Abstract
The main force generators in eukaryotic cilia and flagella are axonemal outer dynein arms (ODAs). During ciliogenesis, these ~1.8 MDa complexes are assembled in the cytoplasm and targeted to cilia via an unknown mechanism. Here we used the ciliate Tetrahymena to identify two factors (Q22YU3 and Q22MS1) that bind ODAs in the cytoplasm and are required for ODA delivery to cilia. Q22YU3, which we named Shulin, locked the ODA motor domains into a closed conformation and inhibited motor activity. Cryo-EM revealed how Shulin stabilized this compact form of ODAs by binding to the dynein tails. Our findings provide a molecular explanation for how newly assembled dyneins are packaged for delivery to the cilia.
Motile cilia play essential roles that range from setting up the left-right body axis to clearing mucus from the lungs (1). These slender cellular projections contain an axoneme built of microtubule doublets. Ciliary beating is powered by arrays of inner and outer dynein arm motors that slide adjacent doublets past each other (2). The outer dynein arms (ODAs) are the main force generators in cilia and the most frequently mutated components in human motile ciliopathies (3). ODAs are multi-subunit complexes (4), which are pre-assembled in the cytoplasm by a collection of nine dynein axonemal assembly factors and associated chaperones (4, 5). Following assembly, ODAs are targeted to cilia, where the intraflagellar transport (IFT) machinery carries them to their docking sites (6, 7). However, the mechanism of ODA delivery to the cilia and whether any additional factors are required for this process are both unclear.
ODAs assembled in the cell body are bound by Q22YU3 and Q22MS1
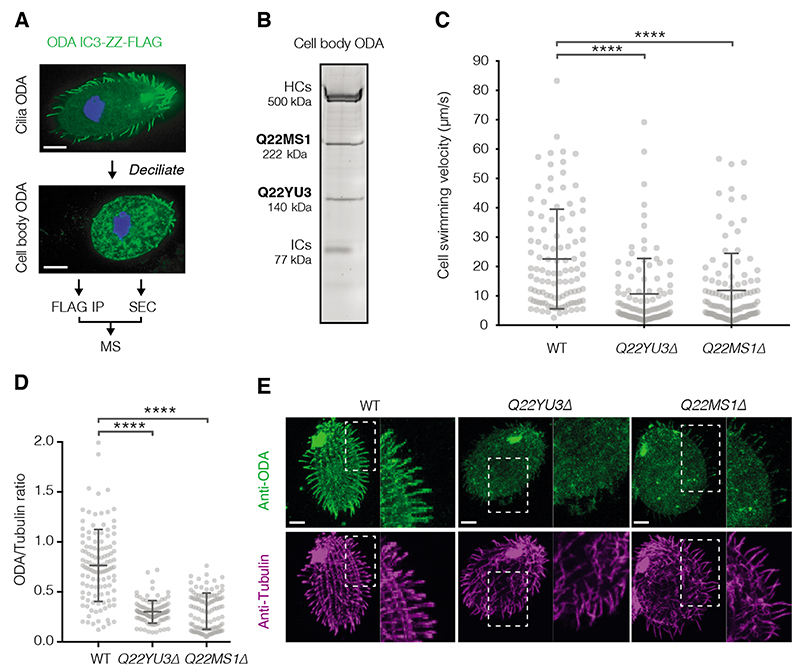
To identify potential ciliary targeting factors, we purified newly-assembled ODAs from the cytoplasm of the protozoan ciliate Tetrahymena thermophila. We de-ciliated Tetrahymena to remove pre-existing axonemes and trigger new ODA synthesis (8) (Fig. 1A). ODA complexes, containing a FLAG-tagged copy of the intermediate chain IC3, were extracted and separated from other assembly intermediates by size exclusion chromatography (SEC). Two factors co-eluted with the new fully-assembled ODAs and were identified by mass spectrometry (MS) as Q22MS1 and Q22YU3 (Fig. 1B, fig. S1A). We also performed label-free quantitative-MS on the immunoprecipitated IC3 subunit and detected an equivalent-fold enrichment of both factors relative to other ODA subunits (fig. S1B, table S1). Taken together, these data suggest Q22MS1 and Q22YU3 interact tightly with ODAs in the cell body.
Fig. 1. Q22MS1 and Q22YU3 deliver ODAs from cytoplasm to cilia.
(A) Scheme used to identify novel interactors of ODAs assembled in the cell body. (B) SDS-PAGE of ODA purified from the cell body by IP-SEC showing co-elution with Q22MS1 and Q22YU3, HC: Heavy chains, IC: Intermediate chains. (C) Cell swimming velocity comparing wildtype (WT n=108) and mutant strains (Q22YU3Δ n=102 and Q22MS1Δ n=110). (D) Ratio of ODA/Tubulin immunofluorescence intensity along individual cilia (WT n=118, Q22YU3Δ n=104, Q22MS1Δ n=129, 3-10 cilia from 14-17 cells/genotype). (E) Representative cells showing immunofluorescence for ODA and tubulin (quantified in D). Insets highlight reduced amount of ODA staining in mutant cilia compared to wildtype cilia. Scale bars:10 μm. Error bars show standard deviation; ns=not significantly different, ****p≤ 0.0001 (ANOVA with Tukey’s test for multiple comparisons).
Loss of Q22MS1 and Q22YU3 impacts cilia motility
These ODA-associated factors lack known functions and have not previously been linked to motile cilia. Q22YU3 shares 24% identity to human C20ORF194 (fig. S2) whereas Q22MS1 has a unique domain architecture and no ortholog outside of Tetrahymena. To investigate their roles, we generated Tetrahymena knockout strains for each factor (fig. S3A). Both strains showed an approximately two-fold decrease in swimming speed compared to wildtype (Q22YU3Δ: 10.6 ± 12 μm/s, Q22MS1Δ: 11.88 ± 12.6 μm/s, WT: 22.5 ± 16.9 μm/s; mean ± SD) suggesting defects in cilia movement (Fig. 1C). Mutants also had decreased accumulation of food vacuoles and a higher frequency of cytokinetic defects, which are hallmarks of defective cilia motility in Tetrahymena (9, 10) (fig. S3B-C). We found the lengths and numbers of cilia in our knockouts were similar to wildtype suggesting our observations were not because of defects in ciliogenesis (fig. S3D-E). However, high-speed imaging showed cilia in mutants beat slower than in wildtype cells (Q22YU3Δ = 7 ± 11 Hz, Q22MS1Δ = 4 ± 6 Hz, WT = 20 ± 12 Hz; mean ± SD), but at similar frequencies to those in an ODA temperature-sensitive mutant (11) (OAD1C11 = 8 ± 11 Hz) (fig. S4, movie S1). We therefore used immunofluorescence to test if loss of Q22YU3 and Q22MS1 affected ODA targeting to cilia. Staining with an antibody against ODAs showed marked reductions in their levels in cilia of mutant strains compared to the wildtype (Fig. 1D-E, fig. S3F). Thus, loss of Q22YU3 or Q22MS1 results in defective ciliary movement owing to reduced delivery of ODAs to the cilia.
Q22YU3 inhibits microtubule gliding by closing the ODAs
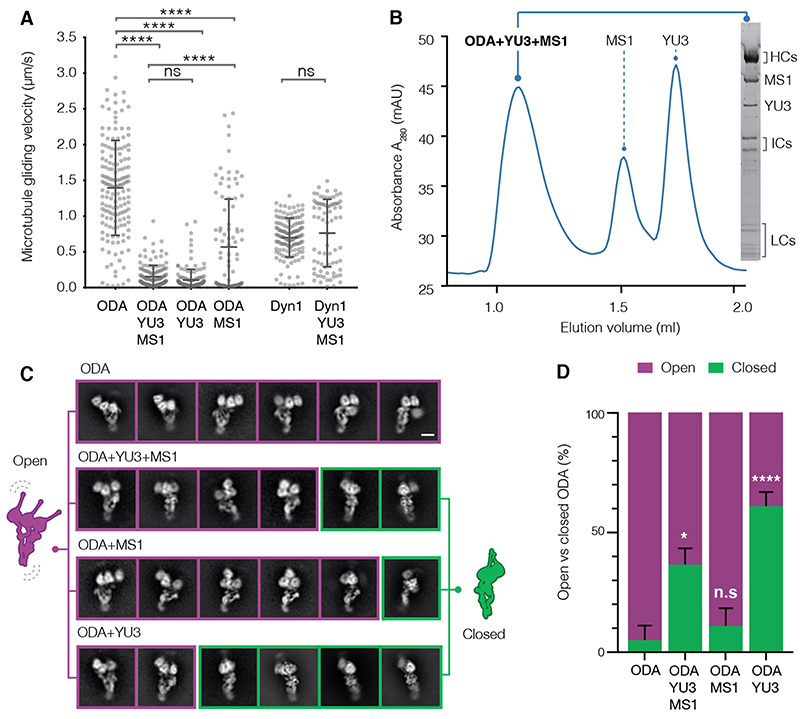
It has been proposed that ODAs need to be held in an inactive state during transport into the axoneme (4). We therefore tested whether Q22YU3 and Q22MS1 inhibited dynein motor activity. We expressed both factors recombinantly and assayed their effect on the microtubule gliding activity of ODAs purified from axonemes (12) (Fig. 2A). In contrast to the cytoplasm-purified sample, these ODAs lacked Q22YU3 or Q22MS1. The axoneme-purified ODAs translocated microtubules at 1.39 ± 0.60 μm/s (mean ± SD). Gliding was severely compromised by the addition of both factors together (0.15 ± 0.15 μm/s) or with Q22YU3 alone (0.10 ± 0.15 μm/s). Q22MS1 reduced microtubule gliding velocities to a lesser extent (0.56 ± 0.76 μm/s). In contrast, addition of both factors to cytoplasmic dynein-1 did not significantly alter its microtubule gliding velocity (0.76 ± 0.47 μm/s for dynein-1 alone vs 0.69 ± 0.27 μm/s with both factors). Thus, Q22YU3 is sufficient to specifically inhibit ODA activity.
Fig. 2. Q22YU3 binding inhibits ODAs by clustering motors.
(A) Microtubule gliding velocities. Individual gliding events from three technical replicates/sample are plotted (ODA n=159, ODA+YU3+MS1 n=136, ODA+YU3 n=146, ODA+MS1 n=94, Dyn1 n=136, Dyn1+YU3+MS1 n=76). YU3: Q22YU3, MS1: Q22MS1, Dyn1: human cytoplasmic dynein 1. Error bars show standard deviation; ns=not significantly different, ****p≤ 0.0001 (ANOVA with Tukey’s test for multiple comparisons). (B) Axonemal-purified open ODA reconstituted with recombinant Q22YU3 and Q22MS1. SDS-PAGE gel of SEC peak fraction. (C) Representative 2D class averages showing the distribution of open (purple) and closed (green) ODA particles from ODAs alone and reconstituted with factors. Scale bars = 10 nm. (D) Quantification of closed and open particles from 18,000 particles/dataset, (n=3 technical replicates) represented as percentage. Error bars show standard deviation. ns=not significantly different, * p≤ 0.01, ****p≤ 0.0001 (Kruskal Wallis test). Refer to Fig. S6 and Table S5.
