Abstract
The ubiquity of tertiary alkylamines in pharmaceutical and agrochemical agents, natural products and smallmolecule biological probes1,2 continues to stimulate enormous efforts towards their streamlined synthesis3–9. Arguably, the most robust method for tertiary alkylamine synthesis is carbonyl reductive amination3: comprising two elementary steps, condensation of a secondary alkylamine with an aliphatic aldehyde forms an all alkyl-iminium ion, which is reduced by a hydride reagent. Chemists have sought to develop direct strategies for a ‘higher order’ variant of this reaction via the union of an alkyl fragment with an in-situ generated all alkyl-iminium ion10–14. However, despite more than 70 years of research, the successful realization of a ‘carbonyl alkylative amination’ has remained elusive. Herein, we report that a practical and general solution can be accomplished by the addition of alkyl-radicals to all alkyl-iminium ions. The process is facilitated by visible-light and a silane reducing agent, which, together with the other reaction components, trigger a distinct radical initiation step to establish a chain process. An attractive feature of this operationally straightforward, metal-free and modular transformation is the unbiased nature of tertiary amines that arise from the traceless union of aldehydes and secondary amines with alkyl-halides. As such, the structural and functional diversity within these classes of abundant feedstocks provides a versatile and flexible strategy for the streamlined synthesis of complex tertiary amines.
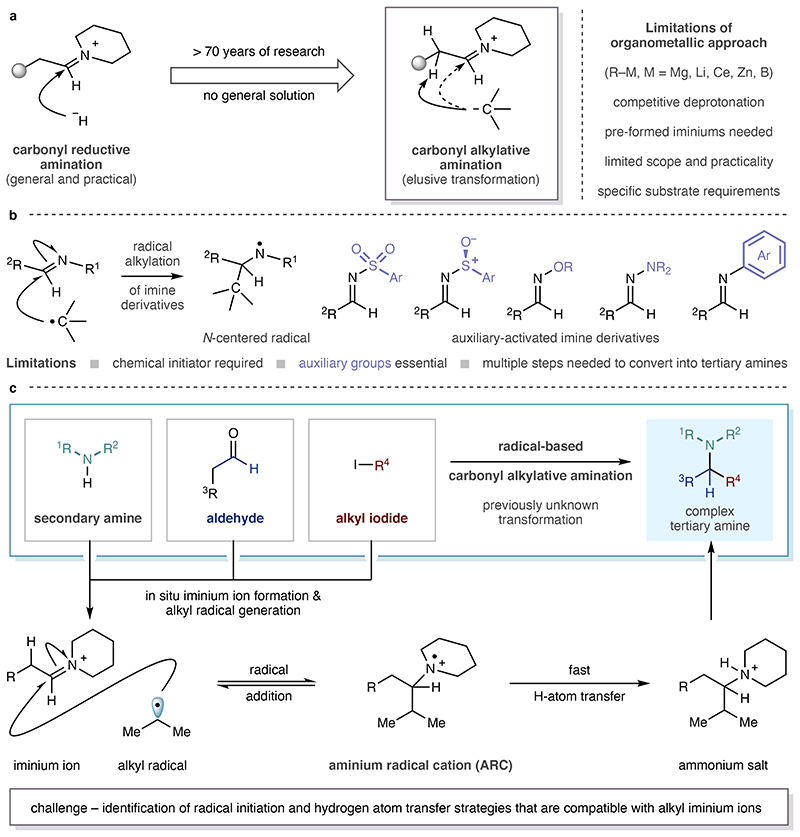
Carbonyl reductive amination is an effective method for the preparation of linear tertiary alkylamines, however, the synthesis of branched variants frequently presents problems3: the condensation of a secondary alkylamine with a dialkylketone is often slow and may require the use of activating reagents; di-alkylketones are also not as readily available as aldehydes and may require multi-step syntheses. A method by which an alkyl group could be directly added to an aldehyde-derived alkyl-iminium ion would circumvent these problems and would increase complexity by leveraging three, rather than two, programmable feedstocks into a tertiary alkylamine synthesis. Unfortunately, the most logical approach to this multi-component strategy - direct addition of common organometallic nucleophiles to alkyl-iminium ions - seldom succeeds in delivering the tertiary alkylamine product11–14: organometallic reagents, such as Grignard and alkyl-lithium reagents, are rarely compatible with in-situ iminium ion formation from alkyl aldehydes and secondary alkylamines, necessitating pre-formation and isolation of an unstable iminium ion12,15. Additionally, their high basicity results in competitive deprotonation of the C-H bond adjacent to the carbon-nitrogen double bond, dramatically restricting their scope16. Less reactive zinc- or cerium-based organometallics also exhibit limited scope and are restricted to activated alkyl fragments12,13,15’ 17–18. The Petasis reaction offers broader scope in the amine component but suffers from specific substrate requirements in the carbonyl and organoboron-derived components19. More