Abstract
■ The afferent branch of the autonomic nervous system contributes with interoception to the multimodal sensory correlation continuously needed to update our representation of the body. To test whether the modulation of body representation would have an impact on the efferent branch of the autonomic nervous system, nonspecific skin conductance has been measured in three rubber hand illusion (RHI) experiments, controlled with asynchronous brush-stroking and incongruent fake hand position. Nonspecific skin conductance standard deviation (SCSD) computed along the whole 90 sec of stroking was found to be increased by the illusion and to correlate with all the typical measures of embodiment. Computing SCSD in shorter time windows strongly enhanced the difference between illusion and controls. The highest difference was found in the 10–55 sec window, being the 14–34 sec window as the most informative one. The higher correlations with the validated measures of embodiment (all but the proprioceptive drift) were found for time windows ranging between 35 and 65 sec. The SCSD was no longer significantly higher when the RHI was repeated twice (two trials each iteration), but it was still significantly higher in synchronous stroking even when considering only the second trial. However, after the first iteration of the RHI paradigm, the effect of the embodiment on nonspecific skin conductance response results to be attenuated, suggesting that novelty in presentation of the RHI can contribute to the effect on nonspecific skin conductance response. Results candidate SCSD as a noninvasive, cheap, easy, and objective measure of embodiment, especially sensible to onset and strength of the illusion. Alike the already known enhanced autonomic reaction to a threatening, SCSD does not interfere with the collection of other behavioral measures. Correlations and their dynamics, presence of the effect in the second presentation of the setup but relative low robustness against multiple repetition, suggest that the increased fluctuations of skin conductance caught by SCSD are not just the effect of different presented sensory stimuli but more likely a stronger arousal response to the novelty of the updated perceptual status. ■
Introduction
Different senses, such as sight and somatosensation, inform on the state of the body. Sensory information is persistently present and updated and exploited to feed a particular perceptual status to feel part of the body as self (Blanke, 2012; Gallagher, 2000), constituting a fundamental aspect of self-awareness.
The constitution of a body representation is mediated by the correlations among coherent multimodal sensory affer-ences (van den Bos & Jeannerod, 2002; Botvinick & Cohen, 1998; Rochat, 1998); for example, the self-attribution of a visible hand is highly dependent on the match between the multimodal afferent somatic signals and the corresponding visual feedback. The rubber hand illusion (RHI) is a body ownership illusion whereby congruently stroking a rubber hand and one’s hidden hand while observing the artificial limb produces the illusion that the rubber hand is part of one’s body (Botvinick & Cohen, 1998). Such paradigm, which has been broadly employed as a tool to study the sense of body ownership and brain plasticity (Di Pino, Maravita, Zollo, Guglielmelli, & Di Lazzaro, 2014), seems to be heavily modulated by interoception (Seth, 2013).
Indeed, those who have worst ability to detect their heartbeat experience stronger RHI, suggesting that interoceptive sensitivity is predictive of the malleability of the body representation (Tsakiris, Tajadura-Jiménez, & Costantini, 2011). Moreover, the ownership of a virtual hand has been shown to be enhanced when it synchronously changed color with the participant’s heartbeat; when experiencing the ownership of a body part, exteroceptive and interoceptive multi-sensory integration matters (Suzuki, Garfinkel, Critchley, & Seth, 2013).
Thus, to experience the body as self, besides extero-ception and proprioception, the brain takes into account also interoception, that is, the state of the inner organs based on cues led by an afferent pathway of the autonomic nervous system (ANS; Tsakiris et al., 2011; Craig, 2009).
The autonomic system is structured as “afferences-CNS node-efferences” reflex loops, as it becomes particularly evident in dysautonomic disorders where it is upregulated (Reichgott, 1990).
The application of such functional organization to the process of building the body representation would suggest that manipulation of the body representation due to the modulation of interoception, as well as due to the modulation of other sensory afferences as in the RHI paradigm, should in turn affect the output of the efferent pathway of ANS.
The skin conductance (SC), a physiological measure of autonomic arousal, is the expression of the electrical conductivity of the skin, which depends on the moisture due to the activity of the sweat glands. Indeed, besides being induced by thermoregulation, secretion can be triggered also by emotional stimulation.
Event-related skin conductance response (ER-SCR) is due to a specific eliciting stimulus, such as a sudden handclap. Physical threats to an embodied artificial hand evoke enhanced ER-SCR (Armel & Ramachandran, 2003); this procedure has been widely used to study and evaluate the strength of the RHI (Tsuji et al., 2013; Yuan & Steed, 2010; Petkova & Ehrsson, 2009; Armel & Ramachandran, 2003). However, the menace-induced ER-SCR may be biased by participants’ prior experiences, which give different emotional valence to different threatening stimuli (e.g., a hammer, a knife, a screwdriver; Ma & Hommel, 2013). Moreover, the menace can induce movements of the tested hand (Kilteni, Normand, Sanchez-Vives, & Slater, 2012), which may invalidate the further collection of the proprioceptive drift.
The nonspecific skin conductance response (NS-SCR) is another information that can be inferred by the SC, that is, the fast fluctuation of the signal, not time-locked with an evoking stimulus, but due to a circumstance that has an influence on one’s general arousal (Braithwaite, Watson, Jones, & Rowe, 2013).
Considering that body representation is influenced by the ANS and that NS-SCR is a measure of the autonomic outflow, the hypothesis of this study is that changes of the body representation would affect the ANS efferent branch and can be revealed by alterations of NS-SCR. To make a long story short, the brain processes involved in the building of body representation, tested with the RHI paradigm, would be accompanied by changes of the NS-SCR.
However, despite NS-SCR being a simple, costeffective, and easy-to-use physiological measure and although it has been found to be altered during challenging stressors such as cognitive or behavioral tasks (Dawson, Schell, & Filion, 2007), it has never been employed to evaluate the embodiment of the rubber hand.
In the RHI, the effectiveness of the illusion depends upon the spatiotemporal congruency of the visuotactile stimuli. Indeed, the RHI does not occur when the rubber hand and the participant’s own hand are asynchronously stroked (Botvinick & Cohen, 1998) or when the rubber hand is not placed in a position congruent with participants’ perspective (Tsakiris & Haggard, 2005). These spatiotemporal constraints are exploited to build the two most common RHI control conditions.
To test the hypothesis that embodiment could affect NS-SCR, we employed the RHI and compared NS-SCR collected during synchronous brush-stroking stimulation with NS-SCR during asynchronous stimulation (Experiment A [ExpA]; Botvinick & Cohen, 1998), and with NS-SCR collected during synchronous brush-stroking but with the fake hand placed in a incongruent position (Experiment B [ExpB]; Tsakiris & Haggard, 2005). Additionally, to assess the persistence of the effect of the embodiment on NS-SCR across multiple presentations of the RHI paradigm, we performed two iterations of synchronous and asynchronous RHI conditions on a new naïve group of participants (Experiment C [ExpC]).
Methods
Participants
Three RHI experiments were performed: ExpA (synchronous vs. asynchronous brushstroke), ExpB (congruent vs. incongruent position), and ExpC (two iterations of synchronous vs. asynchronous brushstroke).
Thirty-seven volunteers took part to ExpA (age = 29 ± 5 years, 18 women, 33 right-handed as by self-report); 18 volunteers took part to ExpB and ExpC (ExpB: age = 26 ± 4 years, 10 women; ExpC: age = 25 ± 3 years, 11 women; all right-handed in both experiments as by selfreport). ExpA was the first to be performed and completed; ExpB was run to test the repeatability of ExpA findings with an additional control condition. ExpC was run to test the persistence of the illusion after multiple repetitions of the experimental conditions. Considered the effect size calculated on the extracted SC index from ExpA (≥0.49) and a medium statistical power of about 0.5 (two tailed test and alpha value equal to .05), we enrolled 18 participants for ExpB and ExpC.
