Abstract
The human nasal epithelium consists of basal stem/progenitor cells that produce differentiated multiciliated and mucosecretory progeny. Basal epithelial cells can be expanded in cell culture and instructed to differentiate at an air-liquid interface using transwell membranes and differentiation media. For basal cell expansion, we have used 3T3- J2 co-culture in epithelial culture medium containing EGF, insulin and a RHO-associated protein kinase (ROCK) inhibitor, Y-27632 (3T3+Y). Here we describe our protocols for ciliated differentiation of these cultures at air-liquid interface and compare four commercially available differentiation media. Across nine donor cell cultures (six healthy, two patients with chronic obstructive pulmonary disease (COPD) and one with primary ciliary dyskinesia (PCD)), brightfield and immunofluorescence imaging suggested broad similarity between differentiation protocols. Subtle differences were seen in transepithelial electrical resistance (TEER), ciliary beat frequency, mucus production and the extent to which basal cells are retained in differentiated cultures. Overall, the specific differentiation medium used in our air-liquid interface culture protocol was not a major determinant of ciliation and our data suggest that the differentiation potential of basal cells at the outset is a more critical factor in air-liquid interface culture outcome. Detailed information on the constituents of the differentiation media was only available from one of the four manufacturers, a factor that may have profound implications in the interpretation of some research studies.
Keywords: in vitro models, cilia, nasal epithelial cells, mucociliary differentiation, air-liquid interface, nasal epithelium, multiciliated cells, primary ciliary dyskinesia, chronic obstructive pulmonary disease
1. Introduction
Air-liquid interface (ALI) culture is a staple technique in respiratory cell biology, allowing the manipulation and interrogation of human cells in the laboratory. In 2D cell cultures on plastic, only keratin 5 (KRT5)/TP63+ nasal, tracheal or bronchial basal stem/progenitor cells are expanded [1]. Of course, these cells represent only a subset of those present in the human airways – which also contain ciliated, mucosecretory and other rare epithelial cell populations – so differentiation using air-liquid interface (or 3D differentiation assays using Matrigel [2, 3]) is necessary to more closely mimic the native epithelium. Although there are likely to be differences in cellular composition between native airways and air-liquid interface cell culture surrogates – for example, due to incomplete and/or squamous differentiation – such cultures have been a useful tool to investigate airway differentiation processes [4, 5], the effects of cigarette smoke [6, 7], infectious stimuli [8] and the pathogenesis of various respiratory diseases, including asthma [9], chronic obstructive pulmonary disease (COPD) [10, 11] and cystic fibrosis [12, 13].
We and others have reported a protocol for human airway epithelial cell expansion in which cells are co-cultured with 3T3-J2 mouse embryonic fibroblasts in epithelial cell culture medium containing EGF, insulin and Y-27632, a RHO-associated protein kinase inhibitor (3T3+Y) [14-16]. In our previous reports, our choice of bronchial epithelial growth medium (BEGM)-based differentiation medium reflected our historical choice of expansion medium and while comparisons of ALI differentiation media have been performed for basal cells isolated and expanded in BEGM, PneumaCult-Ex and PneumaCult-Ex Plus [17], we are not aware of similar methodological comparisons for those isolated and expanded in 3T3+Y co-culture. Here, we describe our air-liquid interface culture protocol in detail and systematically investigate four alternative commercially available differentiation media using early passage human nasal epithelial cells.
2. Materials
2.1. Nasal airway brushing
Medium 199 (M199; Gibco, Thermo Fisher Scientific). Storage at 4°C.
100X penicillin/streptomycin (Gibco, Thermo Fisher Scientific). Storage at -20°C.
100X gentamicin (Gibco, Thermo Fisher Scientific). Storage at 4°C.
100X amphotericin B (Gibco, Thermo Fisher Scientific). Storage at -20°C.
Complete transport medium: 50 ml M199 containing 1X penicillin/streptomycin, 1X amphotericin B. Storage at 4°C (Note 1).
Cytosoft cytology brush (Medical Packaging Corporation).
2.2. 3T3-J2 feeder culture
Dulbecco’s modified Eagle’s medium (DMEM) with high glucose, L-glutamine and pyruvate (Gibco, Thermo Fisher Scientific). Storage at 4°C.
Bovine serum (Gibco, Thermo Fisher Scientific). Long-term storage at -80°C and short-term storage at -20°C.
100X penicillin/streptomycin (Gibco, Thermo Fisher Scientific). Storage at -20°C.
Sterile phosphate-buffered saline (PBS; Sigma-Aldrich). Storage at room temperature.
Mitomycin C (Sigma-Aldrich): 0.4 mg/ml in sterile PBS. Storage at -20°C.
0.05% Trypsin-EDTA (Sigma-Aldrich). Long-term storage at -20°C and short-term storage at 4°C.
Complete fibroblast growth medium – 500 ml DMEM plus 45 ml bovine serum and 1X penicillin/streptomycin. Storage at 4°C for up to a month.
2.3. Nasal epithelial basal cell culture in 3T3-J2 co-culture
Dulbecco’s modified Eagle’s medium (DMEM) with high glucose, L-glutamine and pyruvate (Gibco, Thermo Fisher Scientific). Storage at 4°C.
Ham’s F-12 nutrient mixture with L-glutamine (Gibco, Thermo Fisher Scientific). Storage at 4°C.
Fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific). Long-term storage at -80°C and short-term storage at -20°C.
100X Penicillin/streptomycin (Gibco, Thermo Fisher Scientific). Storage at -20°C.
