Abstract
Background
Coronary artery disease (CAD) is common in patients with severe aortic stenosis (AS). Computed tomography-derived fractional flow reserve (CT-FFR) is a clinically-utilized modality for assessing CAD, however its use has not been validated in patients with severe AS. This study assesses the safety, feasibility and validity of CT-FFR in patients with severe AS.
Methods
Prospectively-recruited patients underwent standard-protocol invasive FFR and coronary CT angiography (CTA). CTA images were analyzed by central core laboratory (HeartFlow, Inc., US) for independent evaluation of CT-FFR. CT-FFR data were compared with FFR (ischaemia defined as FFR<0.80).
Results
42 patients (68 vessels) underwent FFR and CTA; 39 patients (92.3%) and 60 vessels (88.2%) had interpretable CTA enabling CT-FFR computation. Mean age was 76.2±6.7 years (71.8% male). No patients incurred complications relating to pre-medication, CTA or FFR protocol. Mean FFR and CT-FFR were 0.83±0.10 and 0.77±0.14, respectively. CT calcium score was 1373.3±1392.9. On per vessel analysis, there was strong positive correlation between FFR and CT-FFR (Pearson’s correlation coefficient R=0.64, p<0.0001). Sensitivity, specificity, positive predictive and negative predictive values were 73.9%, 78.4%, 68.0% and 82.9%, respectively with 76.7% diagnostic accuracy. The area under the receiver-operating characteristic curve (ROC AUC) for CT-FFR was 0.83 (0.72-0.93, p<0.0001), which was higher than that of CTA and QCA (p=0.01 and p<0.001, respectively). Bland-Altman plot showed mean bias between FFR and CT-FFR as 0.059±0.110. On per patient analysis, the sensitivity, specificity, positive predictive and negative predictive values were 76.5%, 77.3%, 72.2% and 81.0% with 76.9% diagnostic accuracy. The per-patient ROC AUC was 0.81 (0.67-0.95, p<0.0001).
Conclusions
CT-FFR is safe and feasible in patients with severe AS. Our data suggests that the diagnostic accuracy of CT-FFR in this cohort potentially enables its use in clinical practice and provides the foundation for future research into the use of CT-FFR for coronary evaluation pre-AVR.
Keywords: FFR, CT-FFR, FFRct, TAVR, AS
Subject terms: Coronary Artery Disease, Aortic Valve Replacement/Transcatheter Aortic Valve Implantation, Computerized Tomography (CT), Valvular Heart Disease
Introduction
Approximately 25-50% of patients with severe aortic stenosis (AS) have concomitant coronary artery disease (CAD)1-4. Current guidelines recommend revascularization for patients undergoing transcatheter aortic valve replacement (TAVR) with >70% diameter stenosis in proximal coronary segments and it is therefore common practice to perform prior invasive coronary angiography (ICA)5. In addition to revascularization decisions, ICA also serves as a means for procedural risk stratification. However, pre-TAVR ICA is associated with inherent risks, particularly in patients with severe AS whom are usually elderly and with comorbidities6. Additionally, ICA provides no information on the functional impact of coronary stenosis, which may be important further guiding revascularization decisions prior to TAVR7. Given the recognized limitations of ICA in this higher risk cohort, there remains an unmet need for a valid non-invasive alternative that identifies lesion-specific ischaemia.
Coronary computed tomography angiography (CTA) is a well-established non-invasive modality which is used in the diagnosis and management of patients with chest pain of recent onset. Its excellent negative predictive value makes it particularly useful in the assessment of patients with low to intermediate pre-test probability for CAD. Whilst it can provide clinically useful anatomical information regarding the presence and extent of CAD, it does not provide any data on the functional impact of coronary stenosis8. CT-derived fractional flow reserve (CT-FFR) is a more recent development which uses computational flow dynamics to simulate invasive fractional flow reserve (FFR) from a standard CTA acquisition9. CT-FFR now provides a mean for deriving both anatomy and function from a standard CTA and its high diagnostic performance has led to its adoption in clinical guidelines10.
The use of coronary CTA has previously been explored in patients with severe AS11, 12. However, the application of this technology has been limited by the higher burden of calcium within the coronary vasculature in addition to clinicians’ reluctance to use pre-scan medications to optimize image quality (such as nitroglycerin and beta-blockers). However, recent advances and refinements in image-processing techniques have enabled the use of CTA in patients with higher burden of calcium. Improved imaging acquisition in this cohort also permits the possibility of CT-FFR modelling, which provides incremental functional data. However, CT-FFR has not been previously evaluated in the coronary assessment of patients with severe AS.
We therefore designed and conducted a prospective study to assess the clinical safety, feasibility and diagnostic performance of CT-FFR in patients with severe AS, compared against invasively derived FFR.
