Abstract
Easily synthesised and structurally well-defined novel imaging/therapeutic radiopharmaceutical agents for bone metastases are described.
1,1-Bisphosphonates (BPs) are a family of compounds extensively used in the management of disorders of bone metabolism.1 They accumulate in areas of high bone metabolism, such as bone metastases, and consequently have been receiving increasing attention as molecular imaging probes and pain palliation treatments.2 Imaging bone metastases with BPs using single photon emission computed tomography (SPECT) or planar scintigraphy is one of the most often-performed clinical imaging procedures. β-Emitting analogues capable of producing a therapeutic effect have also been developed.3 In particular, the rhenium compounds 186/188Re-hydroxyethylidene-1,1-diphosphonate (186/188Re–HEDP) have shown promise as palliative agents for bone metastases in recent clinical trials.4 The radiochemicals consist of a complex of a BP (e.g. methylene diphosphonate, MDP) with γ-(99mTc) or β-(186/186Re) emitters.
Despite the proven clinical success of 99mTc/188/186Re–BPs, these radiopharmaceuticals are far from optimal from a chemical and pharmaceutical point of view. For example, despite decades of clinical use, their structures and compositions remain unknown. A critical review of the literature reveals that the 99mTc–MDP preparation used in the clinic is composed of a mixture of anionic polymers of different properties.5 A particular concern is that the in vivo stability of 186/188Re–BPs does not adequately match their physical half-life and a large fraction of the injected complex degrades to perrhenate in vivo within 24 h, leading to reduced bone uptake and higher soft-tissue doses. Furthermore, 186/188Re–BPs are not chemically analogous to their Tc-counterparts and do not target bone metastases unless additional “carrier” nonradioactive rhenium is added.7 Consequently there is a need for rational design of 186/188Re-labelled BP derivatives to improve specificity and reduce soft-tissue and bone marrow doses during radionuclide therapy.
In current Tc/Re–BP complexes the BP acts as both chelator and targeting group. Each role, however, may compromise the other as BPs are excellent bone-seeking agents but poor Re chelators. To improve upon current 186/188Re–BPs, a more logical approach is the use of targeted bifunctional ligands in which the targeting (BP) and metal chelating groups are separated within the molecule so that they can each function independently and effectively. A few recent reports describe such a BP–chelator bifunctional approach.8,9 These BP-conjugates, however, require complicated multi-step synthetic strategies, show high plasma protein binding and often form enantiomeric mixtures. Herein we report a new chelator–BP conjugate 3 that is synthesised using mild aqueous conditions in one step from commercially available compounds. In addition, 3 efficiently forms single well-defined isostructural Tc/Re complexes with no detectable protein binding that efficiently accumulate in bone tissue in vivo.
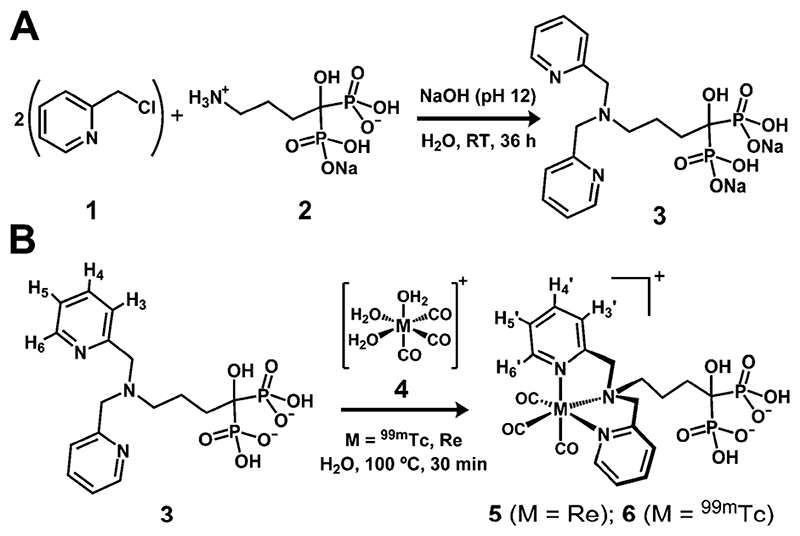
Our approach requires the BP part of the molecule to be separated from the chelator by a spacer, to avoid any BP–metal interactions. In addition, the radionuclide must selectively coordinate with the chelating group, not the BP, and must remain inert under in vivo conditions. The organometallic precursor fac-[M(CO)3(H2O)3]+ (M = Tc, Re) (Scheme 1B, 4), pioneered by Jaouen et al. and Alberto et al., facilitates the latter requirement.10 When the three labile water molecules are displaced by an appropriate ligand system, the d6 low-spin octahedral Tc(I)/Re(I) centre formed is protected from oxidation and ligand substitution. Furthermore, imaging probes containing a coordinatively saturated fac-[M(CO)3]+ core have shown high in vivo inertness and negligible binding to human serum proteins.11 Particularly favourable ligands for [M(CO)3]+ are N3-tridentate chelators containing two sp2 N-heterocycles, such as dipicolylamine (DPA).12 As the targeting vector we selected alendronate (2) (Scheme 1A), a clinically-approved BP that binds avidly to hydroxyapatite (HA), the main component of bone mineral.13 Furthermore, 2 provides an amino group conveniently separated from the BP group by a spacer, allowing facile one-step conjugation of two picolyl units to form a DPA group (Scheme 1A).
