Highlights
-
•
Antiviral activities were comparatively evaluated using copper and silver compounds.
-
•
Solid-state Cu2O showed superior activity against enveloped and non-enveloped viruses.
-
•
Exposure to Cu2O preferentially inactivated infection ability of influenza viruses.
-
•
Cu2O has a unique antiviral mechanism mediated by direct contact.
Keywords: Antiviral, Solid-state copper and silver compounds, Hemagglutinin, Neuraminidase, Protein denaturation
Abstract
Antiviral activities of insoluble solid-state and soluble ionic copper and silver compounds were evaluated against influenza A virus (A/PR8/H1N1) possessing a viral envelope and bacteriophage Qβ lacking an envelope. The viral solutions were exposed on glass samples uniformly loaded with copper and silver compounds. Exposure to solid-state cuprous oxide (Cu2O) efficiently inactivated both influenza A virus and bacteriophage Qβ, whereas solid-state cupric oxide (CuO) and silver sulfide (Ag2S) showed little antiviral activity. Copper ions from copper chloride (CuCl2) had little effect on the activity of bacteriophage Qβ in spite of the fact that copper ions strongly inactivate influenza A in previous studies. Silver ions from silver nitrate (AgNO3) and silver(I) oxide (Ag2O) in solution showed strong inactivation of influenza A and weak inactivation of bacteriophage Qβ. We also investigated the influence of the compounds on the function of two influenza viral proteins, hemagglutinin and neuraminidase. Silver ions from AgNO3 and Ag2O remarkably decreased enzymatic activity of neuraminidase through the breakage of disulfide (S—S) bonds, corresponding to the selective inactivation of influenza A virus. By contrast, exposure to Cu2O markedly reduced the activity of hemagglutinin rather than neuraminidase. These findings suggest that solid-state Cu2O disrupts host cell recognition by denaturing protein structures on viral surfaces, leading to the inactivation of viruses regardless of the presence of a viral envelope.
1. Introduction
Throughout history, infectious diseases caused by viruses have posed a serious threat to human populations worldwide. In recent times, Ebola virus caused more than 11,290 deaths in Africa [1], and over 160 people were infected with Middle East respiratory syndrome coronavirus in South Korea [2], illustrating the devastating effects of viruses on international communities. Influenza A virus, the causative agent of influenza in birds, can also lead to highly infectious respiratory disease in mammals. Viral genome mutations (antigenic drift) and periodic gene segment reassortment between humans and animals (antigenic shift) can result in the emergence of new viral pathogens, with the potential to cause serious pandemics. During the past 100 years, pandemic influenza occurred in 1918, 1957, 1968, and 2009 [3]. The most severe pandemic, in 1918, resulted in approximately 40 million deaths worldwide [4], while the most recent pandemic, the Swine flu pandemic of 2009, emerged in 214 countries and caused more than 18,000 deaths, according to a World Health Organization report [5]. Highly pathogenic H5N1 avian influenza was first identified in Hong Kong [3]. As the high pathogenicity of such a strain and the lack of host protective immunity against such viruses continue to threaten lives, effective infection control measures are crucial for the prevention of serious outbreaks.
Viral particles released from infected patients through coughing or sneezing can survive for prolonged periods in the air and on surfaces. Viral transmission occurs through direct person-to-person contact or through the uptake of contaminated airborne droplets or contact with contaminated surfaces [6]. The use of antiviral materials is an effective way to inactivate viral particles in the environment preventing viral transmission and thereby lowering the risk of infection [7], [8]. For this purpose, solid-state inorganic compounds such as metal oxides are good candidates because of their chemical robustness and their feasibility for use as a coating material. Copper and silver have been recognized for their biocidal properties since ancient times [9], [10]. However, unlike their ionic forms (Cu2+ and Ag+), the biocidal properties of solid-state copper and silver compounds such as CuO, Cu2O and Ag2S, that are poorly soluble in water, remain to be fully elucidated.
We recently reported that solid-state copper(I) compounds, including copper(I) oxide (Cu2O), sulfide (Cu2S), and iodide (CuI), efficiently inactivated bacteriophages and bacteria [11]. The inactivation of bacteriophages is mainly mediated by direct contact with solid-state copper(I) compounds, rather than exposure to reactive oxygen species or leached copper ions. Here, we investigated the antiviral activities of four groups of compounds, solid-state copper compounds (Cu2O and CuO), a copper ionic compound (CuCl2), a solid-state silver compound (Ag2S), and silver ionic compounds (AgNO3 and Ag2O), against pathogenic influenza A virus and bacteriophage Qβ. The effects of these compounds on the surface proteins of influenza viruses, hemagglutinin (HA), and neuraminidase (NA), were also investigated to reveal mechanistic insights into viral inactivation by these compounds.
