Abstract
Pro-protein convertase-2 (PC2) and carboxypeptidase-E (CPE) proteins are two major members of the pro-protein convertases that involve in the maturation of protein precursor. By using PC2 activity, immunocytochemistry (ICC) and Western blot method, PC2, CPE and preproNPY protein expression levels were compared among mature retina tissue, RGC-5 cells and its differentiated cells, or brain cortex tissue, NS20Y tumor cells and its differentiated cells, or mature breast tissue, breast tumor cell RM1 and breast adenocarcinoma tissue. The experimental results indicated that the differentiated cells or tissues had higher or highest PC2 activity. In the comparative experiments, more PC2 protein expression in the mature tissues and more CPE and preproNPY protein expression in the tumor cells or tumor tissue were observed, but no expression of preproNPY protein was observed in the mature tissues. Compared with NS20Y or RGC-5 undifferentiated cells, its differentiated cells showed less proPC2, more proCPE and more preproNPY protein expressions. The results demonstrated that the mature tissues showed stronger PC2/CPE-mediated pro-protein processing ability than the tumor cells or tissue. The results also showed that the artificial differentiation of RGC-5 or NS20Y cells was different from maturation of its corresponding normal tissue.
Keywords: Pro-protein processing system, Tumor cell differentiation, Pro-protein convertase-2, Carboxypeptidase-E, Prepro-neuropeptide-Y
1. Introduction
Subtilisin-like pro-protein convertases (PCs) are the major endoproteolytic processing enzymes that mediate the biosynthesis of a great variety of secreted and membrane proteins. These proteins processed by PCs are involved in embryogenesis, gene expression, cell cycle, programmed cell death, intracellular protein targeting, and endocrine/neural functions (Zhou et al., 1999, Muller et al., 2004). These precursor molecules need to undergo a series of processing steps that involve multiple processing enzymes (Posthaus et al., 2003, Chan et al., 1992). The multi-protein family of mammalian serine proteases is responsible for these proteolytic maturation events. They are expressed together or separately in functional cells from both vertebrates and invertebrates. The mammalian PCs have been demonstrated to be involved in several diseases, i.e. HIV, hepatitis B, severe acute respiratory syndrome (SARS), anthrax, cancer, Alzheimer's disease, arthritis, stroke, glaucoma, and diabetes (Seidah and Prat, 2002, Thomas, 2002, Khatib et al., 2002, Taylor et al., 2003, Molloy et al., 1999, Leak et al., 2007).
Pro-protein convertase-2 (PC2) and carboxypeptidase-E (CPE) are expressed widely in neuroendocrine tissues and play a major role in the proteolytic processing (Furuta et al., 2001). Tanaka (2003) and Sang-Nam et al. (2007) reported that mouse or rat proPC2 with 637 amino acid residues (74 kDa) cleaves into mature PC2 with 529 amino acid residues (64 kDa) in adequate conditions, such as 5.0 pH, higher Ca2+, and other PCs. There is about 96.55% identity between human and mouse PC2, or 99.53% identity between mouse and rat PC2 in amino acid sequence (Smeekens and Steiner, 1990, Seidah et al., 1991, Manser et al., 1990). Fricker et al. (1989) and Hakes et al. (1991) reported that the human and rat proCPE protein sequence with 476 amino acid residues (56 kDa) are highly conserved (99.58% identity between them). The mature CPE protein is about 50 kDa. The soluble CPE (sCPE) is an exo-peptidase that cleaves neuroendocrinopeptide with C-terminal basic amino acid residue and produces active form of peptide hormone and neuropeptide (Fricker and Snyder, 1983, Hook and Loh, 1984). The membrane-bound CPE (mCPE) functions as a sorting receptor in the trans-Golgi network (TGN). It facilitates the sorting of pro-hormones into the regulated secretary pathway (Zhu et al., 2005, Dhanvantari et al., 2003). Brakch et al. (1997) and Miller et al. (2003) reported that the maturation of NPY precursor needed the presence of PC2 and CPE.
In our previous study on neurons and ischemia, we discovered the artificial differentiation of NS20Y or RGC-5 tumor cells caused the increase of PC2 activity. There may seem certain relationship between the PC system and tumor differentiation. The PC2/CPE-mediated processing system in the tumor cells and their corresponding mature tissues was evaluated in the paper.