100X Amphotericin B (Gibco, Thermo Fisher Scientific). Storage at -20°C.
100X Gentamicin (Gibco, Thermo Fisher Scientific). Storage at 4°C.
Insulin (Sigma-Aldrich). Storage at -20°C (Note 2).
Hydrocortisone (Sigma-Aldrich). Storage at -20°C (Note 3).
Recombinant human EGF (Thermo Fisher Scientific). Storage at -20°C (Note 3).
Cholera toxin (Sigma-Aldrich): 1 mg/ml (11.7 μM) in distilled water. Storage at 4°C.
Y-27632 (Cambridge Bioscience): 5 mM in distilled water. Storage at -20°C.
Complete epithelial cell culture medium: 373 ml 10% FBS DMEM (450 ml DMEM containing 50 ml FBS and 1X penicillin/streptomycin), 125 ml Ham’s F-12, 0.5 ml gentamicin, 5 μl amphotericin B, 0.5 ml insulin, 0.5 ml hydrocortisone/EGF, 0.5 ml Y- 27632, 4.3 μl cholera toxin. Storage at 4°C for up to 2 weeks.
PBS (Sigma-Aldrich). Storage at room temperature.
0.05% Trypsin-EDTA (Sigma-Aldrich). Long-term storage at -20°C and short-term storage at 4°C.
TrypLE Express (Thermo Fisher Scientific). Storage at room temperature.
Profreeze chemically defined freezing medium (2X; Lonza). Storage at 4°C.
2.4. Differentiation of nasal epithelial cells at air-liquid interface (ALI)
3-4 mg/ml rat tail collagen I (Corning).
12 mm diameter 0.4 μm pore polyester (PET) membrane transwell (Corning).
All-trans retinoic acid (Sigma-Aldrich). Storage at -80°C (Note 4).
100X penicillin/streptomycin (Gibco, Thermo Fisher Scientific). Storage at 4°C.
Bronchial epithelial basal growth medium with supplement kit (BEGM; Lonza; CC-3170, containing BEBM basal medium (CC-3171) and singleQuotsTM bullet kit (CC-4175)): Remove 40 ml of basal medium (Note 5) before adding supplement kit. Add all supplements except the vial containing gentamicin. Storage at 4°C for up to a month.
Complete ALI medium with BEGM: Mix 1:1 ratio of 25 ml DMEM : 25 ml BEGM and add 100 mM retinoic acid to a final concentration of 1 μM (Note 6). Filter sterilize and add 1X penicillin/streptomycin. Storage at 4°C for up to a week.
Airway epithelial cell growth medium with supplement kit (AECGM; PromoCell; C- 21160, containing AEC basal medium (C-21260) and AEC growth medium supplement pack (C-39160)): Remove 40 ml of basal medium before adding supplement kit. Add all supplement vials. Storage at 4°C for up to a month.
Complete ALI medium with AECGM: Mix 1:1 ratio of 25 ml DMEM : 25 ml AECGM and add 100 mM retinoic acid to a final concentration of 1 μM (Note 6). Filter sterilize and add 1X penicillin/streptomycin. Storage at 4°C for up to a week.
LHC-8 medium without gentamicin (LHC-8; Gibco, Thermo Fisher Scientific Scientific; 12679-015): Add 135 μl 10 mM epinephrine in 500 ml medium to make complete LHC-8. Storage at 4°C for up to a month.
Epinephrine (Sigma-Aldrich): 10 mM solution in sterile distilled water (dH2O). Filter sterilize and store at -20°C.
Complete ALI medium with LHC-8: Mix 1:1 ratio of 25 ml DMEM : 25 ml complete LHC- 8 and add 100 mM retinoic acid to a final concentration of 1 μM (Note 6). Filter sterilize and add 1X penicillin/streptomycin. Storage at 4°C for up to a week.
PneumaCult-ALI medium (STEMCELL Technologies): Follow the manufacturer’s instructions to prepare the basal medium. Long-term storage at -20°C and short-term storage at 4°C.
Hydrocortisone (Sigma-Aldrich): 0.5 mg/ml in 100% ethanol. Storage at -20°C.
Heparin (Sigma-Aldrich): 2 mg/ml solution in sterile dH2O. Long-term storage at -20°C and short-term storage at 4°C.
Complete ALI medium with PneumaCult (STEMCELL Technologies; 05001, containing PneumaCult-ALI Basal medium (05002), PneumaCult-ALI 10X supplement (05003), and PneumaCult-ALI 100X maintenance supplement (05006)): 49.4 mL complete PneumaCult ALI medium containing 500 μl 100X maintenance supplement, 100 μl of 2 mg/ml heparin sulphate and 48 μl 0.5 mg/ml hydrocortisone. Filter sterilize and add 1X penicillin/streptomycin. Storage at 4°C for up to a week.
All complete ALI media require protection from light; wrap falcon tubes with aluminium foil.
2.5. Immunofluorescence staining of ALI cultures
4% (w/v) paraformaldehyde in PBS. Heat to 65°C to dissolve and adjust pH to 7.2.
Blocking and permeabilizing solution: 3% bovine serum albumin (BSA; Sigma-Aldrich) and 0.01% Triton X-100 (Sigma-Aldrich) in PBS.
Primary antibodies: β-tubulin (rabbit, Abcam), MUC5AC (mouse, Thermo Fisher Scientific), Hoechst 33258 (Sigma-Aldrich).
