Abstract
During normal pregnancy, increased insulin resistance acts as an adaptation to enhance materno-foetal nutrient transfer and meet the nutritional needs of the developing foetus, particularly in relation to glucose requirements. However, about one in six pregnancies worldwide is affected by the inability of the mother’s metabolism to maintain normoglycaemia, with the combination of insulin resistance and insufficient insulin secretion resulting in gestational diabetes mellitus (GDM). A growing body of epidemiologic work demonstrates long-term implications for adverse offspring health resulting from exposure to GDM in utero. The effect of GDM on offspring obesity and cardiometabolic health may be partly influenced by maternal obesity; this suggests that improving glucose and weight control during early pregnancy, or better still preconception, has the potential to lessen the risk to the offspring. The consequences of GDM for microbiome modification in the offspring and the impact upon offspring immune dysregulation are actively developing research areas. Some studies have suggested that GDM impacts offspring neurodevelopmental and cognitive outcomes; confirmatory studies will need to separate the effect of GDM exposure from the complex interplay of social and environmental factors. Animal and human studies have demonstrated the role of epigenetic modifications in underpinning the predisposition to adverse health in offspring exposed to suboptimal hyperglycaemic in utero environment. To date, several epigenome-wide association studies in human have extended our knowledge on linking maternal diabetes-related DNA methylation marks with childhood adiposity-related outcomes. Identification of such epigenetic marks can help guide future research to develop candidate diagnostic biomarkers and preventive or therapeutic strategies. Longer-term interventions and longitudinal studies will be needed to better understand the causality, underlying mechanisms or impact of GDM treatments to optimise the health of future generations.
Keywords: DOHaD, Epigenetics, Gestational Diabetes, Life Course Epidemiology, Non-Communicable Disease
Introduction
Gestational diabetes mellitus (GDM) is a glucose tolerance disorder with onset during pregnancy [1]. GDM has been estimated to affect 14.4% of pregnancies globally, ranging from 7.5% in the Middle East and North Africa region to 27.0% in the South-East Asia region [2]. Although dysglycaemia usually improves after delivery, untreated GDM increases the risk of short-term complications including foetal overgrowth, shoulder dystocia, caesarean delivery, and hypertensive disorders [3]. In the long-term, exposure to GDM will likely predispose both the mother and her child to non-communicable diseases (NCDs) later in life.
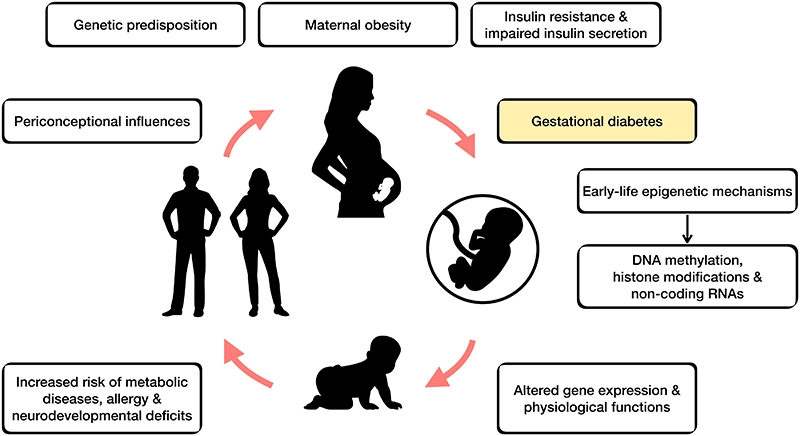
NCDs are often seen as diseases of adult lifestyle and are an important public health issue. Their aetiology is likely multifactorial, involving interactions between environmental and genetic factors and multiple risk pathways. Substantial evidence now suggests that NCDs partly originate through environmental exposures before and during pregnancy [4], which have lasting effects on the developing foetus and serve as potential targets in reversing the epidemic of NCDs. It has become apparent that children born to mothers with GDM have an increased lifetime risk for metabolic diseases compared with unexposed children [5]. This concept of lasting consequences of early life nutrition for later disease risk is widely termed ‘developmental programming’ (Figure 1).
Figure 1. Gestational diabetes mellitus and developmental programming.
There is increasing epidemiological evidence linking the early-life environmental exposures (i.e. maternal malnutrition/overnutrition, environmental chemicals, stress) with later-life health outcomes – conceptualised as the ‘developmental origins of health and disease’ (DOHaD). Compelling studies from animal models have provided strong evidence in support of the DOHaD concept. These have, for example, shown that in utero exposure to maternal diabetes and/or obesity disrupts the development and function of hypothalamus, predisposing offspring to obesity [6,7]. Several decades ago Pedersen proposed that foetal adipogenesis can result from foetal hyperinsulinemia induced by maternal hyperglycaemia [8], with more recent evidence suggesting that the mechanisms involved in lasting effects on obesity risk include epigenetic changes [9].
In this review, we highlight some of the latest findings on the long-term health consequences in offspring born to mothers with GDM, specifically relating to body composition, cardiometabolic, allergic, immune/infections and neurobehavioral outcomes, and elaborate the epigenetic changes as one of the major mechanisms linking GDM with long-term “programmed” adverse effects on the offspring.
Offspring Body composition and Cardiometabolic Health
While GDM has been linked with a higher offspring BMI, several studies have suggested that this association is confounded by higher BMI in the mother. Table 1 summarises selected studies that have examined the association of GDM with offspring body composition and cardiometabolic health. In the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) follow-up study of children aged 10-14 years, no association was found between GDM and overweight/obesity defined by BMI after adjusting for maternal BMI during pregnancy [10]. Similarly, a recent population-based retrospective study of 33,157 children aged 1-6 years showed that the significant associations of GDM coupled with large-for-gestational age on childhood overweight were no longer apparent after adjusting for pre-pregnancy BMI [11]. These findings are consistent with those of a meta-analysis [12], suggesting that GDM was not associated with BMI z-scores when accounting for maternal pre-pregnancy BMI, but few studies have accounted for maternal treatment for GDM as a moderating influence [13]. Higher maternal BMI could be associated with higher childhood adiposity through genetic transmission, shared postnatal lifestyle/environment, and the intrauterine environment [14]. Alternatively, since BMI does not distinguish the contributions of fat and lean mass, using direct measures of child adiposity (based on skinfold or simple imaging measurements) could be feasible options in epidemiological studies [10,15,16]. Positive associations between GDM and skinfold thickness have been observed in children at birth and later childhood (aged 5–10 years), with limited evidence in children aged 2–5 years [17].
