Abstract
α-Chiral amines are pivotal building blocks for chemical manufacturing. Stereoselective amination of alcohols is receiving increased interest due to its higher atom-efficiency and overall improved environmental footprint compared with other chemocatalytic and biocatalytic methods. We previously developed a hydrogen-borrowing amination by combining an alcohol dehydrogenase (ADH) with an amine dehydrogenase (AmDH) in vitro. Herein, we implemented the ADH-AmDH bioamination in resting Escherichia coli cells for the first time. Different genetic constructs were created and tested in order to obtain balanced expression levels of the dehydrogenase enzymes in E. coli. Using the optimized constructs, the influence of several parameters towards the productivity of the system were investigated such as the intracellular NAD+/NADH redox balance, the cell loading, the survival rate of recombinant E. coli cells, the possible toxicity of the components of the reaction at different concentrations and the influence of different substrates and cosolvents. In particular, the cofactor redox-balance for the bioamination was maintained by the addition of moderate and precise amounts of glucose. Higher concentrations of certain amine products resulted in toxicity and cell death, which could be alleviated by the addition of a co-solvent. Notably, amine formation was consistent using several independently grown E. coli batches. The optimized E. coli/ADH-AmDH strains produced enantiopure amines from the alcohols with up to 80% conversion and a molar productivity up to 15 mM. Practical applicability was demonstrated in a gram-scale biotransformation. In summary, the present E. coli-ADH-AmDH system represents an important advancement towards the development of ‘green’, efficient and selective biocatalytic processes for the amination of alcohols.
Introduction
Many fine chemical and pharmaceutical products as well as intermediates either consist of, or are produced from α-chiral amines.1–3 Indeed, α-chiral amines comprise approximately 40% of the optically active drugs that are currently commercialized mainly as single enantiomers. These amines are typically synthesized industrially starting from ketones through multistep processes involving the hydrogenation of an activated intermediate such as an enamide, enamine or pre-formed N-substituted imine.1,2 Such chemical routes are lengthy and atom-inefficient, require the use of an expensive and unsustainable transition metal complex as catalyst (i.e., for stereoselective hydrogenation) and often result in amine products with insufficient chemical and/or optical purity. Therefore, the efficient and sustainable synthesis of enantiomerically pure amines is of critical importance. A high atom-efficient alternative is the recently developed direct conversion of alcohols into amines through a hydrogen-borrowing mechanism using either chemocatalytic or biocatalytic methods.4–15 Compared with organometallic catalysis, biocatalytic processes for amination of alcohols offer additional advantages such as elevated stereoselectivity, maximized atom-efficiency, the use of nontoxic and intrinsically biodegradable catalysts, a requirement of mild reaction conditions (neutral pH, ambient temperature, atmospheric pressure, etc.) and overall reduction of generated waste.16
In this context, we recently developed a biocatalytic stereoselective hydrogen-borrowing cascade for the amination of alcohols via the combination of two enzymes in vitro, namely an alcohol dehydrogenase (ADH) and an amine dehydrogenase (AmDH).11 The former enzyme performs the oxidation of the alcohol to the carbonyl intermediate, whereas the latter performs the subsequent reductive amination. Another research group later presented the same concept using alternative dehydrogenases.12 In this dual-enzyme cascade (Fig. 1A), the coupling of the redox reactions enables an efficient internal recycling of the nicotinamide coenzyme (NAD+/NADH), thereby only requiring ammonia and catalytic quantities of NAD+ coenzyme. This biocatalytic process was applied for the amination of primary and secondary racemic alcohols using isolated enzymes in solution or in immobilized form, thus leading to excellent conversions, chemoselectivities and stereoselectivities.11,12,17,18 Although the hydrogen-borrowing cascade for the amination of alcohols performs efficiently by pairing purified ADH(s) and AmDH in the presence of ca. 2-5 mol% (related to the substrate) of NAD+, the costs and time associated with protein purification and the external supplementation of NAD+ might represent limitations for certain types of large-scale applications (i.e., depending on the product value). For a wider applicability of the bioamination of alcohols, another possibility is the use of whole-cell systems, which provide the advantage of a direct applicability by removing the need for enzyme purification and supplementation of coenzyme while also increasing the stability of enzymes due to the cellular environment.19,20 However, mass transfer limitations of compounds over the cell wall and membrane, possible toxicity of compounds and competition with endogenous metabolic pathways of the host are potential drawbacks to this method.21 In this study, we investigated the applicability of resting cells by co-expressing two or three dehydrogenases for enabling the conversion of alcohols into enantiopure amines (Fig. 1).
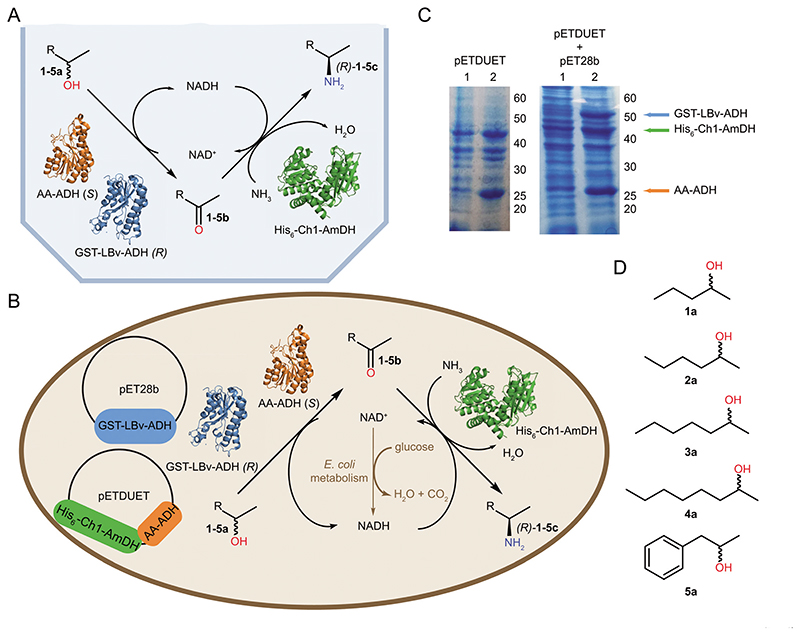
Fig. 1. General overview of the work.
(A) Alcohol amination cascade with internal cofactor recycling employing purified enzymes. (B) E. coli cell transformed with the two plasmids (pET28b and pETDuet) harboring the genes for the enzymes involved in the alcohol amination cascade. The optimal cellular redox-balance is maintained by the addition of minimal amount of glucose, which partially produces NADH by cellular metabolism. (C) SDS-PAGE gels showing balanced expression levels of proteins in E. coli containing pETDuet (Strain 1, left picture) or pETDuet and pET28b (Strain 3, right picture) plasmids, respectively. Lanes numbered 1 and 2 show the samples prior to induction with IPTG and after protein expression, respectively. (D) The substrates explored in this study.
Results and discussion
Co-expression of ADHs and AmDH in E. coli cells
The following dehydrogenases were selected to enable the bioamination cascade starting from secondary (enantiopure or racemic) alcohols: a Prelog alcohol dehydrogenase (ADH) from Aromatoleum aromaticum (AA-ADH);22 an anti-Prelog ADH variant from Lactobacillus brevis (LBv-ADH)23 and a chimeric amine dehydrogenase (Ch1-AmDH).24 AA-ADH and Ch1-AmDH were applied to investigate the bioamination of (S)-configured alcohols. The optimization of expression conditions demonstrated that Ch1-AmDH is efficiently expressed in E. coli only when the gene bears either a His-tag or a GST-tag at its N-terminus, which was not the case for AA-ADH. Further tests of co-expression of AA-ADH and Ch1-AmDH using a Duet plasmid showed that equal mass production of both dehydrogenases can be achieved when the Ch1-AmDH gene precedes the AA-ADH gene in the construct (Strain 1, ESI section 2.2†). Accordingly, this strain—termed E. coli (Ch1-AA)—was used for the continuation of the work. Since the bioamination of racemic alcohols was investigated by applying AA-ADH, LBv-ADH and Ch1-AmDH, Strains 3 and 4 were created by including an additional plasmid for the expression of LBv-ADH into Strain 1 (ESI section 2.2†). Preliminary experiments (not shown) demonstrated that LBv-ADH is efficiently expressed when its gene bears either a His-tag or a GST-tag; however, approximately equal co-expression in mass of AA-ADH, LBv-ADH and Ch1-AmDH was obtained when GST-LBv-ADH was used (Strain 3; ESI section 2.2†). Notably, the selected two- or three-enzyme co-expression systems showed consistent expression levels between various E. coli batches, thus indicating that the plasmids were stably incorporated into the cells. Fig. 1C illustrates typical examples of the expression levels for Strains 1 and 3.
