Abstract
Antibiotic resistance has emerged as a key determinant of outcome in patients with serious infections along with the virulence of the underlying pathogen. Within the intensive care unit (ICU) setting, ventilator-associated pneumonia (VAP) is a common nosocomial infection that is frequently caused by multidrug-resistant bacteria. Antimicrobial resistance is a growing challenge in the care of critically ill patients. Escalating rates of antibiotic resistance add substantially to the morbidity, mortality, and cost related to infection in the ICU. Both gram-positive organisms, such as methicillin-resistant Staphylococcus aureus and vancomycin-intermediate S. aureus , and gram-negative bacteria, including Pseudomonas aeruginosa , Acinetobacter species, carbapenem-resistant Enterobacteriaceae , such as the Klebsiella pneumoniae carbapenemase–producing bacteria, and extended spectrum β-lactamase organisms, have contributed to the escalating rates of resistance seen in VAP and other nosocomial infections. The rising rates of antimicrobial resistance have led to the routine empiric administration of broad-spectrum antibiotics even when bacterial infection is not documented. Moreover, there are several new broader-spectrum antibiotics that have recently become available and others scheduled for approval in the near future. The challenge to ICU clinicians is how to most effectively utilize these agents to maximize patient benefits while minimizing further emergence of resistance. Use of rapid diagnostics may hold the key for achieving this important balance. There is an urgent need for integrating the administration of new and existing antibiotics with the emerging rapid diagnostic technologies in a way that is both cost-effective and sustainable for the long run.
Keywords: rapid diagnostics, antibiotic resistance, microbiology, outcomes
Ventilator-associated pneumonia (VAP) is one of the most common infections occurring in mechanically ventilated patients and is frequently caused by antibiotic-resistant bacteria. 1 Mortality, hospital lengths of stay, and health care costs are typically greater among patients with respiratory failure complicated by VAP compared with patients who do not develop VAP. 2 Moreover, we know that the administration of inappropriate initial antibiotic therapy (IIAT) for VAP, usually attributed to multidrug-resistant (MDR) bacteria, is associated with greater hospital mortality and longer hospital lengths of stay. 3 4 These outcome influencing characteristics of VAP make it an important infection for intensivists to manage in an optimal manner. The ideal management of VAP requires intensive care units (ICUs) and hospitals to have consensus-derived strategies in place for the prevention, diagnosis, and treatment of this important nosocomial infection, which unfortunately are often lacking. Moreover, the overall perceived clinical importance of VAP has diminished in the United States due to the imprecise under-coding of this nosocomial infection using the Centers for Disease Control and Prevention surveillance definitions. 5 This has resulted in the promotion of ventilator-associated events (VAEs) as a preferred surveillance tool for assessing the quality of ICU care in the United States and reducing VAP to a nonreportable condition. 6 This may encourage suboptimal practices for VAP treatment that could be detrimental for patient outcomes and promote further antibiotic resistance.
The clinical importance of VAP is demonstrated by recent surveillance studies showing that it is a common nosocomial infection across all continents. 7 8 9 Moreover, the emerging problem of antibiotic resistance has added a new premium to the importance of accurately diagnosing and more importantly treating VAP with appropriate initial antibiotic therapy. 10 11 It is also imperative to recognize that one of the major clinical issues related to the management of VAP, as well as other nosocomial infections, is the increasing prevalence of MDR or extremely drug-resistant (XDR) pathogens. 12 13 14 15 There appears to be a direct relationship between overall antibiotic consumption for VAP and the emergence of newly resistance bacterial strains. 16 17 The latest and most fearsome example of this trend, due in large part to escalating use of colistin, has been the emergence of plasmid-mediated colistin resistance. 18 The development of colistin resistance in carbapenem-resistant Enterobacteriaceae , including New Delhi metallo-β-lactamase-1 (NDM-1) strains, brings a renewed sense of urgency to minimize any further resistance emergence and to prevent spread of these XDR bacteria. 19 As a result of this trend of increasing antibiotic resistance and boarder spectrum empiric antibiotic treatment of suspected VAP, more precise and rapid microbiologic diagnostic approaches for the antibiotic management of suspected VAP are urgently needed.
