Abstract
The advent of MRI has contributed to increase the interest and awareness in childhood white matter disorders. A major priority is to distinguish transient and self-limited demyelinating syndromes like disseminated encephalomyelitis (DEM), from life-long diseases like multiple sclerosis (MS). However, the term DEM has been inconsistently applied across studies due to the lack of clear clinical and neuroimaging diagnostic criteria. The present review summarizes the available literature on DEM in children, outlines the main clinical and neuroimaging features at presentation, pathogenesis and outcome, and its differentiation from other conditions with acute impact in the CNS. The recently proposed clinical definitions for monophasic disseminated encephalomyelitis and its relapsing variants are discussed, and controversies surrounding the diagnosis of MS in children are addressed.
Keywords: Paediatrics, Acute encephalopathy, Disseminated encephalomyelitis, Multiphasic disseminated encephalomyelitis, Consensus definitions, Treatment, Outcome
1. Introduction
Disseminated encephalomyelitis (DEM) is an immune-mediated inflammatory disorder of the central nervous system (CNS), characterized by a widespread demyelination that predominantly involves the white matter of the brain and spinal cord. The condition is usually preceded by a viral infection o vaccination, and the presenting features include an acute encephalopathy with multifocal neurological signs and deficits [1], [2], [3], [4]. In the absence of specific biological markers the diagnosis of DEM is still based on the clinical and radiological features.
The lack of uniform definitions and clear clinical and neuroimaging diagnostic criteria has led that different conditions being classified as DEM. Although disseminated encephalomyelitis usually has a monophasic course (ADEM), recurrent (RDEM) and multiphasic forms (MDEM) have been reported, raising diagnostic difficulties in distinguishing these cases from multiple sclerosis (MS). The diagnostic differentiation between DEM and MS is important for prognostic reasons and treatment decision. The purpose of this review is to summarize literature data on the clinical and magnetic resonance imaging (MRI) features, pathogenesis and outcome of paediatric DEM. Particular attention on recurrent and multiphasic variants of DEM and its differences from MS are also provided.
2. Epidemiology
DEM is relatively frequent in children, probably due to the higher frequency of immunizations and primary exposure to antigens. The diagnosis of DEM is often made in the setting of a defined viral illness or vaccination. Although there appears to be no gender predominance in DEM [5], [6], a male predominance has been described in two paediatric cohorts, with reported female:male ratios of 0.6 [7] and 0.8 [8] as opposed to a 2:1 female preponderance frequently described for MS. The mean age at DEM presentation reported in recently published paediatric cohorts ranges from 5 to 8 years [5], [8], [9], [10].
A seasonal distribution in the winter and spring months has been reported in two studies [6], [7]. A recent study conducted in San Diego County, USA estimated the mean incidence of DEM as 0.4/100,000/year among persons less than 20 years of age living in that region [6]. Five percent of these patients had received a vaccination within 1 month prior to the DEM event, and 93% reported signs of infection in the preceding 21 days. In contrast with these findings, the number of reported paediatric DEM patients per year in a study conducted in Germany was very low, with only nine children with DEM per year and 44 new paediatric MS cases per year [11]. Despite the lack of clear worldwide studies on epidemiology and distribution of DEM, the reported geographical differences in incidence are important to be considered and could be influenced by environmental triggering factors [11].
The disorder may be classified as either post-vaccinal or post-infectious DEM; however, absence of clear precedent event has been reported in 26% of patients [8]. Rare cases have been described following organ transplantation [12], [13], [14], [15], [16]. Post-infectious forms of DEM typically begin within 2–21 days after an infectious event. Different viral agents, including influenza virus [17], enterovirus, measles [18], [19], mumps [20], rubella [19], varicella-zoster, Epstein Barr virus [21], cytomegalovirus, herpes simplex virus [22], [23], Hepatitis A virus [24], and coxsackie virus [25] have been associated with DEM [26], [27]. Bacterial triggers include Mycoplasma pneumoniae [28], Borrelia burgdorferi [29], Leptospira, and beta-haemolytic Streptococcus [30], [31]. The only epidemiologically and pathologically proven association between vaccination and DEM is with the Semple form of the antirabies vaccine [3], [32], [33], [34], [35], [36], [37], [38], [39]. Other immunizations that have been temporally related to DEM include Hepatitis B, pertussis, diphtheria, measles, mumps, rubella, pneumococcus, varicella, influenza, Japanese encephalitis, and polio [6], [8], [19], [35], [36], [37], [38], [39]. Nevertheless, DEM associated with vaccines may be related to contamination with host animal myelin antigens from CNS culture tissue, as it was identified in certain viral strains of rabies (Semple) and Japanese B encephalitis vaccines [32], [40]. The development of modern formulations based on recombinant proteins has dramatically lowered the incidence of vaccine-related DEM [41].
3. Clinical presentation
DEM is classically described as a monophasic disorder which typically begins within 2 days to 4 weeks after a clinically evident infection or vaccination. The typical symptoms and signs of DEM include a rapid onset encephalopathy associated with a combination of multifocal neurological deficits, leading to hospitalization within a week. A prodromal phase with fever, malaise, headache, nausea, and vomiting may be observed shortly before the development of meningeal signs and drowsiness. The clinical course is rapidly progressive and usually develops over hours to maximum deficits within days (mean, 4.5 days) [8].
The initial neurological features are heterogeneous and related to the distribution of demyelinating lesions within the CNS. The most prevalent neurological symptoms and signs described in recently reported paediatric series are unilateral or bilateral pyramidal signs (60–95%), acute hemiplegia (76%), ataxia (18–65%), cranial nerve involvement (22–45%), visual loss due to optic neuritis (7–23%), seizures (13–35%), spinal cord involvement (24%), impairment of speech [slow, slurred or aphasia] (5–21%), and hemiparesthesias (2–3%) in different combination, with the invariable involvement of mental status, ranging from lethargy to coma [5], [6], [7], [8], [9], [10], [42], [43], [44], [45]. Seizures are mainly seen in children younger than 5 years of age. One study has documented prolonged focal motor seizures going on to status epilepticus in 70% of their younger patients [8].
A unique childhood DEM phenotype was recently reported in association with group A-beta haemolytic streptococcal infection. Ten children between the ages of 3–14 years developed a clinically typical DEM presentation but with a prominent dystonic extrapyramidal syndrome (70%) or behavioral disorder such as emotional lability or inappropriate speech (50%). The syndrome followed an acute pharyngitis but was distinct from rheumatic fever or Sydenham's chorea and was associated with elevated antibasal ganglia antibodies and abnormal basal ganglia imaging [31].
There is a wide variation in the severity of the illness. DEM can present as a subtle disease, with non-specific irritability, headache or somnolence lasting more than 1 day, or may show a rapid progression of symptoms and signs to coma and decerebrate rigidity [26]. Respiratory failure secondary to brainstem involvement or severe impaired consciousness occurs in 11–16% of cases [8], [26]. Clinically relevant features of paediatric DEM are summarized in Table 1 .
Table 1.
Clinically relevant features of monophasic DEM (ADEM)
| Age at onset | Childhood (median 5–8 years of age)a |
| Clinical presentation | Frequent preceding infection or vaccination |
| Headache, fever | |
| Acute–subacute encephalopathy, in | |
| combination with multifocal deficits | |
| Pyramidal syndrome | |
| Cerebellar ataxia | |
| Brainstem involvement | |
| Cerebrospinal fluid | Variable pleocytosis |
| Oligoclonal banding 0–29%b | |
| MRI features | Large, multifocal, poor marginated lesions |
| Bilateral subcortical white matter | |
| Bilateral deep grey matter (thalamus, | |
| basal ganglia) | |
| Absence of previous demyelinating | |
| lesions | |
| Clinical follow-up | Improvement; there may be residual deficits |
| MRI follow-up | Complete or partial resolution of lesions |
| Absence of new clinically silent lesions | |
4. Neuroimaging features
The diagnostic hallmark of DEM is the demonstration of inflammatory-demyelinating white matter lesions. MRI is the most sensitive paraclinical test to show these abnormalities, which are most frequently identified on T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences as patchy, heterogeneous and poorly marginated areas of increased signal intensity. Lesions are typically large, multiple, and asymmetric, involving the white matter of cerebral hemispheres, cerebellum, brainstem and spinal cord [26], [46]. The deep gray matter of the thalami and basal ganglia are frequently involved in a typically symmetrical pattern [8], [47]. The white matter lesions in children tend to involve the subcortical and central white matter; nevertheless involvement of the periventricular white matter has been described in 30–60% of cases [7], [9], [26]. Lesions confined to the corpus callosum are less common. However, large demyelinating lesions of the adjacent white matter may extend into the corpus callosum and cross into the contralateral hemisphere. In a study of 31 children diagnosed with DEM, 90% had lesions in the supratentorial white matter, 29% in the corpus callosum, and 61% had gray matter involvement [9].