Participants, naïve for the study, did not report to be affected by any neurological impairment and claimed to have normal hand sensation and normal or corrected-to-normal vision. Participants were enrolled after having signed a written informed consent, and experimental procedures were approved by the Ethics Committee of the Università Campus Bio-Medico di Roma (EMBODY protocol) and carried out according to the Declaration of Helsinki.
Experimental Procedure
Participants were placed in front of a custom-made experimental setup made of three parallel compartments covered by a two-way mirror. They could see the content of each compartment only when the experimenter turned on the internal light (Mioli, D’Alonzo, Pellegrino, Formica,& Di Pino, 2018).
Then, participants were invited to place their forearms inside the two lateral compartments while their shoulders were covered by a black cloak. A left rubber hand that matched the participants’ gender was placed in the central compartment of the structure 15 cm apart from the real hidden left hand of the participant. The left hand was tested because it seems the side where the RHI is easier to arise (Ocklenburg, Rüther, Peterburs, Pinnow, & Güntürkün, 2011). A well-trained experimenter used two identical paintbrushes to stroke both the second digit of the rubber hand and the corresponding digit of the real hidden hand. The tactile stimulation was delivered at a frequency of about 1 Hz, the brushstroke duration was about 0.6–0.7 sec, and it was delivered from the proximal to the distal phalanx.
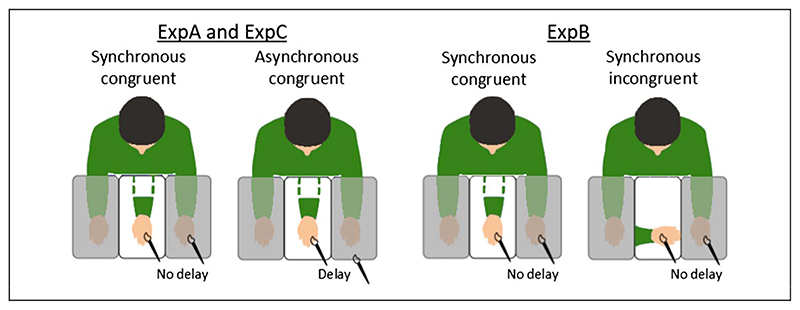
In the asynchronous control condition (ExpA and ExpC), a small temporal delay (about 0.5 sec) was added between the stimulus delivered on the rubber hand and the one delivered on the real hand, whereas in the incongruent control condition (ExpB), the rubber hand was placed at 90° with respect to the real hand’s orientation and the brushstrokes were synchronously administered (Figure 1).
Figure 1. Schematic illustration of the experimental conditions.
For each participant, in each condition, the physiological measures of SC and the electrocardiogram (ECG) were recorded during the whole period of stimulation, along with the most typical and validated measures of the illusion: the responses to the self-evaluation questionnaire and the proprioceptive drift (Botvinick & Cohen, 1998). ECG recording was added to obtain the participants’ heart rate variability (HRV), that is, another index related to autonomic reactivity, generally dependent to both branches of the ANS (Kreibig, 2010; Bradley & Lang, 2000). In such way, it has been attempted to disentangle the single contribution of the two branches of the autonomic system.
In ExpA and ExpB, before the brushstroke stimulation, the participant had to verbally report a number on a measuring tape reflected on the two-way mirror that corresponded to the perceived location of his or her left index finger by maintaining the hands still and relaxed. The measuring tape had the possibility to slide so as to select a random offset before every assessment. The experimenter turned on the light in the central compartment, making the rubber hand visible, and started to stroke the participant’s hand and the rubber hand with the paintbrushes. After this phase, the experimenter switched off the light of the central compartment and asked the participant to repeat the estimation of his or her hand’s location. Post-minus prestimulation positive differences of the estimated position of the hand indicate a drift of the perceived location of the real hand toward the rubber hand.
Subsequently, the experimenter handed to the participant a nine-item questionnaire (Botvinick & Cohen, 1998) aimed at investigating the extent of the self-attribution of the rubber hand (Table 1). The participants were asked to rate the extent to which these items did or did not apply to them, using a 7-point scale. On this scale, −3 meant “absolutely certain that it did not apply,” 0 meant “uncertain whether it applied or not,” and +3 meant “absolutely certain that it applied.” Such questionnaire was provided with two additional items to rate the vividness (0–10) of the perceived illusion (i.e., how realistic the illusion was when it was experienced) and the prevalence (0–100%), which reflected the percentage of time that the illusion was experienced (i.e., how long with respect to the length of section the perception of the illusion was).
Table 1. List of Items of the Questionnaire.
| Questionnaire | Item | Rating |
|---|---|---|
| Statement 1 | It seemed as if I were feeling the tactile stimulation at the location where I saw the visible hand touched | −3 to +3 |
| Statement 2 | It seemed as though the stimulation I felt was caused by the touch on the visible hand | |
| Statement 3 | I felt as if the visible hand was mine | |
| Statement 4 | I felt as if the position of my real hand was drifting toward the visible hand | |
| Statement 5 | It seemed as if I had more than two hand or arm | |
| Statement 6 | It seemed as if the tactile stimulation I was feeling came from somewhere between my own hand and the visible one | |
| Statement 7 | I felt as if my real hand were turning “rubbery” | |
| Statement 8 | It appeared as if the position of the visible hand was drifting toward my real hand | |
| Statement 9 | The visible hand began to resemble my own hand, in terms of shape, skin tone, freckles, or some other visual features | |
| Vividness | How realistic and life-like was the illusion that the visible hand was yours when it was experienced? | 0–10 |
| Prevalence | How long with respect to the length of section the perception of such illusion was? | 0–100% |
SC and ECG were recorded (Biopac MP160, Biopac) all along the stroking time (90 sec) with Ag-AgCl electrodes placed on the fingertips of the second and third digits of the right hand in case of SC signal and near the left (active electrode) and right (reference) midclavicular line below the clavicles and at the level of the left ankle (ground) in case of ECG. SC, which is modulated by a systemic response, was recorded from the right hand, contralateral to the tested hand, so that electrodes did not influence the testing environment.
In case of ExpA and ExpB, the order of the experimental and control conditions was randomized among participants. The overall experimental session lasted about 15 min, including the placement of electrodes, the time to fill in the questionnaires, and a 2-min break to relax between conditions. In case of ExpC, two consecutive iterations of the synchronous and asynchronous conditions were performed on the same participant (Iteration 1, Trial 1 and Trial 2, and Iteration 2, Trial 3 and Trial 4− for a total of four trials). The synchronous versus asynchronous order in both iterations was full-randomized across participants. In this case, the overall experimental session lasted about 20 min. The ExpC was performed to assess the contribution of novelty in the effect of the RHI on NS-SCR.
Data Analysis
The SC signal was band pass filtered (0.05–2 Hz) to eliminate the signal offset and the high-frequency noise (Braithwaite et al., 2013).
The standard deviation of NS-SCR was extracted from the filtered SC signal to estimate signal variability (Boucsein et al., 2012; Bach, Friston, & Dolan, 2010).
SC signal is highly affected by interindividual variability and by the circumstance on which the experiment is run (e.g., the room temperature). To minimize the impact of those factors on the data, the value of the nonspecific skin conductance standard deviation (SCSD) was normalized on each participant by dividing the individual SCSD computed on the trial of interest by the standard deviation computed on his or her entire recording session (both illusion and control condition together).
