Abstract
Background
A polypill comprising statins, multiple blood-pressure–lowering drugs, and aspirin has been proposed to reduce the risk of cardiovascular disease.
Methods
Using a 2-by-2-by-2 factorial design, we randomly assigned participants without cardiovascular disease who had an elevated INTERHEART Risk Score to receive a polypill (containing 40 mg of simvastatin, 100 mg of atenolol, 25 mg of hydrochlorothiazide, and 10 mg of ramipril) or placebo daily, aspirin (75 mg) or placebo daily, and vitamin D or placebo monthly. We report here the outcomes for the polypill alone as compared with matching placebo, for aspirin alone as compared with matching placebo, and for the polypill plus aspirin as compared with double placebo. For the polypill-alone and polypill-plus-aspirin comparisons, the primary outcome was death from cardiovascular causes, myocardial infarction, stroke, resuscitated cardiac arrest, heart failure, or revascularization. For the aspirin comparison, the primary outcome was death from cardiovascular causes, myocardial infarction, or stroke. Safety was also assessed.
Results
A total of 5713 participants underwent randomization, and the mean follow-up was 4.6 years. The low-density lipoprotein cholesterol level was lower by approximately 19 mg per deciliter and systolic blood pressure was lower by approximately 5.8 mm Hg with the polypill and with combination therapy than with placebo. The primary outcome for the polypill comparison occurred in 126 participants (4.4%) in the polypill group and in 157 (5.5%) in the placebo group (hazard ratio, 0.79; 95% confidence interval [CI], 0.63 to 1.00). The primary outcome for the aspirin comparison occurred in 116 participants (4.1%) in the aspirin group and in 134 (4.7%) in the placebo group (hazard ratio, 0.86; 95% CI, 0.67 to 1.10). The primary outcome for the polypill-plus-aspirin comparison occurred in 59 participants (4.1%) in the combined-treatment group and in 83 (5.8%) in the doubleplacebo group (hazard ratio, 0.69; 95% CI, 0.50 to 0.97). The incidence of hypotension or dizziness was higher in groups that received the polypill than in their respective placebo groups.
Conclusions
Combined treatment with a polypill plus aspirin led to a lower incidence of cardiovascular events than did placebo among participants without cardiovascular disease who were at intermediate cardiovascular risk. (Funded by the Wellcome Trust and others; TIPS-3 ClinicalTrials.gov number, NCT01646437.)
C ardiovascular diseases account for approximately 18 million deaths each year worldwide, with more than 80% of the deaths occurring in low-income and middle-income countries.1,2 Elevated blood pressure and an elevated level of low-density lipoprotein (LDL) cholesterol are among the most important modifiable risk factors for cardiovascular disease.3,4 The associations of these risk factors with myocardial infarction and stroke are graded, and so their simultaneous reductions, regardless of initial levels, should, theoretically, lead to substantial reductions in the incidence of cardiovascular disease.5–7 These concepts provide part of the rationale for the use of “polypills,” which combine lipid-lowering and blood-pressure–lowering medications, in populations at increased risk. Aspirin is of proven value in patients with established cardiovascular disease,8 but its role either alone or as part of a polypill for the primary prevention of cardiovascular disease is unclear.9
Therefore, we designed a trial to test separately whether treatment with a polypill consisting of a statin and multiple blood-pressure–lowering drugs, aspirin alone, or their combination would reduce the incidence of cardiovascular events among persons without cardiovascular disease. Here we describe the results of TIPS-3 (the International Polycap Study 3), in which we evaluated the efficacy and safety of the polypill as compared with matching placebo, of aspirin as compared with matching placebo, and of the combination of the polypill plus aspirin as compared with double placebo.10
Methods
Trial Design and Organization
TIPS-3 was a double-blind, randomized, placebo-controlled trial with a 2-by-2-by-2 factorial design.10 In the first randomized comparison, we tested the effect of treatment with a daily polypill comprising 40 mg of simvastatin, 100 mg of atenolol, 25 mg of hydrochlorothiazide, and 10 mg of ramipril (Polycap, Cadila Pharmaceuticals), as compared with placebo, on the incidence of cardiovascular outcomes. In the second randomized comparison, we tested the effect of treatment with enteric-coated aspirin at a dose of 75 mg per day, as compared with placebo, on the incidence of cardiovascular outcomes (primary outcome) and the composite of cardiovascular outcomes and cancer (secondary outcome). We also assessed the effects of the polypill plus aspirin as compared with double placebo. In the third randomized comparison (results not reported here), we tested the effect of treatment with vitamin D at a monthly dose of 60,000 IU, as compared with placebo, on the incidence of fractures and falls.
The trial was conducted at 86 centers in nine countries. The ethics committee at each center and the regulatory authorities in each country approved the protocol (available with the full text of this article at NEJM.org). The trial was coordinated by the Population Health Research Institute, Hamilton Health Sciences and McMaster University, in Hamilton, Ontario, Canada, with national coordinating centers in each country. Funding was provided by the Wellcome Trust and others; Cadila Pharmaceuticals provided the trial drugs and placebos as well as additional support. The trial funders had no role in the design of the trial, its conduct or supervision, in the analysis or interpretation of the data, or in the writing of the manuscript or the decision to submit it for publication. The members of the steering committee (see the Supplementary Appendix, available at NEJM.org) vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol.
Trial Population
Men 50 years of age or older and women 55 years of age or older who did not have known cardiovascular disease but who had an elevated INTERHEART Risk Score11,12 indicating intermediate or high cardiovascular risk were eligible for inclusion in the trial. The scale for the INTERHEART Risk Score ranges from 0 to 48, with higher scores indicating greater cardiovascular risk. Detailed trial inclusion and exclusion criteria are described in Section S2 in the Supplementary Appendix. All the participants provided written informed consent.
Procedures
Eligible participants entered a run-in phase lasting 3 to 4 weeks, during which they received low-dose polypill (half doses of blood-pressure–lowering medications plus 40 mg of simvastatin) and low-dose aspirin (75 mg) daily. Participants who had at least 80% adherence to these medication regimens, who did not have adverse events, and who agreed to undergo randomization were assigned to receive a full-dose polypill or matching placebo, aspirin or matching placebo, and vitamin D or matching placebo. Randomization was performed with the use of an automated central randomization system, with stratification according to center. Follow-up visits occurred at 6 weeks; at 3, 6, 9 and 12 months; and every 6 months thereafter until the end of the trial. A low-dose polypill (or matching placebo) or a polypill with full doses of each component except ramipril (or matching placebo) were available for participants who had dizziness, hypotension, or cough. Blood pressure was recorded at each visit. Fasting blood samples were obtained before the run-in phase and at 6, 12, and 24 months for the local analysis of lipid levels; in addition, central analysis of lipid levels was carried out in a subgroup of participants (Section S3). All the participants received advice on healthy behaviors.
Outcomes
The primary outcome for the evaluations of the polypill and the polypill plus aspirin as compared with their respective placebos was a composite of major cardiovascular events, which included death from cardiovascular causes, stroke, and myocardial infarction, plus heart failure, resuscitated cardiac arrest, and arterial revascularization. Secondary outcomes were major cardiovascular events and the composite of the primary outcome plus angina with evidence of ischemia. For the evaluation of aspirin as compared with placebo, the primary composite outcome was death from cardiovascular causes, myocardial infarction, or stroke. Additional prespecified outcomes included death from any cause and first and recurrent (i.e., all) cardiovascular events; for the evaluation of aspirin as compared with placebo, cancer was an additional prespecified outcome. Definitions of the outcomes are provided in Section S4.
