Highlights
► We design and fabricate a breathing thermal manikin. ► We measure how inhalation influences by pulmonary ventilation rate. ► Pulmonary rate reduces inhalation and the reduction increases with the rate. ► Well-mixed scheme tends to lower the inhalation compared to displacement scheme.
Keywords: Pulmonary ventilation, Breathing thermal manikin, Inhalation, Exposure, Expiratory emission
Abstract
Estimating inhalation dose accurately under realistic conditions can enhance the accuracy of risk assessment. Conventional methods to quantify aerosol concentration that susceptible victims in contaminated environments are exposed to use real time particle counters to measure concentrations in environments without occupancy.
Breathing-induced airflow interacts and influences concentration around nostrils or mouth and alter the ultimate exposure. This subject has not yet been systematically studied, particularly under transient emission. In this work, an experimental facility comprising two manikins was designed and fabricated. One of them mimicked realistic breathing, acting as a susceptible victim. Both steady and episodic emissions were generated in an air-conditioned environmental chamber in which two different ventilation schemes were tested. The scaled-dose of the victim under different expiratory velocities and pulmonary ventilation was measured.
Inferring from results obtained from comprehensive tests, it can be concluded that breathing has very significant influence on the ultimate dose compared with that without breathing. Majority of results show that breathing reduces inhalation quantity and the reduction magnitude increases with breathing rate. This is attributed to the fact that the exhalation process plays a more significant role in reducing the dose level than the enhanced effect during inhalation period. The higher the breathing rate, the sharper the decline of the resultant concentration would be leading to lower dose. Nevertheless, under low pulmonary ventilation, results show that breathing increases dose marginally. Results also reveals that ventilation scheme also affects the exposure.
1. Introduction
After the outbreaks of severe acute respiratory syndrome (SARS) and the new influenza strain H1N1, community preparedness for pandemics has been the focus of much interest and effort in recent years. When a person sneezes, coughs or even talks, expiratory droplets are generated. Both large and small droplets are generated in an expiratory cloud [1]. Although still being debated, recent scientific data support the notion that pathogens can be transmitted by aerosols [2], [3], [4].
Once they are airborne in indoor environments, pathogens can be inhaled by susceptible victims. Inhalation dose depends predominantly on spatial, temporal concentration profiles and the duration of exposure to the microenvironment [5]. Many previous works have evaluated inhalation dose, D (at a specific location) of a specific contaminant by:
| (1) |
where DE is the duration of exposure, Q is the pulmonary ventilation and C is the temporal concentration.
The conventional method to measure concentration of pathogens in a specific enclosure involves putting sampling devices (e.g., counters or impactors) in the environment. If real-time counters are used, such as laser photometers (up to 10 μm), condensation particle counters (up to a few micrometers), or size-resolved particle spectrometers (up to several tens of micrometers), temporal concentration profile can be recorded. Measurement is commonly taken at a reference location and the concentration is considered as representative of the entire indoor microenvironment. This approach completely ignores the influence of human-generated thermal plume effects on concentration profile, which has been reported to be an important factor affecting indoor concentration [6], [7]. Apart from this conventional sampling method, advancement of optical technology had led to more affordable and reliable instruments to measure particle concentration non-invasively. Recently, there have been some works that have used in situ imaging technique for collecting data of exhaled aerosol dispersion [7], [8].
To better estimate inhalation dose caused by exposure to contaminants, breathing thermal manikins (BTMs) have been used. In most scenarios, metabolism rates can be adjusted according to the physical activity level considered. This gives more accurate exposure assessment as influences of thermal and pulmonary ventilation are taken into account. Sampling devices are placed very near or at the nostrils and/or mouth openings [9]. There have been some numerical studies simulating contaminant concentration around BTM. The limitations of these studies were (i) steady inhalation assumed [10]; (ii) only airflow around mouth was considered [11]; and (iii) passive contaminants were used [12].
Experimental studies using BTM have been extremely scarce and most of them were focused on measuring exposure to passive contaminates [9], [13], [14], [15], [16]. Recently a study reported transportation of passive and particulate contaminants in the vicinity of breathing area of a BTM [17]. The authors concluded that thermal plume may play a significant role in contaminant transportation to breathing zone.
If the objective of air sampling is to assess the concentration due to inhalation under transient concentration, temporal concentration measured by the particle sampler or counter should be representative of what would be inhaled by humans. In this regard, characteristics of both expiratory emission and pulmonary ventilation should be considered and their influences on sampling should be quantified.
Typically, the process of generation of expiratory droplets is rapid and the duration is very brief. Velocity of coughing by healthy adults can be up to approximately 30 m/s [12]. Interaction between airflow at the vicinity of the mouth or nose and the incoming expiratory droplets is very complex. Expelled airflow may reduce the contaminant dose or even eliminate it completely by the opposing airflow against the droplet flow. Likewise, inhalation-induced airflow may increase the dose.