Table 1. Selected studies linking GDM with offspring body composition and cardiometabolic health.
| Study | Design | Cohort | Sample size | GDM criteria | Offspring age (years) | Major outcomes for GDM-exposed offspring |
|---|---|---|---|---|---|---|
| Body composition | ||||||
| Chen et al. (2020) [11] China | Cohort | Medical Birth Registry of Xiamen, China; a population-based retrospective cohort | 33,157 | International Association of Diabetes and Pregnancy Study Groups (IADPSG) | Range: 1–6 | GDM and large-for-gestational age not associated with overweight (OR: 1.27, 95% CI; 0.96-1.68), adjusted for maternal pre-pregnancy BMI |
| Kawasaki et al. (2018) [12] Japan | Meta-analysis | Included two cohort studies adjusting for maternal BMI; UK, USA | 5,941 | Carpenter-Coustan, questionnaire | Range: 3–15.5 | Not associated with BMI z-scores (pooled MD: -0.11, 95% CI: -0.33–0.12), adjusted for covariates including maternal pre-pregnancy BMI |
| Lowe et al. (2018) [10] USA | Cohort | Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study | 4,832 | International Association of Diabetes and Pregnancy Study Groups (IADPSG) | Mean (SD): 11.4 (1.2) | Not associated with overweight/obesity (OR: 1.21, 95% CI: 1.00-1.46), adjusted for maternal BMI at OGTT during pregnancy |
| Glucose metabolism | ||||||
| Blotsky et al. (2019) [20] Canada | Matched cohort | A combination of health administrative data with birth registry information from Quebec, Canada | 36,590 mother-child pairs with GDM and matched 1:1 with controls | Two abnormal values on a 75-g OGTT or a 50-g glucose screen ≥10.3 mmol/l | From birth to 22 | Associated with incident diabetes (HR: 1.77, 95% CI: 1.41–2.22), not adjusted for maternal BMI |
| Kawasaki et al. (2018) [12] Japan | Meta-analysis | Included four cohort studies adjusting for maternal BMI; Denmark, Hong Kong SAR, USA | 890 | Self-report, questionnaire, WHO criteria 1999, OGTT | Range: 7–20 | Associated with 2-h plasma glucose (pooled MD: 0.43 mmol/L, 95% CI: 0.18–0.69), adjusted for maternal pre-pregnancy BMI |
| Lowe Jr et al. (2019) [21] USA | Cohort | HAPO Follow-up Study (FUS) | 4,160 | International Association of Diabetes and Pregnancy Study Groups (IADPSG) | Mean (SD): 11.4 (1.2) Range: 10-14 |
|
| Pathirana et al. (2020) [19] Australia | Meta-analysis | Included 11 cohort studies; China, Denmark, Greece, USA | 6,423 | NDDG, self-reported/confirmed with hospital records, Carpenter-Coustan, WHO criteria 1999, IADPSG, based on GDM risk factors followed by OGTT | Range: 7–27 | Associated with fasting glucose (standardized MD: 0.43, 95% CI 0.08–0.77), not adjusted for maternal BMI |
| Cardiovascular outcomes | ||||||
| Øyen et al. (2016) [23] Denmark | Cohort | Data linkage of Denmark’s nationwide registers | 2,025,727 | Medical record | From birth to 34 |
|
| Yu et al. (2019) [22] Denmark | Cohort | Danish national health registries | 26,272 | Medical record | From birth to 40 | Associated with overall CVD (HR: 1.19, 95% CI 1.07–1.32), hypertensive disease (HR 1.77, 1.27–2.48), adjusted for sociodemographic status and maternal/paternal history of cardiovascular disease |
CI, confidence interval; CVD, cardiovascular disease; GDM, gestational diabetes mellitus; HR, hazard ratio; md, mean difference; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NDDG, National Diabetes Data Group; OGTT, oral glucose tolerance test; OR, odds ratio; SD, standard deviation, WHO, World Health Organisation
Evidence for an effect of GDM on offspring abnormal glucose tolerance is mixed as data from several meta-analyses have provided somewhat inconsistent findings. Positive associations between GDM and postnatal abnormal glucose metabolism (fasting plasma glucose, postprandial and diabetes mellitus) in the offspring were reported in a systematic review of prospective cohort studies [18]. In a meta-analysis including 11 studies, marginally higher fasting plasma glucose levels were found in offspring exposed to GDM compared with those who were not (standard mean difference: 0.43, 95% confidence interval CI: 0.08–0.77, 6,423 children) [19]. Likewise, in a retrospective matched cohort study of Canadian mother-offspring pairs, incident diabetes in offspring from birth to 22 years was higher in those born to mothers with GDM (hazard ratio HR: 1.77, 95% CI: 1.41–2.22) [20]. However, the aforementioned studies did not account for a potential confounding effect of maternal BMI. In contrast, an earlier meta-analysis showed no association of GDM with childhood diabetes or fasting plasma glucose but a higher level of 2-h plasma glucose from prepubertal to early adulthood (pooled mean difference: 0.43 mmol/L, 95% CI: 0.18–0.69, 890 children) [12]. This finding was independent of maternal pre-pregnancy BMI. Similarly, GDM was associated with higher risk of impaired glucose tolerance (based on 30-min, 1-h, and 2-h plasma glucose) but not impaired fasting glucose in 4,160 children from the HAPO follow-up study [21].
The observed discrepancies in the relation of GDM with impaired glucose tolerance and impaired fasting glucose may result from distinct pathophysiology induced by in utero exposure to GDM, in which skeletal muscle function (implicated in the insulin resistance of impaired glucose tolerance), not liver, may be more vulnerable to GDM. Also, the HAPO follow-up study found that GDM was associated with lower child insulin sensitivity (Matsuda index) and β-cell compensation for insulin resistance (disposition index) [10]. These associations were independent of maternal BMI during pregnancy and child’s BMI z-score, reinforcing the hypothesis that intrauterine exposure to hyperglycaemia plays a part in glucose intolerance among offspring. Foetal β-cell insulin dysfunction, arising from intrauterine hyperglycaemia and manifest as a decline in β-cell compensation, is likely to contribute to a progressively increasing metabolic load and an increased risk of impaired glucose tolerance in children of mothers with GDM. This does not preclude the possibility that the above associations could be partly due to some overlap in genetic susceptibility to GDM and type 2 diabetes, given that insulin resistance and/or insulin secretory defects are key players in the pathogenesis of these conditions.