Methods
Patient selection
This was a prospective, single-center study carried out at Monash Medical Centre, Melbourne between November 2018 and November 2019. The study protocol was approved by the institutional research ethics committee (Human Research Ethics Committees Australia reference: HREC/43524/MonH-2018-67705v1). All recruited patients provided written informed consent. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Patients with severe AS with an indication for TAVR as per international guidelines 5 and underwent pre-procedural ICA were screened for participation. Inclusion criteria were: (1) aged >18 years and <90 years old and, (2) patients with >30% visual stenosis in at least one coronary artery identified at time of ICA. Exclusion criteria were: (1) severe asthma or resting bradycardia precluding use of adenosine, (2) left ventricular ejection fraction <30%, (3) chronic renal impairment, defined by estimated glomerular filtration rate <30ml/min/1.73m2, (4) myocardial infarction within last 3 months, (5) previous coronary artery bypass surgery (CABG), (6) percutaneous coronary intervention (PCI) in the vessel of interest, (7) >90% visual stenosis in the vessel of interest (8) chronic total occlusions and (9) significant left main coronary disease
Invasive FFR protocol
Cardiac catheterization was performed in accordance to standard practice, via the transfemoral or transradial approach. All patients were anticoagulated using 70-100 IU/kg of unfractionated heparin. Orthogonal plane angiography were acquired at 15 frames per second. Pressure wire assessment was then performed if there was at least one vessel (>2 mm ivabradine to achieve a pre-scan heart rate of <60 beats/min (protocol adopted from with
Statistical analysis
The primary endpoint of this study was per vessel diagnostic performance of CT-FFR to predict ischemia, as defined by invasive FFR <0.80 using the area under the receiveroperating characteristic curve analysis (ROC AUC). Secondary endpoints included diagnostic accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for CT-FFR (<0.80), using FFR <0.80 as reference standard. Additional outcomes included the diagnostic performance on a per patient basis, whereby the lowest values of FFR and CT-FFR were used in patients with complete data in more than one vessel. Diagnostic accuracy was also evaluated based on a median split of CT calcium scores to determine the validity of this approach in patients with high calcium scores. The Shapiro-Wilk test was used to assess normality of continuous variables. Continuous variables are expressed as mean ± standard deviation (SD) or median ± interquartile range. Categorical variables are provided as frequencies (percentages). The correlation between CT-FFR and invasive FFR was assessed with Pearson’s correlation coefficient. Agreement between the two indices was assessed with a Bland-Altman technique. Statistical analyses were performed with Stata v.14.1 (StataCorp, College Station, TX, USA) and GraphPad Prism v.8.1.2 (La Jolla, CA, USA).
Results
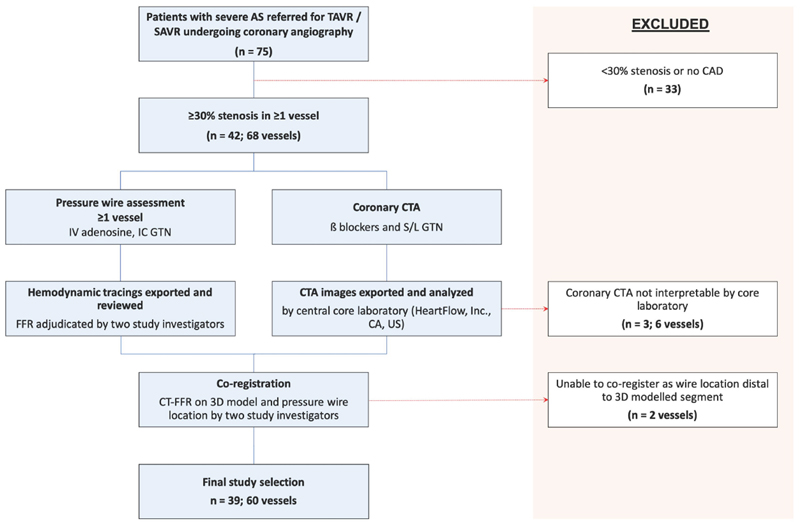
42 patients (68 vessels) underwent invasive FFR and CTA assessment (Figure 1). Of those, 39 patients (92.3%) and 60 vessels (88.2%) had interpretable CTA data enabling CT-FFR computation. Three patients (6 vessels) were not suitable for CT-FFR calculation due to motion artefact on CTA as adjudicated by the central core laboratory. Additionally, 2 vessels were excluded as they could not be co-registered as the angiographic pressure wire sensor location was distal to the 3D modelled segment. The patient and echocardiographic characteristics are presented in Table 1. The mean age was 76.2 ± 6.7 years of whom 71.8% were male, 69.2% had hypertension, 53.8% had diabetes mellitus and 12.8% had previous myocardial infarction. Mean aortic valve gradient, aortic valve area and left ventricular ejection fraction were 45.1 ± 9.5mmHg, 0.89 ± 0.25 cm2 and 62.9 ± 10.7%, respectively. Mean patient CT calcium score was 1373.3 ± 1392.9 Agatston units.
Figure 1. Study Flow Chart.