Secondary antibodies: anti-mouse FITC-conjugated antibody (Abcam), anti-rabbit TRITC-conjugated antibody (Thermo Fisher Scientific).
Anti-fade mounting reagent: 3% N-propyl gallate (PG; Sigma-Aldrich) dissolved in 80% glycerol (Sigma-Aldrich) in PBS. Long-term storage at -20°C; short-term storage at 4°C for up to a week and protect from light by wrapping with aluminium foil.
2.6. Western blotting
RIPA (Merck, Sigma-Aldrich).
Protease inhibitor (cOmplete; Roche).
BCA protein assay kit (Thermo Fisher Scientific).
Laemmli 6X loading buffer (Alfa Aesar, Thermo Fisher Scientific).
4-15% gradient 15 well gel (Bio-Rad).
Running buffer (Tris-Glycine/SDS).
Wet transfer buffer (Tris-Glycine/Methanol).
Immuno-blot PVDF membrane (Bio-Rad).
Filter paper (Thermo Fisher Scientific).
1X Tris-buffered saline (TBS) with 0.1% (v/v) tween buffer (TBS-T).
Blocking buffer (5% (w/v) semi-skimmed milk powder in TBS-T).
Primary antibodies: TP63 (rabbit, Abcam), FOXJ1 (goat, Bio-Techne), DNAI2 (mouse, Bio-Techne), MUC5AC (mouse, Thermo Fisher Scientific), GAPDH (rabbit, Abcam).
Secondary antibodies: anti-mouse (Bio-Techne), anti-rabbit (Cell Signalling Technology), anti-goat (Bio-Techne).
ECL substrate (Bio-Rad).
2.7. Flow cytometry (FACS)
Accutase (Thermo Fisher Scientific).
Fc receptor blocker (BioLegend).
FACS buffer (10% BSA, 0.5 mM EDTA in PBS).
Conjugated antibodies: Integrin α6 (CD49f; PE-conjugated; BioLegend) and nerve growth factor receptor (NGFR/CD271; BV421-conjugated; BD Biosciences).
Viability dye (BioLegend).
3. Methods
3.1. Preparation of 3T3-J2 feeder layers
Feeder layers are prepared according to our previous protocol [3]. Briefly:
3T3-J2 mouse embryonic fibroblasts (below passage 12) are maintained in DMEM plus 9% bovine serum, 100 units/ml penicillin and 100 µg/ml streptomycin (Gibco, Thermo Fisher Scientific).
To prepare feeder layers, cells are inactivated by treatment with 0.4 µg/ml mitomycin C (Sigma-Aldrich) for 3 hours in fibroblast culture medium.
The inactivated cells are collected and seeded at a density of 20,000-30,000 cells/cm2 in fibroblast culture medium to generate feeder layers. Cells are allowed to spread overnight before epithelial cells are added.
3.2. Isolation and expansion of human nasal epithelial cells
3.2.1. Cell seeding from brushings
Nasal brush biopsies are collected from healthy donors and patients with chronic obstructive pulmonary disease (COPD) and primary ciliary dyskinesia (PCD) with informed consent (REC reference 14/NW/0128).
Brushed samples are vigorously agitated manually in 5 ml tubes containing 2 ml transport medium.
Upon arrival at the laboratory, biopsies can be optionally stored overnight at 4°C before processing.
The following day, samples are centrifuged at 1500 rpm for 5 minutes to generate cell pellets.
Cell pellets are resuspended in epithelial cell culture medium by vigorous pipetting to further release cells.
Cell suspensions are seeded onto pre-prepared feeder layers in T25 flasks and incubated at 37°C in incubators with 5% CO2.
Cells are fed with fresh epithelial cell culture medium three times per week.
3.2.2. Maintenance of human nasal basal epithelial cells
Epithelial cell culture is performed according to our previous protocol [3]. Briefly:
Cultures are passaged when epithelial cells reach 80-90% confluence.
Cell culture medium is removed and the cells are washed once with PBS, then 5 ml 0.05% trypsin-EDTA is added for 2 minutes in 37°C incubators. Flasks are removed from incubators and gently tapped to encourage the detachment of feeder cells. Light microscopy confirms the removal of feeder cells, which are substantially more trypsin-sensitive than the epithelial cells.
Trypsin is removed and the cells are washed with PBS to remove any remaining feeder cells.
Cells are incubated with 5 ml TrypLE Express at 37°C until epithelial cells detach from the flask (Note 7).
TrypLE Express solution is neutralized with the same volume of epithelial cell culture medium, then the cell suspension is centrifuged at 1500 rpm for 5 minutes before the pellet is resuspended in fresh epithelial cell culture medium.
For subsequent passages, 5 x 105 epithelial cells are seeded into new T75 flasks containing fresh, inactivated feeder cells.
3.3. Air-liquid interface culture of human nasal epithelial cells
3.3.1. Preparation of collagen-coated transwell inserts
Prepare 1% collagen I in PBS.
250 μl 1% collagen solution is added into each transwell insert and incubated for 1 hour at room temperature in sterile conditions (Note 8).
Collagen solution is gently removed and each well rinsed with 400 μl sterile dH2O.
Inserts are air dried for 15-25 minutes at room temperature in sterile conditions.
Plates can then be sealed using autoclave tape and kept sterile for future use.
3.3.2. Differentiation of nasal epithelial cells at air-liquid interface (ALI)
Following detachment of basal cells from co-cultures (Section 3.2.2), 1.0 x 106 cells / 12 mm well are seeded in 400 μl epithelial culture medium per pre-coated transwell insert and the underlying well is filled with 1 ml epithelial culture medium.