The standard deviation of the inverse of the time between two contiguous R peaks of the ECG signal has been calculated as the index of HRV for each trial and each participant.
The Kolgomorov-Smirnov test (p > .05) was used to verify that the data were normally distributed. On the basis of data distribution, the more adequate statistical test was selected for the following analysis (paired t test or Wilcoxon signed-rank test).
To verify that the results of the questionnaires were not due to participants’ suggestibility, the mean score of the three items employed to measure the illusion was compared against the mean score of the six items that served as control for compliance, suggestibility, and “placebo effect” by using a two-tailed paired t test on the basis of data distribution.
Then, the RHI index, expressed as the difference between the mean score of the illusion items and the mean score of the other ones (Abdulkarim & Ehrsson, 2016), was calculated for each condition and employed as illusion outcome in the following analyses.
All data except for the vividness and prevalence scores in ExpA and vividness scores in the second iteration of ExpC were normally distributed.
Normally distributed data were analyzed with paired t tests to highlight differences between illusion conditions (synchronous congruent one) and control conditions (asynchronous or incongruent one) both for the already validated RHI outcomes (RHI index, vividness, prevalence score, and proprioceptive drift) and for the hypothesized ones (SCSD and HRV).
Wilcoxon signed-rank test was employed to compare the illusion condition to the control for the data with a nonnormal distribution.
For normally distributed data, the effect size (d) was calculated as Cohen’s d, whereas in the other case, effect size (r) was calculated as z/√n, where z is test statistic for signed-rank test and n is the number of observations (Rosenthal, 1994).
For each participant in each condition (i.e., illusion and control conditions of both ExpA and ExpB), the SCSD calculated on the entire trial duration was correlated to the validated measures of illusion (i.e., RHI index, vividness, prevalence scores, and proprioceptive drift). The results of these correlations were indicated with ρtot.
Additionally, to identify the optimal onset and length of the time window suited to highlight differences between illusion and no illusion conditions, a series of statistical comparisons (paired t test) was performed for SCSD between illusion and control conditions (pooling together data from both ExpA and ExpB), calculated for 120 different time windows with window onset (ton) ranging between 0 and 70 sec (step of 5 sec) and window length (wl) ranging between 20 and 90 sec (step of 5 sec). The minimum value of wl (20 sec) was selected on the basis of the dynamic features proper of the SCR peaks (frequency of the NS-SCR peaks ranged between 0.05 and 0.5 Hz; Braithwaite et al., 2013). The statistical comparisons were corrected with Bonferroni adjustment. The p values obtained for the different time windows were employed as intuitive index of the difference between illusion and control condition (i.e., the lower the p value, the higher the difference). Additionally, to monitor the time behavior of SCSD with a high time resolution to identify the most informative time interval (lowest p value), a statistical comparison between illusion and control conditions was performed on the values calculated on the shortest meaningful window (20 sec length), moving in time with a 1-sec step.
The correlation analysis between the SCSD and the validated measures of illusion was also implemented for each of those 120 time windows, and the time window (onset and duration) showing the maximum correlation value (ρmax) was identified for each measure.
A frequentist approach has been used to assess whether ρmax and ρtot are significantly different and whether the ρmax and ρtot of SCSD with RHI index, vividness, prevalence scores, and proprioceptive drift are significantly different among them. The difference is considered significant if the confidence interval of the distribution computed with a percentile bootstrap does not comprise the zero value (Wilcox, 2009).
All the analyses were performed by using MATLAB software (R2015a).
Results
Experiments A and B
In the synchronous condition of both experiments, the mean value of illusion items was higher than the mean value of the ones that served as control items (d = 1.82, t(36) = 11.1,p < .001; d = 2.60, t(17) = 11.0,p < .001 for ExpA and ExpB, respectively); thus, the groups of participants were generally not suggestible.
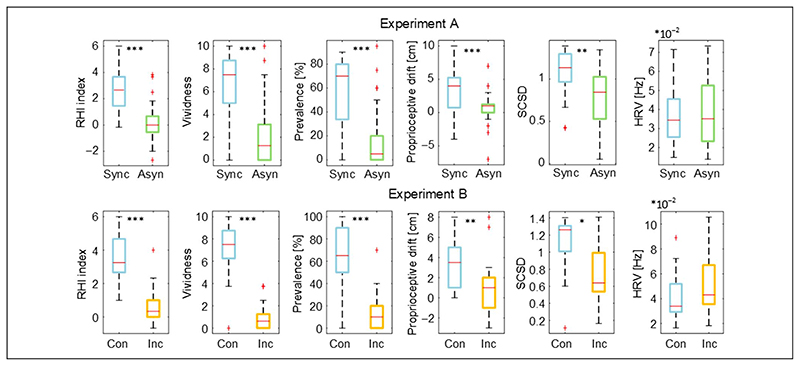
The previously validated illusion outcomes in synchronous congruent conditions were significantly higher than those relative to the asynchronous (ExpA—RHI index: d = 1.22, t(36) = 7.41, p < .001; vividness: r = .77, z = 4.70, p < .001; prevalence: r = .73, z = 4.44, p < .001; proprioceptive drift: d = 0.76, t(36) = 4.62, p < .001; Figure 2) and the incongruent conditions (ExpB—RHI index: d = 2.15, t(17) = 9.14, p < .001; vividness: d = 2.66, t(17) = 11.28, p < .001; prevalence: d = 1.54, t(17) = 6.52, p < .001; proprioceptive drift: d = 0.84, t(17) = 3.58,p = .002; Figure 2). This confirms that participants effectively experienced the RHI during both experiments.
Figure 2.
Box and whisker plots of the illusion outcomes (RHI index, vividness, prevalence rating, and proprioceptive drift), SCSD, and HRV for the synchronous congruent (ExpA and ExpB) and asynchronous congruent (ExpA) and synchronous incongruent conditions (ExpB): median (red lines), first and third quartiles (box), lowest and highest values comprised within 1.5 times the interquartile range from the 25th and 75th percentiles (whisker). *p < .05, **p < .01, ***p < .001.
In the two experiments, SCSD was significantly higher in the illusion condition compared with the control (ExpA: SCSD d = 0.52, t(36) = 3.19, p = .003; Figure 2; ExpB: SCSD d = 0.55, t(17) = 2.34, p < .032; Figure 2).
On the contrary, in the case of HRV, the difference was not significant (ExpA: d = —0.13, t(36) = 0.77, p = .44; ExpB: d = —0.45, t(17) = 1.90, p = .07; Figure 2).
The SCSD computed along the whole duration of the experimental/control session (90 sec) significantly correlated with all the traditional RHI measures (all ps < .01) with correlation coefficient in the range of .36 < ρtot < .40, except for the proprioceptive drift, which was ρtot < .30 (see Table 2). The difference between the correlation of SCSD with proprioceptive drift and the other typical measures was significant (95% confidence intervals of all three bootstrapping distributions were comprised between −0.04 and −0.18). This shows that the correlation between SCSD and proprioceptive drift was significantly lower than the correlations of SCSD with the other embodiment measures.
Table 2. Summary of the Data Relatives to the Correlation Analysis between SCSD and Illusion Outcomes Obtained for Different Time windows and Represented in Figure 4 .
| ρtot | P | ρmax | P | ton(ρmax) | wl(ρmax) | |
|---|---|---|---|---|---|---|
| RHI index | .38 | <.001 | .46 | <.001 | 5 | 50 |
| Vividness | .39 | <.001 | .44 | <.001 | 20 | 35 |
| Prevalence | .37 | <.001 | .41 | <.001 | 5 | 55 |
| Prop. drift | .26 | .006 | .27 | .004 | 0 | 65 |
ρtot indicates the correlation coefficient between the illusion outcomes and the SCSD extracted from the entire length of the trial (t on = 0 sec, wl = 90 sec). The coefficients were calculated pooling together each trial of ExpA (synchronous and asynchronous conditions) and ExpB (congruent and incongruent conditions). t on(ρmax) and wl(ρmax) are the window onset and length relative to time windows with the maximum correlation value (ρmax), respectively.