Table 1. Patient and Echocardiographic Characteristics.
| Patient characteristics (n = 39) | |
|---|---|
| Age, yrs | 76.2 ± 6.7 |
| Male | 28 (71.8) |
| Body mass index | 28.6 ± 6.7 |
| Diabetes mellitus | 21 (53.8) |
| Hypertension | 27 (69.2) |
| Atrial fibrillation | 4 (10.3) |
| Previous MI | 5 (12.8) |
| Dyslipidemia | 26 (66.7) |
| Previous CVA or TIA | 5 (12.8) |
| Smoking | |
| Current smoker | 2 (5.1) |
| Former smoker | 14 (35.9) |
| Creatinine (mmol/L) | 90.5 ± 27.2 |
| Median | 1027 |
| Pre-procedural echocardiographic parameters | |
| LVEF, % | 62.9 ± 10.7 |
| Peak gradient, mmHg | 75.3 ± 15.9 |
| Mean gradient, mmHg | 45.1 ± 9.5 |
| Valve Area, cm2 | 0.89 ± 0.25 |
| Dimensionless index | 0.23 ± 0.04 |
Values are presented as n (%) or mean ± SD; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MR, mitral regurgitation; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
The CT scanning characteristics are presented in Table 2. All patients received 0.4mg sublingual glyceryl trinitrate. Two-thirds of patients received additional pre-scan medications in order to optimize their heart rate. Mean pre-CTA scan heart rate was 54.2 ± 6.6 beats/min. No patients incurred complications relating to the pre-medication, CTA or invasive FFR protocol.
Table 2. CT Scan Acquisition Characteristics.
| CT characteristics | (n = 39) |
|---|---|
| Heart rate, beats/min | 54.2 ± 6.6 |
| Nitrates administered | 39 (100) |
| Pre-scan beta-blocker use | |
| Oral | 26 (66.7) |
| Intravenous | 1 (2.6) |
| Beta-blocker dose, mg (SD) | |
| Oral metoprolol | 78.8 ± 49.3 |
| Intravenous metoprolol | 20 ± 0 |
| Ivabradine (10 mg) use | 16 (41.0) |
| Tube voltage, kV | |
| 100 | 12 (30.8) |
| 120 | 27 (69.2) |
| Tube current, mA | 663.3 ± 123.1 |
| CT protocol B (n=16) | 7.3 ± 6.8 |
Values are presented as n (%) or mean ± SD
The vessel characteristics are presented in Table 3. Of the vessels assessed, 36 were left anterior descending arteries, 5 were diagonal, 13 were circumflex or obtuse marginal, 1 was a ramus and 5 were right coronary arteries. On quantitative coronary angiography (QCA), 10.0% of vessels and 15.4% of patients had diameter stenosis ≥50%. On coronary CTA analysis, 35.0% of vessels and 41.0% of patients had CTA diameter stenosis ≥50%. Mean FFR and CT-FFR were 0.83 ± 0.10 and 0.77 ± 0.14, respectively. 38.3% of vessels had an FFR <0.80 whilst 41.7% of vessels had CT-FFR <0.80.
Table 3. Vessel Characteristics.
| Variable | |
|---|---|
| Vessels studied | |
| LAD or diagonal | 41 (68.3) |
| Cx or OM | 13 (21.7) |
| Ramus intermedius | 1 (1.7) |
| RCA, PDA or R-PLV | 5 (8.3) |
| QCA | |
| Mean diameter stenosis, % | 33.8 ± 12.0 |
| Number of vessels with diameter stenosis ≥50% | 6/60 (10.0) |
| Number of patients with diameter stenosis ≥50 | 6/39 (15.4 |
| Coronary CTA | |
| Number of vessels with CTA maximum stenosis ≥50% | 21 (35.0) |
| Number of patients with CTA maximum stenosis ≥50% | 16 (41.0) |
| Mean FFR | 0.83 ± 0.10 |
| Vessels with FFR ≤0.80 | 23/60 (38.3) |
| Patients with FFR ≤0.80 | 17/39 (43.6) |
| Mean CT-FFR | 0.77 ± 0.14 |
| Vessels with CT-FFR ≤0.80 | 25/60 (41.7) |
| Patients with CT-FFR ≤0.80 | 18/39 (46.1) |
Values are presented as n (%) or mean ± SD; CTA, CT computed tomography angiography; CT-derived fractional flow reserve; Cx, circumflex; FFR, fractional flow reserve; LAD, left anterior descending; OM, obtuse marginal; PDA, posterior descending artery; QCA, quantitative coronary angiography; RCA, right coronary artery; R-PLV, right posterior left ventricular.
