Abstract
Background
A subset of histo-blood group antigens including ABO and Lewis are oligosaccharide structures which may be conjugated to lipids or proteins. They are known to be important recognition motifs not only in the context of blood transfusions, but also in infection and cancer development.
Scope of review
Current knowledge on the molecular background and the implication of histo-blood group glycans in the prevention and therapy of infectious and non-communicable diseases, such as cancer and cardiovascular disease, is presented.
Major conclusions
Glycan-based histo-blood groups are associated with intestinal microbiota composition, the risk of various diseases as well as therapeutic success of, e.g., vaccination. Their potential as prebiotic or anti-microbial agents, as disease biomarkers and vaccine targets should be further investigated in future studies. For this, recent and future technological advancements will be of particular importance, especially with regard to the unambiguous structural characterization of the glycan portion in combination with information on the protein and lipid carriers of histo-blood group-active glycans in large cohorts.
General significance
Histo-blood group glycans have a unique linking position in the complex network of genes, oncodevelopmental biological processes, and disease mechanisms. Thus, they are highly promising targets for novel approaches in the field of personalized medicine. This article is part of a Special Issue entitled "Glycans in personalised medicine" Guest Editor: Professor Gordan Lauc.
Abbreviations: BG, blood group; CA, cancer antigen; FORS, Forssman; FUT, fucosyltransferase (gene); GLOB, globoside; GSL, glycosphingolipid; ISBT, International Society for Blood Transfusion; Le, Lewis; MS, mass spectrometry; RBC, red blood cell; Se, secretor; sICAM-1, soluble intercellular adhesion molecule 1
Keywords: Blood group, Cancer, Glycan, Infection, Personalized medicine, Vaccine
Highlights
-
•
Glycans are well-known for their function as recognition molecules.
-
•
Histo-blood group glycans are derived from allelic glycosyltransferase genes.
-
•
Histo-blood group glycans are associated with disease risk and therapeutic success.
-
•
Pathogens mimic human glycans that are recognized by the host's immune defense.
-
•
Soluble histo-blood group glycans serve as decoy receptors for microbes.
1. Introduction
More than a century after the discovery of the ABO blood group (BG) by Karl Landsteiner [1], currently, over 30 different BG systems and more than 300 recognized BG antigens are defined by the International Society for Blood Transfusion (ISBT) [2]. A recent overview is provided on the society's website [3]. The different BG antigens evolve from genetic polymorphisms of red blood cell (RBC) surface molecules, most of which are peptides, and some are carbohydrates, such as ABO antigens [3]. However, BG antigens including ABO are not only expressed on RBCs, but are also present in many tissues [4]. Therefore, these histo-BG antigens appear to be relevant not only in transfusion medicine, but also in transplantation [5]. Moreover, histo-BG antigens may also occur in glandular secretions. For example, ABO and Lewis antigens are found on saliva mucins, and free oligosaccharides are found in milk and urine [6], [7], [8], [9]. Structurally, these particular histo-BGs are glycans, defined as “any sugar or assembly of sugars, in free form or attached to another molecule” [10].
Glycans in general are known to be “directly involved in the pathophysiology of every major disease”, and it has been concluded that “knowledge from glycoscience will be needed to realize the goals of personalized medicine and to take advantage of the substantial investments in human genome and proteome research and its impact on human health” as stated by a recent report from the US National Academies [11].
Although the lack of expression of BG antigens is not directly resulting in disease, as known so far, the presence or absence of certain BG antigens on RBC surfaces or in other tissues or bodily fluids has been found to be associated with susceptibility to various diseases, beyond their recognized role in incompatibility reactions during transfusion, transplantation or pregnancy. Largest body of evidence for the carbohydrate-based histo-BGs ABO, Lewis (Le) and secretor (Se) is available particularly in the context of infectious diseases and cancer (reviewed in [12], [13], [14], [15], [16]). Nevertheless, histo-BG glycans are far from being exploited for diagnostic or therapeutic applications, apart from the prominent exception of the cancer antigen (CA) 19-9, i.e. sialyl-Lea [17].
Glycan-based BGs, i.e. ABO, P1PK, Le, H and Se, Ii, globoside (GLOB), Forssman (FORS), and the high-incidence antigen Sda, altogether represent more than 20 distinct antigenic structures. An overview of the glycan-based BG systems and the associated RBC phenotypes with their distributions among populations is given in Table 1 . In this review, the potential use of these glycan-based histo-BGs in the context of personalized medicine will be discussed as well as the technology suitable for determining them in a research as well as clinical setting.
Table 1.
Glycan histo-blood groups (BGs) and responsible genes with blood type distributions among populations.
| BG1 | Gene2 | Glycosyltransferase2 | Carrier | Antigens3 | RBC type | Frequencies of blood types in %4 |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Caucasians | China | US blacks | Reference | |||||||
| ABO | ABO | (Inactive) | (H) | O | 39 | (34) | 49 | [12], [18], [19] | ||
| Histo-blood group ABO system transferase (A transferase; alpha 1-3-N-acetylgalactosaminyltransferase; A3GALNT) | GSL, N-/O-glycoprotein, free glycan | A; A1; A2 | A | 42 | (29) | 27 | ||||
| Histo-blood group ABO system transferase (B transferase; alpha 1-3-galactosyltransferase; A3GALT1) | B | B | 13 | (28) | 20 | |||||
| A transferase and B transferase | A; A1; A2; B | AB | 6 | (9) | 4 | |||||
| LE | FUT3 | (Inactive) | Le(a − b −) | 6 | 9 | 22 | [12], [20] | |||
| Galactoside 3(4)-L-fucosyltransferase (Lewis FT; fucosyltransferase 3; CD174); Le-FUT | GSL, N −/O-glycoprotein, free glycan | Lea | Le(a + b −) | 22 | 0 | 23 | ||||
| Leb; (Lea) | Le(a − b +)5 | 72 | 71 | 55 | ||||||
| Lea; (Leb) | Le(a + b +) | 0 | 20 | 0 | ||||||
| Se6 | FUT2 | (Inactive) | (Not applicable)6 | (20) | [21], [22] | |||||
| Galactoside 2-alpha-L-fucosyltransferase 2 (Alpha(1,2)FT 2; secretor factor); Se-FUT | GSL, N-/O-glycoprotein, free glycan | Se (Type 1H) | (80) | |||||||
| H | FUT1 | (Inactive) | Bombay or para-Bombay | Rare | [12] | |||||
| Galactoside 2-alpha-L-fucosyltransferase 1 (fucosyltransferase 1); H-FUT | GSL, N-/O-glycoprotein | H | H | Almost 100% | [12] | |||||
| I | GCNT2 | (Inactive) | i | i | Rare (adults) | [23] | ||||
| N-acetyllactosaminide beta-1,6-N-acetylglucosaminyl-transferase, isoform A (I-branching enzyme) | I; i | I | Almost 100% (adults) | |||||||
| P1PK | A4GALT | (Inactive) | PX2 | p | Rare | [12] | ||||
| Lactosylceramide 4-alpha-galactosyltransferase (Gb3 synthase; P1/Pk synthase) | GSL | P1; Pk; (NOR)7 | P1 | 79 | 27 | 94 | ||||
| Pk; (NOR)7 | P2 | 21 | 73 | 6 | ||||||
| GLOB | B3GALNT1 | (Inactive) | Pk | Rare | [12], [24] | |||||
| UDP-GalNAc:beta-1,3-N-acetylgalactosaminyltransferase 1 (globoside synthase) | GSL | P | High incidence | |||||||
| FORS | GBGT1 | (Inactive) | Almost 100% | [12], [24] | ||||||
| Globoside-3-alpha-N-acetyl-D-galactosaminyltransferase (Fs synthase)8 | GSL | FORS1 | Apae | Rare | ||||||
| Sid | B4GALNT29 | (Inactive) | Sda − | < 10% | [25] | |||||
| Beta-1,4 N-acetylgalactosaminyltransferase 2 | N-glycoprotein | Sda | Sda + | High incidence | [26] | |||||
| (No. 209) | ST3GAL29 | CMP-N-acetylneuraminate-beta-galactosamide-alpha-2,3-sialyltransferase 2 | GSL | LKE | LKE | Almost 100% | [12], [24] | |||
According to Ref. [3].
According to the HUGO Gene Nomenclature Committee at the European Bioinformatics Institute (genenames.org) in case of gene names and/or the recommendations in UniProtKB with a selection of alternative names in parentheses in case of glycosyltransferase names. Short names as used in this article are in bold.
Antigens on red blood cells (RBC), tissues, or in secretions markedly elevated or specific to a certain blood type as compared to the other phenotypes within a BG. List is not extensive, i.e., not including various combinations/extensions of the respective antigens.
Both genotypic and red blood cell phenotypic determinations were included here and genotyping data are shown in parentheses.
Le(a − b +) phenotype only in combination with active FUT2 gene.
Se-gene encoded FUT2 is not expressed in RBCs, and therefore does not represent a classic blood group, but provides type 1H antigens as precursors for BG antigens in secretions and tissues.
NOR antigens expressed if a rare variant of A4GALT gene is present.
Due to its novelty no UniProt entry exists for a human GBGT1-encoded Fs synthase. Nomenclature was used according to Ref. [27].
Genetic background not yet completely understood.
2. Structural basis of glycan histo-blood groups
Glycan histo-BG antigens can occur in various types of glycoconjugates, i.e., on glycosphingolipids (GSLs), N- and O-glycans of cell membrane-bound or secreted glycoproteins and mucins, or on free oligosaccharides from milk or urine (Table 1, Fig. 1 , [23], [28]). As an example, ABO activity on erythrocytes was found to originate from glycoproteins (65–75%), polyglycosylceramides (10–15%) and from other glycoconjugates (10%) [29].
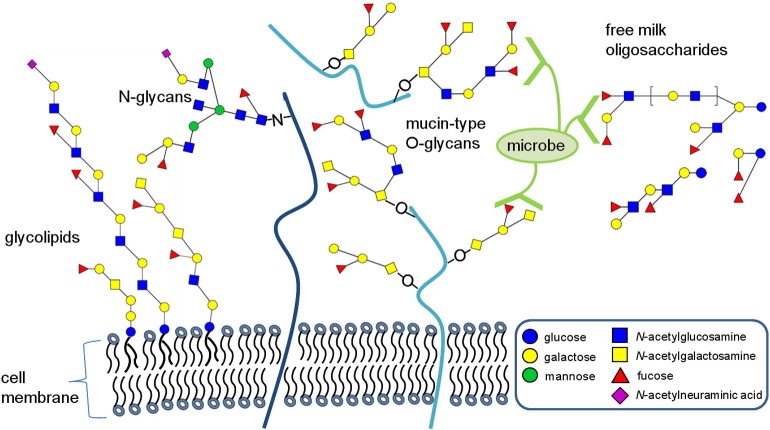
Fig. 1.
Examples of human histo-blood group glycans and their carriers on cell surfaces and in body fluids. Histo-blood group glycans decorate various core glycans attached to lipids (black waves) and proteins (blue lines) anchored in the cell membrane, or to secreted glycoproteins/mucins or free oligosaccharides as found in large quantities in human milk. Blood group antigens are found on both N- and O-glycans of proteins. Microbial receptors can recognize various histo-blood group antigens and can thereby attach to the host's epithelial surfaces. Alternatively, soluble glycans can serve as decoy receptors for pathogens.