Five patterns of cerebral involvement have been proposed to describe the MRI findings in DEM [8], [48]: (a) DEM with small lesions (less than 5 mm); (b) DEM with large, confluent or tumefactive lesions, with frequent extensive perilesional edema and mass effect; (c) DEM with additional symmetric bithalamic involvement; (d) acute hemorrhagic encephalomyelitis (AHEM), when some evidence of haemorrhage can be identified into the large demyelinating lesions and (e) DEM with a pseudo-leukodystrophic pattern, with a diffuse, bilateral, symmetric and usually non-enhanced white matter involvement [49]. The MRI pattern does not appear to correlate with any particular outcome or disability, as observed in a large paediatric cohort [8] since most lesions tend to resolve on follow-up imaging studies [50]. However, this classification may be useful when considering the differential diagnosis of DEM (see Section 8).
Spinal cord involvement in DEM has been described in 11–28% [5], [8], [9], [10], [42]. The typical spinal cord lesion is large, swollen, showing variable enhancement and predominantly affects the thoracic region.
The reported frequency of gadolinium enhancing lesions on T1-weighted sequences is quite variable in DEM (8–100%), depending on the stage of inflammation [8], [9], [47], [51], [52], [53], [54]. The pattern of enhancement is also variable; complete or incomplete ring-shaped, nodular, gyral or spotty patterns have been described [54], [55], [56]. Meningeal enhancement of the brain or spinal cord is unusual.
Complete resolution of white matter abnormalities on sequential follow-up MRI scanning has been described in 37–75% of DEM patients, and partial resolution in 25–53% of patients [5], [8], [51], [53], [57]. The development of new, clinically silent lesions on serial MRI studies is not compatible with the diagnosis of DEM, and is one of the most powerful predictors of future MS diagnosis.
5. Relapsing forms of DEM
A monophasic clinical course is usually described in and identified with ADEM. Nevertheless, cases of DEM showing recurrences had been known since 1932, when van Bogaert published a case classified as “ADEM with relapses” [58]. Several studies have described relapsing episodes in paediatric cohorts with DEM, occurring at different rates: 1/18 (5.5%) [7]; 1/14 (7%) [59]; 8/84 (10%) [8]; 4/31 (13%) [9]; 7/46 (15%) [10]; 24/132 (18%) [60]; 7/35 (20%) [5]; 9/42 (21%) [6]; 5/21 (24%) [61]. The inter-study variability of the relapsing rates may be influenced by the different clinical definitions and neuroimaging diagnostic criteria for DEM and relapses used in these studies. In addition, the mean follow-up reported in some of these series varied from 18 months [9] to 6.6 years [8].
A variety of terms has been used in the scientific literature to describe children who experience a subsequent event after an initial ADEM illness. Recurrent, relapsing, pseudo relapsing, bi- or multiphasic DEM have all been applied as to whether relapses required a monofocal or multifocal presentation, a specific interval from the first event (less than 4 to more than 8 weeks), same or different neurological deficits, and finally MRI lesions as either in the same or different brain regions [5], [8], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], leading to remarkable confusion. However, the terms recurrent (RDEM) and multiphasic (MDEM) had already been well defined by Poser [71]. Recurrent DEM as an initial ADEM event followed by a new one, reproducing the original clinical syndrome, completely or in part [61], [72], [73]. Multiphasic DEM as an initial episode of ADEM followed by bouts of clinical syndromes different from the original one. The clinical concept of relapses of an acute condition like ADEM received support from reports of chronic relapsing experimental encephalomyelitis [46], [74].
To date, there are no clear clinical or radiological parameters that predict which patients with DEM will relapse. One recent study [60] focused on the prognostic factors for relapse in children with DEM. An increased risk of relapse was associated with optic neuritis at first attack (hazard ratio, 5.23; 95% CI, 2–13.65), familial history of CNS inflammatory demyelination (7.79; 1.54–39.5), Barkhof MS criteria on first MRI study (2.52; 1.04–6.12) and with no neurological sequelae after first attack (3.79; 1.12–12.85). But although these relapsing cases might have a final diagnosis of MS, 15 of the 24 ADEM patients with a second event were polysymptomatic, and five of them had changes in mental state, suggesting that these five cases were more probably instances of multiphasic DEM. Additionally, several follow-up studies with a considerable time of follow-up reported only one relapse in most children with MDEM [6], [7], [8], [9], [10], distinguishing the demyelinating process as self-limited and not associated with a lifelong disorder.
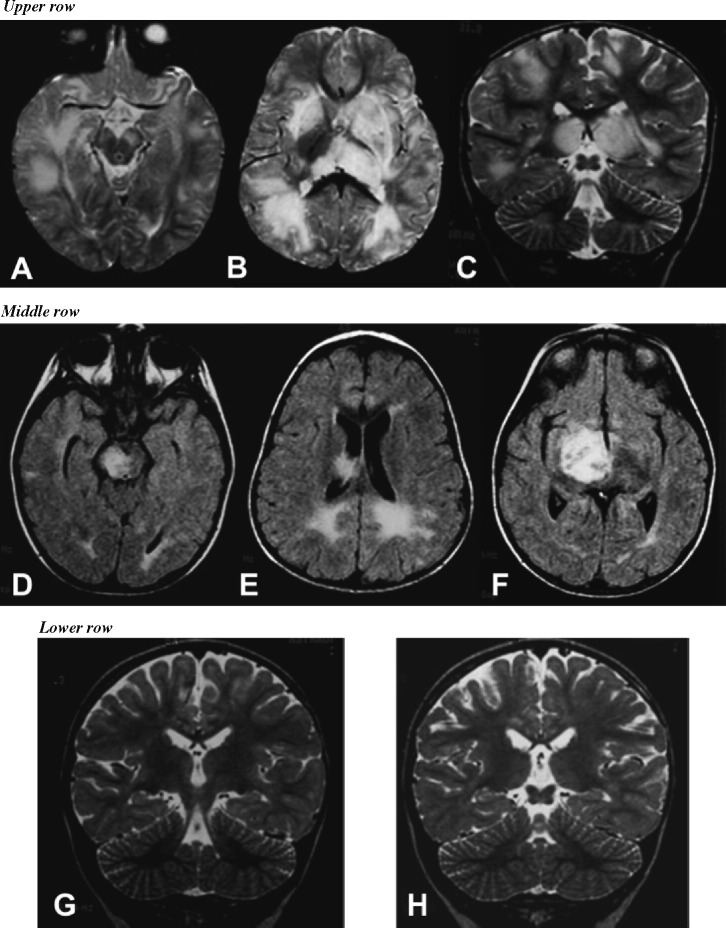
The final outcome of children with definite MDEM has been described in detail in two paediatric cohorts with considerable follow-up. In one study, no impairment was observed in 86% of multiphasic patients [5]. Similarly, eight children with MDEM, who remained relapse-free after a follow-up of 3–16 years (mean 8.2 years), had a median DSS score of 1 (range 0–2.5) at last visit [8]. Serial brain-spinal MRI performed in these children revealed complete or almost complete resolution of demyelinating lesions, and absence of new clinically silent lesions (Fig. 1G and H). Interestingly, relapses in this paediatric subgroup of MDEM were more commonly spontaneous (87%), whereas the first demyelinating episode had been associated with a previous infection in 60% of children [8].
Fig. 1.
Illustration of brain lesions in a child with multiphasic disseminated encephalomyelitis (MDEM). A 21-month-old boy became acutely ill with lowered consciousness, ophthalmoplegia and right-sided hemiparesis. The upper row shows axial (A and B) and coronal (C) T2-weighted brain images at presentation, demonstrating an irregular pattern of focal areas of high signal intensity in the mesencephalon (especially on the left), bilateral thalamic and insular regions, and in the periventricular and subcortical white matter. The patient showed gradual clinical improvement with methylprednisolone pulse-therapy, and fully recovered after 3 weeks. Three months after the initial event and 1 month after completing corticosteroid treatment, this boy developed a left-sided hemiparesis, VI and VII cranial nerve involvement and progressive drowsiness, shortly after an upper respiratory viral infection. The middle row shows axial FLAIR series demonstrating signal abnormalities in the mesencephalon (particularly on the right) (D), in the posterior periventricular white matter (E) and a large-tumefactive right lesion with mass effect over the III ventricle (F). This young boy received a second course of methylprednisolone pulse-therapy followed by full clinical recovery. The lower row shows coronal T2-weighted images obtained 1 year after the first event with complete resolution of prior lesions (G and H). This patient has experienced no further relapses during the 9-year follow-up.
6. Proposed definitions
The International Paediatric MS Study Group has recently proposed operational definitions for acquired CNS demyelinating disorders of childhood, including the monophasic and relapsing forms of DEM [48], [75]:
-
(1)
Acute disseminated encephalomyelitis (ADEM): A first clinical event with a polysymptomatic encephalopathy, of acute or subacute onset, showing focal or multifocal hyperintense lesions predominantly affecting the CNS white matter; no evidence of previous destructive white matter changes should be present and no history of a previous clinical episode with features of a demyelinating event. If a relapse takes place within 4 weeks of tapering steroid treatment or within the first 3 months from the initial event, this early relapse is considered temporally related to the same acute monophasic condition and would replace the terms “steroid dependent ADEM” or “pseudo relapsing ADEM”.
