Highlights
-
•
We tested serum amyloid A and haptoglobin serum levels in Eimeria zuernii infections.
-
•
Haptoglobin levels rose during clinical eimeriosis.
-
•
A rise in serum amyloid A and haptoglobin correlated well with weight loss.
-
•
Acute phase proteins are important in the immunological mechanisms of eimeriosis.
Keywords: Acute phase proteins, Eimeria zuernii, Coccidiosis, Inflammation, Calves, Immunology
Abstract
Acute phase proteins (APPs) have been demonstrated to be useful in evaluating general health stress and diseases in cattle. Serum amyloid A (SAA) and haptoglobin (Hp) are APPs that are produced during inflammation, and likely play a role in host immunological defence against Eimeria infection and the associated intestinal tissue damage. We investigated the involvement of SAA and HP in an experimental study, including three groups of calves: a control group (group 0, n = 11), and two groups infected with either 150,000 or 250,000 Eimeria zuernii oocysts (group 1 (n = 11) and group 2 (n = 12), respectively). The calves were monitored for 28 days and data was collected on oocyst excretion, faecal score, animal weight, and SAA and Hp serum concentrations. Generalized linear mixed models showed that the clinical symptoms, indicated by an increase in the number of oocysts in the faeces and severe diarrhoea, manifested at patency for group 1 and 2. Serum Hp and SAA levels also increased during this period. Hp appeared to be a more sensitive marker than SAA, and differences between groups 1 and 2 were observed only for Hp. Linear regression models showed a negative association between weight gain and Hp concentrations, calculated as the area under the curve (AUC) during the overall experimental period and the patency period. A similar result was seen for SAA only during the patency period. This result supports the assumption that reduced weight gain due to E. zuernii infection is an immunologically driven process that involves an increase in APPs. A random intercept regression model of oocyst shedding groups showed that calves shedding 1–500 oocysts had reduced concentrations of Hp, indicating that a different immunological reaction occurs during mild shedding of E. zuernii oocysts than during more intensive shedding. A similar model was used to examine associations between faecal scores and Hp concentrations for each group. Group 2 calves with haemorrhagic diarrhoea displayed higher Hp levels than calves in that group with lower faecal scores, which may be in response to an increased demand for Hp in the repair process as a result of haemolysis. APPs seem to play an important role in determining the course of E. zuernii infection in calves, which may enhance our understanding of the immunological reaction and development of this disease.
1. Introduction
The acute phase response is an early immune response that is crucial for early immune defence against events such as inflammation, neoplasia, and infections (Cray et al., 2009). Early in infections, or during tissue damage, pro-inflammatory cytokines are released: IFN-γ and IL-6 from affected cells and TNF-α and IL-1β from mononuclear cells (van Miert, 1995, Heinrich et al., 1990). When cytokines reach the liver, they stimulate the hepatocytes to release acute phase proteins (APPs) into the blood stream (Heinrich et al., 1990). APPs circulate in the blood and help to stabilize the internal environment and speed the healing process (Cray et al., 2009). In response to inflammation, APPs may increase (positive APP) or decrease (negative APP) plasma protein levels. APPs in cattle include serum amyloid A (SAA) and haptoglobin (Hp).
In humans, APPs are well established as markers for several physical illnesses, whereas their application in veterinary medicine has developed at a slower pace (Murata et al., 2004, Petersen et al., 2004, Ceron et al., 2005). In calves, APPs have been used to measure the general health of calves, and as indicators of stress, inflammation related to hoof diseases, and various bacterial and viral infections, including coronavirus, bovine respiratory syncytial virus, Escherichia coli, bovine adenovirus, and mastitis related infections (Gånheim et al., 2007, Saco et al., 2008, Suojala et al., 2008, Angen et al., 2009, Kujala et al., 2010, Orro et al., 2011, Pyörälä et al., 2011). Recently reference values of Hp and SAA for clinically healthy dairy calves under natural condition up to 2 months of age have been published (Seppä-Lassila et al., 2013).
Bovine Eimeria species are common and important coccidia of calves that can lead to severe disease (eimeriosis). The clinical disease and subclinical effects can potentially result in long-term losses regarding animal health and production (Fitzgerald, 1980, Stockdale, 1981, Daugschies et al., 1986, Lassen and Ostergaard, 2012). After a sporulated oocyst has excysted in the gut, the parasite undergoes several asexual and sexual cycles of invasion and destruction of the intestinal cells. Eimeriosis due to Eimeria (E.) zuernii manifests as massive tissue damage accompanied by the release of oocysts 2–3 weeks after infection (Daugschies and Najdrowski, 2005). Changes in APP profiles may serve as a useful tool for estimating the health of calves by determining of the impact of pathogens, such as Eimeria, in the clinical and subclinical stages, and may potentially serve as a tool for prognosis of the severity of disease (Hashemnia et al., 2011).
Previous experimental studies investigated the variations in blood chemistry, electrolyte concentrations, acidity, and blood gases over the course of a 28-day experimental period after infecting groups of calves with single doses of 0, 150,000, or 250,000 E. zuernii oocysts (Bangoura and Daugschies, 2007a, Bangoura and Daugschies, 2007b, Bangoura et al., 2007). In this study, we assessed the expression of SAA and Hp in the serum of these experimental groups to test the hypothesis that these levels are unchanged in the early immune response of calves to E. zuernii infection.
