Abstract
Activity in a network of areas spanning the superior temporal sulcus, dorsomedial frontal cortex, and anterior cingulate cortex is concerned with how nonhuman primates negotiate the social worlds in which they live. Central aspects of these circuits are retained in humans. Activity in these areas codes for primates’ interactions with one another, their attempts to find out about one another, and their attempts to prevent others from finding out too much about themselves. Moreover, important features of the social world, such as dominance status, cooperation, and competition, modulate activity in these areas. We consider the degree to which activity in these regions is simply encoding an individual’s own actions and choices or whether this activity is especially and specifically concerned with social cognition. Recent advances in comparative anatomy and computational modeling may help us to gain deeper insights into the nature and boundaries of primate social cognition.
Keywords: social network, dominance, cingulate cortex, superior temporal sulcuS, dorsomedial prefrontal cortex
Introduction
Why Should We Look for Neural Circuits for Social Cognition?
Perhaps the first question to ask is whether it is reasonable to expect that we might identify neural mechanisms for social cognition in the same way that we can for other cognitive, motor, and perceptual processes. Animals possess adaptations that are beneficial for survival in the environments in which they live, and this is also true for primates (Passingham & Wise 2012). Such adaptations occur in the brain just as they do elsewhere in the body. For example, one distinctive primate feature, granular prefrontal cortex, may have evolved as anthropoid primates began to range over large environments to exploit food sources such as fruiting trees available only intermittently and in restricted areas.
For many primates, an important aspect of the environment is that it contains other individuals. Because primates are well adapted to the physical environments they occupy, it is no surprise that they are adapted to their social environments too. According to the social brain hypothesis, the complexity of social life is correlated with brain size across primate species (Dunbar & Shultz 2007, Shultz & Dunbar 2010). Advocates of the social brain hypothesis argue that typical social group sizes or the presence of mating strategies that are more cognitively demanding are associated with larger overall brain size.
One possibility is that primate brain mechanisms are best described simply in relation to basic computational processes, any of which might be pressed into service when an animal negotiates its social environment, just as when it negotiates its spatial environment. According to this view, social cognition is the net output of the aggregate activity of these basic processes, none of which are specifically or exclusively concerned with social cognition (Behrens et al. 2009, Rushworth et al. 2013). The other possibility, however, is that some neural mechanisms are especially and specifically concerned with social cognition (Behrens et al. 2009, Rushworth et al. 2013).
Networks of Social Cognition and Cognition of Social Networks
In humans, a network of brain regions contributes to social cognition. They comprise the superior temporal sulcus (STS) and adjacent temporoparietal junction (TPJ), anterior cingulate cortex (ACC), medial and dorsomedial prefrontal cortex (dmPFC), and subcortical regions such as amygdala and striatum (Amodio & Frith 2006, Apps et al. 2016, Ruff & Fehr 2014, Saxe 2006). What brain regions are related to social cognition in nonhuman primates, and are they similar to those in humans?
Like many animals, several nonhuman primate species, such as macaques, live in groups. Macaque groups are organized in dominance hierarchies where every member has a clear rank position relative to all other group members (Maestripieri & Hoffman 2012). Higher rank enables privileged access to food (Boelkins & Wilson 1972). Social bonds within macaque groups attenuate physiological stress responses (Young et al. 2014a) and facilitate increase of social rank through the formation of alliances (Schülke et al. 2010).
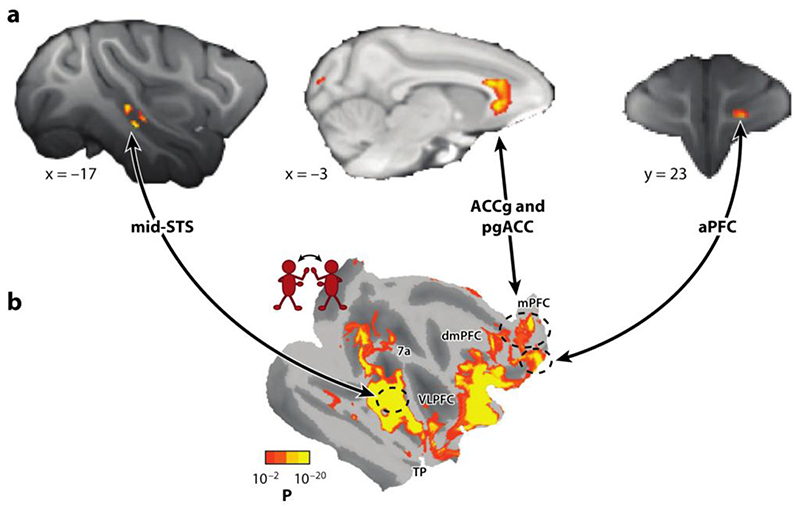
One way to pinpoint the neural network supporting primate social cognition is to identify brain regions that change in relation to variation in group lifestyle. Sallet, Mars, Noonan, and colleagues (Mars et al. 2012, Noonan et al. 2014, Sallet et al. 2011) pursued this question in pseudorandomly composed rhesus macaque groups. They compared gray matter in relation to the complexity of each individual’s social environment. Just as placing greater demands on the motor system results in measurable changes in specific regions of cortex and white matter (Quallo et al. 2009, Scholz et al. 2009), variation in social environments was associated with variation in brain structure; group size was correlated with gray matter in STS and adjacent temporal lobe cortex and in parts of the anterior prefrontal cortex (aPFC). In addition, during rest, functional connectivity between STS and ACC gyrus (ACCg) increased as social group size increased (Figure 1a ).
Figure 1. Brain networks of social cognition in macaques.
Complementary whole-brain neuroimaging approaches identify mid-STS, ACCg, pgACC, and aPFC. (a) These regions are related to the size of the social group a macaque lives in (Sallet et al. 2011), and (b) they are amongst the regions more active when macaques observe conspecifics interact (Sliwa & Freiwald 2017). Panel a and b are reprinted and modified from Sallet and colleagues (2011) and from Sliwa & Freiwald (2017), respectively. Both panels reprinted with permission from AAAS. Abbreviations: ACCg, anterior cingulate gyrus; aPFC, anterior prefrontal cortex; pgACC, perigenual anterior cingulate cortex; STS, superior temporal sulcus.
Sliwa & Freiwald (2017) took a different approach in their attempt to map out a primate circuit for social cognition but identified many of the same brain regions. During MRI, macaques watched short movies of interacting conspecifics and a collection of control videos showing macaques interacting with inanimate objects or moving inanimate objects. The authors found responses in STS, aPFC, ACCg, and dmPFC when macaques viewed social interactions (Figure 1b ).
The STS, aPFC, dmPFC, and ACC are monosynaptically interconnected (Petrides & Pandya 2007, Van Hoesen et al. 1993, Yukie & Shibata 2009), suggesting that they constitute a circuit. Similar or adjacent brain regions also covary with social network size in humans (Bickart et al. 2011, Kanai et al. 2012, Lewis et al. 2011). The fact that certain areas are most affected by an individual’s social group size suggests that it may not be correct to think of the whole brain’s size as determined by social cognitive demands. Instead, social cognitive demands may fall hardest on a subset of brain systems. Such a view would reconcile aspects of the social brain hypothesis with evidence that other factors, such as diet, have a pronounced effect on overall brain size (DeCasien et al. 2017). Furthermore, these findings suggest broad correspondences between brain regions implicated in social cognition in humans and macaques. In the next sections, we discuss these areas in more detail, outlining human-macaque correspondences where appropriate.
Anatomy of Social Cognition in Primates
Superior Temporal Sulcus and Social Cognition
One of the reasons that STS may be an important component of a circuit of social cognition is that it contains patches in which functional MRI (fMRI)-recorded activity increases when macaques look at faces and body parts (Pinsk et al. 2005, 2009; Tsao et al. 2003). Many studies have reported neurons in STS that change their firing rate when macaques look at faces (Perrett et al. 1992, Tsao & Livingstone 2008), and these face-responsive patches in STS identified with fMRI contain high proportions of face-responsive neurons (Bell et al. 2011, Morin et al., 2015; figure 2A; Tsao et al. 2006).
Figure 2. Processing of facial expressions and gaze direction in STS (Morin et al. 2015).
