Abstract
The ventral premotor cortex (PMv) is a key area of the sensorimotor control loop, it subtends complex motor sequences, especially when the hand is involved. However, its specific contribution to simple motor response to sensory cue is still not completely clear. To investigate the role of PMv, we used transcranial magnetic stimulation (TMS) to interfere with its function during a simple reaction time (SRT) task. We ran two experiments where participants were required to respond as fast as possible to a median nerve stimulation (go-signal), while sub-M1-threshold single pulse TMS was delivered either on left (contralateral) PMv or right (ipsilateral to sensory stimulus and motor response) PMv, 5 to 65 ms after the go-signal. TMS delivered on either PMv up to 25 ms after the go-signal shortened reaction time. This is the time window compatible with the arrive of sensory afferences, as if conditioning before sensory afferences arrive lower the threshold needed to release the pre-planned motor program to the primary motor cortex. This is in line with a putative PMv function of buffer of pre-planned motor program not strictly lateralized in one hemisphere.
Keywords: Ventral Premotor Cortex (PMv), Simple reaction time (SRT), Transcranial magnetic stimulation (TMS), Sensorimotor loop, Motor control
1. Introduction
The nervous system relies on neural networks to respond to a sensory stimulus with a movement. Stereotyped responses can be managed by reflex loops involving only first order cortices or lower stations, but when the movement is more complex, a longer control loop involving higher order cortical areas must be put in the play. The ventral premotor cortex (PMv) is a key area of the sensorimotor control loops, as it processes both sensory and motor aspects (Kansaku, Hanakawa, Wu, & Hallett, 2004; Kantak, Stinear, Buch, & Cohen, 2012; Schall, 1997; Wise, Boussaoud, Johnson, & Caminiti, 1997).
PMv studied mostly tested complex motor sequence, especially when the hand is involved (Buch, Mars, Boorman, & Rushworth, 2010; Kantak et al., 2012), but its specific contribute to simple motor response to sensory cue is still not completely clear.
A straightforward tool to investigate a simple sensorimotor loop is to measure the Simple Reaction Time (SRT), which is the minimal time needed to respond with an action to a sensory stimulus (go-signal). SRT is conscious and voluntary, thus it is more than a reflex movement, but the requested motor action is pre-planned in advance, so that action selection, response inhibition and other high demanding cognitive functions are not required (Gescheider, 2013; Pellegrino, Tomasevic, Herz, Larsen, & Siebner, 2018).
Pascual-Leone and colleagues suggested that SRT is subtended by three phases: recognition, initiation and execution. During the ‘time for recognition’, the modality-specific primary sensory cortices detect the go-signal (Pascual-Leone, Brasil-Neto, Valls-SolÉ, Cohen, & Hallett, 1992). This phase lasts about 30 ms and varies in its duration for acoustic, visual and somatosensory stimuli. In the following ‘time for initiation’, the motor program which has been previously prepared and held in a temporary buffer is transferred to M1 in approximately 100-110 ms. Finally, once that the information reaches M1, a ‘time for development’ is needed to activate first and second motor neurons and relevant muscles for the execution of movement. The average duration of a SRT is around 250 ms, with variability that depends on experimental condition, signal intensity, go-signal modality, motivation and level of attention (Luce, 1986).
Most of the studies that investigated the cortical areas involved is SRT focused on M1. Conditioning transcranial magnetic stimulation (TMS) over M1 affects RTs, depending on the timing of stimulation with respect to the go-signal. TMS delivered less than 30 ms after the go-signal shorten simple RTs (Pascual-Leone et al., 1992), conversely TMS delivered more than 30 ms after the go-signal delay the performance (Day et al., 1989; Pascual-Leone et al., 1992; Rothwell, Day, Thompson, & Marsden, 1989; Ziemann, Tergau, Netz, & Homberg, 1997).
At earlier latencies, TMS over M1 activates cortico-cortical connections (Pascual-Leone et al., 1992). At later latencies, TMS delays RTs because of a temporary suppression of the sensorimotor processing, by exhausting M1 neurons and making them unresponsive for the time until they recover from the previous activation (“silent period”). Indeed, high-intensity stimulation induces longer RT (Day et al., 1989), which correlates with the duration of the silent period (Burle, Bonnet, Vidal, Possamai, & Hasbroucq, 2002). Corticospinal excitability is linked with motor performance (Moscatelli et al., 2016), and with RTs, so that greater excitability is associated with faster RTs (Greenhouse, King, Noah, Maddock, & Ivry, 2017; Sinclair & Hammond, 2008). Since M1 excitability increases 80-100 ms before movement onset (Leocani, Cohen, Wassermann, Ikoma, & Hallett, 2000; Rossini, Zarola, Stalberg, & Caramia, 1988; Starr, Caramia, Zarola, & Rossini, 1988), it is likely that the ‘time for development’ takes almost entirely the second half of the whole SRT.
All these studies have focused on the crucial role of M1 in the implementation of the response of SRT, but neurophysiological and neuroimaging studies have revealed a large cortical network involved in mediating sensory cue detection and movement execution in the SRT task. In particular, beyond frontal, temporal and cerebellar, bilateral premotor cortices show a broad activation during the task (Kansaku et al., 2004).
