Abstract
Several shortcomings with currently available pharmacotherapy of epilepsy necessitate the search for new drug targets. Paroxysmal depolarization shifts (PDS) represent the cellular correlates of electrographic (e.g. interictal) spikes. While the ionic basis of PDS is understood in great detail, controversy exists regarding their proposed implication in epilepsy. To address this issue and to consider potential targetability, this mini-review gives an overview of the ionic conductances contributing to PDS and reflects on the hypotheses of their potential pro-epileptic (epileptogenic) and anti-epileptic roles.
Keywords: L-type voltage-gated calcium channels, hippocampal neurons, epileptogenesis, interictal spike
1. Introduction
Epilepsy is an umbrella term for a variety of syndromes whose unifying feature is the unpredictable recurrence of synchronous abnormal discharge activities in central neurons, which are known as seizures. While seizures can be successfully controlled in the majority of epilepsy patients with anti-epileptic drugs (AEDs), there are several shortcomings with currently available therapy (Schmidt and Schachter, 2014). First, seizures remain resistant to AEDs in about one third of the patients. Secondly, AEDs do not provide a cure of the epileptic brain condition and seizures often recur after drug discontinuation. Finally, since a significant proportion of epileptic seizures is not due to genetic abnormalities but results from brain insults such as stroke or trauma, people at risk can be identified. However, to date no drug is available for their prophylactic treatment. Hence, there is an unmet need for basic research, in particular in the field of epileptogenesis. As will be explained below, an electrographic spike termed “interictal”, because of its initial description in seizure-free periods of epilepsy patients, has come into the limelight of the investigations (Staley et al., 2005). However, the interictal spike represents only an EEG phenomenon, and its potential role in epilepsy must be considered in terms of its neuronal correlate, the paroxysmal depolarization shift (PDS).
2. What is a paroxysmal depolarization shift (PDS)?
Paroxysmal depolarization shifts (PDS) were originally identified in the 1960ties in cat cortex as spontaneously occurring abnormal electrical discharges upon application of penicillin (Matsumoto and Ajmone Marsan, 1964; Prince, 1968) (Fig. 1A). Notably, they were not evoked by electrical stimulation via the intracellular recording electrode under the same conditions (Prince, 1968). The PDS was described as a positive shift of membrane voltage “up to 30 mV or occasionally more” with “durations from 40 up to 400 ms or more”, during which “spike generation would progressively decrease in amplitude until only small oscillations remained riding on top of the PDS” (Matsumoto and Ajmone Marsan, 1964). Because penicillin is now known to act as an inhibitor of GABAA receptors at the high doses that were used in these early studies (Antoniadis et al., 1980), the induction of PDS can be ascribed to inhibition of fast GABAergic inhibition (“disinhibition”). In support of this idea, PDS similar to those evoked by penicillin were later recorded upon application of GABAA receptor antagonists, i.e. picrotoxin, pentylenetetrazol and bicuculline (Hablitz, 1984; Bingmann et al., 1988; Straub et al., 1990; Schiller, 2002) (Fig. 1B). Since then, the term PDS has been applied to a various different abnormal discharge patterns including epileptic bursts, segments of seizure-like activity and post-ictal discharges (Silva-Barrat et al., 2001; Sun et al., 2001; Langlois et al., 2010; Dreier et al., 2011). Moreover, PDS-like events were also identified in hippocampal neurons of two epileptic rat models, either together with ictal discharges or prior to the occurrence of the first spontaneous seizures (Momiyama et al., 1995; Hanaya et al., 2002).