To date there are relatively few studies on the association between GDM and cardiovascular morbidity. Nonetheless, a recent 40-year follow-up study of the Danish population based cohort found an increased rate of early-onset cardiovascular disease (HR: 1.19, 95% CI: 1.07–1.32) and hypertensive disease (HR 1.77, 95% CI: 1.27–2.48) in offspring of mothers with GDM [22]. These associations were independent of sociodemographic status and maternal/paternal history of cardiovascular disease. A 34-year follow-up of the Danish cohort with over 2 million births reported a modest increase in risk of specific congenital heart defects in offspring born to mothers with GDM compared with mothers with pregestational diabetes [23]. Interestingly, a systematic review suggested that the association between GDM and congenital heart defects was evident only in women who had both GDM and pre-pregnancy obesity [24].
The effect of GDM on offspring obesity and cardiometabolic health may be in part influenced by maternal obesity; this has led to the notion that improving glycaemia and weight control during early gestation, or better still before conceiving, have the potential to lessen the risk.
Offspring Allergic Diseases
Children born to mothers with GDM may be at risk of immune dysregulation. Table 2 summarises selected studies that have examined the association of GDM and offspring allergy. A recent US study of 97,554 children (median age 7.6 years) reported evidence that the rate of childhood asthma might be influenced by more severe GDM requiring medication use [25]. Compared with no diabetes during pregnancy, an increased risk of childhood asthma was reported only in GDM cases requiring antidiabetic medications (HR: 1.12, 95% CI: 1.01-1.25), but not in those without requiring medications. These findings were independent of maternal asthma. The Boston Birth cohort found that GDM, independently of maternal pregnancy BMI and foetal growth, was associated with atopic dermatitis and allergen sensitisation (driven primarily by food sensitisation) in term births but not preterm, with speculation that term births had longer exposure to the hyperglycaemic insult at a specific point of immunological development [26]. A meta-analysis did not find an association of maternal diabetes (defined as either chronic diabetes before pregnancy or overt diabetes or glucose intolerance in pregnancy) with ever and recurrent wheezing in early childhood from birth up to 1-2 years of age [27].
Table 2. Selected studies linking GDM with offspring allergy.
| Study | Design | Cohort | Sample size | GDM criteria | Offspring age (years) | Major outcomes for GDM-exposed offspring |
|---|---|---|---|---|---|---|
| Kumar et al. (2009) [26] USA | Cohort | Boston Birth Cohort | 680 | Medical record | Mean (SD): 3.2 (2.3) | In term births, GDM associated with atopic dermatitis (OR: 7.2, 95% CI: 1.5-34.5), allergen sensitization (5.7, 1.2-28.0), food sensitization (8.3, 1.6-43.3) |
| Martinez et al. (2020) [25] USA | Cohort | Kaiser Permanente Southern California hospitals (retrospective birth cohort) | 97,554 | Carpenter-Coustan | Median age: 7.6 | GDM requiring antidiabetic medications associated with childhood asthma (HR: 1.12, 95% CI: 1.01-1.25), adjusted for maternal asthma |
| Zugna et al. (2015) [27] Italy | Meta-analysis | 11 European birth cohorts participating in the CHICOS (Developing a Child Cohort Research Strategy for Europe) project | 85,509 | Exposure: maternal diabetes | From birth to 1-2 | Maternal diabetes (regardless of type) associated with ever wheezing (pooled RR: 1.02, 95% CI: 0.98–1.06) and recurrent wheezing (1.24, 0.86–1.79) |
CI, confidence interval; GDM, gestational diabetes mellitus; HR, hazard ratio; SMD, standardised mean difference; RR, risk ratio
Although the immune system is a complex network affected by various environmental and genetic factors, the potential role of the human microbiota in influencing the host immune system has drawn considerable attention. It has been proposed that GDM triggers gut microbiota dysbiosis (i.e. altered gut microbial ecosystem) in both mother and neonate [28], which could lead to alteration of T-cell subpopulations, in turn implicated in maintaining immune tolerance. Indeed, mothers with GDM exhibited higher levels of peripheral Th2, Th17 and regulatory T cells, with these remaining unchanged from the third trimester of pregnancy up to six months postpartum [29]. Hence, it is plausible that altered levels of T-cells in the mother have an epigenetic impact on the immunological function of the offspring.
Offspring Neurocognitive Development and Behavioural Outcomes
While more is known about the association between maternal diabetes (regardless of the type) and offspring neurodevelopmental outcomes, evidence on the adverse effect of GDM is currently inconclusive.
A systematic review reported that while overall intellectual function may be within the normal range in children born to mothers with GDM, they may have an increased risk for problems related to fine and gross motor coordination, attention span and activity level compared to children born to mothers without GDM [30]. A number of important confounding factors, such as socioeconomic status, parental educational level and family upbringing, contribute to children’s cognitive performance [31]. Table 3 summarises selected cohort studies and meta-analyses that have examined the association of GDM and offspring neurodevelopmental outcomes. In a meta-analysis adjusting for parental educational attainment, a deleterious effect of maternal diabetes (encompassing GDM, type 1 and type 2 diabetes) on lower IQ score was observed in children aged 3-12 years, but the authors cautioned against drawing conclusions due to significant heterogeneity in included studies [32]. It is plausible that women with pre-existing diabetes may have received monitoring and counselling prior to pregnancy, and therefore have better controlled glucose levels. Offspring born to mothers with GDM may have a higher exposure to a greater level of circulating glucose during the early stages of pregnancy than those with pre-existing diagnosed diabetes.