Pulmonary ventilation is calculated as the product of tidal volume and respiratory frequency, and both factors depend on many physiological factors such as gender, age and, most importantly, metabolism rates [18]. Average breathing rate of an adult at rest is usually 12 cycles/min. During office work breathing rate can increase to 20 cycles/min. Pulmonary ventilation for resting and moderate work is 6 and 30 liter per minute (LPM), respectively [19]. It is apparent that pulmonary ventilation affects inhalation dose. Nevertheless there is no systematic study that has quantified the effects.
In extant literature there are many studies that quantify aerosol dispersion under various ventilation conditions. A few of them have investigated exposure to expiratory process for studying the influence of different ventilations on aerosol dispersion and mixing [7], [20]; contaminant dispersion in air cabin mock-up [21]; aerosol emitted from a nurse or patient [22]; difference in exposure by a continuous or single expiratory emission [23]; orientation of source-to-receptor and different ventilation schemes of aerosol dispersion and removal [24]; and performance of personalized ventilation [25]. However, only one CFD study has considered breathing when evaluating exposure to droplets [26]. The present authors have measured expiratory droplet profiles spatially and temporally inside a 1:5 scaled chamber after a momentary release of droplets [27], [28].
Recent reviews have highlighted all parameters governing airborne infection in indoor environments [19], [29]. It is shown that ventilation plays an important role in particle concentration indoors. Two ventilation schemes, well-mixing ventilation (MV) and displacement ventilation (DIS), are commonly used in commercial buildings. MV schemes are the most popular ventilation arrangement for commercial buildings. In schemes of this type, high-velocity cooled air is discharged through supply grills and warm air is expelled through return grills. Both grills are located at the ceiling level. DIS schemes exhibit characteristics completely different from those of MV schemes. DIS schemes supply cooled fresh air at a low velocity to occupied zones through supply air grilles located at lower levels of rooms and expel hot air via exhausts located in the ceiling.
To the best of authors’ knowledge, there no study has quantified how pulmonary ventilation affects transient inhalation dose of BTM. In this work, scale of inhalation dose was determined by using a full size BTM inside a full scale environmental chamber. The experiments were conducted under MV and DIS ventilation schemes. A real-time particle counter was used to measure concentration at the mouth under steady and episodic events and interactions of movements of exhaled/inhaled air with the droplets were carefully studied.
2. Experimental setup
The two manikins were located inside a full scale experimental chamber of dimensions 2.30 (L) × 2.25 (W) × 2.3 (H) m3. One is referred to as the “source” and the other as the “susceptible victim.” The distance between the source and the susceptible victim was kept at 60 cm. Location of supply and return air grills for the two ventilation systems are shown in Fig. 1 . For the MV scheme, the air exchange rate per hour was set to 20 h−1 while for the DIS scheme, it was set to 5 ACH. The indoor temperature was 24 ± 0.5 °C.
Fig. 1.
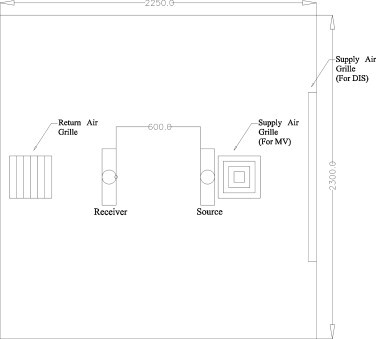
Plan view of the setup inside the environmental chamber.
Two parameters were varied and studied in this work. The first one was pulmonary ventilation of the susceptible victim, set at 7 LPM, 15 LPM and 23 LPM. These values were within the range found in literature and they are classified as “very low”, “low” and “light” pulmonary ventilation in this work. Respiratory frequency was set at 15/min (2 s inhalation and 2 s exhalation). The second one was the emission speed. Two values were selected for tests, 15 m/s and 30 m/s. It was measured by a handheld hot-wire anemometer (VelociCalc 9555-P, TSI) placed at a distance of 5 cm horizontally in front of the source manikin and the velocity measured was treated as a reference.
Fig. 2 depicts the detailed schematic diagram of the experimental setup consisting of an electrical-mechanical breathing and aerosol sampling system for the susceptible victim and sneezing system for the source. The breathing and sampling system was divided into four parts, where Paths A, B and C were involved in breathing circuit while Path D was the sampling system. Each pathway is described in detail herein below.
Fig. 2.
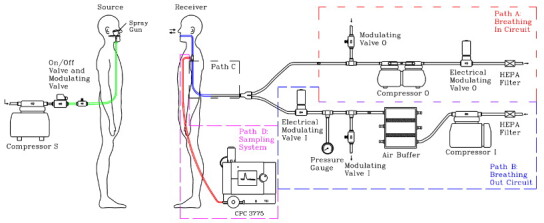
Schematic diagram of the experimental setup and the details of the breathing circuit.