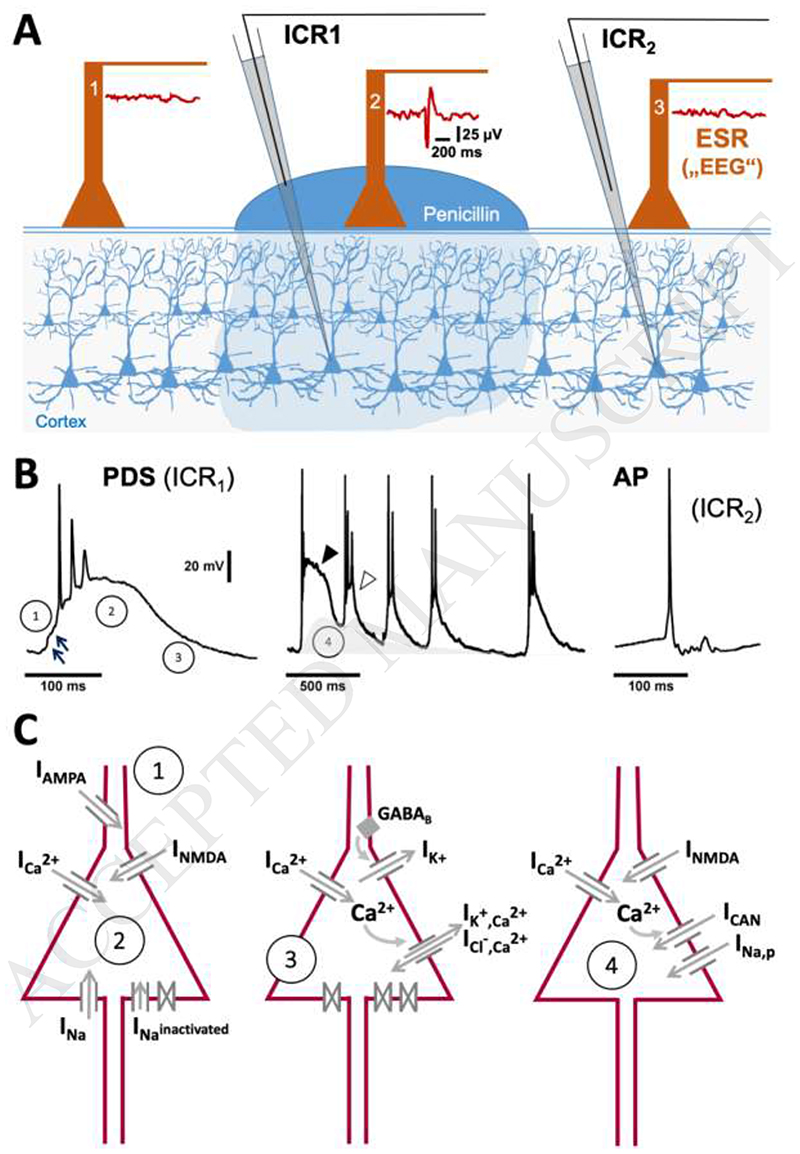
Fig. 1. First identification, typical appearance and ionic basis of paroxysmal depolarization shifts.
A) Matsumoto and Ajmone Marsan (1964) performed simultaneous intracellular (ICR) and extracellular surface recordings (ESR) from cat cortex, on which epileptic foci were generated by topical application of penicillin. Both ictal and interictal manifestations were observed. The illustration shows the situation for interictal manifestations. The extracellular surface electrode (ESR) within the penicillin focus picked up electrographic spikes (an example is shown to the right of ESR electrode 2) at the same time as PDS were detected in subjacent neurons (this is exemplified for a recording with ICR1 in panel B, left trace). Hence, it was concluded that PDS represent the (single-unit) cellular correlate of electrographic (“interictal”) spikes, which result from the synchronous occurrence of PDS in many neurons of the epileptic focus. PDS are distinctly different from normal action potentials (APs) that can be monitored in untreated cortex areas (e.g. with ICR2, see the right trace in panel B). B The exemplary traces highlight the differences between PDS and APs and show that PDS can occur in clusters (middle trace). The circled numbers refer to the schemes in C, which illustrate the ionic currents (I) that contribute to individual PDS formation (1-3), and to the slow depolarizing wave enabling PDS clusters (4).
However, in this mini-review we will mainly deal with PDS as originally identified in the work by Matsumoto and Ajmone Marsan (1964) and Prince (1968), in which the occurrence of PDS was indeed shown to be accompanied by individual electrographic events of similar duration (e.g. “interictal spikes”, IIS). PDS-like events that were seen during certain types of seizures are only briefly mentioned, because the ictal occurrence may come with substantial changes in the ionic basis of these events (e.g. inactivation of contributing ion channels, see chapter 3), thereby potentially also altering their pathophysiological role.