Table 3. Selected studies linking GDM with neurodevelopmental outcomes.
| Study | Design | Cohort | Sample size | GDM criteria | Offspring age (years) | Major outcomes for GDM-exposed offspring |
|---|---|---|---|---|---|---|
| Kong et al. (2018) [36] Sweden | Cohort | Data linkage of Finland’s nationwide registers | 649,043 | Medical record | From birth to 11 |
|
| Nahum Sacks et al. (2016) [38] Israel | Cohort | A university medical center which serves the entire population of the Southern region of Israel | 231,271 | Medical record | Not specified (study population included all patients who delivered between the years 1991 through 2014 and their offspring) |
|
| Robles et al. (2015) [32] Spain | Meta-analysis | Included 7 cohort studies; USA, Israel | 6,140 | Exposure: maternal diabetes (regardless of type) | 1–2 years for mental and psychomotor development; 3-12 years for Intelligence Quotient (IQ) | Maternal diabetes associated with mental development (SMD: -0.41, 95% CI: -0.59 to -0.24), psychomotor development (-0.31, -0.55 to -0.07) and IQ (-0.78, -1.42 to -0.13) |
| Wan et al. (2018) [35] China | Meta-analysis | Included 16 case-control/cohort studies; USA, Canada, Sweden, Israel, Australia, Egypt | Not specified | Exposure: Maternal diabetes | Not specified | Associated with autism spectrum disorders (relative risk: 1.48; 95% CI: 1.26–1.75), adjusted for obesity, maternal age, gestational age |
| Xiang et al. (2018) [34] USA | Cohort | Kaiser Permanente Southern California hospitals (retrospective birth cohort) | 29,534 | Carpenter-Coustan | Median age: 4.9 |
|
| Zhao et al. (2019) [33] China | Meta-analysis | Included 4 cohort studies; Denmark, Greece, USA, China | 985,984 | Medical record, self-report, ADA criteria | Range: 4–19 | Associated with ADHD (RR: 2.00, 95% CI 1.42–2.81) |
ADHD, Attention deficit hyperactivity disorder; CI, confidence interval; GDM, gestational diabetes mellitus; HR, hazard ratio; OR, odds ratio; RR, risk ratio
An increased risk for deficit/hyperactivity disorder (ADHD) in children born to mothers with GDM (risk ratio: 2.00, 95% CI: 1.42–2.81, 985,984 children) has been shown in a meta-analysis [33]. Notably, a large-sample US study suggests that severe GDM requiring antidiabetic medications was associated with increased ADHD risk (HR: 1.26, 95% CI: 1.14–1.41) in children (median age: 4.9 years) compared to the non-exposed group [34]. Neither GDM requiring no medications nor gestational age at GDM diagnosis was associated with offspring ADHD risk. These associations were independent of sociodemographic factors, smoking and alcohol use, maternal history of ADHD and maternal pre-pregnancy BMI.
There are a number of observational epidemiologic studies published on offspring autism spectrum disorders outcome. A meta-analysis detected a positive association between GDM and child autism spectrum disorders even after adjustment for important covariates such as obesity, maternal age and gestational age [35]. However, a Finnish cohort of 649,043 births followed-up to 11 years reported no increased risk of child’s autism spectrum disorders in women with GDM and normal weight, after adjusting for important covariates including maternal psychiatric disorder, maternal age at delivery, maternal smoking and maternal systemic inflammatory disease [36]. Of note, more pronounced risk effects for child autism spectrum disorders were reported in obese mothers with GDM and/or maternal pregestational diabetes [36,37]. Joint effects of maternal obesity and pregestational diabetes were also observed on conduct disorders with onset in childhood as well as mixed disorders of conduct and emotions, disorders of social functioning and tic disorders with onset in childhood and adolescence [36]. Possible explanations for the joint effects between obesity and maternal pregestational diabetes are the stronger neural impact of long-term exposure to concomitant contribution of lipotoxicity, inflammation, metabolic stress and hyperglycaemia.
Limited data exists regarding other offspring neuropsychiatric disorders, with some showing either higher rates [38] or null associations [36] with eating disorders, and positive associations of sleep disorders [36,38] in children exposed to GDM.
Developmental Programming by Epigenetic Mechanisms
In the context of foetal programming, epigenetic processes are thought to be an important mechanism underpinning lasting effects on the offspring [9]. Epigenetic modifications are cell type and tissue-specific, which involve changes in gene expression and genomic structure without altering the DNA sequence. Epigenetic processes include DNA methylation, histone post-translational modifications and expression of non-coding RNAs. GDM, as an example of maternal environmental trigger, can play a role in influencing offspring outcomes through epigenetic regulation of genes. DNA methylation is the classic and most studied epigenetic measure, primarily found in the CpG (cytosine followed by a guanine) sequence contexts. The identification of DNA methylation patterns related to adverse health-related outcomes in offspring is a potentially useful tool to assess individuals at risk for health problems in early life exposed to GDM, representing an important window of opportunity for early interventions during childhood.
Animal Studies
Evidence from non-human animal models suggests that in utero GDM exposure leads, for example, to developmental and functional alterations of the hypothalamus [6,39], heightening the risk of developing overweight/obesity in the offspring. Animal models of developmental programming have to date mainly involved nutritional, toxin exposure, selective breeding and direct genetic manipulations.
A study of streptozotocin-induced maternal diabetes in mice showed an inhibitory effect of intrauterine hyperglycaemia exposure on the development of brown adipose tissue (BAT) in offspring, thereby impairing the glucose uptake function of BAT in adulthood [40]. The authors found a down-regulation of BAT-associated genes, Ucp1, Cox5b, and Elovl3, which are accompanied by disorganised ultrastructural of mitochondria in BAT, probably contributing to intracellular lipid accumulation and fat-induced insulin resistance [40]. Another GDM mouse model showed altered DNA methylation patterns in pancreatic tissues, manifested as dyslipidaemia, impaired glucose tolerance and insulin resistance with advancing age [41]. The authors rationalised that the pancreas has a direct role in regulating blood glucose levels, and should hence serve as an important target tissue to demonstrate the role of DNA methylation as opposed to the more widely studied samples such as placenta, umbilical cord blood or maternal peripheral blood.
However, animal models using chemical approaches such as streptozotocin to induce permanent pancreatic damage with impaired insulin secreting function and irreversible diabetes may be of limited relevance to GDM, which is transient in nature and usually returns to euglycemia after childbirth. A recent mice experiment studied the induction of transient glucose tolerance in pregnant mice with an insulin receptor antagonist (S961), reporting that mice born from S961-treated dams showed no susceptibility to physical or reflexes development in early neonatal period, but had long-term metabolic (glucose intolerance) and cognitive impairment consequences in adulthood when administered a high-fat-diet [42]. The administration high-fat-diets in mice mimics typical energy rich diets in both developing and industrialised countries, implicating epigenetic alterations as an important mechanism underpinning the induction of altered phenotypes in response to environmental cues.