Even when regarding the glycan portion only, a diversity of precursor oligosaccharides together with the various antigenic determinants potentiates the overall structural diversity of histo-BG-active compounds. For instance, ABO determinants are found on type 1 and 2 chains of N-/O-glycans attached to proteins or (neo)lacto-series GSLs (Fig. 2A and B). Furthermore, they decorate O-linked type 3 chains on mucins that are structurally identical to the so-called T antigen (Fig. 2C). Type 4 chains bearing ABO epitopes are part of globo- and ganglio-series GSLs (Fig. 2C and Fig. 3 ; comprehensive reviews in [28], [30], [31], [32]). The expression of histo-BG antigens and their precursors is tissue-specific and has been associated with the embryologic origin of a tissue and the degree of differentiation of the respective cells within a tissue [4], [33], [34], [35]. In the following, the minimal structural features of glycan histo-BG epitopes are briefly described, and a summary of the relevant genes, enzyme names and products is given in Table 2 . For a more extensive overview on the genetic, biochemical, epidemiological, and historical aspects of those readers are referred to literature [4], [12], [23], [24], [26], [28], [36], [37], [38], [39], [40].
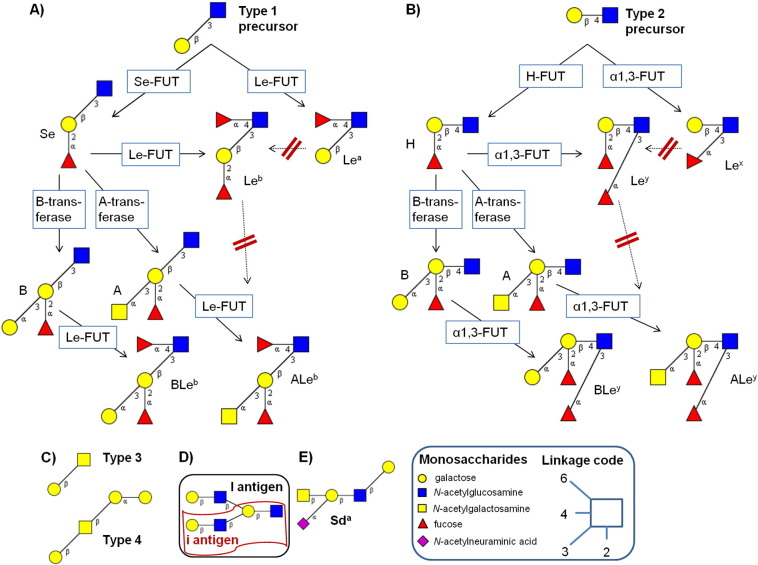
Fig. 2.
Antigenic structural motifs of the histo-blood groups ABO and Lewis (Le) with their precursors. The interaction of glycosyltransferases acting on type 1 and type 2 precursors results in ABO and Lewis a/b (A) or x/y structures (B), respectively. Mucin-type 3 (T antigen) and glycosphingolipid-based type 4 structures are precursors to ABO and Lewis structures (C). The Ii blood group is determined by linear (i) or beta6-branched (I) polylactosamine type 2 chains (D). Sda antigenic determinant (E). FUT, fucosyltransferase; Se, secretor. Dashed, red-crossed arrows indicate inadmissible reactions.
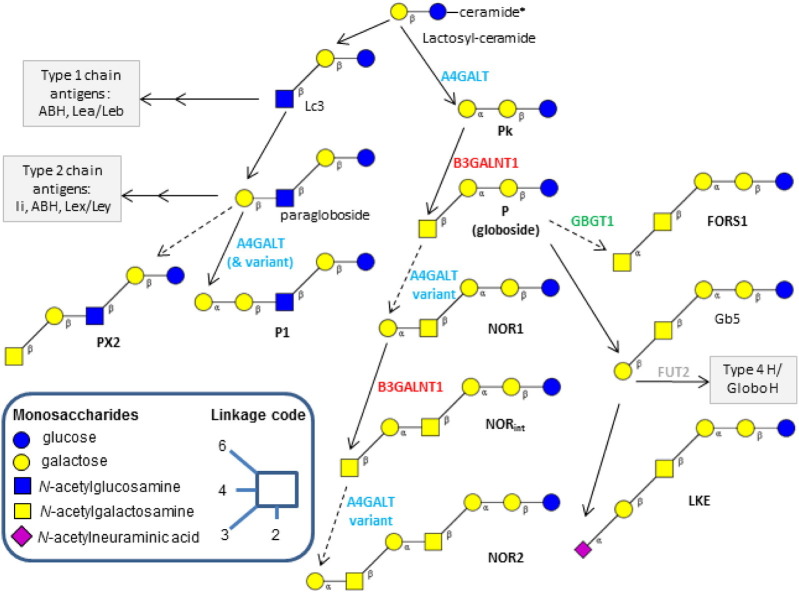
Fig. 3.
The biosynthetic pathways of the glycosphingolipid-based blood group (BG) antigens. The enzymes and resulting antigens linked to the following BGs are depicted: P1PK BG (A4GALT gene, cyan): Pk, P1, NOR; GLOB BG (B3GALNT1 gene, red): P, PX2; FORS BG (GBGT1, green): FORS1; GLOB collection 209: LKE. For full enzyme names see Table 1. *Each of the structures shown carries a beta-linked ceramide residue at the reducing end glucose, which is not depicted here for simplicity reasons. The links to the synthesis pathways of GSL-based blood group antigens of ABO, Le, and H/Se groups are also shown (gray boxes). Solid lines represent common pathways according to common glycosyltransferase gene alleles, whereas dashed lines symbolize very rare ones. Modified from Refs. [12], [24].
Table 2.
Glycan histo-blood groups (BGs) and selected disease associations.
| BG | Type | Disease susceptibility | Reference |
|---|---|---|---|
| ABO | O | H. pylori infection | [63] |
| E. coli O157 infection and death | [67] | ||
| Peptic ulcer | [68] | ||
| A | S. mansoni infection & disease severity | [69] | |
| Gastric cancer | [68] | ||
| Overall cancer | [70] | ||
| B | Salmonellosis | [71] | |
| E. Coli infection | [71] | ||
| Non-O | Severe malaria Exocrine pancreatic cancer Cardiovascular disease |
[72] [73] [74] |
|
| LE | Inactive | Urinary tract infections Invasive ductal breast carcinoma Childhood asthma |
[75] [76] [77] |
| Se | Inactive (non-Se) |
S. pneumonia infection N. meningitidis infection H. influenza infection Urinary tract infections Gram-negative sepsis in premature infants Necrotizing enterocolitis in premature infants Gastric disease Crohn's disease Primary sclerosing cholangitis Chronic pancreatitis Type 1 diabetes Breast axillary lymph nodes metastasis |
[78] [78] [79] [75] [80] [80] [81] [22], [82] [82] [21] [83] [76] |
| Active (Se) | Norovirus infection Rotavirus infection Influenza virus A & B infection Rhinovirus infection Respiratory syncytial virus infection Echovirus infection HIV infection and disease progression |
[84], [85], [86], [87] [88] [89] [89] [89] [89] [90] |
2.1. Ii histo-blood group
Ii epitopes are based on repeating units of either linear or β1,6-branched N-acetyllactosamine chains (Fig. 2D) [41], [42], [43]. The name of the blood group emerged from an abbreviation of ‘individuality’ and thus represents an upper and a lower-case letter ‘i’. Both structures are ubiquitously expressed, with the exception of the very rare adult i phenotype lacking branched I structures either on erythrocytes only or even tissue-wide, depending on the type of mutation [23], [44].
Similar to other polylactosamines, Ii structures are substrates to various glycosyltransferases, including sialyltransferases and ABO and Le BG transferases as described in the following (for review see Refs. [32], [45]).
2.2. ABO, H and Lewis histo-blood groups
The biosynthesis of the antigens from ABO and Le histo-BGs is closely related, although the responsible glycosyltransferases are expressed from several independent genes: ABO gene on chromosome 9, H-gene (FUT1), Se-gene (FUT2), and Le-gene (FUT3) on chromosome 19 [34], [37]. For abbreviated as well as full names including linkage specificities and responsible genes of the fucosyltransferases (FUT), see Table 1. Starting from type 1 lactosamine residues, Le-FUT and Se-FUT are competing to generate an Lea or type 1H epitope (Led), respectively [46], [47] (Fig. 2A). Type 1H (or Se antigen) is a substrate for the ABO gene-encoded A or B transferase, producing the A or B antigen, if one of the active alleles is present. Furthermore, type 1H can be fucosylated by Le-FUT, generating the Leb epitope. Lea and Leb structures cannot be further modified, whereas A and B antigens are again substrates to Le-FUT, resulting in ALeb and BLeb antigens [6], [28], [48], [49]. This pathway takes place in secretory tissues and cells other than erythrocytes, since type 1 structures are highly expressed in outer epithelial layers with higher degree of differentiation, e.g. in the oral or gut mucosa, and are substrates to Se-FUT [4]. Se-FUT is active in about 80% of Caucasians, the so-called secretors (Table 1). If the Le gene is inactive, only the precursor structures Lec (type 1 precursor) and Led (type 1H), that are classified as part of the BG collection 210 [3], are found in plasma or on red blood cells of non-secretors and secretors, respectively [28]. Erythrocytes normally have no Se-FUT or Le-FUT expression [50], and bear ABO antigens mainly on type 2 chains, which are the preferred substrates for H-FUT [4].
On type 2 structures Lex antigens (CD15) in alpha1,3-linkage to the subterminal GlcNAc are being generated by either the Le-dependent alpha1,3/4-FUT or one of the other alpha1,3-FUTs [28] (Fig. 2B). In addition to Se-FUT, if expressed in the respective cell type, the H gene-regulated H-FUT will primarily synthesize type 2H structures, which can be further modified by A or B transferases or alpha1,3-FUTs, incl. Le-FUT3. The action of this type of FUTs results in Ley epitopes, i.e. type 2 isomers of Leb. In analogy to the above-described pathway for type 1 chains, in type 2 ALey and BLey will be the largest end products if all the respective glycosyltransferases are expressed in their active form [6], [28]. The terminal Leb/Ley, A- or B-epitopes can usually not be further modified by elongation or branching; the same applies to the subterminal Lea or Lex [6], [51].
2.3. P1PK, FORS, GLOB and related histo-blood group antigens
The antigens of the P1PK, FORS, GLOB BGs and related collections are all GSLs (reviewed in [12], [24]). The classification of these antigens has been changed several times in the past; the current state according to the ISBT is listed in Table 1. Structurally, GSLs are composed of a lipophilic part containing a long chain fatty acid and a sphingosine anchored in the plasma membrane, and a hydrophilic glycan head group (Fig. 1). Starting from lactosylceramide either Pk antigen (globotriaosylceramide, CD77) is synthesized via the action of the A4GALT gene-encoded galactosyltransferase leading to the globo-series GSL pathway, or P1 antigen is synthesized via the action of the same enzyme following two other (non-BG-related) glycosyltransferases generating neolacto-series GSL (Fig. 3). For P antigen production an active B3GALNT1-gene is required. The structures of the globo- and (neo)lacto-series GSLs act as precursors for GSL-attached ABO, Le and H/Se epitopes, as indicated in Fig. 3. If a null-allele of either A4GALT or B3GALNT1 is apparent (in very rare cases), globo-GSLs cannot be produced [52]. Furthermore, a rare variant of the A4GALT enzyme is linked to the expression of NOR antigens [53]. The FORS1 antigen was found in individuals with an activated GBGT gene, which is normally not active in humans, but in some non-primate animals [27]. Except for P1 antigen, which is RBC-specific, the expression of the other related antigens is common to many tissues and cell types, and expression levels can vary during cell cycle and differentiation [12].
2.4. Other glycan histo-blood group antigens
In addition to the blood group systems and collections as classified by ISBT, two more glycan histo-BGs should be mentioned here, i.e. the high incidence antigen Sda and the T/Tn system. Tn antigen as part of an O-linked mucin-type glycan is primarily a substrate for T-synthase, a ubiquitously expressed beta3-galactosyltransferase responsible for T antigen (O-glycan core 1) formation (for review, see Ref. [54]). T antigen is furthermore identical with type 3 chains, which can be further elongated and/or decorated by other BG antigens (Fig. 2C).
