Abstract
Incineration of medical waste (MW) is an important alternative way for disposal of this type of hazardous waste, especially in China because of the outbreak of severe acute respiratory syndromes (SARs) in 2003. Thus, far, fly ash has received much attention but less attention has been paid to bottom ash. In this study, bottom ash samples were collected from a typical MW incinerator, and typical pollutants including heavy metals and polycyclic aromatic hydrocarbons (PAHs) in the ash were examined. X-ray fluorescence spectroscopy results indicated that CaO, SiO2 and Al2O3 were the main components of the bottom ash. Inductively coupled plasma-optical emission spectroscopy showed that the ash contained large amounts of heavy metals, including Zn, Ti, Ba, Cu, Pb, Mn, Cr, Ni and Sn. Most of the heavy metals (e.g., Ba, Cr, Ni, and Sn) presented in the residual fraction; whereas Mn, Pb and Zn presented in Fe–Mn oxides fraction, and Cu in organic-matter fraction. Toxicity characteristic leaching procedure tests indicated that the leached amounts of heavy metals were well below the limits. The sum of 16 US EPA priority PAHs (ΣPAHs) varied from 10.30 to 38.14 mg kg−1, and the total amounts of carcinogenic PAHs ranged between 4.09 and 16.95 mg kg−1, exceeding the limits regulated by several countries. This research provides basic information for the evaluation of the environmental risk of MW incinerator bottom ash.
Keywords: Medical waste, Heavy metal, Toxic leaching, Sequential extraction, PAHs
1. Introduction
Incineration has become the main method for disposal of medical waste (MW) in China since the nation-wide outbreak of severe acute respiratory syndrome (SARS) in 2003. In recent years, many MW incineration facilities have been established in China. Although incineration can reduce the weight of waste by more than 70%, large amounts of combustion residues, especially bottom ash, still remained after incineration. In some densely populated big cities, disposal of the waste ash is becoming increasingly difficult, owing to high cost, diminishing land availability, more stringent regulation, and frequent public opposition to the sifting of new landfills. Bottom ash is not included in the List of Hazardous Waste published by the Environmental Protection Agency of China, thus, this type of material has the potential to be recycled and reused in the interests of achieving sustainable development. When reused, the leachability of toxic metals is the most concern. A recent paper reported that metals, such as Pb, Cr, Cd, Cu and Zn in bottom ashes from a medical waste incinerator were with high leachability [1]. The useful ways to get is, to put ashes in construction materials, or in cement, or to solid waste landfills.
Bottom ash is reported with less contaminated with heavy metals than is fly ash [2]. Previous studies have shown that bottom ash from incineration of municipal solid waste (MSW) might be a valuable resource because it can be used as a secondary aggregate in roads and construction materials [3], [4]. Due to the chemical compositions of MW ash and MSW ash are similar, MW ash might be reusable in the same way [5], [6]. However, MW bottom ash has some special characteristics that must be taken into consideration before it can be reused. MW contains large amounts of disposal metallic or plastic materials. Therefore, the bottom ash from MW incineration may contain a large proportion of toxic metallic elements or organic compounds that might hinder its reuse. Previous studies have indicated that MW bottom ash contains higher amounts of heavy metals such as Cd, Cr, Ni, Pb and Zn than does MSW bottom ash [5], [7]. In addition, a recent report shows that even though bottom ash is regarded as a non-hazardous material, its TCLP and PBET leachate also showed biotoxicity [8].
When incomplete combustion occurs, persistent organic pollutants (e.g., polychlorinated dibenzo-dioxins, polychlorinated dibenzo-furans, and polychlorinated biphenyls) might be formed [9], [10]. Polycyclic aromatic hydrocarbons (PAHs), which are formed during incomplete combustion and are present in bottom ash [11], [12], persist in the environment, have low biodegradability and are highly carcinogenic to humans [13]. A study of bottom ash from medical waste incineration in Taiwan found that the sum of the amounts of PAHs (ΣPAHs) ranges from 162 to 3480 μg kg−1 [14], and in a study of bottom ash obtained from a clinical waste incinerator, the sum of the amounts of 11 PAHs was found to be 449.3 μg kg−1 [15].