Human Studies
The mechanistic pathways underlying long-term morbidity in offspring exposed to GDM are incompletely understood so far, but a growing number of studies have supported involvement of epigenetic mechanisms in the association of GDM with offspring health. Most human studies on epigenetic mediation examined the associations of in utero GDM exposure and DNA methylation in placentas, offspring cord or infant blood, as summarised in a recent review [43]. Several differentially methylated genes in foetal tissues of babies born to mothers with GDM have been identified using a candidate gene approach; these include loci related to the leptin (LEP), adiponectin (ADIPOQ), mesoderm-specific transcript (Mest), the ATP-binding cassette transporter A1 (ABCA1), SLC2A1/GLUT1 and SLC2A3/GLUT3 genes. Epigenetic modifications at these loci in response to impaired glucose homeostasis during pregnancy might lead to lifelong susceptibility to adiposity development in offspring.
Two epigenome-wide association studies (EWAS) using Illumina 450k methylation arrays have reported associations of maternal diabetes-related DNA methylation marks with childhood adiposity-related outcomes [44,45]. One of these studies included data from two prospective cohorts - the EPOCH (Exploring Perinatal Outcomes in Children) and the Colorado Healthy Start - which identified six GDM-exposure-associated DNA methylation marks that were linked to measures of childhood adiposity and fat distribution [44]. Peripheral/cord blood samples of GDM-exposed and non-GDM-exposed offspring (n=285, aged 10.5 years) were profiled, revealing that DNA methylation of the SH3PXD2A gene was associated with BMI, waist circumference, skinfold thicknesses, subcutaneous adipose tissue and leptin levels, after adjustment for cell proportions [44]. In the second study of 388 Pima Indian children of Arizona (aged 13.0 years) [45], the observed DNA methylation marks altered by intrauterine exposure to maternal diabetes and linked to offspring BMI and insulin secretory were different from those detected by the EPOCH study. The discrepancies in DNA methylation hits could be due to the different population studied, covariates adjusted for, and outcomes of interest.
A causal relation between maternal hyperglycaemia and epigenetic regulation of the leptin gene (with biological relevance to long-term programming of offspring excessive adiposity) in offspring cord blood has been reported based on a 2-step epigenetic Mendelian randomised approach [46]. The epigenetic adaptations triggered by maternal glycaemia, resulted in an association between lower DNA methylation levels at CpG site cg12083122 (in leptin gene of the offspring) and higher cord blood leptin levels [46]. Using mediation analysis, higher DNA methylation levels of the key genes responsible for glycaemic/lipid metabolism (PPARGC1α) were correlated with higher cord blood leptin levels in offspring exposed to maternal hyperglycaemia [47]. DNA methylation (increased methylation of PYGO1 and CLN8) has also been reported to mediate effects of in utero GDM exposure on adverse offspring cardiometabolic traits (increased VCAM-1 levels) [48].
For neurodevelopmental outcome, a recent meta-analyses of EWAS data published by the Pregnancy and Childhood Epigenetics Consortium (with 3677 mother-neonate pairs from seven pregnancy cohorts) showed that GDM was associated with offspring cord blood hypomethylation of the OR2L13 promoter, a gene associated with autism spectrum disorder [49]. Notably, the study accounted for numerous potential confounding influences, including cord blood cell heterogeneity, which is one of the potential sources of variability in DNA methylation.
In a human placenta study [50], maternal dysglycaemia in pregnancy was associated with altered DNA methylation of the serotonin transporter gene (SLC6A4), a principal regulator of serotonin homeostasis. Serotonin, a neurotransmitter, is involved in neurodevelopmental disorders (e.g. depression, anxiety, autism). SLC6A4 methylation levels were negatively associated with maternal glucose levels (both fasting and 2-h plasma glucose) in the 24–28 weeks of gestation, after adjustment for maternal pre-pregnancy BMI and gestational weight gain. Further, placental SLC6A4 methylation was inversely associated with SLC6A4 mRNA levels, suggesting a functional role of the CpG sites in regulating SLC6A4 gene expression, and that epigenetic changes predominates over genetic mechanism in the human placenta. A separate study has shown differential SLC6A4 methylation as a predictive epigenetic marker of adiposity from birth to adulthood [51]. Such studies provide valuable information on epigenetic marks that can guide future research in developing potential diagnostic biomarkers and predictive/treatment strategies for adverse health events.
Transgenerational Epigenetic Inheritance
Increasing research on animal models, mainly in mice and rats, suggests that developmental programming is a transgenerational phenomenon. The programmed phenotype is passed on through several possible mechanisms including persistence of the adverse environmental exposures in subsequent generations, altered maternal phenotype, and inheritance of epigenetic modifications via alteration of the epigenome (germline and/or somatic line). Whilst most literature on transgenerational transmission of traits have focused on the maternal contributions to offspring, impacts of paternal contributions have also been observed [4].
A GDM mice model of intrauterine hyperglycaemia induced by streptozotocin showed a pattern of dysregulation at key methylation sites in the placenta (reflected by down-regulation and up-regulation of Dlk1 and Gtl2 genes, respectively) of the F1 and F2 generations [52]. A reduction in placental weight was found to be transmitted paternally to the F2 offspring, but not maternally, which was believed to be linked to the susceptibility of the sperm under a suboptimal intrauterine environment.
While the transgenerational transmission of traits has been reported through to F2 offspring, evidence on the transmission through to F3 and subsequent generations remains unclear [53]. Studying F3 and succeeding generations is important to eliminate the possible confounding effects by the initial adverse maternal insults on the embryo.
Although there is substantial evidence on the transgenerational inheritance of epigenetic modifications in mice and rats, the application of this concept in humans has been challenged by others [54], mainly due to a complex sum of many confounding factors including ecological and cultural inheritance [55]. Well-controlled experiments in mammalian animal models and large-scale cohorts/well-characterised epidemiological studies are required in the future.