Statistical Analysis
Assuming a cardiovascular event rate of 1.2% per year in the placebo group, with the enrollment of at least 5000 participants over a period of 2 years and a mean follow-up of 5 years, we calculated that the trial would have more than 80% power to detect a 35% lower relative risk of cardiovascular events with the polypill as compared with placebo. We had intended to extend enrollment to 7000 participants in order to enhance the power of the trial, if resources and circumstances had permitted. However, several administrative and regulatory hurdles limited trial recruitment (Section S5).
The primary analysis for each treatment comparison was based on all the participants who had undergone randomization. If no interactions were observed between the main effects of the different treatments being evaluated (Section S6), then each individual comparison was to be performed as described in this article. Survival curves for the primary and secondary outcomes were computed with the use of the Kaplan–Meier method. The primary analyses were based on the time to an unrefuted primary-outcome event (after central adjudication) with the use of Cox proportional-hazards models, stratified according to the other groups in the factorial design. The proportional-hazards assumption was tested for each primary analysis (Section S6). Sensitivity analyses that were limited to events that occurred within 30 days after the stopping of the trial regimen for nonmedical reasons (Section S5) and total-event (first and recurrent events) analyses with the use of proportionalmeans models13,14 were also prespecified in the statistical analysis plan before unblinding. Hazard ratios with 95% confidence intervals are presented. The widths of the confidence intervals have not been adjusted for multiplicity, and therefore the intervals should not be used to infer definitive treatment effects. Treatment effects were also assessed in prespecified subgroups.
Results
Trial Participants
Of 7793 screened persons, 7534 were eligible to participate in the run-in phase. Of these, 1821 persons (24.2%) were not included in the trial; therefore, 5713 participants underwent randomization between July 30, 2012, and August 12, 2017 (Figs. S1 through S3). The most common reasons that participants from the run-in phase did not undergo randomization were side effects attributed to the polypill or aspirin (in 715 participants), nonadherence to the regimen (in 560), and the participant’s decision to not undergo randomization (in 458) (Table S1).
The characteristics of the participants at baseline are shown in Table 1. Most participants were recruited in India (47.9%) and the Philippines (29.3%). The mean age of the participants was 63.9 years, and 52.9% were female. Hypertension or elevated blood pressure was reported in 83.8% of the participants, and diabetes or elevated glucose level in 36.7%. The mean systolic blood pressure was 144.5 mm Hg, the mean heart rate was 77.0 beats per minute, and the mean LDL cholesterol level was 120.7 mg per deciliter (3.1 mmol per liter).
Table 1. Baseline Characteristics of the Participants Randomly Assigned to Receive Double Placebo, Aspirin Alone, Polypill Alone, or Polypill plus Aspirin.* .
| Characteristic | Double Placebo (N = 1421) | Aspirin Alone (N = 1431) | Polypill Alone (N = 1432) | Polypill plus Aspirin (N = 1429) |
|---|---|---|---|---|
| Age — yr | 64.1±6.8 | 63.7±6.7 | 64.1±6.4 | 63.8±6.5 |
| Female sex — no. (%) | 757 (53.3) | 746 (52.1) | 777 (54.3) | 745 (52.1) |
| Geographic distribution — no. (%) | ||||
| India or Bangladesh | 755 (53.1) | 760 (53.1) | 760 (53.1) | 759 (53.1) |
| Philippines, Malaysia, or Indonesia | 479 (33.7) | 477 (33.3) | 478 (33.4) | 479 (33.5) |
| Colombia | 121 (8.5) | 122 (8.5) | 125 (8.7) | 121 (8.5) |
| Canada | 30 (2.1) | 35 (2.4) | 33 (2.3) | 33 (2.3) |
| Tanzania | 10 (0.7) | 10 (0.7) | 10 (0.7) | 9 (0.6) |
| Tunisia | 26 (1.8) | 27 (1.9) | 26 (1.8) | 28 (2.0) |
| Cardiovascular risk factor — no. (%) | ||||
| Reported hypertension or systolic blood pressure >140 mm Hg |
1179 (83.0) | 1220 (85.3) | 1199 (83.7) | 1192 (83.4) |
| Reported diabetes or glucose level >126 mg/dl (7.0 mmol/liter) |
527 (37.1) | 503 (35.2) | 543 (37.9) | 522 (36.5) |
| Impaired fasting glucose ≥110–126 mg/dl (6.1–7.0 mmol/liter) |
97 (6.8) | 101 (7.1) | 109 (7.6) | 98 (6.9) |
| Current smoking | 115 (8.1) | 138 (9.6) | 123 (8.6) | 136 (9.5) |
| INTERHEART Risk Score† | 17.9±4.8 | 17.8±4.7 | 18.0±4.8 | 17.9±4.7 |
| Physiological variables | ||||
| Heart rate — beats/min | 77.1±10.9 | 77.3±10.5 | 77.0±10.5 | 76.6±10.5 |
| Blood pressure — mm Hg | ||||
| Systolic | 144.4±17.2 | 144.7±16.8 | 144.7±16.9 | 144.3±16.6 |
| Diastolic | 83.6±9.6 | 83.7±9.9 | 84.2±9.9 | 84.1±9.4 |
| Cholesterol — mg/dl | ||||
| Total | 196.4±46.9 | 196.1±45.1 | 196.7±45.6 | 195.5±44.9 |
| LDL | 120.7±41.9 | 120.8±40.1 | 121.2±40.7 | 120.0±40.2 |
| HDL | 48.2±13.5 | 47.1±11.9 | 47.7±13.0 | 47.9±13.6 |
| Triglycerides — mg/dl | 143.2±70.8 | 148.7±82.8 | 146.4±70.6 | 144.7±72.3 |
| Fasting plasma glucose — mg/dl | 113.5±43.0 | 114.4±46.6 | 114.9±45.5 | 114.5±44.9 |
| Creatinine — mg/dl | 0.9±0.3 | 0.9±0.3 | 0.9±0.3 | 0.9±0.3 |
| Body-mass index‡ | 25.6±4.6 | 25.7±4.8 | 26.1±4.9 | 25.8±4.6 |
| Waist-to-hip ratio | ||||
| Among women | 0.91±0.07 | 0.91±0.07 | 0.91±0.07 | 0.91±0.08 |
| Among men | 0.96±0.07 | 0.96±0.06 | 0.96±0.06 | 0.96±0.07 |
| Medication use — no. (%) | ||||
| Antihypertensive drug | 155 (10.9) | 156 (10.9) | 161 (11.2) | 157 (11.0) |
| Calcium-channel blocker | 137 (9.6) | 141 (9.9) | 153 (10.7) | 138 (9.7) |
| Aspirin or clopidogrel | 2 (0.1) | 1 (0.1) | 0 | 2 (0.1) |
| Oral anticoagulant | 4 (0.3) | 2 (0.1) | 2 (0.1) | 8 (0.6) |
| Insulin | 35 (2.5) | 29 (2.0) | 24 (1.7) | 29 (2.0) |
| Oral hypoglycemic agent | 302 (21.3) | 292 (20.4) | 310 (21.6) | 314 (22.0) |
| Statin | 0 | 0 | 1 (0.1) | 0 |
| Other lipid-lowering agent | 1 (0.1) | 0 | 0 | 2 (0.1) |
Plus-minus values are means ±SD. Percentages may not total 100 because of rounding. To convert the values for cholesterol to millimoles per liter, multiply by 0.0259. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. To convert the values for glucose to millimoles per liter, multiply by 0.05551. To convert the values for creatinine to micromoles per liter, multiply by 88.4. HDL denotes high-density lipoprotein, and LDL low-density lipoprotein.