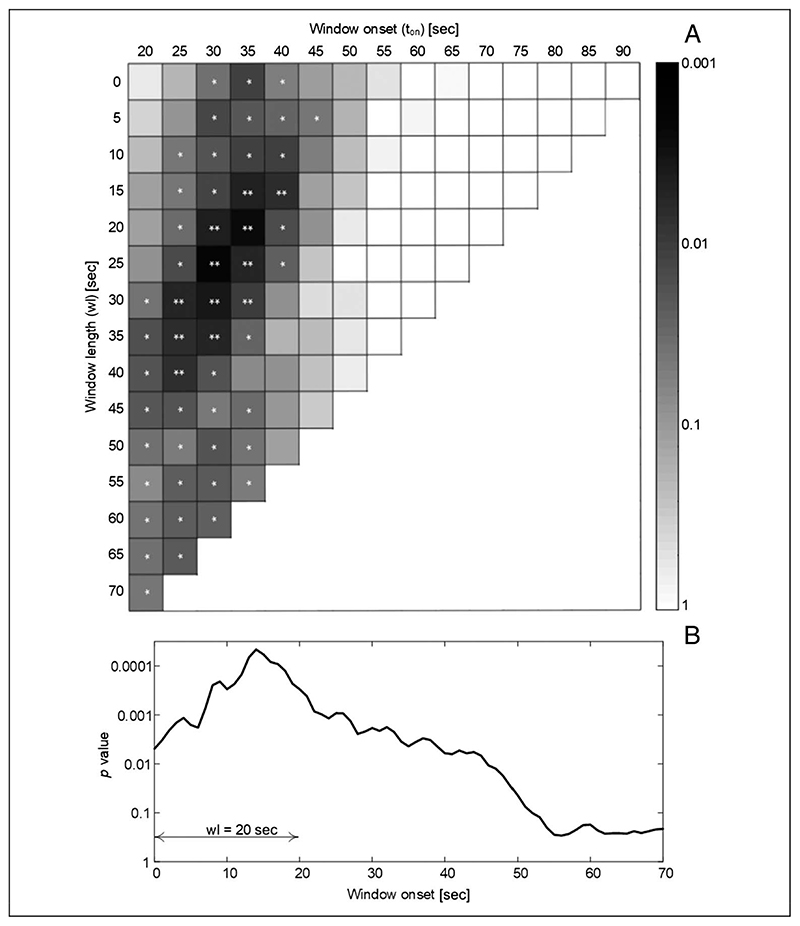
As regard the comparison of SCSD between illusion and control conditions computed for time windows with different lengths, the lowest Bonferroni-corrected p value was obtained for the time window with onset equal to 10 sec and wl equal to 45 sec (Bonferroni-corrected p value equal to .002; Figure 3). In general, the time windows that in Figure 3 are represented by the tiles close to the one with 10-sec onset and 45-sec length showed a Bonferroni-corrected p value of < .01.
Figure 3.
(A) Graphic representation of the results of the SCSD comparison analysis between illusion and control conditions (pulled together) for each time window with onset (t on plotted on x axis) and duration (wl plotted on the y axis). Darker color corresponds to lower Bonferroni-corrected p value. *p < .05, **p < .01. (B) The SCSD comparison analysis between illusion and control conditions calculated on 20-sec moving window with 1-sec steps, the p values are plotted with respect to the onset of the moving window (p values were not corrected because our interest was on their behavior in time).
The differences between illusion and control conditions were statistically significant for all the analyzed windows except for the windows (i) with early onset (≤5 sec) and short lengths, (ii) with 20 sec onset and long lengths, and (iii) with onset later than 20 sec. In particular, focusing on the analysis of the SCSD calculated with 20 sec moving window with 1-sec steps, it is possible to note that the p values (not corrected) lower than .001 were obtained for 20-sec time windows with onset between 7 and 26 sec, and the lowest p value (most informative time interval) was found for the window ranging from 14 to 34 sec (Figure 3B).
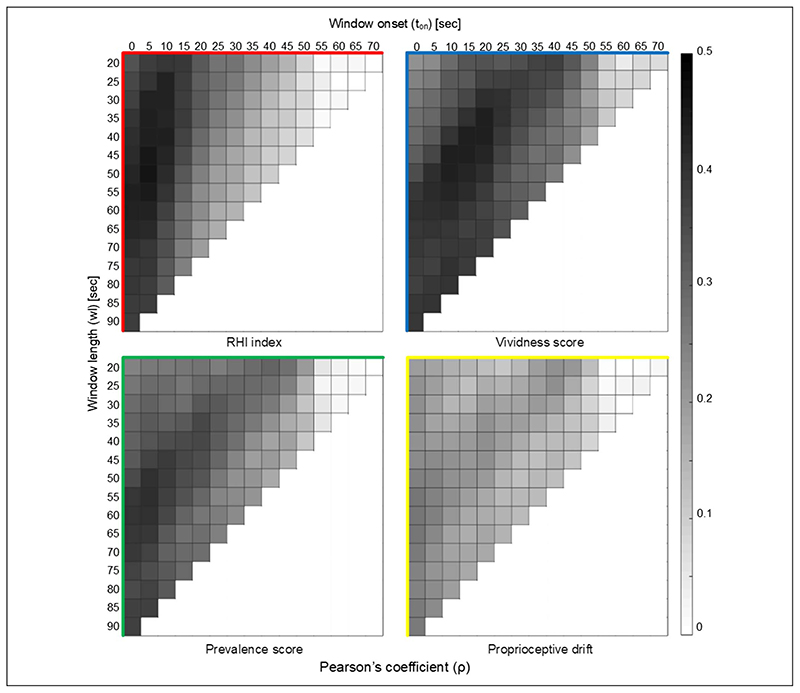
SCSD correlation analysis computed for time windows with different lengths showed that higher correlation coefficients were obtained for time windows shorter than the whole duration of the trial.
In the correlation between SCSD and the measures of embodiment, the maximum correlation coefficients (ρmax equal to .44 and .46, all ps < .001) and the highest differences between the maximum correlation coefficients and those calculated on the whole duration of the trial (ρmax minus ρtot equal to .05 and .08) were obtained for the correlation of SCSD with vividness score and with RHI index, respectively (Table 2). In particular, the correlation coefficient between RHI index and SCSD calculated in the entire 90-sec window (ρtot) was significantly lower than the correlation with SCSD calculated on a window interval comprised between 5 and 55 sec (95% confidence interval [0.02, 0.11]).
Windows with early onset (ranged between 0 and 20 sec) showed higher correlation coefficients, whereas windows with onset later than 50 sec had all correlation coefficients lower than 0.1 (p > .05; Figure 4).
Figure 4.
Graphic representation of the results of correlation analysis between the SCSD and each illusion outcome: RHI index (red graph), vividness score (blue graph), prevalence score (green graph), and proprioceptive drift (yellow graph). The SC standard deviation was calculated for each time window with onset (t on plotted on x axis) and duration (wl plotted on the y axis). The correlation values are summarized in Table 2. Darker color corresponds to higher correlation value.