Plates are incubated at 37°C in incubators with 5% CO2 for 24-48 hours until confluent.
For differentiation culture at air-liquid interface, the medium in the apical transwell chamber is removed and the medium in the underlying well is replaced with 700 μl ALI medium. Plates are then returned to 37°C incubators with 5% CO2 (Note 9).
Medium in the wells underlying each transwell is refreshed three times per week with 700 μl fresh ALI medium.
The apical surface of air-exposed cells is gently rinsed with PBS every 7 days for up to 28 days of ALI culture.
Ciliation can generally be observed between 14-21 days after air exposure and, although cultures can be maintained for much longer, we typically terminate ALI experiments after 28 days of ALI culture.
3.4. Transepithelial electrical resistance (TEER) measurements
In the data shown, measurements of transepithelial electrical resistance (TEER) were obtained after 28 days of culture at an air-liquid interface. Three consecutive readings from two independent wells for each donor and differentiation medium are plotted (i.e. n=6 readings from an individual donor per differentiation medium).
To take TEER measurements, culture medium is removed and 200 µl (apical) and 1 ml (basal) sterile PBS is added to the cultures.
Measurements are performed using an EVOM2 resistance meter and Endohm chamber of the appropriate size for 12 mm culture inserts (World Precision Instruments, U.S.A.).
Three consecutive readings in ohms are taken for each well after allowing values to stabilise for 5-10 seconds.
3.5. Ciliary beat frequency analysis
In the data shown, measurements of ciliary beat frequency were obtained after 28 days of culture at an air-liquid interface.
Cultures are rinsed with 200 µl sterile PBS and maintained at 37°C with 5% CO2 supply for a minimum 2 hours prior to video recording.
We use a Nikon Eclipse Ti-U microscope (Nikon, Japan) with a 20x objective attached to a high-speed video camera (PROMON 501; AOS Technologies AG, Switzerland) and take videos at a frame rate of 248 fps for a region of interest 736 x 736 pixels in size.
We calculate ciliary beat frequency (CBF) using CiliaFA [18], presenting the results in Hz.
3.6. Immunofluorescence
In the data shown, immunofluorescence was performed on cells after 28 days of culture at an air-liquid interface.
Cells are fixed within transwell inserts using 4% paraformaldehyde (Sigma-Aldrich) for 30 minutes at room temperature and stored at 4°C in PBS (apical and basal) until staining.
Cells are permeabilized in PBS containing 3% BSA (Sigma-Aldrich) and 0.1% Triton X- 100 (Sigma-Aldrich) for 30 minutes at room temperature. For this and all remaining staining steps, only the apical side of transwells are treated.
Antibodies (in this case, β-tubulin (1:100 dilution; rabbit anti-human; Abcam) and MUC5AC (1:100 dilution; mouse anti-human; Thermo Fisher Scientific)) are diluted in cold 1% BSA containing 0.1% Triton X-100. Cells are incubated overnight at 4°C.
Cells are rinsed twice with PBS and then secondary antibodies are added to the apical of transwells (in this case, FITC (1:64 dilution; goat anti-mouse; Sigma-Aldrich) and Alexa Fluor 594 (1:250 dilution; donkey anti-rabbit; Thermo Fisher Scientific)) in 1% BSA containing 0.1% Triton X-100. Cells are incubated for 2 hours at room temperature.
In the data shown here, cells are then incubated with the DNA fluorescent stain bisbenzimide H 33258 (Sigma-Aldrich) for 30 minutes.
Cells are washed with PBS twice, then cut the membrane from the transwell using a small scalpel such that the top and bottom surfaces of membrane are known. The membranes are rinsed with sterile H2O and a drop of mounting (N-propyl gallate-based anti-fade solution) solution put onto the upside of the membrane on microscope slides.
Images are then acquired by confocal microscopy. We use a Zeiss LSM710 confocal microscope.
3.7. Western blotting
After 28 days of culture at an air-liquid interface, 200 μl sterile PBS is added to the apical side of transwells and cells are scraped from the transwells using a small spatula. Cells are collected in tubes and centrifuged at 9000 rpm for 3 minutes. Supernatants are removed and the cell pellets can be stored at -20°C.
Total protein is extracted by addition of 30 μl RIPA buffer containing protease inhibitors. Cells are suspended by pipetting and incubated on the ice for 30 minutes for lysis. Lysates are sonicated for 3 minutes, then centrifuged at 11,000 rpm for 10 minutes. Supernatants are transferred to a new tube.
Total protein concentrations are determined using a bicinchoninic acid (BCA) assay following the manufacturer’s instructions. Typically, between 2-9 μg/μl of total protein is extracted from one transwell.
8 µg of total protein is loaded per lane in a 4-15% gradient gel. Gels are initially run at 100 V, then increased to 150 V after 15 minutes.
Once the gel has run, load it onto a stack and transfer for an hour at 100V.
After transferring the proteins onto polyvinylidene fluoride or polyvinylidene difluoride (PVDF), the membranes are blocked with 5% milk in TBS-T for 1 hour at room temperature.
The PVDF membranes are cut into pieces corresponding to the expected protein size (as determined from data sheets), then incubated with 5 ml primary antibody solution in 50 ml tubes overnight at 4°C on a roller.
After overnight incubation, the PVDF membranes are washed with TBS-T three times, then further incubated with 13 ml of HRP-conjugated secondary antibody solution in a 50 ml falcon tube for 1 hour at room temperature.