Currently, there are large amounts of low-standard medical waste incinerators are being operated by some rural and urban medical institutions in China, which are lack of air pollution control devices and without secondary combustion chamber [16] and burning temperature are usually not so high. On the other hand, Chinese medical wastes are collected without sorting, this lead to the large variation in both calorific value and composition. All these factors contribute to uncompleted combustion of medical waste. Thus, the composition and distribution of toxic elements in bottom ash from these incinerators may quite different from that generated from well equipped large incinerator.
Therefore, the properties of MW bottom ash must be extensively investigated before this type of special waste can be reused. The objective of the current study was to obtain basic information about MW bottom ash by examining its chemical properties, heavy metal contents, leaching behavior and PAH concentrations. This information should be useful for evaluating utilization possibilities.
2. Materials and methods
2.1. Sample collection and preparation
Bottom ash samples were collected from a typical incineration facility located in Hangzhou city in northern China. This incinerator is a special type of medium-scale one, which combined more than ten small fixed grate furnaces. Part or all of the furnaces are operated according to the amount of medical wastes collected. Three mixed samples were collected from the incinerator every 10 days during a month. We conducted triplicate analyses of one sample per each kind of MWBA. The samples were dried at 105 °C for 24 h and then ground to a particle diameter of <0.25 mm in an agate mortar for analysis of heavy metals. Samples for PAHs determination were dried at 30 °C for 24 h and ground as mentioned above.
The pH was measured in solutions after 24 h of agitation with distilled water at a liquid to solid ratio of 5. The amount of organic-matter in the samples was calculated by loss of weight on ignition at 550 °C for 6 h.
2.2. Chemical composition analysis
The major elements in the ash MW were determined by means of X-ray fluorescence (XRF) spectroscopy (XRF1700, Shimadzu, Japan). Heavy metals were analyzed by inductively coupled plasma-optical emission spectroscopy (ICP-OES) after HNO3/HClO4/HF digestion [17]. The quality and precision of the data for the metallic element analysis were controlled with NIST 1646, a sediment reference material obtained from the National Institute of Standards and Technology (USA). Comparison of the certified values with the values found in this study indicated that the recoveries of the metallic elements were in the 87–130% range as follows: Al (120%), Ca (108%), Fe (107%), K (117%), Mg (110%), Na (102%), Ti (98%), Ba (130%), Cr (87%), Cu (126%), Mn (91%), Ni (130%), Pb (121%) and Zn (96%).
2.3. Sequential extraction procedure
Chemical speciation of heavy metals in the ash was determined by means of the sequential extraction procedure suggested by Tessier et al. and modified by Tan et al. [18], [19]. The procedure classifies the elements into five fractions:
F1—exchangeable fraction. Approximately 5 g of the ash was extracted at room temperature with 100 ml of 1 mol l−1 sodium acetate (CH3COONa, pH 8.2) with continuous agitation for 1 h. Separation was achieved by centrifugation at 5000 rpm for 30 min.
F2—carbonate fraction. The residue from the exchangeable fraction was leached at room temperature with 100 ml of 1 mol l−1 CH3COONa (adjusted to pH 5.0 with CH3COOH) and continuous shaking for 5 h. Separation was achieved by centrifugation at 5000 rpm for 30 min.
F3—Fe–Mn oxides fraction. To the residue from the carbonate fraction was added 100 ml of 0.04 mol l−1 NH2OH·HCl in 25% CH3COOH, and the mixture was heated at 95 °C for 5 h with occasional agitation. Separation was achieved by centrifugation at 5000 rpm for 30 min.
F4—organic-matter fraction. HNO3 (15 ml, 0.02 mol l−1) and 25 ml of H2O2 (adjusted to pH 2 with HNO3) were added to the residue from the Fe–Mn oxides fraction, and the mixture was heated at 85 °C for 3 h with occasional agitation. After the mixture was cooled, 25 ml of 3.2 mol l−1 CH3COONH4 in 20% HNO3 was added, and the mixture was agitated for an additional 30 min. Separation was achieved by centrifugation at 5000 rpm for 30 min.