PMv exerts powerful facilitation of motor cortex output (Shimazu, Maier, Cerri, Kirkwood, & Lemon, 2004). It is densely interconnected with the ipsilateral primary motor cortex (M1) (Godschalk, Lemon, Kuypers, & Ronday, 1984; Godschalk, Mitz, van Duin, & van der Burg, 1995; Matelli, Camarda, Glickstein, & Rizzolatti, 1986; Muakkassa & Strick, 1979) and has sparse direct corticospinal projections to motor neurons in the cervical spinal cord (Cerri, Shimazu, Maier, & Lemon, 2003; Dum & Strick, 1991). PMv influences the movement directly. Indeed, short (50 ms) electric intracortical pulses delivered to PMv of monkeys evoke simple movements of the wrist and the fingers (Gentilucci et al., 1988; Hepp-Reymond, Husler, Maier, & Ql, 1994), and longer (500 ms) pulses evoke more complex arm postures (Graziano, Taylor, & Moore, 2002). However, premotor cortex role in motor control goes mainly through its connections with M1. Indeed, electrical conditioning stimuli over PMv of the monkey produces a short-latency (1-2 ms) facilitation of intrinsic hand muscles responses evoked by M1 stimulation (Cerri et al., 2003). In humans, TMS delivered about 6 and 8 ms before M1 stimulation induced an inhibition of M1 excitability for high intensity conditioning stimuli (80% of the resting motor threshold) (Davare, Lemon, & Olivier, 2008) and a facilitation for low intensity conditioning stimuli (80% active motor threshold) (Baumer et al., 2009).
PMv exerts a strong time-dependent influence on M1 excitability, which is known to affects RTs. It is rich of preparation-related neurons (Riehle & Requin, 1989), and it keeps a short-term storage of a planned action and it is involved in the intention to make a movement in response to a cue (Wise, Weinrich, & Mauritz, 1983).
Considering data on cortical excitability and the computational role of PMv, it may be hypothesized that interacting with PMv may affects RTs, and in particular its initiation phase. In spite of that, there are no previous studies that test PMv priming on RTs.
In the present randomized sham-controlled study, we investigate whether TMS applied on PMv is able to interfere with the motor performance in a SRT task. Two experiments were run to test whether contralateral or ipsilateral PMv conditioning stimulus change RT, and to identify the ideal time that should separate go-signal from the conditioning stimulus. A further control experiment was run to exclude that a different sensory valence between the employed real and sham stimulation would have determined changes of RT. The knowledge of the direction (i.e. facilitation or inhibition of the performance) and of the timing of any achieved effect could help to disclose PMV role in cued motor control.
2. Material and Methods
2.1. Participants
Twenty-six healthy volunteers (14 males, 12 females) aged 21-37 (mean age 26.5 ± 4 years) participated in the experiment 1. Fifteen healthy volunteers (7 males, 8 females) aged 22-37 (mean age 26.4 ± 4 years) participated in the experiment 2. Eleven healthy volunteers (5 males, 6 females) aged 21-25 (mean age 23.1 ± 1.3 years) participated in an experiment performed to assess the sensory valence of TMS and electrical stimulation.
The three groups were composed by different subjects. Experiment 1 was the first to be performed. The number of subjects recruited in experiment 2 (15) was based on a power analysis starting from the results obtained in experiment 1 with effect size ≥ 0.6 and medium statistical power of 0.6. All the participants were right-handed, as assessed by the Oldfield Handedness Inventory (Oldfield, 1971), had no history of neurological disorders, and had not taken CNS active drugs in four weeks before the testing day. All the subjects were recruited at ‘Università Campus Bio-Medico di Roma’ and gave their written informed consent to participate in the study. The study was run in accordance with the Declaration of Helsinki and future amendments and its protocol was approved by the Campus Bio-Medico University Ethics Committee (EMBODY Protocol).
No part of the study procedures or analyses was pre-registered in a time-stamped, institutional registry prior to the research being conducted. We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study. Data Analysis has been performed with the freeware Jamovi 0.9.0.5.v, without any ad-hoc developed code or algorithm.
All individual anonymised data that are necessary and sufficient to reproduce all procedures, analyses and data presentations reported in the paper are offered in the Supplementary Material or can be downloaded at https://osf.io/2cv4x
Experimental setup
Participants were comfortably seated on an armchair with padded armrests in front of a fixation point, with their right foot placed upon a pedal (X-Keys XF-10 Foot Pedal, P.I. Engineering, Williamston, USA). They were acoustically shielded by wearing over-ear headphones (Vanderfields noise-cancelling headphones NR35X2) and in-ear headphones (SONY MDR-EX15LP) playing white noise at the maximum tolerable volume to prevent hearing any auditory cue (Figure 1). Before beginning the experiments, each participant was explicitly asked if she/he was able to hear a series of TMS “clicks”. Participants were instructed to press as fast as possible the pedal with their right foot when they perceived a somatosensory stimulus (i.e. go-signal). Somatosensory stimuli were square current pulses of 200 μs (Digitimer DS7A, Hertfordshire, UK) delivered on the skin upon the right median nerve at the level of the wrist with the cathode placed in a proximal position for all participants, at minimum intensity needed to evoke a visible twitch on the thenar eminence. The time between the median nerve stimulation and the foot pedal press was recorded as Reaction times (RT) and stored for further analysis.
Fig. 1. Experimental setup.
Placement of the TMS coil on the scalp of one subject from different points of view, the pictures show also the headphone placement and, additionally, it is visible the wire of earphone. The participant provided the consent for use of the image.
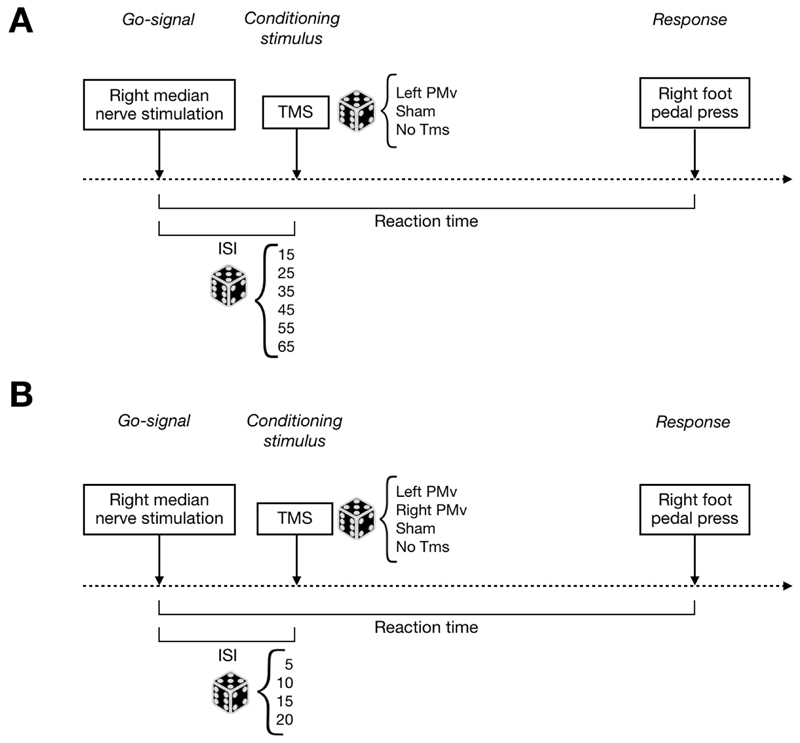
In the experiment 1, TMS was delivered over left PMv and six inter-stimulus intervals (ISIs) (15, 25, 35, 45, 55 and 65 ms) were tested in a random order.