(a) Using fMRI, patches sensitive to faces (red) are found in macaque STS when monkeys observe stimuli from different visual categories. Neurons recorded from these face-selective regions and adjacent cortex (arrow) are sensitive not only to the identity of the observed monkey but also to their gaze direction and facial expression. Example stimuli are shown in panel b, and the distribution of neuron selectivities is shown in panel c. (d) Macaque mid-STS (left) and human TPJ (right) resemble one another in the way in which their activity levels are coupled with those in other brain regions; they share aspects of their connectional fingerprints (Mars et al. 2013). Although macaque mid-STS may have the connectional fingerprint that best matches human TPJs, the areas are not identical (Mars et al. 2013). Macaque STS also resembles another human brain area in anterior STS, suggesting all three areas—human anterior STS, human TPJ, and macaque mid-STS— have some relationship. Both human TPJ and anterior STS are active during social cognitive tasks such as ToM (Deen et al. 2015, Mars et al. 2013). Panels a, b, and c adapted from Morin and colleagues (2015) by permission from Oxford University Press. Panel d adapted from Mars and colleagues (2013). Abbreviations: STS, superior temporal sulcus; ToM, theory of mind; TPJ, temporoparietal junction.
Recent investigations of face processing patches in STS have focused on their role in perceptual discrimination of different face identities (Moeller et al. 2017). Animals living in larger social groups must discriminate between more individuals, and as a result, some form of plasticity may occur in STS that results in increased size. There is, however, evidence that STS’s role in social cognition is not restricted to discriminating face identities. Sliwa & Freiwald (2017) also emphasize that social interaction–related cortex extends beyond face patches. Both fMRI and neurophysiology experiments demonstrate STS cortex adjacent to face patches responds to the social information conveyed by faces such as emotional expressions and gaze direction (Hadj-Bouziane et al. 2008, Morin et al. 2015, figure 2B,C; Perrett et al. 1992). STS may also compute predictive information about the face and body movements that another animal is about to make, suggesting a role in determining current attention (Perrett et al. 2009). Some reports have argued STS lesions cause a greater impairment in gaze direction discrimination than in face discrimination and matching (Campbell et al. 1990, Heywood & Cowey 1992). However, it can be difficult to match the difficulty of gaze and face discrimination tasks.
Comparative studies provide further clues to the role of STS in social cognition and suggest a link with human brain areas such as TPJ that are implicated in high-level social cognitive processes such as theory of mind (ToM) (Saxe 2006). Mars and colleagues (2013) recorded resting-state patterns of activity between brain regions in both humans and macaques. Such activity correlations reflect anatomical connections (O’Reilly et al. 2013). Human TPJ has a distinctive connectional fingerprint characterized by strong functional connections to posterior, mid-, and anterior cingulate cortex and the anterior insula—all areas that have clear correspondences in the macaque. Mid-STS in macaques has a partially similar connectional fingerprint to human TPJ, suggesting a comparable functional role. The mid-STS region is similar to the one that increases with social network size (Sallet et al. 2011) and that is activated during observation of social interactions (Sliwa & Freiwald 2017) (Figure 2d ). In a complementary study, Schwiedrzik and colleagues (2015) have also used functional connectivity measures to estimate connections of face patches in the STS. They also found a functional link with medial frontal cortex areas linked to social cognition.
Theory of Mind Versus Awareness Relations
The human TPJ has been the focus of considerable interest. Accounts of its function focus on its role in sophisticated aspects of social cognition such as ToM, but it is not clear if primates other than humans have ToM.
ToM is the ability to represent the beliefs of other agents. If someone has ToM, then they represent not just stimuli and events in the physical world but another person’s representations, or beliefs, about those stimuli and events. One common way to assess whether a person or animal has ToM is to examine whether they represent false beliefs. For example, if a person’s behavior indicates they simultaneously know about the true disposition of objects in the environment (the banana is in box 1 but not in box 2) and that another agent entertains a false belief about those objects (thinking the banana is in box 2 rather than box 1), then this is strong evidence that they have access both to representations of objects in the world and metarepresentations of what other agents represent, or believe, about those objects. Nonhuman primates have consistently failed false belief tasks (Lurz 2011).
However, nonhuman primates may still be able to track which aspects of the environment another agent is aware of. Martin & Santos (2016) argue that macaques and other nonhuman primates do not track representational relations—others’ beliefs that are not true of the real world—but that they do track awareness relations—aspects of the real world another individual is aware of. In one experiment, macaques watched an experimenter place a food item in a green box and then a white box (Marticorena et al. 2011). The monkeys tracked that the experimenter was aware that the food item was in the white as opposed to the green box. They spent more time looking, indicating surprise, when the experimenter returned to the green than the white box. However, the monkeys could not represent false beliefs. They had no expectation as to where the human experimenter would look when the experimenter’s vision of the food’s transition from green to white box was obscured. Martin and Santos argue an ability to track awareness relations may explain why foraging monkeys behave in a way that reflects knowledge of what competitors are aware of and why they may avoid drawing a competitor’s attention as they approach food. At the same time, the ability to track awareness relations, rather than representational relations, would explain why nonhuman primates do not attempt to hide food that another individual has already been aware of, as they do not attempt to inculcate a false belief in another individual.
Gaze-sensitive neurons in STS may be important for tracking awareness relations. However, ToM and tracking others’ false beliefs may also depend on the ability to represent counterfactual alternatives. It has even been suggested that internally modeling the behavior of another person is akin to counterfactual inference (Lee & Seo 2016). In simple cases, macaques do take counterfactual rewards into account (Hayden et al. 2009), but the degree to which macaques and humans represent all features of counterfactual choice possibilities may vary. Anatomical differences in anterior prefrontal organization in humans and macaques (Neubert et al. 2014, 2015) might indicate that the contribution anterior prefrontal cortex makes to counterfactual choice in humans (Boorman et al., 2009, 2011) does not generalize to macaques in a straightforward way.
Learning from Others and Preventing Others from Learning About You
Several areas in primate medial frontal cortex carry signals necessary for social cognition. These include the gyral portion of the ACC (ACCg), the sulcus of the ACC (ACCs) and dmPFC, dorsal to ACCs. Activity in dmPFC may be especially important for learning from observing another individual and for preventing other individuals from predicting your behavior.
Yoshida and colleagues (2011, 2012) recorded neurons in dmPFC and ACCs during a role-reversal task. In alternation, two macaques performed a deterministic, binary reversal-learning task with one rewarded and one unrewarded option. Such paradigms are frequently used in studies of reward-guided learning and decision making and some simple modifications allowed Yoshida and colleagues to exploit them to study social cognition. One macaque performed two trials while the second observed. The actor and observer roles switched for subsequent trials. Observing macaques tracked the other’s behavior to learn about rule reversals: Without making an error themselves, they correctly switched to the alternative option after observing an error made by the partner, if that error indicated that the rule had reversed. Some of the neurons recorded in ACCs and dmPFC fired more when one macaque observed the other making a choice compared to when the first made a choice itself, and neurons with this firing profile were more frequent in dmPFC than ACCs (Yoshida et al. 2011). Neurons in both areas encoded when the other macaque made an incorrect choice, but in a substantial subset of cases, activity was unrelated to reward omission per se (Yoshida et al. 2012). The finding that dmPFC activity is related to observation of another individual accords well with findings from human fMRI studies that identified dmPFC activity when expectations about how another person would act were violated (Behrens et al. 2008, Suzuki et al. 2012, Wittmann et al. 2016b).
