | BMI, weight, and waist circumference | qt: 168 | Peripheral blood leukocytes | Zhao (2012) | |
| TACSTD2 | Fat mass | qt: 94 | Whole blood | qt: 161 | Groom (2012) |
| EWAS | BMI | qt: 64 | Lymphocytes | Feinberg (2010) | |
| EWAS | Obesity | c/c: 7/7 | Peripheral blood leukocytes | c/c: 46/46 | Wang (2010) |
| EWAS | Obesity | c/c: 23/24 | Whole blood | Almen (2012) |
See Supplementary Data online for the references cited in the table.
BMI, body mass index; c/c, case/control; EWAS, epigenome-wide association study; qt, quantitative trait; T2D, type 2 diabetes.
Pooled samples.
An interesting example of the challenges involved in designing, executing, and interpreting candidate gene methylation studies comes from analyses targeting the leptin-responsive gene POMC.66 POMC (proopiomelanocortin) encodes several key neuropeptides and hormones known to play a central role in body-weight regulation within the hypothalamus. Mutations in POMC are one cause of severe early-onset obesity,67 and the POMC region also harbors common genetic variants that are associated with obesity.25 A hypermethylated CpG site at the intron 2/exon 3 boundary of POMC identified in human peripheral blood leucocytes has shown replicated association with childhood obesity.66 This hypermethylated position overlaps with a putative binding site for the histone acetyltransferase P300 complex, which is known to be involved in chromatin acetylation and gene activation, raising the idea that methylation state influences P300 binding and thereby POMC expression.66 Although peripheral blood leucocytes are not likely to be directly involved in weight regulation, the suggestion is that similar methylation effects are present within the POMC- expressing neurons of the hypothalamic arcuate nucleus. The inaccessibility of this tissue means this hypothesis cannot be directly tested in humans.
In general, the current status of much epigenetic research in humans resembles that of genetics research before the advent of genome-wide association studies, dominated by underpowered candidate gene studies that have proved difficult to replicate and that have led to largely inconclusive results. Consequently, there are no convincing estimates of the extent to which individual differences in the risk of T2D and obesity reflect epigenetic variation. To address this, the field is now preparing to carry out the kinds of large-scale, global studies of epigenetic marks that will provide a more comprehensive and systematic view of the contribution of epigenetics to disease pathogenesis. By analogy with the genetic equivalent, these have been termed epigenome-wide association studies. Some of these studies are targeting diseaserelevant tissues (such as subcutaneous fat or specific blood-cell constituents), whereas others are, for pragmatic reasons, focused on whole blood.
These studies are now feasible because improved understanding of the variably methylated CpG content in the genome has catalyzed development of epigenome-wide DNA methylation profiling platforms such as the Illumina HumanMethylation450 BeadChip68 (widely known as the “450K chip”), which targets ~450,000 methylation sites across the genome. In turn, these array-based assays are increasingly giving way to a range of methods that couple bisulfite treatment with next-generation sequencing to capture regions of differential methylation in more unbiased fashion.
Despite these technological advances, designing appropriate epigenome-wide association studies remains challenging, far more so than genome-wide association studies. Unlike DNA genotypes, epigenetic marks can change over time, and apparent associations with disease are prone to confounding both by environmental influences and by reverse causation due to the disease of interest or its treatment.69 Issues surrounding tissue and patient heterogeneity and differences in sample-collection protocols and laboratory batch effects are all likely to impinge negatively on the ability to deliver conclusive results and to compare epigenetic measurements across studies. Accessing sufficient numbers of samples from disease-relevant tissues and/or cell types remains a major challenge.69
Thus, there have been relatively few epigenome-wide association studies for T2D and obesity to date, all of them on a relatively modest scale (Table 1). The largest70 employed a pooled design to interrogate genome-wide methylation in whole blood from a sample of ~1,150 T2D cases and controls. Analyses revealed that sites differentially methylated between cases and controls were enriched for loci previously implicated in T2D risk through genetic studies. One of the regions highlighted by these studies was the BMI-associated region at FTO, where methylation levels at a CpG site in the first intron of the FTO gene were correlated with genotype at nearby BMI-associated single-nucleotide polymorphisms. Associations of the FTO risk allele with hypermethylation have now been demonstrated and replicated in several studies,70–72 but it remains unclear whether methylation is the causal link between the FTO risk allele and T2D.70
These early studies suggest that the methylation status at FTO may be relevant to the T2D phenotype, and that epigenetic effects at FTO may, at least in part, be driven by underlying variation in the DNA sequence. If so, FTO would represent an example of haplotype-specific methylation, a term describing the situation in which the methylation status of a region is determined directly by the disposition of allelic variants that create or abrogate methylation sites.71 From a genetic (or genome-wide association) perspective, these can be viewed simply as a subset of genetic association signals at which the downstream effects are mediated by DNA sequence-dependent changes in local methylation.