The bioamination of alcohols using purified ADH and AmDH in vitro required a higher molar concentration of the latter enzyme in order to achieve elevated conversions.11,18,25 Although expression levels in vivo might theoretically match the optimal molar ratio found for in vitro experiments, we illustrate in this work that many additional dynamics affect the productivity of the bioamination using engineered E. coli resting cells. Since altering the relative expression levels of ADH and AmDH would also impact the effect of these other factors, further tuning of the expression levels was not considered necessary at this stage.
Influence of intracellular NAD+/NADH redox balance, cells loading and initial substrate concentration on the productivity of the bioaminations of (S)-2-hexanol using E. coli (Ch1-AA)
Initially, we tested various reaction conditions for the bioamination of (S)-2-hexanol ((S)-2a, 20 mM) catalyzed by E. coli (Ch1-AA, 70 mg mL−1 cww) in NH4Cl/NH3 buffer (1 M, pH 8.7; ESI Fig. S1†). The reactions were carried out: (i) with resting or lyophilized cells; (ii) in the presence or absence of externally added nicotinamide cofactor; (iii) in the presence or absence of glucose as additive. Resting and lyophilized E. coli cells performed equally in the absence of externally supplemented NAD+ (ca. 5% conversion to (S)-2-aminohexane, (S)-2c); however, accumulation of a large fraction of ketone intermediate 2b was observed only for the bioamination with lyophilized cells (ca. 80%). Supplementing resting cells or lyophilized cells with NAD+ (1 mM) led to improved conversion into (S)-2c only using lyophilized cells (ca. 30%). This difference compared with the use of resting cells must be attributed to the impermeability of the cell membrane in resting E. coli cells to NAD+,26 whereas lyophilization is known to affect the integrity of the cell membrane. Since most of the intracellular cofactors are bound to enzymes,27,28 it is possible that cofactor shuttling from ADH to AmDH and vice versa is less efficient in the bacterial cytosol than in vitro using isolated enzymes in solution. Depending on environmental factors such as growth medium or aeration,29,30 the oxidative form of the nicotinamide cofactor is predominant in E. coli cells with a NAD+/NADH ratio ranging from 8 to 43.31–34 Therefore, we speculated that the alcohol bioamination could be limited by unbalanced redox equilibrium in the cell due to a higher proportion of oxidized cofactor under physiological conditions. The addition of glucose is a cost-effective means of balancing the NAD+/NADH in resting cells during the alcohol bioamination, as aerobic catabolism of glucose within the cells leads to net and gradual production of NADH,35 which Ch1-AmDH can utilize for the reductive amination step of the cascade reaction. Indeed, the supplementation of glucose (20 mg mL−1, 111 mM) to resting cells increased the conversion to amine up to 80% (ESI Fig. S1†), which is comparable to the level achieved in the in vitro cascade.18 Conversely, the effect of supplementing glucose to the reaction catalyzed by lyophilized cells was mediocre (ca. 10% of (S)-2c), thus demonstrating that in principle, intact metabolism is required for efficient NADH regeneration.
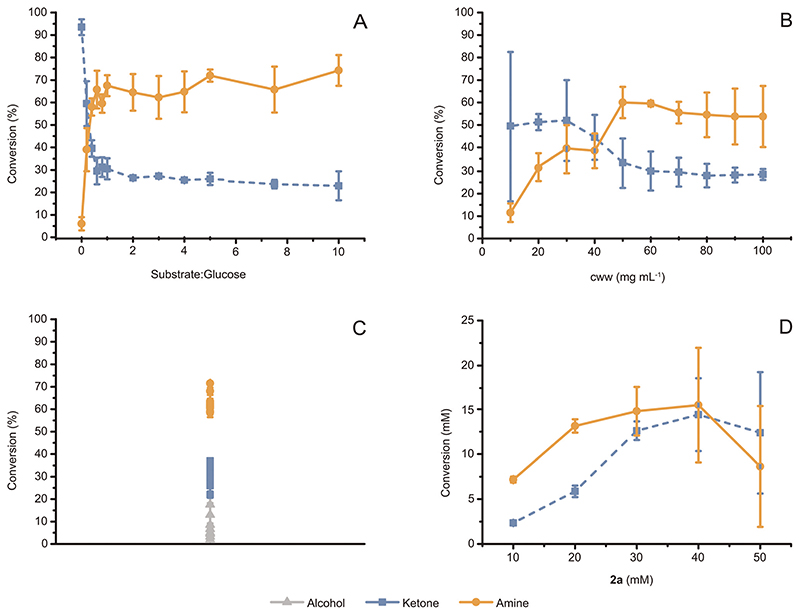
The above-described results were obtained using a substrate : glucose molar ratio of ca. 1 : 5.5. Fig. 2A illustrates an extensive study on the influence of conversion of (S)-2a (20 mM) to (S)-2c versus varied concentration of glucose (up to 10 eq.). Above a threshold of approximately 12 mM glucose (equal to 1: 0.6 substrate : glucose, molar ratio), additional glucose did not lead to further increase of conversion. Therefore, we henceforth used a 1 : 1 molar ratio of substrate : glucose (unless otherwise specified) in order to ensure that sufficient NADH was generated for sustaining the conversion of alcohol to amine. Interestingly, a set of experiments performed at varied but equimolar substrate and glucose concentrations demonstrated that a minimum concentration of glucose (ca. 10 mM) is required in any case for enabling efficient conversion of substrate (ESI Fig. S5†). These data indicated the existence of a certain threshold concentration of glucose that is consumed during aerobic catabolism for cell survival.
Fig. 2. Optimization of the alcohol bioamination catalyzed by E. coli resting cells in NH4Cl/NH3 buffer (1 M, pH 8.7).
(A) Conversion of (S)-2a (20 mM) to (R)-2c catalyzed by E. coli (Ch1-AA) (60 mg mL−1, cww) and at substrate : glucose molar ratio varying from 1: 0 to 1: 10. The maximum conversion was observed at a substrate : glucose molar ratio equal to or above 1: 0.6. (B) Conversion of (S)-2a (20 mM) to (R)-2c catalyzed by E. coli (Ch1-AA) in presence of glucose (20 mM) and at cell wet weight (cww) varying from 10 mg mL−1 to 100 mg mL−1. The maximum conversion was observed at cww equal to or above 50 mg mL−1. (C) Independently grown batches of E. coli (Ch1-AA) showed consistent conversion of (S)-2a (20 mM) to (R)-2c in reactions implemented with 60 mg mL−1 cww of cells, in the presence of glucose (20 mM), and after 24 h of reaction time. (D) Formation of amine product for the bioaminations catalyzed by E. coli (Ch1-AA) (60 mg mL−1, cww), at varied concentrations of (S)-2a (10-50 mM) and at a fixed substrate : glucose molar ratio (1: 1). The maximum amount of (R)-2c produced was approximately 15 mM, independently from the starting concentration of (S)-2a. For (A-D), error bars indicate standard deviations.
Assessing the influence of cell concentration on the conversion of (S)-2a (20 mM) demonstrated that amine production reached a maximum at an E. coli cell concentration of 50 mg mL−1 cww, remaining statistically constant above this value (Fig. 2B). Indeed, the statistical variation (i.e., standard deviation) of the conversion values for the experiments was significantly large at cell concentrations above 70 mg mL−1 cww, which we attributed to the increased viscosity of the samples resulting in less homogenous mixing. In further experiments, the cww was fixed at 60 mg mL−1 for optimal conversion.
Reproducibility of biotransformations is a particular concern when using resting cells versus isolated enzymes. A wide range of side reactions can potentially occur in a cell, which could limit substrate conversion. Additionally, work-up procedures can become more complicated, as the system contains multiple components (e.g., cell membranes, DNA, other proteins and metabolites) that could interfere with—for example—quantitative extraction of products with an organic solvent. Moreover, analytical determination of yield using an internal standard can become difficult due to the viscosity and heterogeneity of the reaction medium, which complicate extraction procedures. Therefore, in this work, we also investigated the efficiency of extraction procedures when using resting E. coli cells and validated that all the components of the reaction mixture (substrates, intermediates and products) can be extracted quantitatively with the optimized procedure (ESI† section 3.6). Another cause of reproducibility issues when using resting cells for biotransformation is batch-to-batch differences among E. coli cultures, particularly variations of protein expression levels. To demonstrate that our system is robust in this sense, we performed replicated experiments for the bioamination of (S)-2a using different batches of independently grown E. coli (Ch1-AA) cells (60 mg mL−1 cww, 20 mM substrate and 20 mM glucose). Fig. 2C depicts a plot of the average conversions per set of experiments with the related standard deviations. Notably, the average conversion into (R)-2c for several independent experiments ranges between 60–75%, thus confirming the consistency and robustness of our system.