The scale for the INTERHEART Risk Score ranges from 0 to 48, with higher scores indicating greater cardiovascular risk.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Follow-Up and Adherence to Trial Regimens
Of the 5713 participants who underwent randomization, 57 were withdrawn early for regulatory and administrative reasons (as described in Section S5 and Figs. S1 through S3). Follow-up events that occurred before the withdrawal of these participants are included in the analyses. For all the other participants, follow-up included events that were known to have occurred by June 30, 2020. The mean follow-up in the overall trial was 4.6 years (median, 4.4 years). At the end of the scheduled follow-up, vital status was available for 5667 of the 5713 participants (99.2%) who had undergone randomization.
For the comparison of the polypill with placebo, 81.1% of the participants in the polypill group and 81.3% of those in the placebo group were adhering to the trial regimen at 24 months; at 48 months, adherence was 67.7% and 69.4%, respectively. For the comparison of aspirin with placebo, 82.0% of the participants in the aspirin group and 82.1% of those in the placebo group were adhering to the trial regimen at 24 months; at 48 months, adherence was 73.0% and 71.0%, respectively. The overall incidence of discontinuation of the trial regimen was 42.2% for the polypill comparison and 39.7% for the aspirin comparison, both of which were higher than the 20% that was anticipated in the trial protocol. Major factors in the discontinuation of trial regimens included administrative, regulatory, and drug-delivery barriers and delays as well as restrictions related to the coronavirus disease 2019 (Covid-19) pandemic. Circumstances contributing to nonadherence and additional information regarding adherence as well as open-label drug use are presented in Sections S5 and S7 and Tables S2 through S11.
Blood Pressure, Heart Rate, and Lipid Levels
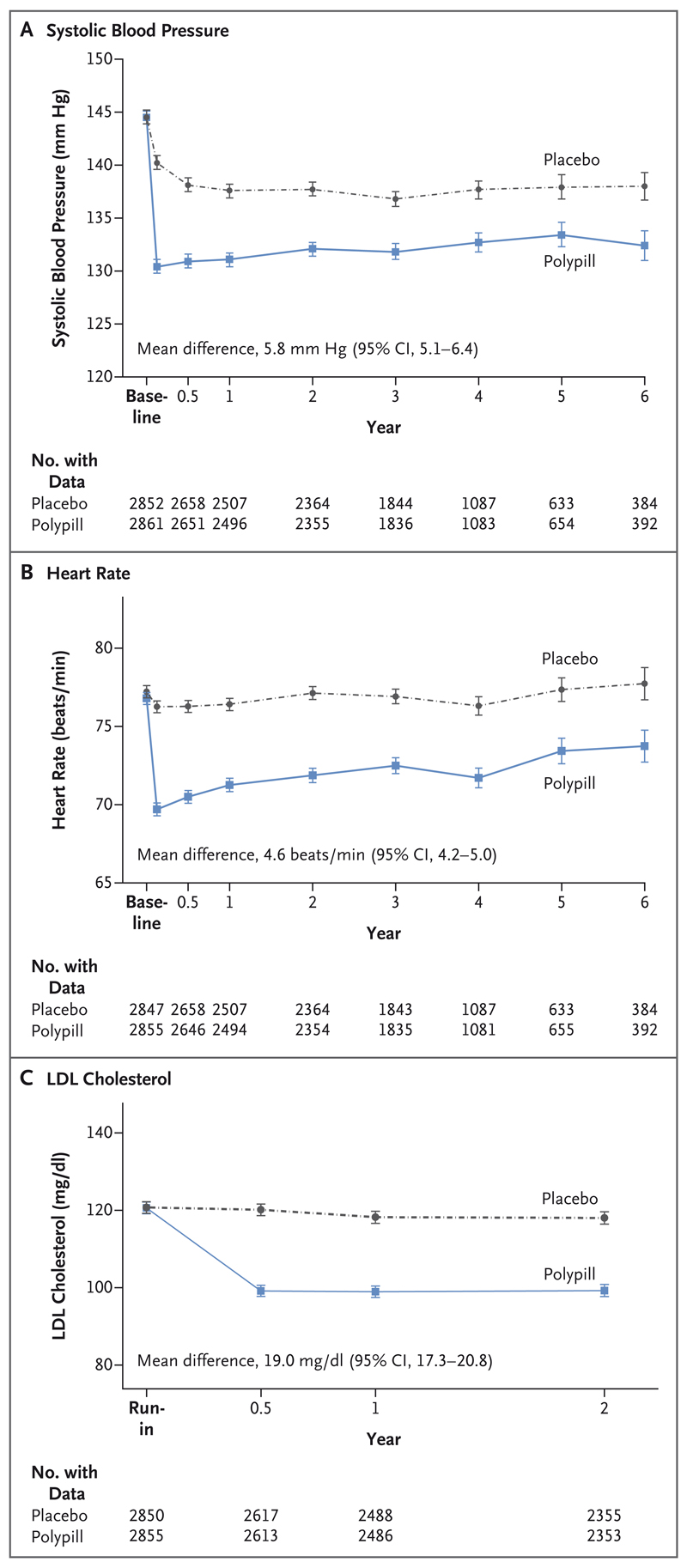
During the trial, the mean systolic blood pressure was 5.8 mm Hg lower in the polypill group than in the placebo group (Fig. 1). This difference was 9.8 mm Hg at 6 weeks and 6.5 mm Hg at 12 months. The mean diastolic blood pressure was 3.2 mm Hg lower in the polypill group than in the placebo group. The mean heart rate was 4.6 beats per minute lower in the polypill group than in the placebo group during the trial; the difference of 6.6 beats per minute at 6 weeks diminished to 5.1 beats per minute at 12 months. The mean LDL cholesterol level was 19.0 mg per deciliter (0.50 mmol per liter) lower in the polypill group than in the placebo group; the between-group difference was 19.2 mg (0.50 mmol per liter) at 12 months and 18.8 mg per deciliter (0.49 mmol per liter) at 24 months. On the basis of central laboratory lipid measurements, the mean between-group difference was 16.2 mg per deciliter (0.42 mmol per liter) at a mean follow-up of 50 months (Table S12). The reductions in blood pressure, the heart rate, and the LDL cholesterol level were similar for the comparison of combined therapy (polypill plus aspirin) with double placebo.
Figure 1. Effects of the Polypill, as Compared with Placebo, on Systolic Blood Pressure, Heart Rate, and the Low-Density Lipoprotein (LDL) Cholesterol Level.