2. Materials and methods
2.1. Study design
Samples originated from 41 Holstein-Mix calves. The calves were vaccinated against Bovine Rota and Corona virus via the dam. The animals were between 10 and 28 days old and were determined to be healthy by a veterinarian at the beginning of the experimental period based on observation of general animal behaviour, good overall health, body temperature between 38.5 °C and 39.5 °C, normal breathing, skin turgidity and heart auscultation, and presence of wounds and diarrhoea. All animals were weighed individually on a mobile scale once weekly in 7-day intervals starting on day 0 or 1 of the study, respectively, until the end of the study. Calves were divided into three infection groups: group 0 (n = 14), uninfected control calves; group 1 (n = 11), calves infected orally with 150,000 sporulated E. zuernii oocysts; and group 2 (n = 16), calves infected orally with 250,000 sporulated E. zuernii oocysts. Interference of E. coli K99 and Giardia were excluded at the onset of diarrhoea, or 18 day post infection (dpi) for controls, using antigen tests (FASTest E. coli K99 strip, and FASTest GIARDIA strip, Megacor, Hoerbranz, Austria). Samples taken simultaneously with the antigen tests were investigated to confirm absence of Cryptosporidium spp. using a carbol fuchsin staining technique (Heine 1982). Calves were kept under the same housing conditions (same feed, two calves per pen, same stable, and air-conditioned rooms). The infective material was isolated from a commercial farm, passaged in calves by experimental infection before use and determined to be comprised of greater than 97% E. zuernii oocysts and less than 3% Eimeria ellipsoidalis oocysts. Blood samples were collected in vacuum tubes (Vacuette® serum clot activator tubes, Greiner bio-one, Kremsmuenster, Austria) on days 0, 1, 3, 7, 9, 12, and 14–28. Blood was drawn from all animals during the experimental period, except on days 3 and 9, upon which 10 and 30 calves were sampled, respectively. Faecal samples (50–100 g) were collected on the sampling days by digital stimulation of the anus. The consistency was scored as: (1) normal to pasty, (2) semiliquid to liquid, (3) watery, and (4) haemorrhagic and/or with tissue according to Mundt et al. (2005). Oocyst excretion was determined using the quantitative McMaster method, as described previously (Thienpont et al., 1990, Bangoura and Daugschies, 2007b).
As the APP response is not disease-specific and diseases other than eimeriosis can influence the results, seven calves were removed from statistical analysis because of clinical signs unrelated to eimeriosis. As a result, the total number of calves in this study was smaller than in the original studies (Bangoura et al., 2007, Bangoura and Daugschies, 2007a, Bangoura and Daugschies, 2007b). From group 0, two calves were removed because of arthritis and one because of conjunctivitis and anorexia. From group 2, four calves were removed because of arthritis. The final number of calves in group 0, group 1 and group 2 were 11, 11 and 12, respectively.
2.2. Acute phase proteins detection
Serum concentrations (mg/l) of Hp were determined using the haemoglobin binding assay described previously by Makimura and Suzuki (1982) and with modifications described by Seppä-Lassila et al. (2013). The working range of the assay was 60–1900 mg/l. The inter-assay and intra-assay coefficients of variation (CV) for Hp analysis were <10% (mean concentrations of control samples 112 mg/l; n = 20 and 458 mg/l; n = 20) and <13% (mean concentrations of control samples 125 mg/l; n = 27 and 471 mg/l; n = 25), respectively.
The serum concentrations of SAA were measured with a commercial multispecies ELISA kit (Phase SAA Assay, Tridelta Development Ltd., Dublin, Ireland) according to the manufacturer’s instructions for cattle. Serum samples were initially diluted 1:1,000. If the concentration was over of the range of standard curve (greater than 150 mg/l), they were diluted as necessary and re-assayed. Intra- and inter-assay CVs were <9% (mean concentrations of control samples 16.4 mg/l; n = 20 and 104.5 mg/l; n = 20) and <12% (mean concentrations of control samples 15.1 mg/l; n = 18 and 119.2 mg/l; n = 18), respectively.
2.3. Statistics
Generalized linear mixed models (GLMM) were used to explore overall time trend differences in APP concentrations, faecal scores, and oocyst shedding between the control and infected groups. The same models allowed investigation of associations between APP concentrations and daily oocyst excretion and faecal scores. Calves were included as random intercepts and polynomials of time (days), with interactions with the infection group added as fixed effects in increasing order. The overall time trend differences between groups were tested with the F-test. Bonferroni coefficients for multiple comparisons were used to correct P-values. As the time between sampling was not the same, an isotropic spatial exponential covariance structure was used for modelling serial correlations of repeated measurements at the within-calf level in all models.
The general equation of the linear mixed models for evaluating changes over time between the infection groups was defined as follows:
| Yi = β0 + β1X1(i) × X4(i) + β2X2(i) × X4(i) + β3X3(i) × X4(i) + ucalf(i) + ϵi |
in which Y is the outcome variable, β0 is the intercept, β1-3 are the sizes of the effects of independent variables X 1-3 (day, day2 and day3, respectively), and X 4 (infection group), u calf(i) is the random effect (calf) with the spatial exponential covariance structure for repeated measures and ϵi is an error term.