F5—residue fraction. This fraction is determined by using total content minus prior four steps.
After each centrifugation, the supernatants were collected by pipette, and the metals in the supernatant were analyzed by ICP-OES.
2.4. Toxicity characteristic leaching procedure (TCLP)
Leaching of hazardous heavy metals from the ash was examined by means of a toxicity characteristic leaching procedure (TCLP), in which two kinds of extraction solutions (solution #1, HOAc, pH 4.90 ± 0.05; solution #2, HOAc, pH 2.88 ± 0.05) were used [20]. The liquid-to-solid ratio was 20:1, and the agitation time was 18 h in a rotary tumbler at 30 ± 2 rpm. After extraction, the leachates were filtered through whatman GF/C glass fiber filter paper (0.45 μm). The leachates were acidified with 1 M HNO3 and subjected to ICP-OES for metal analysis.
2.5. PAHs analysis
Silica gel (100–200 mesh) and alumina (Qingdao, China) were activated at 180 °C and 250 °C, respectively, for 12 h and were then deactivated with 3% water. Sodium sulfate (Beijing Chemical Reagent Co., China) was baked at 450 °C before use. Pesticide-grade acetone, n-hexane and dichloromethane were purchased from Tedia Company (USA). A standard containing 16 US EPA priority PAHs [naphthalene (Nap), acenaphthylene (Ace), acenaphthene (Aceph), fluorene (Fluor), phenanthrene (Phen), anthracene (Anth), fluoranthene (Fanth), pyrene (Pyr), benzo[a]anthracene (B[a]anth), chrysene (Chrys), benzo[b]fluoranthene (B[b]flu), benzo[k]fluoranthene (B[k]flu), benzo[a]pyrene (B[a]pyr), indeno[1,2,3-cd]pyrene (I[123cd]pyr), dibenzo[a,h]anthracene (DB[ah]anth), benzo[g,h,i]perylene (B[ghi]per)] each at 200 μg ml−1 was purchased from Accustandard (USA). Hexamethylbenzene was purchased from Sigma–Aldrich Co.
Ultrasonic extraction was performed by means of US EPA standard method 3550B [21]. The ash sample (1 g) was extracted with 20 ml n-hexane-dichloromethane (3:5) in a glass tube and was placed in an ultrasonic bath for 20 min; the temperature was kept below 30 °C with recycling water. After extraction, the solution was centrifuged for 20 min at 3000 rpm, and the supernatant was decanted. Ultrasonic extraction was repeated twice with two additional 20 ml portions of solvent. After the third extraction, the collected extracts were preconcentrated to a volume of 2 ml with a rotary evaporator, and the solvent was exchanged for hexane. Cleanup and fractionation were achieved with an alumina/silica gel column, as described by Qiao et al. [22]. The extract was then reconcentrated to a volume of 1 ml.
The PAHs concentrations in the extracts were analyzed with an Agilent 6890 gas chromatograph (USA) equipped with a 5973 mass-selective detector operating in selected ion monitoring mode. An HP-5 silica fused capillary column (30 m × 0.25 mm inner diameter × 0.25 μm film thickness) was used; helium at a constant flow rate of 1 ml min−1 was the carrier gas. Splitless injection of 1 μl of sample was conducted with an autosampler. The temperature program for the GC oven was as follows: hold at 50 °C for 2 min; increase to 300 °C at 6 °C min−1; hold at 300 °C for 5 min. The injector and detector temperatures were 300 °C and 230 °C, respectively. Quantification was optimized by means of a five-point calibration curve for the individual components.
An internal standard containing hexa-methyl-benzene was used for the quantification. Calibration curves based on five different concentrations were constructed by means of the internal standard method. The detection limits of the PAHs ranged from 0.02 to 0.03 mg l−1.
