Abstract
A five-year-old male central bearded dragon (Pogona vitticeps) was presented for investigation of blood in the voided urates. A small cutaneous mass was detected in the gular region, but clinical examination was otherwise unremarkable. Fecal parasitology was negative. Initially, further diagnostics were declined, and antimicrobial treatment was initiated. At re-examination one month later, the gular mass had increased in size and an additional mass was detected within the celomic cavity. Both masses were surgically excised and diagnosed by histopathology as a high-grade anaplastic sarcoma (gular mass), resembling a histiocytic sarcoma, and a Sertoli cell tumor (coelomic mass). Neither of these have been previously reported in the central bearded dragon. Twenty months post-surgery, the lizard remains well with no recurrence of clinical signs or evidence of tumor re-growth.
Keywords: Anaplastic sarcoma, central bearded dragon, neoplasia, Pogona vitticeps, reptile, Sertoli cell tumor
Introduction
Reptiles are susceptible to neoplasia, with incidence being comparable to that seen in birds and mammals (Christman et al., 2017). This case report discusses the diagnosis and treatment of ananaplastic sarcoma and Sertoli cell tumor in the same central bearded dragon (Pogona vitticeps), both of which, to the authors’ best knowledge, have not been reported previously in this species.
Presentation
A five-year-old male central bearded dragon was presented for investigation of blood in the voidedurates. Appetite, fecal consistency and frequency of elimination were normal; the dragon’s behaviour at home was also normal. No other concerns were reported by the owner, and the husbandry was considered adequate for the species. The lizard was captive-bred and had no previous medical history. On physical examination, the lizard was bright, alert and responsive and weighed 490g with a body condition score of 5/9. A 5-mm spherical dermal mass was detected in the right caudolateral aspect of the gular region; examination was otherwise unremarkable. Fecal examination, by direct smear and float, was negative for endoparasites. Further investigations were declined and empirical therapy of enrofloxacin (Baytril, 25mg/ml; Bayer; Newbury, UK) at 10mg/kg orally once daily for seven days was initiated to rule out bacterial infection. Ideally, a culture should have been performed prior to the use of a fluoroquinolone to confirm the need for a second-line antibiotic, but this was declined because of cost concerns.
The bearded dragon re-presented one month later with no improvement in clinical signs. The lizard remained bright and had a normal appetite; however, the gular mass had increased in size to 25mm and an approximately 35mm diameter mid-celomic mass was now also palpable. The origin of the blood was suspected to be the urogenital system based on it being passed with the voided urate portion of the droppings. However, intestinal disease was considered a differential given the ability for feces and urate to mix in the cloaca.
Diagnostic Investigations
A blood sample was taken from the ventral coccygeal vein for hematologic and biochemistry testing. All biochemical values were within normal intervals for Pogona vitticeps(Tamukai et al., 2011); however on blood smear examination, marked toxicity and left shift of the heterophils were found (without a heterophilia), indicating high (possibly overwhelming) inflammatory demand and accelerated production of heterophils. Frequent reactive and occasional plasmacytoid lymphocytes were also found, likely because of antigenic stimulation (Figure 1). Other leukocyte, erythrocyte and thrombocyte concentrations and morphologies were unremarkable. Dorsoventral and lateral conscious whole-body radiographs were taken using standard and horizontal beam radiography (Figure 2). Two abnormal areas of soft-tissue opacity were noted, a 25 mm diameter irregular mass within the caudal aspect of the right gular region and a 38mm diameter rounded mass in the mid-coelomic region. No further radiographical abnormalities were observed.
Figure 1.
Blood smear images from this central bearded dragon. A: Normal heterophil with fully granulated cytoplasm. B-E: Toxic heterophils with varying degrees of decreased granulation and basophilic cytoplasm. F: Giant toxic heterophil with decreased granulation and cytoplasmic basophilia. G-J: Severely toxic heterophils with toxic granulation (dark purple). K: Small and medium lymphocytes. L: Medium lymphocyte with basophilic cytoplasm and finely granular chromatin. M: Plasmacytoid lymphocyte. Modified Wright’s stain, 100x oil objective.
Figure 2.
Dorso-ventral radiograph. Arrowhead: gular mass. Arrow: coelomic mass.