2. Materials and methods
2.1. Cell line and differentiation
Retina ganglion cell-5 tumor cell (RGC-5) is transformed from rat retina ganglion cell. NS20Y tumor cell is neuroblastoma one from mouse brain cortex. They were grown in higher glucose DMEM medium containing 10% fetal calf serum (FCS) (Invitrogen Co.), 20 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 500 μM G418 at 37 °C in a 5% CO2 incubator. The differentiation of RGC-5 tumor cells was achieved by incubating cells in the DMEM–FCS medium containing 0.1 μM staurosporine (Sigma Co.) for 24 h and NS20Y cells were differentiated for 24-h in 1 mM dibutylyl cyclic AMP (Yamasa Chiba, Japan). Breast tumor cell line RM1 was cultured in the DMEM medium containing 10% FCS.
2.2. Immunocytochemistry
The cultured cells were performed fluorescent ICC. Because the fluorescent ICC results of tissue sections were not good, the Envision method was used for the section ICC.
2.2.1. Fluorescent ICC
PC2, CPE, or preproNPY fluorescent ICC on cultured RGC-5 or NS20Y cells was analyzed by using FISH protocol (Zhou et al., 2004). Briefly, cultured cells in dish were washed three times with PBS, and then quenched for 5 min in the acidified ethanol solution (ethanol:acetic acid = 1:2) at −20 °C. After incubated for 20 min at room temperature in PBS containing 3% Tween X-100, a 30 min pre-incubation was completed in a blocking solution containing 2% horse serum, 0.1% BSA, and PBS. An appropriate primary antibody (PC2, CPE, or preproNPY antibody 1:1000, 2000, or 2000 diluted with the blocking solution, respectively) were incubated at 4 °C overnight. The next day, after washed three times with PBS for 15 min, they were incubated for 2 h with an appropriate Cy3-conjugated secondary antibody (1:380 diluted with the blocking solution, Vector Co.). After the cells were washed three times for 30 min with PBS, they were mounted with the mounting medium DAPI (4′,6-diamidino-2-phenylindole, Vectashield Co.) to stain nuclei. Fluorescent signals in the culture cells were examined and documented with an epifluorescence microscope (Leica Microsystems Inc.) attached with a magnified digital color camera (ChipCoolers Co.). The fluorescent signals of nuclei and positive portion were merged into our figures.
2.2.2. Envision method for ICC
The Envision method was performed by using an ICC kit (Beijing Zhengshan Biotech Co.). In our study, the method was better to wax tissue sections rather than cultured cells. So the sections used the Envision method for ICC. The wax sections were dried for 2 h at 37 °C in an electric drying oven. After the sections were soaked in turn into 1,2-dimethylbenzene solution three times for 30 min, 100% ethanol two times for 20 min, 90–70% ethanol one time for 10 min, respectively, they were washed one time with water and incubated 20 min in a dark room by adding 3% hydrogen peroxide on tissue. The sections were washed one time for 5 min with water and three times with PBS for 15 min. After incubated for 30 min at room temperature by adding 50 μl of primary antibodies (PC2, and CPE, preproNPY antibodies diluted into 1:1000 and 1:2000 with PBS solution), the sections were washed three times for 15 min with PBS. After incubated for 30 min at room temperature by adding the secondary antibody (ChemMateTMEnVision+/HRP), the sections were washed three times for 15 min with PBS. 50 μl of DAB working solution was added on tissue and incubated for 10 min at room temperature and the sections were washed in water. The sections were stained with hematoxylin and then de-watered in turn with 70–100% ethanol for 10 min each one. The Envision procedure was finished after the sections were soaked into 1,2-dimethylbenzene solution three times for 30 min and mounted with a mounting medium (neutral balsam, Shanghai Biaoben Model Co., China).
2.3. Tissue isolation and sample preparation
Rat retina tissue and mouse brain cortex were taken, respectively from 250–300 g S-D rat and 20–25 g Kunming mouse from Nanfang Medical University. Human breast adenocarcinoma tissues and their normal breast tissues were donated by Dr. Huan-Xin Liu and Dr. Juan-Hui Zhang from Guangdong Armed Police Hospital. The fresh tissues were preserved at −80 °C for Western blot and PC2 activity analysis. PC2, CPE and preproNPY protein expression levels were compared among mature retina tissue, RGC-5 tumor cells and its differentiation cells, or brain cortex tissue, NS20Y tumor cells and its differentiation cells, or mature breast tissue, breast tumor cell RM1, and breast adenocarcinoma tissue.