Initially, the APP models included calf age (days of age) at the beginning of the experiment as a covariate and faecal score (4-level factor variable; normal to pasty, semiliquid to liquid, watery, and haemorrhagic and/or with tissue), oocysts shedding as 3-level factor variable (oocysts per gram (OPG) faeces values: 0, 1–500 and <500), and their interactions with the infection group were added to the models with polynomials of time and experimental group interactions. A backward elimination procedure was performed for the final models. The final model for Hp included day, quadratic and cubic terms of day and their interactions with the infection group, calf age at the beginning of experiment, faecal score and oocyst shedding. The final model for SAA included day, quadratic and cubic terms of day and their interactions with the infection group and calf age at the beginning of experiment. The models assumptions were verified by scatter and normality plots of standardized residuals and logarithmic transformation of oocyst count, SAA and Hp were used. Gaussian link function was used in APP GLMM models and Poisson link function was used in models for faecal scores and oocyst shedding. Model predictions were back-transformed from a logarithmic scale in all models. Generalized linear mixed models were fitted using the GLIMMIX procedure with the SAS/STAT 9.2 (SAS Institute Inc., Cary, NC, USA) software.
For studying the associations between daily weight gain of the calves and APP responses, the area under the curve (AUC) was calculated using a trapezoid method (Microsoft Excel 2010, California, USA) for the overall experimental period (days 0–28), for the prepatent period (days 0–17), and the patent period (days 18–28) for both SAA and Hp. Linear regression models were used in the analysis of AUC data. Daily weight gain (g/day) was used as a response variable, the infection groups were included in all models, and SAA and Hp AUC values separately for the overall period, prepatent, and patent period, and their interactions with the infection groups were included. Interaction terms were excluded from all final models as non-significant. For linear regression models, STATA 11.0 (Stata Corporation, Texas, USA) software was used.
2.4. Ethical compliance statement
All animal experiments were conducted in compliance with the European and German national animal welfare regulations and were approved by the responsible authority (district government Cologne, North Rhine-Westfalia, Germany).
3. Results
3.1. Faecal scores and oocysts excretion
Results for faecal scores and oocyst counts were originally published by Bangoura and Daugschies (2007b). The data given here represent only the calves included in the APP analysis. Changes in faecal scores and oocyst shedding together with the model predictions for the different groups during the experimental period can be found in supplementary material (Fig. 1S). Both infection groups 1 and 2 developed clinical signs (diarrhoea) and a rise in faecal oocyst counts at the onset of the patent period, in contrast to the uninfected control group 0. However, there was no difference between the infection groups with respect to faecal scores and faecal oocyst concentrations.
3.2. Hp and SAA in relation to weight gain
The association between Hp (Table 1 ) and SAA (Table 2 ) and weight gain for the overall (Model 1), prepatent (Model 2), and patent period (Model 3) was investigated by calculating the AUC. Weight gains of the three infection groups can be found in the electronic supplements (Fig. 2S). A negative association between Hp and weight gain was observed during the overall and patent periods, but not during the prepatent period. Similarly, the model for SAA revealed a negative association with weight gain during the patent period. There were no significant interactions between infection groups and APPs in the models.
Table 1.
Results of linear regression models of the association of daily weight gain (g/day) of calves with haptoglobin (Hp): area under the curve (AUC) during all study periods (0–28 days), during the prepatent period (0–17 days) and the patent period (18–28 days). All experimental groups [uninfected control group (group 0; n = 11), calves orally infected with 150,000 Eimeria zuernii oocysts at day 0 (group 1; n = 11) and calves orally infected with 250,000 E. zuernii oocysts at day 0 (group 2; n = 12)] were included to control for possible confounding effects.
| Variable | Coefficient | 95% CI of coef. | P-value |
|---|---|---|---|
| Model 1 (0–28 days) | |||
| Group 1 (vs. group 0) | −90.5 | −182.0; 0.999 | 0.052 |
| Group 2 (vs. group 0) | −134.1 | −225.3; −42.9 | 0.005 |
| Hp AUC (0–28 days; mg/l) | −0.029 | −0.043; −0.014 | 0.000 |
| Intercept | 748.3 | 667.8; 828.9 | 0.000 |
| Model 2 (0–17 days) | |||
| Group 1 (vs. group 0) | −131.8 | −250.1; −13.5 | 0.03 |
| Group 2 (vs. group 0) | −193.6 | −299.3; −87.9 | 0.001 |
| Hp AUC (0-17 days; mg/l) | 0.009 | −0.049; 0.001 | 0.645 |
| Intercept | 667.7 | 551.8; 783.8 | 0.000 |
| Model 3 (18–28 days) | |||
| Group 1 (vs. group 0) | −118.5 | −199.9; −37.1 | 0.006 |
| Group 2 (vs. group 0) | −119.4 | −204.7; −34.0 | 0.008 |
| Hp AUC (18–28 days; mg/l) | −0.039 | −0.055; −0.023 | 0.000 |
| Intercept | 700.8 | 639.5; 762.1 | 0.000 |
Table 2.