-
(2)
Recurrent disseminated encephalomyelitis (RDEM): New demyelinating event fulfilling diagnostic criteria for ADEM, occurring at least 3 months after the initial event and at least 4 weeks after completing steroid therapy, but showing the same clinical presentation and affecting the same areas on MRI as the initial episode.
-
(3)
Multiphasic disseminated encephalomyelitis (MDEM): Refers to one or more DEM relapses, including encephalopathy and multifocal deficits, but involving new areas of the CNS on MRI and neurologic exam (Fig. 1A–F). Relapses take place at least 3 months after initial DEM attack and at least 4 weeks after completing steroid therapy.
7. DEM and MS
It is acknowledged that a proportion of children with DEM may develop MS [5], [6], [42]. However, it remains unclear what proportion of children who present an acute DEM event will be later diagnosed with MS, since numbers from published studies vary from 9.5 [6] to 29% [42]. And it is also unclear if DEM and MS are two distinct clinical disorders or part of a disease spectrum [76].
In the definition proposed by the International Pediatric MS Study Group [75], MS in children requires multiple episodes of CNS demyelination separated in time and space, as specified for adults [77], [78] but including patients under 10 years of age. The MRI findings can be used to meet the dissemination in space requirement and to satisfy criteria for dissemination in time following the initial clinical event, if the McDonald MRI criteria are applied [78].
But it should be noted that in the special circumstance of a child whose initial clinical event was classified as DEM, a second demyelinating event not meeting DEM criteria is considered not enough for a definite diagnosis of MS and additional evidence of further dissemination in time is required, either on MRI with emergence of new lesions at least 3 months or a new clinical attack (Fig. 2 ). Although this statement remains controversial, it seems justifiable to avoid establishing prematurely the diagnosis of MS in children with DEM who relapse, and to extend the clinical and MRI follow-up instead of initiating early immunomodulatory treatment.
Fig. 2.
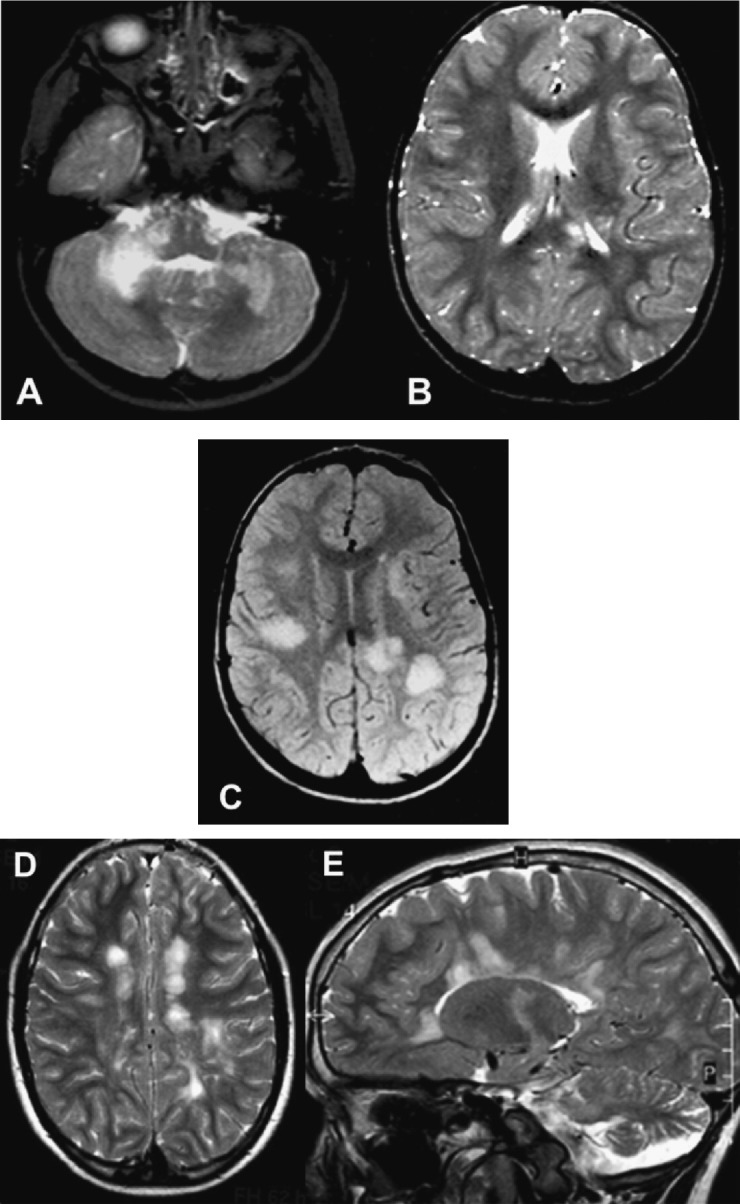
MRI appearance of multiple sclerosis in a child with ADEM-like onset. A 2-year-old boy developed an acute event with ataxia, right hemiparesis and drowsiness; he was unable to walk in the following 3 days. Axial T2-weighted brain images at presentation (A and B) show bilateral involvement of cerebellar white matter, especially on the right side, with additional areas of high signal in the corpus callosum (splenium) and left internal capsule. The patient fully recovered after corticosteroid treatment. Five months later he had difficulties in swallowing with progressive ataxia. On examination he was alert, with cerebellar ataxia, left hemiparesis and bilateral pyramidal signs. Oligoclonal bands were positive in CSF. A new brain MRI showed bilateral ill-defined lesions in the periventricular and subcortical white matter (C). The boy received methylprednisolone pulse-therapy with good recovery. After a new clinical attack 3 months later the diagnosis of paediatric MS was made and treatment with IFN beta-1a was begun. The last two images (D and E) were obtained 6 years after the first event showing well-defined new lesions, perpendicular to the long axis of the corpus callosum, which positively correlate with the diagnosis of MS.
The KIDMUS study group examined children with a first demyelinating event, including CIS and DEM, and reported that overall, 57% developed MS as defined by two demyelinating events [42], and 29% of children with an initial DEM event developed MS. When the diagnosis of DEM was further redefined to include “change in mental status” as a diagnostic criterion, 18% of children were found to develop MS after a mean follow-up of 5.4 years [60]. A recent study examined the clinical presentation and disease course of paediatric MS with onset before the age of 10 years, and a polysymptomatic acute encephalopathy (ADEM-like phenotype) was the most frequent clinical presentation observed in 40% of children [79].
8. Differential diagnosis
The presence of an acute encephalopathy and disseminated demyelination of the CNS in a child represent a diagnostic challenge. Many inflammatory and non-inflammatory disorders may have a similar clinical and radiological presentation and should be considered in the diagnostic evaluation. The most frequently considered disorders in the differential diagnosis of paediatric DEM are summarized in Table 2 .
Table 2.
Diagnostic categories to consider and exclude in children with DEM
| CNS infectious conditions |
| Viral, bacterial or parasitic meningoencephalitis |
| Subacute sclerosing panencephalitis |
| HIV-associated encephalopathy: |
| Subacute HIV encephalitis |
| Opportunistic CNS infections |
| Progressive multifocal leukoencephalopathy |
| CMV subacute encephalitis |
| CNS inflammatory-demyelinating disorders |
| Postinfectious demyelinating cerebellitis |
| Postinfectious demyelinating brainstem encephalitis |
| Neuromyelitis optica |
| Multiple sclerosis |
| Neurosarcoidosis |
| Behçet's disease |
| CNS vascular disorders |
| Prothrombotic conditions |
| Antiphospholipid antibody syndrome |
| Primary isolated CNS angiitis |
| Systemic vasculitis with CNS involvement (systemic lupus erythematosus) |
| Sickle cell anemia |
| Susac syndrome |
| CADASIL |
| Deep sinovenous thrombosis |
| Carotid dissection |
| Intracranial mass lesion |
| Gliomatosis cerebri |
| Primary CNS lymphoma |
| Histiocytosis |
| Toxic, nutritional and metabolic disorders |
| CO poisoning |
| Vitamin B12 deficiency |
| Folate deficiency |
| Mercury poisoning |
| Ibuprofen-induced aseptic meningitis |
| Post-hypoxic-ischemic newborn leukoencephalopathy |
| Central pontine and extrapontine myelinolisis |
| Marchiafava-Bignami disease |
| Radiation induced leukoencephalopathy |
| Mitochondrial encephalopathy with lactic acidosis and stroke like episodes (MELAS) |
| Organic acidurias |
| Inherited leukodystrophies |
| Miscellaneous |
| Reversible posterior leukoencephalopathy |
| Recurrent migraine headache |
| Hashimoto's encephalopathy |
The exclusion of acute CNS infections should be the first and most important diagnostic clue to be considered by lumbar puncture and further microbiological laboratory tests. A gadolinium-enhanced MRI of the brain and spinal cord should be performed to define the regional distribution and MRI lesion appearance. Brain malignancies, Shilder's disease, Marburg's variant of MS, and brain abscess should be considered when large focal tumour-like lesions are detected on MRI [80], [81], [82], [83], [84]. A lesion pattern with symmetric bithalamic involvement may be seen in children with acute necrotizing encephalopathy, deep cerebral venous thrombosis, hypernatremia and extrapontine myelinolysis, as well as in children with ADEM after Japanese B encephalitis vaccination [85], [86], [87], [88], [89], [90], [91]. Lesions in the basal ganglia may be consistent with organic aciduria, infantile bilateral striatal necrosis, M. pneumoniae infection, voltage-gated potassium channel antibody associated encephalitis and poststreptococcal ADEM with auto-reactive antibasal ganglia antibodies [31], [92], [93], [94]. The presence of complete ring-enhanced lesions in the cerebral white matter is unusual in DEM, and brain abscess, tuberculomas, neurocysticercosis, toxoplasmosis and histoplasmosis should be excluded [54], [95].