3. Mechanisms of PDS formation and ion conductances underlying PDS
In addition to GABAA receptor inhibition, PDS were evoked either by modulation of synaptic transmission (e.g. “relief from kynurenate + high Mg2+ model” (Segal, 1990) or by modulation of intrinsic neuronal properties such as calcium homeostasis (caffeine model, Moraidis et al., 1991). Both mechanisms are likely interdependent, as the enhanced intrinsic conductances may only be evoked by synaptic input, whereas enhanced synaptic input may activate abnormal levels of per se unaltered voltage-dependent conductances. PDS are usually studied in neuronal networks, however PDS could also be elicited in microcultures containing only one autaptic excitatory hippocampal neuron (Segal, 1991). In our own work, we found that L-type voltage-gated calcium channel (LTCC)-mediated Ca2+ influx, evidently a single-cell property, is crucial for PDS formation (Rubi et al., 2013). Earlier work by Speckmann et al. (1990) had shown that PDS elicited in-vivo in the rat motor cortex were abolished by intracellular application of the verapamil (an LTCC-antagonist)-derivative D890, and promoted by application of the LTCC potentiator Bay K8644. Under neuropathological conditions, reactive oxygen species may act to abnormally enhance LTCC activity (Akaishi et al., 2004). Potentiated LTCCs, and in particular the Cav1.3 isoform, are required for PDS formation and for its distinct voltage trajectory as was confirmed recently in our lab using genetically modified mice (Stiglbauer et al., 2017). This study also provided evidence arguing against a repeatedly proposed, but never experimentally proven, contribution of Ca2+-dependent nonspecific cation (CAN) channels. In addition to Ca2+ influx carried by LTCCs, there is also evidence that N-methyl-D-aspartate (NMDA)-receptor mediated cation current contributes to PDS (see for example Hwa and Avoli, 1991). However, it is difficult to determine the relative contribution of NMDA- and LTCC-mediated currents, since functional interplay was demonstrated between these two channel classes in neurons, e.g. NMDA receptors providing a critical excitatory component that may ultimately activate LTCCs (Fossat et al., 2007).
In brief, the following picture can be drawn regarding the ionic basis of PDS discharge and its formation (illustrated in Fig. 1C): the PDS emerges from a network-driven giant EPSP mediated by an a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated fast EPSP (arrows in phase 1, Fig.1B). Its depolarizing plateau is mediated by NMDA receptor channel current and current through LTCCs, in particular Cav1.3 channels (phase 2) The action potentials are due to the activation of voltage-gated sodium channels, and the amplitude decline and/or firing cessation is likely due to progressive inactivation of these channels during the continuous depolarization. Of note, when PDS occur in quick succession (Fig. 1B, middle trace), then the depolarizing plateau is transiently reduced (marked by the closed and the open arrowhead), indicating that the channels contributing to the depolarized plateau are also inactivated or desensitized. The termination of PDS may involve different contributions, depending on the expression pattern of various ion channels in the particular type of neuron, for example Ca2+-dependent (e.g. apamin sensitive-) K+ channels, ionotropic GABAA receptors as well as metabotropic GABAB receptor-regulated channels (Witte, 2000). Persistent sodium current (INa,p) and T-type voltage-gated calcium channels (TTCCs, Cav3.* family), two conductances that were shown to contribute to epileptic burst discharges (Chen et al., 2011), do not seem to represent essential components of PDS. Our work using riluzole at low micromolar concentrations, which are known to block INa,p, provided evidence that INa,p is not involved in PDS of hippocampal neurons, although it may play a role – together with current via CAN channels (Schiller, 2004) in triggering runs of PDS and melting of PDS into ictal-like discharges (Stiglbauer et al., 2017) (Fig. 1B, C, phase 4). Moreover, our observation that PDS could also be induced in less polarized hippocampal neurons (e.g. Vm of about -55 to 50 mV) argues against the involvement of TTCCs in PDS. This observation has also been made by others, e.g. in intact brain (de Curtis et al., 1999). Hence, it is highly unlikely that TTCCs, which should be largely inactivated at this potential, can play a significant role in PDS.