Results of linear regression models of the association of daily weight gain (g/day) of calves with serum amyloid A (SAA): area under the curve (AUC) during all study periods (0–28 days), during the prepatent period (0–17 days) and the patent period (18–28 days). All experimental groups [uninfected control group (group 0; n = 11), calves orally infected with 150,000 Eimeria zuernii oocysts at day 0 (group 1; n = 12) and calves orally infected with 250,000 E. zuernii oocysts at day 0 (group 2; n = 12)] were included to control for possible confounding effects.
| Variable | Coefficient | 95% CI of coef. | P-value |
|---|---|---|---|
| Model 1 (0–28 days) | |||
| Group 1 (vs. group 0) | −104.2 | −230.6; 22.1 | 0.102 |
| Group 2 (vs. group 0) | −184.4 | −289.9; −79.3 | 0.001 |
| SAA AUC (0–28 days; mg/l) | −0.052 | −0.144; 0.041 | 0.262 |
| Intercept | 701.4 | 579.9; 822.9 | 0.000 |
| Model 2 (0–17 days) | |||
| Group 1 (vs. group 0) | −141.6 | −257.3; −25.9 | 0.018 |
| Group 2 (vs. group 0) | −195.5 | −301.4; −89.7 | 0.001 |
| SAA AUC (0–17 days; mg/l) | −0.004 | −0.128; 0.119 | 0.946 |
| Intercept | 650.7 | 536.3; 765.0 | 0.000 |
| Model 3 (18–28 days) | |||
| Group 1 (vs. group 0) | −64.1 | −186.6; 58.3 | 0.293 |
| Group 2 (vs. group 0) | −149.5 | −255.6; −43.4 | 0.007 |
| SAA AUC (18–28 days; mg/l) | −0.19 | −0.361; −0.019 | 0.03 |
| Intercept | 714.3 | 621.8; 806.8 | 0.000 |
3.3. Change in Hp and SAA patterns in response to E. zuernii infection
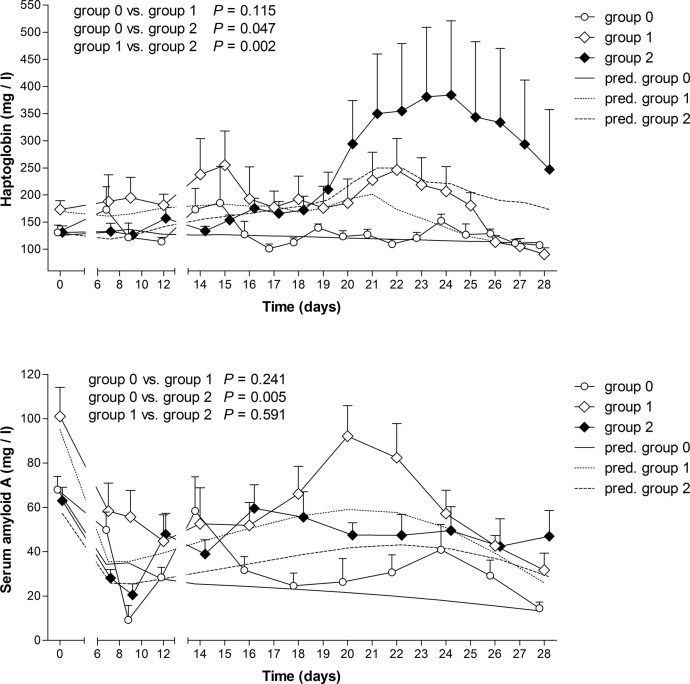
The changes in Hp and SAA concentrations in the blood during the experimental period are shown in Fig. 1 for each of the three infection groups. The Hp response showed large variations between animals. Concentrations of Hp in the blood were generally lower during the prepatent period, but increased for infection groups during the patent period when compared to the control group. A significant increase in Hp concentrations was observed for the 28 days of the experimental period in calves infected with 250,000 oocysts (group 2), mainly due to a rise in the patent period. The Hp concentration in group 2 also was higher than in calves infected with 150,000 oocysts (group 1). As with Hp blood concentrations, there was a relatively large individual variability in SAA concentrations. SAA concentrations were already high at the beginning of the study, in contrast to the Hp concentrations, and during the prepatent period there was no clear increase in SAA levels in the infection groups compared to the control group. There were no associations between SAA concentrations and oocyst shedding or faecal scores of calves. However, there was a significant difference in time trends between calves infected with 250,000 oocysts and non-infected calves (Fig. 1).
Fig. 1.
Mean (±SEM) haptoglobin (Hp) concentrations (upper panel) and serum amyloid A (SAA) concentrations (lower panel) in the two experimental groups and control group during the experimental period. ○, uninfected control group (group 0; n = 11); ◊, calves orally infected with 150,000 Eimeria zuernii oocysts at day 0 (group 1; n = 11); and ♦, calves orally infected with 250,000 E. zuernii oocysts at day 0 (group 2; n = 12). Lines represent model predictions in different groups. P-values are corrected with Bonferroni coefficient for multiple comparisons.
3.4. Hp concentrations in relation to oocyst shedding
Based on the GLMM model for Hp concentrations produced during the experimental period, the association between Hp concentrations and E. zuernii oocyst shedding was examined. Hp concentrations decreased (P = 0.04) in calves with low levels of oocyst shedding (1–500 OPG faeces) compared with calves not shedding oocysts, but not compared to calves shedding more than 500 OPG.