In the experiment 2, TMS was delivered over either left or right PMv and four shorter ISIs (5, 10, 15 and 20 ms) were tested in a random order. To control for data consistency across experiments, the 15ms ISI -tested already in experiment 1- was tested again in experiment 2. In both experiments, Sham stimulation was delivered on the left side of the scalp. Each experiment consisted of four separate blocks, interspersed with 5-minutes breaks. Each of the four blocks consisted of 78 trials presented in a random order. In both experiments, a total of 24 trials for each condition (real and sham) were presented for each participant. Somatosensory stimuli were presented with inter-trial intervals randomly ranging from 3500 to 5000 ms. In case of no response, the following trial would start after 8000 ms. In both experiments, 24 trials were presented when somatosensory stimulation was not followed by any further stimulation (No TMS). Experimental procedure is shown in Figure 2.
Fig. 2. Experimental procedure.
Schematic design of the experimental procedure of Experiment 1 (A – up panel) and Experiment 2 (B – bottom panel). Dotted line represents the timeline. The superficial right median nerve stimulation is the go-signal, transcranial magnetic stimulation (TMS) is the conditioning stimulus and pressing a pedal with the right foot represents the response. The time between go-signal and conditioning stimulus is defined as interstimulus interval (ISI), while the one between go-signal and response is the Reaction Time (RT) exploited as dependent variable of the analysis. The dice is exploited as a symbol of randomization.
In the supplementary control experiment, participants underwent two different sessions (TMS, Sham) in two different days. The order of the sessions was randomized. The experimental setup was the same to the one of both experiment 1 and 2: participants were acoustically shielded by headphones playing white noise and noise-cancelling headphones.
Experiment consisted of two phases. In the first phase, electrodes were mounted on the forehead, FDI RMT was found, three pulses at 90% of RMT were delivered upon PMv and the intensity of the electrical stimulation that subjects referred to match the one of TMS was determined. The first phase was run identical at the beginning of both sessions, either real or sham. In the second phase, participants were asked to complete a RT task in which the right median nerve stimulation (go-signal) was delivered 15 ms before a second stimulus, either a TMS pulse delivered over the left PMv (TMS session) or an electrical stimulation delivered over the forehead (Sham session). A total of 20 trials were administered for each session.
On each session, at the end of the second phase, a visual analogue scale (VAS) questionnaire was handed to the participants, asking them to rate the perceived sensations due to the stimulation of the scalp, by marking a 100 millimetres line for each item. A total of nine items were investigated: pain, tingling, vibration, tolerability, intensity, itching, pressure, warmth and cold.
For all experiments, TMS on PMv was delivered by a BiStim2 stimulator (The Magstim Co. Ltd) equipped with a 40 Alpha BI coil (The Magstim Co. Ltd), pointing backwards at a 15° angle. The induced current flowed in a postero-anterior direction. Stimulation point over PMv was defined with an optoelectronic neuronavigation system (SofTaxic 2.0, EMS, Bologna, Italy) based on a template magnetic resonance image (MRI) modified to fit the subject’s individual head size. We sampled and digitalized the position of reliable anatomical landmarks (nasion, inion, and left and right pre-auricular points) and we obtained the individual scalp reconstruction with approximately 100 uniformly spaced scalp points. The estimated MRI of each participant was obtained with the SofTaxic software, by fitting the high-resolution MRI template to the coordinates of the points collected on their scalp. The coil position was based on Talairach coordinates for Left PMv (x = -52, y = 10, z = 24) and Right PMv (x = 52, y = 10, z = 24) (Jacquet & Avenanti, 2013; Mioli, D'Alonzo, Pellegrino, Formica, & Di Pino, 2018). During the experiment, the experimenter manually maintained the coil position after marking the location onto the scalp. The procedure ensures a global localization accuracy of about 5mm (Carducci & Brusco, 2012). TMS was delivered at various times, i.e. inter-stimulus intervals (ISIs), after the median nerve stimulation.
The TMS intensity used to stimulate PMv was chosen in order to condition PMv without clearly activate it, and it was individualized to each subject’s Rest Motor Threshold (RMT). In particular, 90% RMT was used, according to previous studies that used conditioning stimulation on PMv (Fiori, Chiappini, & Avenanti, 2018). RMT was functionally identified over the primary motor cortex (M1) near the hand-knob on the template brain after transformation onto the participants’ head (Raffin, Pellegrino, Di Lazzaro, Thielscher, & Siebner, 2015), at the optimal scalp position (hotspot) to produce a motor evoked potential (MEP) in the contralateral first dorsal interosseous muscle (FDI). RMT was defined as the lowest intensity to induce a MEP at rest of about 50 μV in at least 5 out of 10 trials.
Electrical stimulation of the skin delivered through two Ag/AgCl electrodes placed on the left side of subject’s forehead was employed as Sham control condition. Sham stimulation intensity was identified with ascending and descending methods of limit (Gescheider, 2013), by asking participant to state the electrical intensity that produced, overall, the closest scalp sensation to the one evoked by three TMS pulses on left PMv given as example. The aim of this phase was just to set the intensity of electrical stimuli; thus, we did not investigate the qualities of sensation. Electrode position was adjusted accordingly to the location of the stimulus that subject declared to match with the TMS one. Timing of electrical somatosensory and transcranial magnetic stimuli were managed by means of the computerized software OpenSesame v3.1.7 (Mathôt, Schreij, & Theeuwes, 2012).