Do GDM treatment interventions improve long-term offspring health?
Infants born to mothers receiving treatment of GDM in the form of dietary advice, blood glucose monitoring and insulin therapy have improved perinatal outcomes compared with those born to women receiving routine care [56]. However, evidence from a Cochrane review of long-term follow-up studies of GDM treatment interventions suggests that treatment may not reduce childhood obesity [13]. In two follow-up studies of children whose mothers participated in pregnancy trials for the treatment of mild GDM, there was no difference in child’s BMI (aged 4-10 years) by treatment and control groups [57,58]. A possible reason for the null finding is that more-pronounced GDM might be necessary to program long-term treatment effects on the development of offspring obesity. Nonetheless, female offspring of mothers treated for mild GDM had lower fasting glucose levels, suggesting a beneficial effect of treatment of mild GDM in relation reducing the risk of offspring insulin resistance in females [57].
Compared with insulin treatment, findings from the Metformin in Gestational diabetes: The Offspring Follow-Up (MiG TOFU) cohort reported no differences in the body fat percent and metabolic measures in children (aged 7-9 years) whose mothers had been randomised to metformin and insulin GDM-treatment [59]. However, metformin-exposed children at 9 years of age were larger than the insulin-exposed group [59]. In line with this, a meta-analysis including three follow-up studies of RCTs reported that children prenatally exposed to metformin-treatment for GDM were heavier than those whose mothers received insulin-treatment [60]. Larger studies with longer follow-up will be needed to better understand the health impact of GDM treatments on offspring to optimise the health of future generations.
There is also evidence for the periconceptional period as an early window for which poor environmental exposures can induce adverse health effects in offspring [4]. Interventions delivered during pregnancy may only partly alter foetal growth and development, and therefore studies examining interventions that begin preconception are warranted. A large multi-centre RCT is underway to investigate the effectiveness of a nutritional (containing myo-inositol, probiotics and additional micronutrients) intervention commencing before conception and continuing during pregnancy to maintain good maternal glycaemic control, with the aim of improving offspring health outcomes [61].
Conclusion
Overall, there is increasing evidence for an impact of in utero GDM exposure on lifetime health in the offspring. However, whether maternal GDM contributes directly to childhood adiposity remains to be elucidated, given that maternal BMI and gestational weight gain are also linked with childhood adiposity. Other observed long-term offspring adverse consequences include cardiovascular abnormalities, glucose/insulin dysfunction, allergic/respiratory health and neurodevelopmental outcomes. Most evidence is based on observational prospective cohorts, and further studies are required to advance our knowledge of the effect of GDM and its treatment on development, function and health in the offspring. Taken together, the adverse health impacts of in utero GDM exposure on offspring may rely upon epigenetic changes in selected genes. Notably, many of these epigenetic modifications may not be reversible and may persist throughout the offspring’s life course. More studies in both animal and human models are needed to replicate the epigenetic findings, with careful consideration of the selection of cell or tissue types for epigenetic analysis because epigenetic mechanisms are generally tissue-specific. There is also a need for larger studies with long-term follow-up to understand the health impact of GDM treatments in preventing adverse programming of health outcomes in offspring.
Key Messages (2-3 messages).
A mother’s glycaemic status and weight during preconception and pregnancy influence the long-term health of the offspring.
The offspring’s future health can be programmed through the role of epigenetic changes induced by a hyperglycaemic environment in utero.
More longitudinal studies are warranted to investigate the causality and underlying mechanisms of GDM on offspring’s long-term health to provide a basis for developing effective interventions during this critical period, with the aim of improving lifelong health and wellbeing.
Footnotes
Disclosure Statement
KMG has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, BenevolentAI Bio Ltd. and Danone. KMG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042) and NIHR Southampton Biomedical Research Centre (IS-BRC-1215-20004)), the European Union (Erasmus+ Project ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP), British Heart Foundation (RG/15/17/3174) and the US National Institute On Aging of the National Institutes of Health (Award No. U24AG047867).
References
- 1.American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2016 Dec;40(Supplement 1):S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas. 9th. Brussels, Belgium: International Diabetes Federation; 2019. Available from: https://diabetesatlas.org/data/en/ [Google Scholar]
- 3.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391(10132):1842–52. doi: 10.1016/S0140-6736(18)30312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burlina S, Dalfrà MG, Lapolla A. Short- and long-term consequences for offspring exposed to maternal diabetes: a review. J Matern Fetal Neonatal Med. 2019;32(4):687–94. doi: 10.1080/14767058.2017.1387893. [DOI] [PubMed] [Google Scholar]
- 6.Steculorum SM, Bouret SG. Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring. Endocrinology. 2011;152(11):4171–9. doi: 10.1210/en.2011-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris MJ, Chen H. Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. Int J Obes. 2009;33:115–22. doi: 10.1038/ijo.2008.213. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen J. Glucose content of the amniotic fluid in diabetic pregnancies; correlations with the maternal blood sugar. Acta Endocrinol. 1954;15:342–54. doi: 10.1530/acta.0.0150342. [DOI] [PubMed] [Google Scholar]