Scheme 1.
Reaction schemes for the syntheses of 3 (A), 5 and 6 (B).
Two major obstacles were encountered during the development of DPA-alendronate (3, Scheme 1A). First is the insolubility of alendronate in organic solvents, which complicates conjugation reactions. Second, the high basicity of its amino group (pK a 12.7) inhibits nucleophilic attack by alendronate using standard organic bases.14 Important factors to overcome these barriers are pH and the concentration of base. Using strong organic bases such as triethylamine was unsuccessful. High concentrations of inorganic bases and high temperatures, however, led to hydrolysis of 2-picolyl chloride (1, Scheme 1A) and rearrangements of the BP.15 We found that using water as solvent and maintaining the pH of the solution at 12 with the minimum amount of NaOH was sufficient to drive the reaction to completion after 36 hours at room temperature, without detectable hydrolysis or BP rearrangements. The yield of 3 was >90% by RP-HPLC.
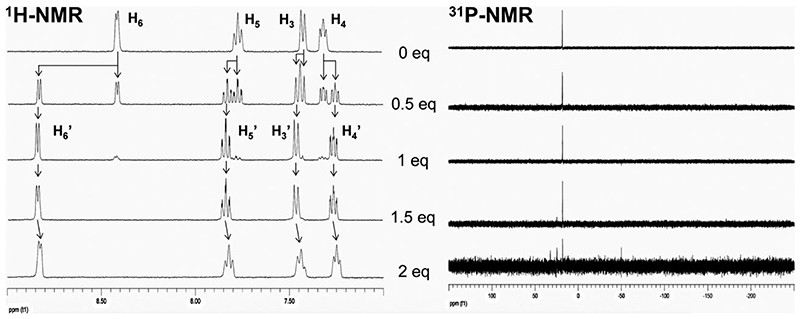
The complexation of 3 with fac-[Re(CO)3]+, and its solution properties, were examined using HPLC and NMR, MS and IR spectroscopies (Fig. 1 and ESI†). The aim was to determine whether the organometallic core selectively coordinated the chelating DPA group. NMR/HPLC titration studies revealed that fac-[Re(CO)3(H2O)3]+ stoichiometrically binds 3 in the designed facial conformation when less than 1.5 equivalents of fac-[Re(CO)3(H2O)3]+ were used. The presence of a single species corresponding to 5 in solution was confirmed by 1H/31P-NMR and HPLC (Fig. 1, Fig. S1(ESI†)). HR-ESI-MS also demonstrates the formation of the desired product. Upon addition of 1.5 equivalents or more of the metal reagent, new 31P-NMR signals appeared, accompanied by a general upfield shift of the aromatic protons in the 1H-NMR spectrum strongly suggesting coordination of metal centres to the BP group. These putative multinuclear species, however, do not form during radiosynthesis as the concentration of ligand always exceeds that of the radionuclide by several orders of magnitude. 3 in concentrations as low as 10 −5 M (0.7 pg per labelling) can be efficiently labelled with fac-[99mTc(CO)3]+ in water to form 6 (>98% radiochemical yield, 22 GBq mg−1). RP-HPLC analyses show that 5 and 6 coeluted, demonstrating their analogous structure (ESI†, Fig. S2).
Fig. 1.