2.2. Analysis
RTs exceeding two standard deviations were considered outliers and therefore excluded from the analysis (less than 5% of trials). Data collected from 2 out 24 subjects were not considered for the analysis of the experiment 1. One participant was excluded because data was not properly recorded due to a system malfunctioning. The other subject was excluded because he spontaneously reported excessive drowsiness during the break among experimental blocks. Data normality was assessed by the Shapiro-Wilk test.
We compared RTs in No TMS to RTs in the other stimulating conditions with paired sample t-tests, by pooling together all ISIs. Then, data were normalized within each subject for each condition, using the formula: RT index = [(Average RT of condition - Average RT of No TMS) / Average RT of No TMS)]. This normalization allowed to test more directly the effect of TMS stimulation as compared to Sham. The analysis of the effects of conditioning stimulus and ISI relied on a repeated measures ANOVA. Significant level was set at p<0.05. The alpha inflation due to multiple comparison was corrected according to Bonferroni procedure whenever appropriate.
In the supplementary control experiment, scores on VAS were obtained by measuring the distance in millimetres from the left end of the line to the mark drawn by the participant, and they were analysed by means of paired-sample t-tests.
3. Results
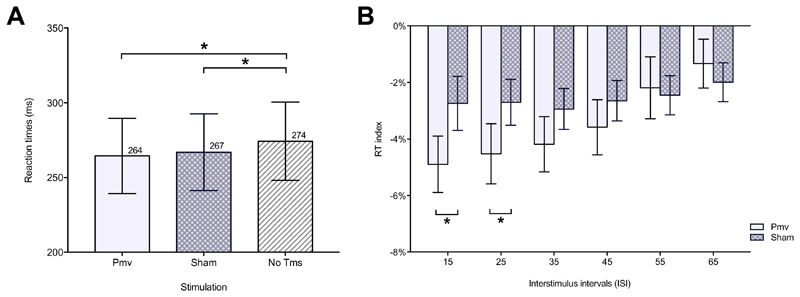
Experiment 1. Pooling together all ISIs, RTs in No TMS (274 ± 26 ms) were significantly slower than RTs in PMv (264 ± 25 ms) [t(23) = 8.85, p < 0.001] and Sham (267 ± 26 ms) [t(23) = 4.14, p < 0.001] conditions, suggesting that the second stimulus always speeds RTs. The repeated measure ANOVA, with factors Stimulation (two levels: PMv, Sham) and ‘ISI’ (six levels: 15, 25, 35, 45, 55, 65) showed a significant main effect of ISI [F(5, 115) = 4.901, p < 0.001], with faster RTs at shorter ISIs, and a significant Stimulation x ISI interaction [F(5, 115) = 2.564, p = 0.031]. No significant stimulation effect was found [F(1, 23) = 3.674, p = 0.068] (Figure 3A). Post-hoc analysis revealed that RTs were shorter when left PMv was stimulated at ISI 15 ms [t(23)=-3.39, p = 0.005] and 25 ms [t(23)=-2.93, p = 0.008] (Figure 3B).
Fig. 3. Results of Experiment 1.
Reaction time (RT) (mean ± Standard Error Mean-(SEM-) to the somatosensory cue due to a superficial right median nerve stimulation (go-signal) of Experiment 1. Panel A on the left shows the three different TMS conditions pooling together all ISIs. Panel B on the right shows paired comparisons run on RT index between left ventral premotor cortex (PMv) and Sham stimulating conditions, for interstimulus interval (ISI) ranging between 15 and 65. RT index is the RT in a stimulating condition normalized on No TMS RT. * means p<0.05.
Experiment 2. To test the reproducibility of our data, we compared RTs at ISI 15 ms collected in experiment 1 with RTs at ISI 15 ms collected in experiment 2 by means of independent sample t-tests, and we did not find any significant difference for PMv [t(37) = -0.319, p = 0.751], Sham [t(37) = -0.477, p = 0.636] and No TMS [t(37) = -0.367, p = 0.716].
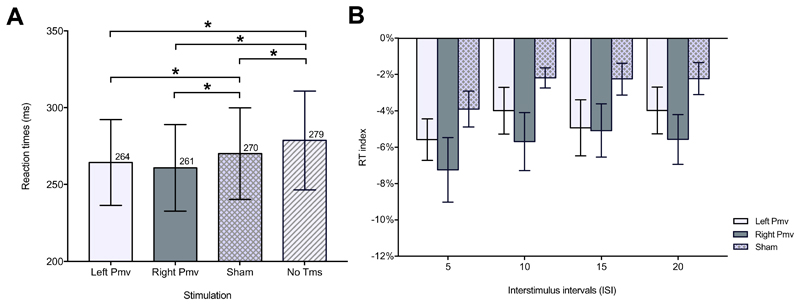
Pooling together all ISIs, RTs in No TMS (279 ± 32 ms) were significantly slower than RTs in Left PMv (264 ± 28 ms) [t(14) = -4.18), p < 0.001], Right PMv (261 ± 28 ms) [t(14) = -4.08, p = 0.001] and Sham (270 ± 30 ms) [t(14) = -4.71, p < 0.001] conditions (Figure 4A). The repeated measure ANOVA with factors Stimulation (three levels: Left PMv, Right PMv, Sham) and ISI (four levels: 5, 10, 15, 20) showed a significant main effect of Stimulation [F(2, 28 = 9.968, p < 0.001)] and ISI [F(3, 42 = 5.232, p = 0.004)]. The interaction Stimulation x ISI was not significant [F(6, 84 = 0.361, p = 0.902)]. This result showed that, when pooling together all ISIs, RTs on both Left PMv [t(28)=2.69, p = 0.036] and Right PMv [t(28)=-4.43, p < 0.001) were faster that RTs on Sham. Importantly, RTs on Left PMv and Right PMv were not significantly different [t(28)=1.74, p = 0.276], meaning that a similar shortening of RTs occurred when either left (contralateral to the side of response) or right PMv (ipsilateral to the side of response) was stimulated (Figure 4B).