Sda antigen is included in the high incidence 901 series according to ISBT classification due to its high prevalence in Caucasians. Similar to the discrepancy of erythrocyte vs. secretions phenotypes in Le BG, individuals having an RBC Sda − phenotype may still display Sda antigens in their secretions and especially urine (for review, see Ref. [26]). The minimal antigenic structure as shown in Fig. 2E is shared by both Sda and CAD antigens, which are assumed to be products of the same B4GALNT gene-encoded beta4-N-acetylgalactosaminyltransferase, however, the latter resulting from a more active enzyme variant. Consequently, Sda + individuals have Sda structures primarily on N-linked glycans, whereas CAD-individuals also express these on type 3 O-linked glycans and long-chain sialyl-paraglobosides [55].
3. Histo-blood group phenotype vs. genotype
The genetically determined repertoire of glycosyltransferases is the basis for an individual's histo-BG phenotype. However, genetic diversity with the ~ 300 alleles found for the ABO locus and ~ 50 alleles for the H, Se, and Le loci each [25], together with zygosity gives rise to an enormous variation of the levels of antigen expression including weak phenotypes leading to blood grouping discrepancies [56], [57]. Moreover, in pregnant women, individuals with different hematologic disorders, and especially in newborns, weak expression of histo-BG antigens on RBCs has been reported [57], [58]. The age-dependency of ABO antigen expression on RBCs is connected to the expression of I antigen, which is the major precursor of ABO structures on RBCs [12]. I antigen expression in RBCs is negligible at birth and reaches the full adult level by the age of 18 months [59], giving an example of the oncodevelopmental nature of histo-BG antigen expression [33]. The expression levels of ABO/Se and Le antigens can also vary tremendously within an adult individual over time [60]. Regulatory mechanisms of histo-BG-related glycogene expression, such as microRNA or transcription factor expression, are now being studied [38], [61].
The vast variability of the actual histo-BG phenotypes furthermore derives from the numerous interrelations between the respective biosynthetic pathways. This is demonstrated by the close relationships between ABO/Se and Le BGs as well as P1PK, GLOB and FORS, in addition to their precursor chains Ii and T/Tn (Fig. 2A–D). Another important modification, which is, however, not directly related to blood groups, is alpha2,3-sialylation of the terminal galactose prior to the action of a FUT on type 1 or 2 chains producing sialyl-Lea and sialyl-Lex antigens, respectively (Fig. 4 ). These combined histo-BG antigens are recognized for their role in the context of cancer as is discussed below. Interestingly, sialyltransferases also compete with FUTs for the same substrates, and can therefore have an impact on the expression levels of the inter-connected ABO and Le glycan BG antigens [12]. The different levels of substrate specificities have an additional impact on the overall complexity of the biosynthetic network of histo-BG glycosyltransferases: Some antigens can be synthesized by more than one enzyme (see H/Se) and in some instances enzymes are not limited to only one type of acceptors (see chain types 1–4 for Se-FUT or the various substrates of Le-FUT), or one type of linkage (s. Le-FUT acting as alpha1,3 and alpha1,4-FUT).
Fig. 4.
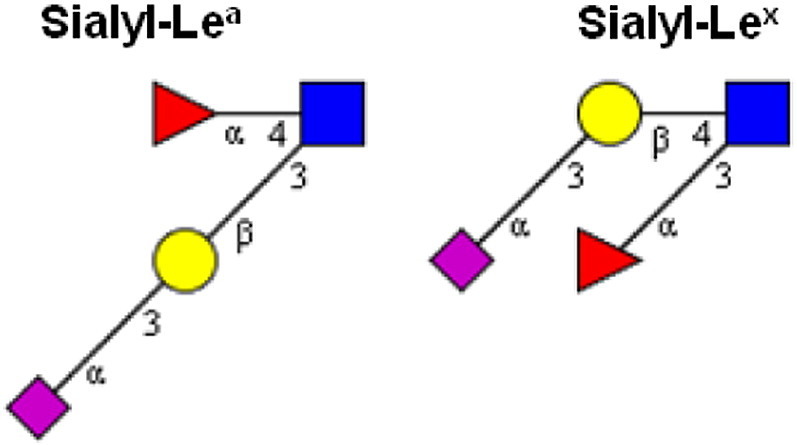
Oncodevelopmental histo-blood group antigens sialyl-Lea (CA19-9) and sialyl-Lex.
Taken together, various factors can have an impact on the final phenotype, i.e. the presence of individual histo-BG glycans on a cell surface or in a body fluid. Obviously, the various associations found between BG glycans and different diseases, disease stages and health-promoting factors are making histo-BG glycans an intriguing topic in the field of personalized medicine as discussed in the following paragraphs. In Table 2 the major conclusions from selected epidemiological studies reporting on associations between different diseases and ABO, Le and Se histo-BGs are summarized.
4. Infectious diseases and glycan histo-blood groups
For all the common and most rare BG phenotypes on RBCs no direct causative relationship is known between the BG null-alleles and inherited diseases [5], [12]. Since glycans are known for their predominant role as recognition molecules, in particular, in infection, the association of glycan histo-BG polymorphisms with various infectious diseases is not surprising. Many vertebrate species have maintained a functional AB gene, however, in humans roughly half of the population has O genotype resulting in a non-functional A/B enzyme. ABO polymorphism has been linked to evolutionary adaptation as defense against inter- and intra-species infections, since individuals produce antibodies against the non-self AB antigens after the exposure to these antigens originating from e.g. pathogens [62]. Masking of pathogen adhesion glycotopes by other glycans is another defense mechanism suggested [63]. Whereas microbial attachment sites on epithelial surfaces can support colonization, histo-BG antigens on soluble glycans such as mucins or free oligosaccharides from human milk may serve as decoy receptors in pathogen defense [64], [65], [66] (Fig. 1).
Glycan epitopes including histo-BGs have an important role in host-pathogen interactions, since glycans act as recognition sites for bacterial adhesins, and secondly, pathogens express surface epitopes to mimic those of the host to evade immune response, as proposed for Helicobacter pylori Le antigens [14]. Remarkably, H. pylori is able to bind to the same antigenic structures on the host's epithelial surface [91]. H. pylori is present in half of the world population and chronic infection is linked to gastritis, peptic ulcer and gastric cancer with a high degree of heterogeneity in disease phenotypes [92]. The bacterium is able to attach only to Leb antigen without additional A or B epitopes, and has therefore been proposed to be linked to BG O [91]. However, clinical data on the association of H. pylori, gastric cancer and ABO/Le BGs are contradictory (Table 2). A higher incidence of H. pylori in O and a lower incidence in A-individuals have been reported in multiple studies [63]. Strikingly, gastric cancer risk was higher in A BG (incidence rate ratio 1.20, 95% CI 1.02–1.42), whereas peptic ulcer risk was found to be higher in O-individuals in a large population-based study [68]. The link to Le and Se phenotype seems to be even more unclear. For instance, Se status and H. pylori infection have shown to be independent risk factors for gastric disease, with a higher risk in non-Se [81], although Leb (Se) seems to play a crucial role in H. pylori adhesion [12], [91]. On tissue level, H. pylori infected patients with gastric ulcer were shown to have increased Lea and loss of H and Leb expression in their inflamed gastric mucosa [7]. The authors claimed ABO and Le antigens to be good indicators for cellular alterations in the gastric epithelium.
Le- and Se-positive women were found to have lower risk for urinary tract infections as compared to Le-negative or non-Se [75]. Since adhesins from uropathogenic Escherichia coli recognize various globo-series GSLs including FORS1, P, and especially the sialylated LKE, antigens in vitro [93], a masking function of antigens competing with sialylation in secretors, i.e. Leb and AB, has been proposed [94]. B and AB individuals in a pediatric cohort were found to be more susceptible to salmonellosis and various E. coli types of infections (meningitis, sepsis, pyelonephritis) as compared to A or O individuals [71]. In contrast, Shigella infections were more prevalent in O individuals [71]. This relates to the higher susceptibility of O-individuals to infection by another Shiga-toxin related bacterial strain, i.e. E. coli O157 [67]. The same study found P-negative individuals to be more likely to develop severe hemolytic uremic syndrome after infection, possibly due to higher induction of TNF-alpha [67]. Pk, LKE and FORS1 histo-BG antigens have been associated with other Shiga toxin- and verotoxin-producing E. coli strains (STEC and EHEC) [24], [95]. Moreover, Pk-knockout mice showed no reaction to verotoxin administration, whereas basal levels of Pk expression were already lethal to wild-type mice [52].
Shiga- and verotoxin-binding Pk antigen is further found in human milk, and may act as decoy receptor in breastfed infants, that are at lower risk for diarrheal diseases as compared to formula-fed ones [96]. The level of Se-positive free oligosaccharides in human milk has also been positively correlated with, e.g., protection against E. coli-induced diarrhea in breastfed infants [97]. Similarly, alpha-1,2-fucosylated oligosaccharides from human milk were associated with protection against Campylobacter jejuni diarrhea [98], possibly due to their function as decoy receptors [99]. In premature infants, non- or low-Se phenotype as well as genotype was furthermore associated with severe outcomes, such as necrotizing enterocolitis or gram-negative sepsis [80].
Expressing AB antigens on epithelia and in secretions, as is the case in Se-positive individuals, might furthermore be protective against pneumonia caused by Streptococci [63], [78]. This pathogen secretes glycosidases acting on type 2 chain A, B and Ley structures, which are known virulence factors [100]. Secretors predominantly express type 1 chains, shielded by A and B epitopes and might therefore be protected against pathogen adhesion.
Non-secretors have also been reported being more susceptible to infections caused by Neisseria meningitidis and Haemophilus influenzae [78], [79]. Se, Le, and AB BGs seem to also play a role in Vibrio cholerae infection and disease severity [101], [102].
Pk, P, ABH antigens and 2′- and 3-fucosyllactose, the latter two being milk oligosaccharides generated by Se- and Le-fucosyltransferases, respectively, may play a role in Pseudomonas aeruginosa adhesion [103], [104]. Pk antigen also binds to adhesin P from Streptococcus suis, a pig pathogen that can cause meningitis in humans [105].
Various studies have demonstrated the stimulation of antibody titers against BG antigens under different conditions of bacterial exposure, thus, supporting the hypothesis of an immunologic defense function of BG antigen (non-)expression [12]. Another indication for the implication of ABO histo-BGs in immunological processes was given by the findings that the efficacy and the vibriocidal antibody response of cholera vaccines was lower in BG O compared to BG A individuals [106], [107]. Therefore, exploring the role of anti-BG antibody response and protection following specific vaccinations is of great interest, as has been shown in a systems biology approach using glycan microarray analysis of immunoglobulin G preparations from different healthy populations [108]. The pooled antibody preparations bound to various self-antigen ligands including especially GLOB, FORS1, Ley, BG A1 and BG B epitopes, but also H2 and sialyl-Lea in some cases. The authors suggested a protective role for these self-antibodies by blocking bacterial attachment sites if released on epithelial surfaces.