To summarize: although there is ample evidence that epigenetic effects are likely to play a role in the pathogenesis of T2D and obesity, as well as to contribute to the observed familial aggregation, experimental studies that conclusively define the key loci responsible in disease-relevant tissues have yet to be performed (Table 1). It is also worth noting that existing studies have focused almost exclusively on DNA methylation, leaving other components of the epigenetic machinery (such as chromatin state) largely unexplored. Given limited access to diseaserelevant human tissues, progress will almost certainly depend on centralized community efforts such as the International Human Epigenome Consortium, which is generating reference epigenomes (including detailed methylome maps) for a panel of human tissues and cell types.69 This epigenetic counterpart of the Human HapMap effort will provide tissue-specific maps of methylation correlation structures, document the positions of variably methylated sites, and empower future epigenome-wide association study efforts.
The Complex Interplay of Genetics, Epigenetics, and Environment
As outlined above, substantial inroads have been made into defining loci and variants contributing to individual risk of T2D and obesity, although modest effect sizes mean that, even when combined, these established loci account for only a small proportion of the observed familial aggregation. Extension of discovery efforts to a more complete set of DNA sequence variants will certainly improve these estimates of variance explained, but a complete explanation of predisposition is unlikely to be achieved.
At the same time, there are strong reasons to believe that epigenetic processes play an important role in the pathogenesis of T2D and obesity, although the evidence implicating specific genomic regions currently falls well short of the robust levels of significance available for genetic data. As a result, there are few reliable estimates concerning the quantitative contribution of altered methylation with respect to either overall variance in disease risk or the proportion of the variance that appears to be segregating within pedigrees.
In fact, as we have highlighted through this review, any attempt to partition individual propensity to develop T2D and/or obesity among genetic, epigenetic, and environmental components is likely to be frustrated by the intimate connections between these different processes. A widespread tendency to apply imprecise terminology and unstated assumptions further complicates matters. For example, it should be clear that familial aggregation is not synonymous with genetic influence, yet measures of familial aggregation, such as the sibling recurrence-risk ratio, are often used as proxies for overall genetic effects, particularly for dichotomous traits.9 In a similar vein, estimates of heritability are often proposed as intrinsic properties of a given disease, whereas, in truth, heritability is simply a ratio of variances that can vary over time and in response to changes in the profile of environmental risk factors. Furthermore, the generation of meaningful estimates of heritability is not trivial for diseases such as T2D that are characterized by a long preclinical prodromal phase and onset in later life (because many apparently healthy relatives will turn out to be diabetic on clinical testing and/or destined to develop diabetes on further follow-up).73 A final concern is that epigenetic influences (such as those presumed to result from an adverse intrauterine environment) and shared family environment can produce nongenetic familial aggregation but may still, in certain study designs, be confused with genetic variance and lead to inflated estimates of heritability.
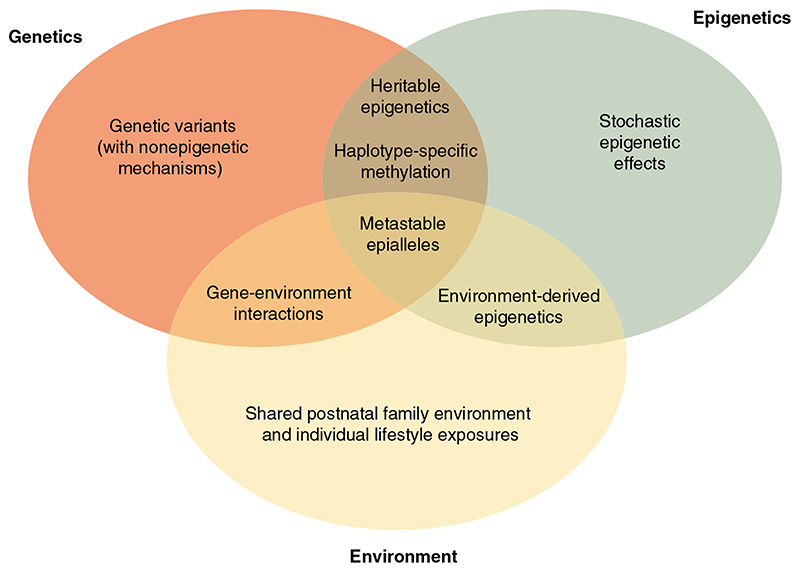
Some elements of this complex interplay among genetics, epigenetics, and environment are summarized in Figure 1. Several of these are mentioned elsewhere in this review.
