Abstract
Halofuginone lactate (HL) is registered in several countries for the prevention of calf cryptosporidiosis, but the compound's utility in the presence of co-infection with other enteropathogens is not well understood. We performed a randomized controlled field trial of the efficacy of HL for the prevention of natural calf cryptosporidiosis, in the presence of co-infection with rotavirus and Salmonella Typhimurium. Newborn calves on one farm were sequentially enrolled and allocated to a full dose (n = 15), half dose (n = 15), or a placebo control group (n = 15), using a randomized block design. The Cryptosporidium oocysts in fecal specimens collected on Days 6, 8, 10, 14 and 20 were counted and the severity of the diarrhea was assessed using fecal consistency scores (solid, semisolid, or liquid). The oocyst numbers and fecal consistency scores were statistically compared between the groups. Ninety one percent of the calves shed Cryptosporidium parvum oocysts during the trial. The full dose group had a longer prepatent period than the control group, but no statistical difference in the number of oocysts was identified between the groups after controlling for the effects of sex and breed. The fecal consistency scores and mortality rates did not differ between the groups. These results indicated that the anti-Cryptosporidium activity and clinical benefit of HL were limited. It is concluded that in order to maximize the clinical efficacy of HL in the field, diagnostic efforts should aim to rule out the presence of other enteropathogens.
Keywords: Cryptosporidium, Halofuginone, Randomized clinical trial, Calf diarrhea
1. Introduction
The cosmopolitan protozoan parasite Cryptosporidium parvum infects humans and a range of other hosts, and is a common agent of calf diarrhea during the first four weeks of life (Gulliksen et al., 2009, Lanz Uhde et al., 2008, Millemann, 2009, Trotz-Williams et al., 2005). The spectrum of severity of cryptosporidiosis in calves varies from subclinical infection to severe diarrhea and dehydration (Fayer et al., 1998, Klein et al., 2008, O’Handley et al., 1999). Typically, natural and experimental infections with C. parvum in calves have a pre-patent period of 3–6 days, followed by a patent period characterized by a bell-shaped oocyst excretion curve, with the number of fecal oocysts peaking and then rapidly decreasing to undetectable levels in a matter of 7–14 days (Fayer et al., 1998, Grinberg et al., 2002). Oocyst numbers as high as 107 oocysts per gram of feces have been reported at the peak of excretion (Fayer et al., 1998, Grinberg et al., 2002). Whereas infection rates as high as 100% have been reported in calves in the first month of life, a lower infection prevalence has been consistently observed in weaned animals (Bartels et al., 2010, Brook et al., 2008, Fayer and Xiao, 2008, Fayer et al., 2000, Garber et al., 1994). Newborn calves are therefore considered major amplifiers of potentially zoonotic C. parvum in nature, and the prevention of calf cryptosporidiosis is relevant from both animal and human health perspectives (Hunter and Thompson, 2005, Kiang et al., 2006, Smith et al., 2007, Grinberg et al., 2008, Xiao and Feng, 2008, Zhou et al., 2008).
Several features of the C. parvum life cycle make the control of cryptosporidiosis on-farms extremely difficult to be achieved by means of hygienic measures alone. Calves can excrete hundreds of millions of oocysts (Naciri et al., 1999, Grinberg et al., 2002), and considering there is a high probability of infection with a dose as low as 50 oocysts (Moore et al., 2003), an infected calf could produce enough oocysts to infect thousands of new animals. Attempts to interrupt the transmission of C. parvum should therefore include the immediate isolation of every infected animal, but this is rarely feasible on commercial farms. In addition, the C. parvum oocysts are insensitive to the action of numerous disinfectants (Chen et al., 2002, Quilez et al., 2005) and are excreted sporulated and fully infectious (Smith et al., 2005), requiring daily cleaning for their removal. Therefore, in the absence of effective immunizing agents, pharmacological control strategies remain central for the prevention of cryptosporidiosis in calves.
A number of compounds, such as halofuginone lactate (HL) (Lefay et al., 2001, Jarvie et al., 2005, Klein, 2008, De Waele et al., 2010, Trotz-Williams et al., 2011), paromomycin sulphate (Fayer and Ellis, 1993, Grinberg et al., 2002), nitazoxanide (Ollivett et al., 2009, Schnyder et al., 2009) and decoquinate (Moore et al., 2003, Lallemond et al., 2006) have been tested for the prevention of cryptosporidiosis in calves, with variable results. HL is a synthetic derivative of a quinazolinone alkaloid with cryptosporidiostatic activity, but a mode of action that is poorly characterized. In naturally and experimentally infected calves, the oral administration of 60 μg/kg HL for seven consecutive days from the first day of life delays the onset of oocyst shedding, reduces the number of oocysts excreted, and lowers the severity of diarrhea (Jarvie et al., 2005, Joachim et al., 2003, Klein, 2008, Lefay et al., 2001, Trotz-Williams et al., 2011, Villacorta et al., 1991). A formulation containing HL (Halocur, Intervet Ltd., Republic of Ireland) is currently the only prescription drug registered for the prevention of cryptosporidiosis of calves in several countries. The use of HL has a number of limitations, including a substantial market price and a narrow therapeutic index, with toxicity observable at approximately twice the recommended dose (Villacorta et al., 1991, Naciri et al., 1993, Trotz-Williams et al., 2005; http://www.msd-animal-health.co.uk/products_public/halocur/090_product_datasheet.aspx, accessed 15 July 2012).
Remarkably, notwithstanding the frequent occurrence of co-infections with Cryptosporidium and other enteropathogens in the field (De la Fuente et al., 1999, Naciri et al., 1999, Tzipori et al., 1980), the utility of HL in the presence of such co-infections is not well understood. Indeed, with some exceptions (Klein, 2008, Lefay et al., 2001), most anti-Cryptosporidium efficacy studies of HL did not analyze or took into account the presence of co-infections. Co-infections might modify the anti-Cryptosporidium effect of HL in different ways. The increased fluid content and intestinal motility determined by the presence of co-infecting pathogens may reduce the activity of HL by dilution, or by reducing the transit time of the drug in the intestinal tract. Furthermore, enteric infection with rotavirus or other agents may cause exfoliation of infected cells, altering intestinal cellular function (Ramig, 2004), potentially increasing the toxicity of HL via systemic absorption.