3.5. Hp concentrations in relation to faecal scores
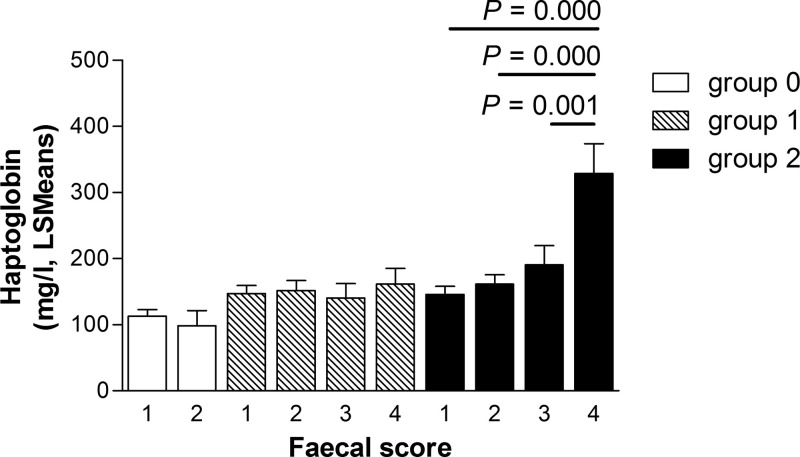
The association between Hp concentrations in the blood of calves and the faecal score categories in the infection groups during the experimental period were also based on the GLMM model for Hp concentrations. Calves infected with 250,000 E. zuernii oocysts that exhibited haemorrhagic diarrhoea during the patent period had increased concentrations of Hp in the blood compared to calves in this group with other faecal scores (Fig. 2 ).
Fig. 2.
Faecal score (1 = normal to pasty; 2 = semiliquid to liquid; 3 = watery and 4 = haemorrhagic and/or with tissue) in relation to haptoglobin (Hp) concentrations was evaluated by a random intercept regression model with uninfected control calves (group 0; n = 11), calves orally infected with 150,000 Eimeria zuernii oocysts at day 0 (group 1; n = 11) and calves orally infected with 250,000 E. zuernii oocysts at day 0 (group 2; n = 12). The graph shows back-transformed (from a logarithmic scale) least squared means (LSMeans) and standard errors (SE). P-values are corrected with Bonferroni coefficient for multiple comparisons.
4. Discussion
According to Levine (1985), the onset of the patent period for E. zuernii is 15–17 days after infection. The observed increase in oocyst numbers in the faeces and increasing faecal scores from day 15 onwards in this study correlated well with the known clinical characteristics of eimeriosis (Fig. 1S). According to this study, a difference of 100,000 E. zuernii oocysts between group 1 and 2 resulted in differences in faecal oocyst concentrations and faecal consistency scores on specific days, but differences in the overall patterns during the experiment period were only borderline significant.
Holst and Svensson (1994) previously explored fibrinogen (Fb) as an APP in the blood during experimental and natural infection with Eimeria alabamensis, but had only limited success. To our knowledge, our study is the first to examine the relationship between Hp and SAA levels and experimental infections with Eimeria in cattle, although studies on SAA and Hp levels in response to Eimeria infection have been performed in rabbits and goats. Rabbits infected with 10,000 Eimeria stiedai oocysts were reported by Freitas et al. (2011) to exhibit increased APP concentrations of ceruloplasmin, transferrin and Hp from 7 to 28 days post-infection. Infection of goat kids with 1,000 or 100,000 sporulated Eimeria arloingi oocysts was also reported to increase blood levels of Hp and SAA (Hashemnia et al., 2011).
Clinical cattle eimeriosis gains its relevance mainly due to the resulting reduction in weight gain and increase in mortality. In the present study, reduced weight gain in infected animals was linked to the expression of both Hp and SAA during the onset of the patent period, and expression of Hp over the entire experimental period. This result supports the theory that the reduced weight gain commonly observed during eimeriosis is the result of an immunological reaction that coincides with the production of Hp and SAA at the time of clinical disease manifestation. Arthington et al. (2005) demonstrated previously the association between higher APP levels and reduced weight gain in beef calves, and studies in growing pigs demonstrated a negative relationship between Hp concentrations and body weight gain (Hiss and Sauerwein, 2003, Sauerwein et al., 2005). The negative association between APPs and weight gain could be explained by a decrease in food intake due to the effect of pro-inflammatory cytokines on the central nervous system (Langhans and Hrupka, 1999). Alternatively, it could result from enhanced catabolism of body tissues (Webel et al., 1997). In experimental infection of goat kids with E. arloingi (Hashemnia et al., 2011), in both infection groups, SAA concentrations and increased levels of TNF-α and IFN-γ were associated. This further supports the hypothesis that APPs and weight gain are associated via the action of pro-inflammatory cytokines during infection.