Figure 1.
The complex relationship among genetics, epigenetics, and the environment in the susceptibility to type 2 diabetes (T2D) and obesity. The schematic Venn diagram illustrates the independent and interacting effects of genetics, epigenetics, and the environment that can give rise to T2D and obesity risk and familial aggregation. The diagram highlights where these effects overlap as well as where overlapping effects can either interact or appear as one of the others.
For instance, haplotype-specific methylation, such as that described at FTO,71 can be considered an example of the intersection of genetics and epigenetics, describing the situation in which segregating DNA sequence variants implicated in disease predisposition exert their downstream effects through epigenetic mechanisms. It also seems likely that many of the environmental exposures influencing T2D and obesity risk act through epigenetic mechanisms—obvious examples are the long-term consequences of adverse intrauterine nutrition and/or gestational hyperglycemia.62,63 Even more complex interactions between environment and epigenetics have been shown to be biologically possible, as in the agouti mouse model.65 The “metastable” Avy epiallele74 displays quasi-genetic intergenerational transmission, but the effect is not persistent and can be modified by maternal diet.65 These and the other overlaps—both real and apparent, as shown in the figure—demonstrate why simplistic attempts at partitioning variance (including many of the classic designs used to estimate heritability) are likely to be flawed and to correspond poorly to underlying mechanistic explanations.
More generally, one attractive perspective is to consider epigenetic modifications a final common pathway through which both genetic and environmental effects can impact T2D and obesity risk. For example, the expression of a given transcript may be influenced by genetic variants that alter promoter methylation (e.g., by creating or abolishing CpG sites) as well as by environmental exposures that have downstream consequences for methylation. These methylation-dependent effects on transcription are likely to be complemented by additional genetic and nongenetic effects on transcription that are not mediated by methylation (e.g., altered transcription factor expression or binding).
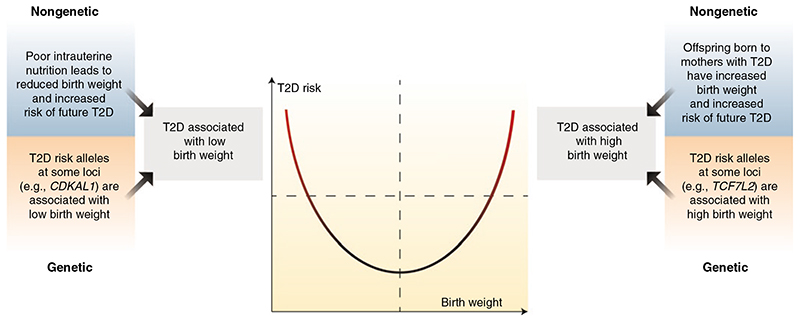
A neat example of this kind of interplay relates to the control of birth weight (Figure 2). In developed societies, it has been shown that the relationship between birth weight and T2D risk is best described through a U-shaped curve (shown in exaggerated form in the figure), such that the future risk of T2D is highest in individuals with either low or high birth weight as compared with those of average birth weight. Both associations with the extremes of birth weight result from a mix of genetic and nongenetic effects. At the lower extreme, the association between low birth weight and later T2D risk reflects both the long-term programming effects of an adverse intrauterine environment (most likely mediated through epigenetic effects)12 and the impact of a subset of T2D-risk variants, such as those at CDKAL1, which have a marked effect on the secretion of insulin in early life (a time at which insulin acts as a major influence on growth).75 At the other extreme, the association between high birth weight and later T2D risk is mediated, at least in part, by exposure to maternal diabetes during pregnancy61,63 and by direct genetic effects, such as those of the T2D risk-variants at TCF7L2, where the dominant effect of allelic variation in the fetomaternal unit appears to be to promote maternal hyperglycemia (and consequent fetal macrosomia).76
Figure 2.
The relationship between risk of type 2 diabetes (T2D) and birth weight. The U-shaped relationship between T2D risk and birth weight is shown in this illustration (not to scale). Both genetic and nongenetic effects are listed on each side of the curve and refer to the upper tails of the curve at high T2D risk (shaded red).
This review highlights evidence to support the notion that individual predisposition to T2D and obesity reflects a complex mix of genetic, epigenetic, and environmental influences. Despite recent progress, the mechanisms driving these interactions remain poorly understood.