Laboratory quality-control procedures included analyses of method blanks, spiked blanks, matrix spike duplicates, and sample duplicates. The recoveries for surrogate standards fell within a fairly narrow range of 72–105%.
3. Results and discussion
3.1. PH and organic-matter
The results from the measurements of pH and amount of organic-matter are given in Table 1 . The pH of MWBA was in the range of 8.6 to 10.7. The content of organic-matter are very high from 21.57 to 30.14%, indicating this type of ash contained high amount of unburned organic-matter, which should be contributed to the low operating temperature of the incinerator during combustion.
Table 1.
pH value and organic-matter content of the MW bottom ashes.
| pH value | Organic-matter content | |
|---|---|---|
| MWBA I | 8.60 | 30.14% |
| MWBA II | 10.70 | 21.57% |
| MWBA III | 9.80 | 27.45% |
The amount of organic-matter in the samples was estimated by loss of weight on ignition at 550 °C for 6 h. It is usually assumed that 50% of the weight loss emanated from organic carbon [23]. However, the method has its limitation when applied on incineration residue. It was reported that this method over-estimated unburned carbon at least 20–44% of coal fly ash samples [11], [24].
3.2. Chemical composition
XRF results (Fig. 1 ) indicated that the MW ash samples were composed mainly of CaO (30.5%), SiO2 (26.1%) and Al2O3 (10.0%). Taken together, the other metal oxides made up about 21% of the ash. Non-metallic elements (P, S and Cl) accounted for approximately 12%. Of the non-metallic elements, Cl was present in the largest quantity, perhaps because increasing amounts of Polyvinyl chloride polymer (PVC), e.g., disposable infusion devices, are used in MW. Moreover, NaCl is reportedly used more frequently in medical treatment in China than in other countries, which might also contribute to the high Cl content in the ash. In this study, the Si/Ca ratio was <1, which is lower than the ratio reported in other studies of MSW incineration ash (Si/Ca > 3) [25], [26]. This result indicates that the compositions of MW and MSW differ in some cases; the MW we studied contained more calcium than silicon, whereas the opposite is true for MSW.
Fig. 1.
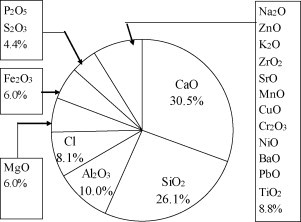
Chemical composition of the MW bottom ashes determined by XRF.
Table 2 lists the metal concentrations in the MW bottom ash obtained by ICP-OES. The data indicate that the ash was enriched with various metallic elements. The major metal elements in the ash were Ca, Fe, Al, Mg, Na and K. There were also large amounts of heavy metals such as Zn, Ti, Ba, Cu, Pb, Mn Cr, Ni, Sn, Sr and Ga. Other metals, such as Li, Co, Ag and Bi, were present in very small amounts. The most toxic heavy metals (e.g., Cd and Sb) were below the detection limit in the ash; these elements and their compounds are generally easily volatile and thus, may have ended up in the fly ash. Compared to values reported in other literature for medical waste incinerator ashes, it is found that Ba concentrations in this study are significantly higher [25], [28]. The possible reason is different compositions of raw MW. Hospital wastes in China usually contain high amount of plastic matter, while Ba are usually used as additive in these plastics.
Table 2.
Metal concentrations of the MW bottom ashes.