4. Role of PDS in epilepsy and epileptogenesis
The PDS can occur both as an individual event as well as in clusters of quick succession (Matsumoto and Ajmone Marsan, 1964; Prince, 1968; Hablitz, 1984; Schiller, 2002; Schiller, 2004). This is similar to interictal spikes, for which sporadic occurrence or the occurrence in brief paroxysmal bursts has been shown in animal models of epilepsy (Zhou et al., 2007). Because of their correlation with epilepsy, interictal spikes have long been used diagnostically, but there are fundamental controversies regarding their actual implication for epileptogenesis and/or epilepsy (Staley et al., 2005), ranging from being asymptomatic and representing simply a byproduct of the epileptic condition, to pro-epileptic (i.e. epileptogenic) and anti-epileptic roles. In the following we will mainly discuss the latter two possibilities in close consideration of the widely accepted view that PDS represent the cellular counterpart of electrographic “interictal” spikes (IIS) (Staley et al., 2005). The proposed close relation between IIS and PDS will be accounted for by using the term “IIS/PDS”, unless information given refers only to the extracellularly detected events (IIS) or events that were recorded solely with intracellular methods (PDS).
4.1. Pro-epileptic role of IIS/PDS
The hypothesis of a pro-epileptic role of IIS/PDS was first formulated in 2005 by Staley and co-workers (Staley et al, 2005) and - in an updated version - can be based on two considerations regarding IIS/PDS: first, in both the post-status epilepticus (post-SE) model of acquired epilepsy as well as in the kindling model, spontaneous unprovoked seizures occur only after a latent period, but IIS can be seen already in this physically “silent” phase, e.g. as early as day one after the insult in post-SE models (Staley et al., 2005). IIS were also found to precede the first seizures in humans who acquired epilepsy as a result of war-related brain insults (Staley et al., 2005). Secondly, PDS strongly resemble giant depolarizing potentials (GDPs), similarly pronounced depolarizing events that occur in the first postnatal days in rat pups, and which are thought to control neuronal development (Ben-Ari et al., 1989). Like GDPs, PDS occur synchronously in a large collective of neurons. And like GDPs (Mohajerani et al., 2007), PDS also involve Ca2+-influx through LTCCs, which are known to feature prominently in excitation-transcription coupling (Fig. 2). Hence, it can be envisaged that PDS recapitulate developmental processes in fully matured neuronal circuits that should remain restricted to newly forming neuronal assemblies (Rubi et al., 2013).
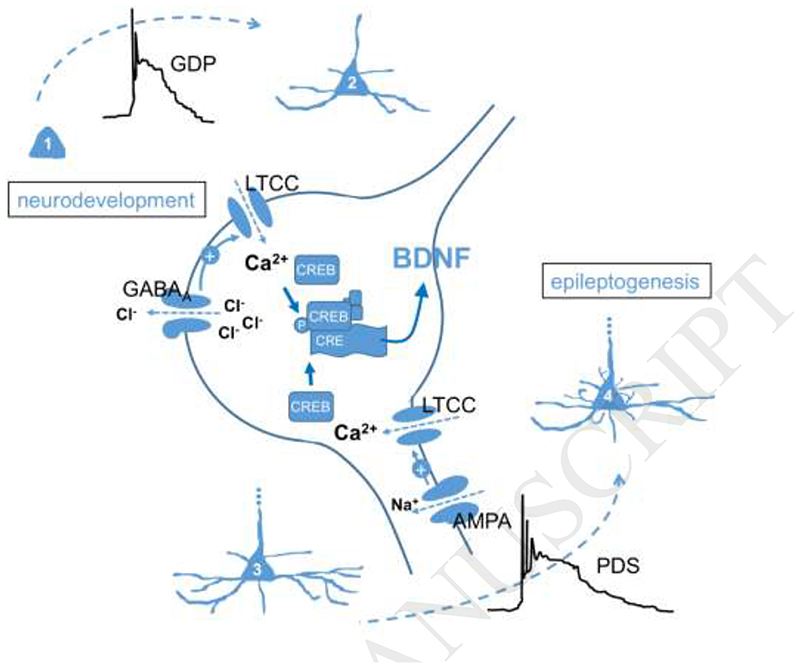
Fig. 2. Putative epileptogenic implication of PDS.
Giant depolarizing potentials (GDP) arise due to excitatory GABAA receptor-mediated signalling in immature neurons (1) and are believed to play a role in neurodevelopment by governing neuronal differentiation (2) (Ben-Ari et al., 1989) (upper left part of the figure). This role was suggested to involve Ca2+ influx via L-type voltage-gated Ca2+ channels (LTCC) and cAMP-responsive element binding protein-regulated gene transcription (CREB, inactive form; pCREB, phosphorylated active form), with brain-derived neurotrophic factor (BDNF) as a likely induced effector protein (Mohajerani et al., 2007). A similar signalling mechanism may be triggered by PDS, which arise due to excitation mediated by ion fluxes through AMPA-type glutamate receptors and LTCCs (the plus sign on an arrow indicates depolarization-induced channel activation), and can thus be envisaged to induce neuronal re-modelling (morphologically, as indicated in the figure, but also in terms of functional synaptic plasticity) in mature neurons (3, 4), thereby contributing to epileptogenesis (lower right part of the figure).