Motivated by the need of more data on the utility HL in the presence of co-infections with other pathogens, we performed a randomized controlled field trial of the anti-Cryptosporidium preventive efficacy of the compound in calves on a New Zealand farm enzootically infected with C. parvum, bovine rotavirus and Salmonella Typhimurium.
2. Materials and methods
2.1. The farm
The study was performed between July and October 2010, on a farm situated in the Taranaki District, New Zealand. Consent for this study was obtained from the farmer and the use of animals was approved by the Animal Ethics Committee, Massey University. The farm managed a seasonal (spring) calving dairy herd of approximately 400 milking cows and a smaller herd of beef cattle. It was recruited following a post-mortem investigation performed in March 2010 on two calves conducted by one of the authors (AG), which indicated the presence of Cryptosporidium oocysts in the feces of two calves. Salmonella was ruled out by culture and rotavirus and coronavirus by antigen ELISA. Sequence analysis of the Cryptosporidium 18S rRNA gene subsequently confirmed C. parvum in both animals. Notwithstanding the microbiological results performed in March, bovine rotavirus and Salmonella spp. were subsequently identified in multiple enrolled calves during the study (see below).
2.2. Study design and sample size calculation
The study was a randomized controlled field trial (see randomization procedure below). A commercial product (Halocur, Intervet Ltd., Republic of Ireland) registered in New Zealand for the prevention and treatment of cryptosporidiosis of calves was used to test the efficacy of HL. The product's recommended preventive dose is 4 mg (8 ml) for calves weighing 35–45 kg, and 6 mg (12 ml) for calves weighing 45–60 kg, for seven consecutive days, from birth. The initial aim was to assess the preventive efficacy of half the recommended dose of HL as compared with the full dose and no treatment, and the following treatment groups were established: Group 1 (full dose regime; n = 15), calves treated orally with 8 ml Halocur; Group 2 (half dose regime; n = 15), calves treated with 4 ml Halocur; Group 3 (placebo control group; n = 15), calves treated with 4 ml water delivered using the product's dispenser. The group sizes were estimated using power analysis for the detection of a difference between means, using PASS software (NCSS, Kaysville, UT). Assumptions for the calculations were a log10-transformed mean number of oocysts per gram of feces of 4.3 for the full dose and 6.2 for the untreated-control group at the peak of shedding, and a common standard deviation of 1.2. These means were estimated from a previously published study of efficacy of paromomycin sulphate (Grinberg et al., 2002). Group sizes of 8 calves per group achieved 84% power to reject the null hypothesis that both group means are 6.2. Thus, assuming a conservative infection rate of ∼50%, 15 calves per group were required.
2.3. Animal husbandry
Management of the enrolled calves followed the same routine procedures used on the farm. Briefly, newborn calves were left on the calving paddock for 10–24 h after birth, as commonly done in New Zealand pasture-based farms to allow for colostrum intake directly from the dam. Then the newborns were transferred to a large shed and allocated to a pen for newborn calves, of a capacity of about 10 calves. Calves were left in this pen for 2–3 days and then transferred to a new pen, in order to create space for new calves in the first pen. Subsequently, the calves progressed to new pens (often adjacent) containing 5–10 animals of the same age group every 2–3 days, until weaning. The allocation of calves to pens was done by caretakers who were not aware of the nature of the treatments given to each calf, effectively creating a commingled pen design. Adjacent pens were separated by slatted wooden fences. Sawdust was used as bedding material. The feeding regimen included the administration of complete commercial milk replacement for the first two weeks using a tank with multiple nipples, followed by feeding ad libitum using automatic feeders until weaning. Roughage was available from the second week of life. No routine vaccinations, preventive treatments or supplementations were administered to the calves during the study. However, inappetent calves could be tube-fed and severely diarrheic calves treated with oral electrolytes and/or other treatments by the caretakers. Severely sick calves were transferred to an isolation shed. The study protocol stipulated that the tube fed calves could continue in the study, but animals treated by the farmer other means or transferred to the isolation pen, were removed.
2.4. Field workflow, randomization and sampling
Newborn calves were sequentially enrolled in the mornings, at arrival from the calving area to the rearing shed. Any calf born on the farm was eligible for enrolment, provided no congenital or pathological conditions were identified. At the day of enrolment (Day 0), calves were identified by the ear or neck tag number, given a sequential number and allocated to one of the three treatment groups. Allocation of calves to treatment groups was done using a randomized block design, with blocks of three (one calf per group), using a random list prepared in advance. The fourth calf presented to the investigator after each block of three was systematically left unenrolled and continued its normal life cycle on the farm. Treatments were administered in the morning using the commercial product (see above). Fecal specimens were collected at the time of treatment from the rectum of each calf using disposable gloves, on Days 6, 8, 10, 14 and 20. At the time of collection, each specimen was scored according to its consistency as 1, solid (specimen conserved its original shape); 2, semi-solid (specimen spread across the bottom of the container but was not liquid); or 3, liquid specimen. Specimens were transported on ice to Massey University and kept in refrigeration and analyzed between December 2010 and April 2011, as described below.
2.5. Laboratory analysis for Cryptosporidium
The fecal specimens were analyzed by a quantitative method that estimated the number of Cryptosporidium oocysts present. Briefly, after mixing the specimen with a spatula, 1 g of feces was suspended in 10 ml tap water and strained through a tea sieve. The filtrate was centrifuged at 900 × g for 10 min and the sediment re-suspended in 4 ml of normal saline. A 10 μl aliquot of this suspension was deposited as a drop on a slide using a micropipette, air-dried and fixed in methanol. Fixed drops were stained using a commercial immunofluoresecent anti-Giardia and Cryptosporidium monoclonal antibody (Aqua-Glo G/C Direct Comprehensive Kit, Waterborne Inc., New Orleans, USA), according to the manufacturer's instructions. The apple-green fluorescent oocysts present on the entire area of the stained drop were counted using a fluorescent microscope using an excitation wavelength of 490 nm and a 200× magnification lens. This total number of oocysts (TON) on the slide was used for statistical analysis (see below). Samples containing >1000 oocysts were difficult to count and were re-processed by a further dilution of the fecal suspension at 10−1 in water, followed by the staining of a 10 μl drop as above. The TON present on each re-diluted specimen was estimated by multiplying the result by 10. When the available fecal material was insufficient for counting, a direct fecal smear was stained and a qualitative result (presence/absence of oocysts) was obtained. These qualitative results could not be used for the statistical comparison of the number of oocysts between the treatment groups, but were used for any comparison between proportions of Cryptosporidium-positive and negative specimens.