Subsequently, we investigated whether higher substrate concentrations (up to 50 mM) could yield an increased absolute product formation. The substrate : glucose ratio was maintained at 1 : 1 to ensure that glucose would not become limiting. Whereas conversion decreased progressively in percentage with the increase of the initial concentration of (S)-2a (ESI Fig. S4†), the absolute amount of (R)-2c formed was stable at approximately 15 mM for the biotransformations at initial substrate concentrations of 20, 30 and 40 mM (Fig. 2D). Conversely, the absolute amount of (R)-2c formed decreased substantially for reactions conducted at substrate concentration below 20 mM and above 40 mM. Notably, besides a maximum productivity at initial ca. 40 mM substrate concentration, the standard deviations of conversion values (i.e., error bars) also increased substantially in the case of reactions at and above 40 mM substrate concentration, which could indicate statistical effects on the cell population during the reaction. Such effects can signify either differences in cell survival and/or cofactor availability/recycling and/or stability of the expressed proteins.
Survival rate, productivity and toxicity assays for E. coli (Ch1-AA): influence of types of substrates, intermediates, products and their concentrations
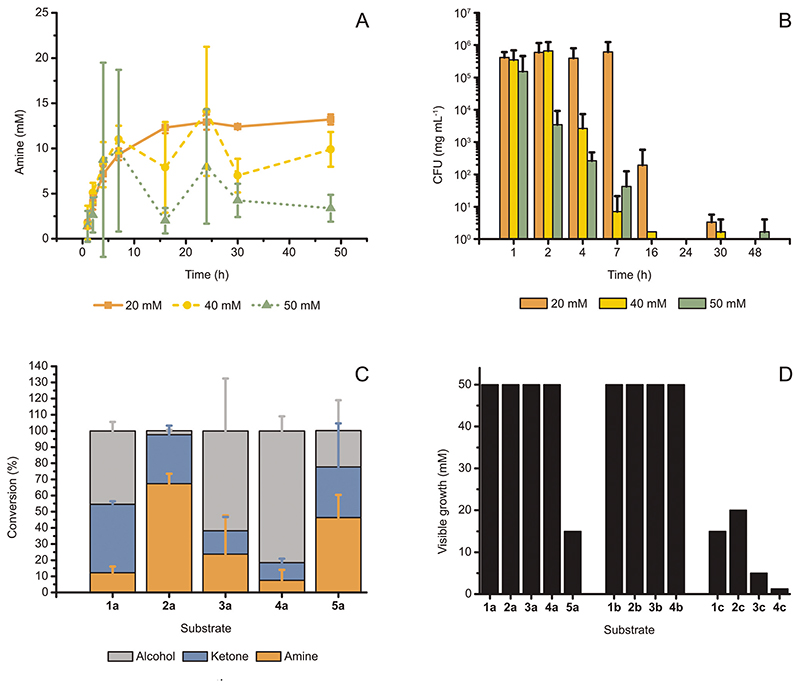
To test whether the reaction suffers from environmental effects, we monitored the conversion over time at varied substrate concentrations and measured the survival rates of the cells in the reaction at each time point. The conversions for the bioamination at 20, 40 and 50 mM of (S)-2a over time are plotted in Fig. 3A. The curve at 20 mM is typical for a successful biotransformation and reached a plateau after 16 h. For all three concentrations, the initial profile was similar; however, from 4 h onwards, the statistical fluctuation of the conversion values for the reactions at 40 and 50 mM increased significantly. Furthermore, after 7 h, curves for the reactions at 20 mM, 40 and 50 mM substrate concentrations started also to diverge. Although the 40 and 50 mM reaction traces showed large differences in conversion for each time point after 2 h, it is noteworthy that the maximum amount of amine formed never consistently exceeded the boundary of 12.5–15 mM established by the 20 mM reaction trace.
Fig. 3.
(A) Progress of the reaction for the conversion of (S)-2a (20-50 mM) to (R)-2c catalyzed by E. coli (Ch1-AA) (60 mg mL−1 cww), at fixed substrate : glucose molar ratio (1: 1) and in NH4Cl/NH3 buffer (1 M, pH 8.7). Increased statistical fluctuation is observed at higher substrate concentrations, whereas the maximum amount of amine formed did not consistently exceed 15 mM. (B) The survival of E. coli (Ch1-AA) cells (measured by CFU) in the reaction samples at varied initial concentrations of (S)-2a, as also indicated in (A). For all applied concentrations of (S)-2a, cell death was observed between 16 and 24 h. (C) Bioamination of substrates (S)-1-5a (20 mM) employing E. coli (Ch1-AA) (60 mg mL−1, cww) in NH4Cl/NH3 buffer (1 M, pH 8.7). (D) Minimal inhibitory (MIC) assays using the available substrates, intermediates and products from this study. The toxicity of the produced amines was evident, whereas most alcohols and all ketones tested did not exhibit inhibition of growth up to at least 50 mM. Note: The precise MIC might be slightly lower than that determined from these experiments because of the volatility of some compounds. However, we assume these possible deviations to be minor or negligible since the 96-well plates, in which the MIC-assays were performed, were sealed during incubation. Error bars in (A-C) indicate standard deviations.
The survival rate of E. coli (Ch1-AA) in the reaction is plotted in Fig. 3B as measures of colony-forming units per mg of cells (CFU mgcells –1). CFU’s are a measure of the number of E. coli cells that survive after being subjected to a certain condition.36 Fig. 3B clarifies that resting cells incubated in a reaction at a 20 mM substrate concentration survive longer and with higher population density than in reactions at 40 or 50 mM substrate concentrations. In fact, reactions with a 50 mM substrate concentration showed a large decrease in cell survival already after 2 h, and both reactions at 40 and 50 mM substrate concentrations exhibited almost no survival after 7 h. In contrast, cells incubated in reactions at a 20 mM substrate concentration still had significant CFU numbers after 16 h. For 20 mM reactions, cell death occurred between 16 and 24 h.
Notably, a correlation was observed between the rapid decrease in survival of E. coli cells (Fig. 3B) and the sharp increase of the standard deviation’s value for conversions in the time range of 2–24 h for bioamination reactions performed at 40 and 50 mM substrate concentrations (Fig. 3A). Indeed, combined with the lower survival rates observed in Fig. 3B and the observation of maximum amine production in Fig. 2C, the large statistical variation at higher substrate concentrations observed in Fig. 3A suggests that the produced amine must be toxic to E. coli cells at a specific concentration.
To expand the substrate scope of the alcohol bioamination in vivo using resting cells (60 mg mL−1, cww), we tested other substrates (20 mM) that were previously studied in the in vitro cascade.11,25 Interestingly, each of these substrates exhibited a different conversion pattern for bioamination in vivo (Fig. 3C). In contrast to in vitro alcohol bioamination, only (S)-2a showed the expected conversion of approximately 75% among the aliphatic substrates (S)-1–4a, whereas the other substrates displayed lower conversions to the amine product (<25%) and partial accumulation of ketone intermediate. In the case of the bioamination of aromatic compound ((S)-5a), the conversion was closer to that for the bioamination of (S)-2a, although the larger standard deviation indicated that (S)-5a is not as easily and consistently converted as (S)-2a. Nevertheless, the stereoselectivities for all tested reactions were perfect (ee >99%, R), and thus identical to the ees obtained for in vitro systems using AA-ADH and Ch1AmDH.11
As mentioned above, large statistical variations of conversions might already indicate toxicity of compounds in the reaction mixture. Moreover, the data on the decrease of E. coli survival at higher concentrations of (S)-2a (Fig. 3B) and the discrepancy in the conversion of chemically similar aliphatic substrates—which showed otherwise similar conversions using purified enzymes in vitro—suggested that either the substrates, intermediates or products were toxic to the cells. Therefore, we thoroughly investigated the probable toxicity of the substrates and/or intermediates and/or products through a minimal inhibitory concentration (MIC) assay, whereby E. coli cells were grown in the presence of varied concentrations of these compounds. The lowest concentration for which no visible growth can be established is defined as the MIC. Fig. 3D shows that most alcohols and all ketones tested did not influence visible E. coli growth up to 50 mM, whereas the amines displayed toxicity at moderate or even low concentrations (e.g., already above 1.25 mM for 4c). Among the tested alcohols, only (S)-5a was found to be toxic. Compounds 5b and 5c could not be tested, as they were unfortunately unattainable in sufficient amounts due to purchase restrictions imposed by drug laws.