In this manner, PDS may promote morphological as well as functional (synaptic plasticity) changes. LTCCs, which play a crucial role in mediating the depolarizing wave of PDS, were suggested to play a privileged role in coupling neuronal membrane depolarization to nuclear transcription (Rubi et al., 2013; Stiglbauer et al., 2017). Moreover, mitochondrial production of reactive oxygen species (ROS), which is augmented during the latent period of epileptogenesis, was shown to boost the activity of the LTCC-regulated gene transcription factor CREB (cAMP-responsive element binding protein) (Hongpaisan et al., 2003). Hence, PDS may not simply represent a by-product of a developing epileptic condition in the brain, but may actually play a key role in the epileptogenic processes. Epileptogenesis, according to this hypothesis, may involve a resumption of neuronal maturation in an already matured neuronal environment, which may eventually lead to the formation of hyperexcitable circuits (Fig. 2). Accordingly, attempts should be made to abolish IIS/PDS in order to prevent the development of epileptic seizures.
4.2. Anti-ictal role of IIS/PDS
However, to date there is no general agreement in the field that PDS should be eliminated. Importantly, in epileptic networks displaying both interictal and ictal electrical activities, the occurrence of IIS has been observed by some authors to stand in inverse relation with the likeliness of seizures, e.g. IIS frequencies remain unaltered or decrease prior to the appearance of seizures and high frequencies were seen after the seizures (de Curtis and Avanzini, 2001). Moreover, action potential firing and seizures were found to be inhibited following interictal spikes (Zhou et al., 2007; Yan et al., 2008). Hence, it was reasoned that IIS/PDS cause a transient refractoriness of the epileptic network and should therefore not be opposed in antiepileptic therapy (de Curtis and Avanzini, 2001). This issue has been heavily debated for quite some time. However, the potential anti-ictal activity may represent a more direct effect of PDS on neuronal discharge activity (electrical refractoriness) and may precede the more long-lasting pro-epileptic effects discussed above (4.1.). The controversy regarding pro-epileptic and anti-ictal effects of PDS can thus partly be attributed to different time scales that had been considered.
5. Future research prospect
More experimental work on PDS is obviously needed to reach a conclusion regarding the predominating pro-epileptic or anti-ictal role. In particular, the potential morphological or functional remodeling effects of PDS that can be envisaged to occur from circumstantial evidence must be directly tested, e.g. in work on primary cell culture or organotypic culture systems. Importantly, the PDS-induced changes need to be thoroughly investigated regarding their role in epileptogenesis. Additionally, the signaling pathways that may mediate these effects need to be clarified in molecular detail to identify suitable targets for therapeutic modulation.
Key facts.
The paroxysmal depolarization shift (PDS) represents the cellular correlate of an interictal spike, which is the electrographic brain surface signal arising from the synchronous occurrence of PDS in many neurons.
Glutamate receptor-mediated currents and currents via L-type voltage gated Ca2+ channels are essential contributors to PDS formation.
In the long term, PDS were suggested to have a pro-epileptic (epileptogenic) role, which may involve excitation-dependent gene transcription and resulting morphological and functional changes.
In the short term, PDS were suggested to have an anti-ictal role, which may involve transient electrical refractoriness of neuronal networks.