In the context of viral infections, associations of non-B as well as Se genotype and phenotype with norovirus or rotavirus infection and gastroenteritis have been reported in numerous studies (Table 2) [84], [85], [86], [87], [88]. In addition, a certain rotavirus genotype, P[11] that mainly infects neonates, was found to attach specifically to saliva from neonates and infants, but not adults, due to high expression of polylactosamines, i.e. the i antigen [109]. Different viral proteins from selected rotaviral genotypes were found to bind to either i epitopes or their type 1 isomers with or without internal Lex of human milk oligosaccharides in a strain- and viral protein-dependent manner [64]. Consequently, infectivity seems to be highly dependent on the virus genotype in interaction with the host ancestry/ethnicity and different histo-BGs. For norovirus, multivalent virus-like particle vaccines have therefore been proposed representing a broad set of viral genotypes and strains [110]. Interestingly, anti-histo-BG, but not immunoglobulin G or total antibody titers prior to the challenge with norovirus were positively correlated with protection against infection in placebo-recipients, while in vaccinees the frequency of severe disease was lower with higher pre-challenge anti-histo-BG antibody titers [111]. For the development of efficient vaccines against norovirus and rotavirus, individualized approaches addressing all the relevant factors such as virus genotype, the specific particles and adjuvants used in the vaccine, and their interaction with the histo-BG antigens and anti-histo-BG antibodies of the recipients will be of particular interest in the future.
O-BG was associated with resistance towards severe acute respiratory syndrome caused by coronavirus [112]. Se phenotype has been linked to other respiratory virus diseases, including influenza, rhinovirus, respiratory syncytial virus, and echovirus [89]. Non-secretor genotype and phenotype are also associated with protective aspects in HIV, such as reduced infection risk (p = .029) [90] and slower disease progression (p < .001) [113]. Likewise, Pk expression has been shown protective against HIV-1 infection in vitro as compared to p phenotype [114]. The role of the P1Pk BG in HIV infection is reviewed in Ref. [115]. In contrast, P antigen might be implicated in parvovirus B19 infection, shown as its primary receptor [116].
BGs have also been associated with parasitic diseases (Table 2), in particular, BG-O is a recognized genetic factor for a lower risk of severe malaria [72]. The high percentage of O-individuals in malaria-endemic regions supports the hypothesis for a selective advantage of having BG O [13]. Furthermore, the incidence of schistosome infection and disease severity has been linked to A-BG [69].
5. Non-communicable diseases and glycan histo-blood groups
Since histo-BG glycans are recognized for their role as oncodevelopmental antigens [33] and are moreover linked to tissue differentiation stage [4], it is not surprising that changes in their expression in the context of malignancies have been described [30], [117] — and more recently other glycosylation changes in cancer (for extensive reviews, see Refs. [15], [118], [119]). Moreover, epidemiological studies found a decreased risk of various types of cancers in BG O and an increased risk in BG A, as found in a meta-analysis [70] (Table 2). However, in another meta-analysis no significant association was found when comparing O vs. non-O BGs, except for exocrine pancreatic cancer (OR = 0.53 and 95% CI 0.33–0.83 for BG-O vs. non-O) [73]. Further associations were found for Le and Se genotypes with breast cancer susceptibility and axillary lymph nodes metastasis, respectively [76].
In clinical samples, an increase of either incomplete/truncated precursor structures (T, Tn, Pk, H, Ii, type 1 chain) or de novo expressed cancer antigens, i.e. increased sialyl-Lea/x (Fig. 4), or FORS1, P, Leb (Se) and A antigens in otherwise null-allelic individuals, is observed in various cancer types depending on the cancer stage [15], [120], [121]. Accordingly, sialyl-Tn is a recognized tumor marker and has been proposed as a target for the design of anticancer vaccines [122], however, with limited success to date [123]. Sialyl-Lea/x appear to play a role in later tumor stages and especially in metastasis [15]. Notably, FUT3 (Le) and alpha2,3-sialyltransferase transcription and sialyl-Lea/x antigen expression was shown to be upregulated in H. pylori infected patients with early onset gastric carcinoma [124]. Moreover, sialyl-Lea (CA19-9) is elevated in various types of gastrointestinal carcinomas and is an approved prognostic marker for pancreatic cancer therapy, however, in Le-positive patients only. Since Le-negative individuals do not express an active alpha1,4-FUT, sialyl-Lea/CA19-9 serum levels are significantly lower or even non-detectable in pancreatic cancer patients with null-allelic Le-gene [17].
Another significant glycomic BG antigen change in cancer is a loss of A and B antigens in premalignant or malignant and metastatic tissue of otherwise A- or B-positive individuals [120], [125], [126], [127]. This switch has furthermore been linked to enhanced cellular motility and poor prognosis [117]. The A/B expression loss goes along with a higher abundance of the precursor structure I antigen in carcinomas from secretors, whereas cancerous tissue from non-secretors experiences a lower I antigen expression [121]. Moreover, an increased distribution of H antigen throughout all the layers of malignant tissue as compared to a more restricted pattern in normal mucosa has been found [126], [128]. Analogously, alpha1,2-FUT activity from both Se- and H-gene encoded enzymes were higher in rectal carcinomas than in normal tissue [129]. Interestingly, Se/H-antigens were found in cecal tumors from Se- as well as non-Se patients, while being absent in normal tissues of non-secretors. A very recent investigation of cyst fluid and tissue from ovarian tumors has shown remarkable differences in ABO BG antigen expression between different tumor stages and subtypes [130]. By using mass spectrometry average compositions of fucosylation (ABO and Le antigens) and sialylation of mucin-type glycans, the authors were able to distinguish three groups of samples: i) low-grade, low malignant potential carcinomas and benign mucinous, ii) all serous epithelial ovarian carcinomas, and iii) serous benign samples. Again, non-secretor samples were exceptions due to a genetically determined lower expression of BG epitopes on epithelia.
The described glycomic changes in cancer have a potential as therapeutic vaccine targets, including histo-BG antigens [16], [131]. Moreover, ABO BG was demonstrated as a possible parameter for detecting responders to a therapeutic prostate cancer vaccine [132]. Compared to BG A/AB participants, BG O and B patients experienced enhanced survival after vaccination, especially if they developed an antibody response against the FORS1 antigen. This was explained by the authors by the presence of FORS1 in the poxviral vector used for the vaccine, showing structural similarity to the A-antigen. If verified, the conclusions drawn from this and similar studies could open an invaluable opportunity for an effective immunization strategy in a blood-group specific manner by designing vaccines with BG epitopes complementary to the BG of the recipients who would thereby develop an enhanced immune response.
In summary, malignancy-associated changes of histo-BG glycans can be different in individuals with different genetic histo-BG backgrounds and may even be tumor stage- and subtype-specific, revealing them as promising biomarkers for (early) cancer diagnostics, as vaccine targets, and as relevant factors to include in personalized oncotherapeutic approaches.
Associations between ABO and especially the non-Se phenotype or genotype with inflammatory and autoimmune diseases such as Crohn's disease, primary sclerosing cholangitis, chronic pancreatitis, and type 1 diabetes have been reported [21], [22], [82], [83]. In addition, childhood asthma was found to have a higher incidence in secretors with BG O and/or Le-negative phenotype [77]. A possible explanation for the higher susceptibility to Crohn's disease in non-secretors is the link via gut microbiota. Intestinal microbiome composition differs between healthy Se and non-Se individuals as well as between different ABO phenotypes among secretors [133], [134]. Moreover, perturbations in gut microbiota metabolism and other factors of the host-microbial environment in non-secretors and FUT2 heterozygous individuals with Crohn's disease have been reported [135]. Fucosylation in the gut, which is primarily mediated by FUT2 and can be induced by certain gut bacteria via yet unknown mechanisms, has a central role in the host-microbe symbiosys and can suppress pathogens [136]. Interestingly, host fucose utilization by specific microbes is only down-regulated in non-Se mice if fed a glucose-rich, polysaccharide-deficient diet, demonstrating an intense interaction between host genetics, diet and gut microbiome [137]. Notably, gastrointestinal malignancies, in particular colorectal cancer, have also been linked to gut microbiota composition [138].
Intriguingly, a previously unrecognized regulatory role of histo-BG antigens in inflammatory adhesion processes has been proposed due to a strong correlation of the ABO locus with plasma concentrations of the soluble intercellular adhesion molecule 1 (sICAM-1) [139]. Moreover, the ABO locus was associated with diabetes type 2 and E-selectin levels, besides sICAM-1 levels [140]. In addition, a lower risk of cardiovascular and associated diseases has been found for BG O individuals and linked to BG antigens on platelet glycoconjugates, as recently reviewed [74]. Interestingly, BG O individuals have lower levels of the blood clotting factors von Willebrand and FVIII due to their shorter plasma half-life [141].
In addition to cancer-related changes in Ii antigen expression mentioned above, a rare disease association has been reported for the adult i phenotype. A correlation was found with congenital cataracts in certain kindreds [44]. This has been putatively explained by a direct effect of the lack of branched type 2 polylactosamines resulting from a certain null-allelic gene variant of the I-antigen producing enzyme in the human lens epithelial cells [23], [38], [44].
Some indications do exist that variants of the T-synthase gene generating T antigen are associated with susceptibility to IgA nephropathy [142].
A downregulation of the B4GALNT2 gene resulting in strong reduction of Sda antigen expression in the colon and a concomitant increase of sialyl-Lex expression is observed in colon cancer and might offer a promising opportunity in cancer therapy in the future [143].
6. Conclusions and future perspectives
As defined by the US Food and Drugs Administration, personalized medicine is the “tailoring of medical treatment to the individual characteristics, needs, and preferences of a patient during all stages of care, including prevention, diagnosis, treatment, and follow-up” [144]. As briefly demonstrated by the example of the interaction of an individual's Se status and gut microbiota, histo-BGs might play a significant role already at the early stage of disease prevention. Another example is the various histo-BG glycans in human milk, which are suggested to provide double protection to the breastfed child via their prebiotic and their antimicrobial activity in the gut [145]. The decoy function of milk glycans, in particular the highly abundant free oligosaccharides and mucin glycans, offers a yet underexamined possibility for a novel preventive and therapeutic antibiotic strategy. Emerging high-throughput analytical technologies such as glycan microarrays, multiplexed capillary electrophoresis, or matrix-assisted laser desorption/ionization (MALDI) mass spectrometry (MS) will pave the way for the discovery of such novel bioactive compounds [64], [65], [146], [147].
Traditionally, BG phenotyping is performed via targeted immunological assays using antisera, lectins, or monoclonal antibodies with or without staining on body fluids or tissues to determine the specific antigenicities [41], [121], [130], [148], [149]. An immunoassay is also used for the determination of CA19-9 (sialyl-Lea) levels in clinics [17]. Genotyping, glycosyltransferase transcript analysis as well as standard proteomic techniques are currently being increasingly used also in the context of BG-typing [27], [130], and do therefore comply with the requirements of modern personalized medicine. Furthermore, they might be useful for patient stratification in settings where the genetic histo-BG background in combination with the natural anti-BG antigen antibodies plays a role, such as vaccine efficacy studies. The reverse ABO-typing method developed by Muthana and colleagues by using only 4 μL of serum with glycan arrays was proven as particularly useful since it enabled retrospective BG typing without having whole blood samples available [132].
Our current knowledge on the significance of histo-BGs in the context of disease as summarized in 5, 6 is thus far mainly based on observational studies stratifying patients and controls by their blood type and showing associations with disease or disease stages (Table 2). Information on the underlying mechanisms is very limited to date, possibly due to the extremely reductionistic approach that emerged from transfusion medicine, i.e. grouping individuals into a few categories deduced from their serological phenotype (Table 1, Table 2). However, the knowledge of a patient's RBC pheno −/genotype or glycosyltransferase expression is only indicative for the final, complex glycomic profile [62], especially if taking into account the vast tissue- and disease-specific variations of the histo-BG antigens, their precursors and conjugates, as well as interactions between different BGs, as discussed in 2, 4. Thus, simultaneous mapping of glycans from different histo-BGs, next to the information on their carriers would provide the increased degree of comprehensiveness that is required to investigate the mechanisms behind the BG-to-disease associations presented above.