2. Experiments
2.1. Preparation of copper and silver compounds
All cuprous, copper and silver compounds were obtained from Wako Pure Chemicals (Tokyo, Japan). The diameters of the particles were in the range of 0.5–5 μm in cuprous oxide (Cu2O), 0.5–40 μm in cupric oxide (CuO), and 3–60 μm in silver sulfide (Ag2S). The measured BET surface areas of Cu2O, CuO, and Ag2S particles were 1.23, 1.20, and 0.14 m2/g, respectively. Ethanol suspensions of the compound particles were uniformly spreading on glass slides (2.5 cm × 2.5 cm), followed by heating at 120 °C for 3 h for sterilization. The particles were spread at 2.1 μmol metal (copper or silver), corresponding to 0.24 g/m2 for Cu2O, 0.27 g/m2 for CuO, 0.36 g/m2 for Ag2O, and 0.42 g/m2 for Ag2S. Although Ag2O is moderately water-soluble, glass samples were prepared with the same procedures as other solid-state compounds. In the case of water-soluble compounds, CuCl2 and AgNO3, the dried particles (0.45 g/m2 for CuCl2 and 0.57 g/m2 for AgNO3) from the ethanol solution on the glass substrate at room temperature were used to avoid oxidation by heating treatment.
2.2. Virus strains
The human influenza A virus reference strain (A/PR8/H1N1) used in this study was purchased from the American Type Culture Collection (Manassas, VA, USA). For viral propagation, 11-day-old embryonated chicken eggs were inoculated with the virus, incubated at 35.5 °C for 48 h, chilled at 4 °C for 12 h, and virus was then harvested. Harvested virus particles were purified and concentrated using a combination of depth filters (5.0 μm polypropylene depth filters, GE Infrastructure, USA) and membrane filters (0.45 μm polypropylene mesh microfiltration and cross-flow ultrafiltration polysulfone hollow-fiber filters, 750-kDa cutoff; GE Infrastructure, USA), as previously described [12]. The concentrated virus particles were further purified by sucrose density gradient centrifugation using a 10–60% linear sucrose gradient [13]. The purified samples contained 10 mg/mL protein, as determined by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Virus stocks were stored at −80 °C between all experimental manipulations.
Viral stocks were thawed for the titer assay, described below, and each 200 μL experimental sample was placed in a pre-sterilized centrifuge tube. All experimental samples were adjusted to 0.1 mg/mL protein and were supplemented with 0.01% bovine serum albumin as a stabilizer prior to performing measurements.
Bacteriophage Qβ (NBRC 20012) was also used to evaluate the antiviral activities of the copper and silver compounds. The stock viral suspension was prepared according to a previous report [11]. A suspension of the bacteriophage was mixed with Escherichia coli (NBRC 13965) cells at 35 °C for 10 min. The mixture was then plated onto agar medium to form a double agar layer. After overnight incubation at 35 °C, the bacteriophage from the top agar layer was extracted into SM buffer (0.1 M NaCl, 8 mM MgSO4, 50 mM Tris–HCl [pH 7.5], and 0.1% gelatin) at 4 °C overnight. The extract solution was centrifuged (8000 × g, 4 °C, 20 min), and the supernatant was collected and filtered using a Millex filter (ϕ = 0.22 μm; Millipore, MA, USA). The stock suspension of bacteriophage Qβ was stored at −80 °C prior to use in the evaluation experiment.
2.3. Virus inactivation assay
To evaluate the effects of these compounds on influenza A virus or bacteriophage Qβ, viral suspensions were inoculated onto sample glass slides at room temperature, and incubated in the dark over a time range between 10 min and 1 h. After incubation, the viruses were harvested by shaking in a plastic case (ϕ 60 mm) in phosphate-buffered saline (PBS) buffer. Influenza A virus was titrated using Madin–Darby canine kidney cells, and a 50% tissue culture infective dose (TCID50)/mL was determined according to the method of Reed and Muench [14]. The viral titers of bacteriophage Qβ were also determined from the number of plaques formed using the double-layer method, as described above.
2.4. Hemagglutination assay
The denaturation of HA after exposure to Cu2O, CuO, AgNO3, and Ag2O was investigated. Twenty-five microliters of purified HA H1 (0.125 ng/μL; Abcam, Cambridge, UK) was mixed with an equal volume of copper or silver compound suspension and then incubated in the dark at room temperature. Solids were then removed by centrifugation at 5000 × g for 3 min using ultra-free tubes (ϕ = 0.22 μm; Millipore). Hemagglutinin samples were serially 2-fold diluted in a 96-well microplate and mixed with 25 μL of 2% chicken red blood cells (Nihon BD, Tokyo, Japan) washed in PBS. The HA titer was determined by calculating the reciprocal of the highest dilution of HA in the well with complete hemagglutination [15].
2.5. Evaluation of neuraminidase activity
The NA activity was also evaluated using the standard protocol of the NA-STAR method (Applied Biosystems, Foster City, CA, USA) [16]. Eighty microliters of neuraminidase (0.357 ng/μL; New England Biolabs, Beverly, MA, USA) was mixed with 80 μL suspensions of copper or silver compounds and incubated in the dark at room temperature. Solids were then removed by centrifugation using ultra-free tubes (ϕ = 0.22 μm; Millipore). Ten microliters of NA-star substrate was added to 50 μL of NA samples in triplicate. After the samples were incubated at room temperature for 30 min, 60 μL enhancer was added and chemiluminescence was measured using a SH-9000 microplate reader (Corona Electric Co., Ltd., Ibaraki, Japan).