Treatment and Outcome
Excisional biopsy of the masses was performed under general anesthesia. Analgesia was provided using 0.5mg/kg morphine (Morphine sulfate, 10mg/ml; Martindale Pharmaceuticals; Romford, UK) and 0.3mg/kg meloxicam (Metacam, 2mg/ml; Boehringer Ingelheim; Ingleheim/Rhein, Germany) by intramuscular injection two-hours prior to surgery. General anesthesia was induced using 10mg/kg alfaxalone (Alfaxan, 10mg/ml; Jurox; Crawley, UK) by intravenous injection into the ventral coccygeal vein. The lizard was intubated with a 2mm uncuffed endotracheal tube and maintained on isoflurane (IsoFlo; Zoetis; London, UK) and oxygen using a ventilator (SAV04; Vetronic Services Ltd.; Abbotskerswell, UK) to perform intermittent positive-pressure ventilation. Capnography (Impact III; Vetronic Services Ltd.; Abbotskerswell, UK) was used to monitor end-tidal CO2 and Doppler ultrasonography was used to monitor heart rate and rhythm. The operating theatre was maintained at 30°C (86°F) and a cloacal temperature probe was used to monitor body temperature. The lizard was placed in dorsal recumbency and the surgical sites were aseptically prepared with warmed, diluted iodine (Vetasept Povidone Iodine Surgical Scrub; Animalcare; Dunnington, UK).
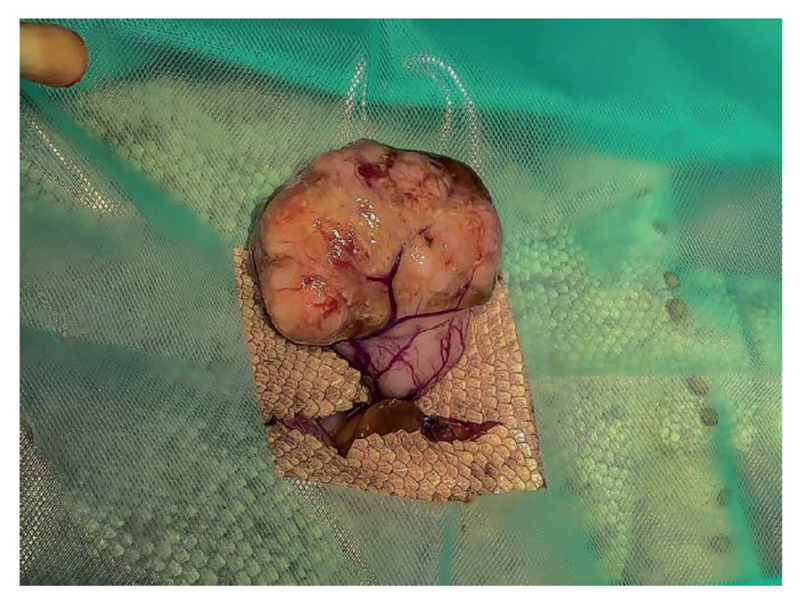
For the coelomic mass, a paramedian celiotomy incision was made, avoiding the midline ventralabdominal vein. Exploratory celiotomy revealed an approximately 35-millimetre cream-colored, irregular, semi-firm mass in the mid-coelom (Figure 3). It was attached via a short pedicle to the aorta. A transfixing suture using 3-0 poliglecaprone 25 (Monocryl; Ethicon; Livingston, UK) was placed as close to the aorta as possible to achieve wide margins and the mass removed. Examination of the celom revealed no further abnormalities. The celom was closed using 3-0 nylon (Ethilon; Ethicon) in a single layer, horizontal mattress pattern. Sutures were removed six weeks post-sugery.
Figure 3.
Sertoli cell tumor expanding the right testicle, exposed via a para-median celiotomy incision.
A circular incision was made around the gular mass (Figure 4). This revealed a firm, cream-colored mass within the empty space of the beard, not attached to deeper structures, originating from the integument. The skin was closed as previously described for closure of the coelom. The two masses were fixed in formalin and submitted for histopathological examination. The lizard made an uneventful recovery from anesthesia. Food was withheld the first night to reduce the risk of regurgitation but was readily accepted the following morning. Morphine was repeated the day following surgery and meloxicam was continued once daily for one week following surgery at the previously noted doses. The lizard quickly returned to normal activity and feeding levels following surgery and no further blood was seen in the urates. Twenty months post-surgery, the lizard remains healthy with no evidence of recurrence of either mass or distant metastasis.
Figure 4.
Anaplastic sarcoma in the gular region.