Experiment C
In the synchronous conditions of both iterations of this experiment, the mean value of illusion items of the questionnaire was higher than the mean value of the ones that served as control items (d = 2.11, t(17) = 8.71,p < .001; d = 2.11, t(17) = 8.70, p < .001 for Iteration 1 and Iteration 2, respectively); thus, participants were generally not suggestible.
The validated illusion outcomes in the synchronous conditions were significantly higher than those relative to the asynchronous conditions in both iterations (Iteration 1—RHI index: d = 1.73, t(17) = 7.33, p < .001; vividness: d = 1.63, t(17) = 5.60, p < .001; prevalence: d = 1.12, t(17) = 4.73, p < .001; Iteration 2—RHI index: d = 1.66, t(17) = 6.92, p < .001; vividness: r = .84, z = 3.47, p < .001; prevalence: d = 1.48, t(17) = 6.30, p < .001; Figure 5). This confirms that participants effectively experienced the RHI during the synchronous conditions of both the first and the second iterations.
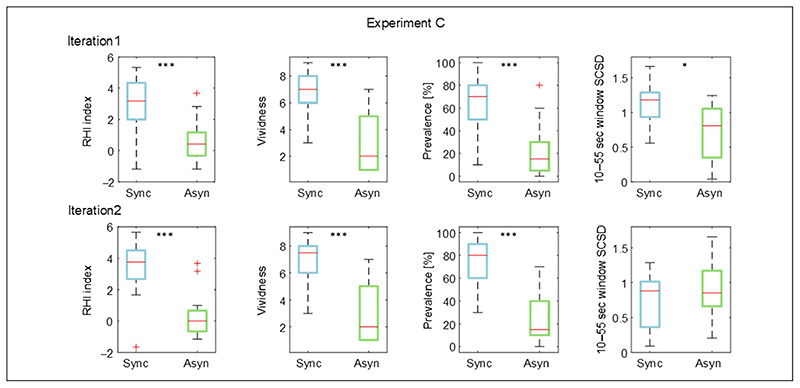
Figure 5.
Box and whisker plots of the illusion outcomes (RHI index, vividness, and prevalence rating) and SCSD for the synchronous and asynchronous congruent conditions for both iterations of ExpC: median (red lines), first and third quartiles (box), lowest and highest values comprised within 1.5 times the interquartile range from the 25th and 75th percentiles (whiskers). *p < .05, *** p < .001.
SCSD, calculated for 10–55 sec time window, was significantly higher in the illusion condition compared with the control for Iteration 1 (10–55 SCSD: d = 0.63, t(17) = 2.69, p = .016), but not for Iteration 2 (10–55 SCSD: d = −0.30, t(17) = −1.28,p = .219).
Furthermore, to assess the impact of novelty on the described effect of embodiment upon SCSD, an analysis on the effect of condition (synchronous vs. asynchronous) has been performed only on the first two trials of the first iteration. Because each participant performed only one condition for each trial, the analysis became unpaired and the sample half-sized. To benefit a larger sample size, data from the first iteration of ExpC were pooled together with data from ExpA. In all those participants, the 10–55 sec window SCSD data of synchronous and asynchronous conditions were compared in the first and second trials of the RHI protocol.
In the two-way ANOVA performed with factors Repetition (two level: Trial 1 vs. Trial 2) and Condition (two level: synchronous vs. asynchronous), significant main effects of both factors were obtained (repetition: F(1, 106) = 13.25, p < .001; condition: F(1, 106) = 22.86, p < .001), and no interaction effect was found (Figure 6). Preplanned two-tailed unpaired Bonferroni-corrected (four comparisons) t tests were employed to perform the comparison between SCSDs.
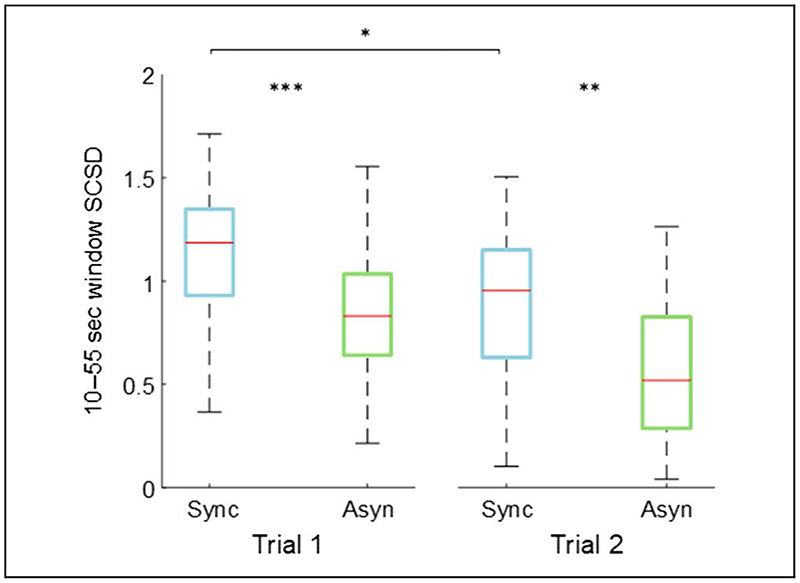
Figure 6.
Box and whisker plots of the SCSD for the synchronous and asynchronous congruent conditions for ExpA and the first two trials of ExpC: median (red lines), first and third quartiles (box), lowest and highest values comprised within 1.5 times the interquartile range from the 25th and 75th percentiles, respectively (whiskers). **p < .01, ***p < .001.
The synchronous 10–55 sec SCSD was significantly higher than the asynchronous one not only in the first trial (d = 0.49, t(53) = 3.55, p < .001) but also in the second one (trial 2: d = 0.45, t(53) = 3.24, p = .008) when participants were no longer exposed to the novelty of the paradigm. With regard to the decreasing of the effect due to repetition of the protocol, the difference was significant only for the synchronous condition (d = 0.37, t(53) = 2.68, p = .040), not for the asynchronous one (d = 0.34, t(53) = 2.48,p > .05).
Discussion
The activity of the afferent branch of the ANS that leads to interoception contributes to build the representation of the body, which is essential to experience the body as self. We argued that an update of such representation, even if due to the modulation of noninteroceptive sensory afferences, would have had an impact on the efferent branch of ANS.
SC is an index of autonomic efferent activation. ER-SCR to a menace has been used as a smart tool to assess how much the fake hand in the RHI is embodied because, similarly to a startle reflex, physical threats to a body part evoke a stronger autonomic reflex. However, having a higher protective response for the body does not imply that the body representation has—by itself—correlates on the efferent autonomic activity.
Indeed, conversely to ER-SCR, NS-SCR has never been employed in studies where the experience of body ownership is manipulated. We hypothesized that, in the RHI paradigm, the arising of the embodiment illusion over the fake hand would have affected the NS-SCR.
To test this hypothesis, we ran three RHI experiments in healthy participants: both ExpA and ExpB compared the NS-SCR collected during the illusion condition with NS-SCR during the control one. ExpA exploited the asynchronous brush-stroking as a control condition, and in ExpB, the fake hand was placed in an incongruent position. In ExpC, the NS-SCR has been evaluated across the repetition of the RHI paradigm presentation.
In ExpA and ExpB, we found that the standard deviation of NS-SCR, employed to extract its variability, was increased by the illusion condition. This is likely due to an induction of a stronger arousal, which recruited the sympathetic division of ANS and increased the fluctuations of SC.
In line with our findings, highly arousing experiences, such as demanding cognitive tasks (Munro, Dawson, Schell, & Sakai, 1987), states of fear and anger (Ax, 1953), or stressful social interactions (Levenson & Gottman, 1983, 1985), increase the frequency of occurrence of peaks of NS-SCR, their amplitude, and their standard deviation value (Boucsein et al., 2012). It has been suggested that the heightened autonomic activation due to an effortful allocation of attentional resources for task accomplishment (Jennings, 1986) increases NS-SCR because it enhances the “energy mobilization” (Dawson et al., 2007).