Most tissue damage occurs at the onset of the patent period, and it is expected that the largest increase in APP levels would occur also at this time point in the experiment. The concentrations of Hp increased as predicted, but only in group 2, and a dose-dependent difference between group 1 and 2 was observed for Hp (Fig. 1). Interestingly, the concentration of Hp also appeared to decline near the end of the 2-week patent period when the faecal oocyst excretion also declined. This supports the hypothesis of a relationship between the release of oocysts, resulting in tissue damage, the production of APPs, and inappetence, resulting in weight loss. However, changes in SAA concentrations during infection showed no clear association between the control group and group 1, or between the infection groups. At the same time, considerable fluctuations in SAA concentrations were observed during the prepatent period (Fig. 1), but generally above the reported median value (32 mg/l, min-max 2–150 mg/l) for calves above 14 days of age using the same assay (Seppä-Lassila et al., 2013). The different patterns of Hp and SAA concentrations observed in this study could be a reflection of the distinctive cytokine response of calves to E. zuernii infection, such as TNF-α, IL-1 and IL-6 that are predominant pro-inflammatory cytokines that stimulate the hepatic synthesis of APPs (Baumann and Gauldie, 1994, Alsemgeest et al., 1996).
The data presented in Fig. 1 may be explained by SAA being a more sensitive inflammatory marker than Hp in cattle (Alsemgeest et al., 1994, Werling et al., 1996, Müller-Doblies et al., 2004), and several factors can affect SAA results. Calves exhibit elevated concentrations of SAA, but not Hp, during the first 3 weeks after birth (Orro et al., 2008). The differences observed between calves infected with 250,000 oocysts compared to non-infected calves may be best explained by the naturally occuring changes in SAA early in the calves life. This appear to coinciding with the the timing of the prepatent period in the experiment, and less likely to be due to a rise in SAA concentrations in infection groups.
The immunological response to intestinal damage is crucial to initiate repair mechanisms and limit the loss of fluids, which has been observed during experimental infection with Eimeria bovis (Daugschies et al., 1988, Daugschies et al., 1997). It is possible that mild Eimeria infections causing less severe tissue damage may interact differently with the local immune response than massive infections associated with more severe tissue destruction. In the case of mild disease, APPs may be used locally to limit infection and initiate the repair process, leading to a transient systemic reduction in APP levels, while more severe damage may initiate a systemic up-regulation of APPs. The main function of Hp is binding of free haemoglobin (Eaton et al., 1982), and increased consumption of circulating Hp will increase during haemolysis. This may also be reflected by the observed reduction in Hp concentrations when less than 500 OPG were shed by calves, resembling mild tissue damage, but not for calves shedding more than 500 OPG. When more severe tissue damage occurs, stronger inflammatory stimulus will up-regulate the hepatic synthesis of Hp, and systemic concentrations will increase despite higher consumption of Hp due to haemorrhage. Higher levels of Hp, above the previously reported reference value for calves up to 2 months of age (<196 mg/l) (Seppä-Lassila et al., 2013), were observed in haemorrhagic and diarrheic faeces in calves infected with 250,000 E. zuernii oocysts compared to calves in the same group with other faecal scores (Fig. 2). The presence of blood in the diarrhoea of calves with E. zuernii infection indicates tissue damage as a consequence of the release of oocysts that expose and potentially rupture intestinal capillaries (Blaxter and Wood, 1958). It is possible that the additional fluid loss, loss of haemoglobin, and presence of an acute inflammatory reaction due to haemorrhage increases the demand for Hp to speed up the repair of the tissue, resulting in the observed rise in Hp.
5. Conclusion
Acute phase proteins appear to be linked with the physiological and immunological mechanisms of eimeriosis in cattle and demonstrate a relationship between the rise in Hp and the development of severe clinical eimeriosis due to E. zuernii infection. This effect was observed mainly during the patent period. The rise in Hp and SAA was correlated with reduced weight gain in infected calves during the patent period. Thus, APPs are an indicator for clinically relevant cases of eimeriosis, but their diagnostic value is limited. In the future, investigation into the relationship between APPs and Eimeria infections at lower experimental infection doses, especially with regard to APP level progression during natural infections, will be necessary.
Conflicts of interest
No authors have been paid for this work by an undisclosed funding source. In vivo trials were performed at Bayer Health Care AG, Division of Animal Health, Monheim, Germany, in 2004, as stated previously (Bangoura and Daugschies 2007a), but this did not impact the study design or evaluation of the presented data.