Path A
It dealt with the inhalation process. There were a total of four components in this pathway. The electrical modulating valve was controlled by user-defined LabVIEW Environment to vary the flow rate as a sinusoidal function. The manual modulating valve was used to regulate the flow rate of inhalation. The compressor was used to drive the air flowing from the manikin to the outlet. The air was forced to pass through the HEPA filter before escaping from the circuit to minimize cross-contamination in the laboratory.
Path B
It dealt with the exhalation process. There were a total of six components in this path. The exhaust of the compressor was connected to the inlet of air buffer tank to stabilize the fluctuation of compressed airflow. The electrical modulating valve was connected to the air buffer tank and it was controlled by the same LabVIEW Environment to generate a sinusoidal output. In between, a manual modulating valve was used for regulating the flow rate of exhalation. Finally, the HEPA filter was used to purify the air to ensure no particles were exhaled to the mouth. In reality, non-deposited inhaled aerosols exhale out. The current approach simplified the problem but the objective of the current work was to investigate how breathing affects inhalation dose and hence the presence of exhalation aerosols does not affect the results. Besides the focus of the current work is an UFP exposure with breathing, so no effect of the CO2 concentration was considered in this study.
Both Path A and B were connected together through the equal Y connector. A common Path C was then connected to the sampling point at mouth.
Path D is the sampling system. The sampling route was made to tee-off from the common Path C of the breathing system. It was directly connected to condensation particle counter (CPC) (3775, TSI) for sampling. To reduce sampling loss, the CPC was put inside the chamber.
A sneezing system was setup for generating testing aerosols, which consisted of a compressor, an on/off electrical modulating valve, a manual electrical modulating valve and a spray gun (180D, Spray-Work, Tamiya). Dilute sodium chloride (NaCl) solution was stored in a small container attached to the spray gun. The size distribution of NaCl was measured to have size ranging from 10 to 200 nm with a mode diameter of 35 nm. The on/off electrical modulating valve governed by LabVIEW was used to control sneezing duration. The manual electrical modulating valve was used to control the flow rate of sneezing.
The chamber door was closed and the breathing system of the susceptible victim was turned on remotely. Before each emission, the background concentration level was checked to ensure the current measurement was not affected by the previous experimental setup. The compressor was then turned on remotely and the whole expiratory process, which lasted 4 s, was initiated by the LabVIEW. Aerosols concentration was sampled by the CPC at the mouth of the susceptible victim. The experiments were repeated at least 25 times under identical conditions. Since the emission was transient and breathing was a periodic function, repeated experimental runs allowed a more practical way for analysis; results of the most frequent range of concentration level (the mode) were used.
3. Results and discussion
3.1. Steady concentration environment
A new parameter was defined to facilitate comparison of effects of pulmonary ventilation on dose under both steady and episodic emission. To facilitate comparison between different data sets, the data were normalized by the background level in the non-breath scenario, defined as:
| (2) |
where B and NB represent breath and non-breath, respectively.
Prior to commencing measurements, the experimental setup was tested, particularly for the breathing circuit. In this regard, the system was tested with and without implementation of the breathing system. To avoid unnecessary uncertainty, the test was done under a steady state environment. Pulmonary ventilation of 7 LPM was utilized. Without any aerosol emission, the background level inside the environmental chamber was maintained at approximately 4000 numbers/cc and this could be regarded as a constant source (Fig. 3 ). The area under the concentration–time profile represents the exposure (referred to as scaled inhalation dose) and it was used to normalize the experimental data to get the BNB ratio, as shown in Eq. (2). The dashed line shows the periodic pattern caused by the breathing cycle. During inhalation cycle, the concentration level was comparable to that without breathing. During exhalation, as expected, the profile exhibits a very sharp dip. However, the profile shows distinct differences between inhalation and exhalation cycles.
Fig. 3.
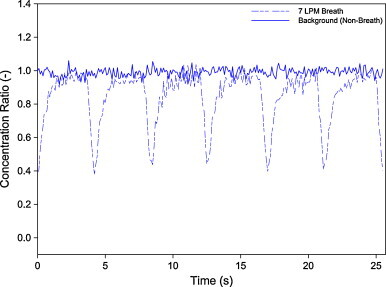
Breath to non-breath ratio under steady concentration with 7 LPM pulmonary ventilation. Non-breath profile is also shown for comparison.
To address the issue, concentration profiles for three breathing rates, 7 LPM, 15 LPM and 23 LPM, with the same breathing frequency, were measured (Fig. 4 ); the BNB is tabulated in Table 1 . These profiles are compared with the ideal breathing pattern with the same breathing frequency and the BNB ratio (Fig. 5 ).
Fig. 4.