Recurrent episodes of CNS demyelination should raise the potential diagnosis of MS or systemic diseases with CNS involvement, such as systemic connective tissue diseases (systemic lupus erythematosus, neuro-Behçet disease, ANCA-associated vasculitis and antiphospholipid syndrome) [96]. The association of symptomatic or asymptomatic brain lesions and recurrent events of demyelination involving optic nerves and spinal cord has been described in children with neuromyelitis optica (NMO), as recently reported [97], [98].
9. Treatment and prognosis
There is no standard therapy for DEM. Corticosteroid treatment is the most widely reported therapy for DEM, typically at high doses. Although there has been great variety in the specific formulations, routes of administration and dosing, most paediatric groups described a high-dose steroid regime using intravenous methylprednisolone (10–30 mg/(kg day) up to maximum dose of 1 g/day) or dexamethasone (1 mg/(kg day)) for 3–5 days [5], [8], [9], [99], [100], [101] followed by oral prednisone taper for 4–6 weeks. In many studies, full recovery following treatment with methylprednisolone pulse-therapy was reported in 50–90% of children [5], [8], [9], [10], [43].
The use of intravenous immunoglobulin (IVIG) has been reported in several case studies as well, either alone [102], [103] or in combination with corticosteroids [100], [104]. The usual total dose of IVIG is 1–2 g/kg, administered over 2–5 days [105], [106], [107]. Additionally, the use of plasma exchange in ADEM has been reported in only a small number of severe cases, usually refractory to corticosteroid or IVIG treatment [43], [108], [109], [110], [111], [112], [113].
With the current use of high-dose corticosteroids, the long-term functional and cognitive prognosis of DEM in children is favorable. The most frequently reported residual problems are focal motor deficits, from mild clumsiness to severe hemiparesis, visual problems to blindness, symptomatic epilepsy, and behavioral and cognitive disturbances [8], [114], [115].
Even children thought to have full recovery demonstrated subtle neurocognitive deficits in attention, executive function and behavior when reevaluated more than 3 years after DEM [114], although these deficits were not as severe as those reported for paediatric patients with MS [116]. In one study, behavioral and cognitive problems were reported as more prominent in children younger than 5 years at DEM diagnosis [115].
10. Immunopathogenesis
ADEM is characterized histologically by perivenular infiltrates of T cells and macrophages, associated with “sleeves” of demyelination that surround venules [117], [118]. A variety of pathological features have been recently described in biopsy and autopsy samples of children with DEM and AHEM. The autopsy of a 5-year-old boy with severe and fatal ADEM grossly described diffuse brain edema, uncal and tonsillar herniation [6], in addition to perivascular lymphocytic infiltrates, fibrin deposition within vascular lumens and adjacent demyelination. Viral inclusion bodies were not seen in HE sections. In contrast, the brain biopsy performed in a 10-year-old girl with severe AHEM, demonstrated perivascular hemorrhagic necrosis with subacute inflammation consisting of macrophages, neurophils and lymphocytes in the subcortical white matter.
Although DEM is typically described as white matter demyelination with relative preservation of axons, axonal damage has been identified in the brain of some patients [119], [120].
The precise pathogenesis of DEM is still unclear; however given its histological features, it has been likened to the animal model of experimental autoimmune encephalomyelitis (EAE) and Theiler murine encephalomyelitis. In EAE, animals are immunized with a combination of encephalitogenic myelin proteins or peptides, developing a monophasic syndrome of motor weakness and incontinence, associated with diffuse CNS inflammatory demyelination [121], [122]. Viral or bacterial epitopes resembling myelin antigens have the capacity to activate myelin-reactive T cell clones through molecular mimicry [123], and can thereby elicit a CNS-specific autoimmune response. Thus, it has been suggested that preceding infections in DEM may elicit a cross-reactive anti-myelin reaction through a mechanism of molecular mimicry. Another animal model, putatively mimicking postinfectious DEM, is created by direct inoculation of genetically susceptible mice with Theiler murine encephalomyelitis virus (TMEV) (picornavirus), inducing widespread CNS inflammatory demyelination through an immune-mediated reaction primarily involving TMEV-specific CD4 and CD8 T cells [124], [125], [126], [127]. The TMEV model highlights the phenomenon of epitope spreading secondary to a destructive CNS viral infection resulting in a secondary autoimmune response and chronic inflammation [128], [129], [130]. Although this model superficially bears some resemblance to DEM, the epitope spreading is more likely to be an important phenomenon in chronic inflammatory disorders such as MS. Alternatively, the molecular mimicry hypothesis suggests that structural similarities between the pathogen (viral/vaccine polypeptides) and the host (central or peripheral nerve myelin) are sufficient to induce T cell activation but not sufficient to induce tolerance.
Sequences in myelin basic protein (MBP) have been shown to resemble several viral sequences, and in some cases, cross-reactive T cell responses have been demonstrated. Examples of cross-reactive T cells with MBP antigens include HHV-6 [131], coronavirus [132], influenza virus hemagglutinin [133], and EBV [134]. Proteolipid protein (PLP) shares common sequences with Haemophilus influenza [135] and myelin oligodendrocyte glycoprotein (MOG) mimics Semliki Forest Virus (SFV) peptides [136].
Elevated titers of anti-myelin antibodies in sera from DEM patients have recently been demonstrated as compared to patients with MS or viral encephalitis [137]. A recently published study reported the presence of circulating conformation-dependent autoantibodies to MOG in a subset of children and adults with DEM, but only rarely in adult-onset MS cases [138]. New available data suggest that enhanced T and B cell-myelin responses play a role in the pathogenesis of postinfectious and postvaccinal DEM, including MOG as the prominent target autoantigen. Furthermore, the response of some DEM patients to plasmapheresis or intravenous immunoglobulins reinforces the pathogenic role of these autoantibodies.
11. Conclusions
Due to the lack of an identified biomarker, the diagnostic of DEM is still based on clinical and neuroimaging presenting features. Hence, to conduct appropriate prospective multicenter studies, the use of standardized definitions and a uniform classification system for disseminated and restricted forms of acquired demyelinating disorders in children is mandatory. Prospective studies are much needed in order to identify biologic mechanisms underlying these disorders as well as relevant prognostic indicators of relapse, which may contribute to an early distinction between DEM/MDEM and definite MS in children.
Conflict of interest
None.