To eliminate any possible leaky expression of ADH and/or AmDH and consequent conversion of compounds which would alter their actual concentration, the E. coli strain used in the initial toxicity assays contained no plasmid. However, both E. coli devoid of exogenous plasmid and E. coli (Ch1-AA) were then tested with several compounds (Fig. S6†). As expected, E. coli (Ch1-AA) exhibited higher resistance to (S)-5a, which is likely due to the partial conversion of (S)-5a to 5b; however, its resistance to 2c was lower than that of E. coli devoid of plasmid. This difference might be due to increased pressure on the E. coli cells to maintain the plasmid and concurrent leaky expression of the genes on the plasmid. Notably, as depicted in Fig. 2D and 3A, the MIC of 15 mM for 2c for E. coli (Ch1-AA) correlates nicely with the observation that the formation of (R)-2c does not exceed ca. 15 mM.
The observed toxicity of the produced amines generally explains the low product titers for some substrates and the large statistical variations between batches observed when operating at substrate concentrations around and above a certain critical value. At high substrate concentrations, even low conversions to product can build-up a toxic level of amine, thus resulting in cell death. As the different cell populations can vary in their resistance to toxic amines from batch to batch (and even per sample), this fact would explain the large variations in conversions observed in these critical situations.
Influence of product toxicity and cellular redox balance on maximum productivity
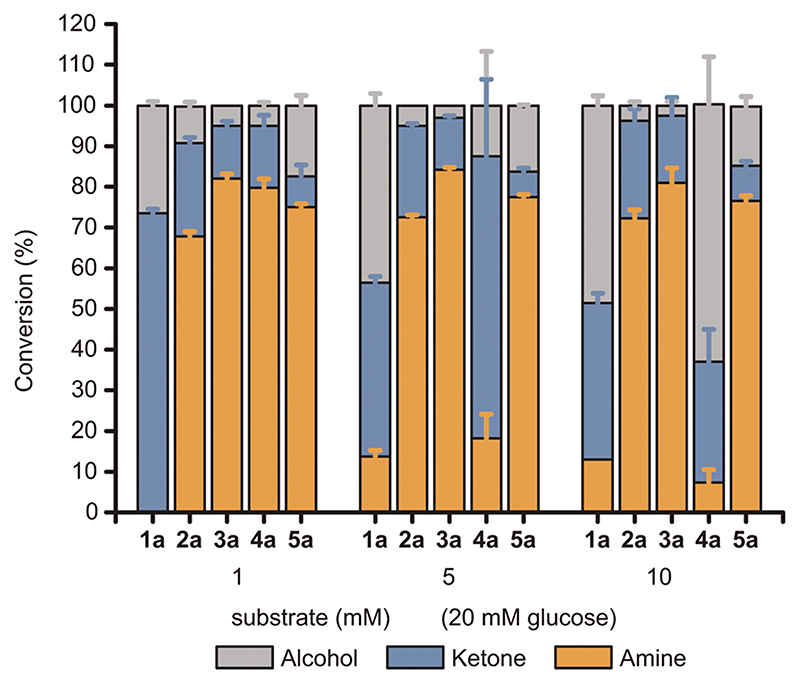
The toxicity level of amines was investigated by repeating the alcohol bioamination at lower substrate concentrations while keeping the 20 mM glucose concentration. Fig. 4 depicts conversions at 1, 5 or 10 mM substrate concentrations, respectively. As expected, lowering the substrate concentration below the lowest MIC (e.g., 2.5 mM for 4c) resulted in highly consistent and elevated conversion values (70–80%) for the amination of (S)-2–5a. Only (S)-1a showed a different behavior, as it was converted solely to ketone. The precise reason why this substrate was not converted in the same manner is unclear; in vitro it was converted similarly to the other aliphatic alcohols.11
Fig. 4.
Influence of the alcohol substrate concentration towards the conversion to the amine product. Bioaminations of (S)-1-5a were conducted using E. coli (Ch1-AA) cells (60 mg mL−1 cww) in NH4Cl/NH3 buffer (1 M, pH 8.7) at various substrate concentrations (1, 5, 10 mM) and a fixed glucose concentration (20 mM). Error bars indicate standard deviations.
Increasing the substrate concentrations to 5 and 10 mM slightly changed the conversion pattern. As expected from the MIC assays, the production of (R)-4c did not rise above approximately 1 mM (e.g., 10% conversion at 10 mM substrate concentration) because higher concentrations led to cell death. The product with the next-lowest MIC was 3c (10 mM). Accordingly, the conversion of (S)-3a exhibited conversions consistent to 80% (R)-3c for reactions at 5 and 10 mM substrate concentrations, as (R)-3c could not reach toxic levels (which was not the case for reactions at 20 mM of (S)-3a). The conversion of (S)-1a was still the outlier in this set with 10–15% amine conversion (i.e., maximum productivity ca. 1 mM). Comparing Fig. 3D and 4, the effect of build-up of toxic amine is well demonstrated, as the production of amines at concentrations above the MIC values was generally not feasible or exhibited large values of standard deviation (i.e., different cell batches can display different tolerance at amine concentration around or slightly above the MIC values). Indeed, when keeping the production of amine below the MIC, conversions were consistently good to excellent, reaching approximately 80%.
Another aspect of the cellular metabolism was revealed when performing this experiment with a 1 : 1 molar ratio of substrate : glucose (ESI Fig. S5†). The reactions at 10 mM alcohol and 10 mM glucose (Fig. S5†) exhibited the same conversions as for those at 10 mM alcohol and 20 mM glucose (Fig. 4). Below 10 mM glucose while keeping the 1 : 1 substrate : glucose molar ratio, the resulting alcohol substrates nearly completely converted into ketones plus amines (except for (S)-5a); however, amine conversion was significantly lower at the glucose and substrate concentration of 5 mM and it was not formed at all at the glucose and substrate concentration of 1 mM. In these cases, accumulation of ketone intermediate occurred. As previously stated, the intracellular cofactor is mostly present as NAD+ at physiological conditions, which is then used by the ADH for oxidation of the alcohol. The NADH generated in the first oxidative step can also be involved in other intracellular reductive processes, thus preventing reductive amination of the ketone intermediate by AmDH. Aerobic respiration of glucose increases NADH levels in cells, thus enabling the reductive amination step. The impaired conversion of ketone to amine in presence of only 1 and 5 mM glucose implies that the cellular metabolism consumes a background level of NADH that has to be regenerated in order to sustain the bioamination in vivo.
Influence of co-solvents on amine product toxicity
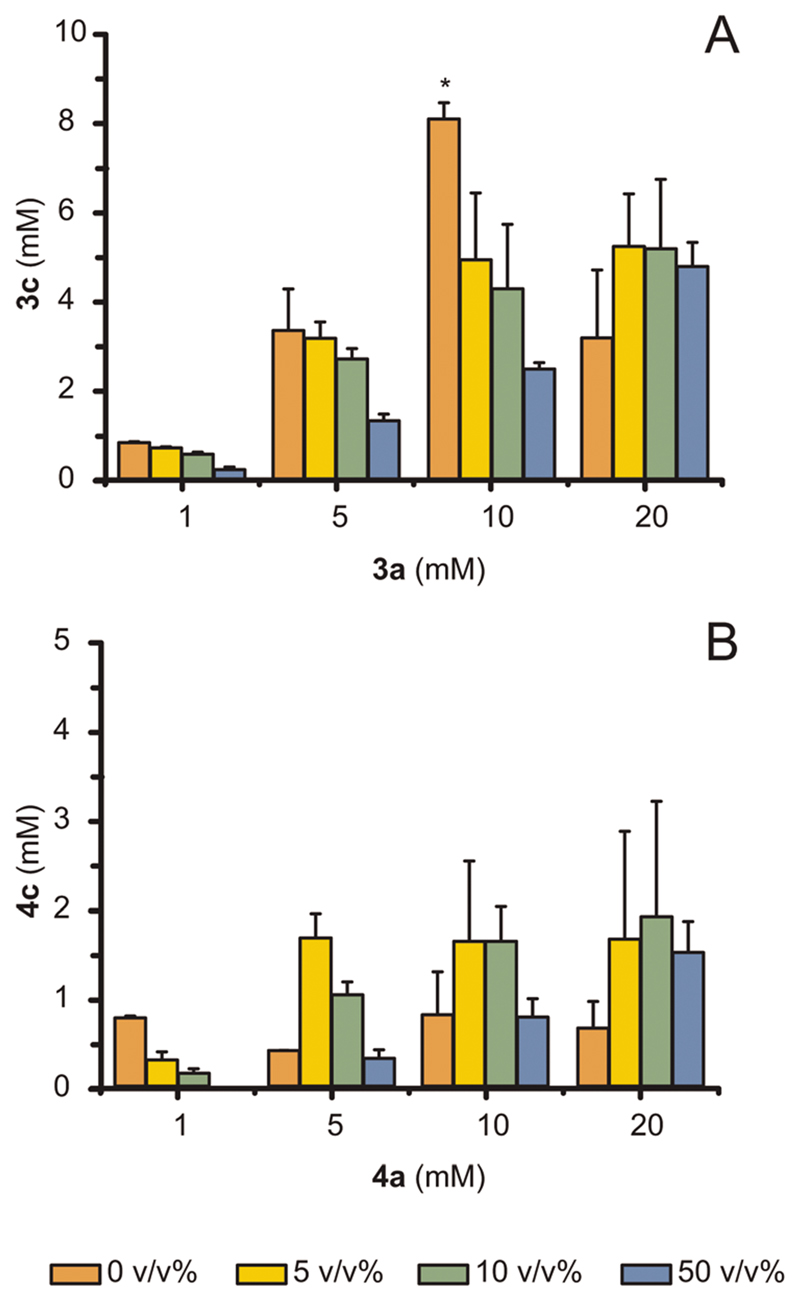
As product titers for the amination of (S)-3a and (S)-4a were particularly moderate, we investigated the use of co-solvents as a reservoir for the produced toxic amines, thereby enhancing cell survival.37,38 Several aspects must be considered for a proper choice of co-solvents for efficient alleviation of toxicity, such as biocompatibility, Log P value (i.e., partition coefficient of amine within liquid phases) and miscibility with the aqueous phase of the reaction.37,38 Operating with resting (living) cells makes co-solvent biocompatibility a critical factor,38,39 particularly considering the intrinsic relatively low kinetics for the conversion of ketones to amines that necessitates longer cell survival. We tested various co-solvents for cell survival at varied volumetric ratios in the aqueous phase, namely n-heptane, n-decane and n-hexadecane, and the latter yielded the highest survival rates for cells after 4 h (data not shown). Consequently, n-hexadecane was used in further experiments. We then investigated the effect of the substrate concentration and different ratios of aqueous phase: cosolvent towards conversion of (S)-3a and (S)-4a, and the results are shown as absolute amounts of amine formed in Fig. 5A and B, respectively.