However, macaque dmPFC is not exclusively concerned with tracking others’ actions. It is also concerned with preventing others from predicting one’s own behavior. Neurons in dmPFC encode the macaque’s own previous choices and whether they were rewarded or not during a matching pennies task (Donahue et al. 2013). In this binary choice task, macaques make choices and receive rewards if a computer opponent makes the same choice (Seo & Lee 2007). The computer is programmed to outplay the macaques based on regularities in their recent choice pattern, so choosing unpredictably is the best strategy., The matching pennies paradigm is similar to paradigms investigating non-social reward learning and choice, and accordingly, the analysis of behavioral and neural data is based on computational models, in particular reinforcement learning (RL) models. For example, Seo and colleagues (Seo et al., 2014) find that often monkeys’ choices are well predicted by a value expectation computed with an RL model. The value expectation is a longer-term estimate of how good it is to choose an option that is revised each time a new outcome is witnessed. However, to earn rewards in the experiment the animals should not behave predictably and thus they also make choices that run counter to what an RL model would predict. Strikingly, the neurons encoding past choices and rewards in dmPFC are especially active when the animal initiates short sequences of choices that systematically deviate from those a reinforcement learner would make (Seo et al. 2014). In such a setting, the animal not only has to prioritize internally generated spontaneous choice over explicitly cued stimulus-response patterns (Nachev et al. 2008), it even has to disregard the type of responses that are directly incentivized by past rewards. Together, the reversal observation (Yoshida et al. 2011, 2012) and matching pennies (Seo et al. 2014) studies suggest dmPFC activity tracks another individual’s performance to predict future behavior and an animal’s attempts to be unpredictable to others (Figure 3).
Figure 3. Locations of mPFC responses during social and nonsocial tasks in humans (top) and macaques (bottom).
(a) Studies that have identified responses in mPFC during fMRI in humans and the responses’ anatomical locations are represented on an MRI scan of the medial surface. Red triangles show anterior cingulate sulcus BOLD response during nonsocial decision making. Circles show locations of BOLD response that tracks social information processing. (b) Studies that have recorded from single neurons in mPFC in macaques and the neurons’ anatomical locations represented on an MRI scan of the medial surface. Red diamond shows recording site for ACCs that contains neurons that respond during nonsocial reward decision-making tasks. Rectangles show locations of neurons that code for social information. Images on the right taken from Apps and colleagues (2016) and adapted from Rushworth and colleagues (2004). Abbreviations: ACCg, anterior cingulate cortex gyrus; ACCs, anterior cingulate sulcus; BOLD, blood-oxygen-level-dependent; mPFC, medial prefrontal cortex; SFG, superior frontal gyrus.
Moreover, neurons recorded in this or an adjacent dorsomedial area track the degree of insight monkeys have into the correctness of their actions (Middlebrooks & Sommer 2012). An fMRI and inactivation study in macaques suggests a role for this region in judging the accuracy of recently encoded memories and the involvement of more anterior dorsomedial regions in metamemory for more remotely encoded memories (Miyamoto et al. 2017). Metacognitive representations relating to one’s own behavior and that of other agents are often considered similar (Carruthers 2009, Frith & Frith 2012). It is therefore plausible that these dmPFC regions are concerned with metacognitive processes relating to other individuals as well to the monkey’s own behavior. In humans, neural activity related to confidence in one’s own judgement is also often found relatively anteriorly in rostrolateral prefrontal cortex (De Martino et al. 2013, Fleming et al. 2010).
The macaque studies highlight co-occurrence of self- and other-related choice signals in dmPFC. However, it should be emphasized that the dmPFC recordings in macaques have been conducted in relatively caudal dmPFC areas such as the supplementary eye fields or the posterior suprapincipal dimple, while human social cognition studies have focused on more anterior dmPFC areas such as area 9 (but see Suzuki et al., 2012, figure 3) that are rarely investigated in macaques. Nevertheless human fMRI studies of dmPFC similarly highlight choice value representations relating to both the self and the other. For example, in one study, participants made choices for themselves on some trials and on behalf of another person, whose preferences were known, on other trials (Nicolle et al. 2012). Activity related to the preference currently not guiding choice (whether one’s own or the other’s) was found in dmPFC. Human studies further suggest that there are not simply two distinct, overlapping neural representations in dmPFC that are recruited in different circumstances, but that—at least in humans—interactions between self and other representations occur in dmPFC. First, Hampton and colleagues (2008) found a signal in dmPFC reflecting the influence that one individual assumes they have on the actions of another player in an interactive strategic game. Second, this area reflects whether our preferences accord with those held by specific other individuals (Izuma & Adolphs 2013) and how much we are influenced by other opinions (De Martino et al. 2017). Finally, Wittmann and colleagues (2016b) reported that dmPFC activity predicts how our estimates of other people’s abilities are biased by how we think about ourselves and vice versa (Figure 3).
Anterior Cingulate Cortex and Tracking Motivational Information About Other Individuals
ACC has been implicated in an array of cognitive processes including motivation, decision making, learning, and conflict and error monitoring (Hayden et al. 2011; Holroyd & McClure 2015; Kolling et al. 2014, 2016; Shackman et al. 2011; Ullsperger et al. 2014; Verguts et al. 2015; Wittmann et al. 2016a). ACC’s role in social cognition may reflect the contribution it makes to these processes but some recent results suggest the existence of a representation within ACC that is concerned not with the choices the individual themselves makes but with the choices that another agent might make. One recent study recorded from neurons in ACC as monkeys played a prisoner’s dilemma game with another monkey. The activity of many neurons was related to the choices that the monkeys themselves made. However, one class of neurons predicted the other monkeys’ choices of whether or not to cooperate. These same neurons largely did not encode features of the monkeys’ own choices (Haroush & Williams 2015). Such an activity pattern may suggest ACC codes others’ actions as well as one’s own.
In such a paradigm it can be difficult to completely disentangle the other individual’s actions from the implications that the other’s actions have for oneself. For example, it is possible that neurons that encode aspects of the other individuals’ choices are signaling a foregone reward because the actions of the other animal have implications for how much reward the first animal itself will receive.
In some other simple paradigms, however, such ambiguities can be reduced and it is clear that ACC neural activity can be related to the behavior and motivation of others. Several of these studies have emphasized a sub-region of the ACC – the ACCg. The ACCg plays the most prominent role in social cognition especially in relation to tracking how motivated another individual is to obtain a goal (Apps et al. 2013b, 2016; Lockwood 2016). In macaques, several lines of evidence also underline ACCg’s importance for social cognition. Lesions to ACCg impair normal patterns of social interest in others and cause a reduction in the execution of social behaviors (Rudebeck et al. 2006). Chang and colleagues (2012) recorded from neurons in ACCs, ACCg, and orbitofrontal cortex (OFC) during a social decision-making task. Monkeys were assigned roles of actor (self) and recipient (other). Intriguingly, they found a greater proportion of neurons in ACCg, compared to ACCs and OFC, responded to cues that predicted rewards for other monkeys and during decisions to allocate rewards to other monkeys (Figure 3).
The link between ACCg and social processing is also supported by fMRI studies in humans. ACCg signals aspects of others’ actions (Behrens et al. 2008), expected rewards for others (Apps & Ramnani 2014, Lockwood et al. 2015), prediction errors when others’ expectations are violated (Apps et al. 2015), whether rewarding outcomes are being delivered to others (Apps et al. 2013a, Zhu et al. 2012), or when painful stimulation is predicted for or delivered to another person (Lamm et al. 2011, Lockwood et al. 2013). These patterns are not seen when similar information is processed with reference to oneself (Figure 3). Again many of these studies explain behavior and neural activity in terms of quantities originating from an RL framework such as decision expectations, decision outcomes, and prediction errors (the positive or negative deviation of outcome from expectation). The RL models employed are structurally very similar to those used when studying non-social reward-guided learning in ACCs.
Although most studies in humans have used fMRI, there is now also evidence that single ACC neurons in humans encode social information. Hill and colleagues (2016) recorded from implanted electrodes in the ACCg in patients who were undergoing surgery for intractable epilepsy. Patients performed a reinforcement learning based task in which they could learn from the outcomes of their own decisions but also from the outcomes of two other players. Activity in ACCg neurons correlated with both the expected outcome and the actual outcome of trials but only when patients monitored the behavior of another player as opposed to their own behavior. Together, these studies suggest that ACCg plays a crucial role in social cognition in humans and macaques.