efficient reactivity has been demonstrated with C(sp)-20,21, C(sp2)-19,22 and allyl-nucleophiles19,23, or with activating auxiliary-derived imines4, however the successful deployment of a suitably reactive and generally available source of unactivated alkyl nucleophile for the addition to an alkyl-iminium ion continues to prove uniquely challenging. Considering the limitations of the organometallic methods, chemists have also sought to develop strategies based on the addition of neutral alkyl-radical species to imine-derivatives10, 24–26. However, the low electrophilicity of the carbon-nitrogen double bond means that the use of an activating group on either the nitrogen atom (R1) or in the carbonyl component (R2) is essential to render the imine-derivative sufficiently reactive towards alkyl-radicals, limiting these reactions’ scope and practical application with respect to the downstream synthesis of tertiary amine targets (Figure 1b)10,24–26.
Figure 1. Evolution of a strategy for carbonyl alkylative amination.
a Addition of alkyl groups to alkyl-iminium ions remains an elusive transformation. b Radical alkylation of auxiliary-activated imine derivatives. c This work: Carbonyl alkylative amination for the synthesis of complex tertiary alkylamines - addition of alkyl radicals to alkyl-iminium ions.
Seeking to develop a direct carbonyl alkylative amination (CAA) method, we hypothesized that the addition of a neutral carbon-centered radical to an in situ generated, positively-charged, alkyl-iminium ion would obviate the requirement for auxiliary-activated imines and provide a single-step synthesis of tertiary alkylamines. To the best of our knowledge, the elementary step comprising the direct intermolecular addition of an alkyl-radical to an alkyl-iminium ion has not been reported, that is, with the exception of a solitary example in 1991 using a mercury salt under UV-light irradiation to generate an alkyl-radical that was added to a formaldehyde-derived iminium ion27. Addition of a neutral nucleophilic alkyl-radical to an alkyl-iminium ion would afford an aminium radical cation (ARC), a species that could then be intercepted through hydrogen atom transfer (HAT) to form the protonated tertiary alkylamine (Figure 1c). A radical-based CAA requires the orchestration of a number of simultaneously occurring events to form reactive intermediates, each of which is capable of following competitive and deleterious reaction pathways. For example, a high concentration of the reactive alkyl-iminium ion would need to be maintained in order to effectively engage the incipient alkyl-radical. Furthermore, the HAT-reagent must be capable of rapidly intercepting the transient ARC while avoiding reduction of the iminium ion. In spite of these challenges, we report the realization of our hypothesis through the development of modular, unbiased and efficient CAA, a new method that combines three abundant feedstocks – secondary amine, aldehyde and alkyl-halide – in a single step. Central to the success of this protocol is the unique effect of visible-light in facilitating a radical initiation step under mild conditions and leads to a practical and general synthesis of complex tertiary alkylamines.