2.6. Evaluation of protein denaturalization
To investigate an influence of cysteine residues in the protein, Cu2O, CuO, Ag2O, and Ag2S particles, and solutions of CuCl2 and AgNO3 (2.8 mmol) were mixed with 10 μg of trypsin inhibitor protein in 0.2 M phosphate buffer (pH 7.4), and incubated for 4 h at room temperature. Solids were precipitated by centrifugation, and 80 μL of filtrate was mixed with 10 μL of 50 μM dithiothreitol. After 1 h incubation at room temperature, 10 μL of 100 μM monobromobimane was added to the mixture, which was then incubated for 15 min at room temperature [17], [18]. The fluorescence at 485 nm exciting at 380 nm was measured using a microplate reader (Infinite M200, Tecan Japan Co., Ltd., Kawasaki, Japan). Each sample was measured in triplicate.
3. Results and discussion
3.1. Viral inactivation of copper and silver compounds
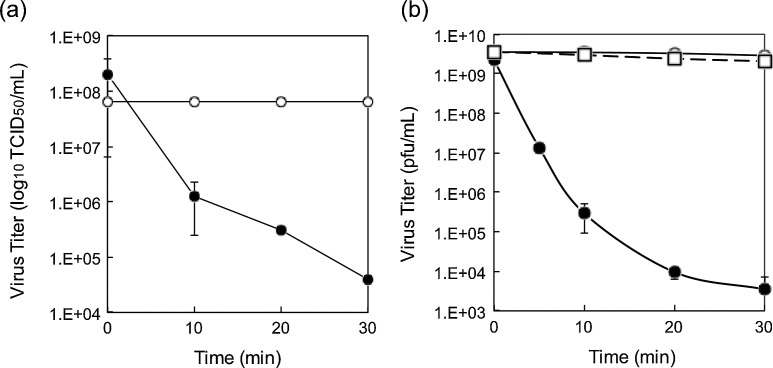
According to our previous study, the biocidal activity of solid-state copper(I) compounds was derived from surface contact with the solids. Thus, the antiviral activity of two types of viruses with different surface structures, H1N1 influenza A virus (A/PR8/H1N1) and bacteriophage Qβ, on the surfaces of solid-state copper or silver compounds was evaluated. Influenza A virus possesses a viral envelope, a cell membrane-like structure that encases its central core, while bacteriophages lack an envelope, instead their surface is composed of protein capsids. These two viral suspensions were inoculated onto glass slides loaded with Cu2O and CuO particles. After incubation for specified time periods, the viruses were harvested and the viral titers were evaluated by determining the TCID50/mL (Fig. 1 a). The titer of influenza A virus drastically decreased on contact with Cu2O particles by over 3 orders of magnitude after 30 min exposure to 2.1 μmol copper amount (log10(N/N0) = −3.7). By contrast, the CuO-loaded sample showed no reduction (log10(N/N0) > −0.01) in influenza A viral titer after 30 min exposure, indicating a significant difference in the antiviral activities of different oxidation states of copper oxide. Borkow et al. reported that copper oxide-impregnated face mask largely inactivate human influenza A and avian influenza virus (log10(N/N0) ∼ −3) within 30 min [9]. Since the oxidation state of copper was not discriminated in their paper, our results suggest that the active compound of the material would be only Cu2O.
Fig. 1.
Viral titers of influenza A virus and bacteriophage Qβ exposed to Cu2O, CuO, and CuCl2. Viruses, (a) influenza A virus and (b) bacteriophage Qβ, were inoculated onto glass substrates loaded with 2.1 μmol copper amount of Cu2O (filled circles), CuO (open circles), or CuCl2 (open squares in (b)). Viral titers were determined. Error bars indicate standard deviations of two or three replicate experiments.
In the case of other copper compounds, a previous study reported that exposure to metallic copper surfaces inactivated influenza virus by an order of magnitude after 1 h and four orders after 6 h [8]. On the other hand, the study on the anti-influenza activity of Cu(II) ions revealed that the virus titer decreased by two orders of magnitude after 1 h even at high concentration (250 μM) [19]. Another study by Imai et al. reported that purified influenza viruses are inactivated by 5.5-log fold reduction after 48 h treatment at high concentration (500 μM) [20]. Among the copper compounds, solid-state Cu2O appears to be the most effective inhibitory agent against influenza virus. Similar findings were observed with the nonenveloped bacteriophage Qβ (Fig. 1b) [11]. In fact, the titer of bacteriophage Qβ decreased by six orders of magnitude after a 30 min incubation with Cu2O particles (log10(N/N0) = −5.8), whereas CuO particles and Cu(II) ions had little effect on viral titer (log10(N/N0) = −0.088 and −0.24 for CuO and CuCl2, respectively). These results showed that solid-state cuprous compounds, in sharp contrast to solid-state cupric compounds or cupric ions, are highly effective at inactivating viruses regardless of the viral envelope.