Social Prediction Areas in Prefrontal Areas Outside the Social Brain
Several studies have identified prediction errors during social interactions in areas beyond dorsal ACC. Subgenual ACC (sgACC) tracks prosocial prediction errors when a person learns how their own action leads to an outcome for another person (Lockwood et al. 2016) and signals high-level prediction errors regarding uncertainty about an adviser’s fidelity or trustworthiness (Diaconescu et al. 2017). Whether this signaling of prediction errors in sgACC is specifically social in nature is unclear because there is initial evidence that this region also tracks prediction errors when learning occurs at a level of abstraction beyond basic stimulus–response association (Iglesias et al. 2013). Moreover, in macaques, this region has been linked to visceromotor control; sgACC lesions disrupt the sustaining of autonomic arousal associated with positive emotional events (Rudebeck et al. 2014). The dorsolateral prefrontal cortex (dlPFC) also tracks social prediction errors in humans (Burke et al. 2010, Suzuki et al. 2012). Although in these studies dlPFC signals were not observed when the players themselves were performing actions, the dlPFC has been linked to a variety of behavioral and cognitive processes beyond social cognition, including strategic decision making, working memory, and attention (Genovesio et al. 2014, Passingham & Wise 2012, Petrides et al. 2012).
Finally, OFC has also been linked to social cognition. Posterior lateral OFC is active during social interaction observation (Sliwa & Freiwald 2017). Macaques have a face-responsive patch in lateral OFC resembling those found in STS (Hadj-Bouziane et al. 2008, Rolls 2007, Tsao et al. 2008b). It has been claimed that a similar region exists in humans (Tsao et al. 2008a), although its location is surprisingly medial. The precise role of OFC face patches remains unclear, but, more generally, OFC plays a central role in learning the reward value of objects and in using these value associations to guide decision making (Rudebeck & Murray 2014). This function of OFC may underlie its recruitment in social situations. Just as OFC learns and infers the values of inanimate objects, other individuals and combinations of individuals are assigned positive or negative values that determine the emotional response they elicit (Rolls 1999). Such values are updated as circumstances change and, in turn, impact on the social interactions that occur between individuals. The lateral OFC is most important for updating such value associations in both humans (Akaishi et al. 2016, Jocham et al. 2016, Noonan et al. 2011) and macaques (Chau et al. 2015, Walton et al. 2010). Lesions of OFC, particularly lateral OFC, disrupt emotional responses (Noonan et al. 2010, Rudebeck et al. 2006), although their impact may depend on adjacent white matter (Rudebeck et al. 2013).
When male monkeys decide between a constant amount of juice or a variable amount of juice and access to a picture of a conspecific, they sometimes pick the variable juice option, particularly if it is accompanied by a picture of a dominant monkey or the perineum of a female monkey (Deaner et al. 2005). This suggests the social stimuli have a value to the male monkeys. OFC neurons respond to such social stimuli more than to other nonsocial stimuli, even if they are associated with reward (Watson & Platt 2012). In some cases, activity levels reflect the varying degree to which male macaques value the opportunity to look at the stimuli, and in other cases, they distinguish between categories of socially significant stimuli, such as faces and female perinea, and the dominance levels of faces (Watson & Platt 2012). Azzi and colleagues (2012) have also identified OFC neurons that track the social rank and identity of other monkeys as well as other OFC neurons tracking the motivational value of rewards obtained in a social context.
Ventral Striatum and Social Behavior
It is well documented that the striatum responds to reward predictions and actual reward outcomes, particularly when outcomes are unexpected. Such a pattern is thought to reflect reinforcement (Schultz 2016). Ventral striatum receives an input from dopamine neurons in the midbrain that carry a prediction error signal, a key feature of RL theory (Schultz et al. 1997). However, the role of this area in social behavior remains incompletely understood (Báez-Mendoza & Schultz 2013).
Like OFC, the striatum may track the value of both social and nonsocial information. Using the paradigm devised by Deaner et al. (2005), Klein & Platt (2013) showed that neurons in the caudate division of the striatum responded more to social reward images, whereas neurons in the putamen division of striatum responded more strongly to juice rewards. They suggest that there may be specific signals of social context and fluid rewards in striatum, even though these are used to guide a single action (Klein & Platt 2013).
In a reward-giving task, neurons in striatum responded mostly to the monkey’s own reward and infrequently to the other monkeys’ reward. However, another class of striatal neurons showed coding of social action without reward, and some of these responses disappeared when a computer replaced the other monkey (Báez-Mendoza et al. 2013). Striatal neurons also signal errors during performances made by the monkeys themselves and by conspecifics that are independent of a negative reward prediction error signal (Báez-Mendoza & Schultz 2016). The striatum may therefore play a role in both identifying social actors and coding one’s own reward.
In humans, striatal responses have been found during the processing of social rewards, such as smiling faces, that overlap with similar striatal responses during the processing of monetary rewards (Izuma et al. 2008, Lin et al. 2012, Spreckelmeyer et al. 2009). The response of the striatum to juice, money, and erotic picture rewards has been taken as support for a common currency of reward processing in striatum (Sescousse et al. 2015). It therefore seems likely that the striatum has a domain-general role in learning and reward. This idea is supported by a recent study in humans that showed a blood-oxygen-level-dependent response in ventral striatum to prediction errors when someone learns to benefit themselves, another person, and no one in particular (Lockwood et al. 2016) (Figure 3).
Cooperation and Affiliation
As we have seen, living in groups shapes neural circuits in primates (Noonan et al. 2014, Sallet et al. 2011). Living in groups is beneficial for primates because it helps increase the reward rates of single individuals over the long term (Pulliam & Caraco 1984). However, for this to happen, primates need to work together to some degree. For example, macaques cooperate when they defend against or attack territorially adjacent macaque communities (Boelkins & Wilson 1972) and when they form alliances to win conflicts within the group (Schülke et al. 2010). Such cooperation relies on simple signals conveyed partly through facial expressions and head or gaze direction, which might be encoded in STS. For example, macaques recruit conspecifics during conflicts with other group members by alternating gaze between the potential recruit and the opponent (Maestripieri 1997, Young et al. 2014b). In this sense, cooperation requires conveying and understanding indicators of impending action, but it does not necessarily require deception or ToM (Devaine et al. 2014). There is, however, debate on how similar cooperation really is in humans and other primates (Drayton & Santos 2014, Kaminski et al. 2008, Lakshminarayanan & Santos 2008, Melis et al. 2006, Suchak et al. 2016).
Recent evidence has suggested that neuropeptides such as oxytocin modulate social behaviors such as cooperation. For example, oxytocin affects social behavior in rodents, monkeys, and humans (Tremblay et al. 2017), although there is debate over the size and nature of effects (Leng & Ludwig 2016, Walum et al. 2016). Macaques are fundamentally interested in their conspecifics and willing to trade off reward to see pictures of them (Deaner et al. 2005, Rudebeck et al. 2006). When macaques have the chance to observe each other, inhalation of oxytocin in combination with opioid antagonists (which are supposed to strengthen the oxytocin effects) leads to an increase in social attention toward another macaque (Figure 4a ) (Dal Monte et al. 2017). Although monkeys rarely sacrifice their own rewards to deliver reward to others (Chang et al. 2012, 2013), they prefer rewarding others compared to no one (Chang et al. 2012, 2013, 2015) as well as rewarding both themselves and another monkey compared to rewarding themselves alone (Ballesta & Duhamel 2015, Chang et al. 2015, Lakshminarayanan & Santos 2008). After intranasal delivery of oxytocin, macaques are more likely to reward conspecifics over no one but also more likely to reward themselves over the conspecific (Chang et al. 2012). Similar effects of increased prosocial choice are observed after direct oxytocin injection in the amygdala (Chang et al. 2015). The amygdala also carries signals related to self and other reward outcomes. One suggestion is that oxytocin might not affect prosociality toward others in general but instead might strengthen the bonds between oneself and one’s own group, which could manifest in prosocial behavior toward group members but also in protective aggression toward potential attackers (De Dreu & Kret 2016, De Dreu et al. 2010).
Figure 4. Self- and other-values in social interactions.