The PVDF membranes are washed with TBS-T three times and developed with ECL substrates.
PVDF membranes are visualized using ChemiDoc™ MP (Bio-Rad).
For quantification, the densities of the bands associated with the protein of interest are normalized with GAPDH in each lane by Image Lab version 6.0.1 (Bio-Rad).
3.8. Flow cytometry
After 28 days of ALI culture, the apical sides of cultures are washed with PBS.
400 μl and 700 μl accutase (Thermo Fisher Scientific) is added to the apical and basal sides of cultures, respectively, and incubated at 37°C for 15 minutes.
5 ml DMEM containing 10% FBS is added to the cell suspension and then cells are pelleted by centrifugation at 1500 rpm for 5 minutes.
Cell pellets are resuspended in a FACS buffer containing Fc receptor blocker for 5 minutes on ice.
Cells are re-centrifuged at 6000 rpm for 3 minutes and re-suspended in fresh FACS buffer containing antibodies for 20 minutes on ice. In the data shown, we used anti-integrin α6 (CD49f-PE; BioLegend) and anti-nerve growth factor receptor (NGFR/CD271-BV421; BD Biosciences).
Cells are centrifuged at 6000 rpm for 3 minutes and the cell pellets resuspended in PBS containing viability dye (BioLegend) for 10 minutes on ice.
After removal of the viability dye by centrifugation at 6000 rpm for 3 minutes, the cells are washed once in FACS buffer. Cells are then resuspended in FACS buffer for analysis.
Cells are run on a BD LSR II flow cytometer (BD Biosciences) and the results analysed using FlowJo 10 (FlowJo LLC).
4. Comparison of commercially available medium
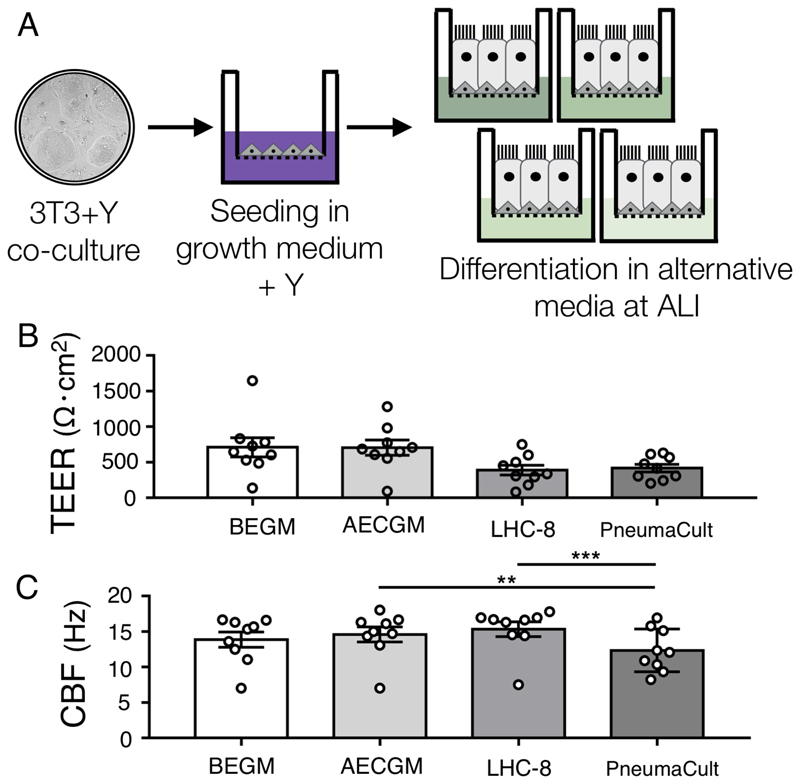
To assess the effect of alternative differentiation media on ALI culture using the method described here, we cultured early passage (P2) human airway nasal epithelial cells, which had been isolated and expanded in 3T3-J2 co-culture in medium containing Y-27632 [19], in four commercially alternative differentiation media at ALI (Figure 1A). In all of the conditions, transepithelial electrical resistance (TEER) values were consistent with the formation of intact epithelia (Figure 1B) and although values were lower in the LHC-8 and PneumaCult conditions, these differences did not reach statistical significance (Figure 1B). Analysis of ciliary beat frequency showed that, for healthy donors and those with COPD, cilia generated in all media conditions had a beat frequency within the expected normal range, while beat frequency in a culture from a donor with PCD, who has a known ciliary defect, was consistently lower (Figure 1C). Despite being within biologically normal range, overall PneumaCult cultures had a statistically lower beat frequency than either AECGM (p < 0.01) or LHC-8 cultures (p < 0.001).
Figure 1. Differentiation of nasal epithelial cells in commercially available differentiation media at air-liquid interface.
(A) Schematic diagram of cell culture methodology. (B) Trans-epithelial electrical resistance (TEER) measurements at day 28 of air-liquid interface (ALI) culture. Values are a mean of three consecutive readings from two wells per donor (n = 9 donors: 6 healthy, 2 with chronic obstructive pulmonary disease (COPD), 1 with primary ciliary dyskinesia (PCD)). There were no significant differences in a one-way ANOVA with Holm-Sidak’s test for multiple comparisons (p > 0.1 for all comparisons). (C) Ciliary beat frequency (CBF) was determined after 28 days of ALI culture. Recordings were from five different regions of interests (ROIs) from two wells per donor (n = 9 donors: 6 healthy, 2 with COPD, 1 with PCD). All data are presented as mean ± SEM; ** indicates p < 0.01 and *** indicates p < 0.001 in a one-way ANOVA with Holm-Sidak’s test for multiple comparisons.