I bars indicate 95% confidence intervals (CIs).
Outcomes for the Polypill as Compared with Placebo
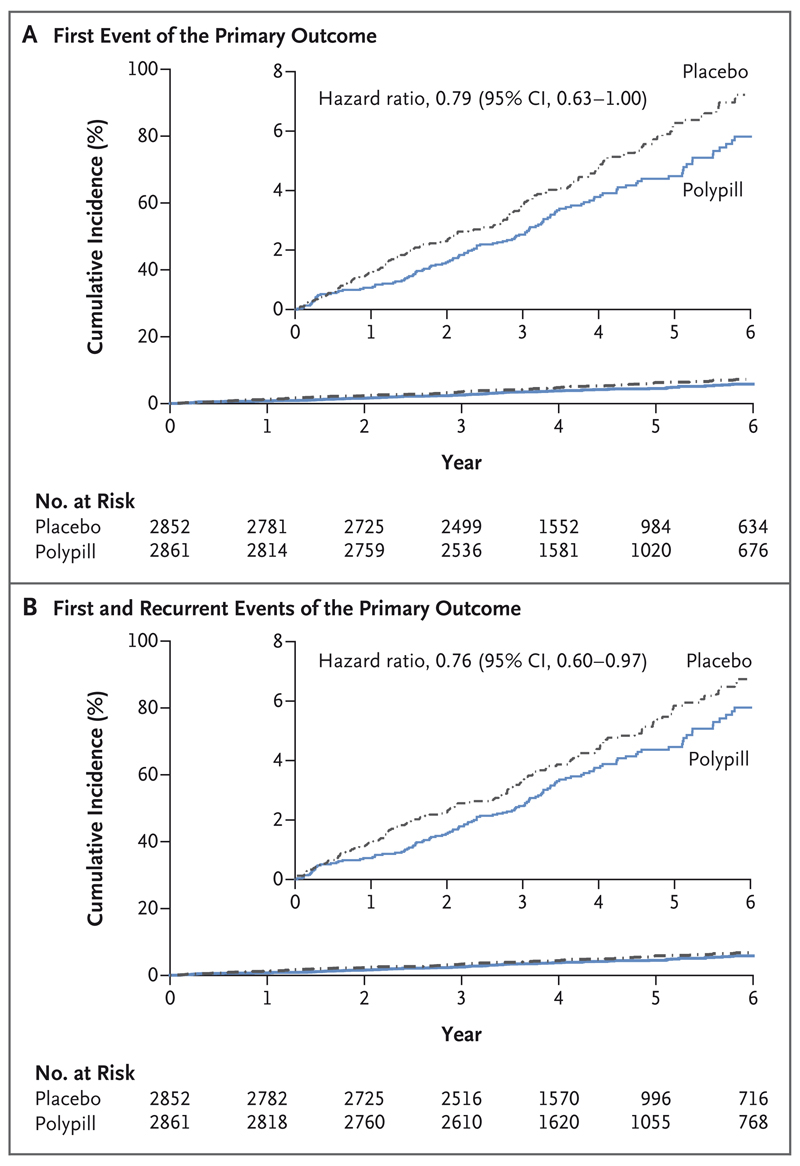
The primary outcome for the polypill comparison (major cardiovascular events plus heart failure, resuscitated cardiac arrest, and arterial revascularization) occurred in 126 participants (4.4%) in the polypill group, as compared with 157 (5.5%) in the placebo group (hazard ratio, 0.79; 95% confidence interval [CI], 0.63 to 1.00) (Fig. 2 and Table 2). Death from cardiovascular causes, myocardial infarction, or stroke (secondary outcome) occurred in 111 participants (3.9%) in the polypill group and in 139 participants (4.9%) in the placebo group (hazard ratio, 0.79; 95% CI, 0.61 to 1.01). The total number of primary-outcome events (including first and recurrent events) was 138 in the polypill group and 179 in the placebo group (hazard ratio, 0.76; 95% CI, 0.60 to 0.97). No interactions were observed between the effects of the different treatments being evaluated, and the proportional-hazards assumption was confirmed for each primary analysis (Section S6).
Figure 2. Effects of the Polypill, as Compared with Placebo, on Clinical Outcomes.
Panel A shows the cumulative incidence of a first composite-primary-outcome event (death from cardiovascular causes, myocardial infarction, stroke, heart failure, resuscitated cardiac arrest, or arterial revascularization) for the comparison of the polypill with placebo. Panel B shows the cumulative incidence of first and recurrent events of the primary composite outcome. Insets show the same data on an enlarged y axis.
Table 2. Clinical Outcomes with the Polypill or Placebo.
| Outcomes | Polypill (N = 2861) |
Placebo (N = 2852) |
Hazard Ratio (95% CI)* |
|---|---|---|---|
| Primary outcome | |||
| Death from cardiovascular causes, myocardial infarction, stroke, heart failure, resuscitated cardiac arrest, or arterial revascularization — no. (%) | 126 (4.4) | 157 (5.5) | 0.79 (0.63–1.00) |
| Secondary outcomes | |||
| Death from cardiovascular causes, myocardial infarction, or stroke — no. (%) | 111 (3.9) | 139 (4.9) | 0.79 (0.61–1.01) |
| Death from cardiovascular causes, myocardial infarction, stroke, heart failure, resuscitated cardiac arrest, arterial revascularization, or angina with evidence of ischemia — no. (%) | 132 (4.6) | 164 (5.8) | 0.79 (0.63–1.00) |
| Components of the primary and secondary outcomes | |||
| Death from cardiovascular causes — no. (%)† | 84 (2.9) | 101 (3.5) | 0.82 (0.61–1.09) |
| Myocardial infarction — no. (%) | 17 (0.6) | 26 (0.9) | 0.66 (0.36–1.22) |
| Stroke — no. (%) | 26 (0.9) | 36 (1.3) | 0.71 (0.43–1.18) |
| Heart failure — no. (%) | 12 (0.4) | 10 (0.4) | 1.19 (0.51–2.74) |
| Resuscitated cardiac arrest — no. (%) | 1(<0.1) | 0 | — |
| Arterial revascularization — no. (%) | 12 (0.4) | 25 (0.9) | 0.48 (0.24–0.95) |
| Angina with evidence of ischemia — no. (%) | 17 (0.6) | 22 (0.8) | 0.77 (0.41–1.44) |
| Other efficacy outcomes | |||
| Death from any cause — no. (%) | 149 (5.2) | 163 (5.7) | 0.90 (0.72–1.13) |
| First and recurrent events of the primary outcome | |||
| No. of participants with ≥1 event | 126 | 157 | — |
| No. of participants with ≥2 events | 12 | 22 | — |
| Total no. of events | 138 | 179 | 0.76 (0.60–0.97)‡ |
The widths of the confidence intervals have not been adjusted for multiplicity, so the intervals should not be used to infer definitive treat ment effects.
Death from cardiovascular causes included confirmed death from cardiovascular causes (in 57 participants in the polypill group and in 80 in the placebo group) and death from an unknown cause, which was categorized according to the protocol definition as presumed death from cardiovascular causes (in 27 and 21 participants, respectively).
The analysis was conducted with the use of a proportional-means model.