Acknowledgements
Work on PDS in the lab of the authors has been funded by the Austrian Science Fund FWF (projects P19710 and P28179) and by a grant from the Herzfelder’sche Familienstiftung.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaishi T, Nakazawa K, Sato K, Saito H, Ohno Y, Ito Y. Hydrogen peroxide modulates whole cell Ca2+ currents through L-type channels in cultured rat dentate granule cells. Neurosci Lett. 2004;356:25–28. doi: 10.1016/j.neulet.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Antoniadis A, Müller WE, Wollert U. Inhibition of GABA and benzodiazepine receptor binding by penicillins. Neurosci Lett. 1980;18:309–312. doi: 10.1016/0304-3940(80)90302-x. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingmann D, Speckmann EJ, Baker RE, Ruijter J, de Jong BM. Differential antiepileptic effects of the organic calcium antagonists verapamil and flunarizine in neurons of organotypic neocortical explants from newborn rats. Exp Brain Res. 1988;72:439–442. doi: 10.1007/BF00250266. [DOI] [PubMed] [Google Scholar]
- Chen S, Su H, Yue C, Remy S, Royeck M, Sochivko D, Opitz T, Beck H, Yaari Y. An increase in persistent sodium current contributes to intrinsic neuronal bursting after status epilepticus. J Neurophysiol. 2011;105:117–129. doi: 10.1152/jn.00184.2010. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–567. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Radici C, Forti M. Cellular mechanisms underlying spontaneous interictal spikes in an acute model of focal cortical epileptogenesis. Neuroscience. 1999;88:107–117. doi: 10.1016/s0306-4522(98)00201-2. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Major S, Pannek HW, Woitzik J, Scheel M, Wiesenthal D, Martus P, Winkler MK, Hartings JA, Fabricius M, Speckmann EJ, et al. COSBID study group Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain. 2012;135:259–275. doi: 10.1093/brain/awr303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossat P, Sibon I, Le Masson G, Landry M, Nagy F. L-type calcium channels and NMDA receptors: a determinant duo for short-term nociceptive plasticity. Eur J Neurosci. 2007;25:127–135. doi: 10.1111/j.1460-9568.2006.05256.x. [DOI] [PubMed] [Google Scholar]
- Hablitz JJ. Picrotoxin-induced epileptiform activity in hippocampus: role of endogenous versus synaptic factors. J Neurophysiol. 1984;51:1011–1027. doi: 10.1152/jn.1984.51.5.1011. [DOI] [PubMed] [Google Scholar]
- Hanaya R, Sasa M, Kiura Y, Ishihara K, Serikawa T, Kurisu K. Epileptiform burst discharges in hippocampal CA3 neurons of young but not mature Noda epileptic rats (NER) Brain Res. 2002;950:317–320. doi: 10.1016/s0006-8993(02)03195-5. [DOI] [PubMed] [Google Scholar]
- Hongpaisan J, Winters CA, Andrews SB. Calcium-dependent mitochondrial superoxide modulates nuclear CREB phosphorylation in hippocampal neurons. Mol Cell Neurosci. 2003;24:1103–1115. doi: 10.1016/j.mcn.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hwa GG, Avoli M. The involvement of excitatory amino acids in neocortical epileptogenesis: NMDA and non-NMDA receptors. Exp Brain Res. 1991;86:248–256. doi: 10.1007/BF00228949. [DOI] [PubMed] [Google Scholar]
- Langlois M, Polack PO, Bernard H, David O, Charpier S, Depaulis A, Deransart C. Involvement of the thalamic parafascicular nucleus in mesial temporal lobe epilepsy. J Neurosci. 2010;30:16523–16535. doi: 10.1523/JNEUROSCI.1109-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Ajmone Marsan C. Cortical cellular phenomena in experimental epilepsy: interictal manifestations. Exp Neurol. 1964;9:286–304. doi: 10.1016/0014-4886(64)90025-1. [DOI] [PubMed] [Google Scholar]
- Mohajerani MH, Sivakumaran S, Zacchi P, Aguilera P, Cherubini E. Correlated network activity enhances synaptic efficacy via BDNF and the ERK pathway at immature CA3 CA1 connections in the hippocampus. Proc Natl Acad Sci U S A. 2007;104:13176–13181. doi: 10.1073/pnas.0704533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama T, Ishihara K, Serikawa T, Moritake K, Sasa M. Effect of nicardipine on abnormal excitability of CA3 pyramidal cells in hippocampal slices of spontaneously epileptic rats. Eur J Pharmacol. 1995;280:119–123. doi: 10.1016/0014-2999(95)00189-r. [DOI] [PubMed] [Google Scholar]