Per vessel analysis
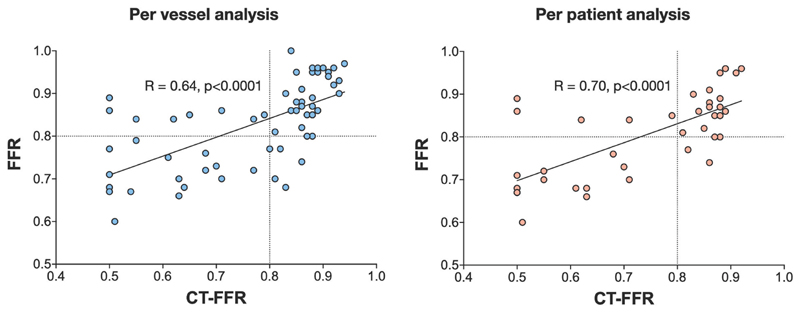
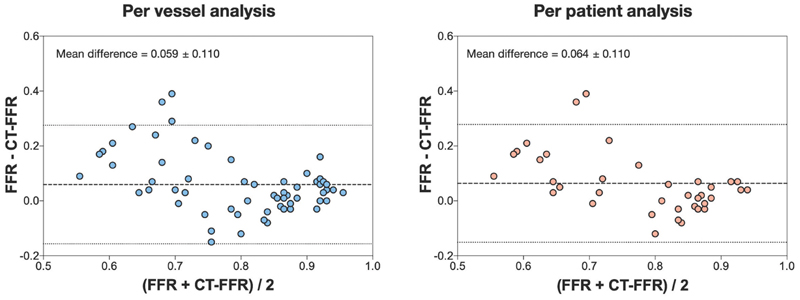
The diagnostic performance of QCA, coronary CTA and CT-FFR against FFR are presented in Table 4. On a per vessel basis, there was a strong positive correlation between FFR and CT-FFR (Pearson’s correlation coefficient R=0.64, p<0.0001, Figure 2). Sensitivity, specificity, positive predictive and negative predictive values were 73.9%, 78.4%, 68.0% and 82.9%, respectively with overall diagnostic accuracy of 76.7%. The ROC AUC for CT-FFR was 0.83 (95% confidence interval [CI] 0.72-0.93, p<0.0001). The Bland-Altman plot showed the mean bias ± standard deviation between FFR and CT-FFR was 0.059 ±0.110 (Figure 3). The ROC AUC for CT-FFR to predict invasive FFR was greater than that of coronary CTA and QCA (both p<0.05).
Table 4. Diagnostic Performance of CT-FFR, Coronary CTA and QCA against Invasive FFR.
| Per vessel analysis | Per patient analysis | |||
|---|---|---|---|---|
| CT-FFR | CCTA (>50%) | QCA (>50%) | CT-FFR | |
| Pearson’s correlation coefficient |
0.64, p<0.0001 | N/A | N/A | 0.70, p<0.0001 |
| True positive | 17 | 12 | 4 | 13 |
| False positive | 8 | 9 | 2 | 5 |
| True negative | 29 | 28 | 35 | 17 |
| False negative | 6 | 11 | 19 | 4 |
| Sensitivity % | 73.9 | 52.2 | 17.4 | 76.5 |
| Specificity % | 78.4 | 75.7 | 94.6 | 77.3 |
| PPV % | 68.0 | 57.1 | 66.7 | 72.2 |
| NPV % | 82.9 | 71.8 | 64.8 | 81.0 |
| Accuracy % | 76.7 | 66.7 | 65.0 | 76.9 |
| ROC AUC (95% CI) Comparison against ROC AUC for CT-FFR to predict FFR |
0.83 (0.72-0.93) | 0.64 (0.51.-0.76) p = 0.01 |
0.56 (0.47-0.65) p <0.001 |
0.81 (0.67 to 0.95) |
| Bland-Altman analysis (mean bias ± SD) | 0.059 ± 0.110 (-0.16-0.27) | N/A | N/A | 0.064 ± 0.110 (-0.15-0.28) |
Values are presented as n (%) or mean ± SD. CCTA, coronary computed tomography angiography; NPV, negative predictive value; PPV, positive predictive value; ROC AUC, area under the receiver-operating characteristic curve.
Figure 2. Correlations of CT-FFR vs FFR on A Per Vessel and Per Patient Basis.
Figure 3. Bland-Altman (Difference Versus Average) of FFR vs CTFFR.
Per patient analysis
On a per patient analysis, there again was a strong positive correlation between FFR and CT-FFR (Pearson’s correlation coefficient 0.70, p<0.0001). Sensitivity, specificity, positive predictive and negative predictive values were 76.5%, 77.3%, 72.2% and 81.0% with overall diagnostic accuracy of 76.9%. On a per patient analysis, the ROC AUC for CT-FFR was 0.81 (CI 0.67-0.95, p = 0.001). The Bland-Altman plot showed the mean bias ± standard deviation between FFR and CT-FFR was 0.064 ±0.110.