Modern glyco-analytical techniques, such as MS and liquid chromatography, are in principle capable to provide this type of information on multiple levels:
-
1)
(semi-)quantitative determination of terminal glycosylation including histo-BG glycans as well as sialylation, the levels of which appear to be inter-related;
-
2)
mapping of the overall glycome by furthermore providing information on the core glycan structures bearing the terminal BG glycans, i.e. N- or O-glycans, or the glycan portion of the GSL-based BG structures;
-
3)
determination of the protein/peptide or lipid carrier.
Thus, instead of just assigning samples to a limited set of categories as based on traditional or genetic BG typing, advanced glyco-analytics will enable an unprecedented insight into the vast complexity and variety of structures (Fig. 1) reflecting the underlying complex biological processes. Therefore, glycomic, together with glycoproteomic and glycolipidomic technologies should be further refined to allow unambiguous (isomer-specific) structural characterization and optimally quantitation of histo-BG glycans that have high potential as diagnostic or prognostic biomarkers in the context of both infectious and non-communicable diseases. Currently applied glyco-analytical techniques in the field of biomarker discovery from cancer tissues are presented in Ref. [150]. Major progress in glycomic approaches distinguishing linkages and/or different monosaccharide species has been made recently in the field of N- and O-glycomics [151], [152]. However, glycomics of histo-BGs has been neglected in the last years except for some few, rather targeted approaches [153], [154], [155]. In a very recent study traditional, targeted techniques in combination with liquid-chromatography-MS/MS were successfully applied showing great potential of mucin-type histo-BG glycans for ovarian tumor staging and classification not only from tissues, but also from cystic fluid [130]. Glycomic approaches on only minute amounts of preferably non-invasively acquired patient samples, such as saliva, tears, milk, urine, or feces [66], [156], [157], [158], [159], will be of particular use in the context of early diagnosis and population-wide screening.
The discovery of novel functions of histo-BGs by applying high-end technologies is warranted. Interestingly, the spatial organization of ABO antigen patches on RBCs from A, B, and O individuals was shown to differ in BG-specific manner resulting in higher or lower binding of sialic acid-binding proteins (Siglecs) to sialic acid-rich clusters that are co-expressed nearby the ABH regions [160]. The authors claimed having found a novel function of ABH antigens, i.e. as indirect stabilizers of other glycan-protein interactions. Further research is warranted to reveal the function relevance of such multi-compound complexes involving the concerted binding of glycans in a multivalent manner.
Finally, a combination of the different OMICs techniques including glycomics will likely enhance the performance of the diagnostic and prognostic approaches [15] and pave the way for a successful translation into clinics. As a first example, in a pioneering combined genomics-glycomics study potential epigenetic regulatory factors of Le-FUT (FUT3), next to other FUTs, were proposed on the basis of plasma protein N-glycome [161]. Studies combining genomic, proteomic, metabolomic and glycomic data containing histo-BG antigen information of different body fluids and/or tissues could help revealing the mechanisms behind the above described disease associations and may provide improved disease biomarkers as well as therapeutic targets in the future.
Conflict of interest statement
The authors declare no conflict of interest.
Transparency Document
Transparency document.
Acknowledgments
This work was supported by the European Union Seventh Framework Programme HighGlycan project, grant number 278535, and the Horizon 2020 GlyCoCan project, grant number 676421.
Footnotes
This article is part of a Special Issue entitled "Glycans in personalised medicine" Guest Editor: Professor Gordan Lauc.
The Transparency document associated with this article can be found, in online version.
References
- 1.Landsteiner K. Ueber Agglutinationserscheinungen normalen menschlichen Blutes. Wien. Klin. Wochenschr. 1901;14:1132–1134. [PubMed] [Google Scholar]
- 2.Storry J.R., Castilho L., Daniels G., Flegel W.A., Garratty G., de Haas M. International Society of Blood Transfusion Working Party on red cell immunogenetics and blood group terminology: Cancun report (2012) Vox Sang. 2014;107:90–96. doi: 10.1111/vox.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ISBT (International Society of Blood Transfusion) Red cell immunogenetics and blood group terminology. 2015. http://www.isbtweb.org/working-parties/red-cell-immunogenetics-and-blood-group-terminology/ (2015) (accessed December 29, 2015)
- 4.Ravn V., Dabelsteen E. Tissue distribution of histo-blood group antigens. APMIS. 2000;108:1–28. doi: 10.1034/j.1600-0463.2000.d01-1.x. [DOI] [PubMed] [Google Scholar]
- 5.Nydegger U.E., Tevaearai H., Berdat P., Rieben R., Carrel T., Mohacsi P. Histo-blood group antigens as allo- and autoantigens. Ann. N. Y. Acad. Sci. 2005;1050:40–51. doi: 10.1196/annals.1313.006. [DOI] [PubMed] [Google Scholar]
- 6.Watkins W.M. Molecular basis of antigenic specificity in the ABO, H and Lewis blood-group systems. In: J. J.V.M., Schachter H., editors. Glycoproteins. Elsevier Science B.V; Amsterdam: 1995. pp. 313–390. [Google Scholar]
- 7.Martins L.C., de Oliveira Corvelo T.C., Oti H.T., Loiola R.d.S.P., Aguiar D.C., Barile K.A.d.S. ABH and Lewis antigen distributions in blood, saliva and gastric mucosa and H. pylori infection in gastric ulcer patients. World J. Gastroenterol. 2006;12:1120–1124. doi: 10.3748/wjg.v12.i7.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundblad A. Excretion of oligosaccharides in the urine of secretors and non-secretors belonging to different blood groups. Biochim. Biophys. Acta, Gen. Subj. 1966;130:130–142. doi: 10.1016/0304-4165(66)90015-8. [DOI] [Google Scholar]
- 9.Kuhn R., Gauhe A. Über ein kristallisiertes, Lea-aktives Hexasaccharid aus Frauenmilch. Chem. Ber. 1960;93:647–651. [Google Scholar]
- 10.Varki A., Cummings R.D. In: Glossary, Essentials Glycobiol. Esko J.D., editor. 2009. http://www.ncbi.nlm.nih.gov/books/NBK1932/ [Google Scholar]
- 11.Walt D., Aoki-Kinoshita K.F., Bertozzi C.R., Boons G.-J. National Academies Press; Washington (DC): 2012. Transforming Glycoscience: A Roadmap for the Future.http://www.ncbi.nlm.nih.gov/books/NBK109958/pdf/Bookshelf_NBK109958.pdf [PubMed] [Google Scholar]
- 12.Cooling L. Blood groups in infection and host susceptibility. Clin. Microbiol. Rev. 2015;28:801–870. doi: 10.1128/CMR.00109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anstee D.J. The relationship between blood groups and disease. Blood. 2010;115:4635–4643. doi: 10.1182/blood-2010-01-261859. [DOI] [PubMed] [Google Scholar]
- 14.Kato K., Ishiwa A. The role of carbohydrates in infection strategies of enteric pathogens. Trop. Med. Health. 2015;43:41–52. doi: 10.2149/tmh.2014-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinho S.S., Reis C.A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 16.Heimburg-Molinaro J., Lum M., Vijay G., Jain M., Almogren A., Rittenhouse-Olson K. Cancer vaccines and carbohydrate epitopes. Vaccine. 2011;29:8802–8826. doi: 10.1016/j.vaccine.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ducreux M., Cuhna A.S., Caramella C., Hollebecque A., Burtin P., Goéré D. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;26:v56–v68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 18.Bugert P., Scharberg E.A., Geisen C., von Zabern I., Flegel W.A. RhCE protein variants in Southwestern Germany detected by serologic routine testing. Transfusion. 2009;49:1793–1802. doi: 10.1111/j.1537-2995.2009.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu F., Tao S., Xu X., Ying Y., Hong X., Zhu H. Distribution of ABO blood group allele and identification of three novel alleles in the Chinese Han population. Vox Sang. 2010;98:554–559. doi: 10.1111/j.1423-0410.2009.01291.x. [DOI] [PubMed] [Google Scholar]
- 20.Race R.R., Sanger R. sixth ed. Blackwell Scientific Publications; Oxford: 1975. Blood Groups in Man. [Google Scholar]
- 21.Weiss F.U., Schurmann C., Guenther A., Ernst F., Teumer A., Mayerle J. Fucosyltransferase 2 (FUT2) non-secretor status and blood group B are associated with elevated serum lipase activity in asymptomatic subjects, and an increased risk for chronic pancreatitis: a genetic association study. Gut. 2015;64:646–656. doi: 10.1136/gutjnl-2014-306930. [DOI] [PubMed] [Google Scholar]
- 22.McGovern D.P.B., Jones M.R., Taylor K.D., Marciante K., Yan X., Dubinsky M. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn's disease. Hum. Mol. Genet. 2010;19:3468–3476. doi: 10.1093/hmg/ddq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooling L. Polylactosamines, there's more than meets the “Ii”: a review of the I system. Immunohematology. 2010;26:133–155. [PubMed] [Google Scholar]
- 24.Kaczmarek R., Buczkowska A., Mikołajewicz K., Krotkiewski H., Czerwinski M. P1PK, GLOB, and FORS blood group systems and GLOB collection: biochemical and clinical aspects. Do We understand it all yet? Transfus. Med. Rev. 2014;28:126–136. doi: 10.1016/j.tmrv.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Patnaik S.K., Helmberg W., Blumenfeld O.O. BGMUT: NCBI dbRBC database of allelic variations of genes encoding antigens of blood group systems. Nucleic Acids Res. 2012;40:D1023–D1029. doi: 10.1093/nar/gkr958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dall'Olio F., Malagolini N., Chiricolo M., Trinchera M., Harduin-Lepers A. The expanding roles of the Sd(a)/Cad carbohydrate antigen and its cognate glycosyltransferase B4GALNT2. Biochim. Biophys. Acta. 2014;1840:443–453. doi: 10.1016/j.bbagen.2013.09.036. [DOI] [PubMed] [Google Scholar]
- 27.Svensson L., Hult A.K., Stamps R., Ångström J., Teneberg S., Storry J.R. Forssman expression on human erythrocytes: biochemical and genetic evidence of a new histo-blood group system. J. Am. Soc. Hematol. 2013;121:1459–1468. doi: 10.1182/blood-2012-10-455055. [DOI] [PubMed] [Google Scholar]
- 28.Daniels G. second ed. Wiley-Blackwell; Hoboken: 2002. Human blood Groups. [Google Scholar]
- 29.Wilczynska Z., Miller-Podraza H., Koscielak J. The contribution of different glycoconjugates to the total ABH blood group activity of human erythrocytes. FEBS Lett. 1980;112:277–279. doi: 10.1016/0014-5793(80)80197-9. [DOI] [PubMed] [Google Scholar]
- 30.Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim. Biophys. Acta, Gen. Subj. 1999;1473:247–266. doi: 10.1016/S0304-4165(99)00183-X. [DOI] [PubMed] [Google Scholar]
- 31.Clausen H., Hakomori S. ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution. Vox Sang. 1989;56:1–20. doi: 10.1111/j.1423-0410.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 32.A. Varki, R.D. Cummings, J.D. Esko, H.H. Freeze, P. Stanley, C.R. Bertozzi, et al., Structures Common to Different Glycans – Essentials of Glycobiology – NCBI Bookshelf, (n.d.). http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=glyco2&part=ch13#ch13.s7.