Fig. 5. The addition of hexadecane at various volumetric ratios (%) with the aqueous phase can alleviate the toxicity of produced amines. Bioamination reactions were run using E. coli (Ch1-AA) cells (60 mg mL−1 cww) in NH4Cl/NH3 buffer (1 M, pH 8.7) at fixed glucose concentration (20 mM).
(A, B) Conversion of (S)-3a above 10 mM and of (S)-4a above 1 mM increased when adding hexadecane as co-solvent. For (A, B), error bars indicate standard deviations. The bar marked with * is replicated from Fig. 4. At 10 mM of (S)-3a in absence of co-solvent, these samples showed low reproducibility among different E. coli batches, which we attribute to the possible varying toxicity of (S)-3c between 5 mM and 10 mM, depending on the E. coli batch population.
Adding the co-solvent was beneficial for the reaction at a 20 mM concentration of (S)-3a but detrimental for reactions at lower concentrations (1, 5, 10 mM). In fact, if the amine does not reach toxic levels, a decrease in conversion is likely due to side-effects of the co-solvent such as disintegration of cell membrane over time,39,40 which also explains the generally lower conversions obtained in the experiments using a 50% v/v of hexadecane rather than with 5 or 10% v/v. Notably, the maximum amount of produced (R)-4c showed a two-fold improvement when using a co-solvent (from <1 mM to 2 mM). In general, the optimal amount of co-solvent was 10% v/v.
Bioamination of racemic alcohols using E. coli (Ch1-AA-LBv)
We have previously demonstrated that the amination of racemic alcohols is feasible by simultaneously using two stereocomplementary ADHs,11 which resulted in the creation of two E. coli (Ch1-AA-LBv) strains (Strains 3 and 4; ESI section 2.2†). Preliminary activity tests evidenced that Strain 3 performed the alcohol bioamination more efficiently than Strain 4 (ESI† section 3.1), and unlike Strain 4, Strain 3 showed equal and elevated expression levels for all three enzymes. Hence, this E. coli (Ch1-AA-LBv) cell strain was tested for further conversion of racemic as well as enantiopure 2a and 5a (20 mM; Fig. 6A and B, respectively).
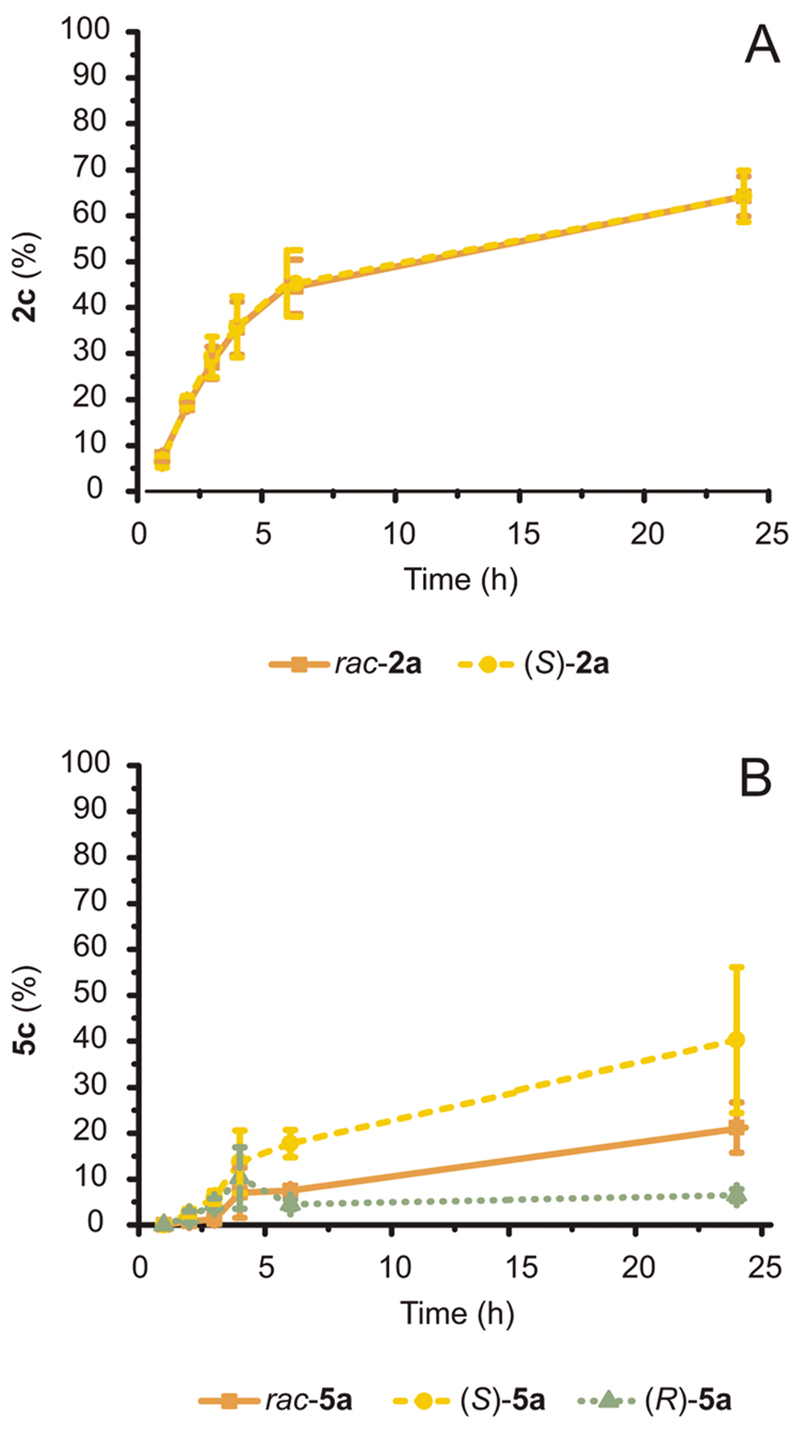
Fig. 6. Conversion of racemic and enantiopure S- or R-configured 2a and 5a (20 mM) catalyzed by E. coli (Ch1-AA-LBv) cells (60 mg mL−1 cww) in NH4Cl/NH3 buffer (1 M, pH 8.7) at fixed glucose concentration (20 mM).
(A) The conversion of rac-2a and (S)-2a over time was identical. (B) The conversion of rac-5a, (S)-5a and (R)-5a diverged over time due to differing enantiomer toxicity. Error bars for both (A) and (B) indicate standard deviations.
rac-2a was converted to 40–65% amine by the three-enzyme E. coli system (depending on the batch of E. coli; ESI Fig. S2†). Notably, both alcohol enantiomers of the racemic mixture were converted to a similar extent because the remaining alcohol 2a at the end of the reaction gave a (R): (S) ratio of 52 : 48. Thus, both stereocomplementary ADHs possess similar apparent activity for their respective 2a enantiomer. The conversion rate was also comparable to that observed for the bioamination employing E. coli (Ch1-AA) strain with enantiopure (S)-2a as a starting material (Fig. 3A and 6A, respectively).