Diagnostic Criteria for VAP
The diagnosis of VAP is problematic because noninfectious conditions can cause pulmonary infiltrates and systemic findings such as leukocytosis, fever, and increased oxygen requirements. 20 Various diagnostic criteria with variable rigor have been developed to assist in the diagnosis of VAP. However, the most stringent criteria available have been associated with the greatest observed mortality and establishing the diagnosis of VAP took significantly longer when applying them compared with less stringent criteria, potentially resulting in delayed therapy. 21 Erring on the side of caution, most clinicians employ the finding of a new or progressive radiographic infiltrate and at least one clinical feature (fever, leukocytosis, worsening oxygenation, or purulent tracheal secretions), which has high sensitivity but low specificity for VAP. 22 The difficulty in relying on clinical criteria for the diagnosis of VAP is the potential for over diagnosis, resulting in the unnecessary administration of antibiotics to noninfected patients. This has the potential to promote further emergence of antibiotic resistance, especially when employed for prolonged time periods, and to dilute out the ability of clinicians to identify the beneficial impact of treating patients with appropriate initial antibiotic therapy. 23 24
Owing to the lack of a proven diagnostic method, two different strategies have been used and compared using clinical or bacteriologic criteria, each associated with advantages and disadvantages. 22 The clinical strategy employs the abovementioned clinical and radiographic criteria in diagnosing VAP. A combination of two out of three clinical criteria and a radiographic infiltrate yielded a sensitivity of 69% and a specificity of 75% for the diagnosis of VAP in 25 mechanically ventilated patients using histology and quantitative lung tissue culture on autopsy as the reference. 25 Increasing the number of clinical criteria resulted in greater specificity but at the cost of lesser overall sensitivity. 25 In a postmortem analysis of 39 mechanically ventilated patients, clinical criteria did not provide reliable predictive accuracy for histologic pneumonia. 26 A semiquantitative endotracheal aspirate culture can be used to identify a causative pathogen of VAP and, if positive, has been shown to correlate with quantitative cultures of the lower respiratory tract obtained via protected specimen brush (PSB). 27 Additionally, a negative endotracheal aspirate culture has good negative predictive value in excluding the presence of VAP if antibiotics have not recently been started or changed. 28 However, semiquantitative cultures are generally not as reliable as quantitative cultures of the lower respiratory tract due to an inability to differentiate between colonization and infection. 29 The use of clinical criteria and a reliance on semiquantitative cultures can result in clinical false-positive results for the diagnosis of VAP resulting in unnecessary antibiotic use.
The bacteriologic strategy uses quantitative cultures obtained from the lower respiratory tract via endotracheal aspirate, PSB, or bronchoalveolar lavage (BAL) to confirm or exclude the diagnosis of nosocomial pneumonia based on thresholds of bacterial growth of ≥10 5 colony forming units (CFU)/mL for an endotracheal aspirate, ≥10 4 CFU/mL for a BAL specimen, and ≥10 3 CFU/mL for a PSB sample. Results of these procedures guide decisions such as when to initiate or stop antibiotics and which drug should be used against the offending agent. There are no definitive data to support the use of one sampling technique over another; however, the cellular analysis of BAL fluid may provide an advantage, as a sample containing less than 50% neutrophils was associated with excellent negative predictive value in one study. 26 Also, given the multifocal nature of VAP, even mini-BAL samples obtained blindly without the use of bronchoscopy can be effective. 30 31 32 However, other studies caution on the use of unilateral cultures even when directed to the side of the dominant radiographic abnormality. 33 The bacteriologic strategy has resulted in less overall prescription and more narrowed antibiotic use, an important point given the surge of antibiotic resistance in the ICU setting. 34 35 36 A major disadvantage of the bacteriologic approach is the concern for false negatives which could result in cases of nosocomial pneumonia going untreated, especially in the setting of recently introduced antibiotics. 37
Multiple studies have compared the clinical and bacteriologic strategies. Only one prospective, randomized trial demonstrated a mortality benefit when using the bacteriologic strategy at 14 days. 34 Others have failed to reproduce these findings, including a large study conducted by the Canadian Critical Care Trials Group and a comprehensive meta-analysis. 38 39 In addition, the bacteriologic strategy does not seem to reduce the duration of mechanical ventilation or ICU length of stay. 39 The decision to employ either the clinical or bacteriologic strategy rests with the clinician on a case-by-case basis. If bronchoscopic sampling can be performed safely and the appropriate personnel is available, it is reasonable to utilize this approach as antibiotic decisions may change based on culture results allowing for more effective antimicrobial deescalation. If the clinical strategy is used, the clinician should reevaluate the patient often for guidance on antibiotic usage. Regardless of the diagnostic strategy, an unstable patient with a high pretest probability of nosocomial pneumonia should be initiated on empiric antibiotics, as a delay in antibiotic administration leads to higher mortality. 40 41 42
The lack of consistency in establishing a precise diagnosis of VAP has led some national guidelines to reflect on the relatively low accuracy of microbiology cultures as a diagnostic tool in VAP. 22 Moreover, contamination with upper respiratory tract pathogens or endotracheal tube colonizers is common and traditional microbiology laboratory flow with Gram staining, cultures, and antibiotic susceptibility testing requires at least 48 to 96 hours for information to be processed for clinical decision making. These current limitations in establishing a rapid and precise microbiologically confirmed diagnosis of VAP serve as the impetus for developing new rapid diagnostic approaches for this important infection.