- 33.Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985;314:53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- 34.Oriol R., Le Pendu J., Mollicone R. Genetics of ABO, H, Lewis, X and related antigens. Vox Sang. 1986;51:161–171. doi: 10.1111/j.1423-0410.1986.tb01946.x. [DOI] [PubMed] [Google Scholar]
- 35.Oriol R. Genetic control of the fucosylation of ABH precursor chains. Evidence for new epistatic interactions in different cells and tissues. J Immunogenet. 1990;17:235–245. doi: 10.1111/j.1744-313x.1990.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 36.Morgan W.T., Watkins W.M. Unravelling the biochemical basis of blood group ABO and Lewis antigenic specificity. Glycoconj. J. 2000;17:501–530. doi: 10.1023/a:1011014307683. [DOI] [PubMed] [Google Scholar]
- 37.Mollicone R., Cailleau A., Oriol R. Molecular genetics of H, Se, Lewis and other fucosyltransferase genes. Transfus. Clin. Biol. 1995;2:235–242. doi: 10.1016/s1246-7820(05)80089-8. [DOI] [PubMed] [Google Scholar]
- 38.Yu L.C., Lin M. Molecular genetics of the blood group I system and the regulation of I antigen expression during erythropoiesis and granulopoiesis. Curr. Opin. Hematol. 2011;18:421–426. doi: 10.1097/MOH.0b013e32834baae9. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto F., Clausen H., White T., Marken J., Hakomori S. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990;345:229–233. doi: 10.1038/345229a0. [DOI] [PubMed] [Google Scholar]
- 40.Hellberg A., Westman J.S., Thuresson B., Olsson M.L. P1PK: the blood group system that changed its name and expanded. Immunohematology. 2013;29:25–33. [PubMed] [Google Scholar]
- 41.Ebert W., Roelcke D., Weicker H. The I Antigen of Human Red Cell Membrane. Eur. J. Biochem. 1975;53:505–515. doi: 10.1111/j.1432-1033.1975.tb04093.x. [DOI] [PubMed] [Google Scholar]
- 42.Koscielak J., Zdebska E., Wilczynska Z., Miller-Podraza H., Dzierzkowa-Borodej W. Immunochemistry of Ii-active glycosphingolipids of erythrocytes. Eur. J. Biochem. 1979;96:331–337. doi: 10.1111/j.1432-1033.1979.tb13044.x. [DOI] [PubMed] [Google Scholar]
- 43.Zdebska E., Krauze R., Koscielak J. Structure and blood-group I activity of poly(glycosyl)-ceramides. Carbohydr. Res. 1983;120:113–130. doi: 10.1016/0008-6215(83)88011-2. [DOI] [PubMed] [Google Scholar]
- 44.Yu L.C., Twu Y.C., Chang C.Y., Lin M. Molecular basis of the adult i phenotype and the gene responsible for the expression of the human blood group I antigen. Blood. 2001;98:3840–3845. doi: 10.1182/blood.V98.13.3840. [DOI] [PubMed] [Google Scholar]
- 45.Renkonen O. Enzymatic in vitro synthesis of I-branches of mammalian polylactosamines: generation of scaffolds for multiple selectin-binding saccharide determinants. Cell. Mol. Life Sci. 2000;57:1423–1439. doi: 10.1007/PL00000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Pendu J., Lemieux R.U., Dalix A.M., Lambert F., Oriol R. Competition between ABO and Le gene specified enzymes. I. A Lewis related difference in the amount of A antigen in saliva of A1 and A2 secretors. Vox Sang. 1983;45:349–358. [PubMed] [Google Scholar]
- 47.Le Pendu J., Oriol R., Lambert F., Dalix A.M., Lemieux R.U. Competition between ABO and Le gene specified enzymes. II. Quantitative analysis of A and B antigens in saliva of ABH nonsecretors. Vox Sang. 1983;45:421–425. [PubMed] [Google Scholar]
- 48.Watkins W.M., Morgan W.T.J. Specific inhibition studies relating to the Lewis blood-group system. Nature. 1957;180:1038–1040. doi: 10.1038/1801038a0. [DOI] [PubMed] [Google Scholar]
- 49.Johnson P.H., Donald A.S., Feeney J., Watkins W.M. Reassessment of the acceptor specificity and general properties of the Lewis blood-group gene associated alpha-3/4-fucosyltransferase purified from human milk. Glycoconj. J. 1992;9:251–264. doi: 10.1007/BF00731137. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1490104 [DOI] [PubMed] [Google Scholar]
- 50.Cartron J.P., Mulet C., Bauvois B., Rahuel C., Salmon C. ABH and Lewis glycosyltransferases in human red cells, lymphocytes and platelets. Rev. Fr. Transfus. 1980;23:271–282. doi: 10.1016/s0338-4535(80)80131-0. [DOI] [PubMed] [Google Scholar]
- 51.Henry S.M., Oriol R., Samuelsson B.E. Detection and characterization of Lewis antigens in plasma of Lewis-negative individuals. Evidence of chain extension as a result of reduced fucosyltransferase competition. Vox Sang. 1994;67:387–396. doi: 10.1111/j.1423-0410.1994.tb01279.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7701811 [DOI] [PubMed] [Google Scholar]
- 52.Okuda T., Tokuda N., Numata S., Ito M., Ohta M., Kawamura K. Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. J. Biol. Chem. 2006;281:10230–10235. doi: 10.1074/jbc.M600057200. [DOI] [PubMed] [Google Scholar]
- 53.Suchanowska A., Kaczmarek R., Duk M., Lukasiewicz J., Smolarek D., Majorczyk E. A Single Point Mutation in the Gene Encoding Gb3/CD77 Synthase Causes a Rare Inherited Polyagglutination Syndrome. J. Biol. Chem. 2012;287:38220–38230. doi: 10.1074/jbc.M112.408286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ju T., Otto V.I., Cummings R.D. The Tn antigen-structural simplicity and biological complexity. Angew. Chem. Int. Ed. Engl. 2011;50:1770–1791. doi: 10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gillard B.K., Blanchard D., Bouhours J.F., Cartron J.P., van Kuik J.A., Kamerling J.P. Structure of a ganglioside with Cad blood group antigen activity. Biochemistry. 1988;27:4601–4606. doi: 10.1021/bi00413a003. [DOI] [PubMed] [Google Scholar]
- 56.Achermann F.J., Julmy F., Gilliver L.G., Carrel T.P., Nydegger U.E. Soluble type A substance in fresh-frozen plasma as a function of ABO and secretor genotypes and Lewis phenotype. Transfus. Apher. Sci. 2005;32:255–262. doi: 10.1016/j.transci.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Olsson M.L., Irshaid N.M., Hosseini-Maaf B., Hellberg A., Moulds M.K., Sareneva H. Genomic analysis of clinical samples with serologic ABO blood grouping discrepancies: identification of 15 novel A and B subgroup alleles. Blood. 2001;98:1585–1593. doi: 10.1182/blood.v98.5.1585. [DOI] [PubMed] [Google Scholar]
- 58.Hammar L., Månsson S., Rohr T., Chester M.A., Ginsburg V., Lundblad A. Lewis phenotype of erythrocytes and Le b-active glycolipid in serum of pregnant women. Vox Sang. 1981;40:27–33. doi: 10.1111/j.1423-0410.1981.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 59.Marsh W.L. Anti-i: a cold antibody defining the Ii relationship in human red cells. Br. J. Haematol. 1961;7:200–209. doi: 10.1111/j.1365-2141.1961.tb00329.x. http://www.ncbi.nlm.nih.gov/pubmed/13767181 [DOI] [PubMed] [Google Scholar]
- 60.Schaeffer A.J., Navas E.L., Venegas M.F., Anderson B.E., Kanerva C., Chmiel J.S. Variation of blood group antigen expression on vaginal cells and mucus in secretor and nonsecretor women. J. Urol. 1994;152:859–864. doi: 10.1016/s0022-5347(17)32591-0. [DOI] [PubMed] [Google Scholar]
- 61.Agrawal P., Kurcon T., Pilobello K.T., Rakus J.F., Koppolu S., Liu Z. Mapping posttranscriptional regulation of the human glycome uncovers microRNA defining the glycocode. Proc. Natl. Acad. Sci. 2014;111:4338–4343. doi: 10.1073/pnas.1321524111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamamoto F., Cid E., Yamamoto M., Saitou N., Bertranpetit J., Blancher A. An integrative evolution theory of histo-blood group ABO and related genes. Sci. Rep. 2014;4:6601. doi: 10.1038/srep06601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koscielak J. The hypothesis on function of glycosphingolipids and ABO blood groups revisited. Neurochem. Res. 2012;37:1170–1184. doi: 10.1007/s11064-012-0734-0. [DOI] [PubMed] [Google Scholar]
- 64.Yu Y., Lasanajak Y., Song X., Hu L., Ramani S., Mickum M.L. Human milk contains novel glycans that are potential decoy receptors for neonatal rotaviruses. Mol. Cell. Proteomics. 2014;13:2944–2960. doi: 10.1074/mcp.M114.039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blank D., Dotz V., Geyer R., Kunz C. Human milk oligosaccharides and Lewis blood group: individual high-throughput sample profiling to enhance conclusions from functional studies. Adv. Nutr. 2012;3:440S–449S. doi: 10.3945/an.111.001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Everest-Dass A.V., Jin D., Thaysen-Andersen M., Nevalainen H., Kolarich D., Packer N.H. Comparative structural analysis of the glycosylation of salivary and buccal cell proteins: innate protection against infection by Candida albicans. Glycobiology. 2012;22:1465–1479. doi: 10.1093/glycob/cws112. [DOI] [PubMed] [Google Scholar]
- 67.Blackwell C.C., Dundas S., James V.S., Mackenzie D.A., Braun J.M., Alkout A.M. Blood group and susceptibility to disease caused by Escherichia coli O157. J. Infect. Dis. 2002;185:393–396. doi: 10.1086/338343. [DOI] [PubMed] [Google Scholar]
- 68.Edgren G., Hjalgrim H., Rostgaard K., Norda R., Wikman A., Melbye M. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: a cohort study. Am. J. Epidemiol. 2010;172:1280–1285. doi: 10.1093/aje/kwq299. [DOI] [PubMed] [Google Scholar]
- 69.Ndamba J., Gomo E., Nyazema N., Makaza N., Kaondera K.C. Schistosomiasis infection in relation to the ABO blood groups among school children in Zimbabwe. Acta Trop. 1997;65:181–190. doi: 10.1016/S0001-706X(97)00671-2. [DOI] [PubMed] [Google Scholar]
- 70.Zhang B.L., He N., Huang Y.B., Song F.J., Chen K.X. ABO blood groups and risk of cancer: a systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2014;15:4643–4650. doi: 10.7314/apjcp.2014.15.11.4643. [DOI] [PubMed] [Google Scholar]
- 71.Robinson M.G., Tolchin D., Halpern C. Enteric bacterial agents and the ABO blood groups. Am. J. Hum. Genet. 1971;23:135–145. [PMC free article] [PubMed] [Google Scholar]
- 72.Timmann C., Thye T., Vens M., Evans J., May J., Ehmen C. Genome-wide association study indicates two novel resistance loci for severe malaria. Nature. 2012;489:443–446. doi: 10.1038/nature11334. [DOI] [PubMed] [Google Scholar]
- 73.Iodice S., Maisonneuve P., Botteri E., Sandri M.T., Lowenfels A.B. ABO blood group and cancer. Eur. J. Cancer. 2010;46:3345–3350. doi: 10.1016/j.ejca.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 74.Zhong M., Zhang H., Reilly J.P., Chrisitie J.D., Ishihara M., Kumagai T. ABO blood group as a model for platelet glycan modification in arterial thrombosis. Arterioscler. Thromb. Vasc. Biol. 2015 doi: 10.1161/ATVBAHA.115.305337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheinfeld J., Schaeffer A.J., Cordon-Cardo C., Rogatko A., Fair W.R. Association of the Lewis blood-group phenotype with recurrent urinary tract infections in women. N. Engl. J. Med. 1989;320:773–777. doi: 10.1056/nejm198903233201205. [DOI] [PubMed] [Google Scholar]
- 76.Teresa D.B., Santos R.A., Takahashi C.S., Carrara H.H., Moreira H.W., Mattos L.C. Polymorphisms of Lewis and secretor genes are related to breast cancer and metastasis in axillary lymph nodes. Tumour Biol. 2010;31:401–409. doi: 10.1007/s13277-010-0048-2. [DOI] [PubMed] [Google Scholar]