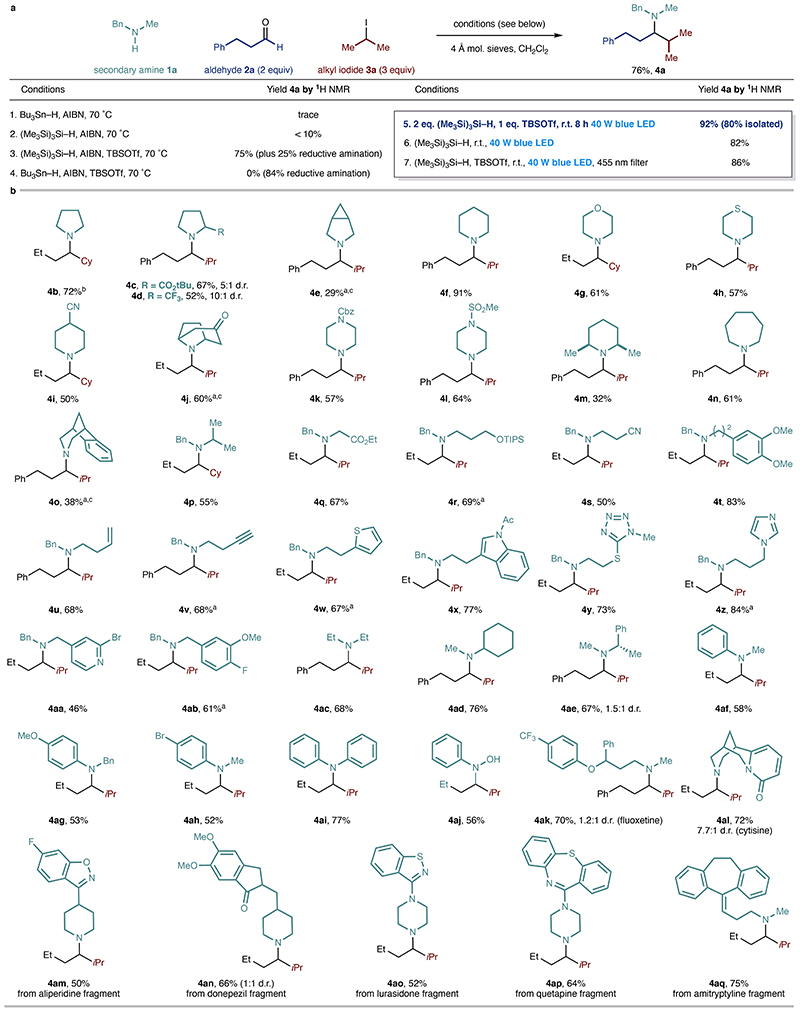
Initial investigations focused on a representative reaction between N-methylbenzylamine, hydrocinnamaldehyde and 2- iodopropane (Figure 2a). Attempts to secure CAA using classical protocols for radical generation failed to produce an efficient reaction28. For example, use of tributyltin hydride (Bu3Sn-H) and azobisisobutyronitrile (AIBN) gave trace amounts of alkylamine 4a (entry 1), which could be modestly improved by using tris(trimethylsilyl)silane [(Me3Si)3Si-H] in place of the tin reagent (entry 2)29. We evaluated different additives to promote the high alkyl-iminium ion concentration needed to effectively intercept the alkyl-radical and found that the presence of tert-butyldimethylsilyl trifluoromethylsulfonate (TBSOTf) secured high conversion, as determined by 1H NMR (S18-S19, Supplementary Information 1). Consequently, a thermal reaction combining (Me3Si)3Si-H, AIBN and TBSOTf produced the desired alkylamine 4a but as a 3:1 mixture with the corresponding reductive amination product 4a’ (entry 3, 4a’ not shown). Interestingly, a reaction that combined TBSOTf with Bu3Sn-H and AIBN, under thermal conditions, resulted exclusively in formal reductive amination to 4a’ (entry 4). Further exploration of the target transformation (S5, Supplementary Information 1) led us to identify a set of unique and much simpler reaction conditions that exploited the effect of visible- light activation, which, after parameter optimization, produced high yields of the desired alkylamine (4a in 92% assay yield and 80% yield after isolation) with only trace amounts of reductive amination (entry 5). When TBSOTf was omitted, 4a was still obtained in 82% assay yield, providing a set of conditions which could be used when acid-sensitive functional groups were present. An important aspect of these mild reaction conditions is the distinct nature of the radical initiation step. While there are a number of possible explanations for this phenomenon, homolysis of the C-I bond seems unlikely given that a control reaction using a 455 nm long-pass filter (removing the minor UV and near-UV components of the blue LED lamp required for the homolysis30’31) still generated 4a in 86% yield (entry 7). We studied systematically the lightabsorbing properties of each component and likely intermediates involved in the CAA reaction – secondary amine, alkylaldehyde, alkyl-iodide, enamine, iminium and (Me3Si)3Si-H – as well as their combinations, in order to probe for the possibility of a photosensitization or electron donor-acceptor (EDA) complex that could be involved in radical generation32. While neither any component alone, nor the combination of any two components showed absorption above or close to 455 nm, the UV-Vis absorption spectrum of a ternary mixture comprising enamine (formed by condensation of 1a and 2a), 2-iodopropane (3a) and (Me3Si)3Si-H revealed a new red-shifted band (400-500 nm). Although at present we do not have sufficient evidence to invoke a specific interaction, this observation potentially supports a multi-component interaction, whose visible-light excitation leads to radical initiation (S12-17, Supplementary Information 1). Regardless, this radical initiation process offers distinct advantages over other light-mediated processes because the (Me3Si)3Si-H is not incorporated in the newly formed bond and thereby permits C(sp3)-C(sp3) bond generation; previous reports have been restricted to the formation of carbon-heteroatom bonds involving the initiating reagent31,33. Furthermore, we believe that the benign nature of the new radical generation step, in the absence of classical chemical initiators, will aid the development of straightforward metal-free radical C(sp3)-C(sp3) bond forming reactions from alkyl-halides.