New Diagnostic Technologies
Multiplex Real-Time Polymerase Chain Reaction
A broad range of viral and bacterial pathogens can cause acute respiratory tract infections including VAP often with similar clinical and radiographic presentations ( Fig. 1 ). Rapid detection of the causative pathogen offers the potential for providing timely administration of appropriate antimicrobial therapy as well as minimizing the use of broad-spectrum antibiotics when they are not justified based on microbiologic evaluation. Multiplex real-time polymerase chain reaction (PCR) offers rapid detection of a broad array of respiratory pathogens to optimize antimicrobial treatment. The FilmArray Respiratory Panel (RP; bioMérieux BioFire, Salt Lake City, UT) assay ( Fig. 2 ) is the first FDA-cleared assay for the qualitative detection of nucleic acid targets from both viruses and bacteria in nasopharyngeal swab specimens. 43 The FilmArray RP can detect 17 viral targets and three bacterial species ( Bordetella pertussis , Chlamydophila pneumoniae , and Mycoplasma pneumoniae ) more typically associated with community-acquired pneumonia with a turnaround time of approximately 1 hour and has been applied to direct respiratory specimens, including BAL specimens from mechanically ventilated patients ( Table 1 ). 44 45 46 More recently, the FilmArray RP has been employed to demonstrate that more than 24% of nonventilated hospital-acquired pneumonia (HAP) episodes were associated with respiratory virus infection alone or concomitant viral and bacterial infection. 47 This type of information could have important implications in terms of modifying or deescalating antibiotic therapy. 44
Fig. 1.
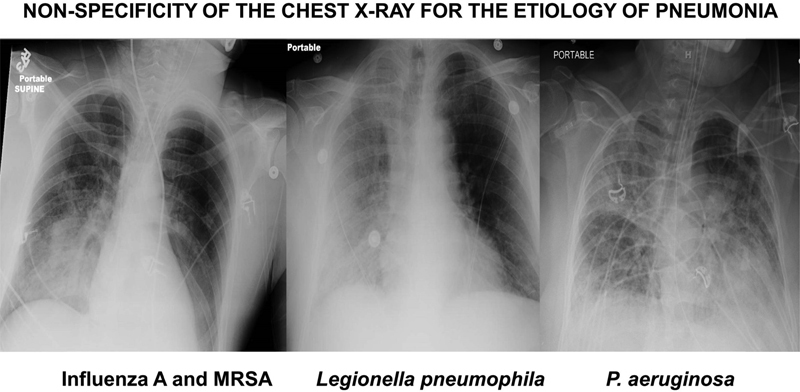
Three chest X-rays of patients with microbiologically confirmed pneumonia showing similar types of infiltrates for different pathogens. These X-rays illustrate the general nonspecificity of the radiographic findings for establishing a precise microbiologic diagnosis of pneumonia. MRSA, methicillin-resistant Staphylococcus aureus .
Fig. 2.
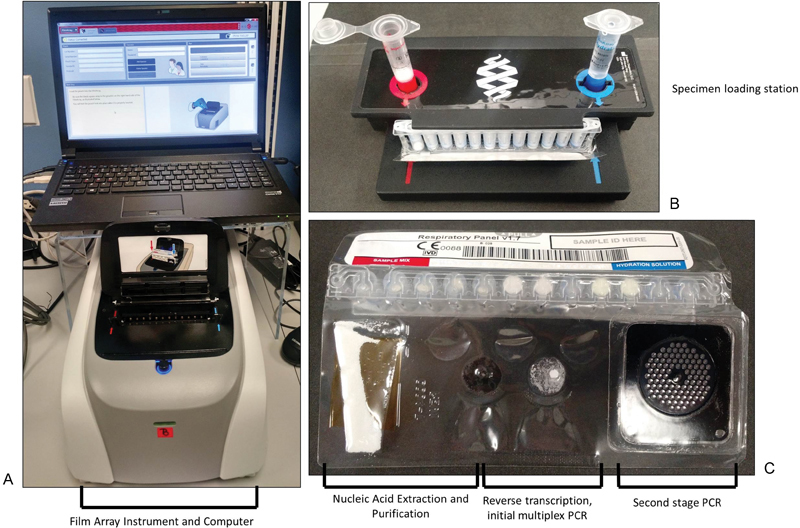
The BioFire FilmArray System. ( A ) The BioFire instrument and computer. Each instrument can run one FilmArray pouch at a time. ( B ) The specimen loading station. The FilmArray pouch is fixed in the station, and rehydrating buffer and specimen are added. ( C ) The FilmArray pouch. The specimen is moved through a series of reagents, including nucleic acid extraction and purification steps, a reverse transcriptase and initial PCR step, and a second-stage PCR. PCR product detection is performed in the “honeycomb” of the second-stage PCR.