Histopathological examination
Histopathological examination of the mid-coelomic mass revealed testicular tissue expanded by an infiltrative neoplasm composed of spindloid to polygonal cells originating from, and expanding, seminiferous tubules that are separated by prominent fibrous tissue, consistent in appearance with a Sertoli cell tumor (Figure 5). Multifocally, neoplastic cells invaded surrounding fibrous tissue resulting in rupture of seminiferous tubules and hemorrhage. This evidence of ongoing destruction of local tissue architecture by the neoplasm was interpreted as the source of the blood that contributed to the presenting clinical sign. It is likely that the hemorrhage drained into the vas deferens, which then pooled in the urodeum before being passed with the urine/urate component. Excision of the mass was judged to be complete and was expected to be curative.
Figure 5.
Seminiferous tubules are expanded by neoplastic Sertoli cells and separated by prominent fibrous tissue, which in some areas is infiltrated by neoplastic cells. Testicular Sertoli cell tumor, bearded dragon, hematoxylin and eosin stain. Bar = 500 μm. Inset: neoplastic Sertoli cells fill seminiferous tubules. Bar = 100 μm.
Histopathological examination of the gular mass revealed a large expansion of the dermis by an infiltrative, non-encapsulated, densely cellular proliferation of polygonal to plump spindloid cells forming streams and irregular sheets supported by an extensive collagenous stroma. Cells were anaplastic with marked cellular pleomorphism and frequent karyomegaly, cytomegaly and multinucleated giant cells (Figure 6). Occasional neoplastic cells appeared to be phagocytosing red blood cells. There were five mitotic figures per ten high power (x400) fields. The gular mass exhibited junctional activity with the overlying epidermis, characterized by multifocal disruption and infiltration of the stratum basale and melanin incontinence. Within the neoplasm, there were multiple areas of necrosis and hemorrhage, and there was a diffuse infiltrate of heterophils and lymphocytes with occasional lymphoid follicle formation. Focally, the overlying epidermis was ulcerated with an associated infiltrate of inflammatory cells. Neoplastic cells extended to the deep margin of the examined sections. The tumor had negative immunoreactivity to multi-cytokeratin antibodies (Cytokeratin AE1/3, Leica Microsystems (UK) Ltd, Milton Keynes, UK) and an assessment of vimentin immunoreactivity, using antibody optimized for mammalian species (Dako, Santa Clara, CA, US), was unsuccessful. The mass was diagnosed as a high-grade anaplastic sarcoma that most closely resembled a histiocytic sarcoma given the cellular morphology, presence of phagocytic activity, and of multinucleated giant cells. Due to the presence of junctional activity, a diagnosis of amelanotic melanoma was also considered, however this was thought to be less likely given the cellular features listed above that were more suggestive of a phagocytic phenotype.
Figure 6.
Sheets of anaplastic polygonal to spindloid cells demonstrating karyomegaly and cytomegalywith occasional multinucleated giant cells. Bi- and tri-nucleated cells indicated by arrowheads. Dermal high-grade anaplastic sarcoma, bearded dragon, hematoxylin and eosin stain. Bar = 50 μm
Discussion
Primary reptilian oncological research is deficient, making the diagnosis and subsequent treatment and prognosis of neoplastic presentations difficult (Christman et al., 2017). In one retrospective pathology analysis, neoplasia accounted for 6% of all saurian samples submitted (Hernandez-Divers & Garner, 2003), and a later study reporteda similar prevalence of 8.5% in all lizards and 8.6% in agamids (Garner et al., 2004). The integumentary system, where the anaplastic sarcoma was found in this case, is the third most common system affected by neoplasia in reptiles (12.8%) (Christman et al., 2017), although this tumor type has only been previously reported in the gallbladder and intracelomic fat of a Madagascar tree boa (Sanzinia madagascariensis) (Sharpe etal., 2013) and the subcutis and muscle of a sand boa (Erixconicus) (Ramsay et al., 1996). In both cases, metastasis was present. Sertoli cell tumors have not been previously reported in reptiles (Effron et al, 1977; Sykes and Trupkiewicz, 2006; Christman et al, 2017).
Previously reported testicular tumors in reptiles include an interstitial cell tumor in a Komodo dragon (Varanus komodoensis) (Harshbarger, 1977) and a seminoma in a spur-thighed tortoise (Testudo graeca) (Christman et al., 2017). As in other species, most tumors in reptiles are considered to occur spontaneously, although viral causes have been reported (Christman et al., 2017), for example fibropapillomatosis caused by herpesvirus in green sea turtles (Chelonia mydas) (Jacobson et al., 1991). There was no evidence of classical histological features of viral infection within the examined sections.