2.4. PC2 enzymatic assay
Analysis of PC2 activity followed a modulated protocol according to Berman et al. (2000). The whole reaction volume was 100 μl. Tissues or cells were homogenized with a buffer containing 50 mM Tris–HCl (pH 7.5), 1% Triton X-100, 10% glycerol and a cocktail of protease inhibitors (aprotinin 1 μM, PMSF 1 mM, benzamidine 1 mM). To control the detergent concentration in the reaction buffer, 10 μl of protein supernatant was incubated with 200 μM l-pyroglutamyl-Arg-Thr-Lys-Arg-7-amino-4-methyl-coumarin in 100 mM sodium acetate (pH 5.0) and 1 mM CaCl2. All incubations were carried out at 37 °C for 4 h. In parallel incubations, 2 μM final concentration of CT peptide (SVNPYLQGKRLDNVVAKK, a PC2-specific inhibitor derived from the C-terminus of 7B2 protein) was used. The release of 7-amino-4-methylcoumarin was measured by using a Spectra Max GEMINI spectrofluorimeter (Molecular Devices Co.; λ ex = 360 nm, λ em = 480 nm). The amount of product formed was calculated by using free 7-amino-4-methylcoumarin as a standard. The activity inhibited by CT peptide was taken as PC2 activity.
2.5. Western blot analysis
To prevent from serum contamination from medium, cells in dishes were washed one time with warm PBS and then suspended softly with cold PBS. After the cell suspension was centrifuged at 6000 rpm for 6 min at 4 °C, the cell pellet was collected. Tissue was homogenized with a 2-ml glass homogenizer. Proteins were extracted from tissue homogenates or cultured cell pellet with the same buffer as PC2 activity. After tissue homogenate or cell lysate was subjected to 1 cycle of freezing–thawing–vortex to further destroy their structures, they were centrifuged at 10,000 × g for 10 min at 4 °C to collect supernatant. Protein concentration of the supernatant was determined by the Bradford method (Bio-Rad Laboratories Co.). Proteins (50 μg per gel lane) were fractionated by SDS–PAGE (150 V for 2 h), blotted onto a PVDF membrane (Millipore Co., USA) (166 mA for 2.5 h), and probed with an appropriate primary antibody. The antibody bound to the membrane was detected with the enzyme-catalyzed chemiluminescence (ECL) method (Pierce Co.). The membrane was cut for ECL according to the molecular weights of the detected proteins. To verify the equality of protein loadings among different samples to be compared, the blotted membrane was detected with β-actin antibody as control. Rabbit antiserum against proPC2 (amino acids 611–638, diluted in 1:1000) (donated by Dr. An Zhou) or antibodies against preproNPY (amino acids 68–97, diluted in 1: 2000) and CPE (diluted in 1:2000) were used. The CPE or preproNPY antibody was purchased from Research Diagnostics Inc.
3. Results
3.1. Differentiation of RGC-5 or NS20Y cell
NS20Y or RGC-5 undifferentiated cells were induced differentiation in 1 mM dibutylyl cyclic AMP or 0.1 μM staurosporine for 24 h. The morphology of RGC-5 cells is similar to that of NS20Y cells. The undifferentiated cells are round and spindle shape in a light microscope. There is no dendrite on the body and granule in the cell (Fig. 1A). The differentiated cells are polygonal shape and have many dendrites on the body. There are many granules in the body and dendrites (Fig. 1B). Dendrites of the different cells linked with each other.
Fig. 1.

Differentiation of NS20Y or RGC-5 cell (mag. 15× 10 times). (A) Undifferentiated cells and (B) differentiated cells.
3.2. ICC results
The ICC results of the cultured cells showed that PC2, CPE and preproNPY had sub-cellular redistribution along the cell body and tips (Fig. 2, Fig. 3, Fig. 4 ). The ICC results of NS20Y or RGC-5 cells were similar. In the differentiated cells, the up-regulations of CPE and preproNPY protein levels were observed (Fig. 3, Fig. 4). Because the ICC results on mouse cortex neuron were similar to those of mature rat retina, the ICC results of mature mouse cortex tissue were only showed in the paper. Compared with the ICC results of breast adenocarcinoma tissue, the mature tissues (rat retina, mouse brain cortex, or normal breast) showed higher PC2 activity, more proPC2, less CPE, and less preproNPY protein expression level (Fig. 5, Fig. 6, Fig. 7, Fig. 8 ).