On the contrary, but in line with (Tieri, Gioia, Scandola, Pavone, & Aglioti, 2017), we did not find differences of HRV between experimental and control conditions. HRV is related to a general autonomic reactivity of both branches of the ANS (Kreibig, 2010; Bradley & Lang, 2000), although it is likely that the described effect on SC is mainly dependent on the activity of cholinergic sympathetic sudomotor fibers innervating the sweat glands (Shields, MacDowell, Fairchild, & Campbell, 1987; Wallin, 1981). The sympathetic and the parasympathetic branches of the ANS have different latent periods and different time courses on HRV; in addition to this, sympathetic effects were found to be much slower than parasympathetic ones (Guzzetti et al., 2005; Warner & Cox, 1962). It is possible that the dynamic of the expression of the sympathetic branch of the ANS over the cardiac dynamics are less suited to highlight the induction of body ownership over a fake limb.
Once established that embodiment impacts on NS-SCR, the neural substrate through which this happens remains to be elucidated. It may be speculated that, at a cortical level, the ACC and particularly the insula play a key role for closing the afferent–efferent ANS loop involved in the process of building our body representation: The results of anatomical, lesional, behavioral and fMRI studies converge on such structures.
Indeed, the afferent activity of vagal, glossopharyngeal, facial, and spinal nerves, which codes the visceral perception, and of small diameter (Aδ and C) primary afferent fibers, which innervate all tissues of the body, is directed to the ACC and to the insula (Craig, 2003, 2008). Besides being a projection site of viscerosensory input from different modalities (Herbert & Pollatos, 2012), the insula is involved in cross-modal visual and somatosensory integration (Hadjikhani & Roland, 1998; Bottini et al., 1995) and in temperature regulation (Maihöfner, Kaltenhäuser, Neundörfer, & Lang, 2002). Accordingly, it has widely demonstrated a link between autonomic mechanisms of temperature regulation and cognitive processes behind body representation (Tieri et al., 2017; Llobera, Sanchez-Vives, & Slater, 2013; Tsakiris et al., 2011). Insular cortex is involved in the modulation of the immune function (Sinha, 2014), which is tightly under control of the sympathetic branch of the ANS (Nance & Sanders, 2007). In line with that, the histamine reactivity increases in the RHI, probably as a sign of rejection of the participants’ real hand (Barnsley et al., 2011). In the RHI, the ownership over a third artificial hand seems to result in a disownership and a decrease of the skin temperature of the real hand (Moseley et al., 2008). However, the consistency of such finding is still under debate (de Haan et al., 2017; Rohde, Wold, Karnath, & Ernst, 2013).
Functional imaging reports showed that the activation of the insula positively correlates to the ownership experience after a synchronous session of RHI (Grivaz, Blanke, & Serino, 2017; Tsakiris, Hesse, Boy, Haggard, & Fink, 2007) and that both insula and ACC become consistently active in response to the rubber hand physical threatening (Ehrsson, Wiech, Weiskopf, Dolan, & Passingham, 2007). Lesions of the insular cortex are very common in stroke patients affected by a pathological alteration of their body ownership, for example, showing asomatognosia (i.e., experiencing their limb as not belonging to them) or so-matoparaphrenia (attributing their limb to someone else; Karnath & Baier, 2010).
The process of embodiment happening in the RHI is known to be paired with statistically significant changes of well-validated measures of illusion; an increase in the mean value of illusion statements of the Botvinick and Cohen (1998)-derived questionnaire, an increase in subjectively and explicitly estimated vividness and prevalence of the illusion and a shift of the estimated position of the real hidden hand toward the fake one. A novel effective measure of embodiment in the RHI procedure should partly correlate with those validated measures, and the extent of specific correlation may be informative of the specific aspect of embodiment to which the measure is more sensible.
SCSD correlated significantly with all the other employed measures of the illusion, but the higher values of correlations were found for the measures designed to rate the strength of the illusion, that is, RHI index, the vividness and prevalence score. In particular, SCSD correlations with those subjective measures of the embodiment were significantly higher than the one with proprioceptive drift. This is in line with the knowledge that different mechanisms of multisensory integration are responsible for the feeling of ownership and for the implicit measure (the proprioceptive drift; Rohde, Di Luca, & Ernst, 2011).
In relation to the evolution in time of NS-SCR, a further hypothesis can be done: If the time needed for the measure to become significant resulted to be short enough to achieve a good time sensitivity, NS-SCR would be a good candidate also to investigate the timing of arising and the dynamic of the illusion.
Thus, we analyzed shorter time windows (wl < 90 sec) with different length and onset to highlight the time windows that held the more pronounced difference between illusion and control condition. No information seems to be present in the first 15 sec of the trial and for the window with onset after 25–30 sec. However, once the period from 15 to 30 sec is included in the analysis, p value decreases for wider windows up to 55 sec. Indeed, the highest difference was found for a 45-sec-long window, from 10 to 55 sec. The shortest time interval (wl = 20 sec) with lowest p value ranged from 14 to 34 sec (see Figure 3).
The onset of the referral of touch sensation on the fake limb has been reported to be between 7 sec (Lloyd, 2007) and 11 sec (Ehrsson, Spence, & Passingham, 2004) from the beginning of paintbrush stroking. Considering that few seconds of delay (2–5 sec) should be taken into account for an SCR peak to occur after an event (Braithwaite et al., 2013), the significant increase of NS-SCR found after 15 sec perfectly fits with the onset of the illusion, which starts to update the representation of the body. Therefore, from the analysis of NS-SCR computed along time windows with different length and onset, it can be argued that NS-SCR can be exploited as a useful and objective tool to evaluate the presence of illusion and to have cues on its arising, but not on its end.
The findings obtained for the p value analysis were indirectly confirmed by the values of correlations between SCSD values and the previous validated measures for the different time windows. For all the measures, the maximum correlation coefficient was obtained for time windows shorter than the whole duration of the trial. The lower correlations (i.e., the ones with proprioceptive drift and with prevalence) were also the ones less affected by the shortening of the window. This suggests that SCSD modulation is more tightly related with the intensity of the illusion than with its duration.
Because NS-SCR is known to be influenced by arousal and the RHI setup is likely to induce surprise, we wanted to test how much the described effect was strong against the multiple repetition of test and control conditions. In ExpC, the second subsequent iteration of a synchronous and an asynchronous RHI condition fails to highlight the difference in NS-SCR, suggesting that the reported effect was at least partially due to a reaction to a novelty, which decreases by repeating the paradigm.
Could this surprise be the reaction to the unexpected feeling of ownership over the artificial limb, or could it be the reaction to the perceptual differences in stimuli presentation between experimental and control condition?
The latter hypothesis could hamper the value of our findings. However, the analysis of the results of both ExpA and ExpB makes it not likely for a number of reasons: (1) The SCSD was subject to the same modulations despite different control conditions; (2) SCSD positively correlates with all the widely-validated measures of the illusion; (3) also, the control conditions can induce surprise in the participants (the unnatural position of the hand and the asynchrony in stimulation) in similar way to tested conditions; and (4) all the circumstantial possible confounding factors that can induce arousal (light, brushstroke, setup, etc.) were maintained as constant as possible between conditions.