(a) Dal Monte and colleagues (2017) measured social attention using eye tracking when two macaques were seated directly across from each other. Administration of OTNAL led to increased fixation on the other’s face and in particular the eyes (left, heat map of fixations under OTNAL compared to a SAL control; right, frequency of eye fixation per condition). (b) Kumaran and colleagues (2016) studied learning about one’s own (yellow) or a close friend’s (blue) place in a dominance hierarchy. For hierarchies including oneself only, the updating of hierarchy position during learning was specifically correlated with pgACC activity. (c) Self-value and other-value can be learned by tracking how well actions are performed. Self-other-mergence occurs when the performance is attributed to the inappropriate agent and people increase confidence in their own abilities after a partner has performed well (Wittmann et al. 2016b). (d) Using logistic General Linear Models (GLMs), self-performance and other-performance were used to predict self-value and other-value as measured by independent ratings. Each data point shows average beta weights (+/− SEMs) for both predictors. GLMs were performed separately for cooperation and competition conditions. Self-value and other-value were largely governed by appropriate value assignment, but self-other-mergence became apparent when considering the cooperative or competitive context. Self-value was significantly more positively influenced by other-performance in cooperation compared to competition. The analogous effect was observed for other-value. (*, p< 0.005) (e) In the same study, pgACC and dmPFC showed distinct patterns of activity. While pgACC tracked the success of one’s own recent actions, dmPFC activity reflected the impact (as shown in panel d) that interacting with others had on self- and other-values. Panel a adapted from Dal Monte and colleagues (2017). Panel b adapted from Kumaran and colleagues (2016; DOI: 10.1016/j.neuron.2016.10.052). Panel d and e adapted from Wittmann and colleagues (2016; DOI: 10.1016/j.neuron.2016.06.022). Abbreviations: dmPFC, dorsomedial prefrontal cortex; GLM, general linear model; NAL, naloxone; OT, oxytocin; OTNAL, oxytocin combined with naloxone; pgACC, perigenual anterior cingulate cortex; SAL, saline, SEM, standard error of the mean.
Navigating the social environment also requires representation of one’s own place within it (Qu et al., 2017). In humans, the most rostral part of the ACC—the perigenual ACC (pgACC)—shows a characteristic pattern of activity related to learning and knowledge of one’s own position within a hierarchy. For example, how much subjects change what they think of their position in a hierarchy on a trial-by-trial basis is correlated with the BOLD signal in pgACC (Kumaran et al. 2016) (Figure 4b ). Such signals might reflect the computation of self-values as opposed to the value of specific choices (Murray et al. 2011). Self-related signals are frequently found in pgACC (Denny et al. 2012, Garvert et al. 2015, Kelley et al. 2002, Mitchell et al. 2006, Sul et al. 2015). Computations of self-value in pgACC could be used to estimate the general success rate of one’s actions and how likely one is to overcome impending challenges (Wittmann et al. 2016b).
In humans, the amygdala tracks learning of a social as opposed to a nonsocial hierarchy but shows no preference for hierarchies that include oneself versus those that do not include oneself (Kumaran et al. 2012, 2016). Similarly, in macaques, dominance status is correlated with gray matter density in the amygdala, hypothalamus, and raphe nuclei (Noonan et al. 2014). Dominance in macaques is not simply a matter of size or aggression but also of the social coalitions that an individual establishes (Schülke et al. 2010), and therefore, social dominance likely reflects how successful macaques are in social situations. Perhaps not surprisingly, social dominance is also related to gray matter in STS and aPFC (Noonan et al. 2014, Sallet et al. 2011).
Social interactions often involve decisions to work with others for a common goal. Working together with others can increase our willingness to invest effort (Le Bouc & Pessiglione 2013) and requires alignment of our own actions with those of others (Stolk et al. 2016, Yoshida et al. 2010). A recent fMRI study in humans investigated how interacting with others affects the representations we hold of self and others (Wittmann et al. 2016b). Participants repeatedly performed games and received performance feedback. Over time, they learned about their own and others’ levels of performance. A simple RL model that computed a longer-term average of the performance feedback was used to approximate subjects’ trial-by-trial representation of their own and others’ performance levels. Importantly, on some trials, they cooperated with each other, and on other trials, they competed. Trial-by-trial performance ratings showed that how subjects rated their own performance was not just a reflection of the feedback they received for their own performance but was also affected by the performance of others. Estimates of one’s own self-value increased when cooperating as opposed to competing with a strong partner (Figure 4c , d ). The same effect was also found when evaluating the partner; during cooperation, strong performances by the players themselves led to higher estimates of the partner’s ability. dmPFC signaled the degree to which the performance feedback observed for the other person had an influence on self-value and vice versa. This suggests that self and other related representations in dmPFC (Seo et al. 2014, Yoshida et al. 2011) not only are used to compute relationships between self and other (Hampton et al. 2008, Izuma & Adolphs 2013) but also that dmPFC adjusts the representations we have of ourselves and others as a consequence of interacting together in groups (Figure 4e ).
Conclusion
A network of brain regions carries information for social interactions. In some cases, such as in the OFC, ventral striatum, and amygdala, this social role may be related to a more basic one in fundamental behavioral processes such as learning the reward associations of objects in the environment. In other cases, potentially for ACCg and dmPFC, the activity may be more specifically linked with social cognition. Computational models, as well as studies drawing on comparative anatomy, will be essential for providing precise and quantitative descriptions of the contribution made by such areas to social cognition.
Acknowledgements
We are grateful to Dr Kentaro Miyamoto for helpful discussions. Funded by the Wellcome Trust and Medical Research Council (MRC). A CC BY or equivalent licence is applied to the author accepted version arising from this submission, in accordance with the grant’s open access conditions. Posted with permission from the Annual Review of Neuroscience, Volume 41©2018 by Annual Reviews, http://www.annualreviews.org.
Footnotes
Disclosure Statement
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Marco K. Wittmann, Email: marco.k.wittmann@gmail.com.
Patricia L. Lockwood, Email: patricia.lockwood@psy.ox.ac.uk.
Matthew F.S. Rushworth, Email: matthew.rushworth@psy.ox.ac.uk.
Literature Cited
- Akaishi R, Kolling N, Brown JW, Rushworth M. Neural mechanisms of credit assignment in a multicue environment. J Neurosci. 2016;36:1096–112. doi: 10.1523/JNEUROSCI.3159-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Apps MAJ, Green R, Ramnani N. Reinforcement learning signals in the anterior cingulate cortex code for others’ false beliefs. NeuroImage. 2013a;64:1–9. doi: 10.1016/j.neuroimage.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Apps MAJ, Lesage E, Ramnani N. Vicarious reinforcement learning signals when instructing others. J Neurosci. 2015;35:2904–13. doi: 10.1523/JNEUROSCI.3669-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps MAJ, Lockwood PL, Balsters JH. The role of the midcingulate cortex in monitoring others’ decisions. Front Neurosci. 2013b;7:251. doi: 10.3389/fnins.2013.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps MAJ, Ramnani N. The anterior cingulate gyrus signals the net value of others’ rewards. J Neurosci. 2014;34:6190–200. doi: 10.1523/JNEUROSCI.2701-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps MAJ, Rushworth MFS, Chang SWC. The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron. 2016;90:692–707. doi: 10.1016/j.neuron.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi JC, Sirigu A, Duhamel JR. Modulation of value representation by social context in the primate orbitofrontal cortex. PNAS. 2012;109:2126–31. doi: 10.1073/pnas.1111715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Mendoza R, Harris CJ, Schultz W. Activity of striatal neurons reflects social action and own reward. PNAS. 2013;110:16634–39. doi: 10.1073/pnas.1211342110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Mendoza R, Schultz W. The role of the striatum in social behavior. Front Neurosci. 2013;7:233. doi: 10.3389/fnins.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Mendoza R, Schultz W. Performance error-related activity in monkey striatum during social interactions. Sci Rep. 2016;6 doi: 10.1038/srep37199. 37199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta S, Duhamel JR. Rudimentary empathy in macaques’ social decision-making. PNAS. 2015;112:15516–21. doi: 10.1073/pnas.1504454112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Hunt LT, Rushworth MFS. The computation of social behavior. Science. 2009;324:1160–64. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Hunt LT, Woolrich MW, Rushworth MFS. Associative learning of social value. Nature. 2008;456:245–49. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AH, Malecek NJ, Morin EL, Hadj-Bouziane F, Tootell RBH, Ungerleider LG. Relationship between functional magnetic resonance imaging-identified regions and neuronal category selectivity. J Neurosci. 2011;31:12229–40. doi: 10.1523/JNEUROSCI.5865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat Neurosci. 2011;14:163–64. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelkins RC, Wilson AP. Intergroup social dynamics of the Cayo Santiago rhesus (Macaca mulatta) with special reference to changes in group membership by males. Primates. 1972;13:125–39. [Google Scholar]