Tetrahymena ODAs contain three dynein heavy chains and, when purified from axonemes, show an open bouquet conformation with the heavy chain motor domains separated (13). However, when we used negative stain electron microscopy (EM) to image ODAs purified from the cytoplasm, we noticed ~40% of intact particles displayed a “closed” conformation where the motor domains were clustered, and the tails were compacted (fig. S5). This closed conformation resembles a form previously observed only after cross-linking (13). To identify the factor responsible, we reconstituted axoneme-purified ODAs with recombinant Q22MS1 and Q22YU3. Both factors formed stable complexes with ODAs either together or individually (Fig. 2B, fig. S6, table S5). Whereas ODAs on their own were mostly in the open conformation (Fig. 2C-D), in the presence of both factors 36 ± 7% (mean ± SD) of particles were closed, similar to the fraction observed for ODAs purified from the cytoplasm. ODAs bound to Q22MS1 alone were closed only 11 ± 8% of the time. In contrast, in the presence of Q22YU3 alone, 61 ± 6% of ODAs were closed (Fig. 2C-D, fig. S6, table S5). Thus, Q22YU3 inhibits ODAs by holding the three heavy chains together into a closed conformation. We propose to name this protein Shulin (Sanskrit: one that holds the trident).
Cryo-EM structure shows molecular mechanism of ODA closure by Shulin (Q22YU3)
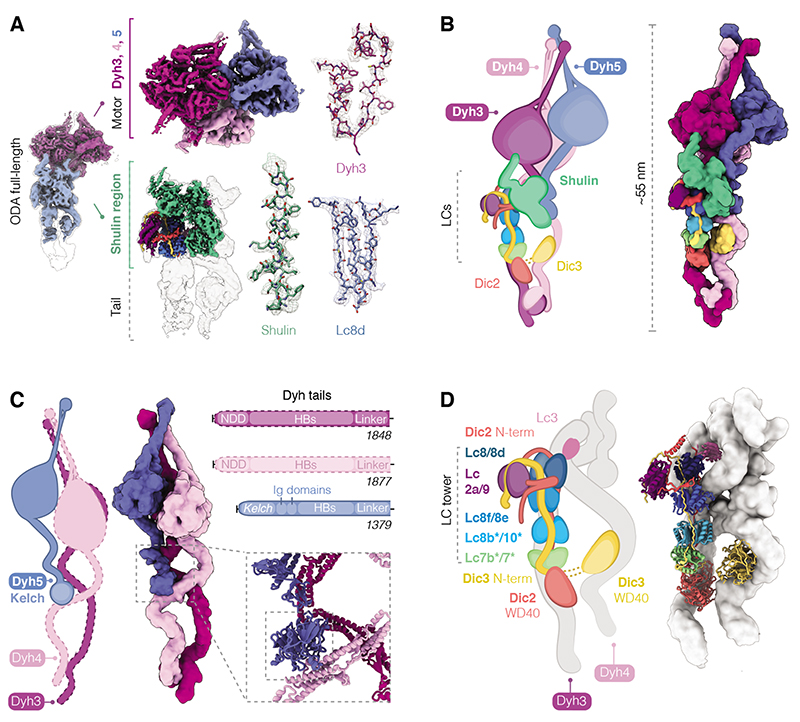
To elucidate how Shulin closes ODAs at a molecular level, we determined the structure of the reconstituted Tetrahymena ODA-Shulin complex by cryo-EM. The resolution of our overall structure was limited to 8.8 Å owing to its flexibility (Fig. 3A, fig. S7-8). However, focused classification and local refinement produced a series of sub-region maps ranging from 4.3-5.9 Å in global resolution (Fig. 3A, fig. S7-S12). The central portion of the Shulin region map has a local resolution range of 3.5-4.2 Å (fig. S9) enabling de novo building of Shulin and its interactions (Fig. 3A, fig. S11A). In combination with MS to identify the ODA subunit composition (fig. S13), our maps allowed us to assign the positions of the three heavy chains (Dyh3, Dyh4, Dyh5), two essential intermediate chains (ICs: Dic2, Dic3) and 11 light chains (LCs) (Fig. 3B, figs. S8-12). We list the names and UniProt IDs of these subunits and provide names for the corresponding orthologs in Chlamydomonas and humans in table S2.
Fig. 3. Cryo-EM reconstruction shows architecture of closed ODA.
(A) Overview of the closed ODA bound by Shulin with head (purple) and tail (blue) maps docked in an overall map (grey). Maps obtained after masked refinements are shown for the head region containing densities for Dyh3, 4 and 5 motor domains and the ordered tail map contains a docked Shulin-region map (green). Representative cryo-EM densities are shown. The following contour levels (σ) are used; right panel-ODA full-length structure: 0.000607, tail: 0.0026, motors: 0.01; middle panel-ordered tail: 0.00448, Shulin region: 0.015, motors: Dyh3 0.01, Dyh4 0.015, Dyh5 0.015. Zoom insets showing side chain densities were at contour level of 0.02. (B) Cartoon and filtered surface representation of all modeled subunits. (C) Dyh5 binds Dyh4 via its N-terminal Kelch domain (inset). HB: Helical bundles, NDD: N-terminal dimerization domain. (D) DIC N-terminal extensions bind dimers of LCs forming a LC tower and followed by globular WD40 domains that contact Dyh3 and Dyh4. Heterodimers of Lc7b/7 and Lc8b/Lc10 are tentatively assigned (*). Lc3 sits on Dyh4 and is not part of the LC tower.
In our structure, the motor domains were clustered in a closed conformation (Fig. 3B) consistent with the negative stain data. At the core of the structure are the Dyh3 (α-HC) and Dyh4 (β-HC) heavy chains, which are conserved across all eukaryotes with motile axonemes (14). They form a heterodimer held together by an N-terminal dimerization domain in an arrangement that is similar to cytoplasmic dyneins (15, 16). The third heavy chain, which is only found in ciliate and algal ODAs, is Dyh5 in Tetrahymena (γ-HC– equivalent to the α-HC in the alga Chlamydomonas, table S2). Our structure showed that Dyh5 is much shorter than the other heavy chains and is anchored to Dyh4 halfway along its length. The N-terminus of Dyh5 contains a Kelch-type β-propeller domain that sits on the helical bundles of Dyh4 (Fig. 3C). The peripheral attachment of this third heavy chain may explain why its loss in Chlamydomonas minimally impacts flagellar motility (17).
In their tail regions, Dyh3 and Dyh4 wrap round the globular WD40 domains of the intermediate chains, with Dyh4 also binding to a small density consistent with the thioredoxin-like Lc3 light chain (Fig. 3D, fig. S14A). The intermediate chains have long N-terminal extensions which are held together by a tower of light chains consisting of a Lc7/Lc7b heterodimer, three dimers of Lc8 orthologs and at the end, bent over to one side, a Tctex like heterodimer of Lc2a/Lc9 (Fig. 3D, fig. S14B). Based on side-chain density we assigned the positions of Lc8 and its orthologs: Lc8d, Lc8e and an unnamed Lc8-like protein (UniProt ID: Q22R86) which we called Lc8f. Our MS analysis on axoneme-purified ODAs showed the additional presence of two more Lc8 orthologs, Lc10 and Lc8b, which we tentatively assigned to the remaining two positions (fig. S13). The bent arrangement of the Lc2a/Lc9 heterodimer is stabilized by the Dic2 N-terminus, which loops out from where it contacts Lc2a and wedges between Lc8d and Lc8e (fig. S14B).
Dynein motors comprise a ring of six AAA+ domains (AAA1-6), a force-producing linker and a long coiled-coil stalk with the microtubule binding domain at the tip. In our structure, the motor regions of all three heavy chains were locked in the pre-power stroke conformation of their catalytic cycle (18) with their linker domains bent through 90° (fig. S15A, B). The density suggests that the stalks of each motor domain are angled to interact with each other close to their microtubule binding domains (Fig. 3A, B, fig. S15B). Clustering of motor domains is further stabilized by interactions between the Dyh3 and Dyh4 linkers, between the Dyh4 AAA4 and the elbow of the Dyh3 linker and between the Dyh5 AAA3 and Dyh3 AAA4 (fig. S15C, D). This clustered conformation is distinct to the one ODAs adopt upon docking onto ciliary doublets where their motor domains are stacked parallel to each other and free to undergo their catalytic cycle (19, 20). Thus, the closed conformation may represent an inactive state of ODAs prior to their final incorporation into cilia.
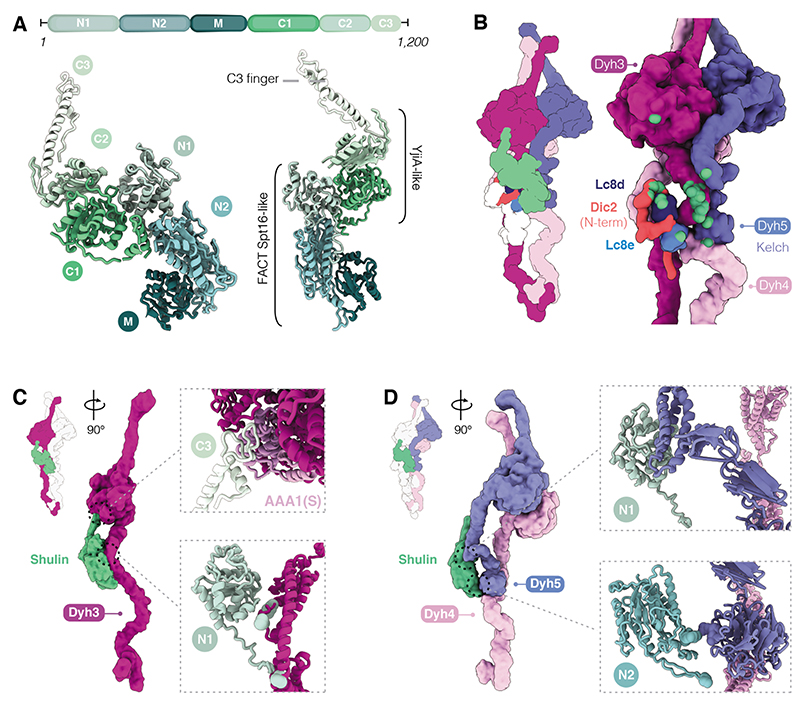
Our Shulin structure shows that it contains N-terminal domains (N1, N2) which are related to the aminopeptidase P domain of Spt16, a core component of the histone chaperone FACT (Fig. 4A). We built the middle (M) domain de novo, revealing that it adopted a pleckstrin homology (PH) fold akin to the Spt16 C-terminus. Thus, the whole N-terminal half of Shulin bears high structural homology to Spt16 (21). Surprisingly Shulin’s C-terminal domains (C1 and C2) are structurally similar to the bacterial GTPase YjiA (22). C1 has a Ras-like fold and C2 comprises of a five-stranded β-sheet. There is a nucleotide between C1 and C2, which plays a structural role holding the two domains together (fig. S16A). Shulin ends in a long C-terminal finger with a helix-loop arrangement that projects away from the core of the molecule (C3) (Fig. 4A). Overall, the structure suggests that Shulin has evolved from a fusion of an ancestral Spt16 and a nucleotide-binding protein.
Fig. 4. Characterization of Shulin structure and its mechanism of ODA inhibition.
(A) Domain architecture of Shulin. N-terminal (N1, N2) and Middle (M) domains share a similar fold with the FACT complex core subunit Spt16. C-terminal (C1, C2) domains are similar to GTPase YjiA and are followed by a C-terminal finger (C3). (B) Cartoon and filtered surface representation with main contacts between Shulin and ODA subunits highlighted in green spheres. (C) Shulin’s N1 domain contacts helical bundles proximal to the linker in Dyh3 tail. The C3 finger projects out to contact Dyh3 AAA1(S) (insets). (D) Shulin’s N1 domain contacts Dyh5 helical bundles and its N2 domain touches the Kelch domain. Shulin contacts Dyh4 just below Dyh5 Kelch-domain (insets).