Figure 2. Scope of the amine component in carbonyl alkylative amination.
a. Optimal reaction conditions. b. Scope of amine component. aNo TBSOTf used bTMSOTf used instead of TBSOTf. cAmine’HCl salt used. TMS: trimethylsilyl; Tf: trifluoromethanesulfonyl; Cy: cyclohexyl.
Having established a viable protocol, we began an extensive investigation of the scope of CAA by first testing its capacity to produce a-branched cyclic tertiary alkylamines, which revealed a wide range of functionalized saturated cyclic and heterocyclic secondary alkylamines could be successfully employed in this radical-based process (Figure 2b), producing the desired alkylamines (4b-4o). Notably, auxiliary-activated radical addition methods cannot be used to directly access this class of tertiary alkylamines, which can be produced in a single step using the new protocol. We found that N,N- dialkylamines displaying a range of both linear and branched functionalized alkyl substituents (including aromatic heterocycles) gave the tertiary alkylamines 4a, 4p-4ae in good yields.
For certain amines bearing electron-withdrawing groups close to the nitrogen atom, reductive amination was observed as a competing side reaction (10% with 4s). N-alkyl anilines and even poorly nucleophilic diarylamines could be employed effectively (4af-4ai), which expands the potential scope of the CAA process, as evidenced by their ubiquity in pharmaceutically-relevant molecules. A reaction using N-phenyl hydroxylamine produced the expected product (4aj) via addition to the corresponding nitrone intermediate. To demonstrate compatibility with features typically encountered in pharmaceutical agents, we showed that a range of drug fragments proved tolerant of the reaction conditions, producing the complex tertiary amine products in synthetically useful yields (4ak-4aq).
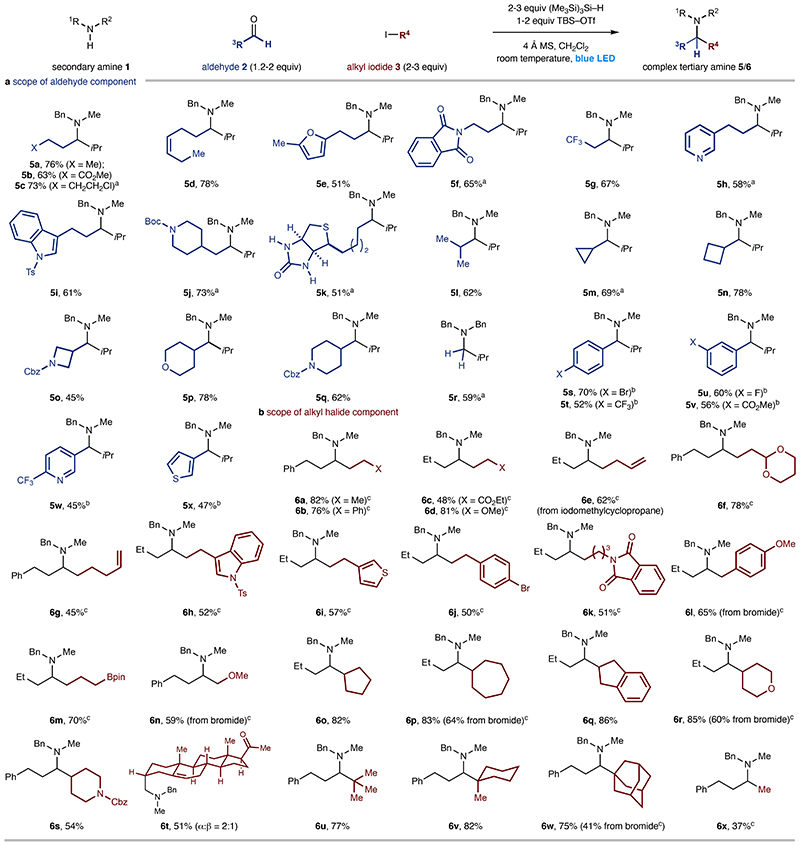
A selection of functionalized linear aldehydes produced the corresponding tertiary alkylamines (Figure 3a, 5a-k). Branched aldehydes, including saturated cyclic and heterocyclic features also performed well, forming hindered tertiary alkylamines 5l-q. Beyond aliphatic aldehydes, we were pleased to observe that formaldehyde (to 5r), substituted benzaldehydes (to 5sv) and heteroaryl aldehydes (5w-x) function well in the reaction and their successful conversion to amine products considerably expands the scope of CAA.