Subgroup analysis
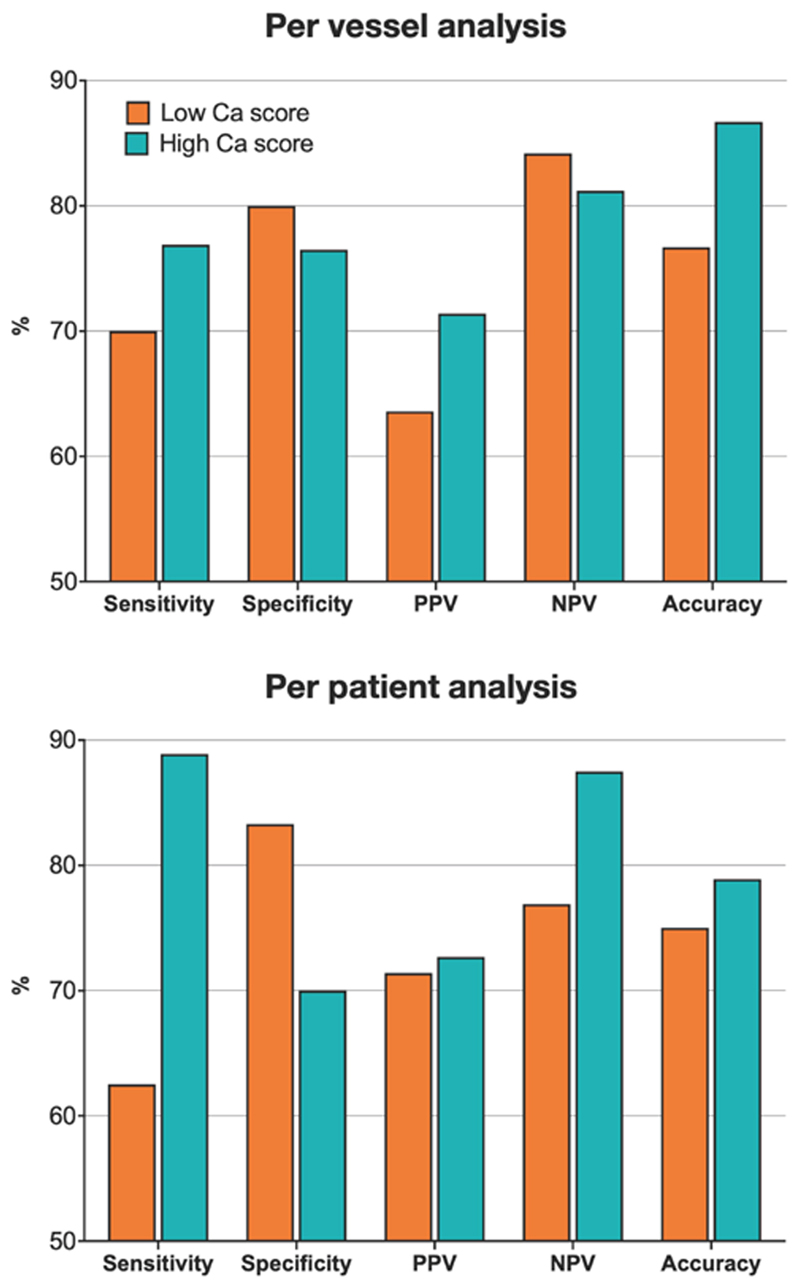
A subgroup analysis was performed to look at the diagnostic performance of CT-FFR according to the magnitude of the CT-derived calcium score. This was on a per vessel and per patient basis and the results are presented in Tables S1 and S2 respectively in the Data Supplement, and in Figure 4. In the per vessel subgroup analysis, the mean vessel calcium scores in the low and high groups were 123.1 ± 94.2 and 735.3 ± 566.5 Agatston units, respectively. The correlation between CT-FFR and FFR in the low calcium score group was stronger (r = 0.85 vs 0.61), however there was no difference between the ROC AUC for CT-FFR between the two groups (0.83 vs 0.82, p = 0.94). In the per patient subgroup analysis, the mean calcium scores in the low and high score groups were 514.8 ± 320.3 and 2277.1 ± 1518.7, respectively. Similarly, the correlation between CT-FFR and FFR was stronger in the low calcium score group (r=0.85 vs r=0.61), however with no difference between the ROC AUC for CT-FFR between the two groups (0.77 vs. 0.80, p = 0.84).
Figure 4. Subgroup Analysis of Diagnostic Performance of CT-FFR According to CT Calcium Score.
Discussion
Our findings demonstrate, for the first time, that coronary CTA and CT-FFR is safe, feasible and has a diagnostic accuracy which potentially enables its practice in patients with severe AS. These results represent significant progress in the non-invasive assessment of the CAD in patients with severe AS. Despite the well-recognized limitations of using coronary CTA in older patient cohorts with greater burden of coronary vascular calcification, we have demonstrated that in this cohort – older and with a notably high average calcium score – a high diagnostic accuracy can be attained using CT-FFR using the protocols described. Importantly, 92% of our cohort had interpretable data highlighting the potential for future translation into clinical practice.