Table 1. Pathogens identified with the FilmArray panels.
| FilmArray respiratory panel | FilmArray blood culture ID panel |
|---|---|
| Adenovirus | Staphylococcus species |
| Coronavirus 229E | Staphylococcus aureus |
| Coronavirus HKU1 | Streptococcus species |
| Coronavirus OC43 | Streptococcus agalactiae |
| Coronavirus NL63 | Streptococcus pyogenes |
| Human Metapneumovirus | Streptococcus pneumoniae |
| Human Rhinovirus/Enterovirus | Enterococcus species |
| Influenza A | Listeria monocytogenes |
| Influenza A/H1 | Klebsiella oxytoca |
| Influenza A/H1–2009 | Klebsiella pneumoniae |
| Influenza A/H3 | Serratia species |
| Influenza B | Proteus species |
| Parainfluenza 1 | Acinetobacter baumannii |
| Parainfluenza 2 | Haemophilus influenzae |
| Parainfluenza 3 | Neisseria meningitidis |
| Parainfluenza 4 | Pseudomonas aeruginosa |
| RSV | Enterobacteriaceae |
| Bordetella pertussis | Escherichia coli |
| Chlamydophila pneumoniae | Enterobacter cloacae complex |
| Mycoplasma pneumoniae | Candida albicans |
| Candida glabrata | |
| Candida krusei | |
| Candida parapsilosis | |
| Candida tropicalis | |
| mecA | |
| vanA/B | |
| bla KPC |
A new Luminex NxTAG Respiratory Pathogen Panel (NxTAG-RPP, Austin, TX) has been introduced as a high-throughput system that can detect nucleic acid from 21 respiratory viruses, including all pathogens detected by the FilmArray RP except B. pertussis plus Legionella pneumophila and human bocavirus. 48 A comparison of these two technologies demonstrated complete concordance in 98.8% (318/322) of positive results (kappa = 0.92). The high sample throughput with reasonable turnaround time of these assays makes them suitable multiplex platforms for routine screening of respiratory specimens in hospital-based laboratories. Moreover, the use of multiplex real-time PCR has been associated with reduced antibiotic utilization in patients evaluated for respiratory tract infections demonstrating their potential value as antibiotic stewardship adjuncts. 49 50 Another potential use of multiplex real-time PCR would be the addition of emerging respiratory viral pathogens to the panel, facilitating surveillance to identify patients with new, and often virulent, respiratory virus syndromes such as Middle East respiratory syndrome coronavirus infection. 51
A preclinical evaluation was recently conducted to evaluate the performance of the Cepheid Xpert MRSA/SA SSTI real-time PCR assay (Cepheid, Sunnyvale, CA) on 135 lower respiratory tract secretions for detection of methicillin-resistant Staphylococcus aureus (MRSA) and S. aureus . 52 Compared with the gold standard quantitative culture, the sensitivity, specificity, and positive and negative predictive values were 99.0, 72.2, 90.7, and 96.3%, respectively. The same assay has been employed to exclude the presence of MRSA and S. aureus in VAP demonstrating negative predictive values of 99.7% (98.1–99.9%) and 99.8% (98.7–99.9%) for methicillin-susceptible S. aureus (MSSA) and MRSA, respectively. 53
Other Nucleic Acid Detection Techniques
New point-of-care PCR systems for rapid identification of pathogens and antibiotic resistance markers are available and show promise for the management of infections like VAP. Kunze et al evaluated point-of-care multiplex PCR (Unyvero, Curetis AG, Holzgerlingen, Germany) for patients with HAP. 54 Mean turnaround test result times were 6.5 hours (4.7–18.3 hours) for multiplex PCR and 71 hours (37.2–217.8 hours) for conventional microbiology. However, they found concordant results in only 45% and nonconcordant results in 45% of all patients. Only 55% of the results were concordant in patients with a clinical pulmonary infection score higher than 5, suggesting a high likelihood for the presence of HAP. These authors concluded that Unyvero allowed point-of-care microbial testing with short turnaround times, but the system performance was poor and what was needed was an improved system with more reliable performance and an extended microbial panel.