Fig. 4. Results of Experiment 2.
Reaction time (RT) (mean ± Standard Error Mean-(SEM-) to the somatosensory cue due to a superficial right median nerve stimulation (go-signal) of Experiment 2. Panel A on the left shows the four different TMS conditions pooling together all ISIs. Panel B on the right shows paired comparisons run on RT index between left or right ventral premotor cortex (PMv) and Sham stimulating conditions, for interstimulus interval (ISI) ranging between 5 and 20. RT index is the RT in a stimulating condition normalized on No TMS RT. * means p<0.05.
Experiment 3. Assessment of the sensory valence of TMS and electrical stimulation. Paired sample t-tests showed no significant difference for all items of the VAS: pain [t(10) = -0.53, p=0.60], tingling [t(10)=1.52, p = 0.15), vibration [t(10)=-1.32, p=0.21], tolerability [t(10)=0.75, p=0.46], intensity [t(10)=-0.76, p=0.46], itching [t(10)=-0.36, p=0.20], pressure [t(10)=1.25, p=0.23], warmth [t(10)=-0.11, p=0.91] and cold [t(10)=0.05, p=0.96] (database available as Supplementary Material). We conclude that participant did not attribute different sensory valence to the two stimulation. Moreover, none of them declared to have been stimulated with different methods.
4. Discussion
In the Experiment 1, when comparing Real and Sham stimulations, we found a shortening of RTs caused by a short-ISI sub-M1-threshold conditioning TMS pulse to the left PMv, contralateral to the side of response. It is likely that, similarly to when M1 is stimulated, the shortening of RTs was obtained by conditioning PMv at ISIs early enough to pre-activate its neurons without interrupting any local cortical process, because at these latencies the transfer of information had yet to begin (or was still at an early stage). Such pre-activation of PMv neurons may act together with the information coming from somatosensory areas, fastening the transfer of information to M1. Accordingly, since previous studies with TMS applied to M1 found a facilitation of RTs at ISIs shorter than 30 ms (Pascual-Leone et al., 1992) and PMv-to-M1 conduction time is about 6 ms (Baumer et al., 2009; Davare et al., 2008), it is reasonable that the ideal ISI between go signal and PMv stimulation that produces a RT shortening effect is 25 ms or less. At ISI < 25 ms, a facilitation can be obtained presumably because the identification of the stimulus occurs in modality-specific brain regions during this window (i.e. ‘time for identification’). TMS delivered on PMv at ISI > 25 ms, when the transfer of information is initiated or is about to begin, are ineffective, or may eventually interfere with RTs at later ISIs or at higher stimulation intensities not tested in our experiments.
Since we have found a facilitation of RTs at the shortest ISIs tested in experiment 1 (15 and 25 ms), we ran Experiment 2 to test ISIs below 20ms. We first demonstrated that the results at ISI 15 did not differ between the two experiments, before concluding that the effect of TMS on RTs occurs for ISIs between 5 and 25 ms. In Experiment 2 we also tested the priming of right PMv, ipsilateral to the go-signal and response, to investigate the lateral specificity of the stimulation, and we found similar RTs as for left PMv.
Ipsilateral PMv and M1 contribute to motor response. Animal studies showed that almost all premotor areas have more extensive connections with the contralateral hemisphere than M1 (Dancause, Barbay, Frost, Mahnken, & Nudo, 2007; Rouiller et al., 1994). While PMv-M1 connections are mostly intra-hemispheric, some trans-hemispheric connections have been shown (Dancause et al., 2007). It is possible that right PMv interacts with left M1, which in turn is engaged in the execution of movements. However, if this was the case, the facilitation of RTs should have been maximal at longer ISIs, since the cortical loop is longer.
Another possibility is that right PMv interacts with ipsilateral M1, which in turn contributes to the execution of voluntary movements via uncrossed descending pathways (McCambridge, Stinear, & Byblow, 2014; Uehara & Funase, 2014). Several studies showed that TMS of ipsilateral M1 could interfere with movements. For example, repetitive TMS induced errors in finger sequences performed with either hand (Chen, Cohen, & Hallett, 1997) and TMS-induced virtual lesions altered the timing of muscle recruitment in motor tasks (Davare, Duque, Vandermeeren, Thonnard, & Olivier, 2007). However, so far a positive contribution of ipsilateral M1 to movement has been demonstrated mainly in stroke patients (Bradnam, Stinear, & Byblow, 2013; Di Pino et al., 2014). Since in healthy adults ipsilateral M1 contributes to movement only minimally (Uehara & Funase, 2014), this hypothesis remains speculative and far to be verified.
Lastly, neurons of both premotor cortices are active long before the onset of movement, without differences for ipsilateral or contralateral movements (Li, Chen, Guo, Gerfen, & Svoboda, 2015; Shenoy, Sahani, & Churchland, 2013). PMv maintains in working memory information from different sources, depending on the task demands (Pardo-Vazquez, Padron, Fernandez-Rey, & Acuna, 2011) and it determines which stimuli are relevant for behaviour (Pardo-Vazquez et al., 2011; Rushworth et al., 2005). This propriety account for its capability to mediate cue detection and movement execution in SRT. Since this preparatory activity is not lateralized in one hemisphere, it may be speculated that conditioning left or right PMv produced a comparable effect on RTs because TMS fastened the transfer of information to M1.