| MWBA I |
MWBA II |
MWBA III |
MWBA I |
MWBA II |
MWBA III |
||
|---|---|---|---|---|---|---|---|
| (g kg−1) | (mg kg−1) | ||||||
| Al | 36.42 | 62.90 | 54.63 | Ag | 24.24 | 21.43 | 27.36 |
| Ba | 2.09 | 1.69 | 2.08 | As | 22.06 | 39.18 | 34.27 |
| Ca | 97.63 | 185.76 | 182.92 | Bi | 1.10 | <1 | 0.87 |
| Cu | 1.16 | 1.45 | 1.26 | Cd | <1 | <1 | <1 |
| Fe | 47.51 | 52.75 | 44.63 | Co | 36.34 | 49.87 | 35.77 |
| K | 8.51 | 6.79 | 6.73 | Cr | 895.37 | 515.19 | 916.50 |
| Mg | 15.18 | 19.24 | 17.03 | Ga | 154.42 | 306.17 | 282.90 |
| Mn | 0.53 | 1.50 | 1.25 | Li | 50.75 | 76.26 | 88.90 |
| Na | 13.07 | 15.16 | 16.84 | Ni | 667.31 | 500.49 | 519.32 |
| Pb | 0.33 | 0.07 | 0.24 | Sb | <1 | <1 | <1 |
| Ti | 7.10 | 15.50 | 7.57 | Sn | 368.47 | 405.91 | 375.39 |
| Zn | 8.43 | 12.70 | 13.72 | Sr | 163.88 | 165.10 | 144.76 |
All data are the average of three triplicate samples.
MW bottom ash contains much higher amounts of Zn (8.43–13.72 g kg−1), Ti (7.10–15.50 g kg−1) and Cr (0.52–0.92 g kg−1) than did the MSW analyzed in previous studies [27], [28]. Other researchers have reported similar results, that is, that Cr and Zn concentrations in medical waste residue are significantly higher than those in MSW [5], [7]. These elements are commonly used in medical facilities; for example, metal alloys containing Zn and Ti are widely used in medical instruments, and Cr is widely used in needles and syringes. Moreover, Ti and Cr have high boiling points and therefore, tend to end up in the bottom ash.
MW bottom ash contains various valuable metallic elements, the recovery of which would be desirable. However, some toxic metals, such as Cr, can have a negative impact at the same time. Thus, before assessing the possibility of reusing MW bottom ash, much attention must be given to avoid heavy metal contamination.
3.3. Fractionation of metals
We conducted sequential extraction to determine the chemical form of some of the metals in the bottom ash (Fig. 2 ). Ba, Cr, Cu, Mn, Ni, Pb, Sn and Zn were determined because they are toxic and are regulated by the US EPA.
Fig. 2.
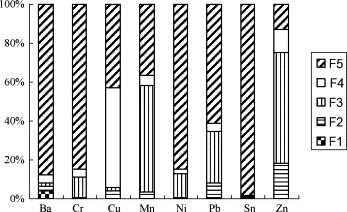
Metal fractionation of the MW bottom ashes. F1—exchangeable fraction; F2—bound to carbonate; F3—bound to Fe–Mn oxides; F4—bound to organic matter; F5—residual fraction.
Only a small percentage of the total metal (0.67–18.18%) was bound to the exchangeable (F1) and carbonate (F2) fractions taken together. The percentages of the metals bound to these two fractions decreased in the order Zn > Pb > Ba > Cu > Mn > Sn > Cr > Ni. Elliot et al. and Sukandar et al. reported that the metal content in the exchangeable and carbonate fractions (the mobile fractions) is indicative of the potential availability and leaching of metals [29], [30]. Therefore, the amounts of the various metals in these two fractions indicate that Zn has the highest leaching potential and Ni the lowest. The percentages of metals associated with the Fe–Mn oxides fraction (F3) in the bottom ash were in the 0.41–57.22% range. The percentages of the metals bound to the Fe–Mn oxides fraction decreased in the order Zn > Mn > Pb > Ni > Cr > Cu > Ba > Sn. Metals associated with the Fe–Mn oxides fraction cannot undergo chemical reaction immediately, but their mobility and availability could be affected by changes in environmental conditions [18]. Thus, Zn, Mn and Pb in MW bottom ash may leach into the environment. The percentages of metals bound to the organic fraction (F4) in the bottom ash were low, except for Cu, and accounted for 50.92% of the total. Residual fractions (stable fractions) are generally less mobile, and thus, the metals in them are difficult to leach out into the environment. The proportions of Ba, Cr, Ni and Sn bound to the residual fractions (F5) were much higher than those of the other metals (84.74–97.99%). Accordingly, we consider these metals to be less mobile and to have low environmental availability.