Figure 3. Reaction scope.
a. Scope of the aldehyde component. b. Scope of alkyl halide component. aNo TBSOTf used. bTMSOTf used instead of TBSOTf. cReaction with 5-10mol% 2-methyl-2-iodopropionate as initiator.
Simple and functionalized primary alkyl iodides proved to be good coupling partners when 5-10mol% of ethyl 2-iodo-2- methylpropionate (which, more easily, generates alkyl radicals than a primary alkyl iodide) was added to the reaction34, with linear alkane fragments added to alkyl-iminium ions to form tertiary alkylamines 6a-n. Although the reaction accommodated a variety of functional groups in the alkyl-halide, slightly lower yields were observed for radicals containing proximal electron withdrawing groups (6c). This is likely to originate from a competitive HAT between the silane and alkyl-radical as well as slower rate of addition of an electrophilic alkyl-radical to the iminium ion. Benzyl bromides and methoxymethyl bromide, the iodides of which are unstable, were found to be suitable alkylating agents and gave the amine products in synthetically useful yields (6l, 6n). As expected, secondary alkyl-halides were competent substrates and formed amines 6o-t in good yields. Tertiary alkyl-iodides were also excellent coupling partners, producing hindered alkylamine products 6u-w (which would be challenging to prepare via reductive amination) in high yield. A selection of unactivated alkyl-bromides reacted under the modified conditions (with the radical chain initiator34) to give reasonable yields of the tertiary alkylamines (6l, 6n, 6p, 6r & 6w) and provides an alternative protocol if an alkyl-iodide is not available. We were pleased to find that iodomethane (acting as a source of methyl-radical) could be added to an iminium ion providing access to an important class of branched tertiary alkylamines (6x). In addition to the 90 examples of successful CAA documented in Figures 2 and 3, we have detailed an extended assessment of the reaction scope (Figure S9, Supplementary Information 1) that includes substrates that give moderate, but still synthetically useable, yields as well as examples where the process is low yielding or unsuccessful.
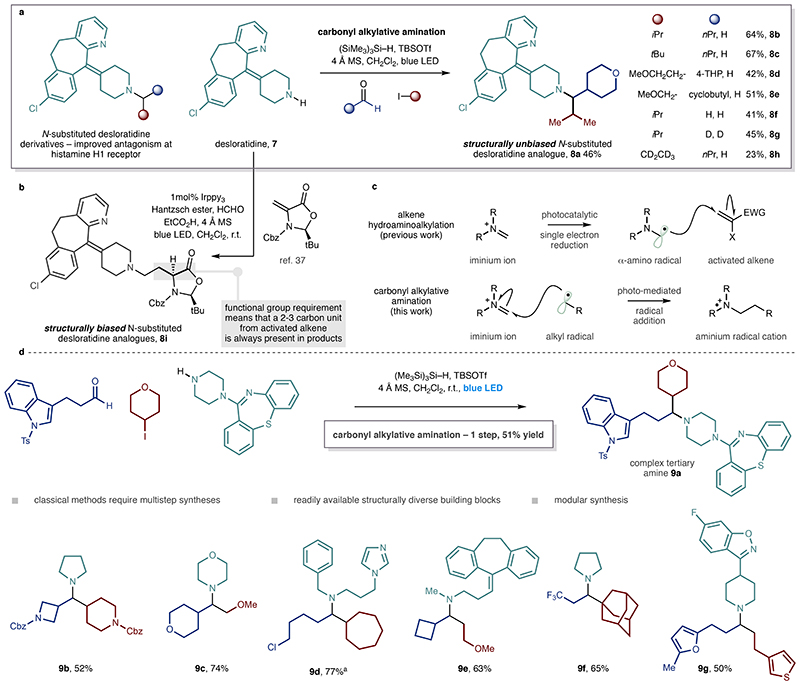
As a further demonstration of this new methodology, we showed that the secondary amine-containing pharmaceutical agent, desloratadine, whose tertiary amine derivatives have recently attracted attention due to their improved pharmacokinetic properties and convey a longer duration of antagonism at the histamine H1 receptor35, can be used as the amine component of this CAA to directly append two interchangeable molecular fragments within the a-branched tertiary alkyl amine product (Figure 4a). Using the standard coupling conditions, we showed that a collection of desloratadine- derived β-branched tertiary amine products can be readily prepared in a modular fashion (8a-g). As a demonstration of the reactions’ efficacy, we were able to prepare a α-d2-A’-alkyl derivative of desloratidine (>95% D incorporation, 8g) in a single step from commercial d2-paraformaldehyde and 2-iodopropane. Furthermore, a similar reaction, but using d5-iodoethane and a linear aldehyde provided direct access to different class of labelled tertiary amine (8h)36.