In the prespecified sensitivity analysis that excluded events that occurred more than 30 days after the trial regimen was stopped for nonmedical reasons, 95 participants (3.3%) in the polypill group had a primary-outcome event, as compared with 126 (4.4%) in the placebo group (hazard ratio, 0.74; 95% CI, 0.57 to 0.97), with similar numbers of events in the two randomized groups (31 and 31, respectively) occurring after the period of censoring (Table S13). There was no evidence of heterogeneity of treatment effect across the prespecified subgroups (Fig. S4).
More participants in the polypill group than in the placebo group stopped the trial regimen because of dizziness (28 vs. 17 participants), hypotension (51 vs. 14), or cough (31 vs. 17); however, the numbers of participants who discontinued the polypill or placebo because of muscle pain (9 and 11 participants, respectively) and weakness (5 and 4, respectively) were similar in the two groups (Table S6). The incidence of permanent discontinuation of the trial regimen because of side effects was 5.4% in the polypill group and 3.9% in the placebo group (Table S7). Serious adverse events and adverse events that led to hospitalization are listed in Tables S14 and S15, respectively.
Outcomes for Aspirin as Compared with Placebo
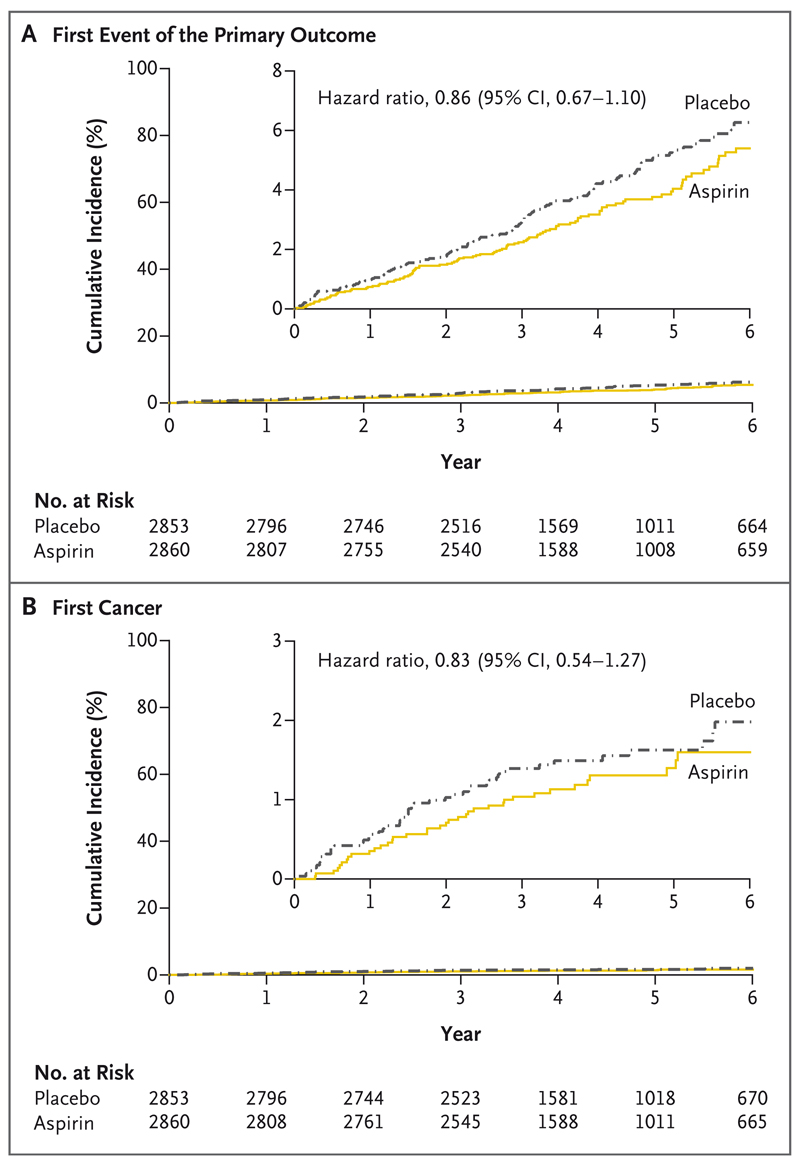
The primary outcome for the aspirin comparison (death from cardiovascular causes, myocardial infarction, or stroke) occurred in 116 participants (4.1%) in the aspirin group and in 134 participants (4.7%) in the placebo group (hazard ratio, 0.86; 95% CI, 0.67 to 1.10) (Fig. 3 and Table 3). Death from cardiovascular causes, myocardial infarction, stroke, or cancer (secondary outcome) occurred in 153 participants (5.3%) in the aspirin group and in 177 participants (6.2%) in the placebo group (hazard ratio, 0.86; 95% CI, 0.69 to 1.07). The total number of primary-outcome events (including first and recurrent events) was 124 in the aspirin group and 144 in the placebo group (hazard ratio, 0.86; 95% CI, 0.67 to 1.11). New cancer occurred in 38 participants (1.3%) in the aspirin group and in 46 (1.6%) in the placebo group (hazard ratio, 0.83; 95% CI, 0.54 to 1.27). The distribution of cancers according to body site is provided in Table S16.
Figure 3. Effects of the Aspirin, as Compared with Placebo, on Clinical Outcomes.
Panel A shows the cumulative incidence of a first composite-primary-outcome event (death from cardiovascular causes, myocardial infarction, or stroke) for the comparison of aspirin with placebo. Panel B shows the cumulative incidence of the first event of cancer (component of the secondary outcome; a prespecified outcome). Insets show the same data on an enlarged y axis.
Table 3. Clinical Outcomes with Aspirin or Placebo.
| Outcome | Aspirin (N = 2860) |
Placebo (N = 2853) |
Hazard Ratio (95% CI)* |
|---|---|---|---|
| Primary outcome | |||
| Death from cardiovascular causes, myocardial infarction, or stroke — no. (%) | 116 (4.1) | 134 (4.7) | 0.86 (0.67–1.10) |
| Secondary Outcome | |||
| Death from cardiovascular causes, myocardial infarction, stroke, or cancer — no. (%) | 153 (5.3) | 177 (6.2) | 0.86 (0.69–1.07) |
| Components of the primary and secondary outcomes | |||
| Death from cardiovascular causes — no. (%)† | 85 (3.0) | 100 (3.5) | 0.85 (0.64–1.14) |
| Myocardial infarction — no. (%) | 22 (0.8) | 21 (0.7) | 1.04 (0.57–1.89) |
| Stroke — no. (%) | 23 (0.8) | 39 (1.4) | 0.58 (0.35–0.98) |
| Cancer — no. (%) | 38 (1.3) | 46 (1.6) | 0.83 (0.54–1.27) |
| Other efficacy outcomes | |||
| Death from any cause — no. (%) | 145 (5.1) | 167 (5.9) | 0.87 (0.70–1.09) |
| First and recurrent events of the primary outcome | |||
| No. of participants with ≥1 event | 116 | 134 | — |
| No. of participants with ≥2 events | 8 | 10 | — |
| Total no. of events | 124 | 144 | 0.86 (0.67–1.11)‡ |
| First and recurrent events of the secondary outcome | |||
| No. of participants with ≥1 event | 153 | 177 | — |
| No. of participants with ≥2 events | 12 | 17 | — |
| Total no. of events | 165 | 194 | 0.85 (0.68–1.06)‡ |
The widths of the confidence intervals have not been adjusted for multiplicity, so the intervals should not be used to infer definitive treatment effects.