Several strategies were employed in order to achieve these results. Besides the use of a 320-slice detector CT scanner, we utilized CT-FFR modelling for better characterization and assessment of the vessels to overcome the inherent limitations in the diagnostic performance of CTA alone in the presence of calcified disease. Additionally, we adhered to a strict prescanning medication protocol in order to optimize image quality. This approach has traditionally been avoided in patients with severe AS due to concerns with hypotension and circulatory collapse. In our study, we ensured all patients were well hydrated prior to drug administration. Nitroglycerin was administered both prior to coronary CTA and invasive FFR measurements. Beta-blockers and ivabradine were used pre-CTA to attain a heart rate of <60 beats/min, which was achieved in 79% of patients. During invasive FFR, intravenous adenosine was used in all patients and there were no adverse effects relating to medication protocols used in our cohort. Whilst complete atrioventricular block remains a greater risk with adenosine in this patient group, a previous report demonstrated preserved coronary hemodynamics despite systemic hypotension in a patient with severe AS14. Despite no adverse outcomes in our cohort, we are unable to quantify the risk for every patient with severe AS and this would need evaluation in further studies, comparing this strategy against the risk of invasive procedures currently used as standard of care.
The use of coronary CTA in patients with severe AS undergoing TAVR has previously been investigated in two retrospective studies11,12. In these studies, drugs for vasodilatation and chronotropic control were not used due to concerns about safety. These medications are otherwise recommended for improving the diagnostic performance of CTA, particularly in older patients with significant and calcified CAD15. In one of these studies, 18% of the patients underwent ICA as they were deemed to have significant or uninterpretable disease (excluding those who had undergone ICA for another indication)12. Of those, more than half the patients had no significant disease on ICA with only a proportion of the remainder (4.5% of the study cohort) undergoing subsequent revascularization. In the second study, all patients underwent ICA following CTA11. Whilst the diagnostic accuracy of CTA was 91% in patients with Agatston scores of <400, the overall accuracy was only 66% in those with Agatston scores >1000. These two studies concluded that the use of coronary CTA in this setting is potentially acceptable in patients with lower calcium scores, with CTA acting as a possible gatekeeper for ICA. Notably, these two studies evaluate CTA diagnostic performance against ICA rather than invasive FFR. In our study, the diagnostic performance of CTA (compared with invasive FFR) demonstrates that the use of this technology - even with strict pre-scanning medication protocols - may be inadequate, even as a gatekeeper for ICA. The use of CT-FFR modelling provides an increment in diagnostic performance and, importantly, this is maintained in both the lower and higher calcium score groups.
The use of CT-FFR also provides important data on the functional impact of coronary disease. Whilst only 10% of vessels in this study cohort had a QCA-defined lesion diameter stenosis >50%, 42% had CT-FFR <0.80 and 38% had invasive FFR <0.80. Similar to observations in non-AS patients16,17, this suggests that diameter stenosis is a poor predictor of FFR <0.80. This may be due to other physiological determinants such as lesion length and the myocardial area subtended.18 The presence of abnormal physiology in the context of AS and left ventricular hypertrophy may also contribute to this apparent discrepancy.
It is key to acknowledge that the overall diagnostic performance of CT-FFR in this severe AS cohort remains lower than that in previously published literature in non-AS, stable CAD cohorts19, 20. Compared to our participants, patients in previous studies were younger and with less coronary calcification. However, the performance of CT-FFR is acceptable in those with high calcium scores and this alone is unlikely to account for all the discrepancy observed. Another contributing factor may be the altered coronary and microcirculatory pathophysiology that occurs in patients with severe AS21. Valvular stenosis results in pressure overload within the left ventricle and resultant left ventricular hypertrophy. The greater myocardial mass of patients with AS results in increased myocardial oxygen demand which is matched with greater resting coronary blood flow. AS patients also exhibit an impaired coronary hyperemic response. This blunted response is not currently accounted for using standard CT-FFR modelling approaches. Overall, the cumulative effect may explain the relative overestimation of translesional gradients by CT-FFR compared with invasive FFR. Further work is now required in describing the abnormal coronary physiology in differing patient cohorts, with the aim of improving the accuracy of CT-FFR and other techniques that use computational fluid dynamic approaches.
The ability to use coronary CTA and CT-FFR to appropriately delineate the anatomy opens the opportunity to using CT as a ‘one-stop-shop’ assessment for patients referred for TAVR. Currently, these patients routinely undergo CTA for pre-TAVR procedural planning for assessment of the left ventricular outflow tract, annulus, ascending and descending aorta and peripheral vasculature 22. Routinely incorporating coronary assessment within this scan would permit comprehensive pre-TAVR assessment in a single scan, which has clear potential benefit for patients. This would potentially include, (1) removing the procedural risks associated with ICA, (2) reducing the risk of nephropathy associated with the additional contrast load of ICA, (3) reducing the discomfort associated with invasive procedures, and (4) potential health-economic advantages associated with fewer invasive tests. Hopefully our data acts as a stimulus for definitive clinical trials that assess the use CT-FFR in procedural planning for patients undergoing TAVR.