Figure 4. One step synthesis of complex tertiary alkyl amines via carbonyl alkylative amination and its comparison with related methods.
a. Rapid and streamlined synthesis of desloratadine analogues made possible by CAA. b. An example of amine synthesis using photocatalytic alkene hydroaminoalkylation highlighting the ‘biased’ nature of the products resulting from the activated alkene acceptor. c. Distinct mechanistic pathways of alkene hydroaminoalkylation and CAA. d. Structurally diverse tertiary alkylamines formed by CAA in one step from readily available building blocks.
CAA on desloratadine provides a striking distinction from our previous work37 on photoredox-mediated alkylamine synthesis, which was based on alkene hydroaminoalkylation via the generation and reaction of a-amino radicals (Figure 4b-c). While we previously showed that this secondary amine can engage highly activated alkenes (to 8i), the tertiary alkylamine products always contain the structural signature derived from the intrinsic requirements of a reaction requiring an activated alkene acceptor. In contrast, the present mechanistically distinct, reaction enables the use of a wide range of primary, secondary and tertiary alkyl-halides, providing direct access to tertiary alkylamines that are unbiased by any functional requirements of the transformation and contain no trace of the activating groups required in the readily available building blocks.
A major advantage of this CAA strategy is its modularity; the vast array of distinct coupling partners within each of the three abundant feedstocks required for this reaction means that structural and functional diversity can be easily programmed into the tertiary amine products (Figure 4d). To illustrate this, we evaluated the cross compatibility of the process by varying each reaction component in order to produce a range of complex tertiary alkylamines with diverse structural and functional properties. The reaction’s robust nature is reflected by the ease with which densely functionalized alkylamines 9a-g can be produced in a single step from readily available building blocks, highlighting their suitability for early-stage drug discovery applications (Figure 4d). The streamlined nature by which these products are prepared, undoubtedly demonstrates the synthetic potential of this new process. Given the intuitive retrosynthetic logic that underpins CAA, this practical multi-component process has the potential to become the benchmark in amine preparation for practitioners of synthetic chemistry in academic and industrial institutions.
Supplementary Material
Acknowledgments
Funding
We acknowledge the Swiss National Science Foundation (R.K.), the Gates Cambridge Trust (N.J.F.), the EPSRC (W.G.W.) and the Royal Society (for Wolfson Merit Award, M.J.G.).
Footnotes
Authors contributions: R.K., N.J.F., W.G.W. and M.J.G. conceived the project; R.K., N.J.F., W.G.W. conducted and analyzed the experiments; and R.K., N.J.F., W.G.W. and M.J.G. wrote the manuscript. † N.J.F. and W.G.W. contributed equally to the project and are listed alphabetically.
Competing interests: no conflicting interests are declared.
Additional information:
Supplementary information is available at XXXXX
Reprints and permissions information is available at http://www.nature.com/reprints
Data and materials availability
Materials and methods, experimental procedures, useful information, mechanistic studies, optimization studies, 1H NMR spectra, 13C NMR spectra and MS data are available in the Supplementary Information. Raw data are available from the corresponding author on reasonable request.