Supplementary Material
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
Acknowledgments
A.W.D. is a Wellcome Trust doctoral student (093933/Z/10/Z). C.M.L. is a Wellcome Trust research career development fellow (086596/Z/08/Z). M.I.M. is a National Institute for Health Research senior investigator.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Roglic G, Unwin N. Mortality attributable to diabetes: estimates for the year 2010. Diabetes Res Clin Pract. 2010;87:15–19. doi: 10.1016/j.diabres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Finucane MM, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9-1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 5.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000;49:2201–2207. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 6.Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H. Heritability of type II (noninsulin-dependent) diabetes mellitus and abnormal glucose tolerance–a population-based twin study. Diabetologia. 1999;42:139–145. doi: 10.1007/s001250051131. [DOI] [PubMed] [Google Scholar]
- 7.Stunkard AJ, Foch TT, Hrubec Z. A twin study of human obesity. JAMA. 1986;256:51–54. [PubMed] [Google Scholar]
- 8.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 9.Kobberling J, Tillil H. Empirical risk figures for first degree relatives of non-insulin dependent diabetics. In: Kobberling J, Tattersall R, editors. The Genetics of Diabetes Mellitus. Academic Press; London: 1982. pp. 201–209. [Google Scholar]
- 10.Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med. 1990;322:1483–1487. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- 11.Bouchard C, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 12.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363:2339–2350. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- 14.Thanabalasingham G, Owen KR. Diagnosis and management of maturity onset diabetes of the young (MODY) BMJ. 2011;343 doi: 10.1136/bmj.d6044. d6044. [DOI] [PubMed] [Google Scholar]
- 15.Boj SF, Parrizas M, Maestro MA, Ferrer J. A transcription factor regulatory circuit in differentiated pancreatic cells. Proc Natl Acad Sci USA. 2001;98:14481–14486. doi: 10.1073/pnas.241349398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansley SJ, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 17.Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273:1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- 18.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altshuler D, et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26:76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 20.Gloyn AL, et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes. 2003;52:568–572. doi: 10.2337/diabetes.52.2.568. [DOI] [PubMed] [Google Scholar]
- 21.Larsen LH, Echwald SM, Sørensen TI, Andersen T, Wulff BS, Pedersen O. Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with juvenile-onset obesity. J Clin Endocrinol Metab. 2005;90:219–224. doi: 10.1210/jc.2004-0497. [DOI] [PubMed] [Google Scholar]
- 22.Hattersley AT, McCarthy MI. What makes a good genetic association study? Lancet. 2005;366:1315–1323. doi: 10.1016/S0140-6736(05)67531-9. [DOI] [PubMed] [Google Scholar]
- 23.Zeggini E, et al. Meta-analysis of genome-wide association data and largescale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40:638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dupuis J, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speliotes EK, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heid IM, et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet. 2010;42:949–960. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voight BF, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42:579–589. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kooner JS, et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet. 2011;43:984–989. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho YS, et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet. 2012;44:67–72. doi: 10.1038/ng.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada Y, et al. Meta-analysis identifies nine new loci associated with rheumatoid arthritis in the Japanese population. Nat Genet. 2012;44:511–516. doi: 10.1038/ng.2231. [DOI] [PubMed] [Google Scholar]
- 31.Wen W, et al. Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet. 2012;44:307–311. doi: 10.1038/ng.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant SF, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 33.Frayling TM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diabetes Genetics Initiative of Broad Institute of Harvard and MIT. Genomewide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 35.Sladek R, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 36.Beer NL, et al. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet. 2009;18:4081–4088. doi: 10.1093/hmg/ddp357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ENCODE Project Consortium. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaulton KJ, et al. A map of open chromatin in human pancreatic islets. Nat Genet. 2010;42:255–259. doi: 10.1038/ng.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 40.Prokopenko I, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyssenko V, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rung J, et al. Genetic variant near IRS1 is associated with type 2 diabetes, insulin resistance and hyperinsulinemia. Nat Genet. 2009;41:1110–1115. doi: 10.1038/ng.443. [DOI] [PubMed] [Google Scholar]