Shulin stabilizes the closed conformation of ODAs by binding all three heavy chains and the LC tower (Fig. 4B). It makes its most extensive interactions with Dyh3 (3216 Å2 surface area). Shulin’s N1 domain contacts multiple sites in the Dyh3 tail and its C3 finger binds the AAA1 small sub-domain (AAA1S) in the Dyh3 motor domain (Fig. 4C). Residues at these two sites are conserved amongst homologs of Dyh3 from multiple species (fig. S17A, B) but not amongst other dynein heavy chains (fig. S17C). Our structure showed that Shulin bridges the Dyh3 tail and motor, holding them in a rigid conformation (fig. S16). It can only make these connections if the motor is in its pre-power stroke state, suggesting it directly locks Dyh3 into its closed conformation (fig. S15B). Truncation of Shulin to remove the motor-binding C3 finger abolished its ability to bind ODAs over gel filtration. Directly mixing the truncated Shulin with ODA also failed to induce closure in a negative stain EM assay (fig. S18). Thus, bridging the Dyh3 tail and motor is key for stabilizing the closed state of ODAs.
Shulin holds the other two motor domains in their closed conformation indirectly by stabilizing the contacts they make with Dyh3 and each other. Its N2 domain makes a small connection (313 Å2) to Dyh4 close to where the Dyh5 Kelch domain is docked (Fig. 4D). This interaction holds Dyh3 and Dyh4 together and reinforces a contact between the helical bundles in their tail regions (fig. S16C). This, in turn, supports the connections, described above, between their motor domains (fig. S15). Shulin’s connection to Dyh5, via its N1 domain, is also small (292 Å2), but sufficient to stabilize the Dyh5 linker binding to the Dyh3 motor domain and motor-motor contacts between Dyh4 and Dyh5 (fig. S15E). In the light chain tower the M-domain of Shulin contacts Lc8e and its C1-domain contacts Lc8d and an α-helix in the Dic2 N-terminus (fig. S16D). These contacts stabilize packing of the LC tower against the Dyh3 tail (fig. S16E). Thus, Shulin makes contacts with multiple ODA subunits and stabilizes the interactions between them that hold the motors in a closed conformation.
Q22MS1 dampens ODA activity by constraining the motors
Here, we identified two proteins, Q22MS1 and Shulin, which co-purify with ODAs in the cytoplasm and are required for their delivery to cilia. Of these two proteins, the mechanism of the Tetrahymena-specific Q22MS1 is less clear. It is 222 kDa in size and contains a catalytically inactive nucleoside diphosphate kinase (NDK) domain. A homologous NDK-domain is found in the recently identified Xenopus protein DAAP1 (23). DAAP1 has also been implicated in ODA delivery to cilia and localizes to membrane-less organelles involved in dynein assembly. Interestingly however, there is no sequence similarity between Q22MS1 and DAAP1 outside the NDK domain and the mammalian orthologs of DAAP1 lack the NDK-domain completely. Q22MS1 mildly reduces ODA motor activity in our microtubule gliding assays. To address how this could occur we sub-classified the open particles of ODAs alone and bound to Q22MS1. In the presence of Q22MS1 we observed a significant increase in the number of particles with their motor domains constrained in a triangular conformation (fig. S19A). Cross-linking mass spectrometry showed that Q22MS1 predominantly interacts with the tails of Dyh3, Dyh4 and Dyh5 on the opposite side of the complex from the Shulin binding interface (fig. S19B). We thus propose that Q22MS1 binds the ODA tails and corrals the heads into a clustered triangular conformation which causes a mild dampening of motor activity.
Shulin keeps ODAs closed during delivery to cilia
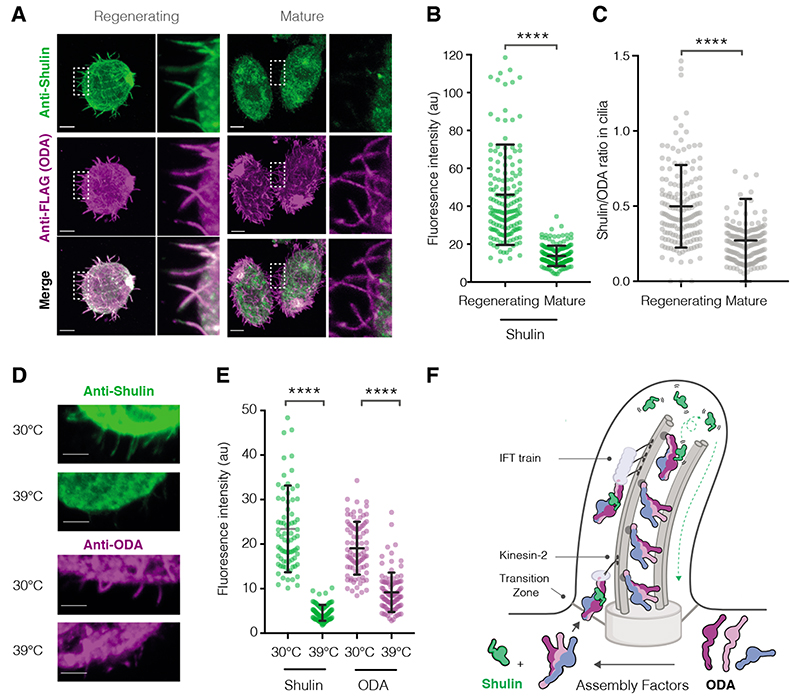
Shulin significantly shuts down ODA motor activity, suggesting it is the proposed inhibitor (4) required during targeting of ODAs to the cilia. Unlike the cytoplasmic dyneins which are auto-inhibited (15, 16), ODAs rely on Shulin to enforce inhibition. Immunostaining revealed that Shulin localized to regenerating cilia (Fig. 5A-C, fig. S20). This is the stage of ciliogenesis when ODAs are being actively imported and incorporated (8). In contrast, in mature cilia with stably incorporated dyneins, the levels of Shulin appeared reduced relative to ODAs. (Fig. 5A-C). To test if Shulin’s entry into cilia was ODA dependent, we used a temperature sensitive mutant (OAD1C11) in which ciliary entry of ODAs is blocked at the restrictive temperature of 39°C (Fig. 5D, E). Shulin accumulated in regenerating cilia as normal at the permissive 30°C, but its entry was prevented at the restrictive temperature (Fig. 5D, E). Thus, Shulin travels with ODAs during cilia growth until they reach their final axonemal location (Fig. 5F).
Fig. 5. Shulin delivers ODAs to regenerating cilia.
(A) Co-immunostaining of ODAs (anti-FLAG) and Shulin in the IC3-FLAG strain. Representative cells regenerating cilia 30-minutes post-deciliation and with mature cilia are shown, insets highlight differences in levels of Shulin and constant levels of ODA in cilia in both conditions. (B, C) Quantification of Shulin levels in regenerating and mature cilia and ratios of per cilium fluorescence intensities for Shulin/ODA (Regenerating n=160, Mature n=162, 9-10 cilia from 16 cells/condition). (D, E) Immunostaining for Shulin and ODA (anti-ODA) in an ODA temperature sensitive mutant strain (OAD1C11) grown at permissive (30°C) and restrictive (39°C) temperatures for 16 hours (Shulin: 30°C n=78, 39°C n=83; ODA: 30°C n=87, 39°C n=95 cilia from 10 cells per condition). Scale bars:10 μm. Error bars show standard deviation, ****p≤ 0.0001 (ANOVA). (F) Model for Shulin’s role in delivering ODAs to cilia. Shulin locks ODAs in their closed state during IFT (intraflagellar transport) in regenerating cilia. Shulin is released after ODAs stably dock and leaves the ciliary compartment (green arrow)
Shulin’s inhibitory effect on ODAs could serve several purposes. During IFT of ODAs, Shulin may prevent aberrant interactions with axonemal microtubules which would hinder their transport. At their final location ODAs interact with the A-tubule via their tail domains and with the B-tubule via their motor domains (20). Shulin’s inhibition could be important to allow the ODA tails to correctly dock before the motors can engage the B-tubule. Finally, as previously proposed, inhibition may also play a role in preventing any free ODAs escaping prematurely from cilia (4).
In addition to its inhibitory role during ciliary trafficking, Shulin is likely to have other functions. Firstly, Shulin knockdown resulted in reduced levels of ODAs in the cell body as well as the cilia (fig. S21), suggesting Shulin is required to prevent ODA degradation in the cytoplasm. Shulin may play a role in ODA assembly, such that in its absence incorrectly assembled ODAs are removed. Alternatively, Shulin binding may directly shield cell body ODAs from constitutive degradation pathways. Secondly, Shulin is also implicated in directly targeting proteins to cilia. Its human ortholog, C20ORF194, binds the small GTPase Arl3 in primary cilia (24). Arl3-GTP exclusively localizes to cilia and regulates targeting of numerous ciliary proteins (25). Because primary cilia lack ODAs, Shulin may target other components in this context. In motile cilia, Arl3 is also important for assembly (26), which raises the possibility that the Shulin-Arl3 interaction plays a direct role in ODA delivery. In summary our work shows that Shulin inhibits and packages ODAs and plays a key role in their targeting to cilia.
Materials and Methods
Tetrahymena thermophila strain engineering and phenotypic analyses
Tetrahymena thermophila wildtype CU428.2 (TSC_SD00178) and ODA temperature sensitive OAD1C11 (TSC_SD01784) strains were acquired from the Tetrahymena Stock Center. Cultures were maintained in SPP medium (1% bacto proteose peptone (Difco), 0.2% glucose, 0.1% yeast extract, 33 μM FeCl3). Transgenic lines were generated using biolistic transformation as previously described (27). For tagging IC3 polypeptide with tandem ZZ and FLAG tags, a construct bearing homology arms to the gene region upstream of the stop codon and the 3’UTR of DIC3 (TTHERM_00079230) flanking a Neomycin resistance cassette, with codon optimized sequences for the tags was used. DNA was precipitated onto 10-micron gold carriers for biolistic bombardment of the macronucleus using a Gene Gun (Bio-Rad). For disruption of Q22YU3/SHULIN (TTHERM_00122270) and Q22MS1 (TTHERM_00030520), constructs with homology arms to the 5’UTR and 3’UTR of the respective genes flanking the resistance cassette were generated. Transformants were transferred to SPP medium for recovery and the promoter driving the Neomycin resistance gene was switched on by addition of cadmium chloride (1 μM). After recovery, bombarded cells were transferred into 96-well plates to isolate transformed clones. Cultures were passaged every few days into medium containing increasing concentrations of Paromomycin for phenotypic assortment of transformants. Successful generation of tagged transgenic strains was assayed by performing genomic PCRs spanning the Neo3 cassette after several generations. Only transgenic strains would amplify this resistance gene. Immunoblotting and immunofluorescence with a monoclonal FLAG antibody (Flag M2 Sigma, 1:100 dilution) further confirmed the endogenous knock-in of the epitope tag at the carboxy terminus of the IC3 polypeptide.