- Boorman ED, Behrens TEJ, Rushworth MFS. Counterfactual choice and learning in a neural network centered on human lateral frontopolar cortex. PLOS Biol. 2011;9 doi: 10.1371/journal.pbio.1001093. e1001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman ED, Behrens TEJ, Woolrich MW, Rushworth MFS. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62:733–43. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Burke CJ, Tobler PN, Baddeley M, Schultz W. Neural mechanisms of observational learning. PNAS. 2010;107:14431–36. doi: 10.1073/pnas.1003111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R, Heywood CA, Cowey A, Regard M, Landis T. Sensitivity to eye gaze in prosopagnosic patients and monkeys with superior temporal sulcus ablation. Neuropsychologia. 1990;28:1123–42. doi: 10.1016/0028-3932(90)90050-x. [DOI] [PubMed] [Google Scholar]
- Carruthers P. How we know our own minds: the relationship between mindreading and metacognition. Behav Brain Sci. 2009;32:121–38. doi: 10.1017/S0140525X09000545. [DOI] [PubMed] [Google Scholar]
- Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) PNAS. 2012;109:959–64. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Fagan NA, Toda K, Utevsky AV, Pearson JM, Platt ML. Neural mechanisms of social decision-making in the primate amygdala. PNAS. 2015;112:16012–17. doi: 10.1073/pnas.1514761112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SWC, Gariepy JF, Platt ML. Neuronal reference frames for social decisions in primate frontal cortex. Nat Neurosci. 2013;16:243–50. doi: 10.1038/nn.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau BK, Sallet J, Papageorgiou GK, Noonan MP, Bell AH, et al. Contrasting roles for orbitofrontal cortex and amygdala in credit assignment and learning in macaques. Neuron. 2015;87:1106–18. doi: 10.1016/j.neuron.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Piva M, Anderson KM, Tringides M, Holmes AJ, Chang SWC. Oxytocin under opioid antagonism leads to supralinear enhancement of social attention. PNAS. 2017;114:5247–52. doi: 10.1073/pnas.1702725114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CKW, Kret ME. Oxytocin conditions intergroup relations through upregulated ingroup empathy, cooperation, conformity, and defense. Biol Psychiatry. 2016;79:165–73. doi: 10.1016/j.biopsych.2015.03.020. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Handgraaf MJJ, Shalvi S, Van Kleef GA, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–11. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- De Martino B, Bobadilla-Suarez S, Nouguchi T, Sharot T, Love BC. Social information is integrated into value and confidence judgments according to its reliability. J Neurosci. 2017;37:6066–74. doi: 10.1523/JNEUROSCI.3880-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Fleming SM, Garrett N, Dolan RJ. Confidence in value-based choice. Nat Neurosci. 2013;16:105–10. doi: 10.1038/nn.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr Biol. 2005;15:543–48. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- DeCasien A, Williams S, Higham J. Primate brain size is predicted by diet but not sociality. Nat Ecol Evol. 2017;1:0112. doi: 10.1038/s41559-017-0112. [DOI] [PubMed] [Google Scholar]
- Deen B, Koldewyn K, Kanwisher N, Saxe R. Functional organization of social perception and cognition in the superior temporal sulcus. Cereb Cortex. 2015;25:4596–609. doi: 10.1093/cercor/bhv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. 2012;24:1742–52. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaine M, Hollard G, Daunizeau J. Theory of mind: Did evolution fool us? Plos One. 2014;9 doi: 10.1371/journal.pone.0087619. e87619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaconescu AO, Mathys C, Weber LAE, Kasper L, Mauer J, Stephan KE. Hierarchical prediction errors in midbrain and septum during social learning. Soc Cogn Affect Neurosci. 2017;12:618–34. doi: 10.1093/scan/nsw171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue CH, Seo H, Lee D. Cortical signals for rewarded actions and strategic exploration. Neuron. 2013;80:223–34. doi: 10.1016/j.neuron.2013.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayton LA, Santos LR. Capuchins’ (Cebus apella) sensitivity to others’ goal-directed actions in a helping context. Anim Cogn. 2014;17:689–700. doi: 10.1007/s10071-013-0700-5. [DOI] [PubMed] [Google Scholar]
- Dunbar RI, Shultz S. Evolution in the social brain. Science. 2007;317:1344–47. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Weil RS, Nagy Z, Dolan RJ, Rees G. Relating introspective accuracy to individual differences in brain structure. Science. 2010;329:1541–43. doi: 10.1126/science.1191883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Mechanisms of social cognition. Annu Rev Psychol. 2012;63:287–313. doi: 10.1146/annurev-psych-120710-100449. [DOI] [PubMed] [Google Scholar]
- Garvert MM, Moutoussis M, Kurth-Nelson Z, Behrens TEJ, Dolan RJ. Learning-induced plasticity in medial prefrontal cortex predicts preference malleability. Neuron. 2015;85:418–28. doi: 10.1016/j.neuron.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovesio A, Wise SP, Passingham RE. Prefrontal-parietal function: from foraging to foresight. Trends Cogn Sci. 2014;18:72–81. doi: 10.1016/j.tics.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Hadj-Bouziane F, Bell AH, Knusten TA, Ungerleider LG, Tootell RBH. Perception of emotional expressions is independent of face selectivity in monkey inferior temporal cortex. PNAS. 2008;105:5591–96. doi: 10.1073/pnas.0800489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, Bossaerts P, O’Doherty JP. Neural correlates of mentalizing-related computations during strategic interactions in humans. PNAS. 2008;105:6741–46. doi: 10.1073/pnas.0711099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroush K, Williams ZM. Neuronal prediction of opponent’s behavior during cooperative social interchange in primates. Cell. 2015;160:1233–45. doi: 10.1016/j.cell.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Pearson JM, Platt ML. Fictive reward signals in the anterior cingulate cortex. Science. 2009;324:948–50. doi: 10.1126/science.1168488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Pearson JM, Platt ML. Neuronal basis of sequential foraging decisions in a patchy environment. Nat Neurosci. 2011;14:933–39. doi: 10.1038/nn.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood CA, Cowey A. The role of the ‘face-cell’ area in the discrimination and recognition of faces by monkeys. Philos Trans R Soc B. 1992;335:31–38. doi: 10.1098/rstb.1992.0004. [DOI] [PubMed] [Google Scholar]
- Hill MR, Boorman ED, Fried I. Observational learning computations in neurons of the human anterior cingulate cortex. Nat Commun. 2016;7 doi: 10.1038/ncomms12722. 12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, McClure SM. Hierarchical control over effortful behavior by rodent medial frontal cortex: a computational model. Psychol Rev. 2015;122:54–83. doi: 10.1037/a0038339. [DOI] [PubMed] [Google Scholar]