- 9.Godfrey KM, Costello PM, Lillycrop KA. The developmental environment, epigenetic biomarkers and long-term health. J Dev Orig Hlth Dis. 2015;6(5):399–406. doi: 10.1017/S204017441500121X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowe WL, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;320(10):1005–1016. doi: 10.1001/jama.2018.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Han L, Shi X, Su W, Liu W, Wang L, et al. Adverse pregnancy outcomes on the risk of overweight offspring: a population-based retrospective study in Xiamen, China. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-58423-7. 1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawasaki M, Arata N, Miyazaki C, Mori R, Kikuchi T, Ogawa Y, et al. Obesity and abnormal glucose tolerance in offspring of diabetic mothers: A systematic review and meta-analysis. PLoS ONE. 2018;13(1):e0190676. doi: 10.1371/journal.pone.0190676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown J, Alwan NA, West J, Brown S, Mckinlay CJD, Farrar D, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5(5) doi: 10.1002/14651858.CD011970.pub2. CD011970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VWV, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endo. 2017;5(1):53–64. doi: 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boeke CE, Oken E, Kleinman KP, Rifas-Shiman SL, Taveras EM, Gillman MW. Correlations among adiposity measures in school-aged children. BMC Pediatr. 2013;13:99. doi: 10.1186/1471-2431-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mooney A, Kelsey L, Fellingham GW, George JD, Hager RL, Myrer JW, et al. Assessing body composition of children and adolescents using dual-energy x-ray absorptiometry, skinfolds, and electrical impedance. Meas Phys Educ Exerc Sci. 2011;15(1):2–17. [Google Scholar]
- 17.Shafaeizadeh S, Harvey L, Abrahamse-Berkeveld M, Muhardi L, van der Beek EM. Gestational diabetes mellitus is associated with age-specific alterations in markers of adiposity in offspring: A narrative review. Int J Environ Res Public Health. 2020;17:3187. doi: 10.3390/ijerph17093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nattero-Chávez L, Luque-Ramírez M, Escobar-Morreale HF. Systemic endocrinopathies (thyroid conditions and diabetes): impact on postnatal life of the offspring. Fertil Steril. 2019;111(6):1076–91. doi: 10.1016/j.fertnstert.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 19.Pathirana MM, Lassi ZS, Roberts CT, Andraweera PH. Cardiovascular risk factors in offspring exposed to gestational diabetes mellitus in utero: systematic review and meta-analysis. J Dev Orig Hlth Dis. 2020;6:1–18. doi: 10.1017/S2040174419000850. [DOI] [PubMed] [Google Scholar]
- 20.Blotsky AL, Rahme E, Dahhou M, Nakhla M, Dasgupta K. Gestational diabetes associated with incident diabetes in childhood and youth: A retrospective cohort study. CMAJ. 2019;191(15):E410–7. doi: 10.1503/cmaj.181001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowe WL, Jr, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42(3):372–80. doi: 10.2337/dc18-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Y, Arah OA, Liew Z, Cnattingius S, Olsen J, Sørensen HT, et al. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ. 2019;367 doi: 10.1136/bmj.l6398. 16398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Øyen N, Diaz LJ, Leirgul E, Boyd HA, Priest J, Mathiesen ER, et al. Prepregnancy Diabetes and offspring risk of congenital heart disease: a nationwide cohort study. Circulation. 2016;133(23):2243–53. doi: 10.1161/CIRCULATIONAHA.115.017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parnell AS, Correa A, Reece EA. Pre-pregnancy obesity as a modifier of gestational diabetes and birth defects associations: a systematic review. Matern Child Nutr. 2017;21(5):1105–20. doi: 10.1007/s10995-016-2209-4. [DOI] [PubMed] [Google Scholar]
- 25.Martinez MP, Lin J, Chow T, Chung J, Wang X, Xiang AH. Maternal gestational diabetes and type 2 diabetes during pregnancy and risk of childhood asthma in offspring. J Pediatr. 2020;219:173–179. doi: 10.1016/j.jpeds.2019.12.053. e1. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Ouyang F, Story RE, Pongracic JA, Hong X, Wang G, et al. Gestational diabetes, atopic dermatitis, and allergen sensitization in early childhood. J Allergy Clin Immunol. 2009;124(5):1031–8. doi: 10.1016/j.jaci.2009.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zugna D, Galassi C, Annesi-Maesano I, Baïz N, Barros H, Basterrechea M, et al. Maternal complications in pregnancy and wheezing in early childhood: a pooled analysis of 14 birth cohorts. Int J Epidemiol. 2015;44(1):199–208. doi: 10.1093/ije/dyu260. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Zheng J, Shi W, Du N, Xu X, Zhang Y, et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut. 2018;67:1614–25. doi: 10.1136/gutjnl-2018-315988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sifnaios E, Mastorakos G, Psarra K, Panagopoulos N-D, Panoulis K, Vitoratos N, et al. Gestational diabetes and T-cell (Th1/Th2/Th17/Treg) immune profile. In Vivo. 2019;33(1):31–40. doi: 10.21873/invivo.11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ornoy A, Reece EA, Pavlinkova G, Kappen C, Miller RK. Effect of maternal diabetes on the embryo, fetus, and children: Congenital anomalies, genetic and epigenetic changes and developmental outcomes. Birth Defects Res C Embryo Today. 2015;105(1):53–72. doi: 10.1002/bdrc.21090. [DOI] [PubMed] [Google Scholar]
- 31.Kelstrup L, Bytoft B, Hjort L, Houshmand-Oeregaard A, Mathiesen ER, Damm P, et al. Diabetes in pregnancy: long-term complications of offsprings. Front Diabetes. 2019;28:201–22. [Google Scholar]
- 32.Robles MC, Campoy C, Fernandez LG, Lopez-Pedrosa JM, Rueda R, Martin MJ. Maternal diabetes and cognitive performance in the offspring: A systematic review and meta-analysis. PLoS ONE. 2015;10(11):e0142583. doi: 10.1371/journal.pone.0142583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao L, Li X, Liu G, Han B, Wang J, Jiang X. The association of maternal diabetes with attention deficit and hyperactivity disorder in offspring: a meta-analysis. Neuropsychiatr Dis Treat. 2019;15:675–84. doi: 10.2147/NDT.S189200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang AH, Wang X, Martinez MP, Getahun D, Page KA, Buchanan TA, et al. Maternal gestational diabetes mellitus, type 1 diabetes, and type 2 diabetes during pregnancy and risk of ADHD in offspring. Diabetes Care. 2018;41(12):2502–8. doi: 10.2337/dc18-0733. [DOI] [PubMed] [Google Scholar]
- 35.Wan H, Zhang C, Li H, Luan S, Liu C. Association of maternal diabetes with autism spectrum disorders in offspring: A systemic review and meta-analysis. Medicine. 2018;97(2):e9438–e9438. doi: 10.1097/MD.0000000000009438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong L, Norstedt G, Schalling M, Gissler M, Lavebratt C. The risk of offspring psychiatric disorders in the setting of maternal obesity and diabetes. Pediatrics. 2018 Sep;142(3):e20180776. doi: 10.1542/peds.2018-0776. [DOI] [PubMed] [Google Scholar]