References
- 1.Rust R.S. Multiple sclerosis, acute disseminated encephalomyelitis, and related conditions. Semin Pediatr Neurol. 2000;7:66–90. doi: 10.1053/pb.2000.6693. [DOI] [PubMed] [Google Scholar]
- 2.Dale R.C. Acute disseminated encephalomyelitis. Semin Pediatr Infect Dis. 2003;14:90–95. doi: 10.1053/spid.2003.127225. [DOI] [PubMed] [Google Scholar]
- 3.Garg R.K. Acute disseminated encephalomyelitis. Postgrad Med J. 2003;79:11–17. doi: 10.1136/pmj.79.927.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones C.T. Childhood autoimmune neurologic diseases of the central nervous system. Neurol Clin. 2003;21:745–764. doi: 10.1016/s0733-8619(03)00007-0. [DOI] [PubMed] [Google Scholar]
- 5.Dale R.C., de Sousa C., Chong W.K., Cox T.C., Harding B., Neville B.G. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123:2407–2422. doi: 10.1093/brain/123.12.2407. [DOI] [PubMed] [Google Scholar]
- 6.Leake J.A., Albani S., Kao A.S., Senac M.O., Billman G.F., Nespeca M.P. Acute Disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr Infect Dis J. 2004;23:756–764. doi: 10.1097/01.inf.0000133048.75452.dd. [DOI] [PubMed] [Google Scholar]
- 7.Murthy K.S.N., Faden H.S., Cohen M.E., Bakshi R. Acute disseminated encephalomyelitis in children. Pediatrics. 2002;110(2):21–28. doi: 10.1542/peds.110.2.e21. [DOI] [PubMed] [Google Scholar]
- 8.Tenembaum S., Chamoles N., Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59:1224–1231. doi: 10.1212/wnl.59.8.1224. [DOI] [PubMed] [Google Scholar]
- 9.Hynson J.L., Kornberg A.J., Coleman L.T., Shield L., Harvey A.S., Kean M.J. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;56:1308–1312. doi: 10.1212/wnl.56.10.1308. [DOI] [PubMed] [Google Scholar]
- 10.Anlar B., Basaran C., Kose G., Guven A., Haspolat S., Yakut A. Acute disseminated encephalomyelitis in children: outcome and prognosis. Neuropediatrics. 2003;34:194–199. doi: 10.1055/s-2003-42208. [DOI] [PubMed] [Google Scholar]
- 11.Pohl D., Hennemuth I., von Kries R., Hanefeld F. Paediatric multiple sclerosis and acute disseminated encephalomyelitis in Germany: results of a nationwide survey. Eur J Pediatr. 2007;166:405–412. doi: 10.1007/s00431-006-0249-2. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz M.B., Comey C., Hirsch W., Marion D., Griffith B., Martinez J. Acute disseminated encephalomyelitis (ADEM) or ADEM-like inflammatory changes in a heart-lung transplant recipient: a case report. Neuroradiology. 1995;37:434–437. doi: 10.1007/BF00600082. [DOI] [PubMed] [Google Scholar]
- 13.Re A., Giachetti R. Acute disseminated encephalomyelitis (ADEM) after autologous peripheral blood stem cell transplant for non-Hodgkin's lymphoma. Bone Marrow Transplant. 1999;24:1351–1354. doi: 10.1038/sj.bmt.1702047. [DOI] [PubMed] [Google Scholar]
- 14.Iwanaga T., Ooboshi H., Imamura T., Mizumasa T., Ibayashi S., Hirakata H. A case of acute disseminated encephalomyelitis after renal transplantation. Rinsho Shinkeigaku. 2001;41:792–796. [PubMed] [Google Scholar]
- 15.Au W.Y., Lie A.K., Cheung R.T., Cheng P.W., Ooi C.G. Acute disseminated encephalomyelitis after para-influenza infection post bone marrow transplantation. Leuk Lymphoma. 2002;43:455–457. doi: 10.1080/10428190290006350. [DOI] [PubMed] [Google Scholar]
- 16.Tomonari A., Tojo A., Adachi D., Iseki T., Ooi J., Shirafuji N. Acute disseminated encephalomyelitis (ADEM) after allogeneic bone marrow transplantation for acute myeloid leukemia. Ann Hematol. 2003;82:37–40. doi: 10.1007/s00277-002-0573-1. [DOI] [PubMed] [Google Scholar]
- 17.Yoshikawa H., Yamazaki S., Watanabe T., Abe T. Study of influenza-associated encephalitis/encephalopathy in children during the 1997 to 2001 influenza seasons. J Child Neurol. 2001;16:885–890. doi: 10.1177/088307380101601204. [DOI] [PubMed] [Google Scholar]
- 18.Johnson R.T., Griffin D.E., Hirsch R.L., Wolinsky J.S., Roedenbeck S., Lindo de Soriano I. Measles encephalomyelitis—clinical and immunologic studies. N Engl J Med. 1984;310:137–141. doi: 10.1056/NEJM198401193100301. [DOI] [PubMed] [Google Scholar]
- 19.Fenichel G.M. Neurological complications of immunization. Ann Neurol. 1982;12:119–128. doi: 10.1002/ana.410120202. [DOI] [PubMed] [Google Scholar]
- 20.Sonmez F.M., Odemis E., Ahmetoglu A., Ayvaz A. Brainstem encephalitis and acute disseminated encephalomyelitis following mumps. Pediatr Neurol. 2004;30:132–134. doi: 10.1016/j.pediatrneurol.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto H., Asaoka K., Imaizumi T., Ayabe M., Shoji H., Kaji M. Epstein-Barr virus infections of the central nervous system. Intern Med. 2003;42:33–40. doi: 10.2169/internalmedicine.42.33. [DOI] [PubMed] [Google Scholar]
- 22.Koenig H., Rabinowitz S.G., Day E., Miller V. Post-infectious encephalomyelitis after successful treatment of herpes simplex encephalitis with adenine arabinoside: ultrastructural observations. N Engl J Med. 1979;300:1089–1093. doi: 10.1056/NEJM197905103001906. [DOI] [PubMed] [Google Scholar]
- 23.De Tiège X., De Laet C., Mazoin N., Christophe C., Mewasingh L.D., Wetzburger C. Postinfectious immune-mediated encephalitis after pediatric herpes simplex encephalitis. Brain Dev. 2005;27:304–307. doi: 10.1016/j.braindev.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Tan H., Kilicaslan B., Onbas O., Buyukavci M. Acute disseminated encephalomyelitis following Hepatitis A virus infection. Pediatr Neurol. 2004;30:207–209. doi: 10.1016/j.pediatrneurol.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 25.David P., Baleriaux D., Bank W.O., Amrom D., De Temmerman D., Babusiaux C. MRI of acute disseminated encephalomyelitis after Coxsackie B infection. J Neuroradiol. 1993;20:258–265. [PubMed] [Google Scholar]
- 26.Wingerchuk D.M. Postinfectious encephalomyelitis. Curr Neurol Neurosci Rep. 2003;3:256–264. doi: 10.1007/s11910-003-0086-x. [DOI] [PubMed] [Google Scholar]
- 27.Bennetto L., Scolding N. Inflammatory/post-infectious encephalomyelitis. J Neurol Neurosurg Psychiatry. 2004;75(Suppl I):22–28. doi: 10.1136/jnnp.2003.034256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riedel K., Kempf V.A., Bechtold A., Klimmer M. Acute disseminated encephalomyelitis (ADEM) due to Mycoplasma pneumoniae infection in an adolescent. Infection. 2001;29:240–242. doi: 10.1007/s15010-001-1173-z. [DOI] [PubMed] [Google Scholar]
- 29.van Assen S., Bosma F., Staals L.M., Kullberg B.J., Melchers W.J., Lammens M. Acute disseminated encephalomyelitis associated with Borrelia burgdorferi. J Neurol. 2004;251:626–629. doi: 10.1007/s00415-004-0415-2. [DOI] [PubMed] [Google Scholar]
- 30.Hahn J.S., Sum J.M., Bass D., Crowley R.S., Horoupian D.S. Acute disseminated encephalomyelitis-like syndrome following group A beta-hemolytic Streptococcal infection. J Child Neurol. 1998;13:354–356. doi: 10.1177/088307389801300711. [DOI] [PubMed] [Google Scholar]
- 31.Dale R.C., Church A.J., Cardoso F., Goddard E., Cox T.C., Chong W.K. Post streptococcal acute disseminated encephalomyelitis with basal ganglia involvement and auto-reactive antibasal ganglia antibodies. Ann Neurol. 2001;50:588–595. doi: 10.1002/ana.1250. [DOI] [PubMed] [Google Scholar]
- 32.Hemachudha T., Griffin D.E., Giffels J.J., Johnson R.T., Moser A.B., Phanuphak P. Myelin basic protein as an encephalitogen in encephalomyelitis and polyneuritis following rabies vaccination. N Engl J Med. 1987;316:369–374. doi: 10.1056/NEJM198702123160703. [DOI] [PubMed] [Google Scholar]
- 33.Hemachudha T., Griffin D.E., Johnson R.T., Giffels J.J. Immunologic studies of patients with chronic encephalitis induced by post-exposure Semple rabies vaccine. Neurology. 1988;38:42–44. doi: 10.1212/wnl.38.1.42. [DOI] [PubMed] [Google Scholar]
- 34.Murthy J.M. Acute disseminated encephalomyelitis. Neurol India. 2002;50:238–243. [PubMed] [Google Scholar]