Acknowledgements
We thank Kadri Kääramees for valuable assistance in the laboratory analysis of APPs. This work was supported by institutional research funding (IUT number 8-1) of the Estonian Ministry of Education and Research and Estonian Science Foundation, ETF9433 grant: Farmers motivation to complex disease control in cattle farms.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vetpar.2015.06.024.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Alsemgeest S.P.M., Kalsbeek H.C., Wensing T., Koeman J.P., van Ederen A.M., Gruys E. Concentrations of serum amyloid A (SAA) and haptoglobin (Hp) as parameters of inflammatory diseases in cattle. Vet. Q. 1994;16:21–23. doi: 10.1080/01652176.1994.9694410. [DOI] [PubMed] [Google Scholar]
- Alsemgeest S.P.M., van't Klooster G.A.E., van Miert A.S.J.P.A.M., Hulskamp-Koch C.K., Gruys E. Primary bovine hepatocytes in the study of cytokine induced acute-phase protein secretion in vitro. Vet. Immunol. Immunopathol. 1996;53:179–184. doi: 10.1016/0165-2427(96)05602-4. [DOI] [PubMed] [Google Scholar]
- Angen O., Thomsen J., Larsen L.E., Larsen J., Kokotovic B., Heegaard P.M., Enemark J.M. Respiratory disease in calves: microbiological investigations on transtracheally aspirated bronchoalveolar fluid and acute phase protein response. Vet. Microbiol. 2009;137:165–171. doi: 10.1016/j.vetmic.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthington J.D., Spears J.W., Miller D.C. The effect of early weaning on feedlot performance and measures of stress in beef calves. J. Anim. Sci. 2005;83:933–939. doi: 10.2527/2005.834933x. [DOI] [PubMed] [Google Scholar]
- Bangoura B., Daugschies A. Influence of experimental Eimeria zuernii infection in calves on electrolyte concentrations, acid-base balance and blood gases. Parasitol. Res. 2007;101:1637–1645. doi: 10.1007/s00436-007-0705-6. [DOI] [PubMed] [Google Scholar]
- Bangoura B., Daugschies A. Parasitological and clinical parameters of experimental Eimeria zuernii infection in calves and influence on weight gain and haemogram. Parasitol. Res. 2007;100:1331–1340. doi: 10.1007/s00436-006-0415-5. [DOI] [PubMed] [Google Scholar]
- Bangoura B., Daugschies A., Fuerll M. Influence of experimental Eimeria zuernii infection on clinical blood chemistry in calves. Vet. Parasitol. 2007;150:46–53. doi: 10.1016/j.vetpar.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Baumann H., Gauldie J. The acute phase response. Immunol. Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Blaxter K.L., Wood W.A. Some observations on the biochemical events associated with diarrhea in calves. Vet. Rec. 1958;65:889. [Google Scholar]
- Ceron J.J., Eckersall P.D., Martynez-Subiela S. Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet. Clin. Pathol. 2005;34:85–99. doi: 10.1111/j.1939-165x.2005.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Cray C., Zaias J., Altman N.H. Acute phase response in animals: a review. Comp. Med. 2009;59:517–526. [PMC free article] [PubMed] [Google Scholar]
- Daugschies A., Akimaru M., Bürger H.J. Experimentelle Eimeria bovis infektionen beim Kalb: 1. Parasitologische und klinische Befunde. Dtsch. Tierürztl. Wschr. 1986;93:377–464. (in German) [PubMed] [Google Scholar]
- Daugschies A., Akimaru M., Bürger H.-J., Rommel M. Experimentelle Eimeria bovis-Infektionen beim Kalb: 2. Kalzium-,Magnesium- und Phosphorhaushalt. Wien. Tierürztl. Monatsschr. 1988;75:480–486. (in German) [Google Scholar]
- Daugschies A., Bürger H.-J., Akimaru M. Effects of experimental infection with Eimeria bovis on the balance of sodium, potassium and water in calves. Parasitol. Int. 1997;46:159–169. [Google Scholar]
- Daugschies A., Najdrowski M. Eimeriosis in cattle: current understanding. J. Vet. Med. B. Infect. Dis. Vet. Public Health. 2005;52:417–427. doi: 10.1111/j.1439-0450.2005.00894.x. [DOI] [PubMed] [Google Scholar]
- Eaton J.W., Brandt P., Mahoney J.R., Lee J.T. Haptoglobin: a natural bacteriostat. Science. 1982;215:691–692. doi: 10.1126/science.7036344. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.R. The economic impact of coccidiosis in domestic animals. Adv. Vet. Sci. Comp. Med. 1980;24:121–143. [PubMed] [Google Scholar]
- Freitas F.L., Yamamoto B.L., Freitas W.L., Fagliari J.J., Almeida Kde S., Machado R.Z., Machado C.R. Systemic inflammatory response indicators in rabbits (Oryctolagus cuniculus) experimentally infected with sporulated oocysts of Eimeria stiedai (Apicomplexa: Eimeriidae) Rev. Bras. Parasitol. Vet. 2011;20:121–126. doi: 10.1590/s1984-29612011000200006. [DOI] [PubMed] [Google Scholar]
- Gånheim C., Alenius S., Persson Waller K. Acute phase proteins as indicators of calf herd health. Vet. J. 2007;173:645–651. doi: 10.1016/j.tvjl.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemnia M., Khodakaram-Tafti A., Razavi S.M., Nazifi S. Changing patterns of acute phase proteins and inflammatory mediators in experimental caprine coccidiosis. Korean J. Parasitol. 2011;49:213–219. doi: 10.3347/kjp.2011.49.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. A simple technic for the demonstration of cryptosporidia in feces. Zentralbl. Veterinarmed. B. 1982;29:324–327. (in German) [PubMed] [Google Scholar]