NMR titration studies in D2O showing the 1H- (aromatic region, left) and 31P-NMR (right) of 3 (top row) upon increasing amounts (from top to bottom) of [Re(CO)3]+ showing the formation of 5 (mid row). See Scheme 1B for 1H-NMR assignments.
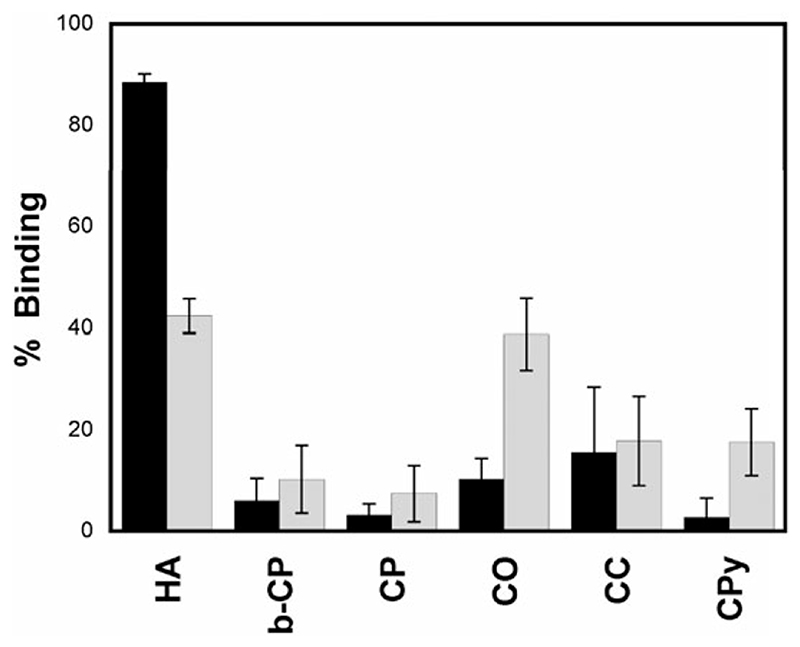
One of the factors that makes 2 one of the most potent BP drugs is its high skeletal uptake and retention, which is directly related to its affinity towards HA.13 The affinities and selectivities of 6 and 99mTc–MDP towards several calcium salts were evaluated using an in vitro assay. As shown in Fig. 2, 6 binds HA selectively with very high affinity (>80% binding). 99mTc–MDP, on the other hand, is less selective and binds HA and calcium oxalate (CO) with lower affinity (~40% binding). Remarkably, 6 shows higher affinity for HA despite having a concentration of competitive inhibitor (in the form of non-labelled BP) ~ 10 times higher than in the 99mTc–MDP preparation.16
Fig. 2.
In vitro calcium salt binding study in 50 mM TRIS pH 6.9 at room temperature to compare the binding of 6 (black bars) and 99mTc-MDP (grey bars) to different calcium salts after 1 h (1 mg ml.−1): hydroxyapatite (HA); β-tricalcium phosphate (b-CP); calcium phosphate (CP); calcium oxalate (CO); calcium carbonate (CC) and calcium pyrophosphate (CPy).
The fate of a targeted imaging probe or radiopharmaceutical in blood is one of the most important factors during pre-clinical development of radiopharmaceuticals. Strong binding to serum proteins such as albumin often delays blood clearance, leading to low target-to-background ratios.11 Previous chelator–BP conjugates have shown high binding to serum proteins.9 Furthermore, human plasma enzymes may decompose exogenous compounds. Complex 6 showed negligible binding to serum proteins and no decomposition after incubation with human plasma for at least 18 h (ESI†, Fig. S4). 99mTc–MDP, on the other hand, remained mostly bound to serum proteins throughout the 18 h incubation.