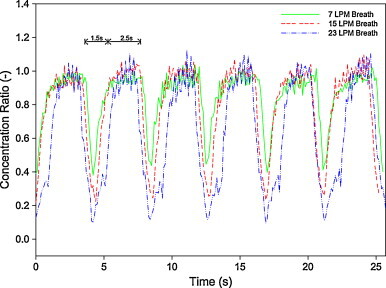
Breath to non-breath ratio under steady concentration with three pulmonary ventilations.
Table 1.
Breath to non-breath ratio under steady concentration.
| Pulmonary ventilation (LPM) | Breath to non-breath ratio under steady concentration (%) |
|---|---|
| 7 | 76.26 |
| 15 | 75.02 |
| 23 | 56.92 |
Fig. 5.
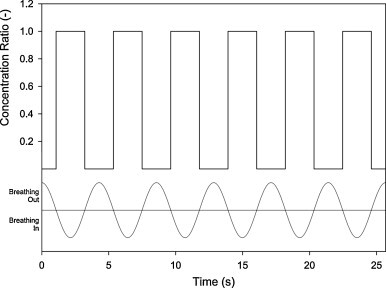
Breath to non-breath ratio under ideal steady concentration.
Based on the results obtained from Fig. 3, Fig. 4, and their comparison with Fig. 5, the experimental results match very well with the ideal profile during the inhalation period, concentration profiles of which were very steady and equaled to the background level; the result was a BNB ratio of 1. This indicates that the inhalation process does not influence the measured concentration. The profiles exhibit almost a vertical drop during exhalation cycle and the rate increasing with breathing rate. Nevertheless, no profile was observed dropping to the “theoretical” zero BNB. This is a distinct difference between the experiments and the ideal pattern which can be plausibly attributed to limitations of the setup.
Some constraints related to the experimental setup caused the ideal concentration profile (Fig. 5) to be unattainable. In the breathing circuit, a common path was used for inhalation and exhalation (Fig. 2, Path C). Although the tubing was kept short, finite time was still required for the filtered air to purge the entire tubing. Thus a certain fraction of particles were retained inside the tube, leading to non-zero concentration during exhalation. Higher breathing rates led to higher purging rates in tubing and hence, fewer particles were retained inside the tube; concentration level dropped further. Comparison of Fig. 4, Fig. 5 shows that the higher the breathing rate, the better the results match with the ideal breathing pattern. Table 1 also confirms that the BNB ratio is inversely proportional to pulmonary ventilation. They may be regarded as a limitation for estimation the dose during breathing. In spite of this constraint, it is believed that it did not affect results of episodic emission shown below.
Besides unattainability of the zero BNB ratio, it is also interesting to notice that temporal profiles for inhalation and exhalation are not symmetrical. During inhalation, all profiles are more steady and last for more than 2 s, while during exhalation, less steady profiles are observed and the holding time was less than 2 s. This can be attributed to the dynamic behavior of motorized valves as it takes finite time for them to open and close mechanically. More experiments to measure the dynamic properties of valves are required to confirm the hypothesis. Inferring from the results shown in Fig. 4, the inhalation period of 2.5 s and the exhalation period of 1.5 s were assumed hereafter. In fact, with such modifications, the results are found to be matching better with the breathing pattern.
3.2. Episodic emission
The source manikin emitted droplets towards the BTM (60 cm). Two emission velocities were tested and the whole expiratory process lasted for 2 s. Fig. 6 shows a concentration profile for non-breathing condition for emission velocity of 15 m/s. It increased steadily and reached the peak at approximately 2.3 s, and then decayed; the entire process lasted approximately 4 s. A similar profile was observed for emission velocity of 30 m/s. They were both used as benchmarks for comparison with concentration profiles with breathing.
Fig. 6.
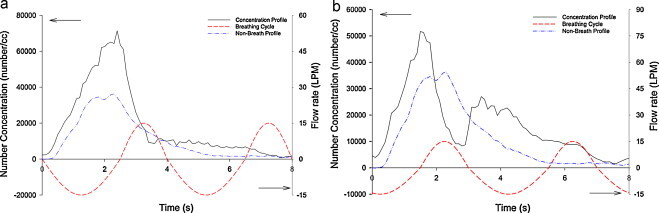
Illustrations the relationship between experimental result and breathing cycle with 15 LPM pulmonary ventilation; (a) single peak and (b) double peaks.
For the transient condition, a similar breath to non-breath ratio was defined to parameterize the effect on application of the breathing circuit. It is defined as
| (3) |
where BG represents the background concentration of the experimental trial.
Most of the experiments were conducted under transient expiratory emission with periodic breathing. Compared to the steady concentration environment, data interpretation for the temporal concentration profile was not straightforward and needed further elaboration. Fig. 7 shows a typical histogram of 25 measurements of concentration taken under identical conditions. Breathing and concentration of the approaching aerosol cloud were both unsteady; the moment when the aerosol enters the physical boundary of the mouth determines the ultimate concentration that enters the physical boundary through the mouth. Hence, distinct concentration levels were observed. In this regard, concentration levels were divided into different bins and the frequency of occurrence was counted for each bin. The results were stochastic but followed a normal-like distribution.