Moreover, considering all the participants that underwent the synchronous versus asynchronous paradigm (ExpA together with ExpC), the SCSD resulted to be significantly higher in the condition eliciting the rubber hand embodiment (synchronous) also when considering only the second administration of the protocol (Trial 2). All the participants that in Trial 2 underwent synchronous stimulation were already exposed to all the novelties of the experiment in the previous first administration of the protocol (i.e., performance anxiety, novelty in presentation of the setup and tactile stimulation), except to the one due to the induction of embodiment. This strongly suggests that the induction of the embodiment is the cause of the enhanced variability of the NS-SCR and that this change is sensible to the novelty.
The dependence to the novelty of our effect is in line with other habituation processes, which have been widely described in electrodermal activity (see Boucsein et al., 2012, for a thorough review). Moreover, the embodiment induced by the RHI procedure suffers by itself of habituation (Convento, Romano, Maravita, & Bolognini, 2018; Bekrater-Bodmann, Foell, Diers, & Flor, 2012). The combination of the habituation of the electrodermal activity and the habituation of the induction of embodiment in the RHI may have contributed to the absence of effect in the second iteration of ExpC.
Conclusion
In conclusion, modifications of the body representation are likely among the processes that increase arousal and sympathetic response of ANS, as shown by the increase of fluctuations of NS-SCR.
This modification goes beyond the already known enhanced autonomic reaction to a threatening stimulus and does not seem to be just due to a differently presented sensory stimulus, but more likely, it seems to be the effect to the novelty due to the update of the perceptual status.
Our results unveil a further link between apparently unrelated complex cognitive activities and low-level peripheral physiological responses by depicting a control loop probably involving interoception and exteroception, the building of the representation of the body, and the output of the efferent autonomic system.
From an applicative point of view, this link can be exploited to impact on the process that builds the representation of the body. For instance, the vagus nerve invasive or noninvasive stimulation may be a good candidate to modulate interoceptive afferences and enhance embodiment.
Moreover, the index of fluctuation of NS-SCR signal, that is, the standard deviation, is an optimal candidate to assess the extent of embodiment over an artificial body limb and seems to be specifically suited to assess the onset of the illusion and its initial development. This physiological measure suffers of habituation; thus, it is not suitable when there are multiple repetitions of the process to induce the embodiment (e.g., the RHI). However, it has several strengths: It is noninvasive, costeffective, easy to employ, and potentially free from subjective biases (such as in self-report evaluations of the illusion), its recording does not interfere with the collection of other behavioral measures (e.g., proprioceptive drift or SCR to threatening event against the fake hand), and it catches quite accurately the strength of the illusion.
Acknowledgments
This work was funded by the European Research Council Starting Grant 2015 RESHAPE: Restoring the Self with Embodiable Hand Prostheses (ERC-2015-STG, Project No. 678908). The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Abdulkarim Z, Ehrsson HH. No causal link between changes in hand position sense and feeling of limb ownership in the rubber hand illusion. Attention, Perception, & Psychophysics. 2016;78:707–720. doi: 10.3758/s13414-015-1016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armel KC, Ramachandran VS. Projecting sensations to external objects: Evidence from skin conductance response. Proceedings of the Royal Society of London, Series B: Biological Sciences; 2003. pp. 1499–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ax AF. The physiological differentiation between fear and anger in humans. Psychosomatic Medicine. 1953;15:433–442. doi: 10.1097/00006842-195309000-00007. [DOI] [PubMed] [Google Scholar]
- Bach DR, Friston KJ, Dolan RJ. Analytic measures for quantification of arousal from spontaneous skin conductance fluctuations. International Journal of Psychophysiology. 2010;76:52–55. doi: 10.1016/j.ijpsycho.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnsley N, McAuley JH, Mohan R, Dey A, Thomas P, Moseley GL. The rubber hand illusion increases histamine reactivity in the real arm. Current Biology. 2011;21:R945–R946. doi: 10.1016/j.cub.2011.10.039. [DOI] [PubMed] [Google Scholar]
- Bekrater-Bodmann R, Foell J, Diers M, Flor H. The perceptual and neuronal stability of the rubber hand illusion across contexts and over time. Brain Research. 2012;1452:130–139. doi: 10.1016/j.brainres.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Blanke O. Multisensory brain mechanisms of bodily self-consciousness. Nature Reviews Neuroscience. 2012;13:556–571. doi: 10.1038/nrn3292. [DOI] [PubMed] [Google Scholar]
- Bottini G, Paulesu E, Sterzi R, Warburton E, Wise RJS, Vallar G, et al. Modulation of conscious experience by peripheral sensory stimuli. Nature. 1995;376:778–781. doi: 10.1038/376778a0. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Cohen J. Rubber hands ‘feel’ touch that eyes see. Nature. 1998;391:756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Boucsein W, Fowles DC, Grimnes S, Ben-Shakhar G, Roth WT, Dawson ME, et al. Publication recommendations for electrodermal measurements. Psychophysiology. 2012;49:1017–1034. doi: 10.1111/j.1469-8986.2012.01384.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: Behavior, feeling, and physiology. In: Lane RD, Nadel L, editors. Cognitive neuroscience of emotion. Oxford University Press; New York: 2000. pp. 242–276. [Google Scholar]
- Braithwaite JJ, Watson DG, Jones R, Rowe M. A guide for analysing electrodermal activity (EDA) and skin conductance responses (SCRs) for psychological experiments. University of Birmingham; Edgbaston, United Kingdom: 2013. [Google Scholar]
- Convento S, Romano D, Maravita A, Bolognini N. Roles of the right temporo-parietal and premotor cortices in self-location and body ownership. European Journal of Neuroscience. 2018;47:1289–1302. doi: 10.1111/ejn.13937. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception and emotion: A neuroanatomical perspective. In: Lewis MJ, Haviland-Jones JM, Barrett LF, editors. Handbook of emotions. Guilford Press; New York: 2008. pp. 272–288. [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. Cambridge University Press; Cambridge: 2007. pp. 200–223. [Google Scholar]
- de Haan AM, Van Stralen HE, Smit M, Keizer A, Van der Stigchel S, Dijkerman HC. No consistent cooling of the real hand in the rubber hand illusion. Acta Psychologica. 2017;179:68–77. doi: 10.1016/j.actpsy.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Di Pino G, Maravita A, Zollo L, Guglielmelli E, Di Lazzaro V. Augmentation-related brain plasticity. Frontiers in SystEMS Neuroscience. 2014;8:109. doi: 10.3389/fnsys.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Spence C, Passingham RE. That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305:875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Wiech K, Weiskopf N, Dolan RJ, Passingham RE. Threatening a rubber hand that you feel is yours elicits a cortical anxiety response. Proceedings of the National Academy of Sciences, U.S.A; 2007. pp. 9828–9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. Philosophical conceptions of the self: Implications for cognitive science. Trends in Cognitive Sciences. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Grivaz P, Blanke O, Serino A. Common and distinct brain regions processing multisensory bodily signals for peripersonal space and body ownership. Neuroimage. 2017;147:602–618. doi: 10.1016/j.neuroimage.2016.12.052. [DOI] [PubMed] [Google Scholar]