Limitations
This a single-center and ongoing validation in a larger, multi-center study is required. With a mean age of 76.2 years, our cohort represents a younger age group than that would currently be undergoing TAVR and it is unclear whether these results can be extrapolated into very elderly patients (>90yrs). However, with expanding indications of TAVR in low and intermediate surgical risk groups, our results may still apply in future patient cohorts. In addition, our subgroup analysis demonstrated that the diagnostic performance of this technology was maintained in patients with higher calcific burden although. Our study also excludes patients who have had previous revascularization (either by CABG or PCI) and LV dysfunction, which represent a sizeable group of patients undergoing TAVR. Finally, the results from this study relate to one commercially available CT-FFR technique and the validity of other non-invasive techniques remains unknown.
Conclusions
Our results demonstrate that CT-FFR is feasible and safe in patients with severe AS. These preliminary data suggest that the diagnostic accuracy of CT-FFR potentially enables its use in clinical practice. These data should act as the foundation for future research into use of CT-FFR during procedural planning for patients with severe AS undergoing valve replacement.
Supplementary Material
What is Known
-
▪
It remains common practice to perform invasive coronary angiography in patients with severe AS undergoing TAVR to identify the severity and extent of CAD.
-
▪
Coronary CTA has had a limited role in this patient group due to significant coronary calcification and clinicians’ reluctance to use pre-scan medications. Additionally, it provides no information on the functional impact of coronary stenosis which may be important in guiding revascularisation pre-TAVR.
-
▪
CT-FFR provides data on the functional impact of coronary stenosis, however, has not previously been validated in patients with severe AS.
What this Study ADDS
-
▪
CT-FFR is safe and feasible in patients with severe AS. CT-FFR in this cohort outperforms coronary CTA to identify FFR-significant lesions.
-
▪
Further research is required to assess the clinical utility of CT-FFR and outcomes in this patient cohort. With further validation, this may reduce the need for invasive coronary angiography in pre-TAVR assessment.
Sources of Funding
AJB is supported by both a National Health and Medical Research Early Career Fellowship and National Heart Foundation Post-Doctoral Scholarship. ADH receives support from the British Heart Foundation (CS/13/1/30327, PG/13/6/29934, PG/15/75/31748, CS/15/6/31468, PG/17/90/33415, IG/18/5/33958), the National Institute for Health Research University College London Hospitals Biomedical Research Centre, the UK Medical Research Council (MR/P023444/1) and works in a unit that receives support from the UK Medical Research Council (MC_UU_12019/1).
Abbreviations
- AS
aortic stenosis
- CABG
coronary artery bypass surgery
- CAD
coronary artery disease
- CTA
computed tomography angiography
- CT-FFR
CT-derived fractional flow reserve
- FFR
fractional flow reserve
- ICA
invasive coronary angiography
- NPV
negative predictive value
- PCI
percutaneous coronary intervention
- PPV
positive predictive value
- ROC AUC
area under the receiver-operating characteristic curve analysis
- TAVR
transcatheter aortic valve replacement
- QCA
quantitative coronary angiography
Footnotes
Disclosures:
ARI has received consulting fees from Boston Scientific and Canon Medical. BK has received consulting fees from Canon Medical and honorarium from Medtronic, Canon Medical and St Jude. AJB has received consultancy fees from Abbott Vascular and Boston Scientific. The remaining authors have nothing to disclose.
References
- 1.Vandeplas A, Willems JL, Piessens J, De Geest H. Frequency of angina pectoris and coronary artery disease in severe isolated valvular aortic stenosis. Am J Cardiol. 1988;62:117–20. doi: 10.1016/0002-9149(88)91375-6. [DOI] [PubMed] [Google Scholar]
- 2.Exadactylos N, Sugrue DD, Oakley CM. Prevalence of coronary artery disease in patients with isolated aortic valve stenosis. Br Heart J. 1984;51:121–4. doi: 10.1136/hrt.51.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortlepp JR, Schmitz F, Bozoglu T, Hanrath P, Hoffmann R. Cardiovascular risk factors in patients with aortic stenosis predict prevalence of coronary artery disease but not of aortic stenosis: an angiographic pair matched case-control study. Heart. 2003;89:1019–22. doi: 10.1136/heart.89.9.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapp AH, Hillis LD, Lange RA, Cigarroa JE. Prevalence of coronary artery disease in patients with aortic stenosis with and without angina pectoris. Am J Cardiol. 2001;87:1216–7. doi: 10.1016/s0002-9149(01)01501-6. A7. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Munoz DR, Rosenhek R, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease: The Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2017;38:2739–2791. [Google Scholar]