- 77.Chen Y.L., Chen J.C., Lin T.M., Huang T.J., Wang S.T., Lee M.F. ABO/secretor genetic complex is associated with the susceptibility of childhood asthma in Taiwan. Clin. Exp. Allergy. 2005;35:926–932. doi: 10.1111/j.1365-2222.2005.02278.x. [DOI] [PubMed] [Google Scholar]
- 78.Blackwell C.C., Jonsdottir K., Hanson M., Todd W.T., Chaudhuri A.K., Mathew B. Non-secretion of ABO antigens predisposing to infection by Neisseria meningitidis and Streptococcus pneumoniae. Lancet. 1986;2:284–285. doi: 10.1016/s0140-6736(86)92103-3. [DOI] [PubMed] [Google Scholar]
- 79.Blackwell C.C., Jonsdottir K., Hanson M.F., Weir D.M. Non-secretion of ABO blood group antigens predisposing to infection by Haemophilus influenzae. Lancet. 1986;2:687. doi: 10.1016/s0140-6736(86)90193-5. [DOI] [PubMed] [Google Scholar]
- 80.Morrow A.L., Meinzen-Derr J., Huang P., Schibler K.R., Cahill T., Keddache M. Fucosyltransferase 2 non-secretor and low secretor status predicts severe outcomes in premature infants. J. Pediatr. 2011;158:745–751. doi: 10.1016/j.jpeds.2010.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dickey W., Collins J.S., Watson R.G., Sloan J.M., Porter K.G. Secretor status and Helicobacter pylori infection are independent risk factors for gastroduodenal disease. Gut. 1993;34:351–353. doi: 10.1136/gut.34.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maroni L., van de Graaf S.F., Hohenester S.D., Oude Elferink R.P., Beuers U. Fucosyltransferase 2: a genetic risk factor for primary sclerosing cholangitis and Crohn's disease—a comprehensive review. Clin. Rev. Allergy Immunol. 2015;48:182–191. doi: 10.1007/s12016-014-8423-1. [DOI] [PubMed] [Google Scholar]
- 83.Smyth D.J., Cooper J.D., Howson J.M., Clarke P., Downes K., Mistry T. FUT2 nonsecretor status links type 1 diabetes susceptibility and resistance to infection. Diabetes. 2011;60:3081–3084. doi: 10.2337/db11-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Currier R.L., Payne D.C., Staat M.A., Selvarangan R., Shirley S.H., Halasa N. Innate susceptibility to norovirus infections influenced by FUT2 genotype in a United States pediatric population. Clin. Infect. Dis. 2015;60:1631–1638. doi: 10.1093/cid/civ165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruvoen-Clouet N., Belliot G., Le Pendu J. Noroviruses and histo-blood groups: the impact of common host genetic polymorphisms on virus transmission and evolution. Rev. Med. Virol. 2013;23:355–366. doi: 10.1002/rmv.1757. [DOI] [PubMed] [Google Scholar]
- 86.Rydell G.E., Kindberg E., Larson G., Svensson L. Susceptibility to winter vomiting disease: a sweet matter. Rev. Med. Virol. 2011;21:370–382. doi: 10.1002/rmv.704. [DOI] [PubMed] [Google Scholar]
- 87.Tan M., Jiang X. Histo-blood group antigens: a common niche for norovirus and rotavirus. Expert Rev. Mol. Med. 2014;16 doi: 10.1017/erm.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Payne D.C., Currier R.L., Staat M.A., Sahni L.C., Selvarangan R., Halasa N.B. Epidemiologic association between FUT2 secretor status and severe rotavirus gastroenteritis in children in the United States. JAMA Pediatr. 2015;1 doi: 10.1001/jamapediatrics.2015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raza M.W., Blackwell C.C., Molyneaux P., James V.S., Ogilvie M.M., Inglis J.M. Association between secretor status and respiratory viral illness. BMJ. 1991;303:815–818. doi: 10.1136/bmj.303.6806.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chanzu N.M., Mwanda W., Oyugi J., Anzala O. Mucosal blood group antigen expression profiles and HIV infections: a study among female sex workers in Kenya. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0133049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boren T., Falk P., Roth K.A., Larson G., Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 92.Suerbaum S., Michetti P. Helicobacter pylori Infection. N. Engl. J. Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 93.Stapleton A.E., Stroud M.R., Hakomori S.I., Stamm W.E. The globoseries glycosphingolipid sialosyl galactosyl globoside is found in urinary tract tissues and is a preferred binding receptor In vitro for uropathogenic Escherichia coli expressing pap-encoded adhesins. Infect. Immun. 1998;66:3856–3861. doi: 10.1128/iai.66.8.3856-3861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stapleton A., Nudelman E., Clausen H., Hakomori S., Stamm W.E. Binding of uropathogenic Escherichia coli R45 to glycolipids extracted from vaginal epithelial cells is dependent on histo-blood group secretor status. J. Clin. Invest. 1992;90:965–972. doi: 10.1172/JCI115973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bitzan M., Richardson S., Huang C., Boyd B., Petric M., Karmali M.A. Evidence that verotoxins (Shiga-like toxins) from Escherichia coli bind to P blood group antigens of human erythrocytes in vitro. Infect. Immun. 1994;62:3337–3347. doi: 10.1128/iai.62.8.3337-3347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Newburg D.S., Ashkenazi S., Cleary T.G. Human milk contains the Shiga toxin and Shiga-like toxin receptor glycolipid Gb3. J. Infect. Dis. 1992;166:832–836. doi: 10.1093/infdis/166.4.832. [DOI] [PubMed] [Google Scholar]
- 97.Newburg D.S., Ruiz-Palacios G.M., Altaye M., Chaturvedi P., Meinzen-Derr J., Guerrero Mde L. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology. 2004;14:253–263. doi: 10.1093/glycob/cwh020. [DOI] [PubMed] [Google Scholar]
- 98.Morrow A.L., Ruiz-Palacios G.M., Altaye M., Jiang X., Guerrero M.L., Meinzen-Derr J.K. Human milk oligosaccharide blood group epitopes and innate immune protection against campylobacter and calicivirus diarrhea in breastfed infants. Adv. Exp. Med. Biol. 2004;554:443–446. doi: 10.1007/978-1-4757-4242-8_61. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15384621 [DOI] [PubMed] [Google Scholar]
- 99.Ruiz-Palacios G.M., Cervantes L.E., Ramos P., Chavez-Munguia B., Newburg D.S. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 2003;278:14112–14120. doi: 10.1074/jbc.M207744200. [DOI] [PubMed] [Google Scholar]
- 100.Higgins M.A., Ficko-Blean E., Meloncelli P.J., Lowary T.L., Boraston A.B. The overall architecture and receptor binding of pneumococcal carbohydrate-antigen-hydrolyzing enzymes. J. Mol. Biol. 2011;411:1017–1036. doi: 10.1016/j.jmb.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 101.Arifuzzaman M., Ahmed T., Rahman M.A., Chowdhury F., Rashu R., Khan A.I. Individuals with Le(a + b −) blood group have increased susceptibility to symptomatic vibrio cholerae O1 infection. PLoS Negl. Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harris J.B., Khan A.I., LaRocque R.C., Dorer D.J., Chowdhury F., Faruque A.S. Blood group, immunity, and risk of infection with Vibrio cholerae in an area of endemicity. Infect. Immun. 2005;73:7422–7427. doi: 10.1128/iai.73.11.7422-7427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weichert S., Jennewein S., Hufner E., Weiss C., Borkowski J., Putze J. Bioengineered 2′-fucosyllactose and 3-fucosyllactose inhibit the adhesion of Pseudomonas aeruginosa and enteric pathogens to human intestinal and respiratory cell lines. Nutr. Res. 2013;33:831–838. doi: 10.1016/j.nutres.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 104.Gilboa-Garber N., Sudakevitz D., Sheffi M., Sela R., Levene C. PA-I and PA-II lectin interactions with the ABO(H) and P blood group glycosphingolipid antigens may contribute to the broad spectrum adherence of Pseudomonas aeruginosa to human tissues in secondary infections. Glycoconj. J. 1994;11:414–417. doi: 10.1007/BF00731276. [DOI] [PubMed] [Google Scholar]
- 105.Haataja S., Tikkanen K., Liukkonen J., Francois-Gerard C., Finne J. Characterization of a novel bacterial adhesion specificity of Streptococcus suis recognizing blood group P receptor oligosaccharides. J. Biol. Chem. 1993;268:4311–4317. [PubMed] [Google Scholar]
- 106.Clemens J.D., Sack D.A., Harris J.R., Chakraborty J., Khan M.R., Huda S. ABO blood groups and cholera: new observations on specificity of risk and modification of vaccine efficacy. J. Infect. Dis. 1989;159:770–773. doi: 10.1093/infdis/159.4.770. [DOI] [PubMed] [Google Scholar]
- 107.Qadri F., Chowdhury M.I., Faruque S.M., Salam M.A., Ahmed T., Begum Y.A. Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine. 2007;25:231–238. doi: 10.1016/j.vaccine.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 108.Schneider C., Smith D.F., Cummings R.D., Boligan K.F., Hamilton R.G., Bochner B.S. The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.3010524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Y., Huang P., Jiang B., Tan M., Morrow A.L., Jiang X. Poly-LacNAc as an age-specific ligand for rotavirus P[11] in neonates and infants. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0078113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Richardson C., Bargatze R.F., Goodwin R., Mendelman P.M. Norovirus virus-like particle vaccines for the prevention of acute gastroenteritis. Expert Rev. Vaccines. 2013;12:155–167. doi: 10.1586/erv.12.145. [DOI] [PubMed] [Google Scholar]
- 111.Atmar R.L., Bernstein D.I., Lyon G.M., Treanor J.J., Al-Ibrahim M.S., Graham D.Y. Serological correlates of protection against a GII.4 norovirus. Clin. Vaccine Immunol. 2015;22:923–929. doi: 10.1128/cvi.00196-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guillon P., Clément M., Sébille V., Rivain J.-G., Chou C.-F., Ruvoën-Clouet N. Inhibition of the interaction between the SARS-CoV Spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology. 2008;18:1085–1093. doi: 10.1093/glycob/cwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kindberg E., Hejdeman B., Bratt G., Wahren B., Lindblom B., Hinkula J. A nonsense mutation (428G–> A) in the fucosyltransferase FUT2 gene affects the progression of HIV-1 infection. AIDS. 2006;20:685–689. doi: 10.1097/01.aids.0000216368.23325.bc. [DOI] [PubMed] [Google Scholar]
- 114.Lund N., Olsson M.L., Ramkumar S., Sakac D., Yahalom V., Levene C. The human Pk histo-blood group antigen provides protection against HIV-1 infection. Blood. 2009;113:4980–4991. doi: 10.1182/blood-2008-03-143396. [DOI] [PubMed] [Google Scholar]
- 115.Branch D.R. Blood groups and susceptibility to virus infection: new developments. Curr. Opin. Hematol. 2010;17:558–564. doi: 10.1097/MOH.0b013e32833ece31. [DOI] [PubMed] [Google Scholar]
- 116.Brown K.E., Anderson S.M., Young N.S. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science. 1993;262:114–117. doi: 10.1126/science.8211117. [DOI] [PubMed] [Google Scholar]
- 117.Le Pendu J., Marionneau S., Cailleau-Thomas A., Rocher J., Le Moullac-Vaidye B., Clement M. ABH and Lewis histo-blood group antigens in cancer. APMIS. 2001;109:9–31. doi: 10.1111/j.1600-0463.2001.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 118.Stowell S.R., Ju T., Cummings R.D. Protein glycosylation in cancer. Annu. Rev. Pathol. 2015;10:473–510. doi: 10.1146/annurev-pathol-012414-040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Drake R., Ball L., editors. Glycosylation and Cancer. first ed. Academic Press; 2015. [Google Scholar]