In March 2012, DNA from three Cryptosporidium-positive specimens from each treatment group was extracted from the stored feces using a DNA extraction kit (QIAamp, DNA Stool Mini Kit, Qiagen, Hilden, GmbH), and Cryptosporidium parasites were identified by means of PCR-sequencing of the 18S rRNA gene. Primers were 5-GTT AAA CTG CGA ATG GCT CA-3 (forward) and 5-CCA TTT CCT TCG AAA CAG GA-3 (reverse) (Learmonth et al., 2004). Amplification was performed in 20 μl containing 2 μl 10 x PCR buffer, 1 μl dNTP (2 mM), 1 μl MgCl2 (50 mM), 2 μl non-acetylated bovine serum albumin (2 mg/ml) (New England Biolabs, USA), 4 picomoles of each primer, and 0.5 μl of Platinum® Taq DNA Polymerase (2 mg/mL) (Invitrogen Corporation, Carlsbad CA, USA). The PCR amplification was carried out in a thermocycler (SensoQuest, Goettingen, Germany) with initial denaturation at 96 °C for 2 min, followed by 40 cycles at 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s. PCR products were purified using an in–house ethanol purification protocol, and bidirectional sequencing of an internal segment of the amplicon was performed using primers 5-CTCGACTTTATGGAAGGGTTG-3 (forward) and 5-CCT CCAATCTCTAGTTG GCATA-3 (reverse). Forward and reverse sequences were aligned and edited manually using Geneious software version 5.6.5 (Biomatters Ltd., http://www.geneious. com/). Distal and proximal segments that could not be verified were trimmed and the resulting edited sequences aligned with sequences deposited in Genbank using the alignment algorithm BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on April 2012).
In addition to the analysis for Cryptosporidium, 23 fecal specimens taken haphazardly from the three treatment groups were analyzed for the presence of rotavirus, coronavirus, enterotoxigenic K99+ Escherichia coli (K99) and Salmonella spp. The analysis for rotavirus, coronavirus and K99-positive E. coli was performed by a commercial diagnostic laboratory using antigen-ELISA (Institut Pourquier, Montpellier, France). The analysis for Salmonella included parallel inoculation of fecal material into tetrathionate and Rappaport-Vassiliadis soy peptone (RVS) broths, and incubation for 24 h at 37 °C and 42 °C, respectively. This was followed by subculture onto xylose lactose deoxycholate agar plates incubated as above. Colonies consistent with Salmonella were sub-cultured into triple-sugar iron agar slopes (TSI) and l-lysine decarboxylase broth. Lysine-positive bacteria exhibiting TSI patterns consistent with Salmonella were subjected to Salmonella poly-O slide agglutination using a commercial antiserum (Institut für Immunpräparate und Nährmedien GmbH Berlin, Berlin, Germany), and agglutinating isolates were sent to the Salmonella Reference Laboratory (Institute of Environmental Science and Research, Porirua, New Zealand) for serotyping.
2.6. Statistical analysis of data
The parasitological and clinical effects of the three treatments were statistically compared. The parasitological effects were analyzed by comparing the TONs between the groups using an analysis of variance for repeated measurements (rmANOVA) implemented by the PROC MIXED procedure of SAS (Statistical Analysis System, 2001, SAS Institute, Cary, NC, USA). TONs were log-transformed as log10 (TON + 1) and analyzed using mixed models. The first model (Model 1) included the fixed effects of treatment (variable ‘treatment Group’), the day of sampling as repeated factor (variable ‘sampling Day’), and the interaction of treatment Group and sampling Day. Model 2 included the fixed effects as in Model 1 plus the fixed effect of sex of the calves, and Model 3 included the same factors as in Model 2 plus the effect of breed of the calves as a co-variable (variable ‘Breed’; Friesian calf = 1; non-Friesian calf = 0). Fixed effects for variable Breed were not included as a class due to unbalanced designs deriving from the uneven distribution of breeds across treatments. Finally, Model 4 was similar to Model 2, but did not include the data obtained from non-Freisian calves, effectively removing any variability due to the breed. All the models included the random effect of calf, to account for the within-calf variability. Using the Akaike's information criterion, an unstructured error structure was determined as the most appropriate residual covariance structure for repeated measures over time, within animals. Finally, the parasitological efficacy was also assessed by comparing the decline of the proportion of parasitologically negative calves as a function of time between the three groups using the non-parametric Kaplan–Meier method (KM) (Kaplan and Meier, 1958). Also in this case, the analysis was repeated after omitting all the data from the non-Friesian calves. The KM analysis was implemented using a code available in “R” (Terry Therneau, 2012; A Package for Survival Analysis in R package version 2.36-14).
The clinical effects of the treatments were assessed by comparing the fecal consistency scores (considered ordinal data) between the groups on each sampling day, using the non-parametric Kruskal–Wallis test. These tests were performed using the codes available in “R” (Kruskal–Wallis: http://stat.ethz.ch/R-manual/R-patched/library/stats/html/kruskal.test.html; Wilcoxon: http://stat. ethz.ch/R-manual/R-patched/library/stats/html/wilcoxon.test.html). Comparisons between proportions of interest were performed using two-tailed Fisher's exact tests, which were interpreted using Bonferroni-adjusted critical P-values for multiple testing. Finally, the existence of association between the fecal consistency and the number of oocysts shed was tested by means of logistic regression, using the log10 (TON + 1) as independent variable and the presence/absence of a liquid specimen (fecal score 3) as outcome variable. This analysis was performed using a code available in “R” (http://www.ats.ucla.edu/stat/r/dae/logit.htm; R package version 2.36–14).