Fig. 2.

PC2 fluorescent ICC analyses of the cultured cells (merged, mag. 40× 10 times). (A) Undifferentiated cells and (B) differentiated cells. White arrowheads indicate positive portion in cell (red portion). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Fig. 3.
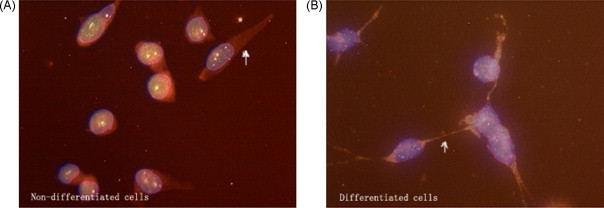
CPE fluorescent ICC analyses of the cultured cells (merged, mag. 40× 10 times). (A) Undifferentiated cells and (B) differentiated cells. White arrowheads indicate positive portion in cell (red portion). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Fig. 4.
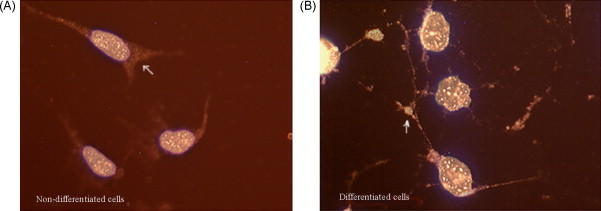
preproNPY fluorescent ICC analyses of the cultured cells (merged, mag. 40× 10 times). (A) Undifferentiated cells and (B) differentiated cells. White arrowheads indicate positive portion in cell (red portion). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Fig. 5.
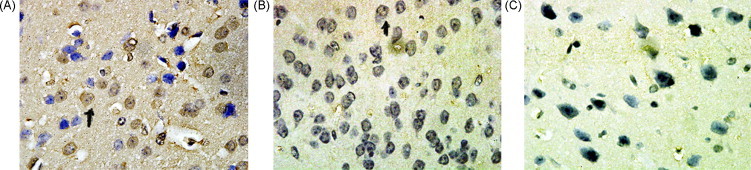
PC2, CPE, and preproNPYICC analyses of mouse cortex neuron (Envision method, mag. 100× 10 times). (A) PC2ICC staining; (B) CPEICC staining; (C) preproNPYICC staining. Black arrowheads indicate positive cell (yellow portion). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Fig. 6.
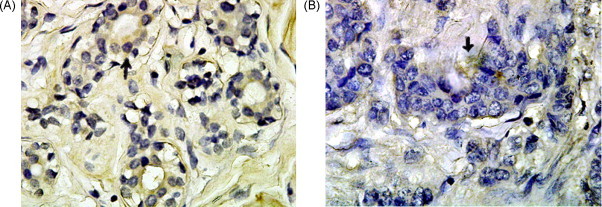
PC2ICC analysis of breast tissue (Envision method, mag. 100× 10 times). (A) Normal tissue and (B) adenocarcinoma tissue. Black arrowheads indicate positive cell (yellow portion). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Fig. 7.
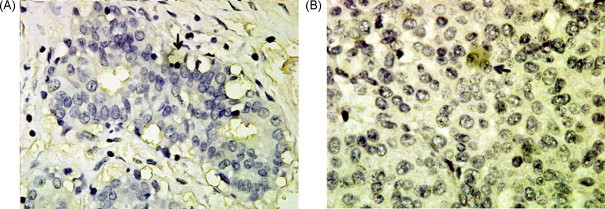
CPEICC analysis of breast tissue (Envision method, mag. 100× 10 times). (A) Normal tissue and (B) adenocarcinoma tissue. Black arrowheads indicate positive cell (yellow portion). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Fig. 8.

preproNPYICC analysis of breast tissue (Envision method, mag. 100× 10 times). (A) Normal tissue and (B) adenocarcinoma tissue. Black arrowheads indicate positive cell (yellow portion). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.3. Western blot results
50 μg of protein was loaded in SDS–PAGE lane and transferred on PVDF membrane. In the comparative experiments, more proPC2 protein expression in the mature tissues and more proCPE protein expression in the tumor cells or tissue were observed (Fig. 9, Fig. 10, Fig. 11 ). preproNPY protein expression were observed in the tumor cells or 5 of 8 adenocarcinoma tissue specimens, but no expression of the protein was observed in the mature tissues (Fig. 9, Fig. 12 ). Compared with the NS20Y or RGC-5 undifferentiated cells, less proPC2, more proCPE and more preproNPY protein expressions were observed in the differentiated cells (Fig. 9A).