Death from cardiovascular causes included confirmed death from cardiovascular causes (in 68 participants in the aspirin group and in 69 in the placebo group) and death from an unknown cause, which was categorized according to the protocol definition as presumed death from cardiovascular causes (in 17 and 31 participants, respectively).
The analysis was conducted with the use of a proportional-means model.
In the prespecified sensitivity analysis that excluded events that occurred more than 30 days after the trial regimen was stopped for nonmedical reasons, primary-outcome events occurred in 83 participants (2.9%) in the aspirin group and in 100 participants (3.5%) in the placebo group (hazard ratio, 0.83; 95% CI, 0.62 to 1.10) (Table S17). There was no evidence of heterogeneity of treatment effect in subgroups defined according to sex, diabetes or hypertension status, lipid or blood-pressure levels, age (in three subgroups), INTERHEART Risk Score, or geographic region either for the primary outcome (Fig. S5) or for the primary outcome plus cancer (Fig. S6).
In the aspirin group and the placebo group, a similar number of participants had major bleeding (21 and 19 participants, respectively), minor bleeding (17 and 14, respectively), and gastrointestinal bleeding (12 and 10); a similar number of participants in the aspirin group and the placebo group had dyspepsia (5 and 4 participants, respectively) or peptic ulcer (5 and 5, respectively) that led to discontinuation of the trial regimen (Tables S8, S9, and S18). Serious adverse events and adverse events that led to hospitalization are listed in Tables S19 and S20, respectively.
Outcomes with Polypill Plus Aspirin as Compared with Double Placebo
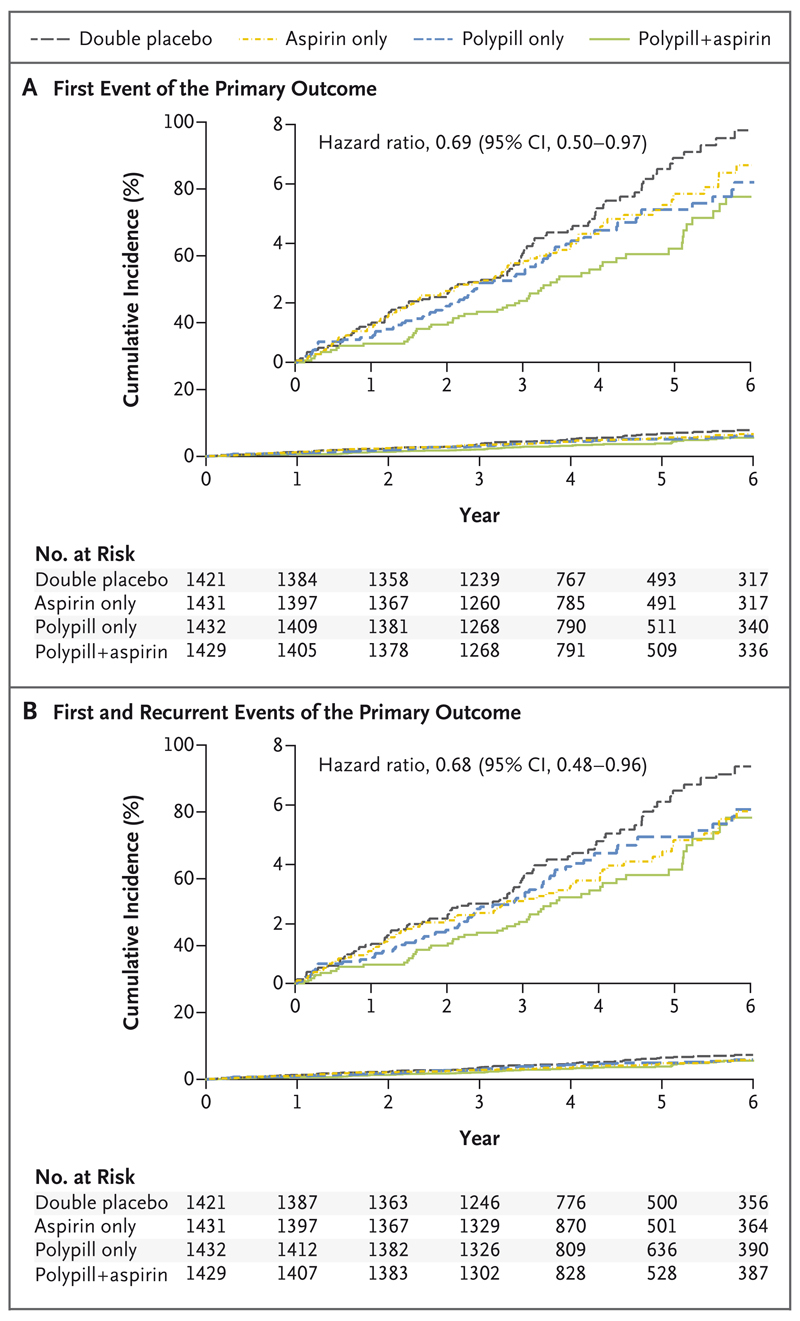
The primary outcome for this comparison (major cardiovascular events plus heart failure, resuscitated cardiac arrest, or arterial revascularization) occurred in 59 participants (4.1%) in the polypill-plus-aspirin group, as compared with 83 (5.8%) in the double-placebo group (hazard ratio, 0.69; 95% CI, 0.50 to 0.97) (Fig. 4 and Table 4). There was a similar effect on death from cardiovascular causes, myocardial infarction, or stroke (secondary outcome; 52 participants [3.6%] in the polypill-plus-aspirin group vs. 75 [5.3%] in the double-placebo group; hazard ratio, 0.68; 95% CI, 0.47 to 0.96). The total number of primary-outcome events (including first and recurrent events) was 64 in the polypill-plus-aspirin group and 93 in the double-placebo group (hazard ratio, 0.68; 95% CI, 0.48 to 0.96).
Figure 4. Effects of the Polypill plus Aspirin, as Compared with Double Placebo, on Clinical Outcomes.
Panel A shows the cumulative incidence of a first composite-primary-outcome event (death from cardiovascular causes, myocardial infarction, stroke, heart failure, resuscitated cardiac arrest, or arterial revascularization) for the comparison of combination therapy with a polypill plus aspirin with placebo. Panel B shows the cumulative incidence of first and recurrent events of the primary composite outcome. Insets show the same data on an enlarged y axis.