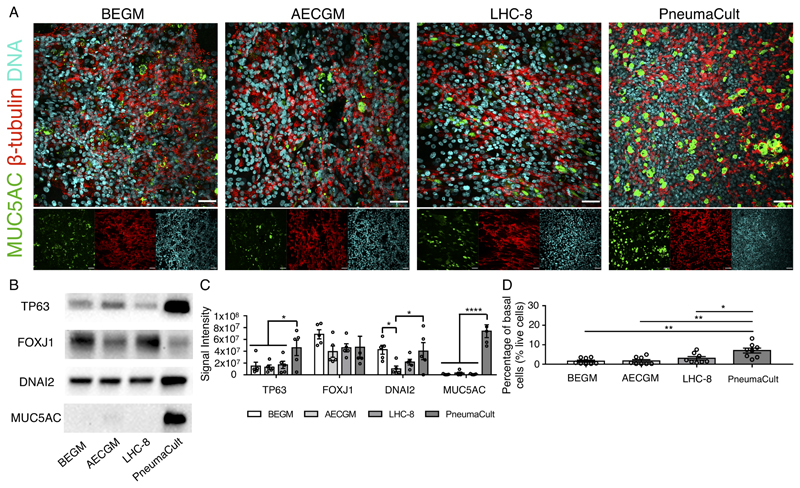
Immunofluorescence analysis using antibodies against MUC5AC (a component of mucus) and β-tubulin (present in cilia) showed that cultures in all conditions were ciliated and contained mucosecretory cells (Figure 2A), although PneumaCult cultures appeared to contain more mucosecretory cells (Figure 2A). Western blot analyses confirmed the expression of ciliated cell-associated proteins FOXJ1 and DNAI2 and similarly suggested that MUC5AC expression was significantly upregulated in PneumaCult cultures compared with the other media tested (p < 0.0001; Figure 2B/2C). We speculate that the greater production of mucus in these cultures might explain the slightly lower CBF observed in PneumaCult cultures (Figure 1C). Interestingly, our western blots also suggested that the abundance of TP63 – a basal cell transcription factor – was higher in PneumaCult ALI cultures (p < 0.05; Figure 2B/2C). To assess whether this reflected changes in expression or higher basal cell abundance, we performed flow cytometry on independent cultures and found that a greater proportion of live cells were ITGA6+/NGFR+ basal cells in PneumaCult cultures than in BEGM (p < 0.01; Figure 2D), AECGM (p < 0.01; Figure 2D) or LHC-8 (p < 0.05; Figure 2D) cultures.
Figure 2. Characterization of epithelial composition in nasal epithelial air-liquid interface cultures generated in commercially available differentiation media.
(A) Immunofluorescence staining for β-tubulin (multiciliated cells; red) and MUC5AC (mucosecretory cells; green) in nasal epithelial air-liquid interface cultures. Nuclei (Hoechst 33258 staining) are shown in cyan. One representative donor is shown (n = 4 donors: 3 healthy, 1 with chronic obstructive pulmonary disease (COPD)). Scale bar = 150 μm. (B) Western blot analysis of cell type-specific marker expression in ALI cultures (TP63 = basal cells; FOXJ1 and DNAI2 = multiciliated cells; MUC5AC = mucosecretory cells) cultured using four ALI differentiation media. 8 µg of total protein extracted was used. One representative image is shown (n = 5 donors: 4 healthy, 1 with primary ciliary dyskinesia (PCD)). (C) Quantification of western blot data. All blots were normalised to a GAPDH loading control. * indicates p < 0.05 and **** indicates p < 0.0001 in a two-way ANOVA with Holm-Sidak’s test for multiple comparisons. (D) Quantification of basal cells at day 28 of air-liquid interface (ALI) culture using flow cytometry. The proportion of live cells that expressed basal cell-associated ITGA6 and NGFR was greater in PneumaCult than in alternative media (n = 8 donors: 5 healthy, 2 with COPD, 1 with PCD). All data are presented as mean ± SEM. * indicates p < 0.05 and ** indicates p < 0.01 in a one-way ANOVA with Holm-Sidak’s test for multiple comparisons.
Overall, we find that all four of the commercially available differentiation media tested supported ciliated differentiation of nasal basal cells derived from 3T3-J2 co-culture in ALI culture. We saw only small differences in TEER and ciliary beat frequency but more pronounced medium-specific changes in mucosecretory differentiation and the retention of basal cells. Importantly for downstream experiments, the detailed medium composition is only available for one of four media that we compared in our method (Table 1) which might guide medium choice for specific applications. Indeed, it presents a fundamental question: how should the results of ALI culture experiments be presented and interpreted? Statements in papers that the constituents of the cell culture media used for the experiments performed are unknown mean that conclusions have to be interpreted with caution; for example, the presence of steroids or catecholamines may have a fundamental effect on results relating to inflammation or bacterial growth, respectively [20].
Table 1.