Conversely, conversion of 5a (20 mM) with E. coli (Ch1-AA-LBv) exhibited a different behavior (Fig. 6B). (S)-5a was converted to ca. 40%, which is somewhat lower than the 50% obtained using E. coli (Ch1-AA) and the same substrate (Fig. 3C). Standard deviation values of conversions between samples were also significantly large, as the experimental conditions are at the toxicity limit for this compound (MIC of 5a is 15 mM, Fig. 3D). Interestingly, (R)-5a was converted very poorly to amine (<10%), whereas rac-5a was converted at intermediate level (ca. 20%) between (R)-5a and (S)-5a. As the enantiomeric ratio of the remaining 5a at the end of the reaction yielded a (R): (S) ratio of 42 : 58, the poor conversion of (R)-5a did not stem from catalytic inefficiency of LBv-ADH. Generally, different enantiomers can have different effects and/or toxicity in biological systems.41 Indeed, (S)-5a was less toxic for our E. coli system than (R)-5a, thus explaining the much higher conversion to amine at 20 mM scale when starting from enantiopure (S)-5a, as well as the halved conversion obtained for the amination starting from rac-5a compared to (S)-5a.
Analysis of molar productivities and potential current limitations of the ‘resting E. coli cells-alcohol bioamination’s system’
Table 1 reports the maximum molar productivity for each substrate tested in this study.
Table 1.
Summary of the highest average molar productivities (mM of amine formed) for the bioamination of the substrates from this study using E. coli strains. Absolute conversion percentages are also reported at the related substrate concentration
| Entry | Strain | Substrate | Substrate concentration [mM] | Amine conversion [%] | Amine formed [mM] | ee [%] |
|---|---|---|---|---|---|---|
| 1 | E. coli (Ch1-AA) | (S)-1a | 20 | 12 ± 4 | 2.5 ± 0.8 | >99 |
| 2 | (S)-2a | 20 | 82 ± 2 | 14.5 ± 0.3 | >99 | |
| 3 | (S)-3a | 10 | 81 ± 4 | 8.1 ± 0.4 | >99 | |
| 4 | (S)-4a | 5 | 33 ± 5 | 1.7 ± 0.3 | >99 | |
| 5 | (S)-5a | 20 | 46 ± 14 | 9.3 ± 2.8 | >99 | |
| 6 | E. coli (Ch1-AA-LBv) | rac-2a | 20 | 64 ± 6 | 12.9 ± 1.1 | >99 |
| 7 | rac-5a | 20 | 21 ± 6 | 4.3 ± 1.1 | >99 |
The amination of alcohol 2a (20 mM) yielded a high molar productivity starting from both enantiopure S-configured and racemic alcohols (Table 1, entries 2 and 6). The reason for the high productivity stems from the relatively low toxicity of all reaction components, including the amine product (R)-2c. The behavior was different in the case of the amination of 5a. Amination of the enantiopure alcohol (S)-5a (20 mM) yielded a molar productivity of (R)-5c of 9.3 mM, whereas the amination of rac-5a (20 mM) produced less than half product concentration (Table 1, entries 5 and 7). We attributed this difference to the higher toxicity of (R)-5a compared to (S)-5a, as also supported by the amination of enantiopure (R)-5a, which afforded less than 2 mM of amine product (Fig. 6B). Finally, the amination of substrate (S)-3a also yielded a remarkable molar productivity above 8 mM (Table 1, entry 3), whereas toxicity was a more limiting factor for the amination of (S)-1a and (S)-4a (entries 1 and 4).
Broadly, we noticed a difference in the maximum attainable conversion between in vitro and in vivo bioamination, one reason for which was the general toxicity of the amine products to E. coli at certain concentrations. The addition of cosolvent could increase product formation in cases of severe toxicity (e.g., amination of (S)-3a and (S)-4a); however, the cosolvent itself seems to have an impact on survivability of the E. coli strain. The influence of the cellular environment on the availability of NAD+, NADH and NH4 + might be another factor that limits bioamination in vivo. On the one hand, in principle, the correct NAD+/NADH redox balance could be set by exploiting the aerobic catabolism of exogenously added glucose. However, as (S)-2a could be converted to 80% in the entire concentration range of 1–20 mM substrate, it seems that the NAD+/NADH cofactor availability was sufficient. On the other hand, it could be that the intracellular NH3/NH4 + concentration is lower than the 1 M value present in the reaction buffer, as intracellular cations concentrations are regulated in vivo.35 Considering that hydrogen-borrowing amination in vitro at 200 mM of NH3/NH4 + buffer afforded typically 75% conversion11 and that the K M of Ch1-AmDH for NH3 is around 350 ± 133 mM,24 it could be that quantitative conversion is partly limited by the actual intracellular NH3/NH4 + concentration. The pH is another factor that can potentially influence the thermodynamics of the system. In fact, the intracellular environment is normally buffered approximately between pH 7.2 and 7.842 regardless the pH of the reaction buffer, which was set at 8.7 in this study because it was found to be optimal for bioamination with purified enzymes. Other, more subtle factors that could prevent quantitative conversion of substrates are either the unavailability of the alcohol substrate and/or ketone intermediate due to partitioning of these compounds to cell membranes, or insufficient shuttling of the intermediate between the ADH and the AmDH.
Scale-up of bioamination with resting E. coli cells
To demonstrate the potential applicability of the bioamination with resting E. coli (Ch1-AA-LBv) for larger scale production of enantiopure amines, we performed a preparative biotransformation on rac-2a (511 mg, 5 mmol). In 250 mL cell suspension, (R)-2c was obtained in 40% conversion. Amine recovery from the reaction mixture was only partial due to amine volatility, giving an isolated yield of approximately 16%; however, the product was isolated with perfect chemical (>99%) and optical purity (ee >99% (R)).
Conclusions
A number of cascades for the bioamination of alcohols using isolated enzymes in vitro have been reported during this decade. One approach entailed the combination of an ADH, a ω-transaminase (ωTA) and an alanine dehydrogenase (AlaDH),14,15,43,44 whereas others utilized either an alcohol oxidase (AOx)45,46 or a laccase/TEMPO system47 for the first oxidative step, and always in combination with a ωTA. In particular, the ADH-ωTA-AlaDH system was co-expressed and tested in resting E. coli cells at a 10 mM concentration of alcohol substrates. Although the supplementation of any cofactor was not required, the addition of 2 to 25 equivalents of L-alanine as amine donor (compared to the substrate) was mandatory to attain elevated conversion.48,49 Notably, the addition of L-alanine could be omitted only when an AOx-AlaDH-ωTA module was applied for the amination of styrene diols to yield the related 1–2 amino-alcohols.50 An alternative approach consisted of an orthogonal enzyme network operating in vitro and comprising four oxidoreductases.25 A similar network was recently implemented in a hybrid system, whereby E. coli cells expressing the ADH and an extracellularly added isolated AmDH were combined.51
In this work, we demonstrated the viability of the hydrogenborrowing amination cascade using an ADH/AmDH combination in resting E. coli cells, thus representing the simplest and most atom-efficient system for the amination of alcohols in vivo. Most of the tested substrates gave conversions to amine of approximately 80%, depending on the substrate concentration. Further studies will focus on improving the system’s toxicity resistance to amines and co-solvents in order to further increase the productivity of the bioamination. Various options are available, such as the use of non-conventional co-solvents52,53 and/or solvent-tolerant bacteria,54 as well as the implementation of a biphasic system with a hollow membrane fiber55 or a constant flow set-up rather than a batch-process. Another challenging and complementary option is the engineering of efflux pumps in E. coli for the selective secretion of the toxic amine products.56–58 Finally, the bioamination reaction could be integrated into longer multistep pathways, whereby the primary amine would become an intermediate rather than the final product; thus, keeping the amine concentration below toxicity levels would prevent cell death and increase the total productivity of the system. In conclusion, the present E. coli-ADH-AmDH system represents an important advancement towards the development of sustainable, efficient and selective biocatalytic processes for the amination of alcohols.