- 120.Hakomori S., Kannagi R. Glycosphingolipids as tumor-associated and differentiation markers. J. Natl. Cancer Inst. 1983;71:231–251. doi: 10.1093/jnci/71.2.231. [DOI] [PubMed] [Google Scholar]
- 121.Kapadia A., Feizi T., Jewell D., Keeling J., Slavin G. Immunocytochemical studies of blood group A, H, I, and i antigens in gastric mucosae of infants with normal gastric histology and of patients with gastric carcinoma and chronic benign peptic ulceration. J. Clin. Pathol. 1981;34:320–337. doi: 10.1136/jcp.34.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Julien S., Picco G., Sewell R., Vercoutter-Edouart A.S., Tarp M., Miles D. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br. J. Cancer. 2009;100:1746–1754. doi: 10.1038/sj.bjc.6605083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Loureiro L.R., Carrascal M.A., Barbas A., Ramalho J.S., Novo C., Delannoy P. Challenges in antibody development against Tn and Sialyl-Tn antigens. Biomolecules. 2015;5:1783–1809. doi: 10.3390/biom5031783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Magalhaes A., Marcos-Pinto R., Nairn A.V., Rosa M.D., Ferreira R.M., Junqueira-Neto S. Helicobacter pylori chronic infection and mucosal inflammation switches the human gastric glycosylation pathways. Biochim. Biophys. Acta. 2015;1852:1928–1939. doi: 10.1016/j.bbadis.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gupta R.K., Schuster R. Isoantigens A, B and H in benign and malignant lesions of breast. Am. J. Pathol. 1973;72:253–257. [PMC free article] [PubMed] [Google Scholar]
- 126.Dabelsteen E., Vedtofte P., Hakomori S., Young W.W., Jr. Accumulation of a blood group antigen precursor in oral premalignant lesions. Cancer Res. 1983;43:1451–1454. [PubMed] [Google Scholar]
- 127.Davidsohn I., Kovarik S., Ni L.Y. Isoantigens A, B, and H in benign and malignant lesions of the cervix. Arch. Pathol. 1969;87:306–314. [PubMed] [Google Scholar]
- 128.Dabelsteen E. ABO blood group antigens in oral mucosa. What is new? J. Oral Pathol. Med. 2002;31:65–70. doi: 10.1046/j.0904-2512.2001.00004.x. [DOI] [PubMed] [Google Scholar]
- 129.Orntoft T.F., Greenwell P., Clausen H., Watkins W.M. Regulation of the oncodevelopmental expression of type 1 chain ABH and Lewis(b) blood group antigens in human colon by alpha-2-L-fucosylation. Gut. 1991;32:287–293. doi: 10.1136/gut.32.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vitiazeva V., Kattla J.J., Flowers S.A., Linden S.K., Premaratne P., Weijdegard B. The O-linked glycome and blood group antigens ABO on mucin-type glycoproteins in mucinous and serous epithelial ovarian tumors. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0130197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Livingston P.O., Hood C., Krug L.M., Warren N., Kris M.G., Brezicka T. Selection of GM2, fucosyl GM1, globo H and polysialic acid as targets on small cell lung cancers for antibody mediated immunotherapy. Cancer Immunol. Immunother. 2005;54:1018–1025. doi: 10.1007/s00262-005-0663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Muthana S.M., Gulley J.L., Hodge J.W., Schlom J., Gildersleeve J.C. 2015. ABO Blood Type Correlates with Survival on Prostate Cancer Vaccine Therapy, Oncotarget. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wacklin P., Tuimala J., Nikkila J., Sebastian T., Makivuokko H., Alakulppi N. Faecal microbiota composition in adults is associated with the FUT2 gene determining the secretor status. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0094863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Makivuokko H., Lahtinen S.J., Wacklin P., Tuovinen E., Tenkanen H., Nikkila J. Association between the ABO blood group and the human intestinal microbiota composition. BMC Microbiol. 2012;12:94. doi: 10.1186/1471-2180-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tong M., McHardy I., Ruegger P., Goudarzi M., Kashyap P.C., Haritunians T. Reprograming of gut microbiome energy metabolism by the FUT2 Crohn's disease risk polymorphism. ISME J. 2014;8:2193–2206. doi: 10.1038/ismej.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pickard J.M., Chervonsky A.V. Intestinal fucose as a mediator of host-microbe symbiosis. J. Immunol. 2015;194:5588–5593. doi: 10.4049/jimmunol.1500395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kashyap P.C., Marcobal A., Ursell L.K., Smits S.A., Sonnenburg E.D., Costello E.K. Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc. Natl. Acad. Sci. U. S. A. 2013;110:17059–17064. doi: 10.1073/pnas.1306070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thomas R.M., Jobin C. The microbiome and cancer: is the “oncobiome” mirage real? Trends Cancer. 2015;1:24–35. doi: 10.1016/j.trecan.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pare G., Chasman D.I., Kellogg M., Zee R.Y., Rifai N., Badola S. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6578 women. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qi L., Cornelis M.C., Kraft P., Jensen M., van Dam R.M., Sun Q. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum. Mol. Genet. 2010;19:1856–1862. doi: 10.1093/hmg/ddq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gallinaro L., Cattini M.G., Sztukowska M., Padrini R., Sartorello F., Pontara E. A shorter von Willebrand factor survival in O blood group subjects explains how ABO determinants influence plasma von Willebrand factor. Blood. 2008;111:3540–3545. doi: 10.1182/blood-2007-11-122945. [DOI] [PubMed] [Google Scholar]
- 142.Li G.S., Zhang H., Lv J.C., Shen Y., Wang H.Y. Variants of C1GALT1 gene are associated with the genetic susceptibility to IgA nephropathy. Kidney Int. 2007;71:448–453. doi: 10.1038/sj.ki.5002088. [DOI] [PubMed] [Google Scholar]
- 143.Groux-Degroote S., Wavelet C., Krzewinski-Recchi M.A., Portier L., Mortuaire M., Mihalache A. B4GALNT2 gene expression controls the biosynthesis of Sda and sialyl Lewis X antigens in healthy and cancer human gastrointestinal tract. Int. J. Biochem. Cell Biol. 2014;53:442–449. doi: 10.1016/j.biocel.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 144.FDA (US Food and Drug Administration) US Department of Health and Human Services, paving the way for personalized medicine. FDA's role in a new era of medical product development, 2015. 2013. http://www.fda.gov/down-loads/ScienceResearch/SpecialTopics/PersonalizedMedicine/UCM372421.pdf
- 145.Georgi G., Bartke N., Wiens F., Stahl B. Functional glycans and glycoconjugates in human milk. Am. J. Clin. Nutr. 2013;98:578S–585S. doi: 10.3945/ajcn.112.039065. [DOI] [PubMed] [Google Scholar]
- 146.Ashline D.J., Yu Y., Lasanajak Y., Song X., Hu L., Ramani S. Structural characterization by multistage mass spectrometry (MSn) of human milk glycans recognized by human rotaviruses. Mol. Cell. Proteomics. 2014;13:2961–2974. doi: 10.1074/mcp.M114.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kottler R., Mank M., Hennig R., Muller-Werner B., Stahl B., Reichl U. Development of a high-throughput glycoanalysis method for the characterization of oligosaccharides in human milk utilizing multiplexed capillary gel electrophoresis with laser-induced fluorescence detection. Electrophoresis. 2013;34:2323–2336. doi: 10.1002/elps.201300016. [DOI] [PubMed] [Google Scholar]
- 148.Watkins W.M., Morgan W.T. Immunochemical observations on the human blood group P system. J. Immunogenet. 1976;3:15–27. [PubMed] [Google Scholar]
- 149.Orntoft T.F., Holmes E.H., Johnson P., Hakomori S., Clausen H. Differential tissue expression of the Lewis blood group antigens: enzymatic, immunohistologic, and immunochemical evidence for Lewis a and b antigen expression in Le(a − b −) individuals. Blood. 1991;77:1389–1396. [PubMed] [Google Scholar]
- 150.Christiansen M.N., Chik J., Lee L., Anugraham M., Abrahams J.L., Packer N.H. Cell surface protein glycosylation in cancer. Proteomics. 2014;14:525–546. doi: 10.1002/pmic.201300387. [DOI] [PubMed] [Google Scholar]
- 151.Everest-Dass A.V., Abrahams J.L., Kolarich D., Packer N.H., Campbell M.P. Structural feature ions for distinguishing N- and O-linked glycan isomers by LC-ESI-IT MS/MS. J. Am. Soc. Mass Spectrom. 2013;24:895–906. doi: 10.1007/s13361-013-0610-4. [DOI] [PubMed] [Google Scholar]
- 152.Reiding K.R., Blank D., Kuijper D.M., Deelder A.M., Wuhrer M. High-throughput profiling of protein N-glycosylation by MALDI-TOF-MS employing linkage-specific sialic acid esterification. Anal. Chem. 2014;86:5784–5793. doi: 10.1021/ac500335t. [DOI] [PubMed] [Google Scholar]
- 153.Ferreira J.A., Domingues M.R., Reis A., Monteiro M.A., Coimbra M.A. Differentiation of isomeric Lewis blood groups by positive ion electrospray tandem mass spectrometry. Anal. Biochem. 2010;397:186–196. doi: 10.1016/j.ab.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 154.Zhang H., Zhang S., Tao G., Zhang Y., Mulloy B., Zhan X. Typing of blood-group antigens on neutral oligosaccharides by negative-ion electrospray ionization tandem mass spectrometry. Anal. Chem. 2013;85:5940–5949. doi: 10.1021/ac400700e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gao C., Zhang Y., Liu Y., Feizi T., Chai W. Negative-ion electrospray tandem mass spectrometry and microarray analyses of developmentally-regulated antigens based on type 1 and type 2 backbone sequences. Anal. Chem. 2015 doi: 10.1021/acs.analchem.5b03471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nguyen-Khuong T., Everest-Dass A.V., Kautto L., Zhao Z., Willcox M.D., Packer N.H. Glycomic characterization of basal tears and changes with diabetes and diabetic retinopathy. Glycobiology. 2015;25:269–283. doi: 10.1093/glycob/cwu108. [DOI] [PubMed] [Google Scholar]
- 157.Vieira A.C., An H.J., Ozcan S., Kim J.H., Lebrilla C.B., Mannis M.J. Glycomic analysis of tear and saliva in ocular rosacea patients: the search for a biomarker. Ocul. Surf. 2012;10:184–192. doi: 10.1016/j.jtos.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Blank D., Gebhardt S., Maass K., Lochnit G., Dotz V., Blank J. High-throughput mass finger printing and Lewis blood group assignment of human milk oligosaccharides. Anal. Bioanal. Chem. 2011;401:2495–2510. doi: 10.1007/s00216-011-5349-9. [DOI] [PubMed] [Google Scholar]
- 159.Dotz V., Rudloff S., Meyer C., Lochnit G., Kunz C. Metabolic fate of neutral human milk oligosaccharides in exclusively breast-fed infants. Mol. Nutr. Food Res. 2014:1–10. doi: 10.1002/mnfr.201400160. [DOI] [PubMed] [Google Scholar]
- 160.Cohen M., Hurtado-Ziola N., Varki A. ABO blood group glycans modulate sialic acid recognition on erythrocytes. Blood. 2009;114:3668–3676. doi: 10.1182/blood-2009-06-227041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lauc G., Essafi A., Huffman J.E., Hayward C., Knezevic A., Kattla J.J. Genomics meets glycomics-the first GWAS study of human N-Glycome identifies HNF1alpha as a master regulator of plasma protein fucosylation. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.