The knockout mutant strains tolerated up to 20-50 mg/ml of Paromomycin concentration. Genomic PCRs spanning Exons 1 to 3 of Q22YU3 (Exon 1 forward primer: atgaatttaaattttgcatgtcttcaataag, Exon 3 reverse primer: ttatacatcatgaactgtacaatcacttgg) and Q22MS1 (Exon 1 forward primer: atgtttggatttgaagatatccattactaacc, Exon 3 reverse primer: attagaggcttagtgaacatgtcttcgtc) confirmed disruption of both loci as only wildtype controls resulted in a robust amplicon whereas both mutants failed to generate a strong PCR product (fig. S3A). A control PCR for β-heavy chain gene was performed to verify the integrity of the genomic DNA. Additionally, immunostaining Q22YU3Δ/SHULINΔ cells with a custom polyclonal antibody against Shulin (Eurogentec, 1:100 dilution) confirmed loss of protein as well as serving as antibody validation (fig. S12C, D).
For assessing ciliary defects in Q22YU3/SHULINΔ and Q22MS1Δ mutant strains, three main phenotypic assays were performed – measurement of cell velocities, counting food vacuole accumulation and quantifying cytokinesis defects. Cell images or movies were acquired using a QIClick camera with QCapture software mounted onto a Leica DM IL LED microscope. Imaging was performed at room temperature. Immunofluorescence images were acquired using LSM710 confocal microscope. Videos of cells swimming close to the plane of imaging (closest to the slide) were acquired at 10 frames per second for 20-40 s using a 20x objective. High speed videos to visualize cilia beating and calculate beat frequencies were acquired (see section below). All cellular phenotyping was done using FIJI (28). Cell velocity was measured using MTrackJ plugin (29). Cell paths were manually traced, and cell velocity was calculated as a function of distance traversed in a given time frame. Food vacuoles were manually counted in phase contrast images. Cilia numbers were counted using central confocal slices in the plane of both the macro and micronucleus. Images were thresholded to segment cilia around the cell circumference for counting using FIJI. Cilia lengths were measured by drawing a line along an individual cilium and measuring its distance. Lengths of 5-15 randomly selected cilia from 14-22 cells, from replicate staining experiments were measured. Cilium lengths per cell were averaged and plotted. Cytokinesis defects were scored in images of cells stained with acetylated α-tubulin (SantaCruz, sc-23950, 1:250 dilution) to mark the cilia and demarcate the cell shape and DAPI to stain for the nuclei. Cells with more than one oral apparatus and/or macronucleus were counted for each genotype. To quantify ciliary loss of ODAs in mutants, indirect immunofluorescence was performed (see section below).
Cilia beat frequency measurements
Differential interference contrast (DIC) time-lapse imaging of beating cilia was performed on a Nikon Eclipse Ti2 inverted microscope equipped with a 20x/0.75 NA Plan Apo VC objective lens and Hamamatsu ORCA-Flash4.0 V2 sCMOS camera (512x512 pixel region of interest, 1024 frames at ~280 Hz). Automated kymograph extraction was performed in ImageJ. In short, the image sequence was stabilized using the plugin stackreg (30) twice with a rigid body motion model: once on the full image and once on a subregion determined by segmenting the moving cell to account for residual rotations. The segmentation was performed by thresholding the absolute value of the difference of the intensity with the mean intensity of the image. A smoothing of the contour was performed by thresholding a Fourier representation of the complex curve represented by x + i y. Kymographs were then extracted by measuring the intensity of the image for each point on the contour over time. The kymographs were further analysed in Matlab (Mathworks). Fourier transform along the temporal dimension was computed (31). Peaks in the max projection of the spectrum along the curvilinear abscissa enabled the calculation of the beat frequency.
Indirect immunofluorescence and fluorescence intensity quantification
Indirect immunofluorescence was performed using a custom generated polyclonal antibody raised against the ODA holocomplex (Eurogentec, 1:150 dilution), an acetylated α-tubulin antibody (SantaCruz, sc-23950, 1:250 dilution) to label ODAs and the ciliary axoneme in wildtype cells or in the OAD1C11 temperature sensitive mutant cells. A monoclonal FLAG antibody (Flag M2 Sigma, 1:100 dilution) and a polyclonal Shulin antibody (Eurogentec, 1:100 dilution) were used to label ODAs and Shulin respectively in the IC3-FLAG strain. The ODA antibody was validated by the manufacturer with ELISA tests against the ODA holocomplex antigen. Additional validation of specificity was performed using a temperature sensitive OAD1C11 mutant strain (Tetrahymena Stock Center) with reduced ciliary ODA staining when grown at the restrictive temperature of 39°C (11). Fluorescence intensity values along the cilium were measured using plot profile tool, averaged and plotted in GraphPad Prism7.
Purification of ODA complexes and interactors from cell body
Large scale cultures of IC3: ZZ: 3xFLAG strains were grown to a high cell density in SPP medium typically for 72 hours. Cultures were starved in Tris-Acetate buffer for 2 hours to reduce numbers of phagocytic vacuoles containing proteases. Cells were deciliated with dibucaine hydrochloride (0.5 M). The extent of deciliation was carefully monitored by visual inspection under a stereomicroscope to ensure minimal cell lysis. Typically, within 5 minutes of adding dibucaine most cells appeared to have lost their cilia. Dibucaine concentration was diluted three-fold by adding more medium and cells were pelleted. Cell pellets were washed in Tris-Acetate buffer and lysed in Lysis Buffer (20 mM HEPES NaOH, pH 8.0, 50 mM NaCl, 1 mM EDTA, 5 mM DTT, and 10% glycerol supplemented with 0.1% Triton X-100, 0.2% IGEPAL CA-630, 1 mM PMSF, 5 μm proteasome inhibitor MG-132, and 3x Complete protease inhibitor tablets (Roche). Lysates were clarified by ultracentrifugation at 70,000 rpm in a Ti70 rotor (Beckman) and flown multiple times over FLAG-M2 affinity beads (Sigma) which were pre-equilibrated in lysis buffer and packed in a gravity flow column. The beads were washed for at least 5 column volumes times in Wash buffer (20 mM HEPES, pH 7.4, 50 mM NaCl, 1mM MgCl2, 1 mM TCEP, 10% glycerol and 0.1% IGEPAL). IC3-ZZ-3xFLAG containing complexes were eluted into 5 fractions by sequentially flowing 5 bed volumes of elution buffer containing FLAG peptide. Efficiency of elution was assessed by running SDS-PAGE gels and staining with Instant Blue (Expedeon). Elution of desired ODA complexes was deemed successful upon detection of high molecular weight bands corresponding to dynein heavy chains. Eluted fractions containing the highest concentration of complexes were further fractionated over a Superose 6 increase 3.2/300 size exclusion column (GE Healthcare) in GF50 gel filtration buffer (25 mM HEPES pH 7.4, 50 mM NaCl, 1 mM MgCl2, 1 mM DTT, 0.1 mM ATP). FLAG eluates and peak fractions from gel filtration run were analysed by mass spectrometry.
Purification of ODA from cilia axonemes
Axonemal ODAs were purified as previously described (13). Large scale wildtype Tetrahymena cultures were deciliated using dibucaine (as above). Deciliated cell pellets were discarded and cilia in the supernatant were centrifuged at 13,500 x g at room temperature. Cilia pellets were washed in Cilia Isolation Buffer (CIB: 20 mM HEPES pH7.4, 100 mM NaCl, 4 mM MgCl2, 0.1 mM EDTA) and centrifuged at 600 x g several times to remove cell bodies and mucus. A final high-speed spin at 13500 x g at 4°C yielded a pure cilia pellet with a white fluffy appearance. Cilia were de-membranated by resuspending the pellet in CIB containing 0.25% Triton-X detergent, freshly added protease inhibitors, 1 mM DTT and 200 mM PMSF followed by a 30-minute incubation on ice. De-membranated cilia axonemes were isolated by centrifugation at 17000 x g at 4°C. Axonemes were washed in CIB buffer to remove residual detergent and re-pelleted. Axoneme pellets were resuspended in high salt buffer (20 mM HEPES pH7.4, 600 mM NaCl, 4 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 0.1 mM ATP, 200 mM PMSF) and incubated for 30 minutes on ice to isolate dynein arms. The dynein containing high salt extract was loaded onto 6 identical 5-25% sucrose density gradients made in CIB and ODA arms were separated from other axonemal complexes over 16 hours by centrifugation at 33,000 rpm in an SW40 rotor at 4°C. The following day, sucrose gradients were manually fractionated into 500 μl fractions. Every alternate fraction was resolved on an SDS-PAGE gel and stained with Instant Blue. ODA isolation was deemed successful upon detection of characteristic high molecular weight bands corresponding to ODA heavy chain polypeptides over several of the denser fractions towards the bottom of the gradient. Sucrose gradient fractions containing ODA complexes were further purified over a MonoQ 5/50 anion exchange column (GE Healthcare) to separate out other axonemal dynein species. ODA complexes eluting at ~300 mM salt off the MonoQ column were verified for intactness and presence of subunits by a further gel filtration step, negative stain electron microscopy and mass spectrometry analyses. ODA complexes purified as above were used in all biochemical reconstitution experiments and subsequent cryo-EM studies.
Insect cell expression and purification of Q22MS1 and Q22YU3/Shulin
Gene sequences coding for Tetrahymena thermophila Q22MS1 (TTHERM_00030520) and Q22YU3 (TTHERM_00122270; C20ORF194-like) were codon-optimized and synthesized (Epoch) for expression in Spodoptera frugiperda derived Sf9 cells. Codon optimized sequences were sub-cloned into pACEBac1-derived vectors containing C-terminal 2xStrep tag. The following constructs were generated pACEBac1-Q22MS1-Psc-2×Strep and pACEBac1-Q22YU3-Psc-2×Strep. Baculoviruses for individual expression of Q22MS1 and Q22YU3/Shulin were prepared using the insect cell-baculovirus system. Cells expressing recombinant proteins were harvested 48 hours after infection and lysed in 50 ml cell lysis buffer (20 mM Hepes-NaOH pH 7.2, 100 mM NaCl, 2 mM MgAc, 1 mM EDTA, 10% (v/v) glycerol, 1 mM DTT). Cells were mechanically lysed in a 40 ml Dounce-type homogenizer (Wheaton) using 15-25 strokes. Lysates were clarified by ultracentrifugation at maximum speed in a Ti70 rotor (503,000 x g) for 45 min, 4°C (Beckman Coulter). Clarified lysates were poured several times over 0.5-1 ml Streptactin resin (IBA) which was applied into a gravity flow column and pre-equilibrated in lysis buffer. The resin was washed for 20 column volumes to remove non-specifically bound contaminants. Recombinant proteins were eluted off the resin in five fractions over sequential incubations in an elution buffer (lysis buffer containing 3 mM D-desthiobiotin). Eluates were resolved on an SDS-PAGE and stained with Instant Blue to assess the purity of the recombinant proteins. Recombinant proteins eluting off the Streptactin beads were further cleaned over gel filtration using GF150 (25 mM HEPES pH 7.4, 150 mM NaCl, 1mM MgCl2, 1 mM DTT, 0.1 mM ATP) buffer. Proteins were snap frozen in liquid nitrogen and stored at -80°C for use in all biochemical reconstitutions which were performed at 4°C.