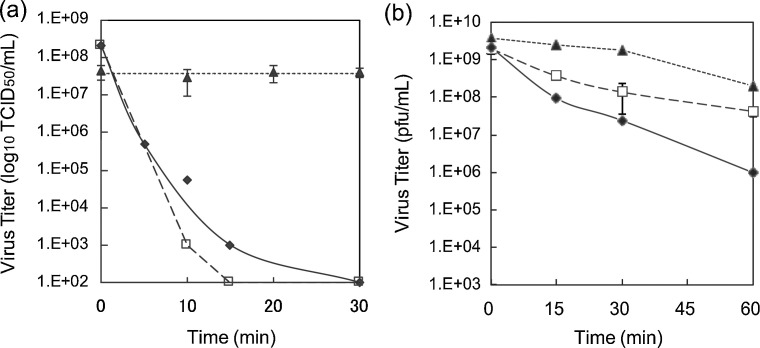
Next, the inactivation of influenza virus and bacteriophage Qβ on silver compounds was investigated. In Fig. 2 a, AgNO3, which has high aqueous solubility, and Ag2O, which has moderate aqueous solubility (0.025 g/L), markedly decreased the titers of influenza virus (log10(N/N0) = −6.3 and −6.3 after 30 min exposure of AgNO3 and AgO, respectively) after 30 min exposure. By contrast, Ag2S, which is insoluble in water, showed no decrease in viral titer (log10(N/N0) = −0.042) after 30 min exposure. These results indicated that the decrease in influenza viral titer depends on the solubility of silver ions from the compounds. Exposure to Ag2O, AgNO3, and Ag2S inactivated bacteriophage Qβ by three (log10(N/N0) = −3.3), two (log10(N/N0) = −1.7), and one (log10(N/N0) = −1.3) orders of magnitude within 60 min, respectively (Fig. 2b). Several studies also showed strong anti-influenza effects of silver nanoparticles [21], [22], [23], which seems similar to our findings. Compared with influenza virus, the inactivation of bacteriophage Qβ by Ag2O and AgNO3 decreased more slowly, indicating that water-soluble silver compounds such as AgNO3 and Ag2O are not effective against the nonenveloped virus. Furthermore, insoluble silver compounds such as Ag2S, were unable to inactivate either virus. These results imply that different inactivation mechanisms exist between influenza and bacteriophage viruses in response to exposure to silver compounds.
Fig. 2.
Viral titers of influenza A virus and bacteriophage Qβ exposed to AgNO3, Ag2O, and Ag2S. Viruses, (a) influenza A virus and (b) bacteriophage Qβ, were inoculated onto glass substrates loaded with 2.1 μmol silver amount of AgNO3 (open squares), Ag2O (filled rhombuses), or Ag2S (filled triangles). Error bars indicate standard deviations of two or three replicate experiments.
In our study, purified viral suspensions were used for evaluation of the inactivation effects among the copper and silver compounds under robust conditions not affected by the protein content, protein amount, and extraction conditions. On the other hand, it would be useful to evaluate the antiviral activity using crude viral suspensions for applications of these materials in real environments [20]. Thus, we evaluated antiviral activities of Cu2O against influenza A virus containing 0.1 and 1 mg/mL bovine serum albumin (BSA), including the protein amount in allantoic fluids (0.2–0.4 mg/mL). Influenza viruses on the exposure to Cu2O were inactivated in the presence 1 mg/mL BSA, whereas the viral inactivation efficiency significantly decreased (log10(N/N0) = −2.8, −2.8 and −1.7 with 0, 0.1 and 1.0 mg/mL BSA, respectively, at 20 min exposure) with the increase of the amount of coexisting protein. Similar tendency has been found in anti-influenza activity of another metal oxide material (TiO2) [12]. These results suggest that the inactivation efficiency of copper and silver compounds would be affected by coexisting proteins and that the antiviral activity in the real environment would be lower than that under controlled laboratory condition.
3.2. Effectiveness to hemagglutinin and neuraminidase on copper and silver compounds
To explain the differences of the inactivation effect of copper and silver compounds against influenza virus, we focused on the viral surface proteins highly involved in the infection process. Influenza virus expresses hundreds of HA and NA proteins on the surface of its envelope. HA is a glycosylated lectin protein that recognizes sialic acid residues on the receptor proteins of host cells [24]. Once influenza viruses bind through the HA-sialic acid interaction, they can enter host cells through endocytosis. NA is an endoglycosidase that is necessary for the release of viruses from the surface of host cells and is also involved in the initiation of influenza infection [25]. Both proteins play important roles in the establishment and spread of influenza infection.