- 35.Takahashi H., Pool V., Tsai T.F., Chen R.T. Adverse events after Japanese encephalitis vaccination: review of post-marketing surveillance data from Japan and the United States. The VAERS Working Group. Vaccine. 2000;18:2963–2969. doi: 10.1016/s0264-410x(00)00111-0. [DOI] [PubMed] [Google Scholar]
- 36.Tourbah A., Gout O., Liblau R., Lyon-Caen O., Bougniot C., Iba-Zizen M.T. Encephalitis after Hepatitis B vaccination: recurrent disseminated encephalitis or MS? Neurology. 1999;53:396–401. doi: 10.1212/wnl.53.2.396. [DOI] [PubMed] [Google Scholar]
- 37.Karaali-Savrun F., Altintas A., Saip S., Siva A. Hepatitis B vaccine related-myelitis? Eur J Neurol. 2001;8(6):711–715. doi: 10.1046/j.1468-1331.2001.00290.x. [DOI] [PubMed] [Google Scholar]
- 38.Sejvar J.J., Labutta R.J., Chapman L.E., Grabenstein J.D., Iskander J., Lane J.M. Neurologic adverse events associated with smallpox vaccination in the United States, 2002–2004. JAMA. 2005;294:2744–2750. doi: 10.1001/jama.294.21.2744. [DOI] [PubMed] [Google Scholar]
- 39.Ozawa H., Noma S., Yoshida Y., Sekine H., Hashimoto T. Acute disseminated encephalomyelitis associated with poliomyelitis vaccine. Pediatr Neurol. 2000;23:177–179. doi: 10.1016/s0887-8994(00)00167-3. [DOI] [PubMed] [Google Scholar]
- 40.Wingerchuk D.M., Lucchinetti C.F. Comparative immunopathogenesis of acute disseminated encephalomyelitis, neuromyelitis optica, and multiple sclerosis. Curr Opin Neurol. 2007;20:343–350. doi: 10.1097/WCO.0b013e3280be58d8. [DOI] [PubMed] [Google Scholar]
- 41.Menge T., Kieseier B.C., Nessler S., Hemmer B., Hartung H.P., Stüve O. Acute disseminated encephalomyelitis: an acute hit against the brain. Curr Opin Neurol. 2007;20:247–254. doi: 10.1097/WCO.0b013e3280f31b45. [DOI] [PubMed] [Google Scholar]
- 42.Mikaeloff Y., Suissa S., Vallée L., Lubetzki C., Ponsot G., Confavreux C. First episode of acute CNS inflammatory demyelination in childhood: prognostic factors for multiple sclerosis and disability. J Pediatr. 2004;144(2):246–252. doi: 10.1016/j.jpeds.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 43.Khurana D.S., Melvin J.J., Kothare S.V., Valencia I., Hardison H.H., Yum S. Acute disseminated encephalomyelitis in children: discordant neurologic and neuroimaging abnormalities and response to plasmapheresis. Pediatrics. 2005;116:431–436. doi: 10.1542/peds.2004-2038. [DOI] [PubMed] [Google Scholar]
- 44.Weng W.C., Peng S.S., Lee W.T., Fan P.C., Chien Y.H., Du J.C. Acute disseminated encephalomyelitis in children: one medical center experience. Acta Paediatr Taiwan. 2006;47:67–71. [PubMed] [Google Scholar]
- 45.Singhi P.D., Ray M., Singhi S., Khandelwal N.K. Acute disseminated encephalomyelitis in North Indian children: clinical profile and follow-up. J Child Neurol. 2006;21:851–857. doi: 10.1177/08830738060210100201. [DOI] [PubMed] [Google Scholar]
- 46.Brinar V.V. Non-MS recurrent demyelinating diseases. Clin Neurol Neurosurg. 2004;106:197–210. doi: 10.1016/j.clineuro.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 47.Baum P.A., Barkovich A.J., Koch T.K., Berg B.O. Deep grey matter involvement in children with acute disseminating encephalomyelitis. AJNR Am J Neuroradiol. 1994;15:1275–1283. [PMC free article] [PubMed] [Google Scholar]
- 48.Tenembaum S., Chitnis T., Ness J., Hahn J.S. For the International Pediatric MS Study Group. Acute disseminated encephalomyelitis. Neurology. 2007;68(Suppl 2):S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 49.Triulzi F. Neuroradiology of multiple sclerosis in children. Neurol Sci. 2004;25:S340–S343. doi: 10.1007/s10072-004-0337-x. [DOI] [PubMed] [Google Scholar]
- 50.Kimura S., Unayama T., Mori T. The natural history of acute disseminated leukoencephalitis. A serial magnetic resonance imaging study. Neuropediatrics. 1992;23:192–195. doi: 10.1055/s-2008-1071339. [DOI] [PubMed] [Google Scholar]
- 51.Kesselring J., Miller D.H., Robb S.A., Kendall B.E., Moseley I.F., Kingsley D. Acute disseminated encephalomyelitis and MRI findings and the distinction from multiple sclerosis. Brain. 1990;113:291–302. doi: 10.1093/brain/113.2.291. [DOI] [PubMed] [Google Scholar]
- 52.Singh S., Alexander M., Korah I.P. Acute disseminated encephalomyelitis: MR imaging features. AJR Am J Roentgenol. 1999;173:1101–1107. doi: 10.2214/ajr.173.4.10511187. [DOI] [PubMed] [Google Scholar]
- 53.Khong P.L., Ho H.K., Cheng P.W., Wong V.C., Goh W., Chan F.L. Childhood acute disseminated encephalomyelitis: the role of brain and spinal cord MRI. Pediatr Radiol. 2002;32:59–66. doi: 10.1007/s00247-001-0582-6. [DOI] [PubMed] [Google Scholar]
- 54.Lim K.E., Hsu Y.Y., Hsu W.C., Chan C.Y. Multiple complete ring-shaped enhanced MRI lesions in disseminated encephalomyelitis. Clin Imag. 2003;27:281–284. doi: 10.1016/s0899-7071(02)00552-1. [DOI] [PubMed] [Google Scholar]
- 55.Caldemeyer K.S., Smith R.R., Harris T.M., Edwards M.K. MRI in acute disseminated encephalomyelitis. Neuroradiology. 1994;36:216–220. doi: 10.1007/BF00588134. [DOI] [PubMed] [Google Scholar]
- 56.Van Meyden C.H., de-Villers J.F.K., Middlecote B.D., Terbalanche J. Gadolinium ring enhancement and mass effect in acute disseminating encephalomyelitis. Neuroradiology. 1994;36:221–223. doi: 10.1007/BF00588135. [DOI] [PubMed] [Google Scholar]
- 57.Amit R., Shapira Y., Blank A., Aker M. Acute, severe, central and peripheral nervous system combined demyelination. Pediatr Neurol. 1986;2:47–50. doi: 10.1016/0887-8994(86)90040-8. [DOI] [PubMed] [Google Scholar]
- 58.Van Bogaert L., Borremans P., Couvreur J. Réflexions sur trois cas d’encéphalomyelite cérébelleuse. Presse Méd. 1932;49:141–144. [Google Scholar]
- 59.Hung K.-L., Liao H.-T., Tsai M.-L. The spectrum of postinfectious encephalomyelitis. Brain Dev. 2001;23:42–45. doi: 10.1016/s0387-7604(00)00197-2. [DOI] [PubMed] [Google Scholar]
- 60.Mikaeloff Y., Caridade G., Husson B., Suissa S., Tardieu M. Acute disseminated encephalomyelitis cohort study: prognostic factors for relapse. Eur J Paediatr Neurol. 2007;11:90–95. doi: 10.1016/j.ejpn.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Cohen O., Steiner-Birmanns B., Biran I., Abramsky O., Honigman S., Steiner I. Recurrence of acute disseminated encephalomyelitis at the previously affected brain site. Arch Neurol. 2001;58:797–801. doi: 10.1001/archneur.58.5.797. [DOI] [PubMed] [Google Scholar]
- 62.Shoji H., Kusuhara T., Honda Y., Hino H., Kojima K., Abe T. Relapsing acute disseminated encephalomyelitis associated with chronic Epstein-Barr virus infection: MRI findings. Neuroradiology. 1992;34:340–342. doi: 10.1007/BF00588198. [DOI] [PubMed] [Google Scholar]
- 63.Khan S., Yaqub B.A., Poser Ch.M., Al Deeb S.M., Bohlega S. Multiphasic disseminated encephalomyelitis presenting as alternating hemiplegia. J Neurol Neurosurg Psychiatry. 1995;58:467–470. doi: 10.1136/jnnp.58.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai M.-L., Hung K.-L. Multiphasic disseminated encephalomyelitis mimicking multiple sclerosis. Brain Dev. 1996;18:412–414. doi: 10.1016/0387-7604(96)00038-1. [DOI] [PubMed] [Google Scholar]
- 65.Hahn J.S., Siegler D.J., Enzmann D. Intravenous gammaglobulin therapy in recurrent acute disseminated encephalomyelitis. Neurology. 1996;46:1173–1174. doi: 10.1212/wnl.46.4.1173. [DOI] [PubMed] [Google Scholar]
- 66.Apak R.A., Anlar B., Saatci I. A case of relapsing acute disseminated encephalomyelitis with high dose corticosteroid treatment. Brain Dev. 1999;21:279–282. doi: 10.1016/s0387-7604(99)00011-x. [DOI] [PubMed] [Google Scholar]
- 67.Revel-Vilk S., Hurvitz H., Klar A., Virozov Y., Korn-Lubetzki I. Recurrent acute disseminated encephalomyelitis associated with acute cytomegalovirus and Epstein-Barr virus infection. J Child Neurol. 2000;15:421–424. doi: 10.1177/088307380001500614. [DOI] [PubMed] [Google Scholar]
- 68.Härtel C., Schilling S., Gottschalk S., Sperner J. Multiphasic disseminated encephalomyelitis associated with streptococcal infection. Eur J Paed Neurol. 2002;6:327–329. doi: 10.1016/s1090-3798(02)90621-5. [DOI] [PubMed] [Google Scholar]