On the basis of the above results, we believe that Zn, Mn, Cu and Pb in MW bottom ash pose comparatively higher leaching risks to the environment, whereas Ba, Cr, Ni and Sn are relatively safe.
3.4. TCLP tests
TCLP results for the bottom ash are given in Table 3 . The leached amounts of the heavy metals were compared with the corresponding US EPA limits. The extracted amounts of all the heavy metals were lower than the limits set by the US EPA. TCLP results showed that the bottom ash produced from MW incinerators can be considered as non-hazardous, and therefore, the risks of reuse are low.
Table 3.
Amounts of heavy metals leached from various MW bottom ash samples determined by USEPA TCLP method (mg l−1).
| As | Ba | Cd | Cr | Cu | Ni | Pb | |
|---|---|---|---|---|---|---|---|
| MWBA I | <0.01 | 0.92 | <0.01 | 3.90 | 4.80 | 2.30 | 0.54 |
| MWBA II | <0.01 | 1.30 | <0.01 | 0.01 | 1.40 | 0.29 | 0.02 |
| MWBA III | <0.01 | 1.06 | <0.01 | 4.13 | 1.38 | 0.67 | 0.43 |
| US EPA Standard | 5.00 | 100.00 | 1.00 | 5.00 | 100.00 | 100.00 | 5.00 |
3.5. PAHs levels in the MW bottom ash
The concentrations of the PAHs in three samples are displayed in Fig. 3 . The total amounts of 16 PAHs in MW bottom ash ranged from 10.30 to 38.14 mg kg−1 ash; this range is substantially higher than that reported in the literature (0.16–3.48 mg kg−1) [14], [15]. The distributions of the three samples were very similar, with phenanthrene being the most abundant PAHs in all the samples, accounting for 19.51–31.44% of the total PAHs. Fluoranthene, pyrene, benzo[a]anthracene and chrysene were also prevalent in all the samples.
Fig. 3.
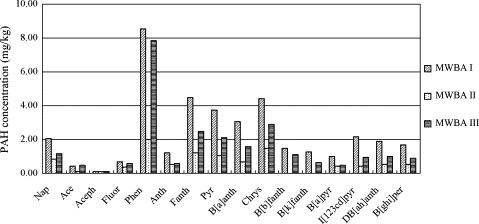
PAHs concentrations of the MW bottom ashes.
The ΣPAHs values for carcinogenic PAHs (B[a]anth, Chrys, B[b]flu, B[k]flu, B[a]pyr, I[123cd]pyr and DB[ah]anth) were between 4.09 and 16.95 mg kg−1 of ash (Table 4 ). Literature values for carcinogenic compounds in MSW ash range from 89 to 438 μg kg−1 in bottom ash [11]. Clearly, both the total PAHs and the levels of carcinogenic compounds in our study were much higher than the literature values. This result may be due to differences in the operating conditions for the incinerators (such as combustion temperature and input MW composition) that are suitable for PAHs formation. The operating temperature of the incinerator in this study is around 700–800 °C, while combustion temperature reported in other literature is over 1000 °C, promoting complete decomposition of PAHs.
Table 4.
Comparison of carcinogenic PAH levels with soil guidelines regulated by various countries.
The Environmental Protection Agency of China has not issued general guidelines for PAHs in soil. Therefore, we used regulated values reported in the literature to evaluate the potential risk of the MW ash (Table 3) [12], [31], [32]. The levels of carcinogenic PAHs in all three samples were above the limits for soil use. Therefore, integrated utilization of MW bottom ash is not advisable.
4. Conclusions
Bottom ash is generally considered to be safer than fly ash in terms of heavy metal contamination. However, our results indicate that MW bottom ash contains high levels of PAHs, highly exceeding the soil limits. Thus, this type of waste ash may cause serious environmental problems if not properly managed.