- Guzzetti S, Borroni E, Garbelli PE, Ceriani E, Della Bella P, Montano N, et al. Symbolic dynamics of heart rate variability: A probe to investigate cardiac autonomic modulation. Circulation. 2005;112:465–470. doi: 10.1161/CIRCULATIONAHA.104.518449. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Roland PE. Cross-modal transfer of information between the tactile and the visual representations in the human brain: A positron emission tomographic study. Journal of Neuroscience. 1998;18:1072–1084. doi: 10.1523/JNEUROSCI.18-03-01072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert BM, Pollatos O. The body in the mind: On the relationship between interoception and embodiment. Topics in Cognitive Science. 2012;4:692–704. doi: 10.1111/j.1756-8765.2012.01189.x. [DOI] [PubMed] [Google Scholar]
- Jennings JR. Bodily changes during attending. In: Coles MGH, Donchin E, Porges SW, editors. Psychophysiology: SystEMS, processes, and applications. Guilford Press; New York: 1986. pp. 268–289. [Google Scholar]
- Karnath H-O, Baier B. Right insula for our sense of limb ownership and self-awareness of actions. Brain Structure and Function. 2010;214:411–417. doi: 10.1007/s00429-010-0250-4. [DOI] [PubMed] [Google Scholar]
- Kilteni K, Normand J-M, Sanchez-Vives MV, Slater M. Extending body space in immersive virtual reality: A very long arm illusion. PLoS One. 2012;7:e40867. doi: 10.1371/journal.pone.0040867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig SD. Autonomic nervous system activity in emotion: A review. Biological Psychology. 2010;84:394–421. doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM. Marital interaction: Physiological linkage and affective exchange. Journal of Personality and Social Psychology. 1983;45:587–597. doi: 10.1037//0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM. Physiological and affective predictors of change in relationship satisfaction. Journal of Personality and Social Psychology. 1985;49:85–94. doi: 10.1037//0022-3514.49.1.85. [DOI] [PubMed] [Google Scholar]
- Llobera J, Sanchez-Vives MV, Slater M. The relationship between virtual body ownership and temperature sensitivity. Journal of the Royal Society Interface. 2013;10:20130300. doi: 10.1098/rsif.2013.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DM. Spatial limits on referred touch to an alien limb may reflect boundaries of visuo-tactile peripersonal space surrounding the hand. Brain and Cognition. 2007;64:104–109. doi: 10.1016/j.bandc.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Ma K, Hommel B. The virtual-hand illusion: Effects of impact and threat on perceived ownership and affective resonance. Frontiers in Psychology. 2013;4:604. doi: 10.3389/fpsyg.2013.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maihöfner C, Kaltenhäuser M, Neundörfer B, Lang E. Temporo-spatial analysis of cortical activation by phasic innocuous and noxious cold stimuli—A magnetoencephalographic study. Pain. 2002;100:281–290. doi: 10.1016/S0304-3959(02)00276-2. [DOI] [PubMed] [Google Scholar]
- Mioli A, D’Alonzo M, Pellegrino G, Formica D, Di Pino G. Intermittent theta burst stimulation over ventral premotor cortex or inferior parietal lobule does not enhance the rubber hand illusion. Frontiers in Neuroscience. 2018;12:870. doi: 10.3389/fnins.2018.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley GL, Olthof N, Venema A, Don S, Wijers M, Gallace A, et al. Psychologically induced cooling of a specific body part caused by the illusory ownership of an artificial counterpart. Proceedings of the National Academy of Sciences, U.S.A; 2008. pp. 13169–13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro LL, Dawson ME, Schell AM, Sakai LM. Electrodermal lability and rapid vigilance decrement in a degraded stimulus continuous performance task. Journal of Psychophysiology. 1987;1:249–257. [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain, Behavior, and Immunity. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocklenburg S, Rüther N, Peterburs J, Pinnow M, Güntürkün O. Laterality in the rubber hand illusion. Laterality. 2011;16:174–187. doi: 10.1080/13576500903483515. [DOI] [PubMed] [Google Scholar]
- Petkova VI, Ehrsson HH. When right feels left: Referral of touch and ownership between the hands. PLoS One. 2009;4:e6933. doi: 10.1371/journal.pone.0006933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichgott MJ. Clinical evidence of dysautonomia. In: Walker HK, Hall WD, Hurst JW, editors. Clinical methods: The history, physical, and laboratory examinations. 3rd. Butterworths; Boston: 1990. [PubMed] [Google Scholar]
- Rochat P. Self-perception and action in infancy. Experimental Brain Research. 1998;123:102–109. doi: 10.1007/s002210050550. [DOI] [PubMed] [Google Scholar]
- Rohde M, Di Luca M, Ernst MO. The rubber hand illusion: Feeling of ownership and proprioceptive drift do not go hand in hand. PLoS One. 2011;6:e21659. doi: 10.1371/journal.pone.0021659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde M, Wold A, Karnath H-O, Ernst MO. The human touch: Skin temperature during the rubber hand illusion in manual and automated stroking procedures. PLoS One. 2013;8:e80688. doi: 10.1371/journal.pone.0080688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges LV, editors. Handbook of research synthesis. Russell Sage Foundation; New York: 1994. pp. 231–244. [Google Scholar]
- Seth AK. Interoceptive inference, emotion, and the embodied self. Trends in Cognitive Sciences. 2013;17:565–573. doi: 10.1016/j.tics.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Shields SA, MacDowell KA, Fairchild SB, Campbell ML. Is mediation of sweating cholinergic, adrenergic, or both? A comment on the literature. Psychophysiology. 1987;24:312–319. doi: 10.1111/j.1469-8986.1987.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Sinha R. Disgust, insula, immune signaling, and addiction. Biological Psychiatry. 2014;75:90–91. doi: 10.1016/j.biopsych.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Garfinkel SN, Critchley HD, Seth AK. Multisensory integration across exteroceptive and interoceptive domains modulates self-experience in the rubber-hand illusion. Neuropsychologia. 2013;51:2909–2917. doi: 10.1016/j.neuropsychologia.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Tieri G, Gioia A, Scandola M, Pavone EF, Aglioti SM. Visual appearance of a virtual upper limb modulates the temperature of the real hand: A thermal imaging study in immersive virtual reality. European Journal of Neuroscience. 2017;45:1141–1151. doi: 10.1111/ejn.13545. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Haggard P. The rubber hand illusion revisited: Visuotactile integration and self-attribution. Journal of Experimental Psychology: Human Perception and Performance. 2005;31:80–91. doi: 10.1037/0096-1523.31.1.80. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. Neural signatures of body ownership: A sensory network for bodily self-consciousness. Cerebral Cortex. 2007;17:2235–2244. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Tajadura-Jiménez A, Costantini M. Just a heartbeat away from one’s body: Interoceptive sensitivity predicts malleability of body-representations. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2011;278:2470–2476. doi: 10.1098/rspb.2010.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Yamakawa H, Yamashita A, Takakusaki K, Maeda T, Kato M, et al. Analysis of electromyography and skin conductance response during rubber hand illusion. Proceedings of the IEEE Workshop on Advanced Robotics and its Social Impacts (ARSO ‘13); IEEE. Tokyo: 2013. pp. 88–93. [Google Scholar]
- van den Bos E, Jeannerod M. Sense of body and sense of action both contribute to self-recognition. Cognition. 2002;85:177–187. doi: 10.1016/s0010-0277(02)00100-2. [DOI] [PubMed] [Google Scholar]
- Wallin BG. Sympathetic nerve activity underlying electrodermal and cardiovascular reactions in man. Psychophysiology. 1981;18:470–476. doi: 10.1111/j.1469-8986.1981.tb02483.x. [DOI] [PubMed] [Google Scholar]
- Warner HR, Cox A. A mathematical model of heart rate control by sympathetic and vagus efferent information. Journal of Applied Physiology. 1962;17:349–355. doi: 10.1152/jappl.1962.17.2.349. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. Comparing Pearson correlations: Dealing with heteroscedasticity and nonnormality. Communications in Statistics—Simulation and Computation. 2009;38:2220–2234. [Google Scholar]
- Yuan Y, Steed A. Is the rubber hand illusion induced by immersive virtual reality?. 2010 IEEE Virtual Reality Conference (VR); Waltham, MA: IEEE; 2010. [Google Scholar]