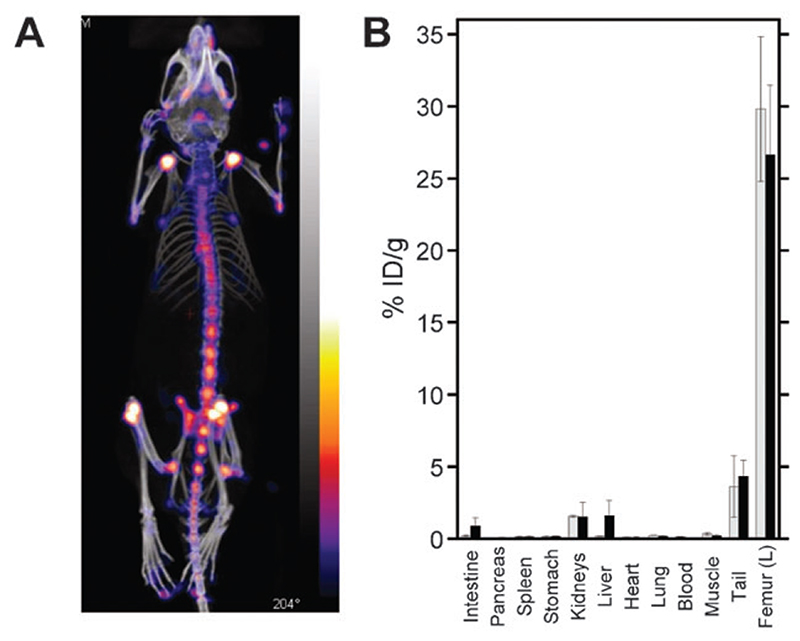
In vivo imaging studies with 6 were carried out with adult Balb/C female mice using a nanoSPECT/CT animal scanner. Control imaging studies were also performed with 99mTc–MDP. 6 shows essentially identical bone uptake to 99mTc–MDP, demonstrating its usefulness as a bone-seeking agent (Fig. 3A, Fig. S5 (ESI†)). Biodistribution studies were performed ex vivo to quantify the uptake of the two tracers in bone and soft-tissue organs (Fig. 3B). As expected, the bone uptake of both compounds was very high, with 27–30% of the injected dose per gram of tissue (% ID g−1) in the femur. Imaging shows that this uptake was in fact confined to the joints, where active remodelling occurs. Liver and lower gastrointestinal uptake, while very low, were slightly higher with 6 (2.5% ID g−1) than with 99mTc-MDP (0.4% ID g−1), consistent with the more lipophilic nature of the tricarbonyl core compared to MDP. An advantage of using bifunctional compounds is that properties like lipophilicity may be tuned by using, for example, different spacers and/or chelators. Thus, our results demonstrate that 3 forms well-defined, well-characterised and stable bone-targeting agents with the fac-[M(CO)3]+ (M = Tc, Re) metal core and deserves further evaluation for the diagnosis and therapy of bone metastases. Biological evaluation of the complex of 3 with [188Re(CO)3]+ compared with 188Re–HEDP is underway to assess the prospects for improving radionuclide therapy by this approach.
Fig. 3.
(A) SPECT (colour)/CT (greyscale) image showing the high uptake of 6 in bone tissue, particularly at the joints. (B) Biodistribution profile of 6 (black bars) and 99mTc-MDP (grey bars) at t = 6.5 h post-injection.
We would like to thank Cancer Research UK (Grant C789/ A7649) for funding, Covidien for providing the Isolink™ kits and Dr Jim Ballinger (Nuclear Medicine, Guy’s and St Thomas’ Hospital, London) for supplying [99mTcO4]Na and for useful discussions.
Supplementary Material
† Electronic supplementary information (ESI) available: Experimental procedures: synthesis and characterisation, HPLC titration studies, radiolabelling, in vitro and in vivo procedures. See DOI: 10.1039/b908652h
Notes and references
- 1.(a) Fleish H. Bisphosphonates in Bone Disease: From the Laboratory to the Patient. Parthenon Publishing Group; London: 1995. [Google Scholar]; (b) Russell RGG. Bone. 2007;40:S21–S25. [Google Scholar]
- 2.Zhang S, Gangal G, Uludag H. Chem Soc Rev. 2007;36:507–531. doi: 10.1039/b512310k. [DOI] [PubMed] [Google Scholar]
- 3.Lewington VJ. J Nucl Med. 2005;46:38S–47. [PubMed] [Google Scholar]