Experimental
General procedure for engineering recombinant E. coli strains
All genetic constructions were implemented using standard molecular biology techniques with Phusion DNA polymerase, FastDigest restriction enzymes and T4 DNA ligase (all from Thermo Scientific). Ch1-AmDH24 and AA-ADH22 were subcloned into a pETDUET plasmid, in the first and second multiple cloning sites, respectively. Ch1-AmDH also contains a N-terminal 6xHis-tag. Transformation of the pETDuet_NHis_Ch1AmDH_AA-ADH plasmid into E. coli resulted in a strain termed E. coli (Ch1-AA). The third protein, LBv-ADH,23 was cloned separately into a pET28 vector with a N-terminal GST-tag. Co-transformation of pETDuet_NHis_Ch1AmDH_AA-ADH and pET28bv_GST_LBv-ADH into E. coli BL21 DE3 resulted in the strain named E. coli (Ch1-AA-LBv).
General procedure for culturing E. coli strains
E. coli BL21 DE3 strains were inoculated in Luria–Bertani (LB) medium with either ampicillin (100 mg L−1) for E. coli (Ch1-AA), or ampicillin (100 mg L−1) and kanamycin (50 mg L−1) for E. coli (Ch1-AA-LBv). Cultures were grown overnight at 37 °C and 170 rpm. The following day, fresh LB medium with the appropriate antibiotic(s) was inoculated with the overnight culture and grown at 37 °C and 170 rpm until reaching an OD600 between 0.6 and 0.8. Protein expression was induced by adding 0.5 mM IPTG and cells were grown overnight at 25 °C and 170 rpm. Cells were harvested at 3400g for 20 minutes.
General optimized procedure for bioamination of alcohols on analytical scale
After harvesting, E. coli cell pellets were washed once with ammonium chloride buffer (1 M NH4Cl/NH3, pH 8.7). Cells were again pelleted by centrifugation at 3400g for 20 minutes and then re-suspended in ammonium chloride buffer. Biotransformations entailed 1 mL cell suspension in ammonium chloride buffer (60 mg mL–1 cells (cww), 20 mM glucose, 20 mM substrate) in 2 mL Eppendorf tubes. Reactions were incubated at 30 °C and 170 rpm for 24 h.
The reaction was quenched by the addition of KOH (200 μL, 10 M), followed by extraction with EtOAc (2 × 500 μL). The organic layer was dried with MgSO4 and conversion was determined by GC with an Agilent DB-1701 column. Details of the GC analysis and methods are reported in the ESI.†
Details about deviations from this general procedure for the various experiments can be found in ESI.† All experiments were performed, at least, with independent biological duplicates (two different batches of E. coli), each of which consisted of a technical duplicate (each reaction was performed twice). Therefore, each sample point is averaged from at least four samples.
Derivatization of samples
The enantiomeric excess of the amine product was determined after derivatization to acetamido. Samples were derivatized by adding a solution of 4-dimethylaminopyridine dissolved in acetic anhydride (20 μL of 50 mg mL–1 stock solution) to 500 μL of sample. The samples were shaken in an incubator at RT for 30 minutes, after which water (600 μL) was added and the samples were shaken for an additional 30 minutes. After centrifugation, the organic layer was dried with MgSO4. Enantiomeric excess was determined by GC with a Variant Chiracel DEX-CB column. Details of the GC analysis and methods are reported in the ESI.†
Minimal inhibitory concentration assays
E. coli BL21 DE3 (devoid of plasmid) cells were inoculated in LB medium without antibiotic. Cultures were grown overnight at 37 °C and 170 rpm. The following day, fresh LB medium was inoculated with the overnight culture and grown at 37 °C and 170 rpm until reaching an OD600 between 0.5 and 0.9. Cells were diluted to a titer of 105 cells per mL in LB medium. In a 96-well plate, 150 μL of diluted cells were added to 150 μL of the tested compounds in an appropriate concentration. Tested concentrations were 0, 5, 10, 15, 20, 30, 40 and 50 mM, respectively for all compounds except 4c, which was tested with 0, 0.08, 0.16, 0.31, 0.63, 1.25, 2.5 and 5 mM, respectively. Plates were covered with Easyseal (Greiner Bio-ONE) and incubated for 24 h at 37 °C. Each compound was tested in triplicate per 96-well plate, with at least two 96-well plates per compound (i.e., at least six samples per compound). Growth was determined by visual inspection and MIC was defined as the lowest concentration that prevents a visible growth in all vials. In the case of less reproducible results for a specific compound and/ or experimental condition, the concentration in which at least half of the samples showed no visible growth was taken as the MIC concentration. Assays with E. coli (Ch1-AA) were performed by growing the cells with 50 mg L−1 kanamycin in the LB medium.
General optimized procedure for bioamination of alcohols in biphasic aqueous–organic media on analytical scale
Preparation of resting cell suspension was performed as described above. Biotransformations consisted of 1 mL cell suspension in ammonium chloride buffer (60 mg mL−1 cells (cww), 20 mM glucose, 20 mM substrate) and 0.5 mL of n-hexadecane (C16) in 4 mL glass vials. Reactions were incubated at 30 °C and 170 rpm for 24 h. For the various experiments, details about deviations from this general procedure can be found in the ESI.†
Before extraction, the total volume of co-solvent was adjusted to 500 μL. The reaction was quenched by the addition of KOH (200 μL, 10 M). The co-solvent was removed and the aqueous phase was extracted once with 500 μL EtOAc. The EtOAc and co-solvent fractions were combined and dried with MgSO4, and conversion was determined by GC with an Agilent DB-1701 column. Details of the GC analysis and methods are reported in the ESI.†
Preparative biotransformation of racemic 2-hexanol
E. coli (Ch1-AA-LBv) was cultured (3.2 L) and harvested as described above. The preparative biotransformation consisted of 250 mL cell suspension in ammonium chloride buffer (60 mg mL−1 cells (cww), 20 mM glucose, 20 mM rac-2a (0.511 g)) in a 500 mL baffled flask. The reaction was incubated at 30 °C and 170 rpm for 24 h and monitored by GC by taking 1 mL samples (worked-up and measured as described above). Additionally, an analytical biotransformation was run in parallel with the same E. coli batch as the control experiment.
The preparative reaction mixture was acidified to pH 2–4 through the addition of a concentrated HCl solution. The reaction was extracted with methyl tert-butyl ether (3 × 60 mL) to remove the unreacted alcohol and ketone intermediate. The pH of the reaction was increased to basic pH through the addition of KOH (10 M) and extraction was performed with methyl tert-butyl ether (3 × 60 mL). The organic fractions containing the amine product were combined and dried with MgSO4. After filtration and evaporation of the solvent, the product was obtained with >99% chemical purity and >99% enantiomeric excess (R).
The authenticity of the product was confirmed by 1H-NMR (400 MHz, CDCl3, see ESI†).
Supplementary Material
† El I) available. See DOI: 10.1039/c9gc01059a
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation programme (grant agreement no 638271, BioSusAmin). Dutch funding from the NWO Sector Plan for Physics and Chemistry is also acknowledged.
Footnotes
Conflicts of interest
The authors declare to have no competing interests, or other interests that might be perceived to influence the results and/ or discussion reported in this article.