Fig. 7.
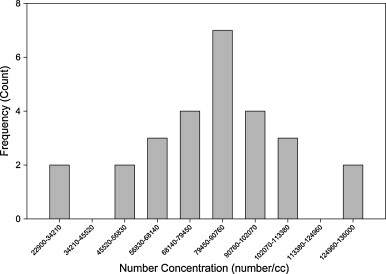
A typical concentration frequency distribution.
There were two extreme cases, depending on the moment when the aerosol cloud reached the mouth, whether it started inhalation or exhalation. For the former, aerosols can enter the respiratory system while for the latter, no aerosol can cross the physical boundary (see Fig. 3, Fig. 4). Summarizing all experimental results, two types of concentration profiles, single peak and double peak, were observed. Fig. 6(a) and (b) shows concentration profiles with emission velocity of 15 m/s under the condition of 15 LPM pulmonary ventilation together with a measured concentration without breathing. To plausibly explain the characteristics of the profiles, possible breathing cycle at the mouth was added.
As explained above, there was evidence to show the existence of “lagging” response of the motorized valves, i.e. the opening and closing times were different. Inferring from Fig. 4, exhalation cycles lasted less than 2 s while inhalation cycles lasted longer than 2 s. Hence asymmetrical cycling time was adapted for Fig. 6. The modified cycle of 2.5 s inhalation and 1.5 s of exhalation matches well with measured concentration profile. Fig. 6(a) shows a single peak profile and the concentration starts to decline when the inhalation cycle begins. On the contrary, Fig. 6(b) shows a double peak profile and it seems it was counter-intuitive as there was only one peak in the non-breath profile. It was attributed to occurrence of inhalation–exhalation during incoming of the aerosol cloud. The existence of the double peaks and the timing of decline and rise of peaks match very well with the breathing pattern. This shows that breathing can drastically alter the concentration profile (and concentration).
Fig. 8, Fig. 9 show the typical concentration profiles from the experimental run of 15 m/s emission velocity, and pulmonary rate of 15 LPM, under the DIS scheme. In Fig. 8, although each of the three measured concentration profiles show double peaks, the profiles are very different from each other as the second peak occurred at different times, early, middle and later. It is plausible to attribute this to interaction of the incoming aerosol plume with respect to the various instantaneously breathing cycles. The differential time from the first peak to the local minimum is shown in the figure and they are all approximately 1.5 s. This observation shows how the starting time of exhalation affects the ultimate concentration profile at the mouth. Similar observations were found in the MV scheme.
Fig. 8.
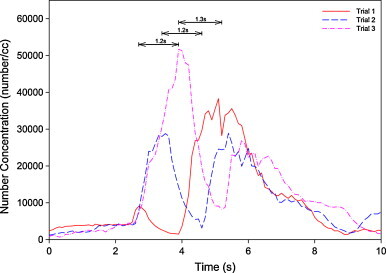
Comparison of concentration profiles of double peaks under condition of 15 m/s emission, 15 LPM pulmonary ventilation under displacement ventilation.
Fig. 9.
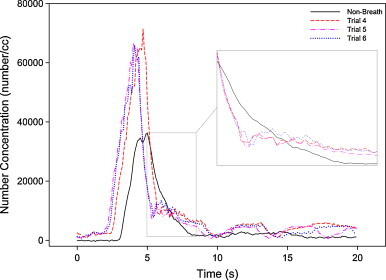
Comparison of concentration profiles of single peak under condition of 15 m/s emission, 15 LPM pulmonary ventilation under well-mixed ventilation.
Fig. 9 depicts another comparison between three concentration profiles sampled with active breathing and one profile without breathing. It can be observed that each peak was substantially higher than that without breathing, with the peak-to-peak ratio ranging from 1.3 to 1.6.
Another interesting point is the behavior of the decay. Profiles with breathing were observed to have sharp dips at the beginning of the decay part but the decay slowed afterwards (see the insert in Fig. 9). This can be explained by commencement of the exhalation cycle, which accelerates the decay, whereas the beginning of the inhalation cycle leads to slower decay. This observation was very different from the profile of the non-breath system in which concentration drops gradually to the background level.
Fig. 10 further quantifies effects of pulmonary ventilation on the decay. Three profiles of different pulmonary ventilations are shown in Fig. 10. The insert in Fig. 10 shows the decay part of the profiles in detail; best-fitted exponential decay rates are shown. It shows distinctly that the higher the pulmonary ventilation, the faster the decay during exhalation would be.
Fig. 10.