- Iglesias S, Mathys C, Brodersen KH, Kasper L, Piccirelli M, et al. Hierarchical prediction errors in midbrain and basal forebrain during sensory learning. Neuron. 2013;80:519–30. doi: 10.1016/j.neuron.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Izuma K, Adolphs R. Social manipulation of preference in the human brain. Neuron. 2013;78:563–73. doi: 10.1016/j.neuron.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–94. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Jocham G, Brodersen KH, Constantinescu AO, Kahn MC, Ianni AM, et al. Reward-guided learning with and without causal attribution. Neuron. 2016;90:177–90. doi: 10.1016/j.neuron.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski J, Call J, Tomasello M. Chimpanzees know what others know, but not what they believe. Cognition. 2008;109:224–34. doi: 10.1016/j.cognition.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Roylance R, Rees G. Online social network size is reflected in human brain structure. Proc Biol Sci. 2012;279:1327–34. doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Klein JT, Platt ML. Social information signaling by neurons in primate striatum. Curr Biol. 2013;23:691–96. doi: 10.1016/j.cub.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N, Wittmann MK, Behrens TEJ, Boorman ED, Mars RB, Rushworth MFS. Anterior cingulate cortex and the value of the environment, search, persistence, and model updating. Nat Neurosci. 2016;19:1280–85. doi: 10.1038/nn.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N, Wittmann MK, Rushworth MFS. Multiple neural mechanisms of decision making and their competition under changing risk pressure. Neuron. 2014;81:1190–202. doi: 10.1016/j.neuron.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Banino A, Blundell C, Hassabis D, Dayan P. Computations underlying social hierarchy learning: distinct neural mechanisms for updating and representing self-relevant information. Neuron. 2016;92:1135–47. doi: 10.1016/j.neuron.2016.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Melo HL, Duzel E. The emergence and representation of knowledge about social and nonsocial hierarchies. Neuron. 2012;76:653–66. doi: 10.1016/j.neuron.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarayanan VR, Santos LR. Capuchin monkeys are sensitive to others’ welfare. Curr Biol. 2008;18:R999–1000. doi: 10.1016/j.cub.2008.08.057. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Le Bouc R, Pessiglione M. Imaging social motivation: distinct brain mechanisms drive effort production during collaboration versus competition. J Neurosci. 2013;33:15894–902. doi: 10.1523/JNEUROSCI.0143-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Seo H. Neural basis of strategic decision making. Trends Neurosci. 2016;39:40–48. doi: 10.1016/j.tins.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Ludwig M. Intranasal oxytocin: myths and delusions. Biol Psychiatry. 2016;79:243–50. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Rezaie R, Brown R, Roberts N, Dunbar RI. Ventromedial prefrontal volume predicts understanding of others and social network size. NeuroImage. 2011;57:1624–29. doi: 10.1016/j.neuroimage.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Adolphs R, Rangel A. Social and monetary reward learning engage overlapping neural substrates. Soc Cogn Affect Neurosci. 2012;7:274–81. doi: 10.1093/scan/nsr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL. The anatomy of empathy: vicarious experience and disorders of social cognition. Behav Brain Res. 2016;311:255–66. doi: 10.1016/j.bbr.2016.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL, Apps MAJ, Roiser JP, Viding E. Encoding of vicarious reward prediction in anterior cingulate cortex and relationship with trait empathy. J Neurosci. 2015;35:13720–27. doi: 10.1523/JNEUROSCI.1703-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL, Apps MAJ, Valton V, Viding E, Roiser JP. Neurocomputational mechanisms of prosocial learning and links to empathy. PNAS. 2016;113:9763–68. doi: 10.1073/pnas.1603198113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL, Sebastian CL, McCrory EJ, Hyde ZH, Gu X, et al. Association of callous traits with reduced neural response to others’ pain in children with conduct problems. Curr Biol. 2013;23:901–5. doi: 10.1016/j.cub.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurz RW. Mindreading Animals: The Debate over What Animals Know about Other Minds. MIT Press; Cambridge, MA: 2011. [Google Scholar]
- Maestripieri D. Gestural communication in macaques: usage and meaning of nonvocal signals. Evol Commun. 1997;1:193–222. [Google Scholar]
- Maestripieri D, Hoffman CL. Behavior and social dynamics of rhesus macaques on Cayo Santiago. In: Wang Q, editor. Bones, Genetics, and Behavior of Rhesus Macaques. Springer; New York: 2012. pp. 247–62. [Google Scholar]
- Mars RB, Neubert FX, Noonan MP, Sallet J, Toni I, Rushworth MFS. On the relationship between the “default mode network” and the “social brain.”. Front Hum Neurosci. 2012;6:189. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Neubert FX, Rushworth MFS. Connectivity profiles reveal the relationship between brain areas for social cognition in human and monkey temporoparietal cortex. PNAS. 2013;110:10806–11. doi: 10.1073/pnas.1302956110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marticorena DC, Ruiz AM, Mukerji C, Goddu A, Santos LR. Monkeys represent others’ knowledge but not their beliefs. Dev Sci. 2011;14:1406–16. doi: 10.1111/j.1467-7687.2011.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Santos LR. What cognitive representations support primate theory of mind? Trends Cogn Sci. 2016;20:375–82. doi: 10.1016/j.tics.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Melis AP, Hare B, Tomasello M. Chimpanzees recruit the best collaborators. Science. 2006;311:1297–300. doi: 10.1126/science.1123007. [DOI] [PubMed] [Google Scholar]
- Middlebrooks PG, Sommer MA. Neuronal correlates of metacognition in primate frontal cortex. Neuron. 2012;75:517–30. doi: 10.1016/j.neuron.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Osada T, Setsuie R, Takeda M, Tamura K, et al. Causal neural network of metamemory for retrospection in primates. Science. 2017;355:188–93. doi: 10.1126/science.aal0162. [DOI] [PubMed] [Google Scholar]
- Moeller S, Crapse T, Chang L, Tsao DY. The effect of face patch microstimulation on perception of faces and objects. Nat Neurosci. 2017;20:743–52. doi: 10.1038/nn.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin EL, Hadj-Bouziane F, Stokes M, Ungerleider LG, Bell AH. Hierarchical encoding of social cues in primate inferior temporal cortex. Cereb Cortex. 2015;25:3036–45. doi: 10.1093/cercor/bhu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Wise SP, Drevets WC. Localization of dysfunction in major depressive disorder: prefrontal cortex and amygdala. Biol Psychiatry. 2011;69:e43–54. doi: 10.1016/j.biopsych.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–69. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Sallet J, Rushworth MFS. Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. PNAS. 2015;112:E2695–704. doi: 10.1073/pnas.1410767112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Thomas AG, Sallet J, Rushworth MFS. Comparison of human ventral frontal cortex areas for cognitive control and language with areas in monkey frontal cortex. Neuron. 2014;81:700–13. doi: 10.1016/j.neuron.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Nicolle A, Klein-Flügge MC, Hunt LT, Vlaev I, Dolan RJ, Behrens TEJ. An agent independent axis for executed and modeled choice in medial prefrontal cortex. Neuron. 2012;75:1114–21. doi: 10.1016/j.neuron.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Mars RB, Rushworth MFS. Distinct roles of three frontal cortical areas in reward-guided behavior. J Neurosci. 2011;31:14399–412. doi: 10.1523/JNEUROSCI.6456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Sallet J, Mars RB, Neubert FX, O’Reilly JX, et al. A neural circuit covarying with social hierarchy in macaques. PLOS Biol. 2014;12 doi: 10.1371/journal.pbio.1001940. e1001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Walton ME, Behrens TEJ, Sallet J, Buckley MJ, Rushworth MFS. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. PNAS. 2010;107:20547–52. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Croxson PL, Jbabdi S, Sallet J, Noonan MP, et al. Causal effect of disconnection lesions on interhemispheric functional connectivity in rhesus monkeys. PNAS. 2013;110:13982–87. doi: 10.1073/pnas.1305062110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE, Wise SP. The Neurobiology of the Prefrontal Cortex: Anatomy, Evolution, and the Origin of Insight. Oxford Univ. Press; Oxford, UK: 2012. [Google Scholar]
- Perrett DI, Hietanen JK, Oram MW, Benson PJ. Organization and functions of cells responsive to faces in the temporal cortex. Philos Trans R Soc B. 1992;335:23–30. doi: 10.1098/rstb.1992.0003. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Xiao D, Barraclough NE, Keysers C, Oram MW. Seeing the future: Natural image sequences produce “anticipatory” neuronal activity and bias perceptual report. Q J Exp Psychol. 2009;62:2081–104. doi: 10.1080/17470210902959279. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya D. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–86. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex. 2012;48:46–57. doi: 10.1016/j.cortex.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Pinsk MA, Arcaro M, Weiner KS, Kalkus JF, Inati SJ, et al. Neural representations of faces and body parts in macaque and human cortex: a comparative FMRI study. J Neurophysiol. 2009;101:2581–600. doi: 10.1152/jn.91198.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsk MA, DeSimone K, Moore T, Gross CG, Kastner S. Representations of faces and body parts in macaque temporal cortex: a functional MRI study. PNAS. 2005;102:6996–7001. doi: 10.1073/pnas.0502605102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam HR, Caraco T. Living in groups: Is there an optimal group size? In: Krebs JR, Davies NB, editors. Behavioral Ecology: An Evolutionary Approach. 2nd. Sinauer Associates; Sunderland, MA: 1984. pp. 122–47. [Google Scholar]