Fig. 9.
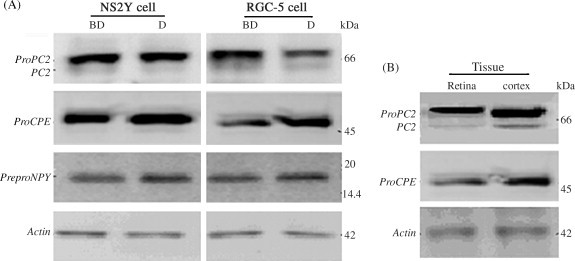
Western blot analyses of PC2, CPE and preproNPY proteins. (A) Expression in NS20Y or RGC-5 cells. BD: undifferentiated cells; D: differentiated cells. (B) Expression in mature retina or brain cortex tissue. Protein markers 14.4–97 kDa or 3.5–45 kDa were used for PC2 and CPE or preproNPY analysis.
Fig. 10.
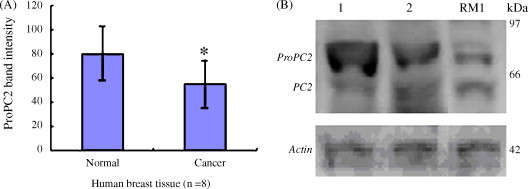
Western blot analysis of proPC2 protein in breast tissue. (A) ProPC2 protein band intensity statistics (n = 8). *P < 0.05 vs. normal breast tissue (paired t-test). (B) Western blot picture of proPC2 protein. Lane 1: normal breast tissue; Lane 2: breast adenocarcinoma tissue; Lane RM1: breast tumor cell RM1; protein markers 14.4–97 kDa.
Fig. 11.

Western blot analysis of proCPE protein in breast tissue. (A) proCPE protein band intensity statistics (n = 8). *P < 0.05 vs. normal breast tissue (paired t-test). (B) Western blot picture of proCPE protein. Lane 1: normal breast tissue; Lane 2: breast adenocarcinoma tissue; Lane RM1: breast tumor cell RM1; protein markers 14.4–97 kDa.
Fig. 12.
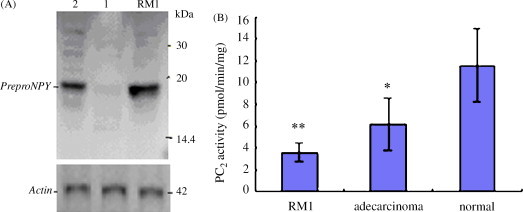
(A) Western blot analysis of preproNPY protein in breast tissue (n = 8). Lane 1: normal breast tissue; Lane 2: breast adenocarcinoma tissue; Lane RM1: breast tumor cell RM1; protein markers 3.5–45 kDa. (B) PC2 activity analysis of breast cells (n = 3) and tissues (n = 8). *P < 0.05 and **P < 0.01 vs. normal breast tissue (paired t-test).
3.4. PC2 activity
PC2 activity showed significant difference between NS20Y or RGC-5 undifferentiated cells and its differentiated cells or the corresponding mature tissues (P < 0.05, P < 0.01) (Fig. 13 ). Compared with the undifferentiated cells, the artificially differentiated cells showed higher PC2 activity (P < 0.05). Compared with the normal breast tissue, the breast adenocarcinoma tissue or breast tumor cell RM1 showed lower PC2 activity (P < 0.05, P < 0.01) (Fig. 12B). The differentiated cell or the mature tissues showed higher or the highest PC2 activity whereas the tumor tissue or cells showed lower PC2 activity.
Fig. 13.
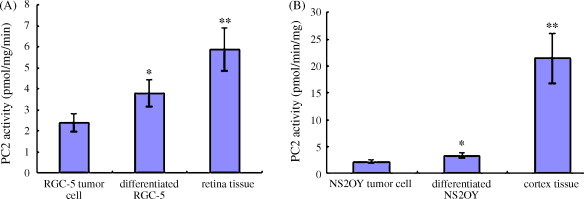
PC2 activity analysis of tumor cells or tissues (n = 3). (A) RGC-5 cells and retina tissue. (B) NS20Y cells and mouse brain cortex tissue. *P < 0.05 and **P < 0.01 vs. result of tumor cell (t-test).