Finally, our results demonstrate that the RT was shorter in both Real and Sham stimulation, as compared to trials with the go-signal only (No TMS). It is important to note that TMS beside activating cortical neurons produces peripheral auditory and cutaneous sensations that provide supplementary sensory cues, even if irrelevant for the task. The shortening of RTs that occurs when a second stimulus of another modality is presented temporally close the go-signal is an established phenomenon known as “intersensory facilitation” (Diederich, 1995; Diederich & Colonius, 1987; Nickerson, 1973). For example, RTs to visual stimuli are 20 to 40 ms faster when the go-signal is followed by an additional auditory or kinaesthetic stimulus (Gielen, Schmidt, & Van Den Heuvel, 1983). This effect occurs before and independently from the conscious detection of the additional stimulus and even when the accessory stimulus is irrelevant for the task (Nickerson, 1973). Moreover, greater facilitation occurs when two stimuli are close in time, in accordance with the ‘temporal rule’ of intersensory facilitation. When piloting the study (data not reported) we employed sham TMS on the vertex, but subjects reported to be able to perceive additional peripheral auditory and cutaneous sensations with real TMS upon PMv, as magnetic stimuli over PMv often cause involuntary facial muscles contractions (Meteyard & Holmes, 2018).
In order to avoid any possible additional sensory cue that the real TMS could have provided compared to Sham stimulation, we employed an improved Sham method, based on individually-threshold skin electrical stimulation, in-ear white noise and noise-cancelling headphones. Similar methods are reported to be very effective in producing perceived sensation like real TMS (Arana et al., 2008; Borckardt et al., 2008; Duecker & Sack, 2015; Mennemeier et al., 2009).
However, once acknowledged the importance of intersensory facilitation in speeding RT, we had to be sure that the sensory valence of the stimulations employed for test and control were not different. Thus, we ran a further control experiment (Experiment 3) to evaluate the presence of any difference in the perceived cutaneous sensations. None of the participants declared to have been stimulated with different methods, and results from the VAS scale showed no significant difference in the nine investigated qualities, suggesting that participant did not attribute different sensory valence to the two stimulations. In addition, the absence of a relative facilitation when the stimulus was delivered on one PMv compared to the other is in favour of a peculiar neural contribute of PMv to the described effect. Indeed, accordingly to the spatial rule of intersensory facilitation is ruled by: two stimuli close in space have a stronger additive effect, especially when they came from the same side of the peripersonal space (Holmes & Spence, 2005). In the experiment 2, a speeding of RT completely due to intersensory facilitation would have been more present when TMS was delivered over right PMv because it is closer and ipsilateral to the median nerve stimulation. The cautions used in designing sham stimulation, the findings of the two experiments and the absence of a different sensory valence of real and sham stimulation allow us to charge to the effect on PMv neurons any further difference between Real and Sham stimulations.
Conclusion
We performed two experiments where we applied single pulse TMS over PMv to investigate the behavioural effects of PMv-to-M1 connectivity during a simple RT task. After having normalized the data to rule out the effect intersensory facilitation, we found a shortening of RTs when TMS was delivered on either left or right PMv at ISIs < 25 ms. This, together with knowledge of the timing of effect due to M1 priming, and the known role of PMv in mediating sensory cue detection and movement execution, is strongly in favour of the theory that PMv is a temporary buffer where pre-planned motor programs are stored. In SRT, the information coming from sensory cortices is likely to be the trigger needed to release the program to primary motor cortex, which implements the response. Preconditioning PMv before sensory afferences arrive, makes PMv more prone to release the motor program. Thus, afferences from sensory cortices achieve the threshold needed to release the motor program in a shorter period, fastening the response.
Supplementary Material
Funding
This work was funded by the European Research Council (ERC) Starting Grant 2015 RESHAPE: REstoring the Self with embodiable Hand ProsthesEs (ERC-2015-STG, project n. 678908).
Footnotes
Financial disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Credit Author Statement
Giovanni Di Pino designed the study, wrote the manuscript and revised it. Andrea Zangrandi wrote the manuscript. Andrea Zangrandi Alessandro Mioli and Marco D’Alonzo performed the experiments and the analysis. Giovanni Pellegrino and Domenico Formica performed the analysis and revised the manuscript.
All Authors have read and approved the final version of the manuscript.
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arana AB, Borckardt JJ, Ricci R, Anderson B, Li X, Linder KJ, et al. George MS. Focal electrical stimulation as a sham control for repetitive transcranial magnetic stimulation: Does it truly mimic the cutaneous sensation and pain of active prefrontal repetitive transcranial magnetic stimulation? Brain Stimul. 2008;1(1):44–51. doi: 10.1016/j.brs.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumer T, Schippling S, Kroeger J, Zittel S, Koch G, Thomalla G, et al. Munchau A. Inhibitory and facilitatory connectivity from ventral premotor to primary motor cortex in healthy humans at rest - A bifocal TMS study. Clinical Neurophysiology. 2009;120(9):1724–1731. doi: 10.1016/j.clinph.2009.07.035. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Walker J, Branham RK, Rydin-Gray S, Hunter C, Beeson H, et al. George MS. Development and evaluation of a portable sham transcranial magnetic stimulation system. Brain Stimul. 2008;1(1):52–59. doi: 10.1016/j.brs.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradnam LV, Stinear CM, Byblow WD. Ipsilateral motor pathways after stroke: implications for non-invasive brain stimulation. Frontiers in human neuroscience. 2013;7:184. doi: 10.3389/fnhum.2013.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch ER, Mars RB, Boorman ED, Rushworth MFS. A Network Centered on Ventral Premotor Cortex Exerts Both Facilitatory and Inhibitory Control over Primary Motor Cortex during Action Reprogramming. Journal of Neuroscience. 2010;30(4):1395–1401. doi: 10.1523/Jneurosci.4882-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burle B, Bonnet M, Vidal F, Possamai CA, Hasbroucq T. A transcranial magnetic stimulation study of information processing in the motor cortex: Relationship between the silent period and the reaction time delay. Psychophysiology. 2002;39(2):207–217. doi: 10.1017/S0048577201392119. [DOI] [PubMed] [Google Scholar]