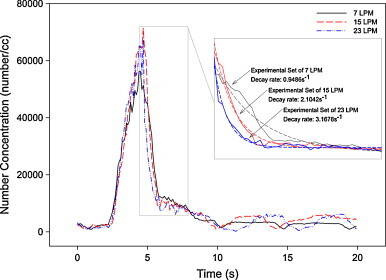
Comparison of concentration profiles of single peak of three pulmonary ventilations.
It should also be noted that different profile characteristics are exhibited and the most remarkable one is the existence of the second peak. Fig. 11 shows two concentration profiles of 15 m/s emission velocity and pulmonary rate of 7 LPM under DIS scheme to illustrate how they influence on dose. It can be observed that when the double peak concentration profile (Trial 1) started to decline, concentration profile of the single peak (Trial 2) continued to rise. This contrasting behavior of the two profiles leads to a large difference in inhaled dose (shaded area in the figure). For instance, in this case, the measured scaled-dose of inhalation in Trial 1 is only about 72% of that in Trial 2.
Fig. 11.
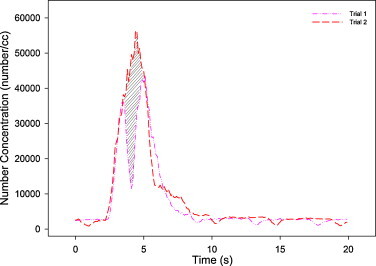
Comparison of concentration profiles of single peak and double peaks under condition of 15 m/s emission, 7 LPM pulmonary ventilation under displacement ventilation. The shaded area represents the difference in scaled-dose inhaled.
Fig. 12, Fig. 13 show values of BNB ratio for three different pulmonary rates with sneezing velocity of 30 m/s under the two ventilation schemes. Owing to the large variation in concentration profiles (e.g., Fig. 7), it can be seen that the ratio varies significantly when the breathing system is applied (for instance, see Fig. 11). Detailed comparison of Fig. 12, Fig. 13 reveals that the maximum to minimum ratio increases with pulmonary ventilation, i.e. 2.9–6.4 times for 7 LPM to 23 LPM, respectively, for the MV scheme, and 1.6–1.9 times for the DIS scheme. The variation of ratio under the MV scheme is larger than the DIS scheme. Under the MV scheme (Fig. 12), high-velocity, high-turbulence supply air disturbs the aerosol-laden jet from the expiratory source. Nevertheless, this would not be the case in the DIS scheme, where low-velocity airflow is not expected to disturb the aerosol-laden jet. Thus, the results under the MV scheme fluctuate more and a larger range of variation was caused.
Fig. 12.
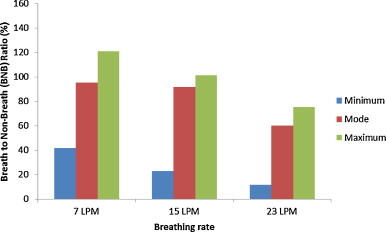
Example variation of breath to non-breath ratio of breathing flow rate of 30 m/s emission under well-mixed ventilation.
Fig. 13.
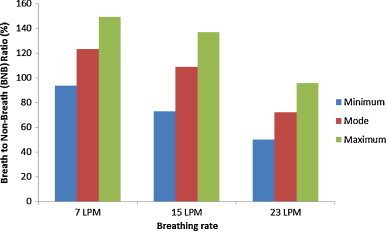
Example variation of breath to non-breath ratio of breathing flow rate of 30 m/s emission under displacement ventilation.
Fig. 14 summarizes BNB ratio for mode values for all tested scenarios. The ratio is inversely proportional to the pulmonary rate, regardless of the ventilation mode and emission velocity. This is attributed to the influence of the exhalation process. Under light physical activities where pulmonary ventilation equals 23 LPM, exhalation process plays a significant role in reducing the inhalation level. It overwhelms the enhanced inhalation in the process. The higher the breathing rate, the sharper the resultant drop of concentration would be, leading to lower the dose. However, it is possible that dose would be increased under very low and low pulmonary ventilation, where some results show BNB ratios higher than 100%. This may be attributed to the effect of dose reduction is smaller for very low and low pulmonary ventilation and, therefore, the reduction of dose caused by the exhalation process was not high enough, compared to the enhancement of the dose during inhalation. Besides, the BNB ratio under the MV scheme is lower than that of the DIS scheme under the same emission velocity and pulmonary ventilation.
Fig. 14.
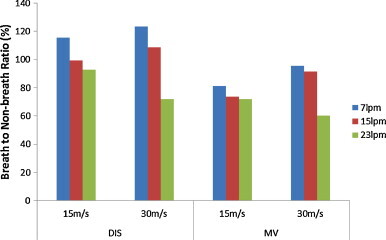
Breath to non-breath ratio of scenarios for the mode value.