3. Results
3.1. Animals’ characteristics and adherence to the protocol
All the calves were enrolled within 24 h from birth, over a 26-day period, between 16 July and 06 August 2010. A total of 24/45 (53.3%) calves were females and there were no statistical differences between the proportion of males and females among the treatment groups. There were 27 (60%) Friesian, 11 (25%) Angus, 6 (11%) Hereford calves and one (2%) Jersey × Angus crossbred calf (Table 1 ). Six calves haphazardly selected at enrolment and weighted using electronic scales had bodyweights between 35 and 40 kg (mean = 36.5, standard deviation = 1), indicating under/over-dosing of calves was unlikely to have occurred. One calf that died on the same day of enrolment (calf number 1; Table 1) was substituted by the subsequent calf presented to the investigator (calf number 46; Table 1). All the enrolled calves remained in their pens during the study and no calf was withdrawn due to concomitant treatment. Thirty eight calves (84.5%) were followed up for the entire observation period, and seven (15.5%) died at different stages during the study. The causes of death were defined by the farmer as following: two calves from Group 1 died on Days 6 and 7, two from Group 3 on Days 7 and 9, and one from Group 2 on Day 13, from severe diarrhea. Two calves (Group 1 and Group 3) died from improper tube-feeding (possibly, milk inhalation) on Day 7 and Day 13. There were 3 deaths in Group 1, three in Group 3 and one in Group 2, and these rates were not statistically different (Table 1). Only 6/225 (2.6%) fecal specimens could not be retrieved and were not analyzed (Table 1).
Table 1.
Raw data from 46 calves enrolled in this study. F, female; M, male; D, dead calf – specimen not available; +I, sample positive for Cryptosporidium oocysts but fecal material insufficient for counting; MS, missing specimen (not analyzed).
| Enrolment sequence | Treatment group | Gender/breed | Oocyst count (fecal consistency score) |
||||
|---|---|---|---|---|---|---|---|
| Day 6 | Day 8 | Day 10 | Day 14 | Day 20 | |||
| 1 | 1 | F/Friesian | D | D | D | D | D |
| 2 | 2 | F/Friesian | 1(2) | 22260(1) | 41940(2) | 12850(3) | 0(1) |
| 3 | 3 | F/Hereford | 0(3) | 585(3) | 44850(1) | 256(1) | 0(1) |
| 4 | 1 | M/Friesian | 0(1) | 0(1) | 7(1) | 146(1) | 0(1) |
| 5 | 3 | M/Friesian | 0(1) | 652(1) | 0(1) | 635(1) | 0(1) |
| 6 | 2 | M/Friesian | 0(2) | +I(1) | 7260(2) | 6870(2) | 0(1) |
| 7 | 2 | M/Angus | 0(1) | 0(1) | 0(1) | 0(1) | 0(1) |
| 8 | 3 | F/Hereford | 0(1) | 452(1) | D | D | D |
| 9 | 1 | F/Crossbreed | 0(2) | 0(2) | 0(2) | 856(1) | 156(1) |
| 10 | 2 | M/Friesian | +I(1) | 0(1) | 0(1) | 573(2) | 70(1) |
| 11 | 1 | M/Friesian | 0(3) | +I(1) | 0(1) | 224(1) | 9(1) |
| 12 | 3 | F/Friesian | 0(1) | 487(3) | 9660(3) | 459(2) | 0(1) |
| 13 | 3 | M/Friesian | MS(1) | 610(2) | 998(1) | 29610(2) | 154(2) |
| 14 | 1 | M/Angus | 0(1) | 0(2) | 0(1) | 0(1) | 986(2) |
| 15 | 2 | M/Friesian | 0(1) | 19(2) | 55(1) | 488(3) | 0(1) |
| 16 | 3 | F/Angus | 0(1) | 0(1) | 16000(1) | +I(1) | 0(1) |
| 17 | 2 | M/Friesian | 0(1) | 0(1) | 0(2) | 0(1) | 0(1) |
| 18 | 1 | F/Friesian | MS(1) | 1(1) | 0(1) | 752(1) | 4550(1) |
| 19 | 1 | M/Angus | 0(1) | 0(1) | 0(2) | 365(1) | 9050(1) |
| 20 | 2 | M/Angus | 0(1) | 0(1) | 12180(3) | D | D |
| 21 | 3 | F/Friesian | 0(3) | 478(3) | 12850(2) | 365(1) | +I(1) |
| 22 | 1 | M/Angus | 0(3) | 0(1) | 0(2) | 9450(1) | 879(2) |
| 23 | 3 | F/Friesian | 0(1) | 399(1) | 41510(1) | 24010(3) | 0(1) |
| 24 | 2 | M/Hereford | +I(1) | 935(1) | 44450(1) | 37000(1) | 0(1) |
| 25 | 2 | F/Angus | 0(3) | 0(2) | 38950(1) | 11070(3) | 0(1) |
| 26 | 3 | M/Friesian | 0(1) | 303(3) | 986(3) | D | D |
| 27 | 1 | F/Friesian | 0(3) | 0(2) | 368(3) | 8460(3) | 0(3) |
| 28 | 2 | F/Angus | 0(1) | 0(1) | 0(1) | 7(3) | 0(1) |
| 29 | 1 | F/Hereford | +I | D | D | D | D |
| 30 | 3 | M/Friesian | MS | D | D | D | D |
| 31 | 3 | F/Friesian | 0(3) | 569(2) | 997(3) | 478(1) | 0(1) |
| 32 | 1 | M/Angus | 0(1) | 0(1) | 0(1) | 154(2) | 2(1) |
| 33 | 2 | M/Hereford | 0(3) | 0(2) | 7670(2) | 15820(2) | 0(2) |
| 34 | 3 | F/Friesian | 0(1) | 390(1) | 33560(1) | 11920(1) | 0(1) |
| 35 | 2 | M/Angus | 0(3) | 0(2) | 8680(1) | +I(1) | 0(1) |
| 36 | 1 | F/Friesian | 0(3) | D | D | D | D |
| 37 | 1 | F/Friesian | 0(1) | 125(3) | 568(3) | +I(3) | 0(1) |
| 38 | 2 | F/Friesian | 0(3) | 0(2) | 234(1) | 18650(2) | 0(2) |
| 39 | 3 | F/Friesian | 0(1) | 253(1) | 10010(1) | 478(1) | 0(1) |
| 40 | 1 | F/Friesian | +I(1) | D | D | D | D |
| 41 | 3 | F/Friesian | 510(1) | 7(3) | MS(3) | 0(2) | 0(2) |
| 42 | 2 | F/Friesian | 26(1) | 5810(3) | +I(2) | 11330(1) | 0(1) |
| 43 | 2 | M/Friesian | MS(1) | 6080(1) | +I(1) | I(1) | 1(1) |
| 44 | 3 | M/Friesian | 245(3) | 5(3) | 989(2) | 314(1) | 0(1) |
| 45 | 1 | F/Angus | 0(3) | 5(2) | +I(3) | MS(3) | 0(2) |
| 46 | 1 | F/Hereford | 0(1) | 0(2) | 115(1) | 97(1) | 0(2) |
3.2. Laboratory results
A total of 41/45 (91%) calves were Cryptosporidium-positive by immunofluorescence at some stage during the study. Two calves belonging to Group 2, that were able to be followed up for the entire observation period, remained parasitologically negative throughout the study (Table 1). Sequence analysis of the 18SrRNA gene of the 9 fecal specimens analyzed by PCR-sequencing indicated the presence of C. parvum in all cases. Out of 23 specimens analyzed for other enteropathogens, 21 (91%) were positive for rotavirus and 3 (13%) for Salmonella Typhimurium. As expected for calves of this age, no E. coli K99-positive specimens were identified. Although analysis for Giardia spp. was not the subject of this study (as the parasite it is not widely considered pathogenic for calves), we note for completeness that Giardia cysts were observed in 20 calves by immunofluorescence.