Comparison of media composition. Lechner and LaVeck refers to the composition of LHC-9 medium published by those authors [21]. The difference between LHC-8 and LHC-9 is the presence of retinoic acid and epinephrine in the latter (Thermo Fisher Scientific). * In our experiments 100 nM retinoic acid was added to any already present for differentiation media; r = recombinant, h = human.
| Growth | Differentiation | Growth | Differentiation | Lechner & LaVeck (LHC-9) | Growth | Differentiation | Differentiation |
|---|---|---|---|---|---|---|---|
| ? | ? | 0.4% | 0.2% | 0.5% | ? | ? | |
| ? | ? | 0.5 μg /ml | 0.25 μg/ml | 0.5 μg /ml | 2.7 μMa | 1.35 μMa | ? |
| ? | ? | 0.5 μg /ml | 0.25 μg/ml | 0.72 μg/ml | ? | ? | 0.48 μg/ml |
| ?rh | ?rh | 10 ng/mlrh | 5 ng/mlrh | 5 ng/ml | ? | ? | ? |
| ?h | ?h | 10 μg/mlrh | 5 μg/mlrh | 10 μg/ml | ? | ? | ? |
| ?rh | ?rh | 5 μg/ml rh | 2.5 μg/mlrh | 5 μg/ml | ? | ? | ? |
| ? | ? | 6.7 ng/ml | 3.35 ng/ml | 6.5 ng/ml | ? | ? | ? |
| ? | ? + 100 nMa | 0.1 ng/ml | 100.17 nMa | 0.33 nM | 100 nMa | ? | |
| ? | ? | 50 μg/ml | ? | ||||
| ? | ? | ? | ? | ? | |||
| ? | ? | 6mM | 5 mM | ? | ? | ? | ? |
| ? | ? | ? | ? | 0.5 μM | ? | ? | ? |
| ? | ? | ? | ? | 0.5 μM | ? | ? | ? |
| ? | ? | 4 μg /ml | |||||
| ? | ? | ? | ? | ? |
 Not present
Not present
 Present
Present
 Unknown
Unknown
Acknowledgements
We thank Dr. Gabriel Gata (Lonza), Lisa Schmidtke (PromoCell), Dr. Frankie Vanterpool (Thermo Fisher Scientific Scientific) and Dr. Angela Zhang (STEMCELL Technologies) for their feedback on this chapter and for assistance with establishing media composition. We are grateful to Prof. Sam Janes (University College London, U.K.) and Prof. Fiona Watt (Kings College London, U.K.) for providing the 3T3-J2 mouse embryonic fibroblasts used in our study. We also thank Dr. Kate Gowers (University College London, U.K.) and Dr. Kyren Lazarus (University College London, U.K.) for proof-reading the manuscript.
This work was supported by the NIHR GOSH BRC and the Living Airway Biobank (C.O.). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. R.E.H. is supported by a Sir Henry Wellcome Postdoctoral Fellowship (Wellcome Trust; WT209199/Z/17/Z).
Notes
- We typically also include 1X gentamicin as a precaution for samples where we judge the contamination risk to be high, e.g. if the patient has an ongoing infection.
- Dissolve 100 mg insulin in 20 ml dH2O. Gradually add glacial acetic acid until clearly dissolved. Sterilize using 0.2 μm syringe filters; aliquot 550 μl in Eppendorf tubes and store at -20°C.
- Dissolve hydrocortisone in 100% ethanol at 0.5 mg/ml. Mix 1 ml of this solution with 19 ml DMEM containing 2.5 μg EGF. Sterilize using 0.2 μm syringe filters; aliquot 550 μl in Eppendorf tubes to store at -20°C.
- For retinoic acid preparation, resuspend 100 mg dry powder in 3.33 ml of 100% ethanol (100 mM). Given the light sensitivity of retinoic acid, work with hood lights off, aliquot 100 μl into amber tubes and store at -80°C.
- We retain some basal medium for use in downstream experiments and for washing of the apical surface of cultures.
- For the addition of retinoic acid in ALI medium, mix 5 μl 100 mM retinoic acid into 500 μl 1:1 basal cell epithelial medium:DMEM, then add 5 μl of this mixture to 50 ml ALI medium.
- Complete detachment of basal cells usually occurs within 5-10 minutes.
- To maintain the sterile condition of transwells, take care not spill the collagen solution into any part of the well except the well surface.
- High humidity should be maintained in the incubators for ALI cultures by the inclusion of additional water reservoirs. Further, minimal opening of incubator doors is recommended to maintain the conditions within the incubators. We find that this supports differentiation and reduces contamination of cultures.