Table 4. Clinical Outcomes with Polypill plus Aspirin or Double Placebo.
| Outcome | Polypill plus Aspirin (N = 1429) | Double Placebo (N = 1421) | Hazard Ratio (95% CI)* |
|---|---|---|---|
| Primary outcome | |||
| Death from cardiovascular causes, myocardial infarction, stroke, heart failure, resuscitated cardiac arrest, or arterial revascularization — no. (%) | 59 (4.1) | 83 (5.8) | 0.69 (0.50–0.97) |
| Secondary outcomes | |||
| Death from cardiovascular causes, myocardial infarction, or stroke — no. (%) | 52 (3.6) | 75 (5.3) | 0.68 (0.47–0.96) |
| Death from cardiovascular causes, myocardial infarction, stroke, heart failure, resuscitated cardiac arrest, arterial revascularization, or angina with evidence of ischemia — no. (%) | 61 (4.3) | 86 (6.1) | 0.69 (0.50–0.96) |
| Components of the primary and secondary outcomes | |||
| Death from cardiovascular causes — no. (%)† | 38 (2.7) | 54 (3.8) | 0.69 (0.46–1.05) |
| Myocardial infarction — no. (%) | 10 (0.7) | 14 (1.0) | 0.69 (0.31–1.56) |
| Stroke — no. (%) | 10 (0.7) | 23 (1.6) | 0.42 (0.20–0.89) |
| Heart failure — no. (%) | 7 (0.5) | 3 (0.2) | 2.30 (0.60–8.90) |
| Resuscitated cardiac arrest — no. (%) | 0 | 0 | — |
| Arterial revascularization — no. (%) | 5 (0.3) | 12 (0.8) | 0.40 (0.14–1.14) |
| Angina with evidence of ischemia — no. (%) | 6 (0.4) | 10 (0.7) | 0.59 (0.22–1.63) |
| Other outcomes | |||
| Death from any cause — no. (%) | 75 (5.2) | 93 (6.5) | 0.80 (0.59–1.08) |
| Cancer — no. (%) | 19 (1.3) | 24 (1.7) | 0.78 (0.43–1.42) |
| Primary-outcome event or cancer — no. (%) | 76 (5.3) | 106 (7.5) | 0.70 (0.52–0.94) |
| First and recurrent events of the primary outcome | |||
| No. of participants with ≥1 event | 59 | 83 | |
| No. of participants with ≥2 events | 5 | 10 | |
| Total no. of events | 64 | 93 | 0.68 (0.48–0.96)‡ |
The widths of the confidence intervals have not been adjusted for multiplicity, so the intervals should not be used to infer definitive treatment effects.
Death from cardiovascular causes included confirmed death from cardiovascular causes (in 24 participants in the polypill-plus-aspirin group and in 36 in the double-placebo group) and death from an unknown cause, which was categorized according to the protocol definition as presumed death from cardiovascular causes (in 14 and 18 participants, respectively).
The analysis was conducted with the use of a proportional-means model.
In the prespecified sensitivity analysis that excluded events that occurred more than 30 days after the trial regimen was stopped for nonmedical reasons, primary-outcome events occurred in 40 participants (2.8%) in the polypillplus-aspirin group and in 64 participants (4.5%) in the double-placebo group (hazard ratio, 0.61; 95% CI, 0.41 to 0.91) (Table S21). The results were consistent across subgroups defined according to diabetes status, initial LDL cholesterol level, systolic blood pressure, and geographic region (Fig. S7).
The number of participants who discontinued the trial medications or placebo because of side effects was similar in the polypill-plus-aspirin group and the double-placebo group (5 and 7, respectively because of muscle symptoms; 3 and 1 because of gastrointestinal bleeding; 3 and 3 because of dyspepsia; 19 and 22 because of gastritis; and 3 and 3 because of peptic ulcer). More participants in the polypill-plus-aspirin group than in the double-placebo group discontinued the trial regimen because of dizziness or hypotension (45 vs. 22 participants). Major bleeding was reported in 9 participants in the polypill-plus-aspirin group and in 12 participants in the double-placebo group. Details of the safety outcomes and discontinuations of the trial regimen are provided in Tables S10, S11, and S22 through S26.
Discussion
In TIPS-3, the combination of a polypill (consisting of simvastatin and three blood-pressure–lowering drugs) plus aspirin administered for a mean of 4.6 years in a primary-prevention population at intermediate risk led to a 31% lower relative risk of cardiovascular events (absolute risk difference, 1.7 percentage points) in an intention-to-treat analysis. The benefit was similar in an analysis that included both first and recurrent events and in an analysis that excluded events that occurred more than 30 days after the trial regimen was stopped for nonmedical reasons. Both interventions appear to have contributed to the overall benefit of the combination, with a larger portion of the benefit attributable to the polypill.
The reduction in the LDL cholesterol level with the polypill was half the value that we had anticipated on the basis of previous trials of simvastatin at a dose of 40 mg per day,15 of the TIPS-1 and TIPS-2 (the Indian Polycap Study 1 and 2) pilot studies,16,17 or of trials of the newer statins (atorvastatin or rosuvastatin).18,19 The degree of blood-pressure lowering was also less by approximately one third, as compared with the findings of previous short-term trials.20 However, the reduction in systolic blood pressure that was observed in TIPS-3 (with full doses of three blood-pressure–lowering drugs) was similar to that seen in the Heart Outcomes Prevention Evaluation (HOPE)–3 trial, which used two blood-pressure–lowering drugs at half doses,21 and was larger than that observed in the long-term PolyIran trial, in which no difference in diastolic blood pressure was observed at the end of the trial.22 An attenuation of both a shortterm reduction in the LDL cholesterol level and a short-term reduction in blood pressure during long-term follow-up has been observed in previous trials.21–23 The observed benefits on cardiovascular events were consistent with what would be expected from the modest reduction in the LDL cholesterol level and blood pressure, with added benefits from aspirin.
The smaller-than-expected reductions in risk factors in this trial may have contributed to the fact that the observed clinical benefit was smaller than the 40 to 45% lower relative risk that we had expected on the basis of risk-factor changes that had been observed in our pilot studies. However, our results are consistent with the 29% lower relative risk (absolute risk difference, 1.4 percentage points) of cardiovascular events among participants receiving a statin plus two blood-pressure–lowering drugs in the HOPE-3 trial23 and the 34% lower relative risk (absolute risk difference, 2.9 percentage points) that was seen in the PolyIran trial, which used a polypill that included a statin, two blood-pressure–lowering drugs, and aspirin.22 Exploratory analyses (data not shown) suggested that the group of centers that had the greatest differences in risk factors had the greatest differences in the incidence of cardiovascular events with the polypill plus aspirin. Therefore, future formulations of a polypill that can result in greater differences in risk factors may be expected to lead to greater benefits than were observed in the current trial.