Microtubule gliding assays and quantification
Gliding assays were performed as previously described (32). Microtubules were polymerized at 37°C using Alexa-647 and unlabeled tubulin at 3 μm and 11 μm concentration respectively in polymerization mix (BRB80: 80 mM potassium PIPES pH 6.8, 1 mM MgCl2 and 1 mM EGTA with 10mM GTP). Polymerized microtubules were stabilized with 2 μM Taxol in BRB80 (Sigma T1912). 10-20 μl of freshly prepared or freshly thawed ODA at ~100 μg/ml concentration was applied to a flow chamber and allowed to adhere to glass for 2 min at room temperature. Flow chamber was washed in buffer (50 mM KAc, 10 mM HEPES pH 7.4, 4 mM MgAc, 1 mM EGTA) containing 1% BSA. Taxol stabilized microtubules were flowed in with a motility mix (20 mM HEPES pH 7.4, 5 mM MgSO4, 1 mM DTT, 1 mM EGTA) and allowed to bind to motors. Finally, motility mix containing 1mM ATP was flowed in prior to TIRF imaging. Imaging was performed at room temperature using a Nikon microscope with a 100× oil-immersion objective (Nikon, 1.49 NA Oil, APO TIRF).
The imaging system used the 100 mW 641 nm (Coherent Cube) laser. Images were acquired with a back illuminated EMCCD camera (iXonEM+ DU‐897E, Andor, UK) controlled with μManager software. Imaging was performed with 100 ms exposures taken at 2 s intervals with a pixel size of 160 nm. For testing effect of factors, ODAs were pre-incubated on ice for 30 minutes with Q22YU3/Shulin, Q22MS1 or both in 10-25x molar excess and these complexes were applied to the flow chamber as above. These molar ratios resulted in successful reconstitutions as above and enforced closure of ODAs (by Shulin) and were therefore also used in gliding assays. As control, full-length human cytoplasmic dynein-1 (33) was used at 100 μg/ml with or without both factors. Microtubule gliding velocities for each condition tested were calculated by manually tracking the leading edge of moving microtubules using the FIJI plugin MTrackJ. The average velocity for the track of each microtubule was used to calculate the average velocity for the entire population of microtubules recorded. Experiments were performed in triplicate technical repeats.
Reconstitution of ODA with Shulin and/or Q22MS1
ODA complexes (~0.5-1 mg/ml) purified over a MonoQ column were mixed with 10-25x molar excesses of purified Q22YU3/Shulin, Q22MS1 (~1-1.5 mg/ml) or both. Complexes formed by overnight dialysis into 50 mM or 150 mM NaCl buffer were used for negative stain EM analyses and complexes reconstituted by dialysis into 150 mM salt buffer were used for cryo-EM grid freezing and analyses. Complex formation was assessed by fractionating dialysates over a Superose 6 increase 3.2/300 size exclusion column (GE) in GF50 (25 mM HEPES NaOH pH7.4, 50 mM NaCl, 1mM MgCl2, 1 mM DTT, 0.1 mM ATP) or GF150 (25 mM HEPES NaOH pH 7.4, 150 mM NaCl, 1mM MgCl2, 1 mM DTT, 0.1 mM ATP) buffer. Fractions spanning the entire column volume were resolved on an SDS-PAGE gel and stained with Coomassie or SYPRO Ruby gel stain (Bio-Rad). The primary peak consisted of ODA subunits with bound factors confirming successful reconstitution. Secondary peaks contained molar excesses of individual proteins unbound to ODAs.
Reconstitution of ODA with ShulinΔC3 truncation
ODA complexes were mixed with 5-10x molar excesses of recombinant insect cell expressed full-length Shulin or Shulin truncations lacking the C3 finger (ShulinΔC3 contains only the first 1104 amino acids – Methionine 1 – Valine 1104, and lacks the C-terminal extension i.e., ‘C-3 finger’). Complex formation was assessed by fractionation over a Superose 6 increase 3.2/300 size exclusion column (GE) in GF150 as above. The primary peak consisted of ODA subunits either bound by Shulin (only the full-length protein) or unbound (Shulin ΔC3). Secondary peaks contained molar excesses of the individual proteins unbound to ODAs. For negative stain EM analyses, ~0.5 mg/ml ODA complexes were mixed with 5x molar excesses of recombinant full-length or truncated forms of Shulin, incubated on ice for 30 mins and used at a final concentration of ~0.01-0.05 mg/ml for negative stain electron microscopy analysis (see section below). Datasets of particles acquired (same parameters as was used for ODA open vs. closed data collection, see section below) for each set of reconstitutions were subjected to rounds of 2D classification to assess conformational states of ODAs in the presence of full-length Shulin or its truncated forms. Particle numbers for ODA+ Shulin ΔC3: (initially) 150,905, (final classes) 28,501. Particles numbers for ODA+ full-length Shulin (initially) 29,480 (final classes) 5,267.
Negative stain electron microscopy analysis
Negative stain microscopy analyses were performed on ODAs from axonemes, ODAs from cell bodies and ODA complexes reconstituted with Q22YU3/Shulin, Q22MS1 or both. In each case, 3 μl of sample at ~0.05-0.1 mg/ml concentration were applied for 1 minute to freshly glow-discharged (Edwards Sputter Coater S150B, 15-30 s at 35 mA) 400 mesh copper grids coated with a continuous carbon support layer (Agar Scientific) followed by application of 2% uranyl acetate for a minute and air-dried after wicking away excess liquid. For statistical analysis of open versus closed ODAs, triplicate datasets per condition were collected automatically using FEI EPU on a FEI F20 electron microscope (equipped with a FEI Flacon II direct electron detector) operated at 200 kV with a 0.996 second exposure and a pixel size of 3.58 Å/pix (or 4.36 Å/pix for cell body dataset). A typical dose of ~ 20 e−1/Å2 and a range of defoci between 1.5-3.0 μm were used. Number of micrographs collected for axonemal datasets: ODA only, n=1: 646; n=2: 1,691; n=3: 1,278; ODA-YU3-MS1, n=1: 1,234; n=2: 518; n=3: 1,772; ODA-MS1, n=1: 4,338; n=2: 672; n=3: 2,279; ODA-YU3, n=1: 553; n=2: 1,915; n=3: 616.
Statistical analysis of open and closed ODA conformations
To quantify the effect of factors on ODA structure relative to isolated ODAs, particles were first semi-automatically picked using EMAN2 (v2.07) e2boxer.py (Swarm function) and thereafter processed using RELION 3.1 (34). Particles were extracted using a 256 pixel box size, yielding the following particle numbers for each dataset: ODA only, n=1: 93,756; n=2: 41,467; n=3: 56,679; ODA-YU3-MS1, n=1: 42,040; n=2: 98,443; n=3: 169,297; ODA-MS1, n=1: 127,030; n=2: 90,402; n=3: 393,961; ODA-YU3, n=1: 45,478; n=2: 79,255; n=3: 110,055.
Two rounds of 2D classification were performed with 30-75 classes (depending on dataset size) to remove ‘junk’ particles and stain artefacts. Intact ODA particles showing both heads and tails were selected and subjected to random extraction of 18,000 particles using a python script (https://github.com/sami-chaaban/StarParser). This step was performed to ensure equal number of particles for subsequent analysis across the different datasets. These ‘final’ 18,000 particles were used for a reference-free 30 class 2D classification, selected and subjected to alignment against a reference such that all classes adopted the same orientation (using EMAN2 [v.2.31] e2align2d.py). The four datasets per repeat were combined (giving 120 classes) and randomized using a custom script (Sami Chaaban) for subsequent double-blind analysis by six lab members. The categories for analysis were: (1) open-separated (3 motors splayed apart in an open bouquet conformation); (2) open-clustered (3rd motor docks in against two lower motors in a ‘triangle’ formation) (3) closed (motors tightly shut, identified by a ‘phi’-like conformation or stacking of motors on top of each other) (4) ambiguous (unidentifiable conformation). For each set mean frequency of open versus closed and standard deviations were calculated. A Kruskal Wallis statistical test was performed on the closed and open-clustered conformations using Prism.
For cell body ODAs, a large dataset of 279,106 particles acquired from a cell body purification of ODAs was sub-classified as above. Non-ODA particles such as ribosomes, other cellular complexes and broken ODAs were classed in an ambiguous class. Intact ODA particles were sub-classified as above until closed and open classes were clearly distinguishable (fig. S5)
Cryo-EM grid preparation
ODA were reconstituted with Q22YU3/Shulin using 1:10-1:25 molar ratios (ODA:Shulin; ODA at ~0.5-1 mg/ml and Shulin at ~1-1.5 mg/ml) as described above. Reconstituted complexes were purified over a Superose 6 increase 3.2/300 size exclusion column SEC in GF150 buffer and immediately crosslinked in 0.025% Glutaraldehyde (Sigma-Aldrich) for 30 minutes on ice followed by quenching with 1 mM Tris-HCl (pH 7.4). This mild crosslinking with 0.025% glutaraldehyde minimized complex dissociation during grid freezing. To assess that crosslinking did not cause gross artefacts to reconstituted complexes, crosslinked complexes were negatively stained and had an appearance indistinguishable to non-crosslinked complexes freshly applied to EM grids.
ODA:Shulin complexes were applied at a concentration of ~0.1-0.2 mg/ml to graphene oxide (GO) grids. GO grids were prepared a day prior to freezing as previously described (35). Briefly, gold 300 mesh Quantifoil R2/2 holey carbon grids (Quantifoil Micro Tools) were glow-discharged using an Edwards Sputter Coater S150B for 60 s at 35 mA). GO dispersion (Sigma-Aldrich; 2 mg/mL in H2O) was diluted ten-fold with ddH2O to a final concentration of 0.2 mg/ml and subsequently spun down at 600 x g for ~15 sec to remove large aggregates of GO flakes. 3 μl of GO flake solution from the top was applied to grids. After incubation for one minute with graphene oxide dispersion, the GO solution was removed by blotting briefly with Whatman No.1 filter paper and washed by absorbing 20 μl ddH2O onto the GO coated side twice and once on the back side of the grid with blotting steps in between. Grids were air-dried and used the next day for cryo-EM grid freezing without further glow discharging as GO grids were already hydrophilic. Freezing was performed at 4°C with 100% humidity using a Vitrobot IV (ThermoFisher Scientific). 3 μl of sample were applied to the GO side of the grids. After a wait time of 45 s, excess liquid was blotted away for 2-2.5 s with Whatman filter papers pre-equilibrated in the humidity chamber. Grids were immediately plunge-frozen into liquid ethane. Grids were transferred into grid-boxes and stored in liquid nitrogen for future screening and data collection.
Cryo-EM data collection and initial processing of whole ODA molecule
Electron micrograph movies were recorded using a Titan Krios (Thermo Fisher Scientific) equipped with an energy filtered K3 detector (Gatan) at 81,000x magnification in EFTEM mode. Six datasets were collected at the LMB using Serial EM at a pixel size of 1.11 Å/pixel, 300 kV, 66 frames, 2.6 s exposure, ~52 e-/Å2. A script was used to collect data in a 3x3 hole pattern, 3 images/hole, using beam-tilt to speed up data collection. A further dataset was collected at the Electron Bio-Imaging Centre (eBIC), Diamond Light Source, UK using EPU v2.7 at 0.53 Å/pixel, 300 kV, 54 frames, 3 s exposure, 54 e-/Å2 (table S3). Aberration-free image shift (AFIS) collection was used to speed up data collection (4 images/hole).