- 4.(a) de Klerk JMH, van het Schip AD, Zonnenberg BA, van Dijk A, Quirijnen J, Blijham GH, van Rijk PP. J Nucl Med. 1996;37:244–249. [PubMed] [Google Scholar]; (b) Hsieh BT, Hsieh JF, Tsai SC, Lin WY, Wang SJ, Ting G. Nucl Med Biol. 1999;26:973–976. doi: 10.1016/s0969-8051(99)00075-x. [DOI] [PubMed] [Google Scholar]; (c) Liepe K, Kropp J, Runge R, Kotzerke J. Br J Cancer. 2003;89:625–629. doi: 10.1038/sj.bjc.6601158. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Maxon HR, Schroder LE, Hertzberg VS, Thomas SR, Englaro EE, Samaratunga R, Smith H, Moulton JS, Williams CC, Ehrhardt GJ, Schneider HJ. J Nucl Med. 1991;32:1877–1881. [PubMed] [Google Scholar]; (e) Palmedo H, Grunwald F, Wagner U, Kohler S, Krebs D, Biersack HJ. Clin Nucl Med. 1998;23:501–504. doi: 10.1097/00003072-199808000-00001. [DOI] [PubMed] [Google Scholar]; (f) Tian M, Zhang H, Li S, Endo K, Tanada S. J Clin Oncol. 2004;22:766S–766S. [Google Scholar]
- 5.Handeland Å, Lindegaard MW, Heggli D-E. Eur J Nucl Med Mol Imaging. 1989;15:609–611. doi: 10.1007/BF00256939. [DOI] [PubMed] [Google Scholar]
- 6.de Klerk JMH, van Dijk A, van het Schip AD, Zonnenberg BA, van Rijk PP. J Nucl Med. 1992;33:646–651. [PubMed] [Google Scholar]
- 7.Chung YS, Jeong JM, Lee DS, Chung JK, Lee MC, Koh CS, Yu HJ, Cho JH, Knapp FF. J Nucl Med. 1998;39:1037. [Google Scholar]
- 8.(a) Ogawa K, Mukai T, Arano Y, Ono M, Hanaoka H, Ishino S, Hashimoto K, Nishimura H, Saji H. Bioconjugate Chem. 2005;16:751–757. doi: 10.1021/bc040249w. [DOI] [PubMed] [Google Scholar]; (b) Ogawa K, Mukai T, Arano Y, Otaka A, Ueda M, Uehara T, Magata Y, Hashimoto K, Saji H. Nucl Med Biol. 2006;33:513–520. doi: 10.1016/j.nucmedbio.2006.03.006. [DOI] [PubMed] [Google Scholar]; (c) El-Mabhouh A, Angelov C, McEwan A, Jia G, Mercer J. Cancer Biother Radiopharm. 2004;19:627–640. doi: 10.1089/cbr.2004.19.627. [DOI] [PubMed] [Google Scholar]; (d) El-Mabhouh A, Mercer JR. Appl Radiat Isot. 2005;62:541–549. doi: 10.1016/j.apradiso.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa K, Mukai T, Inoue Y, Ono M, Saji H. J Nucl Med. 2006;47:2042–2047. [PubMed] [Google Scholar]
- 10.(a) Salmain M, Gunn M, Gorfti A, Top S, Jaouen G. Bioconjugate Chem. 1993;4:425–433. doi: 10.1021/bc00024a003. [DOI] [PubMed] [Google Scholar]; (b) Alberto R, Schibli R, Egli A, Schubiger AP, Abram U, Kaden TA. J Am Chem Soc. 1998;120:7987–7988. doi: 10.1021/ic980112f. [DOI] [PubMed] [Google Scholar]
- 11.Schibli R, La Bella R, Alberto R, Garcia-Garayoa E, Ortner K, Abram U, Schubiger PA. Bioconjugate Chem. 2000;11:345–351. doi: 10.1021/bc990127h. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee SR, Levadala MK, Lazarova N, Wei L, Valliant JF, Stephenson KA, Babich JW, Maresca KP, Zubieta J. Inorg Chem. 2002;41:6417–6425. doi: 10.1021/ic020476e. [DOI] [PubMed] [Google Scholar]
- 13.Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RGG, Ebetino FH. Bone. 2006;38:617–627. doi: 10.1016/j.bone.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Kubíček V, Kotek J, Hermann P, Lukeš I. Eur J Inorg Chem. 2007:333–344. [Google Scholar]
- 15.Lecouvey M, Leroux Y. Heteroat Chem. 2000;11:556–561. [Google Scholar]
- 16.The concentration of 3 in the solution of 6 used in these studies was b 10 times higher than that of MDP in the 99mTc-MDP solution. To test the inhibition properties of 3, the same binding studies were carried out with HA and CO in the presence of an excess of 3, resulting in the complete inhibition of binding of 6.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.