- 69.Mariotti P., Batocchi A.P., Colosimo C., Lo Monaco M., Caggiula M., Colitto F. Multiphasic demyelinating disease involving central and peripheral nervous system in a child. Neurology. 2003;60:348–349. doi: 10.1212/01.wnl.0000044051.79305.dd. [DOI] [PubMed] [Google Scholar]
- 70.Alper G., Schor N.F. Toward the definition of acute disseminated encephalitis of childhood. Curr Opin Pediatr. 2004;16:637–640. doi: 10.1097/01.mop.0000136120.83277.72. [DOI] [PubMed] [Google Scholar]
- 71.Poser C. The epidemiology of multiple sclerosis: a general overview. Ann Neurol. 1994;36(S2):S231–S243. doi: 10.1002/ana.410360805. [DOI] [PubMed] [Google Scholar]
- 72.Divekar D., Bhosale S., Divate P. Recurrent acute disseminated encephalomyelitis. Indian Pediatr. 2007;44:138–140. [PubMed] [Google Scholar]
- 73.Ohtake T., Hirai S. Recurrence of acute disseminated encephalomyelitis after a 12-year symptom-free interval. Intern Med. 2004;43:746–749. doi: 10.2169/internalmedicine.43.746. [DOI] [PubMed] [Google Scholar]
- 74.Lassmann H., Wisniewksi H. Chronic relapsing experimental allergic encephalomyelitis. Arch Neurol. 1979;36:490–497. doi: 10.1001/archneur.1979.00500440060011. [DOI] [PubMed] [Google Scholar]
- 75.Krupp L.B., Banwell B., Tenembaum S. For the International Paediatric MS Study Group. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(Suppl 2):S7–S12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 76.Dale R.C., Branson J.A. Acute disseminated encephalomyelitis or multiple sclerosis: can the initial presentation help in establishing a correct diagnosis? Arch Dis Child. 2005;90:636–639. doi: 10.1136/adc.2004.062935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poser C.M., Paty D.W., Scheinberg L., McDonald W.I., Davis F.A., Ebers G.C. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 78.McDonald W.I., Compston A., Edan G., Goodkin D., Hartung H.P., Lublin F.D. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 79.Tenembaum S.N., Segura M.J. Childhood multiple sclerosis: clinical presentation and disease course. J Neurol. 2007;254(Suppl 3):39–40. [Abstract] [Google Scholar]
- 80.Poser C.M., Goutières F., Carpentier M.A., Aicardi J. Shilder's myelinoclastic diffuse sclerosis. Pediatrics. 1986;77:107–112. [PubMed] [Google Scholar]
- 81.Kepes J.J. Large focal tumor-like demyelinating lesions of the brain: intermediate entity between MS and acute disseminated encephalomyelitis? A study of 31 patients. Ann Neurol. 1993;33:18–27. doi: 10.1002/ana.410330105. [DOI] [PubMed] [Google Scholar]
- 82.Yapici Z., Eraksoy M. Bilateral demyelinating tumefactive lesions in three children with hemiparesis. J Child Neurol. 2002;17:655–660. doi: 10.1177/088307380201700901. [DOI] [PubMed] [Google Scholar]
- 83.Poser C. Pseudo-tumoral multiple sclerosis. Clin Neurol Neurosurg. 2005;107:535. doi: 10.1016/j.clineuro.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 84.McAdam L.C., Blaser S.I., Banwell B.L. Pediatric tumefactive demyelination: case series and review of the literature. Pediatr Neurol. 2002;26:18–25. doi: 10.1016/s0887-8994(01)00322-8. [DOI] [PubMed] [Google Scholar]
- 85.Mizuguchi M., Abe J., Mikkaichi K., Noma S., Yoshida K., Yamanaka T. Acute necrotizing encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry. 1995;58:555–561. doi: 10.1136/jnnp.58.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997;19:81–92. doi: 10.1016/s0387-7604(96)00063-0. [DOI] [PubMed] [Google Scholar]
- 87.Suwa K., Yamagata T., Momoi M.Y., Kawakami A., Kikuchi Y., Miyao M. Acute relapsing encephalopathy mimicking acute necrotizing encephalopathy in a 4-year-old boy. Brain Dev. 1999;21:554–558. doi: 10.1016/s0387-7604(99)00078-9. [DOI] [PubMed] [Google Scholar]
- 88.Ruggieri M., Polizzi A., Pavone L., Musumeci S. Thalamic syndrome in children with measles infection and selective, reversible thalamic involvement. Pediatrics. 1998;101:112–119. doi: 10.1542/peds.101.1.112. [DOI] [PubMed] [Google Scholar]
- 89.Hartfield D., Loewy J., Yager J. Transient thalamic changes on MRI in a child with hypernatremia. Pediatr Neurol. 1999;20:60–62. doi: 10.1016/s0887-8994(98)00092-7. [DOI] [PubMed] [Google Scholar]
- 90.Ohtaki E., Murakami Y., Komori H., Yamashita Y., Matsuishi T. Acute disseminated encephalomyelitis after Japanese B encephalitis vaccination. Pediatr Neurol. 1992;8:137–139. doi: 10.1016/0887-8994(92)90036-x. [DOI] [PubMed] [Google Scholar]
- 91.Cusmai R., Bertini E., Di Capua M., Ricci S., Vigevano F., Milani L. Bilateral, reversible, selective thalamic involvement demonstrated by brain MR and acute severe neurological dysfunction with favorable outcome. Neuropediatrics. 1994;25:44–47. doi: 10.1055/s-2008-1071582. [DOI] [PubMed] [Google Scholar]
- 92.Goutières F., Aicardi J. Acute neurological dysfunction associated with destructive lesions of the basal ganglia in children. Ann Neurol. 1982;12:328–332. doi: 10.1002/ana.410120403. [DOI] [PubMed] [Google Scholar]
- 93.Green C., Riley D. Treatment of dystonia in striatal necrosis caused by Mycoplasma pneumoniae. Pediatr Neurol. 2002;26:318–320. doi: 10.1016/s0887-8994(01)00396-4. [DOI] [PubMed] [Google Scholar]
- 94.Hiraga A., Kuwabara S., Hayakawa S., Ito S., Arimura K., Kanai K. Voltage-gated potassium channel antibody-associated encephalitis with basal ganglia lesions. Neurology. 2006;66:1780–1781. doi: 10.1212/01.wnl.0000218157.53333.79. [DOI] [PubMed] [Google Scholar]
- 95.Klein C.J., Dinapoli R.P., Temesgen Z., Meyer F.B. Central nervous system histoplasmosis mimicking a brain tumor: difficulties in diagnosis and treatment. Mayo Clin Proc. 1999;74:803–807. doi: 10.4065/74.8.803. [DOI] [PubMed] [Google Scholar]
- 96.Cikes N. Central nervous system involvement in systemic connective tissue diseases. Clin Neurol Neurosurg. 2006;108:311–317. doi: 10.1016/j.clineuro.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 97.Pittock S.J., Lennon V.A., Krecke K., Lucchinetti C.F., Weinshenker B.G. Brain abnormalities in Neuromyelitis Optica. Arch Neurol. 2006;63:390–396. doi: 10.1001/archneur.63.3.390. [DOI] [PubMed] [Google Scholar]
- 98.Banwell B, Tenembaum S, Lennon VA, Ursell E, Kennedy J, Bar-Or A, et al. Neuromyelitis Optica-IgG in childhood inflammatory demyelinating CNS disorders. Neurology; 2007; Dec 19: Epub. [DOI] [PubMed]
- 99.Gupte G., Stonehouse M., Wassmer E., Coad N.A., Whitehouse W.P. Acute disseminated encephalomyelitis: a review of 18 cases in childhood. J Paediatr Child Health. 2003;39:336–342. doi: 10.1046/j.1440-1754.2003.00154.x. [DOI] [PubMed] [Google Scholar]
- 100.Shahar E., Andraus J., Savitzki D., Pilar G., Zelnik N. Outcome of severe encephalomyelitis in children: effect of high-dose methylprednisolone and immunoglobulins. J Child Neurol. 2002;17:810–814. doi: 10.1177/08830738020170111001. [DOI] [PubMed] [Google Scholar]
- 101.Kotlus B.S., Slavin M.L., Guthrie D.S., Kodsi S.R. Ophthalmologic manifestations in pediatric patients with acute disseminated encephalomyelitis. J AAPOS. 2005;9:179–183. doi: 10.1016/j.jaapos.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 102.Nishikawa M., Ichiyama T., Hayashi T., Ouchi K., Furukawa S. Intravenous immunoglobulin therapy in acute disseminated encephalomyelitis. Pediatr Neurol. 1999;21:583–586. doi: 10.1016/s0887-8994(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 103.Kleiman M., Brunquell P. Acute disseminated encephalomyelitis: response to intravenous immunoglobulin. J Child Neurol. 1995;10:481–483. doi: 10.1177/088307389501000612. [DOI] [PubMed] [Google Scholar]
- 104.Straussberg R., Schonfeld T., Weitz R., Karmazyn B., Harel L. Improvement of atypical acute disseminated encephalomyelitis with steroids and intravenous immunoglobulins. Pediatr Neurol. 2001;24:139–143. doi: 10.1016/s0887-8994(00)00229-0. [DOI] [PubMed] [Google Scholar]