Utilizing the current experimental setup can facilitate many detailed measurements to determine what parameters affect dose level. Nevertheless, due to some technical constraints, there were some limitations. Synchronization of the instant of the aerosol plume and the breathing cycle was not feasible and hence whether dose would be enhanced or reduced could not be predicted in advance. A more advanced electronic circuit is required. Furthermore, the current study focused only on ultrafine particles. However, the risk of exposure depends not only on emission speeds but also on the size of aerosols [30]. Estimation of dose in different aerosol size levels can be studied in the future.
4. Conclusions
Research interest in a diverse range of fields in control and reduction of human-to-human transmission of airborne pathogens indoors has increased. Within the space of a few years, transportation of droplets exhaled indoors has become an important topic in aerosol science, building science and public health.
The influence of breathing on estimation of inhalation level or exposure risk under expiratory process has not been studied previously. In this work, an experimental approach was adopted to investigate how breathing affects inhalation level under ventilated environments, in case of steady and episodic emission. Two thermal-regulated manikins were designed and fabricated one of which was made with regulated breathing function. A new parameter, breath to non-breath ratio, was defined to quantify the effect of breathing on concentration.
Under a steady concentration environment, inhalation does not alter the concentration profile compared to non-breathing. On the contrary, the exhalation cycle leads to substantial reduction in concentration and the reduction increases with pulmonary rate.
Inferring from the results obtained by mimicking an expiratory emission, it can be seen that the operation of the breathing system has a very substantial influence on the ultimate dose under episodic emission. Depending on whether the mouth is exhaling or inhaling when the aerosol cloud reaches it, concentration is smaller or larger than that without breathing. Although it is not a direct measurement of the amount of aerosol inhaled by the manikin, the results indicate how breathing affects the dose level.
Under light-load pulmonary ventilation (23 LPM), the exhalation process plays a significant role in reducing the dose level. During an exhalation cycle, aerosol concentration decreases considerably and the effect of exhalation overwhelms the “enhanced” dose during the inhalation process. The higher the breathing rate, the sharper is the resultant decay of concentration, leading to lower dose. Most of the times, under low pulmonary ventilation, dose is increased, compared to non-breath situations.
The MV scheme tends to lower the dose compared to the DIS scheme, and has a higher variation. Besides, the unidirectional airflow characteristics of the DIS scheme show small variations in results.
References
- 1.Blachere F.M., Lindsley W.G., Pearce T.A., Anderson S.E., Fisher M., Khakoo R., Meade B.J., Lander O., Davis S., Thewlis R.E., Celik I., Chen B.T., Beezhold D.H. Measurement of airborne influenza virus in a hospital emergency department. Clin. Infect. Dis. 2009;48:438–440. doi: 10.1086/596478. [DOI] [PubMed] [Google Scholar]
- 2.Fennelly K.P., Martyny J.W., Fulton K.E., Orme I.M., Cave D.M., Heifets L.B. Cough-generated aerosols of Mycobacterium tuberculosis – a new method to study infectiousness. Am. J. Respir. Crit. Care Med. 2004;169:604–609. doi: 10.1164/rccm.200308-1101OC. [DOI] [PubMed] [Google Scholar]
- 3.Tellier R. Review of aerosol transmission of influenza a virus. Emerg. Infect. Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stelzer-Braid S., Oliver B.G., Blazey A.J., Argent E., Newsome T.P., Rawlinson W.D., Tovey E.R. Exhalation of respiratory viruses by breathing, coughing, and talking. J. Med. Virol. 2009;81:1674–1679. doi: 10.1002/jmv.21556. [DOI] [PubMed] [Google Scholar]
- 5.Evans G.J., Peers A., Sabaliauskas K. Particle dose estimation from frying in residential settings. Indoor Air. 2008;18:499–510. doi: 10.1111/j.1600-0668.2008.00551.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z., Chen Q. Experimental measurements and numerical simulations of particle transport and distribution in ventilated rooms. Atmos. Environ. 2006;40:3396–3408. [Google Scholar]
- 7.Chao C.Y.H., Wan M.P., Sze-To G.N. Transport and removal of expiratory droplets in hospital ward environment. Aerosol Sci. Technol. 2008;42:377–394. [Google Scholar]
- 8.Wan M.P., Chao C.Y.H. Transport characteristics of expiratory droplets and droplet nuclei in indoor environments with different ventilation airflow patterns. J. Biomech. Eng. 2007;129:341–353. doi: 10.1115/1.2720911. [DOI] [PubMed] [Google Scholar]