References
- 1.Roughley SD, Jordan AM. The medicinal chemist’s toolbox: An analysis of reactions used in the pursuit of drug candidates. J Med Chem. 2011;54:3451–3479. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- 2.Blakemore DC, et al. Organic synthesis provides opportunities to transform drug discovery. Nat Chem. 2018;10:383–394. doi: 10.1038/s41557-018-0021-z. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Magid AF, Mehrman SJ. A review on the use of sodium triacetoxyborohydride in the reductive amination of ketones and aldehydes. Org Process Res Dev. 2006;10:971–1031. [Google Scholar]
- 4.Robak MT, Herbage MA, Ellman J. A Synthesis and applications of tert-butanesulfinamide. Chem Rev. 2011;110:3600–3740. doi: 10.1021/cr900382t. [DOI] [PubMed] [Google Scholar]
- 5.Huang L, Arndt M, GooBen K, Heydt H, GooBen LJ. Late transition metal-catalyzed hydroamination and hydroamidation. Chem Rev. 2015;115:2596–2697. doi: 10.1021/cr300389u. [DOI] [PubMed] [Google Scholar]
- 6.Pirnot MT, Wang Y-M, Buchwald SL. Copper hydride-catalyzed hydroamination of alkenes and alkynes. Angew Chem Int Ed. 2016;57:48–57. doi: 10.1002/anie.201507594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musacchio AJ, et al. Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science. 2017;355:727–730. doi: 10.1126/science.aal3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matier CD, Schwaben J, Peters JC, Fu GC. Copper-catalyzed alkylation of aliphatic amines induced by visible light. J Am Chem Soc. 2017;139:17707–17710. doi: 10.1021/jacs.7b09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grogan G. Synthesis of chiral amines using redox biocatalysis. Curr Opin Chem Biol. 2018;43:15–22. doi: 10.1016/j.cbpa.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Friestad GK. Addition of carbon-centered radicals to imines and related compounds. Tetrahedron. 2001;57:5461–5496. [Google Scholar]
- 11.Reiber HG, Stewart TD. The tetra alkyl methylene immonium salts. J Am Chem Soc. 1940;62:3026–3030. [Google Scholar]
- 12.Paukstelis JW, Cook A. In: G In enamines: Synthesis, structure and reactions. Cook AG, editor. Vol. 6. Marcel Dekker Inc; New York: 1988. pp. 275–346. [Google Scholar]
- 13.Bloch R. Additions of organometallic reagents to CN bonds: Reactivity and selectivity. Chem Rev. 1998;98:1407–1438. doi: 10.1021/cr940474e. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser EM. Lithium: Annual survey covering the year 1975. J Organomet Chem. 1977;130:1–131. [Google Scholar]
- 15.Werner V, Ellwart M, Wagner AJ, Knochel P. Preparation of tertiary amines by the reaction of iminium ions derived from unsymmetrical aminals with zinc and magnesium organometallics. Org Lett. 2015;17:2026–2029. doi: 10.1021/acs.orglett.5b00801. [DOI] [PubMed] [Google Scholar]
- 16.Saidi MR, Nazari M. Aminoalkylation with aldehydes mediated by solid lithium perchlorate. Monatshefte fur Chemie. 2004;135:309–312. [Google Scholar]
- 17.Agosti A, Britto S, Renaud P. An efficient method to convert lactams and amides into 2,2-dialkylated amines. Org Lett. 2008;10:1417–1420. doi: 10.1021/ol800145h. [DOI] [PubMed] [Google Scholar]
- 18.Haurena C, LeGall E, Sengmany S, Martens T. Chiral amines in the diastereoselective Mannich-related multicomponent synthesis of diarylmethylamines, 1,2-diarylethylamines, and b-arylethylamines. Tetrahedron. 2010;66 99029911. [Google Scholar]
- 19.Wu P, Givskov M, Nielson TE. Reactivity and synthetic applications of multicomponent Petasis reactions. Chem Rev. 2019;119:11245–11290. doi: 10.1021/acs.chemrev.9b00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aschwanden P, Carreira EM. Diederich F, Stang PJ, Tykwinski RR, editors. Addition of terminal acetylides to C=O and C=N electrophiles in acetylene chemistry. Chemistry, biology and materials. Wiley-VCH. 2005:101–138. [Google Scholar]
- 21.Lauder K, Toscani A, Scalacci N, Castagnolo D. Synthesis and reactivity of propargylamines in organic chemistry. Chem Rev. 2017;117:14091–14200. doi: 10.1021/acs.chemrev.7b00343. [DOI] [PubMed] [Google Scholar]