Notes and references
- 1.Nugent TC, editor. Chiral Amine Synthesis: Methods, Developments and Applications. Wiley-VCH; Weinheim, Germany: 2010. [Google Scholar]
- 2.Li W, Zhang X, editors. Asymmetric Reductive Amination. Springer; 2014. [Google Scholar]
- 3.Ghislieri D, Turner NJ. Top Catal. 2013;57:284–300. [Google Scholar]
- 4.Leonard J, Blacker AJ, Marsden SP, Jones MF, Mulholland KR, Newton R. Org Process Res Dev. 2015;19:1400–1410. [Google Scholar]
- 5.Imm S, Bahn S, Zhang M, Neubert L, Neumann H, Klasovsky F, Pfeffer J, Haas T, Beller M. Angew Chem, Int Ed. 2011;50:7599–7603. doi: 10.1002/anie.201103199. [DOI] [PubMed] [Google Scholar]
- 6.Pingen D, Muller C, Vogt D. Angew Chem, Int Ed. 2010;49:8130–8133. doi: 10.1002/anie.201002583. [DOI] [PubMed] [Google Scholar]
- 7.Bähn S, Imm S, Neubert L, Zhang M, Neumann H, Beller M. ChemCatChem. 2011;3:1853–1864. [Google Scholar]
- 8.Zhang Y, Lim CS, Sim DS, Pan HJ, Zhao Y. Angew Chem, Int Ed. 2014;53:1399–1403. doi: 10.1002/anie.201307789. [DOI] [PubMed] [Google Scholar]
- 9.Oldenhuis NJ, Dong VM, Guan Z. J Am Chem Soc. 2014;136:12548–12551. doi: 10.1021/ja5058482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knaus T, Mutti FG. Chim Oggi - Chem Today. 2017;35:34–37. [PMC free article] [PubMed] [Google Scholar]
- 11.Mutti FG, Knaus T, Scrutton NS, Breuer M, Turner NJ. Science. 2015;349:1525–1529. doi: 10.1126/science.aac9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen F-F, Liu Y-Y, Zheng G-W, Xu J-H. ChemCatChem. 2015;7:3838–3841. [Google Scholar]
- 13.Montgomery SL, Mangas-Sanchez J, Thompson MP, Aleku GA, Dominguez B, Turner NJ. Angew Chem, Int Ed. 2017;56:10491–10494. doi: 10.1002/anie.201705848. [DOI] [PubMed] [Google Scholar]
- 14.Sattler JH, Fuchs M, Tauber K, Mutti FG, Faber K, Pfeffer J, Haas T, Kroutil W. Angew Chem, Int Ed. 2012;51:9156–9159. doi: 10.1002/anie.201204683. [DOI] [PubMed] [Google Scholar]
- 15.Palacio CM, Crismaru CG, Bartsch S, Navickas V, Ditrich K, Breuer M, Abu R, Woodley JM, Baldenius K, Wu B, Janssen DB. Biotechnol Bioeng. 2016;113:1853–1861. doi: 10.1002/bit.25954. [DOI] [PubMed] [Google Scholar]
- 16.Au SK, Groover J, Feske BD, Bommarius AS. Chapter 7 – Organic Synthesis with Amino Acid Dehydrogenases, Transaminases, Amine Oxidases, and Amine Dehydrogenases. In: Goswami A, Stewart JD, editors. Organic synthesis using biocatalysis. Elsevier Inc; 2015. [Google Scholar]
- 17.Thompson MP, Turner NJ. ChemCatChem. 2017;9:3833–3836. [Google Scholar]
- 18.Böhmer W, Knaus T, Mutti FG. ChemCatChem. 2018;10:731–735. doi: 10.1002/cctc.201701366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klatte S, Lorenz E, Wendisch VF. Bioengineered. 2014;5:56–62. doi: 10.4161/bioe.27151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladkau N, Schmid A, Buhler B. Curr Opin Biotechnol. 2014;30:178–189. doi: 10.1016/j.copbio.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Kratzer R, Woodley JM, Nidetzky B. Biotechnol Adv. 2015;33:1641–1652. doi: 10.1016/j.biotechadv.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Höffken HW, Duong M, Friedrich T, Breuer M, Hauer B, Reinhardt R, Rabus R, Heider J. Biochemistry. 2006;45:82–93. doi: 10.1021/bi051596b. [DOI] [PubMed] [Google Scholar]
- 23.Schlieben NH, Niefind K, Müller J, Riebel B, Hummel W, Schomburg D. J Mol Biol. 2005;349:801–813. doi: 10.1016/j.jmb.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Bommarius BR, Schurmann M, Bommarius AS. Chem Commun. 2014;50:14953–14955. doi: 10.1039/c4cc06527a. [DOI] [PubMed] [Google Scholar]
- 25.Knaus T, Cariati L, Masman MF, Mutti FG. Org Biomol Chem. 2017;15:8313–8325. doi: 10.1039/c7ob01927k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou YJ, Yang W, Wang L, Zhu Z, Zhang S, Zhao ZK. Microb Cell Fact. 2013;12:103. doi: 10.1186/1475-2859-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin SJ, Guarente L. Curr Opin Cell Biol. 2003;15:241–246. doi: 10.1016/s0955-0674(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 28.Sun F, Dai C, Xie J, Hu X. PLoS One. 2012;7:e34525. doi: 10.1371/journal.pone.0034525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wimpenny JW, Firth A. J Bacteriol. 1972;111:24–32. doi: 10.1128/jb.111.1.24-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee IY, Kim MK, Park YH, Lee SY. Biotechnol Bioeng. 1996;52:707–712. doi: 10.1002/(SICI)1097-0290(19961220)52:6<707::AID-BIT8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 31.Bautista J, Satrustegui J, Machado A. FEBS Lett. 1979;105:333–336. doi: 10.1016/0014-5793(79)80642-0. [DOI] [PubMed] [Google Scholar]
- 32.Chassagnole C, Noisommit-Rizzi N, Schmid JW, Mauch K, Reuss M. Biotechnol Bioeng. 2002;79:53–73. doi: 10.1002/bit.10288. [DOI] [PubMed] [Google Scholar]
- 33.Henry CS, Broadbelt LJ, Hatzimanikatis V. Biophys J. 2007;92:1792–1805. doi: 10.1529/biophysj.106.093138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, Rabinowitz JD. Nat Chem Biol. 2009;5:593–599. doi: 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Molecular Cell Biology. 4th edn. W. H. Freeman; New York: 2000. [Google Scholar]
- 36.Goldman E, Green LH. Practical Handbook of Microbiology. 2nd. CRC Press; 2008. [Google Scholar]
- 37.Straathof AJ. Biotechnol Prog. 2003;19:755–762. doi: 10.1021/bp025750m. [DOI] [PubMed] [Google Scholar]
- 38.Heipieper HJ, Neumann G, Cornelissen S, Meinhardt F. Appl Microbiol Biotechnol. 2007;74:961–973. doi: 10.1007/s00253-006-0833-4. [DOI] [PubMed] [Google Scholar]
- 39.Aono R, Kobayashi H, Joblin KN, Horikoshi K. Biosci, Biotechnol, Biochem. 1994;58:2009–2014. [Google Scholar]
- 40.Pfruender H, Jones R, Weuster-Botz D. J Biotechnol. 2006;124:182–190. doi: 10.1016/j.jbiotec.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Ariëns EJ. Eur J Clin Pharmacol. 1984;26:663–668. doi: 10.1007/BF00541922. [DOI] [PubMed] [Google Scholar]
- 42.Zilberstein D, Agmon V, Schuldiner S, Padan E. J Bacteriol. 1984;158:246–252. doi: 10.1128/jb.158.1.246-252.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tauber K, Fuchs M, Sattler JH, Pitzer J, Pressnitz D, Koszelewski D, Faber K, Pfeffer J, Haas T, Kroutil W. Chem – Eur J. 2013;19:4030–4035. doi: 10.1002/chem.201202666. [DOI] [PubMed] [Google Scholar]
- 44.Lerchner A, Achatz S, Rausch C, Haas T, Skerra A. ChemCatChem. 2013;5:3374–3383. [Google Scholar]
- 45.Fuchs M, Tauber K, Sattler J, Lechner H, Pfeffer J, Kroutil W, Faber K. RSC Adv. 2012;2:6262. [Google Scholar]
- 46.Pickl M, Fuchs M, Glueck SM, Faber K. ChemCatChem. 2015;7:3121–3124. doi: 10.1002/cctc.201500589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martínez-Montero L, Gotor V, Gotor-Fernández V, Lavandera I. Green Chem. 2017;19:474–480. [Google Scholar]
- 48.Klatte S, Wendisch VF. Bioorg Med Chem. 2014;22:5578–5585. doi: 10.1016/j.bmc.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Klatte S, Wendisch VF. Microb Cell Fact. 2015;14:9. doi: 10.1186/s12934-014-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu S, Zhou Y, Wang T, Too HP, Wang DI, Li Z. Nat Commun. 2016;7:11917. doi: 10.1038/ncomms11917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J, Li Z. Biotechnol Bioeng. 2019;116:536–542. doi: 10.1002/bit.26896. [DOI] [PubMed] [Google Scholar]
- 52.Sheldon RA. Chem – Eur J. 2016;22:12984–12999. doi: 10.1002/chem.201601940. [DOI] [PubMed] [Google Scholar]
- 53.Guajardo N, Muller CR, Schrebler R, Carlesi C, de Maria PD. ChemCatChem. 2016;8:1020–1027. [Google Scholar]
- 54.Nicolaou SA, Gaida SM, Papoutsakis ET. Metab Eng. 2010;12:307–331. doi: 10.1016/j.ymben.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Gao P, Wu S, Praveen P, Loh KC, Li Z. Appl Microbiol Biotechnol. 2017;101:1857–1868. doi: 10.1007/s00253-016-7954-1. [DOI] [PubMed] [Google Scholar]
- 56.Jezierska S, Van Bogaert INA. J Ind Microbiol Biotechnol. 2017;44:721–733. doi: 10.1007/s10295-016-1858-z. [DOI] [PubMed] [Google Scholar]
- 57.Jones CM, Hernandez Lozada NJ, Pfleger BF. Appl Microbiol Biotechnol. 2015;99:9381–9393. doi: 10.1007/s00253-015-6963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyarskiy S, Tullman-Ercek D. Curr Opin Chem Biol. 2015;28:15–19. doi: 10.1016/j.cbpa.2015.05.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.