Chemical analysis showed that the major components of MW bottom ash were CaO, SiO2 and Al2O3. High concentrations of metallic elements, such as Al, Cu, Fe, Mg, Mn, Pb, Ti, Zn and Cr were determined, and most of these metals were associated with the stable residual fraction. The results of US EPA leaching tests verified that all the metals met the standard leaching limits set by the US EPA. The ash could be recyclable as construction material, but it must be treated at high temperature (850–1000 °C) so as to destroy the PAHs before or during the recycling process.
Acknowledgements
This work was made possible by financial support from National Key Technology R&D Program (2008BAC32B03), National Basic Research Program (2007CB407303) and Water Pollution Control and Management Program (2009ZX07212-002) of China. The authors wish to thank C.Y. He, Z.W. Ma, B. H. Chen and H.Y. Liu for their assistance during sample collection.
References
- 1.Valavanidis A., Iliopoulos N., Gotsis G., Fiotakis K. Leachability, heavy metals, PAHs and PCBs in fly and bottom ash of a medical waste incineration facility. Waste Manage. Res. 2008;26:247–255. doi: 10.1177/0734242X07083345. [DOI] [PubMed] [Google Scholar]
- 2.Stegemann J.A., Scneider J., Baetz B.W., Murphy K.L. Lysimeter washing of MSW incinerator bottom ash. Waste Manage. Res. 1995;13:149–165. [Google Scholar]
- 3.Chandler A.J., Eighmy T.T., Hartlen J., Hjelmar O., Kosson D.S., Sawell S.E., Vehlow J. Municipal solid waste incinerator residues. Stud. Environ. Sci. 1997;67:974–979. [Google Scholar]
- 4.Hubscher V.B., Lagarde F., Leroy M.J.F., Coughanowr C., Enguehard F. Utilisation of bottom ash in road construction: a lysimeter study. Waste Manage. Res. 2001;6:557–566. doi: 10.1177/0734242X0101900612. [DOI] [PubMed] [Google Scholar]
- 5.Ibanez R., Andres A., Viguri J.R., Ortiz I., Irabien J.A. Characterisation and management of incinerator wastes. J. Hazard. Mater. 2000;79:215–227. doi: 10.1016/s0304-3894(00)00268-5. [DOI] [PubMed] [Google Scholar]
- 6.Izquierdo M., Lopez-Soler A., Ramonich E.V., Barra M., Querol X. Characterisation of bottom ash from municipal solid waste incineration in Catalonia. J. Chem. Technol. Biotechnol. 2002;77:576–583. [Google Scholar]
- 7.Kuo M.W., Shu S.L., Wu C.C., Lai J.S. Characteristics of medical waste in Taiwan. Water Air Soil Pollut. 1999;114:413–421. [Google Scholar]
- 8.Chou J.D., Wey M.Y., Liang H.H., Chang S.H. Biotoxicity evaluation of fly ash and bottom ash from different municipal solid waste incinerators. J Hazard Mater. 2009;168:197–202. doi: 10.1016/j.jhazmat.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Wagner J.C., Green A.E.S. Correlation of chlorinated organic compound emissions from incineration with chlorinated organic input. Chemosphere. 1993;26:2039–2054. [Google Scholar]
- 10.Levendis Y.A., Atal A., Carlson J.B. PAH and soot emissions from burning components of medical waste: examination/surgical gloves and cotton pads. Chemosphere. 2001;42:775–783. doi: 10.1016/s0045-6535(00)00251-4. [DOI] [PubMed] [Google Scholar]
- 11.Johansson I., Bavel B.V. Levels and patterns of polycyclic aromatic hydrocarbons in incineration ashes. Sci. Total Environ. 2003;311:221–231. doi: 10.1016/S0048-9697(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 12.Johansson I., Bavel B.V. Polycyclic aromatic hydrocarbons in weathered bottom ash from incineration of municipal solid waste. Chemosphere. 2003;53:123–128. doi: 10.1016/S0045-6535(03)00299-6. [DOI] [PubMed] [Google Scholar]
- 13.IARC. Polynuclear aromatic compounds, Part 1: chemical, environmental and experimental data. International Agency for Research on Cancer, 32 (1983). [PubMed]