References
- [1].Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol. 2013;945:109–21. doi: 10.1007/978-1-62703-125-7_8. [DOI] [PubMed] [Google Scholar]
- [2].Danahay H, Pessotti AD, Coote J, Montgomery BE, Xia D, Wilson A, Yang H, Wang Z, Bevan L, Thomas C, Petit S, et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep. 2015;10(2):239–52. doi: 10.1016/j.celrep.2014.12.017. [DOI] [PubMed] [Google Scholar]
- [3].Hynds RE, Butler CR, Janes SM, Giangreco A. Expansion of Human Airway Basal Stem Cells and Their Differentiation as 3D Tracheospheres. Methods Mol Biol. 2016 doi: 10.1007/7651_2016_5. [DOI] [PubMed] [Google Scholar]
- [4].Plasschaert LW, Zilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, Klein AM, Jaffe AB. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018 doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Garcia SR, Deprez M, Lebrigand K, Paquet A, Cavard A, Arguel M-J, Magnone V, Caballero I, Leroy S, Marquette C-H, Marcet B, et al. Single-cell RNA sequencing reveals novel cell differentiation dynamics during human airway epithelium regeneration. bioRxiv. 2018 451807. [Google Scholar]
- [6].Amatngalim GD, Schrumpf JA, Dishchekenian F, Mertens TCJ, Ninaber DK, van der Linden AC, Pilette C, Taube C, Hiemstra PS, van der Does AM. Aberrant epithelial differentiation by cigarette smoke dysregulates respiratory host defence. Eur Respir J. 2018;51(4) doi: 10.1183/13993003.01009-2017. [DOI] [PubMed] [Google Scholar]
- [7].Li X. In vitro toxicity testing of cigarette smoke based on the air-liquid interface exposure: A review. Toxicol In Vitro. 2016;36:105–113. doi: 10.1016/j.tiv.2016.07.019. [DOI] [PubMed] [Google Scholar]
- [8].Smith CM, Kulkarni H, Radhakrishnan P, Rutman A, Bankart MJ, Williams G, Hirst RA, Easton AJ, Andrew PW, O’Callaghan C. Ciliary dyskinesia is an early feature of respiratory syncytial virus infection. Eur Respir J. 2014;43(2):485–96. doi: 10.1183/09031936.00205312. [DOI] [PubMed] [Google Scholar]
- [9].Vries M, Bedke N, Smithers NP, Loxham M, Howarth PH, Nawijn MC, Davies DE. Inhibition of Pim1 kinase, new therapeutic approach in virus-induced asthma exacerbations. Eur Respir J. 2016;47(3):783–91. doi: 10.1183/13993003.00171-2015. [DOI] [PubMed] [Google Scholar]
- [10].Faiz A, Weckmann M, Tasena H, Vermeulen CJ, Van den Berge M, Ten Hacken NHT, Halayko AJ, Ward JPT, Lee TH, Tjin G, Black JL, et al. Profiling of healthy and asthmatic airway smooth muscle cells following interleukin-1beta treatment: a novel role for CCL20 in chronic mucus hypersecretion. Eur Respir J. 2018;52(2) doi: 10.1183/13993003.00310-2018. [DOI] [PubMed] [Google Scholar]
- [11].Mertens TCJ, Karmouty-Quintana H, Taube C, Hiemstra PS. Use of airway epithelial cell culture to unravel the pathogenesis and study treatment in obstructive airway diseases. Pulm Pharmacol Ther. 2017;45:101–113. doi: 10.1016/j.pupt.2017.05.008. [DOI] [PubMed] [Google Scholar]
- [12].de Courcey F, Zholos AV, Atherton-Watson H, Williams MT, Canning P, Danahay HL, Elborn JS, Ennis M. Development of primary human nasal epithelial cell cultures for the study of cystic fibrosis pathophysiology. Am J Physiol Cell Physiol. 2012;303(11):C1173–9. doi: 10.1152/ajpcell.00384.2011. [DOI] [PubMed] [Google Scholar]
- [13].Brewington JJ, Filbrandt ET, LaRosa FJ. Brushed nasal epithelial cells are a surrogate for bronchial epithelial CFTR studies. Moncivaiz JD, Ostmann AJ, Strecker LM, Clancy JP, editors. JCI Insight. (3rd) 2018;3(13) doi: 10.1172/jci.insight.99385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Butler CR, Hynds RE, Gowers KH, Lee Ddo H, Brown JM, Crowley C, Teixeira VH, Smith CM, Urbani L, Hamilton NJ, et al. Rapid Expansion of Human Epithelial Stem Cells Suitable for Airway Tissue Engineering. Am J Respir Crit Care Med. 2016;194(2):156–68. doi: 10.1164/rccm.201507-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, Haddad BR, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180(2):599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Reynolds SD, Rios C, Wesolowska-Andersen A, Zhuang Y, Pinter M, Happoldt C, Hill CL, Lallier SW, Cosgrove GP, Solomon GM, Nichols DP, et al. Airway Progenitor Clone Formation Is Enhanced by Y-27632-Dependent Changes in the Transcriptome. Am J Respir Cell Mol Biol. 2016;55(3):323–36. doi: 10.1165/rcmb.2015-0274MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rayner RE, Makena P, Prasad GL, Cormet-Boyaka E. Optimization of Normal Human Bronchial Epithelial (NHBE) Cell 3D Cultures for in vitro Lung Model Studies. Sci Rep. 2019;9(1):500. doi: 10.1038/s41598-018-36735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Smith CM, Djakow J, Free RC, Djakow P, Lonnen R, Williams G, Pohunek P, Hirst RA, Easton AJ, Andrew PW, O’Callaghan C. ciliaFA: a research tool for automated, high-throughput measurement of ciliary beat frequency using freely available software. Cilia. 2012;1:14. doi: 10.1186/2046-2530-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gowers KHC, Hynds RE, Thakrar RM, Carroll B, Birchall MA, Janes SM. Optimized isolation and expansion of human airway epithelial basal cells from endobronchial biopsy samples. J Tissue Eng Regen Med. 2018;12(1):e313–e317. doi: 10.1002/term.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Freestone PP, Hirst RA, Sandrini SM, Sharaff F, Fry H, Hyman S, O’Callaghan C. Pseudomonas aeruginosa-catecholamine inotrope interactions: a contributory factor in the development of ventilator-associated pneumonia? Chest. 2012;142(5):1200–1210. doi: 10.1378/chest.11-2614. [DOI] [PubMed] [Google Scholar]
- [21].Lechner JF, LaVeck M. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. Journal of Tissue Culture Methods. 1985;9(2):43–48. [Google Scholar]