Vincent et al employed culture-independent polymerase chain reaction/electrospray ionization-mass spectrometry (PCR/ESI-MS) to test 616 bloodstream infection samples, 185 pneumonia samples, and 110 sterile fluid and tissue specimens from 529 patients. 55 From the 616 bloodstream samples, PCR/ESI-MS identified a pathogen in 228 cases (37%) and conventional culture methods in just 68 (11%). Conventional cultures were positive and PCR-ESI-MS was negative in 13 cases, and both were negative in 384 cases, giving PCR/ESI-MS a sensitivity of 81%, specificity of 69%, and negative predictive value of 97% at 6 hours from sample acquisition. Similar observations were made for pneumonia and sterile fluid and tissue specimens. An independent clinical analysis of results suggested that PCR/ESI-MS technology could potentially have resulted in altered treatment in up to 57% of patients. The findings of this study were promising in suggesting that clinical decision making could potentially be influenced in a positive manner with PCR/ESI-MS by allowing more rapid and accurate modifications in antibiotic therapy.
Banerjee et al performed a randomized trial in a total of 617 patients with positive blood culture bottles (BCBs) who underwent stratified randomization into three arms: standard BCB processing (control, n = 207), rapid multiplex PCR reported with templated comments (rmPCR, n = 198), or rmPCR reported with templated comments and real-time audit and feedback of antimicrobial orders by an antimicrobial stewardship team (rmPCR/AS, n = 212). 56 The primary outcome was antimicrobial therapy duration. The rmPCR panel used in both intervention arms was the FilmArray Blood Culture ID Panel (BioFire Diagnostics/bioMérieux BioFire), which was performed as soon as a BCB signaled positive, 24 hours a day, 7 days a week. This assay detects the pathogens and resistance genes shown in Table 1 . Compared with the control group, both intervention groups had decreased broad-spectrum piperacillin-tazobactam use and increased narrow-spectrum β-lactam antibiotic use, and fewer instances of antibiotic therapy for contaminants. Time from Gram stain to appropriate antimicrobial deescalation or escalation was shortest in the rmPCR/AS group. The aim would be to replicate these types of findings in patients with pneumonia using lower respiratory specimens.
The Verigene Nanosphere system is a multiplex nucleic acid detection assay that is being used in clinical laboratories for pathogen identification and resistance gene detection in positive blood culture broth and for respiratory pathogen detection ( Table 2 , Fig. 3 ). 57 58 59 Similar to the BioFire blood culture assay, use of the Verigene assay for bacteremic patients has been associated with reduced length of stay, reduced mortality, and improvement in time to optimization of antimicrobial therapy. 57 59 60 Panels directed toward lower respiratory tract pathogens are in development.
Table 2. Pathogens detected with the Verigene panels.
| Verigene respiratory pathogen panel | Gram-positive blood culture test | Gram-negative blood culture test |
|---|---|---|
| Adenovirus Human Metapneumovirus Influenza A Influenza A (subtype H1) Influenza A (subtype H3) Influenza B Parainfluenza 1 Parainfluenza 2 Parainfluenza 3 Parainfluenza 4 Rhinovirus RSV A RSV B Bordetella pertussis Bordetella parapertussis/B. bronchiseptica Bordetella holmesii |
Staphylococcus aureus
Staphylococcus epidermidis Staphylococcus lugdunensis Streptococcus anginosus Group Streptococcus agalactiae Streptococcus pneumoniae Streptococcus pyogenes Enterococcus faecalis Enterococcus faecium Staphylococcus spp. Streptococcus spp. Listeria spp. mecA vanA vanB |
Escherichia coli
Klebsiella pneumoniae Klebsiella oxytoca Pseudomonas aeruginosa Acinetobacter spp. Citrobacter spp. Enterobacter spp. Proteus spp. CTX-M IMP KPC NDM OXA VIM |
Fig. 3.

The Nanosphere Verigene System, consisting of instrumentation ( A, B ) and the test cartridge ( C ). Sample and reagents are added to the processing unit. After analysis is completed, the cartridge is moved briefly to the reading unit for interpretation.
Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry
Traditionally, the identification of microbes recovered in culture has relied on microbial growth and metabolism in the presences of various biochemical substrates. In contrast, matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) uses proteomic profiling to assign an identification; this can be applied to a variety of microbes, including bacteria, yeast, mold, and mycobacteria. 61 62 63 64 65 66 It is primarily ribosomal proteins that are detected using this method. The MALDI BioTyper system (Bruker Daltonics, Billerica, MA) and the VITEK MS (bioMerieux, Durham, NC) are the commercially available MALDI-TOF MS instrumentation/database platforms for microorganism identification. While MALDI-TOF MS has been used most frequently for expediting the identification of microbes recovered on solid culture media, it has also been used to identify some microbes from clinical specimens, including positive blood culture broth and urine. 67 68 69 In addition, proof-of-principle studies have demonstrated the power of this method to simultaneously identify important resistance determinants during routine organism identification, such as a vancomycin-intermediate S. aureus and certain KPC-containing plasmids. 70 71 As this technology becomes more widespread, it is likely that the rapid and accurate identification of pathogens will facilitate optimization of antimicrobial therapy in patients with all types of infection, including respiratory infection.
Fluorescence In-Situ Hybridization
The fluorescence in-situ hybridization (FISH) technique is based on fluorescently labeled oligonucleotide probes that complementarily bind to specific target ribosomal RNA sequences of bacteria, yeasts, or other microorganisms. Target sequences are naturally present in bacteria at a concentration high enough to enable visual detection of the specific fluorescent signal. 72 FISH can be used to detect pathogens that are difficult or time consuming to identify with traditional culture methods, especially when more than one species is present in the sample, as in the case of polymicrobial infections including VAP. RespiFISH HAP Gram (−) Panel (miacom diagnostics GmbH, Duesseldorf, Germany) is a classic FISH technology employing fluorescently labeled DNA molecular beacons as probes to develop a simple procedure known as the beacon-based FISH technology. 73 This panel is able to detect most gram-negative bacterial pathogens and has been shown to be accurate in detecting the causative pathogens in patients with pneumonia, including VAP. 74
Automated Microscopy
Douglas et al employed a real-time multiplexed FISH-based microscopy ID/AST system (Accelerate Diagnostics, Tucson, AZ), capable of evaluating antibiotic sensitivity and resistance against live pathogenic organisms from blood cultures or respiratory samples using automated phenotypic growth pattern analysis ( Fig. 4 ), to study surveillance for potential preempted treatment of VAP. 75 Seventy-seven mini-BAL specimens were obtained in 33 patients. One patient (3%) was clinically diagnosed with VAP. Of 73 paired samples, conventional culture methods identified seven, containing pneumonia panel bacteria (>10 4 colony-forming units/mL) from five patients (four S. aureus [three MRSA], two Stenotrophomonas maltophilia , one Klebsiella pneumoniae ) and resulted in antimicrobial changes/additions to two of five of those patients. Microscopy identified seven of seven microbiologically positive organisms and 64 of 66 negative samples compared with culture. Antimicrobial changes/additions would have occurred in three of seven microscopy-positive patients had those results been clinically available in 5 hours, including one patient diagnosed later with VAP despite negative mini-BAL cultures. Overall, automated microscopy was 100% sensitive and 97% specific for high-risk pneumonia organisms compared with clinical cultures suggesting that rapid microscopy-based surveillance may be informative for treatment and antimicrobial stewardship in patients at risk for VAP. In addition, this system has been demonstrated to rapidly detect carbapenem resistance in K. pneumoniae , and, if present, predict if the resistance can be attributed to KPC carbapenemase. 76
Fig. 4.
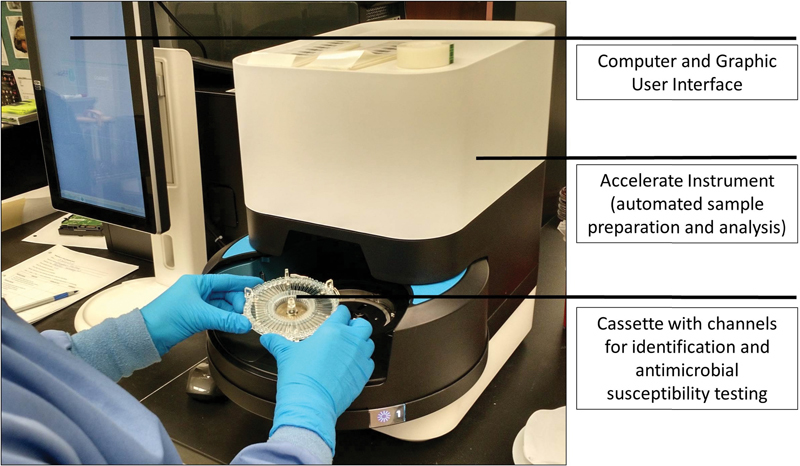
The Accelerate System. A cassette, regent pack, and clinical sample are loaded into the analyzer. Following automated sample preparation, organism identification and antimicrobial susceptibility testing are performed. The results are available via the graphic user interface.