- 37.Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, et al. The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics. 2016;137:e20152206. doi: 10.1542/peds.2015-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nahum Sacks K, Friger M, Shoham-Vardi I, Abokaf H, Spiegel E, Sergienko R, et al. Prenatal exposure to gestational diabetes mellitus as an independent risk factor for long-term neuropsychiatric morbidity of the offspring. Am J Obstet Gynecol. 2016;215(3) doi: 10.1016/j.ajog.2016.03.030. 380.e1-7. [DOI] [PubMed] [Google Scholar]
- 39.Franke K, Harder T, Aerts L, Melchior K, Fahrenkrog S, Rodekamp E, et al. “Programming” of orexigenic and anorexigenic hypothalamic neurons in offspring of treated and untreated diabetic mother rats. Brain Res. 2005;1031(2):276–83. doi: 10.1016/j.brainres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Yu DQ, Lv PP, Yan YS, Xu GX, Sadhukhan A, Dong S, et al. Intrauterine exposure to hyperglycemia retards the development of brown adipose tissue. FASEB Journal. 2019;33(4):5425–39. doi: 10.1096/fj.201801818R. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Z, Chen X, Xiao Y, Wen J, Chen J, Wang K, et al. Gestational diabetes mellitus alters DNA methylation profiles in pancreas of the offspring mice. J Diabetes Complicat. 2019;33(1):15–22. doi: 10.1016/j.jdiacomp.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 42.de Sousa RAL, de Lima Ev, da Silva TP, de Souza Rv, Figueiredo CP, Passos GF, et al. Late Cognitive Consequences of Gestational Diabetes to the Offspring, in a New Mouse Model. Mol Neurobiol. 2019;56(11):7754–64. doi: 10.1007/s12035-019-1624-0. [DOI] [PubMed] [Google Scholar]
- 43.Elliott HR, Sharp GC, Relton CL, Lawlor DA. Epigenetics and gestational diabetes: a review of epigenetic epidemiology studies and their use to explore epigenetic mediation and improve prediction. Diabetologia. 2019;62:2171–2178. doi: 10.1007/s00125-019-05011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Iv, Zhang W, Davidson EJ, Fingerlin TE, Kechris K, Dabelea D. Epigenetic marks of in utero exposure to gestational diabetes and childhood adiposity outcomes: the EPOCH study. Diabet Med. 2018;35(5):612–20. doi: 10.1111/dme.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen P, Piaggi P, Traurig M, Bogardus C, Knowler WC, Baier LJ, et al. Differential methylation of genes in individuals exposed to maternal diabetes in utero. Diabetologia. 2017;60(4):645–55. doi: 10.1007/s00125-016-4203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allard C, Desgagné V, Patenaude J, Lacroix M, Guillemette L, Battista MC, et al. Mendelian randomization supports causality between maternal hyperglycemia and epigenetic regulation of leptin gene in newborns. Epigenetics. 2015;10(4):342–351. doi: 10.1080/15592294.2015.1029700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Côté S, Gagné-Ouellet V, Guay SP, Allard C, Houde AA, Perron P, et al. PPARGC1α gene DNA methylation variations in human placenta mediate the link between maternal hyperglycemia and leptin levels in newborns. Clin Epigenetics. 2016;62:2171–2178. doi: 10.1186/s13148-016-0239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West NA, Kechris K, Dabelea D. Exposure to Maternal Diabetes in Utero and DNA Methylation Patterns in the Offspring. Immunometabolism. 2013;1:1–9. doi: 10.2478/immun-2013-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howe CG, Cox B, Fore R, Jungius J, Kvist T, Lent S, et al. Maternal gestational diabetes mellitus and newborn DNA Methylation: Findings from the pregnancy and childhood epigenetics consortium. Diabetes Care. 2020;43(1):98–105. doi: 10.2337/dc19-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blazevic S, Horvaticek M, Kesic M, Zill P, Hranilovic D, Ivanisevic M, et al. Epigenetic adaptation of the placental serotonin transporter gene (SLC6A4) to gestational diabetes mellitus. PLoS ONE. 2017;12(6):e0179934. doi: 10.1371/journal.pone.0179934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lillycrop KA, Garratt ES, Titcombe P, Melton PE, Murray RJS, Barton SJ, et al. Differential SLC6A4 methylation: a predictive epigenetic marker of adiposity from birth to adulthood. Int J Obes. 2019;43:974–988. doi: 10.1038/s41366-018-0254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang Y, Yu YC, Ding GL, Gao Q, Chen F, Luo Q. Intrauterine hyperglycemia induces intergenerational Dlk1-Gtl2 methylation changes in mouse placenta. Oncotarget. 2018;9(32):22398–22405. doi: 10.18632/oncotarget.23976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aiken CE, Ozanne SE. Transgenerational developmental programming. Hum Reprod Update. 2014;20:63–75. doi: 10.1093/humupd/dmt043. [DOI] [PubMed] [Google Scholar]
- 54.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell. 2014;157(1):95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horsthemke B. A critical view on transgenerational epigenetic inheritance in humans. Nat Commun. 2018;9 doi: 10.1038/s41467-018-05445-5. 2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 57.Landon MB, Rice MM, Varner MW, Casey BM, Reddy UM, Wapner RJ, et al. Mild gestational diabetes mellitus and long-term child health. Diabetes Care. 2015;38(3):445–52. doi: 10.2337/dc14-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care. 2010;33(5):964–8. doi: 10.2337/dc09-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rowan JA, Rush EC, Plank LD, Lu J, Obolonkin V, Coat S, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6(1):e000456. doi: 10.1136/bmjdrc-2017-000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Weelden W, Wekker V, de Wit L, Limpens J, Ijäs H, van Wassenaer-Leemhuis AG, et al. Long-Term Effects of Oral Antidiabetic Drugs During Pregnancy on Offspring: A Systematic Review and Meta-analysis of Follow-up Studies of RCTs. Diabetes Ther. 2018;9(5):1811–29. doi: 10.1007/s13300-018-0479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Godfrey KM, Cutfield W, Chan SY, Baker PN, Chong YS, NiPPeR Study Group Nutritional Intervention Preconception and During Pregnancy to Maintain Healthy Glucose Metabolism and Offspring Health (“NiPPeR”): Study protocol for a randomised controlled trial. Trials. 2017;18:131. doi: 10.1186/s13063-017-1875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, et al. Association of maternal diabetes with autism in offspring. JAMA. 2015;313(14):1425–34. doi: 10.1001/jama.2015.2707. [DOI] [PubMed] [Google Scholar]