Cryo-EM image processing
All image processing was performed using RELION-3.1 and software wrapped within (34). Inter-frame motion in each movie was corrected using RELION’s own implementation of motion correction as described above, using 5x5 patches and a B-factor of 150 Å2 applied to the micrographs (36). Defocus parameters were estimated on non-dose weighted micrographs either using GCTF v1.18 (37) or CTFFIND4 v4.1.13 (38). For each individual dataset, particles were picked on non-dose weighted micrographs using Gautomatch v0.56 using permissive picking parameters (cc_cutoff=0.1, 400 Å diameter) and projections from an initial structure of full-length ODA. This initial model was obtained from a preliminary round of processing of the eBIC and first two LMB datasets where the phi-dynein structure was used as a reference (EMD-3705).
Particles were extracted from dose weighted micrographs with bin 4 parameters (768-pixel box size re-scaled to 192, yielding a final pixel size of 4.24-4.44 Å/pixel). 2D classification was subsequently performed with 75-100 classes, T=4, 750 Å circular mask, limiting resolution E-step to 15 Å and ignoring CTFs until first peak. A further 2-3 rounds of 2D classification without alignment (50-70 classes) was performed to remove graphene oxide layer artifacts, ice contamination and aggregated particles refractory to averaging. Particles from 2D classes showing projections of recognizable ODA views were selected and joined from all datasets (1,300,000 particles).
The combined particles were subjected to global auto-refinement with a loose mask around the whole ODA and an initial model of the whole molecule filtered to 50 Å giving a 15 Å structure. Considerable flexibility was observed in this overall structure (hereafter referred to as overall-1), particularly the Dyh4 and Dyh5 motors and the lower tail section. To resolve the tail and motors of ODA separately, we employed a combination of focused classification, masked refinement and signal subtraction as outlined in fig. S6. All masks used were created using volumes of sub-regions generated in Chimera (UCSF) using ‘volume eraser’ or ‘color zone’ (39). These sub volumes were low pass filtered to 15 Å with a soft edge and binary map extension (both 6-8 pixels).
Processing of full-length and Dyh4/Dyh5 motors
To improve upon the resolution of the full-length ODA structure, the overall-1 refinement data.star was used as input for masked 3D classification without alignment (5 classes, T=4). Particles from the class showing the most complete density (evidence for Dyh4 and Dyh5 at lower threshold levels) were selected for masked global 3D refinement, yielding a 9.7 Å structure. At this point particles were re-extracted to their bin by 2 and unbinned parameters in parallel: 384-pixel box size at 2.22 Å/pixel and 768-pixel box size at 1.11 Å/pixel, respectively. These particles were used for local refinements, yielding full-length ODA structures that resolved to 8.9 Å (bin by 2) (overall-2) and 8.8 Å (unbinned) (overall-3; EMD-11576). A mask was applied to the tail of the overall-3 map and a local refinement was performed resulting in a 6.7 Å map of the entire ODA tail (EMD-11577). The overall-2 map provided higher signal to noise ratio for the flexible Dyh4 and Dyh5 motor domains and was thus used to resolve these regions through signal subtraction and focused refinements. To this end, particle subtraction was performed by centering the subtracted images on a mask encompassing both Dyh4 and Dyh5 motors and re-boxing to 256 pixels (2.22 Å/pixel). Subtracted particles were subjected to a local refinement, giving an overall 12.9 Å structure of Dyh4-Dyh5 motors. This reconstruction was used as the basis for signal subtraction of Dyh4 and Dyh5 individually. Each motor was subsequently locally refined (Dyh4, 10.5 Å and Dyh5, 11.8 Å with no post processing) and 3D classified without alignment (5 classes, T=50). A final local refinement with particles from the best 3D classes (selection criteria: ordered motor, presence of stalk and limited noise) was performed, resolving to 5.0 Å (57,761 particles) and 5.6 Å (49,756 particles) for Dyh4 (EMD-11582) and Dyh5 (EMD-11583, EMD-11584), respectively.
Processing of the tail and Shulin region
To get high resolution information on the Shulin region, a mask for the full tail was first generated based on the overall-1 structure. Using this mask, a round of 3D classification without alignment was performed on bin by 4 particles (8 classes, T=8) with the data.star from overall-1 as input. Particles from the class containing clear density for the Shulin finger, mid- and low tail region were selected for global refinement, resolving to 9.0 Å. This was subsequently used as an input for 3D classification without alignment with a mask focusing on the Shulin region (8 classes, T=100). Six classes containing clear density for the Shulin region were selected for a local refinement that produced an 8.9 Å reconstruction. At this point, 123,484 particles were unbinned and re-boxed to a smaller 384-pixel box size (1.11 Å/pixel). A more specific mask of Shulin was created encompassing its core N-terminal and C-terminal domains, the C3 finger as well as contacts to the uppermost LC tower and portions of the contacting Dyh3-5 tails. Masked local refinement of this region produced a 4.8 Å structure. Finally, the Shulin region refinement parameters were used for a round of 3D classification without alignment to sort out remaining heterogeneity (5 classes, T=50). 43,338 particles from two overlapping classes were combined for two separate masked local refinements of the Shulin region: Shulin region and the DYH3 tail contact (4.6 Å; EMD-11580), and Shulin region excluding C3 finger (4.3 Å; EMD-11579). A larger mask of the tail was also applied to the earlier 123,484 particle subset to get an overview of the lower tail region (refined to 5.9 Å after signal subtraction, re-centering and masked 3D classification; EMD-11578).
Processing of the Dyh3 region
The overall-1 map indicated that Dyh3 is rigid relative to the two other motors, enabling direct masked 3D classification without alignment of this motor and region of the upper tail (based on overall-1 data.star, 8 classes, T=10). Selection of particles from the best class and global 3D refinement resulted in a 9.0 Å structure, at which point particles were unbinned and re-boxed (512-pixel box size). A tighter Dyh3 motor only mask was generated and used for a round of local 3D refinement, producing a 4.8 Å structure. This was used as the basis for 3D classification without alignment to separate out conformational heterogeneity (5 classes, T=50), resulting in only one class showing complete density. 49,397 particles from this class were selected for a final round of local 3D refinement and re-centering in the box, yielding a 4.4 Å resolution structure (Dyh3 region; EMD-11581).
Model building and refinement
Homology models were generated for ODA subunits using PHYRE2 (40), using the sequences of chains found in mass spectrometry. These were supplemented by homology models from sequences for all ODA light chain subunits. Initially models were fit into density using rigid body fitting, followed by jiggle fitting in Coot (41). For the dynein heavy chain, we could assign the shorter Dyh5 based on its shorter length. We could then distinguish between Dyh3 and Dyh4 by using side-chain density in the Dyh3 motor map (EMD-11581), which allowed us to assign and build this motor as Dyh3. This density allowed us to build sidechains for the following residues: 1490-1641; 1652-1744; 1750-2782; 2814-2972; 2980-3045; 3057-3114; 3434-3553; 3624-3674; 3739-3836; 3841-3852; 3907-3922; 3934-4170; 4189-4225; 4275-4305; 4316-4486; 4495-4513; 4520-4545; 4547-4563; 4573-4620. An extended Dyh5 region map containing the complete Dyh5 motor region and parts of the interacting Dyh3 motor allowed us to model the stalks for these two motors. For the LC tower, side-chain density allowed us to confidently assign four of the LC8-like light chains (W7XJB1_Lc8, Q24CE5_Lc8d, Q24DI9_Lc8e, Q22R86 (named Lc8f), and the Tctex-like light chains (A4VEB3_Lc9 and Q1HGH8_Lc2a). The roadblock light-chains Lc7 and Lc7b were tentatively assigned based on their differing C-terminal sequence lengths. The final two LC8-like chains identified in the MS data were assigned to the remaining positions based on their N-terminal sequence lengths. Models in higher resolution density were manually refined into maps using Coot (EMD-11579, EMD-11581), rebuilding regions when necessary. Side-chain resolution density also allowed us to distinguish the N-termini of the two intermediate chains, Dic2 and Dic3. The orientation of the two N-termini allowed us to distinguish the IC WD40 domains. We then fit homology models of these two domains into density. We could also assign the registry of Dyh3 and Dyh4 in the tail regions that contacts Shulin (EMD-11580).
For Shulin, PHYRE2 initially generated two models. The N-terminus residues (21-500 aligned with c5ce6A) were predicted to adopt a fold similar to Spt16 from the FACT complex, whilst the residues (726-1104 aligned with c1nijA) were predicted to fold similar to the GTPase YjiA. These two domains were fit into EMD-11579 in Coot (41). We then rebuilt the majority of both domains into our map and built the middle domain of Shulin de novo. The C terminal finger was initially fit into lower resolution density in EMD-11579. We then built the fold at the tip of the C3 finger, where it contacts the Dyh3 motor domain, into higher resolution density in EMD-11581. Density for nucleotide between the C1 and C2 domains was fitted with a GTP analogue from PDB 2HF8, changed to GTP and manually refined in Coot. Once all the subunits were modeled or built, the structure was split, with subunits refined against the highest resolution maps using PHENIX (42) and REFMAC5 (43) (table S4). Regions were refined until their model validation statistics, calculated using PHENIX, no longer improved. In other regions, homology models were docked into density (EMD-11577, EMD-11578, EMD-11582, EMD-11583, EMD-11584) then refined using PHENIX, including secondary structure restraints. For all three motor domains, microtubule binding domains were tentatively placed based on the angles and registries of the stalks. All regions were then re-assembled into one model, with boundaries refined using PHENIX. For the overall model, all side chains were removed for deposition. Furthermore, we represent the N terminal region of Dyh3 (amino acids 1-579; 618-642) and Dyh4 (amino acids 1-424) as Cα-trace only, as the homology model did not match the low-resolution density in this region.
Mass spectrometry
Protein identification by mass-spectrometry was done either using in-gel or in-solution tryptic digestion. Three separate types of MS analyses were performed. 1) For identifying novel interactors of cell body ODAs, IC3-ZZ-FLAG pulldowns were performed on deciliated cells (as described above) in quadruplicates. Eluates were run on SDS-PAGE gels, Coomassie stained and excised gel slices were analysed by mass spectrometry. 2) For identifying proteins in the cell body ODA peak fraction following pulldowns and gel filtration, samples were resolved on SDS-PAGE and stained with SYPRO ruby protein gel stain (Bio-Rad). Polyacrylamide gel slices containing the bands for ODA holocomplex and bound factors were prepared for mass spectrometric analysis. 3) For precisely identifying subunit composition of ODAs in cilia, IP/MS experiments as above were performed on the isolated ciliary fraction of the IC3-ZZ-FLAG strains. Additionally, MonoQ fractions containing ODAs purified from cilia of wildtype strains (as described above) were tryptically digested in-solution for mass spectrometric analysis. Both these latter mass spectrometry experiments identified the same subunit composition for ODAs.