- 9.Qian H., Li Y., Nielsen P.V., Hyldgaard C.E., Wong T.W., Chwang A.T.Y. Dispersion of exhaled droplet nuclei in a two-bed hospital ward with three different ventilation systems. Indoor Air. 2006;16:111–128. doi: 10.1111/j.1600-0668.2005.00407.x. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi T., Ishizu Y., Kato S., Murakami S. CFD analysis on characteristics of contaminated indoor air ventilation and its application in the evaluation of the effects of contaminant inhalation by a human occupant. Build. Environ. 2002;37:219–230. [Google Scholar]
- 11.Anthony T.R., Flynn M.R. Computational fluid dynamics investigation of particle inhalability. J. Aerosol Sci. 2006;37:750–765. [Google Scholar]
- 12.Zhu S.W., Kato S., Murakami S., Hayashi T. Study on inhalation region by means of CFD analysis and experiment. Build. Environ. 2005;40:1329–1336. [Google Scholar]
- 13.Brohus H., Nielsen P.V. Personal exposure in displacement ventilated rooms. Indoor Air. 1996;6:157–167. doi: 10.1034/j.1600-0668.2002.08126.x. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer I.M., Marr D.R., Glauser M.N. Impact of manikin motion on particle transport in the breathing zone. J. Aerosol Sci. 2010;41:373–383. [Google Scholar]
- 15.Bjorn E., Nielsen P.V. Dispersal of exhaled air and personal exposure in displacement ventilated rooms. Indoor Air. 2002;12:147–164. doi: 10.1034/j.1600-0668.2002.08126.x. [DOI] [PubMed] [Google Scholar]
- 16.Melikov A., Kaczmarczyk J. Influence of geometry of thermal manikins on concentration distribution and personal exposure. Indoor Air. 2007;17:50–59. doi: 10.1111/j.1600-0668.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 17.Rim D., Novoselac A. Transport of particulate and gaseous pollutants in the vicinity of a human body. Build. Environ. 2009;44:1840–1849. [Google Scholar]
- 18.Astrand P.O., Rodahl K. third edition. McGraw-Hill; New York: 1986. Textbook of Work Physiology. [Google Scholar]
- 19.Li Y., Leung G.M., Tang J.W., Yang X., Chao C.Y.H., Lin J.Z., Lu J.W., Nielsen P.V., Niu J., Qian H., Sleigh A.C., Su H.-J.J., Sundell J., Wong T.W., Yuen P.L. Role of ventilation in airborne transmission of infectious agents in the built environment—a multidisciplinary systematic review. Indoor Air. 2007;17:2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 20.Lai A.C.K., Cheng Y.C. Study of expiratory droplet dispersion and transport using a new Eulerian modeling approach. Atmos. Environ. 2007;41:7473–7484. doi: 10.1016/j.atmosenv.2007.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z., Chen X., Mazumdar S., Zhang T., Chen Q. Experimental and numerical investigation of airflow and contaminant transport in an airliner cabin mockup. Build. Environ. 2009;44:85–94. [Google Scholar]
- 22.Zhao B., Yang C.Q., Chen C., Feng C., Yang X.D., Sun L.C., Gong W., Yu L. How many airborne particles emitted from a nurse will reach the breathing zone/body surface of the patient in ISO Class-5 single-bed hospital protective environments?.—a numerical analysis. Aerosol Sci. Technol. 2009;43:990–1005. [Google Scholar]
- 23.Richmond-Bryant J. Transport of exhaled particulate matter in airborne infection isolation rooms. Build. Environ. 2009;44:44–55. doi: 10.1016/j.buildenv.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mui K.W., Wong L.T., Wu C.L., Lai A.C.K. Numerical modeling of exhaled droplet nuclei dispersion and mixing in indoor environments. J. Hazard. Mater. 2009;167:736–744. doi: 10.1016/j.jhazmat.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pantelic J., Sze-To G.N., Tham K.W., Chao C.Y.H., Khoo Y.C.M. Personalized ventilation as a control measure for airborne transmissible disease spread. J. R. Soc. Interface. 2009;6:715–726. doi: 10.1098/rsif.2009.0311.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao N.P., Niu J.L. Transient CFD simulation of the respiration process and inter-person exposure assessment. Build. Environ. 2006;41:1214–1222. doi: 10.1016/j.buildenv.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai A.C.K., Wong S.L. Experimental investigation of exhaled aerosol transport under two ventilation systems. Aerosol Sci. Technol. 2010;44:444–452. [Google Scholar]
- 28.Lai A.C.K., Wong S.L. Expiratory aerosol transport in a scaled chamber under a variety of emission characteristics: an experimental study. Aerosol Sci. Technol. 2011;45:909–917. [Google Scholar]
- 29.Chao C.Y.H., Wan M.P., Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S., Li Y., Xie X., Katoshevski D. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. J. Aerosol Sci. 2009;40:122–133. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sze-To G.N., Chao C.Y.H. Review and comparison between the Wells–Riley and dose–response approaches to risk assessment of infectious respiratory diseases. Indoor Air. 2010;20:2–16. doi: 10.1111/j.1600-0668.2009.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