4. Conclusion
PC2 is one major endoproteolytic enzyme and CPE is an exoproteolytic one. When precursor molecule is endoproteolyzed by PCs, the soluble CPE recognizes the specific basic residue at the C-terminus and processes it. NPY molecule is one of the most abundant peptides found in the mammalian tissue (Chronwall et al., 1985, De Quidt and Emson, 1986). Its maturation is subjected to the processing of PC2 and CPE. preproNPY has 97 amino acid residues in mouse and human, 98 amino acid residues in rat. The isoelectric point (pI) of preproNPY is 11.1. The Western blot result of preproNPY peptide indicated that its specific band on SDS–PAGE was close to 17 kDa, which is because the higher pI of the peptide formed a larger molecule by combining with more SDS molecule. The electric mobility is very similar with the GHRH analogs we ever reported (Tang et al., 2004). In the study on PC2 activity, the leupeptin in the Berman buffer strongly inhibits PC2 activity and there is lower PC2 activity in the tumor cells. In order to obtain the reliable results, the Berman protocol was modulated.
RGC-5 or NS20Y cells were usually used in the in vitro study on retina or brain cortex neuron. For the cell cultures, 500 μM of G418 was added in the DMEM medium, which was because the cell lines were thought not to be in purity. The cells were kept in purity by adding G418. In our previous publication (Tang et al., 2009), we discovered that there was a close relationship between the PC system and tumor. So the PC2/CPE-mediated processing was evaluated in mature tissues and tumor cells or tissues.
The experimental results indicated that the differentiated cells or tissues showed higher or highest PC2 activity. In the comparative experiments, more proPC2 protein expression in the mature tissues and more proCPE and high preproNPY protein expression in the tumor cells or tissue were observed, but no expression of preproNPY was observed in the mature tissue, which suggested the mature tissues have a stronger PC2/CPE–mediated pro-protein processing ability. As long as intracellular proNPY protein occurs, the strong PC2/CPE-mediated processing system processes the precursor into NPY, whereas the weak PC2/CPE-mediated pro-protein processing level in the tumor tissues or cells leads to the aggregations of proCPE and preproNPY proteins. In the research the cell or tissue ICC results collaborated with the Western blot ones. We predict that there is a weak or uneven PCs system in tumor. The microenvironment formed in tumor was different from normal mature tissue. So the profile of tumor may result from not only up- or down-regulation(s) of component(s), but also the difference of the construct ratio of the functional molecules in tumor tissue should be considered. We predict that the increase of PC2 activity in tumor cell may induce its differentiation.
The differentiated cells in our research showed higher PC2 activity, less proPC2, more proCPE and high preproNPY protein expressions compared with RGC-5 or NS20Y undifferentiated cells, which suggested that the artificial differentiations were different from the differentiation of mature tissue. The staurosporine or dbcAMP reagent promoted the maturation of proPC2 into PC2 and induced the aggregations of proCPE and preproNPY proteins. The differentiations are more similar with “cell stressing induced by oxygen and glucose deprivation” (Tang et al., 2009). The artificial differentiations may be false. But we also did not know why the increase of PC2 in the differentiated cells accompanied the aggregations of proCPE and preproNPY proteins.
proPC2 and proCPE or preproNPY protein in the experiments appeared to express differently in the cells or tissues, which may be that the mature PC2 and CPE function accordingly. Because the transformation of proPC2 into PC2 is far slower than that of proCPE into CPE, when high PC2 activity (more proPC2) is necessary in function, more CPE (less proCPE) should be accompanied. The more PC2-CPE quickly processes the substrate proNPY molecule. Similarly, less PC2 causes the aggregations of proCPE and preproNPY proteins.
Acknowledgements
We thank and appreciate for Dr. An Zhou's help and supervision in method and antibody. The research work was supported by Guangdong Pharmaceutical University Grant 2005SMK22 and Key-Teacher Training Grant.