It is likely that the lower relative risk that was associated with combined treatment with the polypill plus aspirin than with double placebo that was observed in the intention-to-treat analysis in our trial is an underestimate of the true effects of treatment because of the high incidence of discontinuation of the trial regimen, which was mostly for reasons unrelated to side effects. During the trial, complexities in the processes of drug distribution resulted in drug-supply delays to many sites (Section S5). These delays not only limited the initial access to the trial medications and placebos for participants but often impeded resupply, leading to discontinuation after the initiation of the regimen. In addition, the Covid-19 pandemic restricted site operations in virtually all countries beginning in March 2020. This situation restricted the ability of the participants to physically return to sites, to obtain trial medications and placebo, and to complete the end-of-trial visits. As a consequence, the overall trial follow-up was somewhat shorter than we had planned. TIPS-3 had a median of 4.4 years of follow-up, as compared with the planned 5 years, whereas the median follow-up in the HOPE-3 trial was 5.6 years23 and in the PolyIran trial all the participants were followed for 5 years.22
The magnitude of risk reduction that we observed was substantially lower than the 80% lower relative risks of stroke and myocardial infarction that were hypothesized by Wald and Law in their advocacy for the polypill concept in 2003.7 Wald and Law assumed that polypill treatment would reduce blood pressure and the LDL cholesterol level to a much larger extent than the effects observed in TIPS-3 and in most other long-term trials.15,19,21,24 They also assumed that aspirin therapy would reduce the risk of cardiovascular events by 30% and that lowering the homocysteine level (with vitamin B12, vitamin B6, and folate therapy) would further reduce cardiovascular risk. The effects of lowering the homocysteine level with regard to reducing cardiovascular risk are unclear,25,26 so we chose not to include this component in the polypill we tested. The benefits we observed are nonetheless likely to be of clinical and population-wide importance, especially if the polypill is inexpensive. A similar formulation of the Polycap in India costs approximately $15 U.S. dollars per month. We have not yet completed a formal economic analysis. The polypill was given in addition to regular advice on lifestyle modification; such an approach could be part of a comprehensive strategy for the prevention of cardiovascular diseases in the community.27
There was a higher incidence of hypotension, dizziness, and cough, but no excess of bleeding, with the use of the polypill plus aspirin than with double placebo. In considering the relatively low incidence of adverse events, it is important to note that the trial had an active run-in phase during which participants received a low-dose polypill plus aspirin. Of the 7534 participants who entered the run-in phase, 715 (9.5%) did not undergo randomization because of side effects. An additional 560 participants (7.4%) did not undergo randomization because they had less than 80% adherence to the trial-drug regimen — a figure that was not related primarily to drug-distribution issues. Therefore, TIPS-3 may underestimate the number of persons who would stop treatment for side effects or poor adherence if a polypill including aspirin were used widely among eligible persons in the general population.
In this large, randomized trial, we found that combination treatment with a polypill (consisting of a statin plus three blood-pressure–lowering drugs) plus aspirin led to a lower incidence of cardiovascular events than did placebo among participants without established cardiovascular disease who were at intermediate cardiovascular risk.
Supplementary Material
Acknowledgments
Supported by grants from the Wellcome Trust (089725/B/09/Z), the Canadian Institutes of Health Research (IPR-119993), and the Heart and Stroke Foundation of Canada (000448) and by the Population Health Research Institute and Hamilton Health Sciences Research Institute, St. John’s Research Institute, Cadila Pharmaceuticals, the Philippine Council for Health Research and Development, and Secretaria de Salud del Departamento de Santander, Colombia.
Dr. Yusuf reports receiving lecture fees and travel support from AstraZeneca and grant support, lecture fees, and travel support from Bayer; Dr. D. Xavier, receiving grant support from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Coca-Cola India, and Pfizer and lecture fees from Eli Lilly and Sanofi; Dr. Dagenais, receiving lecture fees from Bayer; and Dr. Bosch, receiving advisory board fees and fees for adjudication from Bayer. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank Judy Lindeman for administrative assistance.
Contributor Information
A. Dans, University of the Philippines, Manila
D. Xavier, St. John’s Medical College, Bangalore, India
P. López-Jaramillo, Fundación Oftalmológica de Santander, Universidad de Santander, Bucaramanga, Colombia, Malaysia
K. Yusoff, Universiti Teknologi MARA Selayang, Selangor, and UCSI University, Cheras, Kuala Lumpur, Malaysia
A. Santoso, Universitas Indonesia, National Cardiovascular Center, Jakarta
H. Gamra, Fattouma Bourguiba Hospital and University of Monastir, Monastir, Tunisia
S. Talukder, Eminence, Dhaka, Bangladesh
C. Christou, Population Health Research Institute, McMaster University and Hamilton Health Sciences, Hamilton, Canada
P. Girish, St. John’s Medical College, Bangalore, India
K. Yeates, Queen’s University, Kingston, Canada
F. Xavier, St. John’s Medical College, Bangalore, India
G. Dagenais, Université Laval Institut Universitaire de Cardiologie et de Pneumologie de Québec, Quebec, QC, Canada
C. Rocha, Fundación Oftalmológica de Santander, Universidad de Santander, Bucaramanga, Colombia, Malaysia
References
- 1.GBD 2017 Causes of Death Collaborators. Global, regional, and national agesex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–88. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagenais GR, Leong DP, Rangarajan S, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2020;395:785–94. doi: 10.1016/S0140-6736(19)32007-0. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–94. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middleincome, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395:795–808. doi: 10.1016/S0140-6736(19)32008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a metaanalysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 7.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326:1419. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events; a systematic review and metaanalysis. JAMA. 2019;321:277–87. doi: 10.1001/jama.2018.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph P, Pais P, Dans AL, et al. The International Polycap Study-3 (TIPS-3): design, baseline characteristics and challenges in conduct. Am Heart J. 2018;206:72–9. doi: 10.1016/j.ahj.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGorrian C, Yusuf S, Islam S, et al. Estimating modifiable coronary heart disease risk in multiple regions of the world: the INTERHEART Modifiable Risk Score. Eur Heart J. 2011;32:581–9. doi: 10.1093/eurheartj/ehq448. [DOI] [PubMed] [Google Scholar]
- 12.Joseph P, Yusuf S, Lee SF, et al. Prognostic validation of a non-laboratory and a laboratory based cardiovascular disease risk score in multiple regions of the world. Heart. 2018;104:581–7. doi: 10.1136/heartjnl-2017-311609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat SocB. 2000;62:711–30. [Google Scholar]
- 14.Wang M-C, Chang S-H. Nonparametric estimation of a recurrent survival function. J Am Stat Assoc. 1999;94:146–53. doi: 10.1080/01621459.1999.10473831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heart Protection Study Collaborative Group. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757–67. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 16.The Indian Polycap Study (TIPS) Effects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double-blind, randomised trial. Lancet. 2009;373:1341–51. doi: 10.1016/S0140-6736(09)60611-5. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Pais P, Sigamani A, et al. Comparison of risk factor reduction and tolerability of a full-dose polypill (with potassium) versus low-dose polypill (Polycap) in individuals at high risk of cardiovascular diseases: the Second Indian Polycap Study (TIPS-2) investigators. Circ Cardiovasc Qual Outcomes. 2012;5:463–71. doi: 10.1161/CIRCOUTCOMES.111.963637. [DOI] [PubMed] [Google Scholar]
- 18.Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–31. doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 20.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2009–20. doi: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]
- 22.Roshandel G, Khoshnia M, Poustchi H, et al. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial. Lancet. 2019;394:672–83. doi: 10.1016/S0140-6736(19)31791-X. [DOI] [PubMed] [Google Scholar]
- 23.Yusuf S, Lonn E, Pais P, et al. Bloodpressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med. 2016;374:2032–43. doi: 10.1056/NEJMoa1600177. [DOI] [PubMed] [Google Scholar]
- 24.Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressurelowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591–8. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 25.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 26.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- 27.Schwalm JD, McCready T, Lopez-Jaramillo P, et al. A community-based comprehensive intervention to reduce cardiovascular risk in hypertension (HOPE 4): a cluster-randomised controlled trial. Lancet. 2019;394:1231–42. doi: 10.1016/S0140-6736(19)31949-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.