- Heinrich P.C., Castell J.V., Andus T. Interleukin-6 and the acute phase response. Biochem. J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiss S., Sauerwein H. Influence of dietary ss-glucan on growth performance, lymphocyte proliferation, specific immune response and haptoglobin plasma concentrations in pigs. J. Anim. Physiol. Anim. Nutr. Berl. 2003;87:2–11. doi: 10.1046/j.1439-0396.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- Holst H., Svensson C. Changes in the blood composition of calves during experimental and natural infections with Eimeria alabamensis. Res. Vet. Sci. 1994;57:377–383. doi: 10.1016/0034-5288(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Kujala M., Orro T., Soveri T. Serum acute phase proteins as a marker of inflammation in dairy cattle with hoof diseases. Vet. Rec. 2010;166:240–241. doi: 10.1136/vr.b4770. [DOI] [PubMed] [Google Scholar]
- Langhans W., Hrupka B. Interleukins and tumor necrosis factor as inhibitors of food intake. Neuropeptides. 1999;33:415–424. doi: 10.1054/npep.1999.0048. [DOI] [PubMed] [Google Scholar]
- Lassen B., Ostergaard S. Estimation of the economical effects of Eimeria infections in Estonian dairy herds using a stochastic model. Prev. Vet. Med. 2012;106:258–265. doi: 10.1016/j.prevetmed.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Levine N. The Iowa University State Press; Iowa: 1985. Veterinary Protozoology; pp. 148–149. [Google Scholar]
- Makimura S., Suzuki N. Quantitative determination of bovine serum haptoglobin and its elevation in some inflammatory diseases. Jpn. J. Vet. Sci. 1982;44:15–21. doi: 10.1292/jvms1939.44.15. [DOI] [PubMed] [Google Scholar]
- van Miert A.S. Pro-inflammatory cytokines in a ruminant model: pathophysiological, pharmacological, and therapeutic aspects. Vet. Q. 1995;17:41–50. doi: 10.1080/01652176.1995.9694530. [DOI] [PubMed] [Google Scholar]
- Mundt H.C., Bangoura B., Rinke M., Rosenbruch M., Daugschies A. Pathology and treatment of Eimeria zuernii coccidiosis in calves: investigations in an infection model. Parasitol. Int. 2005;54:223–230. doi: 10.1016/j.parint.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H., Shimada N., Yoshioka M. Current research on acute phase proteins in veterinary diagnosis: an overview. Vet. J. 2004;168:28–40. doi: 10.1016/S1090-0233(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Müller-Doblies D., Arquint A., Schaller P., Heegaard P.M., Hilbe M., Albini S., Abril C., Tobler K., Ehrensperger F., Peterhans E., Ackermann M., Metzler A. Innate immune responses of calves during transient infection with a noncytopathic strain of bovine viral diarrhea virus. Clin. Diagn. Lab. Immunol. 2004;11:302–312. doi: 10.1128/CDLI.11.2.302-312.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orro T., Jacobsen S., Lepage J.-P., Niewold T., Alasuutari S., Soveri T. Temporal changes in serum concentrations of acute phase proteins in newborn dairy calves. Vet. J. 2008;176:182–187. doi: 10.1016/j.tvjl.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Orro T., Pohjanvirta T., Rikula U., Huovilainen A., Alasuutari S., Sihvonen L., Pelkonen S., Soveri T. Acute phase protein changes in calves during an outbreak of respiratory disease caused by bovine respiratory syncytial virus. Comp. Immunol. Microbiol. Infect. Dis. 2011;34:23–29. doi: 10.1016/j.cimid.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen H.H., Nielsen J.P., Heegaard P.M. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 2004;35:163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- Pyörälä S., Hovinen M., Simojoki H., Fitzpatrick J., Eckersall P.D., Orro T. Acute phase proteins in milk in naturally acquired bovine mastitis caused by different pathogens. Vet. Rec. 2011;168:535. doi: 10.1136/vr.d1120. [DOI] [PubMed] [Google Scholar]
- Saco Y., Fina M., Gimenez M., Pato R., Piedrafita J., Bassols A. Evaluation of serum cortisol, metabolic parameters, acute phase proteins and fecal corticosterone as indicators of stress in cows. Vet. J. 2008;177:439–441. doi: 10.1016/j.tvjl.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Sauerwein H., Schmitz S., Hiss S. The acute phase protein haptoglobin and its relation to oxidative status in piglets undergoing weaning-induced stress. Redox Rep. 2005;10:295–302. doi: 10.1179/135100005X83725. [DOI] [PubMed] [Google Scholar]
- Stockdale P.H.G. Effects of monensin on coccidiosis in ruminants. Vet. Med. Small Anim. Clin. 1981;76:1575–1578. [PubMed] [Google Scholar]
- Suojala L., Orro T., Järvinen H., Saatsi J., Pyörälä S. Acute phase response in two consecutive experimentally induced E. coli intramammary infections in dairy cows. Acta Vet. Scand. 2008;50:18. doi: 10.1186/1751-0147-50-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä-Lassila L., Orro T., Lepage J.P., Soveri T. Reference values for acute phase proteins in calves and its clinical application. Vet. Rec. 2013;173:319. doi: 10.1136/vr.101233. [DOI] [PubMed] [Google Scholar]
- Thienpont D., Rochette F., Vanparijs O.F.J. Janssen Pharmaceutica Beerse; 1990. Diagnose von Helminthosen durch korposkopische Untersuchung; p. 187. (in German) [Google Scholar]
- Webel D.M., Finck B.N., Baker D.H., Johnson R.W. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 1997;75:1514–1520. doi: 10.2527/1997.7561514x. [DOI] [PubMed] [Google Scholar]
- Werling D., Sutter F., Arnold M., Kun G., Tooten P.C.J., Gruys E., Kreuzer M., Langhans W. Characterisation of the acute phase response of heifers to a prolonged low dose infusion of lipopolysaccharide. Res. Vet. Sci. 1996;61:252–257. doi: 10.1016/s0034-5288(96)90073-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.