3.3. Statistical results
3.3.1. Parasitological efficacy
The calves in Group 3 (control Group) showed an oocyst shedding curve which was typical for natural cryptosporidiosis, with the majority of the animals becoming parasitologically positive by Day 8 and again negative by Day 20 (Table 1, Table 2 ). Group 3 started shedding earlier than the other two groups. In this group, the highest mean log10 (TON + 1) was observed on Day 10, and on Day 20 oocysts were observed only in two calves (Table 1). In contrast, in Group 1 the peak mean log10 (TON + 1) was lower and occurred later (Day 14) than in Group 3, and on Day 20, the calves in this group were shedding more oocysts than the other two groups. However, when only Friesian calves were considered, Groups 1 and 3 showed a very similar oocyst shedding curve, which peaked in both groups on Day 10. Comparisons between the crude mean log10 (TON + 1) of the three treatment groups at the various sampling days are reported in Fig. 1 .
Table 2.
The number of Cryptosporidium-negative/positive fecal specimens stratified by the treatment Groups and sampling Days. On the same Day, similar superscripts indicate comparisons between proportions resulting in two-tailed Fisher's exact test P < 0.01 (less than Bonferroni-adjusted critical probability).
| All calves |
Friesian calves |
|||||
|---|---|---|---|---|---|---|
| Sampling day | Group 1 (full dose) | Group 2 (half dose) | Group 3 (placebo-control) | Group 1 (full dose) | Group 2 (half dose) | Group 3 (placebo-control) |
| Day 6 | 13/0 | 13/2 | 12/2 | 7/0 | 6/2 | 10/2 |
| Day 8 | 9/3* | 10/5+ | 1/13*+ | 5/2 | 4/4* | 0/12* |
| Day 10 | 7/5* | 4/11 | 1/11* | 4/3 | 2/6 | 3/9 |
| Day 14 | 1/11 | 2/12 | 1/11 | 2/5 | 1/7 | 3/9 |
| Day 20 | 5/7* | 12/2* | 10/2 | 5/2 | 6/2 | 10/2 |
Fig. 1.
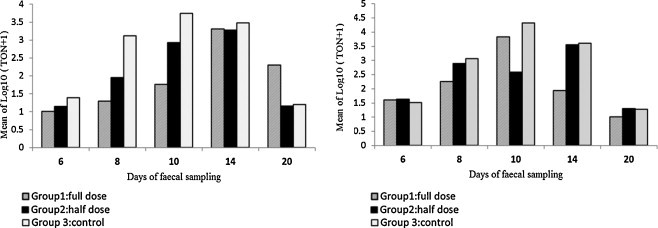
Crude mean log10 of the total number of oocysts +1 (TON+1) present on slides, stratified by treatment groups and sampling days in calves of all breeds (left graph) and Friesian calves only (right graph).
Model 1 (which included the effects of treatment Group, sampling Day and their interaction term), indicated a significant difference between the three treatments (P = 0.04). Post hoc comparisons identified a significant difference between Group 1 (full dose) and Group 3 (control) (Group 1 < log10(TON + 1) than Group 3; P = 0.01), and no significant difference between Group 2 and the other two groups. In Model 2, which included also the fixed effect of sex, the significance between the treatment Groups was preserved (P = 0.02), but there was no significant effect of sex on the outcome (P = 0.17). When variable ‘Breed’ was introduced as a covariate in Model 3, the P-value of the effect of treatment increased to P = 0.098, and the covariable ‘Breed’ was also significant (Friesian < log10 (TON + 1) than non-Friesian; P = 0.02). This increase in the P-value was supported by Model 4 (Friesian only), which showed P = 0.73 for the effect of treatment. All these models produced similar P-values when the interaction of treatment × day was removed (not shown). In order to cross-validate these results we compared the mean log10 (TON + 1) between the treatment groups using bivariate ANOVA for each sampling day separately (not shown), with consistent results: whereas the inclusion of all the animals resulted in statistically significant difference between treatment Groups 1 and 3 on Days 8, 10 and 20 (P < 0.05), all the P-values were > 0.05 when only Friesian calves were analyzed.
The results of the Kaplan–Meier test indicated a significantly longer prepatent period in Group 1 as compared with Group 3. In fact, 13 calves (93%) in Group 3 were parasitologically positive on Day 8, whereas only three calves from Group 1 (25%) were shedding oocysts on the same Day (Table 1, Table 2). The duration of the prepatent period in Group 2 was intermediate, although not statistically different from the other groups. Similar results were observed when only data from Friesian calves were analyzed (Fig. 2 ). The results of the Fisher's exact tests showed that on Days 8 and 10, the proportion of Cryptosporidium-positive calves was significantly greater in Group 3 than in Group 1 (two-tailed Fisher's exact test P < Bonferroni-adjusted critical value of 0.003). On the other hand, most calves (58%) in Group 1 and only two calves in Group 2 and two in Group 3 were shedding oocysts on that day (Table 2).