For polyacrylamide gel slices (1-2 mm) containing the purified proteins were prepared for mass spectrometric analysis by manual in situ enzymatic digestion. Briefly, the excised protein gel pieces were placed in a well of a 96-well microtitre plate and destained with 50% v/v acetonitrile and 50 mM ammonium bicarbonate, reduced with 10 mM DTT, and alkylated with 55 mM iodoacetamide. After alkylation, proteins were digested with 6 ng/μL Trypsin (Promega, UK) overnight at 37 °C. The resulting peptides were extracted in 2% v/v formic acid, 2% v/v acetonitrile. Protein samples in solution were reduced with 10 mM DTT and alkylated with 50 mM iodoacetamide. Following alkylation, the proteins were digested with trypsin (Promega, UK) at an enzyme-to-substrate ratio of 1:100, for 1 hour at room temperature and then further digested overnight at 37 °C following a subsequent addition of trypsin at a ratio of 1:20. The digests were analysed by nano-scale capillary LC-MS/MS using a Ultimate U3000 HPLC (ThermoScientific Dionex, San Jose, USA) to deliver a flow of approximately 300 nL/min. A C18 Acclaim PepMap100 5 μm, 100 μm x 20 mm nanoViper (ThermoScientific Dionex, San Jose, USA), trapped the peptides prior to separation on a C18 Acclaim PepMap100 3 μm, 75 μm x 150 mm nanoViper (ThermoScientific Dionex, San Jose, USA). Peptides were eluted with a gradient of acetonitrile. The analytical column outlet was directly interfaced via a modified nano-flow electrospray ionization source, with a hybrid dual pressure linear ion trap mass spectrometer (Orbitrap Velos, ThermoScientific, San Jose, USA). Data dependent analysis was carried out, using a resolution of 30,000 for the full MS spectrum, followed by ten MS/MS spectra in the linear ion trap. MS spectra were collected over a m/z range of 300–2000. MS/MS scans were collected using a threshold energy of 35 for collision induced dissociation. LC-MS/MS data were then searched against an in-house protein sequence database, containing Swiss-Prot and the protein constructs specific to the experiment, using the Mascot search engine program (Matrix Science, UK) (44).
Database search parameters were set with a precursor tolerance of 5 ppm and a fragment ion mass tolerance of 0.8 Da. Two missed enzyme cleavages were allowed and variable modifications for oxidized methionine, carbamidomethyl cysteine, pyroglutamic acid, phosphorylated serine, threonine, tyrosine, tert-butyloxycarbonyl-lysine, norbornene-lysine and prop-2-yn-1-yloxycarbonyl-lysine were included. MS/MS data were validated using the Scaffold program (Proteome Software Inc., USA) (45). All data were additionally interrogated manually. For quantitative analysis of replicate runs, NSAF (Normalized Spectral Abundance Factor) values for each protein hit were used as a proxy of protein abundance (46). NSAF values were used to calculate significance and fold-changes (i.e. consistent enrichment of a given protein in test sample over control sample).
Cross-linking Mass Spectrometry (CLMS) analysis
The precipitated protein sample was resolubilized in digestion buffer (8M urea in 100 mM ammonium bicarbonate) to an estimated protein concentration of 1 mg/ml. Dissolved protein sample was reduced by addition of Dithiothreitol (DTT) to a final concentration of 5 mM. Reduction was carried out at room temperature for 30 minutes. The free sulfhydryl groups in the sample were then alkylated by adding iodoacetamide to a final concentration of 15 mM. Alkylation was incubated at room temperature for 20 minutes in dark. After alkylation, excess of iodoacetamide was quenched by addition of DTT to a total centration of 10 mM. Next, the protein sample was digested with LysC (at a 50:1 (m/m) protein to protease ratio) at room temperature for six hours. The sample was then diluted with 100 mM ammonium bicarbonate to reach a urea concentration of 1.5 M. Trypsin was added at a 50:1 (m/m) protein to protease ratio to further digest proteins overnight (~15 hours) at room temperature. Resulting peptides were desalted using C18 StageTips (47).
The resulting peptides were fractionated using size exclusion chromatography in order to enrich for crosslinked peptides (48). Peptides were separated using a Superdex 30 Increase 3.2/300 column (GE Healthcare) at a flow rate of 10 μl/minute. The mobile phase consisted of 30% (v/v) acetonitrile and 0.1% trifluoroacetic acid. The earliest seven peptide-containing fractions (50 μl each) were collected. Solvent was removed using a vacuum concentrator. The fractions were then analysed by LC-MS/MS.
LC-MS/MS analysis was performed using an Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Fisher Scientific), connected to an Ultimate 3000 RSLCnano system (Dionex, Thermo Fisher Scientific). Each size exclusion chromatography fraction was resuspended in 1.6% v/v acetonitrile 0.1% v/v formic acid. For each fraction, four AmU (at 214 nm) equivalent amount of material were injected for a LC-MS/MS acquisition. When there is enough material in a fraction, a replicate LC-MS/MS acquisition was carried out. Peptides were injected onto a 50-centimetre EASY-Spray C18 LC column (Thermo Scientific) that is operated at 50 °C column temperature. Mobile phase A consists of water, 0.1% v/v formic acid and mobile phase B consists of 80% v/v acetonitrile and 0.1% v/v formic acid. Peptides were loaded and separated at a flowrate of 0.3 μl/min. Peptides were separated by applying a gradient ranging from 2% to 55% B over 92.5 minutes. The gradient was optimized for each fraction. Following the separating gradient, the content of B was ramped to 55% and 95% within 2.5 minutes each. Eluted peptides were ionized by an EASY-Spray source (Thermo Scientific) and introduced directly into the mass spectrometer.
The MS data is acquired in the data-dependent mode with the top-speed option. For each 2.5 s acquisition cycle, the full scan mass spectrum was recorded in the Orbitrap with a resolution of 120,000. The ions with a charge state from 3+ to 7+ were isolated and fragmented using higher-energy collisional dissociation (HCD). For each isolated precursor, a stepped collision energy (26%, 28% or 30%) was applied. 4+ or higher charged precursors were prioritized over 3+ precursors in selection for fragmentation. The fragmentation spectra were then recorded in the Orbitrap with a resolution of 60,000. Dynamic exclusion was enabled with single repeat count and 60 s exclusion duration.
MS2 peak lists were generated from the raw mass spectrometric data files using the MSConvert module in ProteoWizard (version 3.0.11729). The default parameters were applied. Precursor and fragment m/z values were recalibrated. Identification of crosslinked peptides was carried out using xiSEARCH software (https://www.rappsilberlab.org/software/xisearch) (version 1.7.6.1) (49). The peak lists from all LC-MS/MS acquisitions were searched against the sequence and the reversed sequence of ODA-MS1 subunits. The following parameters were applied for the search: MS accuracy = 3 ppm; MS2 accuracy = 5 ppm; enzyme = trypsin (with full tryptic specificity); allowed number of missed cleavages = 2; missing monoisotopic peak = 2; cross-linker = BS3 (the reaction specificity for BS3 was assumed to be for lysine, serine, threonine, tyrosine and protein N termini); fixed modifications = carbamidomethylation on cysteine; variable modifications = oxidation on methionine and BS3 modification on lysine, serine, threonine, tyrosine and protein N termini with the NHS ester on the other side hydrolyzed or amidated.. Identified crosslinked peptide candidates were filtered using xiFDR (50). A false discovery rate of 2% on residue-pair level was applied with “boost between” option selected.
Conservation analysis of Shulin contact sites
Dyh3 homologues were retrieved using BLASTp, and were curated to remove duplicate sequences. Tetrahymena dyneins were retrieved from the TGD. Sequences were aligned using MUSCLE (51). Aligned Dyh3, or Tetrahymena dynein sequences were submitted to the CONSURF server (52), along with the PDB structure, in order to plot residue conservation on the structure. For visualisation of the conservation of solvent-facing residues, CONSURF scores were plotted onto the Dyh3 structure using a 15 Å surface rendering of the model in ChimeraX.
Bioinformatics
Predicted homology models were generated using Phyre2 (40). For ortholog searches and sequence alignments PSI-BLAST and align tools embedded in UniProt, NCBI, ENSEMBL and Tetrahymena Genome Databases were used. Sequence alignments were visualized using ESPript (53).
Image processing and structure representation tools
FIJI (28) used for all phenotyping and gliding assays. RELION-3.1 (54) was used for all EM data processing. Cryo-EM maps were resampled using EMDA (https://www2.mrc-lmb.cam.ac.uk/groups/murshudov/content/emda/emda.html). Chimera (39) and ChimeraX (55) used for model fitting and density map figure making.
Statistical tests and data representation
Usage of one-way ANOVA with Tukey’s test for multiple comparisons is indicated wherever applied and used for parametric data. A Kruskal Wallis test was used for non-parametric data. High speed videos of cells to visualize cilia movement were composited using the kapwing online tool. All graphical representations were generated using GraphPad Prism 7.
Supplementary Material
One Sentence Summary.
Outer dynein arm motors are shutoff by Shulin which locks them closed and facilitates their targeting to cilia.
Acknowledgments
We thank B. Santhanam for help with comparative genomic analyses. We acknowledge, J. Grimmett and T. Darling for scientific computing support and the MRC LMB EM Facility (G. Sharov, G. Cannone) for microscopy data acquisition time and support. We also acknowledge Diamond Light Source for access and support of the cryo-EM facilities at the UK’s national Electron Bio-imaging Centre (eBIC: proposal bi23268), funded by the Wellcome Trust, MRC and BBRSC. We thank K. Nguyen and V. Chandrasekaran for microscopy time, F. Coscia for graphene oxide grid assistance, F. O’Reilly for guidance optimising crosslinking, S. Bullock and members of the Carter lab for comments on the manuscript, S. Chaaban for sharing custom randomization and angular-distribution representation scripts, S. Chaaban, A. Fellows and G. Manigrasso for double-blind counting of ODA conformations, C. Stone for help with figures and S. Utekar for suggesting the name Shulin.
Funding sources
Medical Research Council, UK (MRC_UP_A025_1011) and Wellcome Trust (WT210711) to A.P.C., (218653/Z/19/Z) to F.A.A. and (103139) to J.R, Deutsche 5 Forschungsgemeinschaft project no. 426290502.
Footnotes
Author contributions G. R. M. performed all cell biological, biochemical and with F. A. A. negative stain EM analyses. G. R. M. prepared ODA-Q22MS1 BS3 crosslinking samples and ODA-Shulin cryo-EM samples. C. K. L collected all cryo-EM datasets and performed conservation analysis for ODA-Shulin contacts. G. R. M. determined an initial cryo-EM structure. F. A. A. with guidance from C. K. L. determined the higher resolution cryo-EM structures. A. P. C., C. K. L. and F. A. A. built and refined models. J. D. H. and J. B. performed cilia beat frequency analysis. M. S. and F. B. performed mass spectrometry. J. R. and Z. A. C processed ODA-Q22MS1 crosslinked samples by mass spectrometry and analysed the data. A. P. C. and G. R. M. conceived the project. A. P. C. guided the project. A. P. C., G. R. M., C. K. L. and F. A. A. wrote the manuscript with help from all authors.
Competing interests The authors declare no competing interests.
Data and materials availability
Mass spectrometry data has been deposited to ProteomeXchange (PRIDE) database under accession code PXD022396. Crosslinking mass spectrometry data has been deposited via the ProteomeXchange (JPOST) database under accession code PXD022936. Atomic coordinates and cryo-EM maps have been deposited in the Protein Data Bank under accession codes 6ZYW (Overall structure), 6ZYX (Shulin region), 6ZYY (Dyh3 motor region) and in the Electron Microscopy Data Bank under accession codes EMD-11576 (Full-length), EMD-11577 (Tail), EMD-11578 (Ordered tail), EMD-11579 (Shulin region), EMD-11580 (Extended Shulin region), EMD-11581 (Dyh3 motor region), EMD-11582 (Dyh4 motor), EMD-11583 (Dyh5 motor), EMD-11584 (Extended Dyh5 motor).