References
- Berman Y., Mzhavia N., Polonskaia A., Furuta M., Steiner D.F., Pintar J.E., Devi L.A. Defective prodynorphin processing in mice lacking prohormone convertase PC2. J. Neurochem. 2000:1763–1770. doi: 10.1046/j.1471-4159.2000.0751763.x. [DOI] [PubMed] [Google Scholar]
- Brakch N., Rist B., Beck-Sickinger A.G., Goenaga J., Wittek R., Bürger E., Brunner H.R., Grouzmann E. Role of prohormone convertases in pro-neuropeptide Y processing: coexpression and in vitro kinetic investigations. Biochemistry. 1997;36(51):16309–16320. doi: 10.1021/bi9714767. [DOI] [PubMed] [Google Scholar]
- Chan S.J., Oliva A.A., Jr., LaMendola J., Grens A., Bode H., Steiner D.F. Conservation of the prohormone convertase gene family in metazoa: analysis of cDNAs encoding a PC3-like protein from hydra. Proc.Natl. Acad. Sci. 1992;89:6678–6682. doi: 10.1073/pnas.89.15.6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronwall B.M., DiMaggio D.A., Massari V.J., Pickel V.M., Ruggiero D.A., O’Donohue T.L. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985;15(4):1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- De Quidt M.E., Emson P.C. Neuropeptide Y in the adrenal gland: characterization, distribution and drug effects. Neuroscience. 1986;19(3):1011–1022. doi: 10.1016/0306-4522(86)90313-1. [DOI] [PubMed] [Google Scholar]
- Dhanvantari S., Shen F.S., Adams T., Snell C.R., Zhang C., Mackin R.B., Morris S.J., Loh Y.P. Disruption of a receptormediated mechanism for intracellular sorting of proinsulin in familial hyperproinsulinemia. Mol. Endocrinol. 2003;17:1856–1867. doi: 10.1210/me.2002-0380. [DOI] [PubMed] [Google Scholar]
- Fricker L.D., Snyder S.H. Purification and characterization of enkephalin convertase, an enkephalin-synthesizing carboxypeptidase. J. Biol. Chem. 1983;258:10950–10955. [PubMed] [Google Scholar]
- Fricker L.D., Adelman J.P., Douglass J., Thompson R.C., von Strandmann R.P., Hutton J. Isolation and sequence analysis of cDNA for rat carboxypeptidase E [EC 3.4.17.10], a neuropeptide processing enzyme. Mol. Endocrinol. 1989;3(4):666–673. doi: 10.1210/mend-3-4-666. [DOI] [PubMed] [Google Scholar]
- Furuta M., Zhou A., Webb G., Carroll R., Ravazzola M., Orci L., Steineri D.F. Severe defect in proglucagon processing in islet A-cells of prohormone convertase 2 null mice. J. Biol. Chem. 2001;276(29):27197–27202. doi: 10.1074/jbc.M103362200. [DOI] [PubMed] [Google Scholar]
- Hakes D.J., Birch N.P., Mezey A., Dixon J.E. Isolation of two complementary deoxyribonucleic acid clones from a rat insulinoma cell line based on similarities to Kex2 and furin sequences and the specific localization of each transcript to endocrine and neuroendocrine tissues in rats. Endocrinology. 1991;129(6):3053–3063. doi: 10.1210/endo-129-6-3053. [DOI] [PubMed] [Google Scholar]
- Hook V.Y., Loh Y.P. Carboxypeptidase B-like converting enzyme activity in secretory granules of rat pituitary. Proc. Natl. Acad. Sci. 1984;81:2776–2780. doi: 10.1073/pnas.81.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatib A.M., Siegfried G., Chretien M., Metrakos P., Seidah N.G. Proprotein convertases in tumor progression and malignancy: novel targets in cancer therapy. Am. J. Pathol. 2002;160:1921–1935. doi: 10.1016/S0002-9440(10)61140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak T.S., Keene K.L., Langefeld C.D., Gallagher C.J., Mychalecky J.C., Freedman B.I., Bowden D.W., Rich S.S., Sale M.M. Association of the proprotein convertase subtilisin/kexin-type 2 (PCSK2) gene with type 2 diabetes in an African American population. Mol. Genet. Metab. 2007;92(1–2):145–150. doi: 10.1016/j.ymgme.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang-Nam L., Magdalena M., Kacprzak R., Lindberg I. Processing and trafficking of a prohormone convertase 2 active site mutant. Biochem. Biophys. Res. Commun. 2007;355(3):825–829. doi: 10.1016/j.bbrc.2007.