Fig. 2.
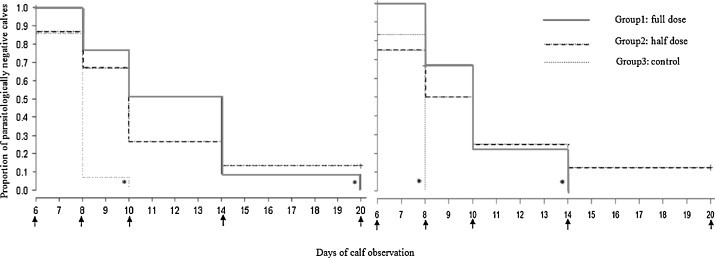
Reduction of the proportion of parasitologically negative calves (Y axis) as a function of time (X axis), in calves of all breeds (left) and in Friesian calves (right). The sampling days are indicated by arrows. Asterisks indicate a Kaplan Meier P < 0.05, to observe such a difference between the groups by chance alone.
3.3.2. Clinical efficacy
Twenty three calves (51%) passed at least one liquid specimen in the course of the study, and the proportion of liquid specimens on Day 6 was relatively high, and very similar in the three treatment groups (Table 3 ). The results of Kruskal–Wallis and Wilcoxon tests test did not indicate any significant difference (P > 0.05) between the fecal consistency scores of the three groups on any sampling day (not shown). Except one significant difference between Group 2 and Group 3 on Day 8, the proportion of liquid specimens did not differ between the groups (Table 3), and these result persisted when only Friesian calves were included in the analysis (not shown). There was no association between the log10 (TON + 1) and the presence of liquid specimen by logistic regression (P > 0.05).
Table 3.
The number of liquid feces (fecal score 3)/total specimens assessed, stratified by sampling Days, treatment Groups (the corresponding proportions are in brackets). On the same sampling Day, an asterisk indicates a two-tailed Fisher's exact test P < 0.003 (Bonferroni-adjusted critical probability).
| Sampling day | Group 1 (full dose) | Group 2 (half dose) | Group 3 (placebo-control) |
|---|---|---|---|
| Day 6 | 5/14 (0.36) | 4/15 (0.27) | 4/14 (0.28) |
| Day 8 | 1/12 (0.08) | 1/15 (0.07)* | 6/14 (0.43)* |
| Day 10 | 3/12 (0.25) | 1/15 (0.07) | 4/13 (0.31) |
| Day 14 | 3/12 (0.25) | 4/14 (0.28) | 1/12 (0.08) |
| Day 20 | 1/12 (0.08) | 0/14 (0) | 0/12 (0) |
4. Discussion
Halofuginone lactate is registered for the prevention of calf cryptosporidiosis in several countries, but the compound has a relatively narrow therapeutic index and a substantial market price. This study was initially designed to assess the efficacy of a reduced dosage regime of HL on a farm infected with C. parvum, with no evidence for the presence of co-infection. However, the identification of rotavirus and Salmonella Typhimurium in multiple calves provided an opportunity to collect much needed data of the utility of the compound in the presence of such common co-infections.
When the study was designed, we predicted a longer prepatent period and a decreased number of oocysts and fecal consistency scores in the full dose group, as reported for HL in the absence of documented co-infections. The co-infections with rotavirus and Salmonella Typhimurium did not affect the ability of HL (full dose) to delay the onset of shedding. Whereas most calves (5/12) in Group 1 were still shedding oocysts on Day 20, the majority of the calves in the control group were already parasitologically negative by that day (Table 2). The prolongation of the prepatent period was not coupled with statistically significant differences between the mean log10 (TON + 1) of the treatment Groups after controlling for the repeated measurements, the sex, and the breed of the calves (Models 3 and 4). We hypothesize that HL suppressed the parasite's life cycle during the first days of treatment, when the diarrhea caused by the other pathogens was not yet overt, effectively prolonging the prepatent period in Group 1. Conversely, about 30% of the calves in Group 1 were passing liquid feces on Day 6, and this early diarrhea (probably caused by the co-infecting pathogens) could have shortened the intestinal transit time of HL in the last days of treatment, effectively abolishing its anti-Cryptosporidium effect.
Some authors suggested that co-infections with multiple agents could cause a more severe diarrhea than mono-infections (De la Fuente et al., 1999, Garcia et al., 2000). Consequently, it could be hypothesized that suppression of one organism could reduce the severity of the diarrhea. In this study, there was no significant difference between the fecal consistency scores of the treated and untreated groups, and no association between the TON and the presence of liquid feces was found by logistic regression. Furthermore, in agreement with the results of a metaanalysis of the literature (Silverlås et al., 2009), the mortality rates did not differ between the groups. Therefore, the results do not allow conclusions to be drawn on a clinical benefit of HL in the presence of co-infections.
Finally, the statistically significant effect of breed on the intensity of oocyst shedding observed in this study was intriguing. Other studies have previously reported lower Cryptosporidium infection prevalence in beef calves than in dairy calves (Geurden et al., 2006, Kváč et al., 2006).
5. Conclusions
The anti-Cryptosporidium activity of HL was not fully preserved and the use of the drug was not associated with a clinical benefit in the presence of enzootic co-infection with rotavirus and Salmonella Typhimurium in calves. Diagnostic efforts should therefore aim to rule out the presence of other common enteropathogens in order to maximize the clinical efficacy of HL in the field.
Conflicts of interest statement
One year after the completion of this study, the authors requested and obtained funding from MSD Animal Health New Zealand (the new distributors of Halocur) to perform an epidemiological study of neonatal calf diarrhea in dairy farms. The current study was performed before such funds were requested and the company was not consulted during the preparation of this manuscript.
Acknowledgements
The authors thank the farm owners and staff for their co-operation, and Mark Stevenson (Massey University) for contributing to the statistical analysis. This work was funded by the Lewis Fitch Veterinary Research Fund, New Zealand. The funding body had no active role in this study. BH was funded by a Pfizer (currently Zoetis) undergraduate summer scholarship.