- Qu C, Ligneul R, Van der Henst JB, Dreher JC. An Integrative Interdisciplinary Perspective on Social Dominance Hierarchies. Trends in cognitive sciences. 2017;21:893–908. doi: 10.1016/j.tics.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Quallo MM, Price CJ, Ueno K, Asamizuya T, Cheng K, et al. Gray and white matter changes associated with tool-use learning in macaque monkeys. PNAS. 2009;106:18379–84. doi: 10.1073/pnas.0909751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The Brain and Emotion. Oxford Univ. Press; Oxford, UK: 1999. [Google Scholar]
- Rolls ET. The representation of information about faces in the temporal and frontal lobes. Neuropsychologia. 2007;45:124–43. doi: 10.1016/j.neuropsychologia.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Buckley MJ, Walton ME, Rushworth MFS. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313:1310–12. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron. 2014;84:1143–56. doi: 10.1016/j.neuron.2014.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Putnam PT, Daniels TE, Yang T, Mitz AR, et al. A role for primate subgenual cingulate cortex in sustaining autonomic arousal. PNAS. 2014;111:5391–96. doi: 10.1073/pnas.1317695111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci. 2013;16:1140–45. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CC, Fehr E. The neurobiology of rewards and values in social decision making. Nat Rev Neurosci. 2014;15:549–62. doi: 10.1038/nrn3776. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Mars RB, Sallet J. Are there specialized circuits for social cognition and are they unique to humans? Curr Opin Neurobiol. 2013;23:436–42. doi: 10.1016/j.conb.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–17. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Noonan MP, Andersson JL, O’Reilly JX, et al. Social network size affects neural circuits in macaques. Science. 2011;334:697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16:235–39. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in whitematter architecture. Nat Neurosci. 2009;12:1370–71. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schülke O, Bhagavatula J, Vigilant L, Ostner J. Social bonds enhance reproductive success in male macaques. Curr Biol. 2010;20:2207–10. doi: 10.1016/j.cub.2010.10.058. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine reward prediction-error signalling: a two-component response. Nat Rev Neurosci. 2016;17:183–95. doi: 10.1038/nrn.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–99. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schwiedrzik CM, Zarco W, Everling S, Freiwald WA. Face patch resting state networks link face processing to social cognition. PLOS Biol. 2015;13 doi: 10.1371/journal.pbio.1002245. e1002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H, Cai X, Donahue CH, Lee D. Neural correlates of strategic reasoning during competitive games. Science. 2014;346:340–43. doi: 10.1126/science.1256254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H, Lee D. Temporal filtering of reward signals in the dorsal anterior cingulate cortex during a mixed-strategy game. J Neurosci. 2007;27:8366–77. doi: 10.1523/JNEUROSCI.2369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Li Y, Dreher JC. A common currency for the computation of motivational values in the human striatum. Soc Cogn Affect Neurosci. 2015;10:467–73. doi: 10.1093/scan/nsu074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz S, Dunbar R. Encephalization is not a universal macroevolutionary phenomenon in mammals but is associated with sociality. PNAS. 2010;107:21582–86. doi: 10.1073/pnas.1005246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwa J, Freiwald WA. A dedicated network for social interaction processing in the primate brain. Science. 2017;356:745–49. doi: 10.1126/science.aam6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, et al. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc Cogn Affect Neurosci. 2009;4:158–65. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk A, Verhagen L, Toni I. Conceptual alignment: how brains achieve mutual understanding. Trends Cogn Sci. 2016;20:180–91. doi: 10.1016/j.tics.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Suchak M, Eppley TM, Campbell MW, Feldman RA, Quarles LF, de Waal FBM. How chimpanzees cooperate in a competitive world. PNAS. 2016;113:10215–20. doi: 10.1073/pnas.1611826113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul S, Tobler PN, Hein G, Leiberg S, Jung D, et al. Spatial gradient in value representation along the medial prefrontal cortex reflects individual differences in prosociality. PNAS. 2015;112:7851–56. doi: 10.1073/pnas.1423895112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Harasawa N, Ueno K, Gardner JL, Ichinohe N, et al. Learning to simulate others’ decisions. Neuron. 2012;74:1125–37. doi: 10.1016/j.neuron.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Sharika KM, Platt ML. Social decision-making and the brain: a comparative perspective. Trends Cogn Sci. 2017;21:265–76. doi: 10.1016/j.tics.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RBH. Faces and objects in macaque cerebral cortex. Nat Neurosci. 2003;6:989–95. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RBH, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–74. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Livingstone MS. Mechanisms of face perception. Annu Rev Neurosci. 2008;31:411–37. doi: 10.1146/annurev.neuro.30.051606.094238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. PNAS. 2008a;105:19514–19. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Schweers N, Moeller S, Freiwald WA. Patches of face-selective cortex in the macaque frontal lobe. Nat Neurosci. 2008b;11:877–79. doi: 10.1038/nn.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Fischer AG, Nigbur R, Endrass T. Neural mechanisms and temporal dynamics of performance monitoring. Trends Cogn Sci. 2014;18:259–67. doi: 10.1016/j.tics.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Morecraft RJ, Vogt BA. Connections of the monkey cingulate cortex. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus. Birkhauser; Boston: 1993. pp. 249–84. [Google Scholar]
- Verguts T, Vassena E, Silvetti M. Adaptive effort investment in cognitive and physical tasks: a neurocomputational model. Front Behav Neurosci. 2015;9:57. doi: 10.3389/fnbeh.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Behrens TEJ, Buckley MJ, Rudebeck PH, Rushworth MFS. Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron. 2010;65:927–39. doi: 10.1016/j.neuron.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Waldman ID, Young LJ. Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biol Psychiatry. 2016;79:251–57. doi: 10.1016/j.biopsych.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK, Platt ML. Social signals in primate orbitofrontal cortex. Curr Biol. 2012;22:2268–73. doi: 10.1016/j.cub.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann MK, Kolling N, Akaishi R, Chau BK, Brown JW, et al. Predictive decision making driven by multiple time-linked reward representations in the anterior cingulate cortex. Nat Commun. 2016a;7 doi: 10.1038/ncomms12327. 12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann MK, Kolling N, Faber NS, Scholl J, Nelissen N, Rushworth MFS. Self-other mergence in the frontal cortex during cooperation and competition. Neuron. 2016b;91:482–93. doi: 10.1016/j.neuron.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Saito N, Iriki A, Isoda M. Representation of others’ action by neurons in monkey medial frontal cortex. Curr Biol. 2011;21:249–53. doi: 10.1016/j.cub.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Saito N, Iriki A, Isoda M. Social error monitoring in macaque frontal cortex. Nat Neurosci. 2012;15:1307–12. doi: 10.1038/nn.3180. [DOI] [PubMed] [Google Scholar]
- Yoshida W, Seymour B, Friston KJ, Dolan RJ. Neural mechanisms of belief inference during cooperative games. J Neurosci. 2010;30:10744–51. doi: 10.1523/JNEUROSCI.5895-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Majolo B, Heistermann M, Schülke O, Ostner J. Responses to social and environmental stress are attenuated by strong male bonds in wild macaques. PNAS. 2014a;111:18195–200. doi: 10.1073/pnas.1411450111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Majolo B, Schülke O, Ostner J. Male social bonds and rank predict supporter selection in cooperative aggression in wild Barbary macaques. Anim Behav. 2014b;95:23–32. [Google Scholar]
- Yukie M, Shibata H. Temporocingulate interactions in the monkey. In: Vogt B, editor. Cingulate Neurobiology and Disease. Oxford Univ. Press; New York: 2009. pp. 95–162. [Google Scholar]
- Zhu L, Mathewson KE, Hsu M. Dissociable neural representations of reinforcement and belief prediction errors underlie strategic learning. PNAS. 2012;109:1419–24. doi: 10.1073/pnas.1116783109. [DOI] [PMC free article] [PubMed] [Google Scholar]