References and notes
- 1.Drummond IA. Cilia functions in development. Curr Opin Cell Biol. 2012;24:24–30. doi: 10.1016/j.ceb.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodenough UW, Heuser JE. Outer and inner dynein arms of cilia and flagella. Cell. 1985;41:341–342. doi: 10.1016/s0092-8674(85)80003-9. [DOI] [PubMed] [Google Scholar]
- 3.Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell SD, Davis SD, Knowles MR, Zariwala Ma. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med. 2009;11:473–487. doi: 10.1097/GIM.0b013e3181a53562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King SM. Axonemal dynein arms. Cold Spring Harb Perspect Biol. 2016;8:a028100. doi: 10.1101/cshperspect.a028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowkes ME, Mitchell DR. The Role of Preassembled Cytoplasmic Complexes in Assembly of Flagellar Dynein Subunits. Mol Biol Cell. 1998;9:2337–2347. doi: 10.1091/mbc.9.9.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed NT, Gao C, Lucker BF, Cole DG, Mitchell DR. ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. J Cell Biol. 2008;183:313–322. doi: 10.1083/jcb.200802025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai J, Barbieri F, Mitchell DR, Lechtreck KF. In vivo analysis of outer arm dynein transport reveals cargo-specific intraflagellar transport properties. Mol Biol Cell. 2018;29:2553–2565. doi: 10.1091/mbc.E18-05-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbaum JL, Carlson K. Cilia regeneration in Tetrahymena and its inhibition by colchicine. J Cell Biol. 1969;40:415–425. doi: 10.1083/jcb.40.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams NE, Tsao CC, Bowen J, Hehman GL, Williams RJ, Frankel J. The actin gene ACT1 is required for phagocytosis, motility, and cell separation of Tetrahymena thermophila. Eukaryot Cell. 2006;5:555–567. doi: 10.1128/EC.5.3.555-567.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood CR, Hard R, Hennessey TM. Targeted gene disruption of dynein heavy chain 7 of Tetrahymena thermophila results in altered ciliary waveform and reduced swim speed. J Cell Sci. 2007;120:3075–3085. doi: 10.1242/jcs.007369. [DOI] [PubMed] [Google Scholar]
- 11.Attwell GJ, Bricker CS, Schwandt A, Gorovsky MA, Pennock DG. A Temperature-Sensitive Mutation Affecting Synthesis of Outer Arm Dyneins in Tetrahymena thermophila. J Protozool. 1992;39:261–266. doi: 10.1111/j.1550-7408.1992.tb01312.x. [DOI] [PubMed] [Google Scholar]
- 12.Porter ME, Johnson KA. Characterization of the ATP-sensitive binding of Tetrahymena 30 S dynein to bovine brain microtubules. J Biol Chem. 1983;258:6575–81. [PubMed] [Google Scholar]
- 13.Goodenough U, Heuser J. Structural comparison of purified dynein proteins with in situ dynein arms. J Mol Biol. 1984;180:1083–1118. doi: 10.1016/0022-2836(84)90272-9. [DOI] [PubMed] [Google Scholar]
- 14.Wickstead B, Gull K. Dyneins across eukaryotes: A comparative genomic analysis. Traffic. 2007;8:1708–1721. doi: 10.1111/j.1600-0854.2007.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K, Foster HE, Rondelet A, Lacey SE, Bahi-Buisson N, Bird AW, Carter AP. Cryo-EM Reveals How Human Cytoplasmic Dynein Is Auto-inhibited and Activated. Cell. 2017;169:1303–1314. doi: 10.1016/j.cell.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toropova K, Zalyte R, Mukhopadhyay AG, Mladenov M, Carter AP, Roberts AJ. Structure of the dynein-2 complex and its assembly with intraflagellar transport trains. Nat Struct Mol Biol. 2019;26:823–829. doi: 10.1038/s41594-019-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakakibara H, Mitchell DR, Kamiya R. A Chlamydomonas outer arm dynein mutant missing the α heavy chain. J Cell Biol. 1991;113:615–622. doi: 10.1083/jcb.113.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt H, Zalyte R, Urnavicius L, Carter AP. Structure of human cytoplasmic dynein-2 primed for its power stroke. Nature. 2015;518:435–438. doi: 10.1038/nature14023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J, Nicastro D. Asymmetric distribution and spatial switching of dynein activity generates ciliary motility. Science (80-) 2018;360 doi: 10.1126/science.aar1968. aar1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubo S, Yang SK, Black C, Dai D, Valente M, Gaertig J, Ichikawa M, Bui KH. bioRxiv. doi: 10.1101/2020.11.30.404319. in press. [DOI] [Google Scholar]
- 21.Liu Y, Zhou K, Zhang N, Wei H, Tan YZ, Zhang Z, Carragher B, Potter CS, D’Arcy S, Luger K. FACT caught in the act of manipulating the nucleosome. Nature. 2020;577:426–431. doi: 10.1038/s41586-019-1820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sydor AM, Jost M, Ryan KS, Turo KE, Douglas CD, Drennan CL, Zamble DB. Metal binding properties of escherichia coli YjiA, a member of the metal homeostasis-associated COG0523 family of GTPases. Biochemistry. 2013;52:1788–1801. doi: 10.1021/bi301600z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee C, Cox R, Papoulas O, Horani A, Drew K, Devitt C, Brody SL, Marcotte EM, Wallingford JB. Functional partitioning of a liquid-like organelle during assembly of axonemal dyneins. bioRxiv Cell Biol. 2020 doi: 10.1101/2020.04.21.052837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright KJ, Baye LM, Olivier-Mason A, Mukhopadhyay S, Sang L, Kwong M, Wang W, Pretorius PR, Sheffield VC, Sengupta P, Slusarski DC, et al. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev. 2011;25:2347–2360. doi: 10.1101/gad.173443.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alkanderi S, Molinari E, Shaheen R, Elmaghloob Y, Stephen LA, Sammut V, Ramsbottom SA, Srivastava S, Cairns G, Edwards N, Rice SJ, et al. ARL3 Mutations Cause Joubert Syndrome by Disrupting Ciliary Protein Composition. Am J Hum Genet. 2018;103:612–620. doi: 10.1016/j.ajhg.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuvillier A, Redon F, Antoine JC, Chardin P, DeVos T, Merlin G. LdARL-3A, a Leishmania promastigote-specific ADP-ribosylation factor-like protein, is essential for flagellum integrity. J Cell Sci. 2000;113:2065–2074. doi: 10.1242/jcs.113.11.2065. [DOI] [PubMed] [Google Scholar]
- 27.Chalker DL. Transformation and Strain Engineering of Tetrahymena. Methods Cell Biol. 2012;109:327–345. doi: 10.1016/B978-0-12-385967-9.00011-6. [DOI] [PubMed] [Google Scholar]
- 28.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meijering E, Dzyubachyk O, Smal I. Methods for cell and particle tracking. Methods Enzymol. 2012;504:183–200. doi: 10.1016/B978-0-12-391857-4.00009-4. [DOI] [PubMed] [Google Scholar]
- 30.Thévenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- 31.Smith CM, Djakow J, Free RC, Djakow P, Lonnen R, Williams G, Pohunek P, Hirst RA, Easton AJ, Andrew PW, O’Callaghan C. CiliaFA: A research tool for automated, high-throughput measurement of ciliary beat frequency using freely available software. Cilia. 2012;1:14. doi: 10.1186/2046-2530-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vale RD, Yano Toyoshima Y. Rotation and translocation of microtubules in vitro induced by dyneins from Tetrahymena cilia. Cell. 1988;52:459–469. doi: 10.1016/s0092-8674(88)80038-2. [DOI] [PubMed] [Google Scholar]
- 33.Schlager MA, Hoang HT, Urnavicius L, Bullock SL, Carter AP. In vitro reconstitution of a highly processive recombinant human dynein complex. EMBO J. 2014;33:1855–1868. doi: 10.15252/embj.201488792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheres SHW. RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol. 2012;180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pantelic RS, Meyer JC, Kaiser U, Baumeister W, Plitzko JM. Graphene oxide: A substrate for optimizing preparations of frozen-hydrated samples. J Struct Biol. 2010;170:152–156. doi: 10.1016/j.jsb.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Zivanov J, Nakane T, Scheres SHW. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ. 2019;6:5–17. doi: 10.1107/S205225251801463X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang K. Gctf: Real-time CTF determination and correction. J Struct Biol. 2016;193:1–12. doi: 10.1016/j.jsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohou A, Grigorieff N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J Struct Biol. 2015;192:216–221. doi: 10.1016/j.jsb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera - A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 40.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr Sect D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr Sect D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr Sect D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 44.Perkins DN, Pappin DJC, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 46.Florens L, Carozza MJ, Swanson SK, Fournier M, Coleman MK, Workman JL, Washburn MP. Analyzing chromatin remodeling complexes using shotgun proteomics and normalized spectral abundance factors. Methods. 2006;40:303–311. doi: 10.1016/j.ymeth.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc. 2007;2 doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- 48.Leitner A, Aebersold R. XSnapShot: Mass spectrometry for protein and proteome analyses. Cell. 2013:252–252. doi: 10.1016/j.cell.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 49.Mendes ML, Fischer L, Chen ZA, Barbon M, O’Reilly FJ, Giese SH, Bohlke-Schneider M, Belsom A, Dau T, Combe CW, Graham M, et al. An integrated workflow for crosslinking mass spectrometry. Mol Syst Biol. 2019;15:e8994. doi: 10.15252/msb.20198994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer L, Rappsilber J. Quirks of Error Estimation in Cross-Linking/Mass Spectrometry. Anal Chem. 2017;89:3829–3833. doi: 10.1021/acs.analchem.6b03745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashkenazy H, Abadi S, Martz E, Chay O, Mayrose I, Pupko T, Ben-Tal N. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016;44:W344–50. doi: 10.1093/nar/gkw408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gouet P, Courcelle E, Stuart DI, Métoz F. ESPript: Analysis of multiple sequence alignments in PostScript. Bioinformatics. 1999;15:305–308. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]
- 54.Zivanov J, Nakane T, Scheres SHW. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ. 2020;7:253–267. doi: 10.1107/S2052252520000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goddard TD, Huang CC, Meng EC, Pettersen EF, Couch GS, Morris JH, Ferrin TE. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2018;27:14–25. doi: 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilkes DE, Rajagopalan V, Chan CWC, Kniazeva E, Wiedeman AE, Asai DJ. Dynein light chain family in Tetrahymena thermophila. Cell Motil Cytoskeleton. 2007;64:82–96. doi: 10.1002/cm.20165. [DOI] [PubMed] [Google Scholar]
- 57.Penque D, Galego L, Rodrigues-Pousada C. Multiple α-tubulin isoforms in cilia and cytoskeleton of Tetrahymena pyriformis generated by post-translational modifications: Studies during reciliation. Eur J Biochem. 1991;195:487–494. doi: 10.1111/j.1432-1033.1991.tb15729.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Mass spectrometry data has been deposited to ProteomeXchange (PRIDE) database under accession code PXD022396. Crosslinking mass spectrometry data has been deposited via the ProteomeXchange (JPOST) database under accession code PXD022936. Atomic coordinates and cryo-EM maps have been deposited in the Protein Data Bank under accession codes 6ZYW (Overall structure), 6ZYX (Shulin region), 6ZYY (Dyh3 motor region) and in the Electron Microscopy Data Bank under accession codes EMD-11576 (Full-length), EMD-11577 (Tail), EMD-11578 (Ordered tail), EMD-11579 (Shulin region), EMD-11580 (Extended Shulin region), EMD-11581 (Dyh3 motor region), EMD-11582 (Dyh4 motor), EMD-11583 (Dyh5 motor), EMD-11584 (Extended Dyh5 motor).