- Moraidis I, Bingmann D, Lehmenkühler A, Speckmann EJ. Caffeine-induced epileptic discharges in CA3 neurons of hippocampal slices of the guinea pig. Neurosci Lett. 1991;129:51–54. doi: 10.1016/0304-3940(91)90718-9. [DOI] [PubMed] [Google Scholar]
- Prince DA. The depolarization shift in “epileptic” neurons. Exp Neurol. 1968;21:467–485. doi: 10.1016/0014-4886(68)90066-6. [DOI] [PubMed] [Google Scholar]
- Rubi L, Schandl U, Lagler M, Geier P, Spies D, Gupta KD, Boehm S, Kubista H. Raised activity of L-type calcium channels renders neurons prone to form paroxysmal depolarization shifts. Neuromolecular Med. 2013;15:476–492. doi: 10.1007/s12017-013-8234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller Y. Inter-ictal- and ictal-like epileptic discharges in the dendritic tree of neocortical pyramidal neurons. J Neurophysiol. 2002;88:2954–2962. doi: 10.1152/jn.00525.2001. [DOI] [PubMed] [Google Scholar]
- Schiller Y. Activation of a calcium-activated cation current during epileptiform discharges and its possible role in sustaining seizure-like events in neocortical slices. J Neurophysiol. 2004;92:862–872. doi: 10.1152/jn.00972.2003. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Schachter SC. Drug treatment of epilepsy in adults. BMJ. 2014;348:g254. doi: 10.1136/bmj.g254. [DOI] [PubMed] [Google Scholar]
- Segal MM. Epileptiform activity in microcultures containing one excitatory hippocampal neuron. J Neurophysiol. 1991;65:761–770. doi: 10.1152/jn.1991.65.4.761. [DOI] [PubMed] [Google Scholar]
- Segal MM, Furshpan EJ. Epileptiform activity in microcultures containing small numbers of hippocampal neurons. J Neurophysiol. 1990;64:1390–1399. doi: 10.1152/jn.1990.64.5.1390. [DOI] [PubMed] [Google Scholar]
- Silva-Barrat C, Szente M, Menini C, Velluti JC, Champagnat J. Muscarinic depression of synaptic transmission in the epileptogenic GABA withdrawal syndrome focus. J Neurophysiol. 2001;85:2159–2165. doi: 10.1152/jn.2001.85.5.2159. [DOI] [PubMed] [Google Scholar]
- Speckmann EJ, Walden J, Bingmann D. Contribution of calcium ions to epileptogenesis. J Basic Clin Physiol Pharmacol. 1990;1:95–105. doi: 10.1515/jbcpp.1990.1.1-4.95. [DOI] [PubMed] [Google Scholar]
- Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11:272–276. doi: 10.1177/1073858405278239. [DOI] [PubMed] [Google Scholar]
- Stiglbauer V, Hotka M, Ruiß M, Hilber K, Boehm S, Kubista H. Cav1.3 channels play a crucial role in the formation of paroxysmal depolarization shifts in cultured hippocampal neurons. Epilepsia. 2017;58:858–871. doi: 10.1111/epi.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub H, Speckmann EJ, Bingmann D, Walden J. Paroxysmal depolarization shifts induced by bicuculline in CA3 neurons of hippocampal slices: suppression by the organic calcium antagonist verapamil. Neurosci Lett. 1990;111:99–101. doi: 10.1016/0304-3940(90)90351-9. [DOI] [PubMed] [Google Scholar]
- Sun DA, Sombati S, DeLorenzo RJ. Glutamate injury-induced epileptogenesis in hippocampal neurons: an in vitro model of stroke-induced “epilepsy”. Stroke. 2001;32:2344–2350. doi: 10.1161/hs1001.097242. [DOI] [PubMed] [Google Scholar]
- Witte OW. Physiological basis of pathophysiological brain rhythms. Acta Neurobiol Exp (Wars) 2000;60:289–297. doi: 10.55782/ane-2000-1347. [DOI] [PubMed] [Google Scholar]
- Yan W, Mitzelfelt JD, Principe JC, Sanchez JC. The effects of interictal spikes on single neuron firing patterns in the hippocampus during the development of temporal lobe epilepsy. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:4134–4137. doi: 10.1109/IEMBS.2008.4650119. [DOI] [PubMed] [Google Scholar]
- Zhou JL, Lenck-Santini PP, Zhao Q, Holmes GL. Effect of interictal spikes on single-cell firing patterns in the hippocampus. Epilepsia. 2007;48:720–731. doi: 10.1111/j.1528-1167.2006.00972.x. [DOI] [PubMed] [Google Scholar]