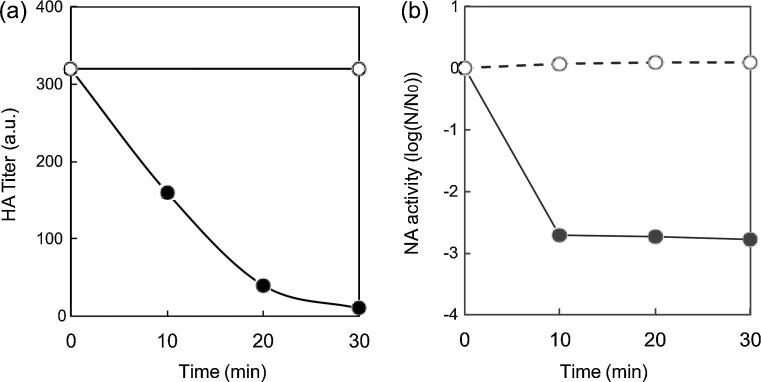
To determine HA activity after treatment with copper compounds, purified H1 protein was incubated with Cu2O and CuO suspensions at the same concentration as the antiviral evaluation experiments. After removal of the solid particles by filtration, the samples were then mixed with chicken red blood cells. Treatment with Cu2O drastically decreased the HA titer, and the HA titer fell below the detection limit within 30 min. By contrast, the HA titer after treatment with CuO did not change over 30 min (Fig. 3 a). For highly sensitive detection of NA activity, the 1,2-dioxetane derivative of sialic acid (NA-STAR) was used as a chemiluminescence substrate. As shown in Fig. 3b, Cu2O-treated NA showed a large decrease in chemiluminescence after 10 min. By contrast, treatment with CuO did not affect the activity of NA, even after 30 min of exposure (Fig. 3b). These results indicated that both the hemagglutination ability of HA and the enzymatic activity of NA are disrupted by incubation with Cu2O particles only, corresponding with the inactivation rate of influenza viruses on Cu2O-loaded glass substrates. Therefore, the loss of HA and NA function following exposure to Cu2O inactivates the ability of influenza viruses to infect host cells.
Fig. 3.
Hemagglutinin (HA) titer and neuraminidase (NA) activity exposed to Cu2O and CuO suspensions. Effect on (a) HA titer and (b) NA activity of Cu2O (filled circles) and CuO (open circles) as determined by a hemagglutination test and chemiluminescence using the NA-Star method, respectively. N0 in panel (b) is the initial NA activity.
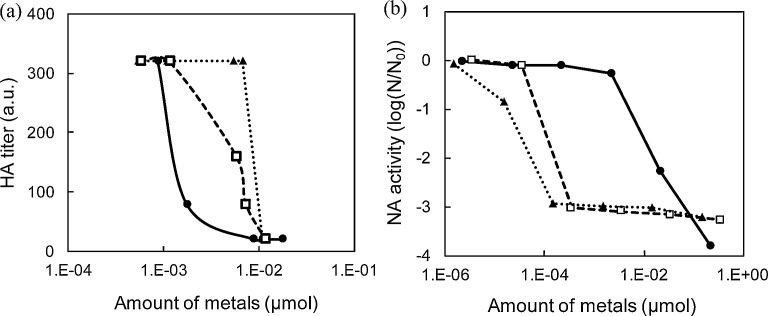
Next, the activities of HA and NA after exposure to Cu2O, AgNO3, and Ag2O were determined at different metal concentrations. Exposure to Cu2O decreased the HA titer at lower metal concentrations than the two silver compounds (AgNO3 and Ag2O) (Fig. 4 a). In addition, HA activity was significantly decreased over a range of Cu2O concentrations (0.001–0.01 μmol), whereas NA activity was not affected under the same conditions (Fig. 4a, b). This difference in the dose response between HA and NA activities suggests that influenza virus inactivation by solid-state Cu2O at low concentration mainly originates from the inhibition of HA protein. On exposure to silver ionic compounds, NA activity was lost at a lower metal concentration than observed with Cu2O (Fig. 4b), suggesting that silver ionic compounds inactivate the virus by preferentially losing the enzyme activity of NA. Imai et al. reported that treatment with Cu2+-containing zeolite decreased NA activity, while did not affect HA titers [20], which is similar tendency to our findings of silver ionic compounds.
Fig. 4.
Hemagglutinin (HA) titers and neuraminidase (NA) activity exposed to AgNO3, Ag2O and Cu2O suspensions. Effect on (a) HA titer (b) NA activity of viruses exposed to AgNO3 (open squares), Ag2O (filled triangles), and Cu2O (filled circles) suspensions, as determined by a hemagglutination test and chemiluminescence using the NA-Star method, respectively. N0 in panel (b) is the initial NA activity.
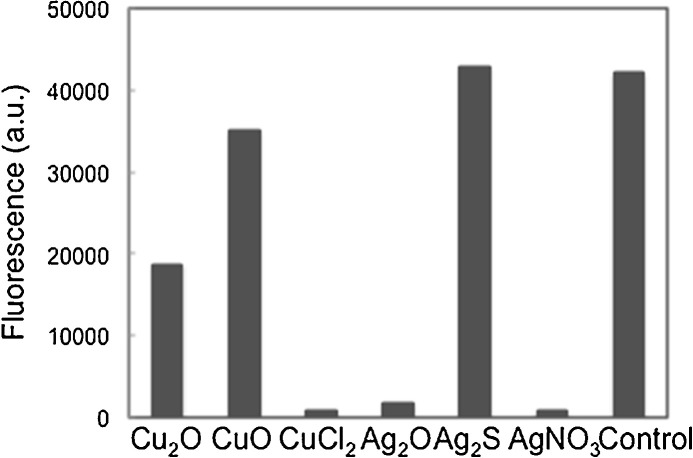
Furthermore, we asked whether the structure of viral proteins would be destabilized by contact with copper and silver compounds by evaluating the total thiol (R—SH) concentration from both disulfide (S—S) bonds and cysteine residues. Disulfide bond formation is essential for protein folding and for maintenance of the structure and function of proteins. Like other proteins, HA and NA proteins possess multiple disulfide bonds that stabilize their structure. A previous report detailed a method for determining the total concentration of thiols by evaluating by fluorescence of monobromobimane using trypsin inhibitor protein, which possesses two S—S bonds and one cysteine residue per molecule, as a model protein [26]. As shown in Fig. 5 , CuO and Ag2S, which cannot inactivate both influenza virus and bacteriophage Qβ, did not affect the thiol concentration. By contrast, copper ion from CuCl2 and silver ion from AgNO3 or Ag2O clearly decreased the thiol from trypsin inhibitor protein, suggesting that copper and silver ions denature proteins by breakage of S—S bonds according to the following reactions [27], [28]:
| R—S—S—R + Ag+ → 2R—S—Ag, R—SH + Ag+ → R—S—Ag + H+ |
| R—S—S—R + Cu2+ → R—S—Cu—S—R, 2R—SH + Cu2+ → R—S—Cu—S—R + 2H+ |
(R—S—S—R and R—SH represent disulfide (S—S) bond and cysteine residues in trypsin inhibitor protein.)
Fig. 5.
Level of fluorescence, corresponding to total thiol (R-SH) from trypsin inhibitor protein, after incubation with different copper and silver compounds. Incubations were carried out for 4 h with the compound suspension (2.8 mmol). A control lacking the compound was included.
As Ag2S or CuS were formed at the end of these reactions, the fluorescence generated by the reaction of –SH and monobromobimane was hardly detectable.
3.3. Summary and mechanistic implication of antiviral effects on copper and silver compounds
Table 1 summarizes the antiviral activity of each test compound against influenza virus and bacteriophage Qβ, and the effect on the function and structure of viral proteins. Copper and silver ions such as CuCl2, AgNO3, and Ag2O inactivate influenza viruses and also inhibit NA enzyme activity at low concentrations, indicating that the biocidal activity of such ionic compounds is mainly mediated by NA inhibition. In addition, these ions were able to induce protein destabilization through the breakage of S—S bonds. Disruption of the disulfide bonds in NA may result in loss of enzymatic activity because mutation of cysteine residues in NA has been reported to significantly impair enzyme activity [29]. By contrast, solid state Cu2O inactivated viruses with or without viral envelopes (influenza virus and bacteriophage Qβ) and preferentially inhibited HA activity. HA is involved in host cell recognition through ligand–receptor interactions on the cell surface. Our previous study revealed that the major biocidal effect of Cu2O is mediated when viruses come in direct contact with metal-coated solid surfaces [11]. Thus, direct contact of the protein with Cu2O would cause the structural denaturation of HA, resulting in impaired ligand–receptor recognition. This idea is supported by the efficient inactivation mediated by solid-state Cu2O of bacteriophage Qβ, a virus possessing capsid proteins on its surface instead of a viral envelope. Like HA inactivation, the denaturation of the surface proteins in bacteriophage Qβ can inhibit infection into host bacteria. Such inactivation effects are distinct from those observed for ionic copper and silver compounds in our study. The denaturation of protein structure is partially caused by the cleavage of S—S bonds; however, Cu2O decreased the thiol concentration by approximately half despite the high antiviral activity, which is weaker than the effects of ionic copper and silver compounds. These results suggested that Cu2O can denature protein structures by other mechanisms, such as disorder of hydrogen binding networks or ionic interactions in protein surfaces. We found that there are significant differences of protein adsorption and denaturation between Cu2O and CuO [11], which might cause the opposite antiviral activities. Although interactions between proteins and solid surfaces have been studied [30], the effects of solid-state Cu2O on viral protein structure following direct contact require further clarification.
Table 1.