The anaplastic sarcoma removed in this case was thought to most likely be a localized histiocytic sarcoma. A histiocytic sarcoma is a focal proliferation of neoplastic cells from the histiocytic cell lineage, which includes macrophages and dendritic cells (Moore, 2002). These tumors are found commonly in dogs and less so in cats andmay derive from most dendritic cell lineages. In these species, histiocytic sarcomas originate from interstitial dendritic cells that are present in most tissues within the body. They originate from a single site but can rapidly metastasize (Moore, 2014), especially via the lymphatic route. Cutaneous histiocytic sarcomas are often found on the extremities, with the best prognosis achieved by early surgical resection (Moore, 2002). A genetic mutation within the gene TP53 that encodes p53, a protein involved in regulation of the cell cycle, has been identified in dogs with histiocytic sarcoma, resulting in speculation that this mutation could affect p53 function and may be involved in the pathogenesis of canine histiocytic sarcoma (Asada et al., 2017). However, associations between specific genetic mutations and histiocytic sarcomas have not yet been investigated in reptiles. Anaplastic sarcomas, diagnosed histologically by cell morphology, have been reported in snakes to arise from skin, muscle and connective tissue and are highly malignant (Ramsay et al., 1996), however to the authors’ knowledge these tumors have not been characterized as histiocytic sarcomas by use of immunohistochemical (IHC) cell lineage markers. Histiocytic cell lineage can be characterised using IHC by immunoreactivity to vimentin, CD18 and Iba1 and concurrent negative immunoreactivity to cytokeratin, CD3, CD20, CD79a. Confirmation of the cellular origin of anaplastic sarcomas can be attempted by IHC, however the anaplastic nature of the neoplasms may result in aberrant marker expression, hindering interpretation of immunohistochemical results.
The infiltrative histological growth pattern and anaplastic cytologic features of the mass suggested that it was locally aggressive with the potential for metastasis, however vascular invasion was not observed in the examined sections. Given the anatomy of the beard, with empty space between the skin and gular cartilage that allows the lizard to expand the beard, for example as a threat display, and the observation at surgery that the mass was not connected to underlying soft tissues, it was interpreted that the deep surgical margins were likely to be adequate. There was no evidence of gross metastasis at exploratory coeliotomy.
One review paper found that 93% of resected saurian tumors did not achieve adequate margins (Hernandez-Divers & Garner, 2003), possibly suggesting that a surgeon should take wider surgical margins than would be thought necessary in more commonly seen species. Wide margins were achieved in this case, with the skin margins free of neoplastic cells, however the owner has been informed to monitor the surgical site closely and to present the lizard for re-examination if there are any concerns of tumor re-growth.
The presentation of two concurrent neoplasms within the same animal is unusual and there was no obvious connection between the two masses presented in this case. No husbandry factors were noted that could have made this lizard more susceptible to developing neoplastic disease and there are no known genetic predispositions to neoplasia identified in bearded dragons.
When the lizard first presented, further investigations were advised, however these were declined due to cost concerns. As fecal analysis was negative for endoparasites, the animal was dispensed a course of antibiotics to rule out bacterial infection. Enrofloxacin was not the most appropriate antibiotic in this case, as guidelines for responsible antibiotic use state that fluoroquinolones as first-line agents should be avoided and should only be used when other agents are ineffective (BSAVA 2017).
A morphine dose of 1mg/kg in bearded dragons is reported to provide some degree of analgesia (Greenacre et al., 2008). As multimodal analgesia was provided with the addition of meloxicam, a lower morphine dose was used in this case to utilize its sedative effects in preparation for induction of anesthesia.
Cloacal endoscopy was considered to assess for cloacal bleeding or tumors, however the celomic mass identified on radiography reduced the index of suspicion for cloacal pathology and therefore cloacal endoscopy was not performed. Ideally further imaging such as coelomic ultrasonography or computed tomography could have been performed to determine the anatomical origin of the mass, as well as its morphology and blood supply. These diagnostics were discussed with the owner, but due to financial constraints, the decision was taken to prioritize excisional biopsy. Follow up imaging to stage the tumor was also declined. Radiation therapy has been demonstrated to be useful for the treatment of sarcomas in other species and could have been considered, but in this case the owner preferred just to monitor for signs of recurrence.
Blood smear examination found marked toxic changes and left-shift of the heterophils, indicating inflammatory disease and an excessive peripheral use of those cells (Campbell, 2004). The multiple areas of necrosis with infiltration of inflammatory cells within the gular mass, and the ulcerated surface, were presumed the most probable cause of the large demand for heterophils; no other inflammatory foci were found during the various clinical examinations.
Sertoli cell tumors should be considered in reptiles with blood in the urates. Surgical excision is expected to be curative. Anaplastic sarcomas should also be considered as differentials for cutaneous masses. Prompt surgical removal is recommended and can carry a good prognosis.