- 22.Heinz C, et al. Ni-catalyzed carbon-carbon bond-forming reductive amination. J Am Chem Soc. 2018;140:2292–2300. doi: 10.1021/jacs.7b12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen T-Y, Tsutsumi R, Montgomery TP, Volchkov I, Krische MJ. Ruthenium catalyzed C-C coupling of amino alcohols with dienes via transfer hydrogenation: Redox-triggered imine addition and related hydroaminoalkylations. J Am Chem Soc. 2015;137:1798–1801. doi: 10.1021/ja5130258. [DOI] [PubMed] [Google Scholar]
- 24.Miyabe H, Yoshioka E, Kohtani S. Progress in intermolecular carbon radical addition to imine derivatives. Curr Org Chem. 2010;14:1254–1264. [Google Scholar]
- 25.Friestad GK. Radical additions to chiral hydrazones: stereoselectivity and functional group compatibility. Top Curr Chem. 2012;320:61–92. doi: 10.1007/128_2011_163. [DOI] [PubMed] [Google Scholar]
- 26.Tauber J, Imbri D, Opatz T. Radical addition to iminium ions and cationic heterocycles. Molecules. 2014;19:16190–16222. doi: 10.3390/molecules191016190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell GA, Yao C-F, Rajaratnam R, Kim BH. Electron transfer processes. 52. Promotion of electron transfer by protonation of nitrogen-centered free radicals. The addition of radicals to iminium ions. J Am Chem Soc. 1991;113:373–375. [Google Scholar]
- 28.Baguley PA, Walton JC. Flight from the tyranny of tin: The quest for practical radical sources free from metal encumbrances. Angew Chem Int Ed. 1998;37:3072–3082. doi: 10.1002/(SICI)1521-3773(19981204)37:22<3072::AID-ANIE3072>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Chatgilialoglu C, Ferreri C, Landais Y, Timokhin VI. Thirty years of (TMS)ßSiH: A milestone in radical-based synthetic chemistry. Chem Rev. 2018;118:6516–6572. doi: 10.1021/acs.chemrev.8b00109. [DOI] [PubMed] [Google Scholar]
- 30.Le C, Chen TQ, Liang T, Zhang P, MacMillan DWC. A radical approach to the copper oxidative addition problem: Trifluoromethylation of bromoarenes. Science. 2018;360:1010–1014. doi: 10.1126/science.aat4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng Y, Mück-Lichtenfeld C, Studer A. Metal-free radical borylation of alkyl and aryl iodides. Angew Chem Int Ed. 2018;57:16832–16836. doi: 10.1002/anie.201810782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahamonde A, Melchiorre P. Mechanism of the stereoselective a-alkylation of aldehydes driven by the photochemical activity of enamines. J Am Chem Soc. 2016;138:8019–8030. doi: 10.1021/jacs.6b04871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fawcett A, Pradeilles J, Wang Y, Mutsuga T, Myers EL, Aggarwal VK. Photoinduced decarboxylative borylation of carboxylic acids. Science. 2017;357:283–286. doi: 10.1126/science.aan3679. [DOI] [PubMed] [Google Scholar]
- 34.Lei L, et al. Systematic study on alkyl iodide initiators in living radical polymerization with organic catalysts. Macromolecules. 2014;47:6610–6618. [Google Scholar]
- 35.Bosma R, et al. Route to prolonged residence time at the Histamine Hi receptor: growing from Desloratadine to Rupatadine. J Med Chem. 2019;62:6630–6644. doi: 10.1021/acs.jmedchem.9b00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atzrodt J, Derdau V, Kerr WJ, Reid M. Deuterium- and tritium-labelled compounds: applications in the life sciences. Angew Chem Int Ed. 2018;57:1758–1784. doi: 10.1002/anie.201704146. [DOI] [PubMed] [Google Scholar]
- 37.Trowbridge A, Reich D, Gaunt MJ. Multicomponent synthesis of tertiary alkylamines by photocatalytic olefinhydroaminoalkylation. Nature. 2018;561:522–527. doi: 10.1038/s41586-018-0537-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Materials and methods, experimental procedures, useful information, mechanistic studies, optimization studies, 1H NMR spectra, 13C NMR spectra and MS data are available in the Supplementary Information. Raw data are available from the corresponding author on reasonable request.