Analysis of Exhaled Breath Condensate Fluid and Volatile Organic Compounds
May et al employed a novel strategy for the rapid diagnosis of VAP utilizing exhaled breath condensate fluid (EBCF) obtained from heat moisture exchangers to provide a substrate for testing with PCR to identify bacterial DNA. 77 These investigators showed in critically ill surgical patients excellent concordance between pathogen identification using PCR of EBCF and pathogens isolated from BAL fluid using conventional microbiology techniques. Additionally, they found that increasing DNA load among serial EBCF samples preceded the clinical suspicion of VAP. The potential advantages of this type of diagnostic approach include noninvasive sampling of EBCF, ease of acquiring serial samples to potentially allow preemptive or targeted preventative treatment of early VAP or tracheobronchitis, and pathogen-specific characterization. The latter could help direct antibiotic therapy limiting the unnecessary use of broad-spectrum antibiotics for pathogens that are not identified, thus promoting antibiotic stewardship principles. The main disadvantage of this type of PCR-directed diagnostic approach is that it does not provide true antimicrobial susceptibility testing of the causative pathogens.
Volatile organic compound (VOC) detection is another promising diagnostic technology with probably the greatest applicability in VAP. Both humans and bacteria produce VOCs (volatile carbon molecules) as part of their metabolism. The VOCs vary depending on disease states, growth environment, and the presence of other bacteria. This technology is particularly appealing to lung diseases, as it can be monitored noninvasively analyzing exhaled breath (similar to EBCF). Changes in VOC patterns can trigger an early workup and also can be monitored to assess response to treatment. Mass spectrometry can swiftly identify and quantify VOCs. New technologies like electronic noses and optical spectra systems can describe the VOC patterns or fingerprints of bacteria. 78 79 In a study that included 38 ventilated patients, electronic nose–derived VOC fingerprints showed good correlation with clinical pneumonia scores. 80 A recent study monitored 45 ventilated patients thrice weekly using electronic nose technology. 81 The obtained VOC fingerprints were able to differentiate between infected, colonized, and noninfected patients. The potential for VOC detection in diagnosing lung infections using either few specific biomarkers or the whole VOC fingerprint is currently being actively pursued. 82 83
Potential Limitations and Implications of Novel Diagnostics for VAP
As suggested earlier, 56 experiences with rapid diagnostics for the evaluations of blood culture specimens suggest that rapid diagnostics may play an important role in enhancing antimicrobial prescribing practices in hospitalized patients. The benefits to this can be numerous, including optimizing clinical outcomes, reducing toxicity, and facilitating clinical trials for new anti-infective agents by stratifying patients eligible for the trial at the earliest possible opportunity. However, it is also important to understand the limitations of these new technologies including that they cannot differentiate colonization from infection, which could be highly problematic in mechanically ventilated patients, nor give us the true susceptibility patterns of the responsible pathogens. The latter is true with the exception of a few specific mechanisms of resistance provided by the previously described molecular techniques and automated microscopy which has the potential to provide real susceptibility data.
Further illustrating the potential role of rapid diagnostics in improving antimicrobial therapy and outcome when embedded in a well-organized antimicrobial stewardship program is the study by Huang et al from the University of Michigan. 84 These investigators performed a quasi-experimental study to analyze the impact of MALDI-TOF MS in conjunction with an antimicrobial stewardship team intervention in patients with bloodstream infections. 84 The antimicrobial stewardship team provided antibiotic recommendations after receiving real-time notification following blood culture Gram stain, organism identification, and antimicrobial susceptibilities using conventional microbiology methods in the before-period and MALDI-TOF MS in the after-period. Use of MALDI-TOF MS significantly decreased time to organism identification, and improved time to effective antibiotic therapy as well as optimal directed antibiotic therapy. Mortality, length of ICU stay, and recurrent bacteremia were also lower during the intervention period. Similarly, the PCR-based GeneXpert MRSA/SA diagnostic platform (Cepheid, Sunnyvale, CA) was studied at the Veterans Affairs Medical Center in Houston demonstrating that for MSSA bacteremia, the mean time to initiation of appropriate therapy was reduced from 49.8 to 5.2 hours and the duration of unnecessary MRSA drug therapy was reduced by 61 hours per patient. 85 It is hoped that the application of rapid diagnostic methods to respiratory specimens could have a similar impact on patients with pneumonia including VAP.