- 105.Sahlas D.J., Miller S.P., Guerin M., Veilleux M., Francis G. Treatment of acute disseminated encephalomyelitis with intravenous immunoglobulin. Neurology. 2000;54:1370–1372. doi: 10.1212/wnl.54.6.1370. [DOI] [PubMed] [Google Scholar]
- 106.Pradhan S., Gupta R.P., Shashank S., Pandey N. Intravenous immunoglobulin therapy in acute disseminated encephalomyelitis. J Neurol Sci. 1999;165:56–61. doi: 10.1016/s0022-510x(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 107.Pittock S.J., Keir G., Alexander M., Brennan P., Hardiman O. Rapid clinical and CSF response to intravenous gamma globulin in acute disseminated encephalomyelitis. Eur J Neurol. 2001;8:725. doi: 10.1046/j.1468-1331.2001.00195.x. [DOI] [PubMed] [Google Scholar]
- 108.Keegan M., Pineda A.A., McClelland R.L., Darby C.H., Rodriguez M., Weinshenker B.G. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58:143–146. doi: 10.1212/wnl.58.1.143. [DOI] [PubMed] [Google Scholar]
- 109.Stricker R.B., Miller R.G., Kiprov D.D. Role of plasmapheresis in acute disseminated (postinfectious) encephalomyelitis. J Clin Apheresis. 1992;7:173–179. doi: 10.1002/jca.2920070403. [DOI] [PubMed] [Google Scholar]
- 110.Ramachandrannair R., Rafeequ M., Girija A.S. Plasmapheresis in childhood acute disseminated encephalomyelitis. Indian Pediatr. 2005;42:479–482. [PubMed] [Google Scholar]
- 111.Miyazawa R., Hikima A., Takano Y., Arakawa H., Tomomasa T., Morikawa A. Plasmapheresis in fulminant acute disseminated encephalomyelitis. Brain Dev. 2001;23:424–426. doi: 10.1016/s0387-7604(01)00256-x. [DOI] [PubMed] [Google Scholar]
- 112.Balestri P., Grosso S., Acquaviva A., Bernini M. Plasmapheresis in a child affected by acute disseminated encephalomyelitis. Brain Dev. 2000;22:123–126. doi: 10.1016/s0387-7604(99)00115-1. [DOI] [PubMed] [Google Scholar]
- 113.Newton R. Plasma exchange in acute post-infectious demyelination. Dev Med Child Neurol. 1981;23:538–543. doi: 10.1111/j.1469-8749.1981.tb02030.x. [DOI] [PubMed] [Google Scholar]
- 114.Hahn C.D., Miles B.S., MacGregor D.L., Blaser S.I., Banwell B.L., Hetherington C.R. Neurocognitive outcome after acute disseminated encephalomyelitis. Pediatr Neurol. 2003;29:117–123. doi: 10.1016/s0887-8994(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 115.Jacobs R.K., Anderson V.A., Neale J.L., Shield L.K., Kornberg A.J. Neuropsychological outcome after acute disseminated encephalomyelitis: impact of age at illness onset. Pediatr Neurol. 2004;31:191–197. doi: 10.1016/j.pediatrneurol.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 116.Banwell B.L., Anderson P.E. The cognitive burden of multiple sclerosis in children. Neurology. 2005;64:891–894. doi: 10.1212/01.WNL.0000152896.35341.51. [DOI] [PubMed] [Google Scholar]
- 117.Hart M.N., Earle K.M. Haemorrhagic and perivenous encephalitis: a clinical–pathological review of 38 cases. J Neurol Neurosurg Psychiatry. 1975;38:585–591. doi: 10.1136/jnnp.38.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Prineas J., McDonald W.I., Franklin R. Demyelinating diseases. In: Graham D., Lantos P., editors. Greenfield's Neuropathology. 7th ed. Arnold; London: 2002. pp. 471–550. [Google Scholar]
- 119.Ghosh N., DeLuca G.C., Esiri M.M. Evidence of axonal damage in human acute demyelinating diseases. J Neurol Sci. 2004;222:29–34. doi: 10.1016/j.jns.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 120.DeLuca G.C., Ebers G.C., Esiri M.M. Axonal loss in multiple sclerosis: a pathological survey of the corticospinal and sensory tracts. Brain. 2004;127:1009–1018. doi: 10.1093/brain/awh118. [DOI] [PubMed] [Google Scholar]
- 121.Kuchroo V.K., Sobel R.A., Yamamura T., Greenfield E., Dorf M.E., Lees M.B. Induction of experimental allergic encephalomyelitis by myelin proteolipid-protein-specific T cell clones and synthetic peptides. Pathobiology. 1991;59:305–312. doi: 10.1159/000163668. [DOI] [PubMed] [Google Scholar]
- 122.Linington C., Engelhardt B., Kapocs G., Lassman H. Induction of persistently demyelinated lesions in the rat following the repeated adoptive transfer of encephalitogenic T cells and demyelinating antibody. J Neuroimmunol. 1992;40:219–224. doi: 10.1016/0165-5728(92)90136-9. [DOI] [PubMed] [Google Scholar]
- 123.Wucherpfennig K.W., Strominger J.L. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lipton H.L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clatch R.J., Lipton H.L., Miller S.D. Characterization of Theiler's murine encephalomyelitis virus (TMEV)-specific delayed-type hypersensitivity responses in TMEV-induced demyelinating disease: correlation with clinical signs. J Immunol. 1986;136:920–927. [PubMed] [Google Scholar]
- 126.Miller S.D., Mc Rae B.L., Vanderlugt C.L., Nikcevich K.M., Pope J.G., Pope L. Evolution of the T-cell repertoire during the course of experimental immune-mediated demyelinating diseases. Immunol Rev. 1995;144:225–244. doi: 10.1111/j.1600-065x.1995.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 127.Rodriguez M., Pavelko K.D., Njenga M.K., Logan W.C., Wettstein P.J. The balance between persistent virus infection and immune cells determines demyelination. J Immunol. 1996;157:5699–5709. [PubMed] [Google Scholar]
- 128.Miller S.D., Vanderlugt C.L., Begolka W.S., Pao W., Yauch R.L., Neville K.L. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 129.Katz-Levy Y., Neville K.L., Girvin A.M., Vanderlugt C.L., Pope J.G., Tan L.J. Endogenous presentation of self myelin epitopes by CNS-resident APCs in Theiler's virus-infected mice. J Clin Invest. 1999;104:599–610. doi: 10.1172/JCI7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McMahon E.J., Bailey S.L., Castenada C.V., Waldner H., Miller S.D. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 131.Tejada-Simon M.V., Zang Y.C., Hong J., Rivera V.M., Zhang J.Z. Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosis. Ann Neurol. 2003;53:189–197. doi: 10.1002/ana.10425. [DOI] [PubMed] [Google Scholar]
- 132.Talbot P.J., Paquette J.S., Ciurli C., Antel J.P., Ouellet F. Myelin basic protein and human coronavirus 229E cross-reactive T cells in multiple sclerosis. Ann Neurol. 1996;39:233–240. doi: 10.1002/ana.410390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Markovic-Plese S., Hemmer B., Zhao Y., Simon R., Pinilla C., Martin R. High level of cross-reactivity in influenza virus hemagglutinin-specific CD4+ T-cell response: implications for the initiation of autoimmune response in multiple sclerosis. J Neuroimmunol. 2005;169:31–38. doi: 10.1016/j.jneuroim.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 134.Lang H.L., Jacobsen H., Ikemizu S., Andersson C., Harlos K., Madsen L. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol. 2002;3:940–943. doi: 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- 135.Olson J.K., Croxford J.L., Miller S.D. Virus-induced autoimmunity: potential role of viruses in initiation, perpetuation, and progression of T-cell-mediated autoimmune disease. Viral Immunol. 2001;14:227–250. doi: 10.1089/088282401753266756. [DOI] [PubMed] [Google Scholar]
- 136.Mokhtarian F., Zhang Z., Shi Y., Gonzales E., Sobel R.A. Molecular mimicry between a viral peptide and a myelin oligodendrocyte glycoprotein peptide induces autoimmune demyelinating disease in mice. J Neuroimmunol. 1999;95:43–54. doi: 10.1016/s0165-5728(98)00254-9. [DOI] [PubMed] [Google Scholar]
- 137.O’Connor K.C., Robinson W.H., De-Jager P.L., Fukaura H., Chitnis T., Wong S.J. High-throughput analysis of autoantibodies recognizing myelin antigens in acute disseminated encephalomyelitis. Neurology. 2005;64(Suppl 1):A417. [Abstract] [Google Scholar]
- 138.O’Connor K., McLaughlin K.A., De Jager P., Chitnis T., Bettelli E., Xu C. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med. 2007;13:211–217. doi: 10.1038/nm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]