- 14.Lee W.J., Liow M.C., Tsai P.J., Hsieh L.T. Emission of polycyclic aromatic hydrocarbons from medical waste incinerators. Atmos. Environ. 2002;36:781–790. [Google Scholar]
- 15.Wheatley A.D., Sadhra S. Polycyclic aromatic hydrocarbons in solid residues from waste incineration. Chemosphere. 2004;55:743–749. doi: 10.1016/j.chemosphere.2003.10.055. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y., Li P.J., Lupi C., Sun Y.Z., Xu D.D., Feng Q., Fu S.S. Sustainable management measures for healthcare waste in China. Waste Manage. 2009;29:1996–2004. doi: 10.1016/j.wasman.2008.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamasaki S. Soil Environment Analysis. Japanese Society of Soil Science and Plant Nutrition; Hakuyusya, Tokyo: 1997. Digestion method for total element analysis. pp. 278–288 (in Japanese) [Google Scholar]
- 18.Tessier A., Campbell P.G.C., Bisson M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979;51:844–851. [Google Scholar]
- 19.Tan L.C., Choa V., Tay J.H. The influence of pH on mobility of heavy metals from municipal solid waste incinerator fly ash. Environ. Monit Assess. 1997;44:275–284. [Google Scholar]
- 20.USEPA, Tests methods for evaluating solid waste, Physical Chemical Methods, 1992, SW-846, Method 1311, US Environmental Protection Agency, Washington, DC. http://www.epa.gov/epaoswer/hazwaste/test/pdfs/1311.pdf.
- 21.USEPA, 1996A. Ultrasonic extraction, EPA standard method 3550B.
- 22.Qiao M., Wang C.X., Huang S.D., Wang M., Wang Z.J. Composition, sources, and potential toxicological significance of PAHs in the surface sediments of the Meiliang Bay, Taihu Lake, China. Environ. Int. 2006;32:28–33. doi: 10.1016/j.envint.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 23.McBride MB. Environmental chemistry of soils. New York: Oxford University Press, 1994. p. 406.
- 24.Fan M., Brown R. Comparison of the loss-on-ignition and thermogravimetric analysis techniques in measuring unburned carbon in coal fly ash. Energy Fuel. 2001;15:1414–1417. [Google Scholar]
- 25.Idris A., Saed K. Characteristics of slag produced from incinerated medical waste. J. Hazard. Mater. 2002;B93:201–208. doi: 10.1016/s0304-3894(02)00010-9. [DOI] [PubMed] [Google Scholar]
- 26.Li M., Xiang J., Hu S., Sun L.S., Li P.S., Sun X.X. Characterization of solid residues from municipal solid waste incinerator. Fuel. 2004;83:1397–1405. [Google Scholar]
- 27.Herck P.V., Vandecasteele C. Evaluation of the use of a sequential extraction procedure for the characterization and treatment of metal containing solid waste. Waste Manage. 2001;21:685–694. doi: 10.1016/s0956-053x(01)00011-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhang F.S., Yamasaki S., Kimura K. Waste ashes for use in agricultural production: II. Contents of minor and trace metals. Sci. Total Environ. 2002;286:111–118. doi: 10.1016/s0048-9697(01)00968-8. [DOI] [PubMed] [Google Scholar]
- 29.Elliot H.A., Dempsey B.A., Maille P.J. Content and fractionation of heavy metals in water treatment sludges. J. Environ. Quality. 1990;19:330–334. [Google Scholar]
- 30.Sukandar S., Yasuda K., Tanaka M., Aoyama I. Metal leachability from medical waste incinerator fly ash: a case study on particle size comparison. Environ. Pollut. 2006;144:726–735. doi: 10.1016/j.envpol.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Environment Canada, 2003. Canadian Soil Quality Guidelines, Environment Canada.
- 32.Vane C.H., Harrison I., Kim A.W. Polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in sediments from the Mersey Estuary, U.K. Sci. Total Environ. 2007;374:112–126. doi: 10.1016/j.scitotenv.2006.12.036. [DOI] [PubMed] [Google Scholar]