It is clear that we are entering a new era in the management and treatment of serious infections such as VAP. Spellberg et al made a recent plea to change our current patterns of managing patients with proven and presumed infections to reverse the spiraling trend of antibiotic resistance that has occurred over the last century. 86 Within the next 3 to 5 years, new antibiotics directed against MDR Gram-negative bacteria, in addition to the recently approved ceftolozane–tazobactam and ceftazidime-avibactam, will likely become available, including carbavance, plazomicin, eravacycline, relebactam, brilacidin, BAL30072, aztreonam-avibactam, carbapenems with ME 1071, and S-649266—a novel siderophore cephalosporin. These agents can provide enhanced activity against β-lactamase producers, carbapenem-resistant bacteria, and in some cases even metallo-β-lactamase–producing bacteria.
The challenge to ICU clinicians is how to most effectively utilize these agents once they become available to maximize patient benefits while minimizing the emergence of resistance ( Table 3 ). This is an especially important challenge in resource-limited countries that have often been at the forefront of the emergence of novel antimicrobial resistance mechanisms due to local patterns of antibiotic use. The use of rapid diagnostics may hold the key for achieving this important balance. There is an urgent need for clinical studies aimed at understanding how to best integrate the use of these new antibiotics with the emerging rapid diagnostic technologies in a way that is cost-effective and sustainable for the long run. 87 In addition, the microbiology laboratory must work closely with their clinical partners to deploy these new diagnostic tools in a manner that will afford the maximum benefit of these new technologies, including incorporation of the antimicrobial stewardship team and interpretative report comments, when applicable. Clinical outcome studies demonstrating the benefit of these new technologies on patient outcomes are needed. VAP may be an ideal infection to demonstrate the impact of rapid diagnostics as a means of enhancing antimicrobial treatment and stewardship. 88
Table 3. Characteristics of diagnostic methods for ventilator-associated pneumonia.
| Diagnostic method | Conventional culture time (h) | Pathogen/Biochemical identification time (h) | True antibiotic susceptibility available | Antibiotic susceptibility time (h) | Total diagnostic time (h) |
|---|---|---|---|---|---|
| Conventional culture method | 24–36 | n/a | Yes | 12–24 | 36–72 |
| BioFire/Luminex | n/a | 2–4 a | No | n/a | 2–4 |
| PNA-FISH | n/a | 2–4 a | No | n/a | 2–4 |
| AXDX ID/AST | n/a | 2–4 a | Yes | 3–6 | 6–10 |
| VOC fingerprints | n/a | 2–4 a | No | n/a | 2–4 |
Abbreviations: FISH, fluorescence in situ hybridization; ID/AST, identification/antibiotic susceptibility testing via automated microscopy; n/a, not applicable; VOC, volatile organic compounds.
Assumes direct specimen inoculation from respiratory samples including endotracheal aspirates and bronchoalveolar lavage samples.
References
- 1.Chastre J, Fagon J-Y. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(07):867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 2.Kollef M H, Hamilton C W, Ernst F R. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol. 2012;33(03):250–256. doi: 10.1086/664049. [DOI] [PubMed] [Google Scholar]
- 3.Kollef K E, Schramm G E, Wills A R, Reichley R M, Micek S T, Kollef M H. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest. 2008;134(02):281–287. doi: 10.1378/chest.08-1116. [DOI] [PubMed] [Google Scholar]
- 4.Guillamet C V, Kollef M H. Update on ventilator-associated pneumonia. Curr Opin Crit Care. 2015;21(05):430–438. doi: 10.1097/MCC.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 5.Skrupky L P, McConnell K, Dallas J, Kollef M H. A comparison of ventilator-associated pneumonia rates as identified according to the National Healthcare Safety Network and American College of Chest Physicians criteria. Crit Care Med. 2012;40(01):281–284. doi: 10.1097/CCM.0b013e31822d7913. [DOI] [PubMed] [Google Scholar]
- 6.Magill S S, Klompas M, Balk R et al. Developing a new, national approach to surveillance for ventilator-associated events. Crit Care Med. 2013;41(11):2467–2475. doi: 10.1097/CCM.0b013e3182a262db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kollef M H, Chastre J, Fagon J Y et al. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit Care Med. 2014;42(10):2178–2187. doi: 10.1097/CCM.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 8.Micek S T, Wunderink R G, Kollef M H et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance . Crit Care. 2015;19:219. doi: 10.1186/s13054-015-0926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung D R, Song J-H, Kim S H et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184(12):1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Loeches I, Torres A, Rinaudo M et al. Resistance patterns and outcomes in intensive care unit (ICU)-acquired pneumonia. Validation of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) classification of multidrug resistant organisms. J Infect. 2015;70(03):213–222. doi: 10.1016/j.jinf.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Nseir S, Martin-Loeches I, Makris D et al. