Abstract
The objectives were to determine the median infective dose (ID50) of Cryptosporidium parvum and to describe the dose–response relationship including associated clinical illness in experimentally challenged dairy calves. Within the first 24 h of life, 27 test calves were experimentally challenged with C. parvum oocysts and 3 control calves were sham dosed. Test calves received 1 of 8 possible doses (25, 50, 100, 500, 1 × 103, 1 × 104, 1 × 105, and 1 × 106 oocysts). All 27 test calves developed diarrhea. Fecal oocyst shedding occurred in 25 (92.6%) test calves and in 0 control calves. The 2 non-shedding test calves both received 25 oocysts. There was an inverse relationship between dose and time to onset of fecal oocyst shedding (P = 0.005). There was no relationship found between dose and duration (P = 0.2) or cessation (P = 0.3) of fecal oocyst shedding. In addition, there was not a significant relationship between log-dose and the log-peak oocysts (P = 0.2) or log-total oocysts (P = 0.5) counted/g of feces across the dose groups. There was a positive dose–response relationship between log-dose and diarrhea (P = 0.01). However, when controlling for other factors, such as onset and cessation of fecal oocyst shedding, dose was not a significant predictor of diarrhea (P = 0.5). Onset and cessation of fecal oocyst shedding were found to be the best predictors of diarrhea (P = 0.0006 and P = 0.04, respectively). The ID50 for fecal oocyst shedding was 5.8 oocysts, for diarrhea was 9.7 oocysts, and for fecal oocyst shedding with diarrhea was 16.6 oocysts. Given that the ID50 of C. parvum is far less than would be excreted into the environment by a naturally infected calf, prevention and control of cryptosporidiosis is a formidable challenge.
Keywords: Cryptosporidium, Infectious dose, Dose–response, Calf, Bovine
1. Introduction
Cryptosporidium is a genus of apicomplexan protozoal parasites that are globally distributed and are known to infect several species of animals (Fayer, 2004). Cryptosporidium parvum and Cryptosporidium hominis, which are respectively host-adapted to cattle and people, are recognized as being among the most pathogenic species. Cryptosporidiosis, refers to infection with Cryptosporidium spp., and is primarily characterized by villus atrophy and fusion, intestinal crypt inflammation, and a resultant malabsorptive, maldigestive, osmotic diarrhea. C. parvum infection typically occurs in calves less than 1 month old and impacts calf morbidity and mortality (O’Handley et al., 1999). Infected calves shed large numbers of readily infective oocysts in their feces which impacts environmental parasite loading (Nydam et al., 2001).
C. parvum infection is an important zoonosis, causing similar clinical symptoms in people, and is more likely to be acquired by immune suppressed individuals (Anderson, 1998). Transmission is typically via the fecal–oral route. Common risk factors for cryptosporidiosis include ingestion of contaminated water (i.e., fecal accidents in public swimming pools or use of unprotected water sources), poor hygiene, contact with livestock, and failures at municipal water-treatment facilities (MacKenzie et al., 1995, Valderrama et al., 2009, Ng et al., 2012).
There are currently no consistently effective and commercially available treatments or vaccines. Nitazoxanide (NTZ) has shown efficacy in HIV-seronegative patients, but not in HIV-seropositive patients (Amadi et al., 2002). NTZ does reduce fecal oocyst shedding in calves but is not commercially available for use in cattle (Ollivett et al., 2009). Given these challenges, cryptosporidiosis is not only a calf management concern, but is also a global public health concern (O’Handley et al., 1999, Thompson et al., 2008).
The dose–response relationship has been described and median infective dose (ID50) has been determined for some Cryptosporidium spp. through investigations carried out in healthy human volunteers. For both C. parvum and C. hominis, a positive relationship between the size of the inoculum and occurrence of enteric symptoms has been confirmed, thus, the larger the exposure to infective oocysts, the worse the clinical outcome (DuPont et al., 1995, Chappell et al., 2006). In people experimentally infected with C. parvum and C. hominis, 61% developed enteric symptoms (DuPont et al., 1995, Chappell et al., 2006). In human subjects who developed diarrhea and excreted oocysts, the median infective dose (ID50) for C. parvum was determined to be 132 oocysts, and for C. hominis was 83 oocysts (DuPont et al., 1995, Chappell et al., 2006).
Similar studies in animals have been restricted to laboratory species. The reported ID50 for C. parvum in immunocompetent neonatal mice is 100–500 oocysts, and in CD1 neonatal mice is 79 oocysts, which is consistent with the doses reported in people (Ernest et al., 1986, Finch et al., 1993). While experimental infection of livestock is not reported in the literature, studies of natural infection have been conducted. Naturally infected calves experience diarrhea, have a mean onset of fecal oocyst shedding of 16.3 days, and a mean duration of fecal oocyst shedding of 10.5 days (O’Handley et al., 1999). Though not shown conclusively, a study of naturally infected calves suggested that reduced exposure to C. parvum oocysts resulted in reduced duration of fecal oocyst shedding (Moore et al., 2003).
Even though studies of natural infection provide important information on disease ecology, they are limited by confounders such as co-infection and lack of experimental controls. Given that C. parvum is a known zoonosis, is up to 95% prevalent in US dairy herds, contributes to increased calf mortality, and that there is not an available pharmacologic intervention; it is important to improve our understanding of C. parvum epidemiology, through experimental studies of infection, in order to develop appropriate disease control and prevention strategies (Lefay et al., 2001, Trotz-Williams et al., 2008, Tzipori and Widmer, 2008). The objectives of this study were to determine the median infective dose (ID50) of C. parvum and to describe the dose–response relationship and associated clinical illness in experimentally challenged dairy calves.
2. Materials and methods
2.1. Challenge model
2.1.1. Calf enrollment
Calves used in this study were cared for in compliance with the Cornell University Institutional Animal Care and Use Committee (IACUC). This randomized, double-blinded study was performed at the College of Veterinary Medicine, Cornell University (Ithaca, NY) from February to March 2011. Thirty calves were purchased at birth from a local dairy farm and enrolled in the study as they were born. Control calves (n = 3) were enrolled concurrently with test calves (n = 30). At least one study author attended all calvings. The perineum of the dam was thoroughly cleaned with povidone–iodine scrub and calves were delivered onto single-use plastic sheets to prevent manure contamination. Immediately after birth, a physical examination was performed and an identification tag was placed in the right ear. All calves were fed 4 L of ≥50 g IgG/L commercial colostrum replacer (Bovine IgG, Colostrum Replacement, Land O’Lakes Inc., St. Paul, MN) within the first 4 h of life via an oroesophageal feeding tube in order to replicate conventional calf management on commercial dairies as best as possible. Commercial colostrum replacer was fed instead of colostrum in order to minimize variability between calves, to limit potential pathogen exposure, and to provide adequate passive transfer of immunity without providing anti-Cryptosporidium specific antibodies. The calves were then transported from the source farm to Cornell University.
2.1.2. Calf management, sampling, and inoculation
At Cornell University, all calves were housed in a Biosafety Level 2 facility in individual concrete box stalls. Blood samples were collected from each calf within 24–48 h of life and the serum total protein was measured in order to assess adequacy of passive transfer. Calves were fed commercial 22% protein/20% fat non-medicated milk replacer (Nursing Formula NT Calf Milk Replacer, Land O’Lakes Inc.) with at least 0.68 kg of dry matter per day, split into 2 feedings, for the duration of the study and water was provided ad libitum. At enrollment, both control and test calves were randomized to a dose group by a number generator. Calves received an oral challenge of C. parvum oocysts within the first 24 h of life. Three calves served as controls and were sham dosed with 0 oocysts. Twenty-seven calves were inoculated with one of eight possible doses of a genotyped field strain of C. parvum oocysts. Five calves received 25 oocysts, 4 calves received 50 oocysts, and 3 calves each received 100, 500, 1000, 10,000, 100,000, or 1,000,000 oocysts. All study personnel were blinded to dose group.
Control calves (n = 3) were housed in the same facility as test calves, sham dosed, and managed as if they were test calves in order to maintain blinding. Control calves also served as sentinels for cross contamination from test calves, and to help maintain quality assurance in data collection and husbandry practices. To prevent cross contamination, calves were fed and bedded in the same order (youngest to oldest) each day, each calf stall had dedicated equipment and supplies, and all study personnel used single-use personal protective equipment when entering each calf stall.
A fecal sample was collected from each calf every 24 h after oral challenge. Clinical data including rectal temperature, general health status, and fecal consistency were recorded every 10–12 h for each calf. Health status was assessed on a scale of 1–4 and fecal consistency was assessed on a scale of 1–5 in accordance with previously described methods (Table 1 ) (Bellosa et al., 2011). Diarrhea was defined as having at least 2 consecutive fecal consistency scores ≥3. Calves that did not shed oocysts in their feces were enrolled in the study for 21 days. Calves that did have oocysts present in their feces were enrolled in the study until 2 consecutive negative fecal exams were recorded after the onset of fecal shedding. All calves were tested for the presence of rotavirus, coronavirus, and Salmonella spp.
Table 1.
Health status and fecal consistency rubric used for evaluating severity of calf illness and diarrhea.
| Score | Health status | Fecal consistency |
|---|---|---|
| 1 | Normal. The calf is alert, hungry, and watches the caretakers. It may stretch when it gets up. The calf will eat greedily, often twitching its tail as it eats | Normal feces; feces retain form. The feces may be pasty but do not flow across a surface |
| 2 | Mildly depressed. The calf drinks without coaxing, but not aggressively. The calf pays some attention to caretakers and assessment for dehydration (skin tent ≤4 s, eyes normal) produces equivocal results | Mild diarrhea; form is a puddle, not a patty. Sufficient water content to slowly flow across or down a surface |
| 3 | Severely depressed. The calf must be coaxed to get up, and has difficulty rising or standing, does not pay attention to caretakers when touched, may refuse to eat, and is clearly dehydrated (e.g., skin tent >9 s, separation between eyeball and orbit ≥0.5 cm, dry mucous membranes). The calf is unlikely to recover without supportive treatment | Moderate diarrhea; feces with sufficient water content to easily flow across or down a surface, while leaving some adherent material |
| 4 | Moribund or dead. The calf cannot stand or is dead | Severe diarrhea; part or all of feces are very watery. Feces can drain away leaving little or no residual on a smooth surface (a calf may have very watery feces followed by some solid material and still have severe diarrhea) |
| 5 | N/A | Not observed |
The oocysts used to dose the calves were purified using a procedure previously described (Jenkins et al., 1997). In brief, feces were collected from naturally infected 6- to 14-day-old calves from a separate commercial dairy operation and processed by continuous-flow differential density flotation. They were stored until needed at 4 °C in suspension with 100 U of penicillin G sodium per ml, 100 mg of streptomycin sulfate per ml, and 0.25 mg of amphotericin B per ml. The oocyst DNA was genotyped as C. parvum by sequence and restriction fragment length polymorphism analysis via amplification of the small subunit (SSU) rRNA gene in a nested polymerase chain reaction (PCR) as described previously (Jiang et al., 2005). In brief, the primary PCR step amplifies a fragment of approximately 1325 base pairs, whereas the secondary PCR step results in a fragment of approximately 823 base pairs. In the primary PCR, the following forward and reverse primers were used, respectively: 5′-TTCTAGAGCTAATACATGCG-3′ and 5′-CCCATTTCCTTCGAAACAGGA-3′. In the secondary PCR, the following forward and reverse primers were used, respectively: 5′-GGAAGGGTTGTATTTATTAGATAAAG-3′ and 5′-CTCATAAGGTGCTGAAGGAGTA-3′.
2.1.3. Preparation of inoculum
Before inoculation, oocysts were first cleaned for one minute in 0.6% sodium hypochlorite to inactivate viruses and bacteria co-purified with the oocysts, then washed four times with phosphate buffered saline to remove the sodium hypochlorite, quantified using a hemocytometer and finally viability determined using a dye permeability assay as described previously (Campbell et al., 1992, Anguish and Ghiorse, 1997, Jenkins et al., 1997). Viable oocysts were the sum of 4,6-diamidino-2-phenylindole-negative (DAPI−) propidium iodide-negative (PI−) oocysts and DAPI-positive (DAPI+) PI− oocysts; DAPI+ PI+ oocysts were considered inactivated (Jenkins et al., 1999). Oocysts used for dosing were at least 87% viable. Doses were calculated based on the percent viable. Each dose was administered in a 5 ml suspension of C. parvum oocysts in reverse osmosis water via the rigid portion of an oroesophageal feeding tube. Followed by 120 ml of water to ensure all of the oocyst suspension was delivered to the calf.
2.2. Fecal sample analysis
Quantitative analysis of C. parvum oocysts in the fecal samples collected was performed using Merifluor Crypto/Giardia immunofluorescence antibody detection reagent from Meridian Diagnostics (Cincinnati, OH) (Xiao and Herd, 1993). The immunofluorescence procedure was modified from the kit instructions. Briefly, a 0.10 g portion of feces was mixed into 10 ml of PBS (pH = 7.4) in a 15 ml conical centrifuge tube. Then, 100 μl of the mixture was removed and 5 μl of Merifluor immunofluorescence antibody reagent was added. The solution was vortexed and incubated in the dark at room temperature for at least 30 min and stored at 4 °C until examination. Following incubation, 10.5 μl of the sample was placed on a slide and covered with a coverslip. The 20× objective on a fluorescent compound binocular microscope (460–490 wavelength fluorescent compound binocular microscope Olympus BX41, Olympus America Inc., Center Valley, PA) was used to count the number of oocysts observed. The number of oocysts observed in 10.5 μl was then multiplied by 10,000 to give the number of oocysts per gram of feces. This count was standardized by the dry weight percentage. Dry weight analysis of fecal samples was obtained by taking a 10–20 g portion of each original fecal sample, drying it at 108 °C for a minimum of 24 h (Thermolyne Mechanical Oven, Barnstead International, Dubuque, IA), then weighing it directly (Precision Standard Scale, Ohaus Corporation, Pine Brook, NJ) (Bellosa et al., 2011).
2.3. Data analysis
Data were analyzed using descriptive and inferential methods. Using the Shapiro–Wilk test, data were determined to be non-Gaussian. A Wilcoxon Rank Sum test was used to compare sets of continuous data. For each dose group, the probability of shedding on a given day after challenge was estimated using the Kaplan–Meier product limit method (Kaplan and Meier, 1958). Post hoc analysis of the percentage of fecal scores ≥3 across the dose groups was carried out with Dunn's Post Test. Analysis of variance was used to assess differences in fecal oocyst counts across dose groups. Simple linear regression analysis was used to evaluate the relationship between individual explanatory variables and diarrhea (percent of fecal scores ≥3) as well as onset of fecal oocyst shedding. Explanatory variables having P ≤ 0.1 were selected for analysis using multiple linear regression. Manual backward-stepwise regression was used to remove explanatory variables and their interactions from the model when P > 0.05. The ID50 for fecal oocyst shedding, diarrhea, and fecal oocyst shedding with diarrhea was estimated using linear regression analysis. The percent of dosed calves that shed oocysts in their stool, developed diarrhea, or both was compared with the total number of calves receiving each dose of oocysts (log transformed) (DuPont et al., 1995). Data were analyzed using JMP 9.0 (SAS Institute Inc., 1989–2007).
3. Results
3.1. Description of study animals
There were 30 calves enrolled in the study; 3 were control calves and 27 were experimentally challenged with Cryptosporidium parvum oocysts. Calves were enrolled in the study for 20.5 ± 1.1 days, during which time the mean number of fecal scores recorded was 38.2 ± 2.7 and the mean number of health scores recorded was 40.9 ± 2.5. Across all groups, the mean serum total protein measurement (g/dl) was 4.8 ± 0.4 (95% CI 4.6–4.9) and there were no differences between groups (P = 1.0). None of the calves tested positive for coinfection with rotavirus, coronavirus, or Salmonella spp.
3.2. Description of fecal oocyst shedding
None of the 3 control calves developed diarrhea or fecal oocyst shedding. All 27 experimentally challenged calves developed diarrhea (at least 2 consecutive fecal consistency scores ≥3). Twenty-five calves shed C. parvum oocysts in their feces and 2 calves did not. The 2 non-shedding calves were both challenged with 25 oocysts. The mean time to onset of fecal oocyst shedding was 5.6 ± 2 days (95% CI 4.8–6.5) (n = 25) (Table 2 ). There was an inverse relationship between dose and time to onset of fecal oocyst shedding, i.e., calves that received smaller doses began shedding later (P = 0.005). As the log-dose increased by 1, the time to onset of fecal oocyst shedding was shortened by 1 day (P < 0.0001) (Fig. 1 a). Between dose groups, calves that received 25 or 50 oocysts shed later than calves that received 1,000,000 oocysts (P = 0.06 and P = 0.005, respectively) (Fig. 2 ). The mean duration of fecal oocyst shedding was 11.1 ± 2.4 days (95% CI 10.1–12.1) (n = 25) (Table 2). The mean time to cessation of fecal oocyst shedding was 16.8 ± 2.2 days (95% CI 15.8–17.7) (n = 25) (Table 2). Dose did not influence duration of fecal oocyst shedding (P = 0.2) or cessation of fecal oocyst shedding (P = 0.3). However, at higher doses more variability was observed in both duration and cessation of fecal oocyst shedding (Table 2).
Table 2.
The mean, median, and range for the number of days post-challenge until the onset of fecal shedding, the number of days duration of fecal shedding post-challenge, and the number of days post-challenge until the cessation of fecal shedding, in calves experimentally challenged with 25, 50, 100, 500, 1 × 103, 1 × 104, 1 × 105, or 1 × 106 oocysts of C. parvum.
| Dose of C. parvum oocysts | 25 | 50 | 100 | 500 | 1000 | 10,000 | 100,000 | 1,000,000 |
|---|---|---|---|---|---|---|---|---|
| Onset of fecal oocyst shedding (days) | ||||||||
| Mean | 7.3 | 8.8 | 5.7 | 5.3 | 4.7 | 4.7 | 5 | 2.7 |
| Median | 7 | 9 | 6 | 5 | 4 | 4 | 5 | 3 |
| Range | (7–8) | (7–10) | (5–6) | (5–6) | (4–6) | (4–5) | (4–6) | (2–3) |
| Duration of fecal oocyst shedding (days) | ||||||||
| Mean | 11 | 9.5 | 11.7 | 9.7 | 10.7 | 13.3 | 10.7 | 13 |
| Median | 11 | 9 | 12 | 9 | 11 | 13 | 9 | 12 |
| Range | – | (9–11) | (11–12) | (8–12) | (9–12) | (13–14) | (8–15) | (9–18) |
| Cessation of fecal oocyst shedding (days) | ||||||||
| Mean | 18 | 18.3 | 17.3 | 15.3 | 15.3 | 18 | 15.7 | 15.7 |
| Median | 18 | 18.5 | 18 | 15 | 15 | 18 | 14 | 14 |
| Range | (17–19) | (16–20) | (16–18) | (14–17) | (15–16) | (17–19) | (14–19) | (12–21) |
Fig. 1.
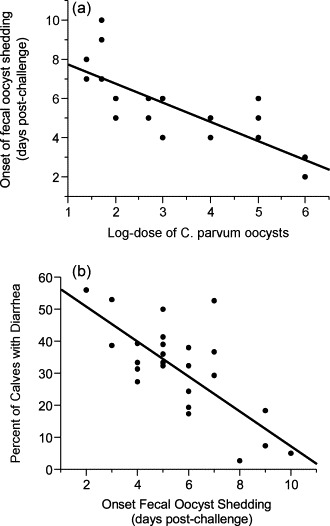
(a) The relationship between log-dose and the onset of fecal oocyst shedding in calves experimentally challenged with C. parvum oocysts. (b) The relationship between the onset of fecal oocyst shedding and the percent of calves with diarrhea in calves experimentally challenged with C. parvum oocysts.
Fig. 2.
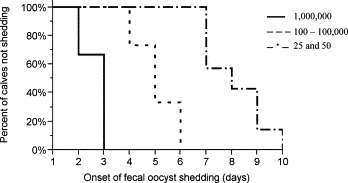
Probability of onset of fecal shedding post-challenge in calves dosed with 25 and 50, 100–100,000, and 1,000,000 C. parvum oocysts.
When the dose groups are collapsed into 3 dose levels (25 and 50 oocysts, 100–100,000 oocysts, and 1,000,000 oocysts) there is a difference in time to onset across the 3 levels (P < 0.0001). Between dose levels, calves that received 25 and 50 oocysts shed later than calves that received 100–100,000 and 1,000,000 oocysts (P = 0.003 and P = 0.0002, respectively). Fig. 2 shows the probability of onset of fecal oocyst shedding on any given day post-challenge for the 3 dose levels.
3.3. Fecal oocyst shedding and diarrhea
Fecal shedding and diarrhea occurred at all doses. There was an inverse relationship between onset of fecal oocyst shedding and diarrhea, i.e., calves that began to shed oocysts sooner after dosing experienced more days of diarrhea (P < 0.0001) (Fig. 1b). The log-peak oocysts counted was 7.8 ± 0.4 (95% CI 7.6–8.0) and the log-total oocysts counted was 7.9 ± 0.4 (95% CI 7.7–8.1). There was no difference in log-peak or log-total oocysts counted/g of feces across the dose groups (P = 0.2 and P = 0.5, respectively) or among the 3 dose levels (P = 0.3 and P = 0.9, respectively). Among the 5 calves dosed with 25 oocysts, 3 calves shed oocysts, 2 of which also developed diarrhea. For the remaining doses, all calves shed and developed diarrhea (Table 3 ). Health scores >1 occurred in 2 calves, but only occurred once for each calf. Calf 207 had health scores >1 for several consecutive days. This calf had a septic stifle joint secondary to an umbilical abscess, and was excluded from health score analysis, but included in all other analysis.
Table 3.
Risk of fecal oocyst shedding, diarrhea, and fecal oocyst shedding with diarrhea in calves experimentally challenged with C. parvum oocystsa.
| Dose of C. parvum oocysts | Log dose of C. parvum oocysts | No. calves challenged | Fecal shedding (%) | Diarrhea (%) | Fecal shedding + diarrhea (%) |
|---|---|---|---|---|---|
| 0 | 0 | 3 | 0 (0) | 0 (0) | 0 (0) |
| 25 | 1.4 | 5 | 3 (60) | 3 (60) | 2 (40) |
| 50 | 1.7 | 4 | 4 (100) | 3 (75) | 3 (75) |
| 100 | 2 | 3 | 3 (100) | 3 (100) | 3 (100) |
| 500 | 2.7 | 3 | 3 (100) | 3 (100) | 3 (100) |
| 1000 | 3 | 3 | 3 (100) | 3 (100) | 3 (100) |
| 10,000 | 4 | 3 | 3 (100) | 3 (100) | 3 (100) |
| 100,000 | 5 | 3 | 3 (100) | 3 (100) | 3 (100) |
| 1,000,000 | 6 | 3 | 3 (100) | 3 (100) | 3 (100) |
Linear regression analysis of the data yielded an ID50 of 5.8 oocysts for fecal oocyst shedding, 9.7 oocysts for diarrhea, and 16.6 oocysts for fecal oocyst shedding with diarrhea.
The explanatory variables log-dose, total protein on day 2 of enrollment, weight on day 5 of enrollment, weight on the final day of enrollment, onset of fecal oocyst shedding, duration of fecal oocyst shedding, cessation of fecal oocyst shedding, log-peak number of fecal oocysts counted, and log-total number of fecal oocysts counted were all evaluated via simple linear regression to evaluate a possible relationship with the diarrhea (Table 4 ). The variables log-dose, weight on final day of enrollment, onset of fecal oocyst shedding, and cessation of fecal oocyst shedding all had P-values ≤ 0.1 and were retained for analysis via multiple linear regression. In the final model, onset and cessation of fecal oocyst shedding were found to be the best predictors of diarrhea (P = 0.0006 and P = 0.04, respectively) (Table 5 ). Simple linear regression was also conducted using the same explanatory variables to evaluate the relationship with onset of fecal oocyst shedding (Table 4). Log-dose, duration of fecal oocyst shedding, and cessation of fecal oocyst shedding were found to have P-values ≤ 0.1 and were retained for analysis via multiple linear regression. The best predictors of onset of fecal oocyst shedding were duration and cessation of fecal oocyst shedding (P < 0.0001 for both predictors) (Table 5).
Table 4.
Simple linear regression analysis evaluating the relationship between individual explanatory variables and two possible outcomes, diarrhea and the onset of fecal oocyst shedding.
| Explanatory variable | Outcome = Diarrhea |
Outcome = Onset of oocyst shedding |
||||
|---|---|---|---|---|---|---|
| B0 and B1 | SE | P-value | B0 and B1 | SE | P-value | |
| Diarrhea | ||||||
| Intercept | – | – | – | 8.8 | 0.7 | <0.0001 |
| Regression coefficient | −0.1 | 0.02 | <0.0001* | |||
| Onset of fecal oocyst shedding | ||||||
| Intercept | 61.0 | 6.3 | <0.0001 | – | – | – |
| Regression coefficient | −5.2 | 1.1 | <0.0001* | |||
| Log dose | ||||||
| Intercept | 10.5 | 4.5 | 0.03 | 8.7 | 0.6 | <0.0001 |
| Regression coefficient | 6.2 | 1.4 | 0.0001* | −1.0 | 0.2 | <0.0001* |
| Total protein on day 2 | ||||||
| Intercept | 67.4 | 36.0 | 0.07 | 5.2 | 6.1 | 0.4 |
| Regression coefficient | −8.4 | 7.5 | 0.3 | 0.09 | 1.3 | 0.9 |
| Weight on day 5 | ||||||
| Intercept | 3.9 | 17.3 | 0.8 | 9.5 | 2.5 | 0.0009 |
| Regression coefficient | 0.2 | 0.2 | 0.2 | −0.04 | 0.02 | 0.1* |
| Weight at study completion | ||||||
| Intercept | −6.6 | 17.9 | 0.7 | 10.0 | 2.7 | 0.001 |
| Regression coefficient | 0.3 | 0.2 | 0.06* | −0.04 | 0.02 | 0.1* |
| Duration of fecal oocyst shedding | ||||||
| Intercept | 28.6 | 14.4 | 0.06 | 9.8 | 1.8 | <0.0001 |
| Regression coefficient | 0.3 | 1.3 | 0.8 | −0.4 | 0.2 | 0.02* |
| Cessation of fecal oocyst shedding | ||||||
| Intercept | 91.2 | 18.7 | <0.0001 | −0.18 | 2.9 | 1.0 |
| Regression coefficient | −3.5 | 1.1 | 0.004* | 0.3 | 0.2 | 0.05* |
| Log peak oocysts counted | ||||||
| Intercept | −13.4 | 51.8 | 0.8 | 17.4 | 6.9 | 0.02 |
| Regression coefficient | 5.8 | 6.6 | 0.4 | −1.5 | 0.9 | 0.1* |
| Log total oocysts counted | ||||||
| Intercept | −38.5 | 52.3 | 0.5 | 13.5 | 7.3 | 0.08 |
| Regression coefficient | 8.9 | 6.6 | 0.2 | −1.0 | 0.9 | 0.3 |
Explanatory variables with a P-value ≤ 1.0 were selected for inclusion in the multiple linear regression model.
Table 5.
Final models following manual backward stepwise regression to analyze the relationship between the explanatory variables onset, cessation, and duration of fecal oocyst shedding and the outcome variables diarrhea and onset of fecal oocyst shedding.
| Model 1, Outcome = Diarrheaa |
Model 2, Outcome = Days to onset of oocyst shedding |
||||||
|---|---|---|---|---|---|---|---|
| Explanatory variable | Regression coefficient | SE | P-value | Explanatory variable | Regression coefficient | SE | P-value |
| Intercept | 90.5 | 14.5 | <0.0001 | Intercept | −0.3 | 0.5 | 0.6 |
| Onset of fecal shedding | −4.3 | 1.1 | 0.0006 | Duration of fecal shedding | −1.0 | 0.03 | <0.0001 |
| Cessation of fecal shedding | −2.1 | 0.9 | 0.04 | Cessation of fecal shedding | 1.0 | 0.04 | <0.0001 |
a Diarrhea is defined as 2 or more consecutive feedings with a fecal score ≥3.
3.4. Determination of ID50
There was a positive relationship between log-dose and fecal oocyst shedding with diarrhea (P = 0.01) (Fig. 3 ) as well as between log-dose and diarrhea alone (P = 0.007). The ID50 for shedding was 5.8 oocysts, for diarrhea was 9.7 oocysts, and for shedding and diarrhea was 16.6 oocysts (Table 6 ).
Fig. 3.
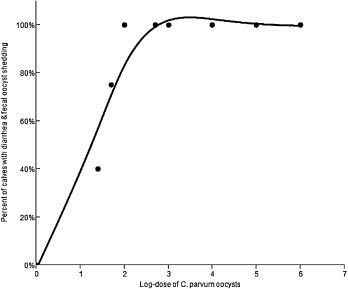
The relationship between dose of C. parvum oocysts and the percent of calves with diarrhea and fecal oocyst shedding.
Table 6.
Regression analysis to determine the ID50 for fecal oocyst shedding, diarrhea, and fecal oocyst shedding with diarrhea in calves experimentally challenged with C. parvum oocysts.
| Factor | SE | P-value | R2 | ID50 (oocysts) | |
|---|---|---|---|---|---|
| Shedding | |||||
| Intercept | 114 | 9.5 | <0.0001 | 0.45 | 5.8 |
| Regression coefficient | −48.8 | 22.0 | 0.07 | ||
| Diarrhea | |||||
| Intercept | 118.2 | 7.6 | <0.0001 | 0.71 | 9.7 |
| Regression coefficient | −67.4 | 17.5 | 0.009 | ||
| Shedding and diarrhea | |||||
| Intercept | 125.2 | 11.2 | <0.0001 | 0.67 | 16.6 |
| Regression coefficient | −91.8 | 26.1 | 0.01 | ||
4. Discussion
In this study we have demonstrated a positive dose–response relationship between C. parvum infection and fecal oocyst shedding with diarrhea. We have determined that in neonatal dairy calves the ID50 of C. parvum is 16.6 oocysts, depending on which clinical definition of infection is applied. This dose is orders of magnitude less than the number of oocysts typically shed into the environment by naturally infected calves, which has been reported to be in excess of 3 × 1010 oocysts in a calf that sheds oocysts for 6 days (Nydam et al., 2001). In our current study, calves that received the lowest doses of C. parvum oocysts began fecal oocyst shedding later, and this was associated with fewer days of diarrhea. Given that C. parvum is prevalent in dairy calves, ubiquitous in the environment, and very difficult to kill, its control poses a challenge to dairy farmers (Fayer et al., 1997, Trotz-Williams et al., 2005, Trotz-Williams et al., 2007). In the fecal–oral transmission cycle, knowing that small levels of exposure can result in a large amount of fecal oocyst shedding, and not knowing exactly how many oocysts a calf is exposed to on any given day, poses the greatest challenge for control. However, it is very likely that calf exposure greatly exceeds the ID50 we have reported. Therefore, findings from this study suggest that unless C. parvum is completely eliminated from the environment, it will be very difficult to reduce the incidence of cryptosporidiosis.
Previous studies report a mean onset of fecal oocyst shedding of 7.4 days in experimentally challenged calves, and 16.3 days in naturally infected calves (O’Handley et al., 1999, Moore et al., 2003). A study of healthy human volunteers reported an average onset of fecal oocyst shedding of 9 days (DuPont et al., 1995). We found an inverse relationship between onset of fecal oocyst shedding and dose. Calves that received smaller doses began shedding later, which was also found in a prior study (Zambriski et al., 2013). Likewise, a DuPont et al. (1995) report a relationship between onset of fecal oocyst shedding in people and size of oral inoculum, i.e., at higher doses, infection tended to occur sooner and last longer. Other studies in calves and people have reported different relationships between duration of fecal oocyst shedding and dose (DuPont et al., 1995, Moore et al., 2003). In a study by Moore et al. (2003), an association between dose and duration of fecal oocyst shedding is reported. In that study, 32 of 75 calves died, and among the 8 calves necropsied, all were found to have co-infection with Salmonella spp., rotavirus, and coronavirus, in addition to C. parvum. Therefore, it is possible that the relationship reported between dose and duration of fecal shedding was influenced by co-infection. In the study conducted in healthy human volunteers challenged with C. parvum, a non-statistically significant relationship was reported between dose and duration of oocyst excretion, but the authors do not discuss possible reasons for this association. With respect to enteric symptoms, Moore et al. (2003) report an inverse relationship between onset of diarrhea and duration of diarrhea in calves, and DuPont et al. (1995) report a relationship between dose and occurrence of enteric signs. Our study found a positive relationship between dose and diarrhea, but in the final model, onset and cessation of fecal oocyst shedding were found to be better predictors of diarrhea. This can be explained in part by the parasite life cycle and associated pathology of the gastrointestinal (GI) tract. Calves that shed sooner or longer, may be more likely to experience a longer auto-infective stage, or may experience more and recurrent villus damage over time, both of which could result in a longer time to recovery and healing of the GI tract and a longer and more severe episode of diarrhea.
Depending on the definition of infection applied, the ID50 of C. parvum in calves in our study ranged from 5.8 to 16.6 oocysts. To our knowledge, this is the first study to report this information in calves. In other species, the reported ID50 of C. parvum is also very low. In outbred neonatal CD1 mice it is 79 oocysts, and is as low at 60 oocysts in wild-type neonatal mice (Finch et al., 1993, DuPont et al., 1995). Immunosuppressed Mongolian gerbils become infected when challenged with 100 C. parvum oocysts (Baishanbo et al., 2005). In healthy human volunteers, the ID50 is reported to be 132 oocysts, but infections occurred at all dose levels, including 30 oocysts (DuPont et al., 1995). For infection with C. hominis in healthy human volunteers, the ID50 is estimated to be 10–83 oocysts (Chappell et al., 2006). Our study findings are consistent with those reported by other researchers, and again illustrate the point that the dose of C. parvum oocysts required to induce illness and fecal oocyst shedding is relatively low compared to the environmental load.
Since this study was a controlled experimental trial, our study calves were maintained in clean dry housing, received appropriate dry matter intake, were not challenged with other GI parasites, bacteria, or viruses, and may therefore be considered to be in better overall health than the general population of calves on North American dairy farms. Thus, when this research is translated to field settings, it is possible that it is an overestimation of ID50 and the dose–response relationship, and that more severe clinical outcomes could occur. Given that none of the control calves developed diarrhea or fecal oocyst shedding, it is unlikely that cross contamination occurred between challenged calves or that the challenged calves were exposed to oocysts in any quantity greater than their initial challenge dose.
This study demonstrates that calves are susceptible to C. parvum infection at very low doses, and will experience clinical illness and fecal oocyst shedding. The degree of fecal oocyst shedding associated cryptosporidiosis is dramatically disproportionate to the ID50 of C. parvum, which contributes to environmental loading and infection of subsequent calves. This study showed that calves receiving a lower dose began to shed oocysts later, and this was associated with less diarrhea. However, while these calves fared better from a clinical perspective, there was no difference in the log-total or log-peak oocysts counted/g of feces across all doses, meaning that regardless of the level of exposure, the degree of environment loading is unaffected. Therefore, the best method of controlling cryptosporidiosis is to prevent exposure entirely, however, in settings where this is not feasible, keeping exposed calves clean, dry, and nourished will minimize the impact of disease on calf health and wellbeing (Ollivett et al., 2012).
Acknowledgments
The authors thank the pre-veterinary and veterinary students for their invaluable help feeding and managing the calves throughout the course of the study.
Cornell University discretionary research funds supported this research.
Contributor Information
J.A. Zambriski, Email: jaz44@cornell.edu.
D.V. Nydam, Email: dvn2@cornell.edu.
References
- Amadi B., Mwiya M., Musuku J., Watuka A., Sianongo S., Ayoub A., Kelly P. Effect of nitazoxanide on morbidity and mortality in Zambian children with Cryptosporidiosis: a randomised controlled trial. Lancet. 2002;360(9343):1375–1380. doi: 10.1016/S0140-6736(02)11401-2. [DOI] [PubMed] [Google Scholar]
- Anderson B.C. Cryptosporidiosis in bovine and human health. J. Dairy Sci. 1998;81(11):3036–3041. doi: 10.3168/jds.S0022-0302(98)75868-0. [DOI] [PubMed] [Google Scholar]
- Anguish L.J., Ghiorse W.C. Computer-assisted laser scanning and video microscopy for analysis of Cryptosporidium parvum oocysts in soil, sediment, and feces. Appl. Environ. Microbiol. 1997;63(2):724–733. doi: 10.1128/aem.63.2.724-733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baishanbo A., Gargala G., Delaunay A., Francois A., Ballet J.J., Favennec L. Infectivity of Cryptosporidium hominis and Cryptosporidium parvum genotype 2 isolates in immunosuppressed Mongolian gerbils. Infect. Immun. 2005;73(8):5252–5255. doi: 10.1128/IAI.73.8.5252-5255.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellosa M.L., Nydam D.V., Liotta J.L., Zambriski J.A., Linden T.C., Bowman D.D. A comparison of fecal percent dry matter and number of Cryptosporidium parvum oocysts shed to observational fecal consistency scoring in dairy calves. J. Parasitol. 2011;97(2):349–351. doi: 10.1645/GE-2475.1. [DOI] [PubMed] [Google Scholar]
- Campbell A.T., Robertson L.J., Smith H.V. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl. Environ. Microbiol. 1992;58(11):3488–3493. doi: 10.1128/aem.58.11.3488-3493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell C.L., Okhuysen P.C., Langer-Curry R., Widmer G., Akiyoshi D.E., Tanriverdi S., Tzipori S. Cryptosporidium hominis: experimental challenge of healthy adults. Am. J. Trop. Med. Hyg. 2006;75(5):851–857. [PubMed] [Google Scholar]
- DuPont H.L., Chappell C.L., Sterling C.R., Okhuysen P.C., Rose J.B., Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 1995;332(13):855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- Ernest J.A., Blagburn B.L., Lindsay D.S., Current W.L. Infection dynamics of Cryptosporidium parvum (Apicomplexa: Cryptosporiidae) in neonatal mice (Mus musculus) J. Parasitol. 1986;72(5):796–798. [PubMed] [Google Scholar]
- Fayer R. Cryptosporidium: a water-borne zoonotic parasite. Vet. Parasitol. 2004;126(1–2):37–56. doi: 10.1016/j.vetpar.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Fayer R., Speer C.A., Dubey J.P. The General Biology of Cryptosporidium. In: Fayer R., editor. Cryptosporidium and Cryptosporidiosis. CRC Press; Boca Raton, FL: 1997. pp. 1–47. [Google Scholar]
- Finch G.R., Daniels C.W., Black E.K., Schaefer F.W., 3rd, Belosevic M. Dose response of Cryptosporidium parvum in outbred neonatal CD-1 mice. Appl. Environ. Microbiol. 1993;59(11):3661–3665. doi: 10.1128/aem.59.11.3661-3665.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M.B., Anguish L.J., Bowman D.D., Walker M.J., Ghiorse W.C. Assessment of a dye permeability assay for determination of inactivation rates of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 1997;63(10):3844–3850. doi: 10.1128/aem.63.10.3844-3850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M.B., Walker M.J., Bowman D.D., Anthony L.C., Ghiorse W.C. Use of a sentinel system for field measurements of Cryptosporidium parvum oocyst inactivation in soil and animal waste. Appl. Environ. Microbiol. 1999;65(5):1998–2005. doi: 10.1128/aem.65.5.1998-2005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Alderisio K.A., Xiao L. Distribution of Cryptosporidium genotypes in storm event water samples from three watersheds in New York. Appl. Environ. Microbiol. 2005;71(8):4446–4454. doi: 10.1128/AEM.71.8.4446-4454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958;53:457–481. [Google Scholar]
- Lefay D., Naciri M., Poirier P., Chermette R. Efficacy of halofuginone lactate in the prevention of cryptosporidiosis in suckling calves. Vet. Rec. 2001;148(4):108–112. doi: 10.1136/vr.148.4.108. [DOI] [PubMed] [Google Scholar]
- MacKenzie W.R., Schell W.L., Blair K.A., Addiss D.G., Peterson D.E., Hoxie N.J., Kazmierczak J.J., Davis J.P. Massive outbreak of waterborne Cryptosporidium infection in Milwaukee, Wisconsin: recurrence of illness and risk of secondary transmission. Clin. Infect. Dis. 1995;21(1):57–62. doi: 10.1093/clinids/21.1.57. [DOI] [PubMed] [Google Scholar]
- Moore D.A., Atwill E.R., Kirk J.H., Brahmbhatt D., Herrera Alonso L., Hou L., Singer M.D., Miller T.D. Prophylactic use of decoquinate for infections with Cryptosporidium parvum in experimentally challenged neonatal calve. J. Am. Vet. Med. Assoc. 2003;223(6):839–845. doi: 10.2460/javma.2003.223.839. [DOI] [PubMed] [Google Scholar]
- Ng J.S., Eastwood K., Walker B., Durrheim D.N., Massey P.D., Porigneaux P., Kemp R., McKinnon B., Laurie K., Miller D., Bramley E., Ryan U. Evidence of Cryptosporidium transmission between cattle and humans in northern New South Wales. Exp. Parasitol. 2012;130(4):437–441. doi: 10.1016/j.exppara.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Nydam D.V., Wade S.E., Schaaf S.L., Mohammed H.O. Number of Cryptosporidium parvum oocysts or Giardia spp. cysts shed by dairy calves after natural infection. Am. J. Vet. Res. 2001;62(10):1612–1615. doi: 10.2460/ajvr.2001.62.1612. [DOI] [PubMed] [Google Scholar]
- O’Handley R.M., Cockwill C., McAllister T.A., Jelinski M., Morck D.W., Olson M.E. Duration of naturally acquired giardiosis and cryptosporidiosis in dairy calves and their association with diarrhea. J. Am. Vet. Med. Assoc. 1999;214(3):391–396. [PubMed] [Google Scholar]
- Ollivett T.L., Nydam D.V., Bowman D.D., Zambriski J.A., Bellosa M.L., Linden T.C., Divers T.J. Effect of nitazoxanide on cryptosporidiosis in experimentally infected neonatal dairy calves. J. Dairy Sci. 2009;92(4):1643–1648. doi: 10.3168/jds.2008-1474. [DOI] [PubMed] [Google Scholar]
- Ollivett T.L., Nydam D.V., Linden T.C., Bowman D.D., Van Amburgh M.E. Effect of nutritional plane on health and performance in dairy calves after experimental infection with Cryptosporidium parvum. J. Am. Vet. Med. Assoc. 2012;241(11):1514–1520. doi: 10.2460/javma.241.11.1514. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc., 1989–2007. JMP. 7.0.
- Thompson R.C., Palmer C.S., O’Handley R. The public health and clinical significance of Giardia and Cryptosporidium in domestic animals. Vet. J. 2008;177(1):18–25. doi: 10.1016/j.tvjl.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotz-Williams L.A., Jarvie B.D., Martin S.W., Leslie K.E., Peregrine A.S. Prevalence of Cryptosporidium parvum infection in southwestern Ontario and its association with diarrhea in neonatal dairy calves. Can. Vet. J. 2005;46(4):349–351. [PMC free article] [PubMed] [Google Scholar]
- Trotz-Williams L.A., Martin S.W., Leslie K.E., Duffield T., Nydam D.V., Peregrine A.S. Association between management practices and within-herd prevalence of Cryptosporidium parvum shedding on dairy farms in southern Ontario. Prev. Vet. Med. 2008;83(1):11–23. doi: 10.1016/j.prevetmed.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotz-Williams L.A., Wayne Martin S., Leslie K.E., Duffield T., Nydam D.V., Peregrine A.S. Calf-level risk factors for neonatal diarrhea and shedding of Cryptosporidium parvum in Ontario dairy calves. Prev. Vet. Med. 2007;82(1–2):12–28. doi: 10.1016/j.prevetmed.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Widmer G. A hundred-year retrospective on cryptosporidiosis. Trends Parasitol. 2008;24(4):184–189. doi: 10.1016/j.pt.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valderrama A.L., Hlavsa M.C., Cronquist A., Cosgrove S., Johnston S.P., Roberts J.M., Stock M.L., Xiao L., Xavier K., Beach M.J. Multiple risk factors associated with a large statewide increase in cryptosporidiosis. Epidemiol. Infect. 2009;137(12):1781–1788. doi: 10.1017/S0950268809002842. [DOI] [PubMed] [Google Scholar]
- Xiao L., Herd R.P. Quantitation of Giardia cysts and Cryptosporidium oocysts in fecal samples by direct immunofluorescence assay. J. Clin. Microbiol. 1993;31(11):2944–2946. doi: 10.1128/jcm.31.11.2944-2946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambriski J.A., Nydam D.V., Bowman D.D., Bellosa M.L., Burton A.J., Linden T.C., Liotta J.L., Ollivett T.L., Tondello-Martins L., Mohammed H.O. Description of fecal shedding of Cryptosporidium parvum oocysts in experimentally challenged dairy calves. Parasitol. Res. 2013;112(3):1247–1254. doi: 10.1007/s00436-012-3258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