References
- Bartels C.J.M., Holzhauer M., Jorritsma R., Swart W.A.J.M., Lam T.J.G.M. Prevalence, prediction and risk factors of enteropathogens in normal and non-normal feces of young Dutch dairy calves. Prev. Vet. Med. 2010;93:162–169. doi: 10.1016/j.prevetmed.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook E., Hart C.A., French N., Christley R. Prevalence and risk factors for Cryptosporidium spp. infection in young calves. Vet. Parasitol. 2008;152:46–52. doi: 10.1016/j.vetpar.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Chen X.-M., Keithly J.S., Paya C.V., LaRusso N.F. Cryptosporidiosis. N. Engl. J. Med. 2002;346:1723–1731. doi: 10.1056/NEJMra013170. [DOI] [PubMed] [Google Scholar]
- De la Fuente R., Luzón M., Ruiz-Santa-Quiteria J.A., Garcia A., Cid D., Orden J.A., Garcia S., Sanz R., Gómez-Bautista M. Cryptosporidium and concurrent infections with other major enterophatogens in 1 to 30-day-old diarrheic dairy calves in central Spain. Vet. Parasitol. 1999;80:179–185. doi: 10.1016/S0304-4017(98)00218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waele V., Speybroeck N., Berkvens D., Mulcahy G., Murphy T.M. Control of cryptosporidiosis in neonatal calves: use of halofuginone lactate in two different calf rearing systems. Prev. Vet. Med. 2010;96:143–151. doi: 10.1016/j.prevetmed.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R., Xiao L. second ed. CRC Press; Boca Raton, FL, USA: 2008. Cryptosporidium and Cryptosporidiosis. [Google Scholar]
- Fayer R., Ellis W. Paromomycin is effective as prophylaxis for cryptosporidiosis in dairy calves. J. Parasitol. 1993;79:771–774. [PubMed] [Google Scholar]
- Fayer R., Gasbarre L., Pasquali P., Canals A., Almeria S., Zarlenga D. Cryptosporidium parvum infection in bovine neonates: dynamic clinical, parasitic and immunologic patterns. Int. J. Parasitol. 1998;28:49–56. doi: 10.1016/s0020-7519(97)00170-7. [DOI] [PubMed] [Google Scholar]
- Fayer R., Morgan U., Upton S.J. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 2000;30:1305–1322. doi: 10.1016/s0020-7519(00)00135-1. [DOI] [PubMed] [Google Scholar]
- Garber L.P., Salman M.D., Hurd H.S., Keefe T., Schlater J.L. Potential risk factors for Cryptosporidium infection in dairy calves. J. Am. Vet. Med. Assoc. 1994;205:86–91. [PubMed] [Google Scholar]
- Garcia A., Ruiz-Santa-Quiteria J.A., Orden J.A., Cid D., Sanz R., Gómez-Bautista M., de la Fuente R. Rotavirus and concurrent infections with other enteropathogens in neonatal diarrheic dairy calves in Spain. Comp. Immunol. Microbiol. Infect. Dis. 2000;23:175–183. doi: 10.1016/S0147-9571(99)00071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurden T., Goma F.Y., Siwila J., Phiri I.G.K., Mwanza A.M., Gabriel S., Claerebout E., Vercruysse J. Prevalence and genotyping of Cryptosporidium in three cattle husbandry systems in Zambia. Vet. Parasitol. 2006;138:217–222. doi: 10.1016/j.vetpar.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Grinberg A., Lopez-Villalobos N., Markovics A., Kosak A., Galindez J., Tranquillo V.M. Controlling the onset of natural cryptosporidiosis in calves with paromomycin sulphate. Vet. Rec. 2002;151:606–608. doi: 10.1136/vr.151.20.606. [DOI] [PubMed] [Google Scholar]
- Grinberg A., Learmonth J., Kwan E., Pomroy W., Lopez Villalobos N., Gibson I., Widmer G. Genetic diversity and zoonotic potential of Cryptosporidium parvum causing foal diarrhoea. J. Clin. Microbiol. 2008;46:2396–2398. doi: 10.1128/JCM.00936-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliksen S.M., Jor E., Lie K.I., Hamnes I.S., Løken T., Åkerstedt J., Østerås O. Enteropathogens and risk factors for diarrhea in Norwegian dairy calves. J. Dairy Sci. 2009;92:5057–5066. doi: 10.3168/jds.2009-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P.R., Thompson R.C.A. The zoonotic transmission of Giardia and Cryptosporidium. Int. J. Parasitol. 2005;35:1181–1190. doi: 10.1016/j.ijpara.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Jarvie B.D., Trotz-Williams L.A., McKnight D.R., Leslie K.E., Wallace M.M., Todd C.G., Sharpe P.H., Peregrine A.S. Effect of halofuginone lactate on the occurrence of Cryptosporidium parvum and growth of neonatal dairy calves. J. Dairy Sci. 2005;88:1801–1806. doi: 10.3168/jds.S0022-0302(05)72854-X. [DOI] [PubMed] [Google Scholar]
- Joachim A., Krull T., Schwarzkopf J., Daugschies A. Prevalence and control of bovine cryptosporidiosis in German dairy herds. Vet. Parasitol. 2003;112:277–288. doi: 10.1016/S0304-4017(03)00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958;53:457–481. [Google Scholar]
- Kiang K.M., Scheftel J.M., Leano F.T., Taylor C.M., Belle-Isle P.A., Cebelinski E.A., Danila R., Smith K.E. Recurrent outbreaks of cryptosporidiosis associated with calves among students at an educational farm programme, Minnesota, 2003. Epidemiol. Infect. 2006;134:878–886. doi: 10.1017/S0950268805005649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. Preventive and therapeutic efficacy of halofuginone-lactate against Cryptosporidium parvum in spontaneously infected calves: a centralised, randomised, double-blind, placebo-controlled study. Vet. J. 2008;177:429–431. doi: 10.1016/j.tvjl.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P., Kleinová T., Volek Z., Šimůnek J. Effect of Cryptosporidium parvum infection on the absorptive capacity and paracellular permeability of the small intestine in neonatal calves. Vet. Parasitol. 2008;152:53–59. doi: 10.1016/j.vetpar.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kváč M., Kouba M., Vítovec J. Age-related and housing-dependence of Cryptosporidium infection of calves from dairy and beef herds in South Bohemia, Czech Republic. Vet. Parasitol. 2006;137:202–209. doi: 10.1016/j.vetpar.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Lallemond M., Villeneuve A., Belda J., Dubreuil P. Field study of the efficacy of halofuginone and decoquinate in the treatment of cryptosporidiosis in veal calves. Vet. Rec. 2006;159:672–676. doi: 10.1136/vr.159.20.672. [DOI] [PubMed] [Google Scholar]