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E., Fernandez D., Loo L., Goh P.Y., Monfries C., Hall C., Lim L. Human carboxypeptidase E. Isolation and characterization of the cDNA, sequence conservation, expression and processing in vitro. Biochem. J. 1990;267(2):517–525. doi: 10.1042/bj2670517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R., Toneff T., Vishnuvardhan D., Beinfeld M., Hook V.Y. Selective roles for the PC2 processing enzyme in the regulation of peptide neurotransmitter levels in brain and peripheral neuroendocrine tissues of PC2 deficient mice. Neuropeptides. 2003;37(3):140–148. doi: 10.1016/s0143-4179(03)00027-1. [DOI] [PubMed] [Google Scholar]
- Molloy S.S., Anderson E.D., Jean F., Thomas G. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- Muller E.J., Caldelari R., Posthaus H. Role of subtilisin-like convertases in cadherin processing or the conundrum to stall cadherin function by convertase inhibitors in cancer therapy. J. Mol. Histol. 2004;35:263–275. doi: 10.1023/b:hijo.0000032358.51866.a2. [DOI] [PubMed] [Google Scholar]
- Posthaus H., Dubois C.M., Muller E. Novel insights into cadherin processing by subtilisin-like convertases. FEBS Lett. 2003;536:203–208. doi: 10.1016/s0014-5793(02)03897-8. [DOI] [PubMed] [Google Scholar]
- Seidah N.G., Prat A. Precursor convertases in the secretory pathway, cytosol and extracellular milieu. Essays Biochem. 2002;38:79–94. doi: 10.1042/bse0380079. [DOI] [PubMed] [Google Scholar]
- Seidah N.G., Mattei M.G., Gaspar L., Benjannet S., Mbikay M., Chrétien M. Chromosomal assignments of the genes for neuroendocrine convertase PC1 (NEC1) to human 5q15-21, neuroendocrine convertase PC2 (NEC2) to human 20p11.1-11.2, and furin (mouse 7[D1-E2] region) Genomics. 1991;11(1):103–107. doi: 10.1016/0888-7543(91)90106-o. [DOI] [PubMed] [Google Scholar]
- Smeekens S.P., Steiner D.F. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J. Biol. Chem. 1990;265(6):2997–3000. [PubMed] [Google Scholar]
- Tanaka S. Comparative aspects of intracellular proteolytic processing of peptide hormone precursors: studies of proopiomelanocortin processing. Zoolog. Sci. 2003;20(10):1183–1198. doi: 10.2108/zsj.20.1183. [DOI] [PubMed] [Google Scholar]
- Tang S.S., Chen Z.L., Liu J.J. Production and enhanced biological activity of a novel GHRH analog, hGHRH with an N-terminal Pro–Pro extension. Protein Expr. Purif. 2004;34:296–301. doi: 10.1016/j.pep.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Tang S.S., Zhang J.H., Liu H.X., Zhou D., Qi R. Pro-protein convertase-2/carboxypeptidase-E mediated neuropeptide processing of RGC-5 cell after in vitro ischemia. Neurosci. Bull. 2009;25(1):1–8. doi: 10.1007/s12264-009-1027-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Taylor N.A., Van De Ven W.J., Creemers J.W. Curbing activation: proprotein convertases in homeostasis and pathology. FASEB J. 2003;17:1215–1227. doi: 10.1096/fj.02-0831rev. [DOI] [PubMed] [Google Scholar]
- Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A., Webb G., Zhu X.P., Steiner D.F. Proteolytic processing in the secretory pathway. J. Biol. Chem. 1999;274(30):20745–20748. doi: 10.1074/jbc.274.30.20745. [DOI] [PubMed] [Google Scholar]
- Zhou A., Minami M., Zhu X., Bae S., Minthorne J., Lan J., Xiong Z.G., Simon R.P. Altered biosynthesis of neuropeptide processing enzyme carboxypeptidase E after brain ischemia: molecular mechanism and implication. J. Cereb. Blood Flow Metab. 2004;24(6):612–622. doi: 10.1097/01.WCB.0000118959.03453.17. [DOI] [PubMed] [Google Scholar]
- Zhu X.M., Wu K., Lawrence R., Niamh X., Cawley B.B., Tiffany A., Karen T., Concepcion L., David S., Williams Y., Peng L., Cheryl M.C. Carboxypeptidase E is required for normal synaptic transmission from photoreceptors to the inner retina. J. Neurochem. 2005;95:1351–1362. doi: 10.1111/j.1471-4159.2005.03460.x. [DOI] [PubMed] [Google Scholar]