Funding
HW is funded by the Wellcome Trust [RG63078]. The Wellcome Trust had no involvement in the data collection or manuscript preparation.
References
- Asada H, Tsuboi M, Chambers JK, Uchida K, Tomiyasu H, Goto-Kishino Y, Ohno K, Tsujimoto H. A 2-base insertion in exon 5 is a common mutation of the TP53 gene in dogs with histiocytic sarcoma. Journal of Veterinary Medical Science. 2017;79(10):1721–1726. doi: 10.1292/jvms.17-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BSAVA 2017 PROTECT. 2017 https://www.bsava.com/Resources/Veterinary-resources/PROTECT.
- Campbell TW. Hematology of Lower Vertebrates. Middleton, WI, USA, American College of Veterinary Pathologists & American Society for Veterinary Clinical Pathology. 2004:1104–1108. [Google Scholar]
- Christman J, Devau M, Wilson-Robles H, Hoppes S, Rech R, Russel KE, Heatley JJ. Oncology of Reptiles: Diseases, Diagnosis and Treatment. Veterinary Clinicsof North America: Exotic Animal Practice. 2017;20(1):87–110. doi: 10.1016/j.cvex.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Effron M, Griner L, Benirschke K. Nature and rate of neoplasia found in captive wild mammals, birds, and reptiles at necropsy. J Natl Cancer Inst. 1977;59(1):185–198. doi: 10.1093/jnci/59.1.185. [DOI] [PubMed] [Google Scholar]
- Garner MM, Hernandez-Divers SM, Raymond JT. Reptile Neoplasia: A Retrospective Study of Case Submissions to a Specialty Diagnostic Service. Veterinary Clinics of North America: Exotic AnimalPractice. 2004;7(3):653–671. doi: 10.1016/j.cvex.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Greenacre CB, Massi K, Schumacher JP, Harvey RC. Comparative Antinociception of Various Opioids and Non-Steroidal Anti-Inflammatory Medications Versus Saline in the Bearded Dragon (Pogona vitticeps) Using Electrostimulation. 2008 Proceedings Association of Reptilian and Amphibian Veterinarians; 2008. pp. 87–88. [Google Scholar]
- Harshbarger JC. Washington DC: Smithsonian Institution; 1977. Activities report of the registry of tumors in lower animals; p. 298. [Google Scholar]
- Hernandez-Divers SM, Garner MM. Neoplasia of Reptiles with an Emphasis on Lizards. The Veterinary Clinics of North America: Exotic Animal Practice. 2003;6(1):251–273. doi: 10.1016/s1094-9194(02)00028-2. [DOI] [PubMed] [Google Scholar]
- Jacobson ER, Buergelt C, Williams B, Harris RK. Herpesvirus in Cutaneous Fibropapillomas of the Green Turtle (Chelonia mydas) Diseases of Aquatic Organisms. 1991;12:1–6. [Google Scholar]
- Moore PF. Canine histiocytic neoplasia: cell lineages and disease classification. New York, International Veterinary Information Service. 2002 [Google Scholar]
- Moore PF. A Review of Histiocytic Diseases of Dogs and Cats. Veterinary Pathology. 2014;51(1):167–184. doi: 10.1177/0300985813510413. [DOI] [PubMed] [Google Scholar]
- Ramsay EC, Munson L, Lowenstine L, Fowler ME. A Retrospective Study of Neoplasiain a Collection of Captive Snakes. Journal of Zoo and Wildlife Medicine. 1996;27(1):28–34. [Google Scholar]
- Sharpe S, Lamm CG, Killick R. Intracoelomic anaplastic sarcoma in an intersex Madagascar tree boa (Sanzinia madagascariensis) Journal of Veterinary Diagnostic Investigation. 2013;25(1):153–157. doi: 10.1177/1040638712468432. [DOI] [PubMed] [Google Scholar]
- Sykes JM, Trupkiewicz JG. Reptile neoplasia at the Philadelphia Zoological Garden, 1901-2002. J Zoo Wildl Med. 2006;37(1):11–19. doi: 10.1638/04-112.1. [DOI] [PubMed] [Google Scholar]
- Tamukai K, Takami Y, Akabane Y, Kanazawa Y, Une Y. Plasma biochemical reference values inclinically healthy captive bearded dragons (Pogona vitticeps) and the effects of sex and season. Veterinary Clinical Pathology. 2011;40(3):368–373. doi: 10.1111/j.1939-165X.2011.00329.x. [DOI] [PubMed] [Google Scholar]