- Lanz Uhde F., Kaufmann T., Sager H., Albini S., Zanoni R., Schelung E., Meylan M. Prevalence of four enteropathogens in the feces of young diarrhoeic dairy calves in Switzerland. Vet. Rec. 2008;163:362–366. doi: 10.1136/vr.163.12.362. [DOI] [PubMed] [Google Scholar]
- Learmonth J.J., Ionas G., Ebbett K.A., Kwan E.S. Genetic characterization and transmission cycles of Cryptosporidium species isolated from humans in New Zealand. Appl. Environ. Microbiol. 2004;70:3973–3978. doi: 10.1128/AEM.70.7.3973-3978.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefay D., Naciri M., Poirier P., Chermette R. Efficacy of halofuginone lactate in the prevention of cryptosporidiosis in suckling calves. Vet. Rec. 2001;148:108–112. doi: 10.1136/vr.148.4.108. [DOI] [PubMed] [Google Scholar]
- Millemann Y. Diagnosis of neonatal calf diarrhea. Rev. Med. Vet. 2009;160:404–409. [Google Scholar]
- Moore D.A., Atwill E.R., Kirk J.H., Brahmbhatt D., Alonso L.H., Hou L., Singer M.D., Miller T.D. Prophylactic use of decoquinate for infections with Cryptosporidium parvum in experimentally challenged neonatal calves. J. Am. Vet. Med. Assoc. 2003;223:839–845. doi: 10.2460/javma.2003.223.839. [DOI] [PubMed] [Google Scholar]
- Naciri M., Mancassola R., Yvore P., Peeters J.E. The effect of halofuginone lactate on experimental Cryptosporidium parvum infections in calves. Vet. Parasitol. 1993;45:199–207. doi: 10.1016/0304-4017(93)90075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naciri M., Paul Lefay M., Mancassola R., Poirier P., Chermette R. Role of Cryptosporidium parvum as a pathogen in neonatal diarrhea complex in suckling and dairy calves in France. Vet. Parasitol. 1999;85:245–257. doi: 10.1016/S0304-4017(99)00111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollivett T.L., Nydam D.V., Bowman D.D., Zambriski J.A., Bellosa M.L., Linden T.C., Divers T.J. Effect of nitazoxanide on cryptosporidiosis in experimentally infected neonatal dairy calves. J. Dairy Sci. 2009;92:1643–1648. doi: 10.3168/jds.2008-1474. [DOI] [PubMed] [Google Scholar]
- O’Handley R.M., Cockwill C., McAllister T.A., Jelinski M., Morck D.W., Olson M.E. Duration of naturally acquired giardiosis and cryptosporidiosis in dairy calves and their association with diarrhea. J. Am. Vet. Med. Assoc. 1999;214:391–396. [PubMed] [Google Scholar]
- Quilez J., Sanchez-Acedo C., Avendaño C., Del Cacho E., Lopez-Bernad F. Efficacy of two peroxygen-based disinfectants for inactivation of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 2005;71:2479–2483. doi: 10.1128/AEM.71.5.2479-2483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig R.F. Pathogenesis of intestinal and systemic rotavirus infection. J. Virol. 2004;78:10213–10220. doi: 10.1128/JVI.78.19.10213-10220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverlås C., Björkman C., Egenvall A. Systematic review and meta-analyses of the effects of halofuginone against calf cryptosporidiosis. Prev. Vet. Med. 2009;91:73–84. doi: 10.1016/j.prevetmed.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Smith H.V., Cacciò S.M., Cook N., Nichols R.A.B., Tait A. Cryptosporidium and Giardia as foodborne zoonoses. Vet. Parasitol. 2007;149:29–40. doi: 10.1016/j.vetpar.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Smith H.V., Nichols R.A.B., Grimason A.M. Cryptosporidium excystation and invasion: getting to the guts of the matter. Trends Parasitol. 2005;21:133–142. doi: 10.1016/j.pt.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Schnyder M., Kohler L., Hemphill A., Deplazes P. Prophylactic and therapeutic efficacy of nitazoxanide against Cryptosporidium parvum in experimentally challenged neonatal calves. Vet. Parasitol. 2009;160:149–154. doi: 10.1016/j.vetpar.2008.10.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotz-Williams L.A., Jarvie B.D., Martin S.W., Leslie K.E., Peregrine A.S. Prevalence of Cryptosporidium parvum infection in southwestern Ontario and its association with diarrhea in neonatal dairy calves. Can. Vet. J. 2005;46:349–351. [PMC free article] [PubMed] [Google Scholar]
- Trotz-Williams L.A., Jarvie B.D., Peregrine A.S., Duffield T.F., Leslie K.E. Efficacy of halofuginone lactate in the prevention of cryptosporidiosis in dairy calves. Vet. Rec. 2011;168:509. doi: 10.1136/vr.d1492. [DOI] [PubMed] [Google Scholar]
- Tzipori S., Campbell I., Sherwood D., Snodgrass D.R., Whitelaw A. An outbreak of calf diarrhea attributed to cryptosporidial infection. Vet. Rec. 1980;107:579–580. [PubMed] [Google Scholar]
- Villacorta I., Peeters J.E., Vanopdenbosch E., Ares-Mazas E., Theys H. Efficacy of halofuginone lactate against Cryptosporidium parvum in calves. Antimicrob. Agents Chemother. 1991;35:283–287. doi: 10.1128/aac.35.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Feng Y. Zoonotic cryptosporidiosis. FEMS Immunol. Med. Microbiol. 2008;52:309–323. doi: 10.1111/j.1574-695X.2008.00377.x. [DOI] [PubMed] [Google Scholar]
- Zhou P., Chen N., Zhang R.-L., Lin R.-Q., Zhu X.-Q. Food-borne parasitic zoonoses in China: perspective for control. Trends Parasitol. 2008;24:190–196. doi: 10.1016/j.pt.2008.01.001. [DOI] [PubMed] [Google Scholar]