- 43.Loos RJ, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freathy RM, et al. Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes. 2008;57:1419–1426. doi: 10.2337/db07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freathy RM, et al. Variants in ADCY5 and near CCNL1 are associated with fetal growth and birth weight. Nat Genet. 2010;42:430–435. doi: 10.1038/ng.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frayling TM. Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet. 2007;8:657–662. doi: 10.1038/nrg2178. [DOI] [PubMed] [Google Scholar]
- 47.Small KS, et al. Identification of an imprinted master trans regulator at the KLF14 locus related to multiple metabolic phenotypes. Nat Genet. 2011;43:561–564. doi: 10.1038/ng.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearson ER, et al. Variation in TCF7L2 influences therapeutic response to sulfonylureas: a GoDARTs study. Diabetes. 2007;56:2178–2182. doi: 10.2337/db07-0440. [DOI] [PubMed] [Google Scholar]
- 49.Zhou K, et al. The role of ATM in response to metformin treatment and activation of AMPK. Nat Genet. 2012;44:361–362. doi: 10.1038/ng.2234. [DOI] [PubMed] [Google Scholar]
- 50.Willems SM, Mihaescu R, Sijbrands EJ, van Duijn CM, Janssens AC. A methodological perspective on genetic risk prediction studies in type 2 diabetes: recommendations for future research. Curr Diab Rep. 2011;11:511–518. doi: 10.1007/s11892-011-0235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manolio TA, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willer CJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walters RG, et al. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature. 2010;463:671–675. doi: 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lupski JR, Belmont JW, Boerwinkle E, Gibbs RA. Clan genomics and the complex architecture of human disease. Cell. 2011;147:32–43. doi: 10.1016/j.cell.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stahl EA, et al. Bayesian inference analyses of the polygenic architecture of rheumatoid arthritis. Nat Genet. 2012;44:483–489. doi: 10.1038/ng.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J, et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat Genet. 2012;44:369–375, S1. doi: 10.1038/ng.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zuk O, Hechter E, Sunyaev SR, Lander ES. The mystery of missing heritability: Genetic interactions create phantom heritability. Proc Natl Acad Sci USA. 2012;109:1193–1198. doi: 10.1073/pnas.1119675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolffe AP, Guschin D. Review: chromatin structural features and targets that regulate transcription. J Struct Biol. 2000;129:102–122. doi: 10.1006/jsbi.2000.4217. [DOI] [PubMed] [Google Scholar]
- 59.Handel AE, Ebers GC, Ramagopalan SV. Epigenetics: molecular mechanisms and implications for disease. Trends Mol Med. 2010;16:7–16. doi: 10.1016/j.molmed.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Heijmans BT, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pettitt DJ, Aleck KA, Baird HR, Carraher MJ, Bennett PH, Knowler WC. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes. 1988;37:622–628. doi: 10.2337/diab.37.5.622. [DOI] [PubMed] [Google Scholar]
- 62.Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med. 1983;308:242–245. doi: 10.1056/NEJM198302033080502. [DOI] [PubMed] [Google Scholar]
- 63.Dabelea D, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 64.Seki Y, Williams L, Vuguin PM, Charron MJ. Minireview: Epigenetic programming of diabetes and obesity: animal models. Endocrinology. 2012;153:1031–1038. doi: 10.1210/en.2011-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23:314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 66.Kuehnen P, et al. An Alu element-associated hypermethylation variant of the POMC gene is associated with childhood obesity. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002543. e1002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 68.Bibikova M, et al. Genome-wide DNA methylation profiling using Infinium® assay. Epigenomics. 2009;1:177–200. doi: 10.2217/epi.09.14. [DOI] [PubMed] [Google Scholar]
- 69.Rakyan VK, Down TA, Balding DJ, Beck S. Epigenome-wide association studies for common human diseases. Nat Rev Genet. 2011;12:529–541. doi: 10.1038/nrg3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Toperoff G, et al. Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum Mol Genet. 2012;21:371–383. doi: 10.1093/hmg/ddr472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bell CG, et al. Integrated genetic and epigenetic analysis identifies haplotype-specific methylation in the FTO type 2 diabetes and obesity susceptibility locus. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0014040. e14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Almén MS, et al. Genome wide analysis reveals association of a FTO gene variant with epigenetic changes. Genomics. 2012;99:132–137. doi: 10.1016/j.ygeno.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 73.Visscher PM, Hill WG, Wray NR. Heritability in the genomics era–concepts and misconceptions. Nat Rev Genet. 2008;9:255–266. doi: 10.1038/nrg2322. [DOI] [PubMed] [Google Scholar]
- 74.Rakyan VK, Blewitt ME, Druker R, Preis JI, Whitelaw E. Metastable epialleles in mammals. Trends Genet. 2002;18:348–351. doi: 10.1016/s0168-9525(02)02709-9. [DOI] [PubMed] [Google Scholar]
- 75.Freathy RM, et al. Type 2 diabetes risk alleles are associated with reduced size at birth. Diabetes. 2009;58:1428–1433. doi: 10.2337/db08-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Freathy RM, et al. Type 2 diabetes TCF7L2 risk genotypes alter birth weight: a study of 24,053 individuals. Am J Hum Genet. 2007;80:1150–1161. doi: 10.1086/518517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.