Summary of the antiviral activity of copper and silver compounds against influenza virus and bacteriophage Qβ, and the denaturation of HA and NA proteins.
| Copper and silver compounds | Virus with envelope (Influenza virus) |
Virus without envelope (Bacteriophage Qβ) |
HA | NA | S—S bonds cleavage |
|---|---|---|---|---|---|
| Solid-state Cu(I) compounds (Cu2O) |
Strong inactivation | Strong inactivation | Denaturation at low concentration | Denaturation at high concentration | Weak |
| Solid-state Cu(II) compounds (CuO) |
No effect | No effect | No denaturation | No denaturation | No |
| Water-soluble Cu(II) compounds (CuCl2) |
Inactivationa | No effect | No denaturationb | Denaturationb | Strong |
| Solid-state Ag compounds (Ag2S) |
No effect | No effect | –c | –c | No |
| Water-soluble Ag compounds (AgNO3, Ag2O) |
Strong inactivation | Weak inactivation | Denaturation at high concentration | Denaturation at low concentration | Strong |
4. Conclusion
In conclusion, we evaluated viral activity in response to exposure to copper and silver compounds and demonstrated that the antiviral mechanism of these metals against influenza viruses is mediated by inactivation of HA and NA surface proteins. Comparative analysis of these compounds revealed that the antiviral effect of Cu2O significantly differs from that of other ionic copper and silver compounds. With respect to influenza virus, one of the major strategies to control virus transmission is vaccination. However, vaccines are typically not effective against newly emerged viruses owing to the lack of suitable target antigens [31]. Inhibitors of NA, such as oseltamivir (Tamiflu®) and zanamivir (Relenza®), are also currently used as anti-influenza drugs. Despite their reported efficacy, it is likely that influenza viruses will gradually develop resistance to these drugs [32]. Combined with current strategies, the utilization of inorganic chemicals containing copper and silver as anti-influenza materials would potentially reduce the risk viral transmission in the environment. In particular, solid-state Cu2O has superior activity against both types of viruses, enveloped and nonenveloped compared with silver compounds, because of its unique inactivation mechanism mediated by direct contact. Furthermore, Cu2O is low-cost and widely available as a surface coating agent in various material surfaces and air-cleaning filters. Cu2O can also be combined with other biocidal coating chemicals such as photocatalytic TiO2 nanoparticles [33]. The use of Cu2O for the treatment of both public and living spaces may help limit, or even prevent, future influenza pandemics.
Acknowledgment
This study was supported by the New Energy and Industrial Technology Development Organization (NEDO) of Japan.
References
- 1.World Health Organization Ebola Situation Reports. http://who.int/csr/disease/ebola/en/
- 2.World Health Organization Middle East Respiratory Syndrome Coronavirus (MERS-CoV) http://www.who.int/emergencies/mers-cov/en/
- 3.Webby R.J., Webster R.G. Are we ready for pandemic influenza? Science. 2003;302:1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- 4.Reid A.H., Taubenberger J.K., Fanning T.G. The 1918 Spanish influenza: integrating history and biology. Microbes Infect. 2001;3:81–87. doi: 10.1016/s1286-4579(00)01351-4. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . 2009. Evolution of a Pandemic A(H1N1)http://www.who.int/influenza/resources/publications/evolution_pandemic_Ah1n1/ [Google Scholar]
- 6.Brankston G., Gitterman L., Hirji Z., Lemieux C., Gardam M. Transmission of influenza A in human beings. Lancet Infect. Dis. 2007;7:257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 7.Borkow G., Gabbay J. Putting copper into action: copper-impregnated products with potent biocidal activities. FASEB J. 2004;18:1728–1730. doi: 10.1096/fj.04-2029fje. [DOI] [PubMed] [Google Scholar]
- 8.Noyce J.O., Michels H., Keevil C.W. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 2007;73:2748–2750. doi: 10.1128/AEM.01139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borkow G., Zhou S.S., Page T., Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PLoS One. 2010;5:e11295. doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurman R.B., Gerba C.P. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. CRC Crit. Rev. Environ. Control. 1989;18:295–315. [Google Scholar]
- 11.Sunada K., Minoshima M., Hashimoto K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012;235–236:265–270. doi: 10.1016/j.jhazmat.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 12.Nakano R., Ishiguro H., Yao Y.Y., Kajioka J., Fujishima A., Sunada K., Minoshima M., Hashimoto K., Kubota Y. Photocatalytic inactivation of influenza virus by titanium dioxide thin film. Photochem. Photobiol. Sci. 2012;11:1293–1298. doi: 10.1039/c2pp05414k. [DOI] [PubMed] [Google Scholar]