- 6.Tavakol M, Ashraf S, Brener SJ. Risks and complications of coronary angiography: a comprehensive review. Glob J Health Sci. 2012;4:65–93. doi: 10.5539/gjhs.v4n1p65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lunardi M, Scarsini R, Venturi G, Pesarini G, Pighi M, Gratta A, Gottin L, Barbierato M, Caprioglio F, Piccoli A, Ferrero V, et al. Physiological Versus Angiographic Guidance for Myocardial Revascularization in Patients Undergoing Transcatheter Aortic Valve Implantation. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012618. e012618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danad I, Raijmakers PG, Driessen RS, Leipsic J, Raju R, Naoum C, Knuuti J, Maki M, Underwood RS, Min JK, Elmore K, et al. Comparison of Coronary CT Angiography, SPECT, PET, and Hybrid Imaging for Diagnosis of Ischemic Heart Disease Determined by Fractional Flow Reserve. JAMA Cardiol. 2017;2:1100–1107. doi: 10.1001/jamacardio.2017.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min JK, Taylor CA, Achenbach S, Koo BK, Leipsic J, Norgaard BL, Pijls NJ, De Bruyne B. Noninvasive Fractional Flow Reserve Derived From Coronary CT Angiography: Clinical Data and Scientific Principles. JACC Cardiovasc Imaging. 2015;8:1209–22. doi: 10.1016/j.jcmg.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence. HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography. NICE Medical technologies guidance [MTG32] 2017 Available from: https://www.nice.org.uk/guidance/mtg32 [Published date: February 2017]
- 11.Rossi A, De Cecco CN, Kennon SRO, Zou L, Meinel FG, Toscano W, Segreto S, Achenbach S, Hausleiter J, Schoepf UJ, Pugliese F. CT angiography to evaluate coronary artery disease and revascularization requirement before trans-catheter aortic valve replacement. JCardiovasc Comput Tomogr. 2017;11:338–346. doi: 10.1016/j.jcct.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Chieffo A, Giustino G, Spagnolo P, Panoulas VF, Montorfano M, Latib A, Figini F, Agricola E, Gerli C, Franco A, Giglio M, et al. Routine Screening of Coronary Artery Disease With Computed Tomographic Coronary Angiography in Place of Invasive Coronary Angiography in Patients Undergoing Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.114.002025. e002025. [DOI] [PubMed] [Google Scholar]
- 13.Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK, Marwan M, Naoum C, Norgaard BL, Rubinshtein R, Schoenhagen P, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI) J Cardiovasc Comput Tomogr. 2016;10:435–449. doi: 10.1016/j.jcct.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Michail M, Asrress KN, Cameron JD, Gooley R, McCormick LM, Hughes AD, Brown AJ. Adaptations to Coronary Physiology in a Patient With Severe Aortic Stenosis and Complete Heart Block Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2019;12:687–689. doi: 10.1016/j.jcin.2019.01.215. [DOI] [PubMed] [Google Scholar]
- 15.Raff GL, Chinnaiyan KM, Cury RC, Garcia MT, Hecht HS, Hollander JE, O’Neil B, Taylor AJ, Hoffmann U, Society of Cardiovascular Computed Tomography Guidelines C SCCT guidelines on the use of coronary computed tomographic angiography for patients presenting with acute chest pain to the emergency department: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:254–71. doi: 10.1016/j.jcct.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Park SJ, Kang SJ, Ahn JM, Shim EB, Kim YT, Yun SC, Song H, Lee JY, Kim WJ, Park DW, Lee SW, et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. JACC: Cardiovasc Interv. 2012;5:1029–1036. doi: 10.1016/j.jcin.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Ko BS, Wong DT, Cameron JD, Leong DP, Leung M, Meredith IT, Nerlekar N, Antonis P, Crossett M, Troupis J, Harper R, et al. 320-row CT coronary angiography predicts freedom from revascularisation and acts as a gatekeeper to defer invasive angiography in stable coronary artery disease: a fractional flow reserve-correlated study. Eur Radiol. 2014;24:738–47. doi: 10.1007/s00330-013-3059-8. [DOI] [PubMed] [Google Scholar]
- 18.Michail M, Dehbi HM, Nerlekar N, Davies JE, Sharp ASP, Talwar S, Cameron JD, Brown AJ, Wong DT, Mathur A, Hughes AD, et al. Application of the DILEMMA score to improve lesion selection for invasive physiological assessment. Catheter CardiovascInterv. 2019;94:E96–E103. doi: 10.1002/ccd.28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, Jensen JM, Mauri L, De Bruyne B, Bezerra H, Osawa K, et al. Group NXTTS Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps) J Am Coll Cardiol. 2014;63:1145–1155. doi: 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 20.Ko BS, Cameron JD, Munnur RK, Wong DTL, Fujisawa Y, Sakaguchi T, Hirohata K, Hislop-Jambrich J, Fujimoto S, Takamura K, Crossett M, et al. Noninvasive CT-Derived FFR Based on Structural and Fluid Analysis: A Comparison With Invasive FFR for Detection of Functionally Significant Stenosis. JACC Cardiovasc Imaging. 2017;10:663–673. doi: 10.1016/j.jcmg.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Michail M, Davies JE, Cameron JD, Parker KH, Brown AJ. Pathophysiological coronary and microcirculatory flow alterations in aortic stenosis. Nat Rev Cardiol. 2018;15:420–431. doi: 10.1038/s41569-018-0011-2. [DOI] [PubMed] [Google Scholar]
- 22.Jilaihawi H, Kashif M, Fontana G, Furugen A, Shiota T, Friede G, Makhija R, Doctor N, Leon MB, Makkar RR. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol. 2012;59:1275–86. doi: 10.1016/j.jacc.2011.11.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.