- Carducci F, Brusco R. Accuracy of an individualized MR-based head model for navigated brain stimulation. Psychiatry Research: Neuroimaging. 2012;203(1):105–108. doi: 10.1016/j.pscychresns.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Cerri G, Shimazu H, Maier M, Lemon R. Facilitation from ventral premotor cortex of primary motor cortex outputs to macaque hand muscles. Journal of neurophysiology. 2003;90(2):832–842. doi: 10.1152/jn.01026.2002. [DOI] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallett M. Role of the ipsilateral motor cortex in voluntary movement. Canadian Journal of Neurological Sciences. 1997;24(4):284–291. doi: 10.1017/s0317167100032947. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Mahnken JD, Nudo RJ. Interhemispheric connections of the ventral premotor cortex in a new world primate. The Journal of Comparative Neurology. 2007;505(6):701–715. doi: 10.1002/cne.21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Duque J, Vandermeeren Y, Thonnard JL, Olivier E. Role of the ipsilateral primary motor cortex in controlling the timing of hand muscle recruitment. Cereb Cortex. 2007;17(2):353–362. doi: 10.1093/cercor/bhj152. [DOI] [PubMed] [Google Scholar]
- Davare M, Lemon R, Olivier E. Selective modulation of interactions between ventral premotor cortex and primary motor cortex during precision grasping in humans. J Physiol. 2008;586(11):2735–2742. doi: 10.1113/jphysiol.2008.152603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, De Noordhout AM, Nakashima K, Shannon K, Marsden CD. Delay in the Execution of Voluntary Movement by Electrical or Magnetic Brain Stimulation in Intact Man. Brain. 1989;112(3):649–663. doi: 10.1093/brain/112.3.649. [DOI] [PubMed] [Google Scholar]
- Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, et al. Rothwell JC. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nature Reviews Neurology. 2014;10(10):597. doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- Diederich A. Intersensory facilitation of reaction time: Evaluation of counter and diffusion coactivation models. Journal of Mathematical Psychology. 1995;39(2):197–215. [Google Scholar]
- Diederich A, Colonius H. Intersensory facilitation in the motor component? Psychological Research. 1987;49(1):23–29. [Google Scholar]
- Duecker F, Sack AT. Rethinking the role of sham TMS. Front Psychol. 2015;6:210. doi: 10.3389/fpsyg.2015.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11(3):667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori F, Chiappini E, Avenanti A. Enhanced action performance following TMS manipulation of associative plasticity in ventral premotor-motor pathway. Neuroimage. 2018;183:847–858. doi: 10.1016/j.neuroimage.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Functional organization of inferior area 6 in the macaque monkey. I. Somatotopy and the control of proximal movements. Exp Brain Res. 1988;71(3):475–490. doi: 10.1007/BF00248741. [DOI] [PubMed] [Google Scholar]
- Gescheider GA. Psychophysics: the fundamentals. Psychology Press; 2013. [Google Scholar]
- Gielen SC, Schmidt RA, Van Den Heuvel PJ. On the nature of intersensory facilitation of reaction time. Perception & Psychophysics. 1983;34(2):161–168. doi: 10.3758/bf03211343. [DOI] [PubMed] [Google Scholar]
- Godschalk M, Lemon RN, Kuypers HGJM, Ronday HK. Cortical afferents and efferents of monkey postarcuate area: an anatomical and electrophysiological study. Experimental Brain Research. 1984;56(3) doi: 10.1007/bf00237982. [DOI] [PubMed] [Google Scholar]
- Godschalk M, Mitz AR, van Duin B, van der Burg H. Somatotopy of monkey premotor cortex examined with microstimulation. Neurosci Res. 1995;23(3):269–279. doi: 10.1016/0168-0102(95)00950-7. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34(5):841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Greenhouse I, King M, Noah S, Maddock RJ, Ivry RB. Individual Differences in Resting Corticospinal Excitability Are Correlated with Reaction Time and GABA Content in Motor Cortex. J Neurosci. 2017;37(10):2686–2696. doi: 10.1523/JNEUROSCI.3129-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp-Reymond MC, Husler EJ, Maier MA, Ql HX. Force-related neuronal activity in two regions of the primate ventral premotor cortex. Can J Physiol Pharmacol. 1994;72(5):571–579. doi: 10.1139/y94-081. [DOI] [PubMed] [Google Scholar]
- Holmes NP, Spence C. Multisensory integration: space, time and superadditivity. Current Biology. 2005;15(18):R762–R764. doi: 10.1016/j.cub.2005.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet PO, Avenanti A. Perturbing the action observation network during perception and categorization of actions' goals and grips: state-dependency and virtual lesion TMS effects. Cerebral cortex. 2013;25(3):598–608. doi: 10.1093/cercor/bht242. [DOI] [PubMed] [Google Scholar]
- Kansaku K, Hanakawa T, Wu T, Hallett M. A shared neural network for simple reaction time. Neuroimage. 2004;22(2):904–911. doi: 10.1016/j.neuroimage.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Kantak SS, Stinear JW, Buch ER, Cohen LG. Rewiring the brain: potential role of the premotor cortex in motor control, learning, and recovery of function following brain injury. Neurorehabil Neural Repair. 2012;26(3):282–292. doi: 10.1177/1545968311420845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123(6):1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Li N, Chen TW, Guo ZV, Gerfen CR, Svoboda K. A motor cortex circuit for motor planning and movement. Nature. 2015;519(7541):51–56. doi: 10.1038/nature14178. [DOI] [PubMed] [Google Scholar]
- Luce RD. Response times: Their role in inferring elementary mental organization. Oxford University Press on Demand; 1986. [Google Scholar]
- Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol. 1986;251(3):281–298. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- Mathôt S, Schreij D, Theeuwes J. OpenSesame: An open-source, graphical experiment builder for the social sciences. Behavior research methods. 2012;44(2):314–324. doi: 10.3758/s13428-011-0168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge AB, Stinear JW, Byblow WD. A dissociation between propriospinal facilitation and inhibition after bilateral transcranial direct current stimulation. Journal of neurophysiology. 2014;111(11):2187–2195. doi: 10.1152/jn.00879.2013. [DOI] [PubMed] [Google Scholar]
- Mennemeier M, Triggs W, Chelette K, Woods A, Kimbrell T, Dornhoffer J. Sham Transcranial Magnetic Stimulation Using Electrical Stimulation of the Scalp. Brain Stimul. 2009;2(3):168–173. doi: 10.1016/j.brs.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meteyard L, Holmes NP. TMS SMART - Scalp mapping of annoyance ratings and twitches caused by Transcranial Magnetic Stimulation. J Neurosci Methods. 2018;299:34–44. doi: 10.1016/j.jneumeth.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Mioli A, D'Alonzo M, Pellegrino G, Formica D, Di Pino G. Intermittent Theta Burst Stimulation Over Ventral Premotor Cortex or Inferior Parietal Lobule Does Not Enhance the Rubber Hand Illusion. Front Neurosci. 2018;12:870. doi: 10.3389/fnins.2018.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli F, Messina G, Valenzano A, Petito A, Triggiani AI, Messina A, et al. Capranica L. Differences in corticospinal system activity and reaction response between karate athletes and non-athletes. Neurological Sciences. 2016;37(12):1947–1953. doi: 10.1007/s10072-016-2693-8. [DOI] [PubMed] [Google Scholar]
- Muakkassa KF, Strick PL. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized 'premotor' areas. Brain Res. 1979;177(1):176–182. doi: 10.1016/0006-8993(79)90928-4. [DOI] [PubMed] [Google Scholar]
- Nickerson RS. Intersensory facilitation of reaction time: energy summation or preparation enhancement? Psychological review. 1973;80(6):489. doi: 10.1037/h0035437. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pardo-Vazquez JL, Padron I, Fernandez-Rey J, Acuna C. Decision-making in the ventral premotor cortex harbinger of action. Front Integr Neurosci. 2011;5:54. doi: 10.3389/fnint.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Brasil-Neto JP, Valls-SolÉ J, Cohen LG, Hallett M. Simple Reaction Time to Focal Transcranial Magnetic Stimulation. Brain. 1992;115(1):109–122. doi: 10.1093/brain/115.1.109. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Brasil-Neto J, Cohen LG, Hallett M. Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual and somatosensory stimuli. Brain. 1992;115(Pt 4):1045–1059. doi: 10.1093/brain/115.4.1045. [DOI] [PubMed] [Google Scholar]
- Pellegrino G, Tomasevic L, Herz DM, Larsen KM, Siebner HR. Theta activity in the left dorsal premotor cortex during action re-evaluation and motor reprogramming. Frontiers in human neuroscience. 2018;12 doi: 10.3389/fnhum.2018.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffin E, Pellegrino G, Di Lazzaro V, Thielscher A, Siebner HR. Bringing transcranial mapping into shape: sulcus-aligned mapping captures motor somatotopy in human primary motor hand area. Neuroimage. 2015;120:164–175. doi: 10.1016/j.neuroimage.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Riehle A, Requin J. Monkey primary motor and premotor cortex: single-cell activity related to prior information about direction and extent of an intended movement. J Neurophysiol. 1989;61(3):534–549. doi: 10.1152/jn.1989.61.3.534. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Zarola F, Stalberg E, Caramia M. Pre-movement facilitation of motor-evoked potentials in man during transcranial stimulation of the central motor pathways. Brain research. 1988;458(1):20–30. doi: 10.1016/0006-8993(88)90491-x. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Day BL, Thompson PD, Marsden CD. Interruption of motor programmes by electrical or magnetic brain stimulation in man. 1989;80:467–472. doi: 10.1016/s0079-6123(08)62244-x. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu X-H, Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Experimental Brain Research. 1994;102(2):227–243. doi: 10.1007/BF00227511. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Gough PM, Alexander IH, Kyriazis D, McDonald KR, Passingham RE. Attentional selection and action selection in the ventral and orbital prefrontal cortex. Journal of Neuroscience. 2005;25(50):11628–11636. doi: 10.1523/JNEUROSCI.2765-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD. Extrastriate cortex in primates. Springer; 1997. Visuomotor areas of the frontal lobe; pp. 527–638. [Google Scholar]
- Shenoy KV, Sahani M, Churchland MM. Cortical control of arm movements: a dynamical systems perspective. Annu Rev Neurosci. 2013;36:337–359. doi: 10.1146/annurev-neuro-062111-150509. [DOI] [PubMed] [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J Neurosci. 2004;24(5):1200–1211. doi: 10.1523/jneurosci.4731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair C, Hammond GR. Reduced intracortical inhibition during the foreperiod of a warned reaction time task. Exp Brain Res. 2008;186(3):385–392. doi: 10.1007/s00221-007-1241-4. [DOI] [PubMed] [Google Scholar]
- Starr A, Caramia M, Zarola F, Rossini PM. Enhancement of motor cortical excitability in humans by non-invasive electrical stimulation appears prior to voluntary movement. Electroencephalogr Clin Neurophysiol. 1988;70(1):26–32. doi: 10.1016/0013-4694(88)90191-5. [DOI] [PubMed] [Google Scholar]
- Uehara K, Funase K. Contribution of ipsilateral primary motor cortex activity to the execution of voluntary movements in humans: A review of recent studies. The Journal of Physical Fitness and Sports Medicine. 2014;3(3):297–306. doi: 10.7600/jpfsm.3.297. [DOI] [Google Scholar]
- Wise S, Weinrich M, Mauritz K-H. Motor aspects of cue-related neuronal activity in premotor cortex of the rhesus monkey. Brain research. 1983;260(2):301–305. doi: 10.1016/0006-8993(83)90685-6. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Netz J, Homberg V. Delay in simple reaction time after focal transcranial magnetic stimulation of the human brain occurs at the final motor output stage. Brain research. 1997;744(1):32–40. doi: 10.1016/S0006-8993(96)01062-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.