- 13.Kalbfuss B., Genzel Y., Wolff M., Zimmermann A., Morenweiser R., Reichl U. Harvesting and concentration of human influenza A virus produced in serum-free mammalian cell culture for the production of vaccines. Biotechnol. Bioeng. 2007;97:73–85. doi: 10.1002/bit.21139. [DOI] [PubMed] [Google Scholar]
- 14.Reed L., Muench H. A simple method of estimating 50% endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- 15.Donald H.B., Isaacs A. Counts of influenza virus particles. J. Gen. Microbiol. 1954;10:457–464. doi: 10.1099/00221287-10-3-457. [DOI] [PubMed] [Google Scholar]
- 16.Buxton R.C., Edwards B., Juo R.R., Voyta J.C., Tisdale M., Bethell R.C. Development of a sensitive chemiluminescent neuraminidase assay for the determination of influenza virus susceptibility to zanamivir. Anal. Biochem. 2000;280:291–300. doi: 10.1006/abio.2000.4517. [DOI] [PubMed] [Google Scholar]
- 17.Newton G.L., Dorian R., Fahey R.C. Analysis of biological thiols: derivatization with monobromobimane and separation by reverse-phase high-performance liquid chromatography. Anal. Biochem. 1981;114:383–387. doi: 10.1016/0003-2697(81)90498-x. [DOI] [PubMed] [Google Scholar]
- 18.Yano H., Wong J.H., Lee Y.M., Cho M.J., Buchanan B.B. A strategy for the identification of proteins targeted by thioredoxin. Proc. Natl. Acad. Sci. 2001;98:4794–4799. doi: 10.1073/pnas.071041998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horie M., Ogawa H., Yoshida Y., Yamada K., Hara A., Ozawa K., Matsuda S., Mizota C., Tani M., Yamamoto Y., Yamada M., Nakamura K., Imai K. Inactivation and morphological changes of avian influenza virus by copper ions. Arch. Virol. 2008;153:1467–1472. doi: 10.1007/s00705-008-0154-2. [DOI] [PubMed] [Google Scholar]
- 20.Imai K., Ogawa H., Bui V.N., Inoue H., Fukuda J., Ohba M., Yamamoto Y., Nakamura K. Inactivation of high and low pathogenic avian influenza virus H5 subtypes by copper ions incorporated in zeolite-textile materials. Antiviral Res. 2012;93:225–233. doi: 10.1016/j.antiviral.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Mehrbod P., Motamed N., Tabatabaian M., Soleimani Estyar R., Amini E., Shahidi M., Kheiri M.T. In vitro antiviral effect of nanosilver on influenza virus. DARU. 2009;17:88–93. [Google Scholar]
- 22.Xiang D.-X., Chen Q., Pang L., Xiang C.-l. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J. Viol. Methods. 2011;178:137–142. doi: 10.1016/j.jviromet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Mori Y., Ono T., Miyahara Y., Nguyen V.Q., Matsui T., Ishihara M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013;8:93. doi: 10.1186/1556-276X-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das K., Aramini J.M., Ma L.C., Krug R.M., Arnold E. Structures of influenza A proteins and insights into antiviral drug targets. Nat. Struct. Mol. Biol. 2010;17:530–538. doi: 10.1038/nsmb.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matrosovich M.N., Matrosovich T.Y., Gray T., Roberts N.A., Klenk H.D. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J. Virol. 2004;78:12665–12667. doi: 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koide T., Ikenaka T. Studies on soybean trypsin inhibitors: 3 amino acid sequence of the carboxyl-terminal region and the complete amino acid sequence of soybean trypsin inhibitors (Kunitz) Eur. J. Biochem. 1973;32:417–431. doi: 10.1111/j.1432-1033.1973.tb02624.x. [DOI] [PubMed] [Google Scholar]
- 27.Russell A.D., Path C., Pharm S., Hugo W.B. Antimicrobial activity and action of silver. Prog. Med. Chem. 1994;31:351–370. doi: 10.1016/s0079-6468(08)70024-9. [DOI] [PubMed] [Google Scholar]
- 28.Gevondyan N.M., Volynskaia A.M., Gevondyan V.S. Four free cysteine residues found in human IgG1 of healthy donors. Biochem. (Moscow) 2006;71:279–284. doi: 10.1134/s0006297906030072. [DOI] [PubMed] [Google Scholar]
- 29.Warda C.W., Colmana P.M., Laverb W.G. The disulphide bonds of an Asian influenza virus neuraminidase. FEBS Lett. 1983;153:29–33. doi: 10.1016/0014-5793(83)80113-6. [DOI] [PubMed] [Google Scholar]
- 30.Wahlgren M., Arnebrant T. Protein adsorption to solid surfaces. Trends Biotechnol. 1991;9:201–208. doi: 10.1016/0167-7799(91)90064-o. [DOI] [PubMed] [Google Scholar]
- 31.Wright P.F. Vaccine preparedness—are we ready for the next influenza pandemic? N. Engl. J. Med. 2008;358:2540–2543. doi: 10.1056/NEJMp0803650. [DOI] [PubMed] [Google Scholar]
- 32.Weinstock D.M., Zuccotti G. The evolution of influenza resistance and treatment. J. Am. Med. Assoc. 2009;301:1066–1069. doi: 10.1001/jama.2009.324. [DOI] [PubMed] [Google Scholar]
- 33.Qiu X.Q., Miyauchi M., Sunada K., Minoshima M., Liu M., Lu Y., Shimodaira Y., Hosogi Y., Kuroda Y., Hashimoto K. Hybrid CuxO/TiO2 nanocomposites as risk-reduction materials in indoor environments. ACS Nano. 2012;6:1609–1618. doi: 10.1021/nn2045888. [DOI] [PubMed] [Google Scholar]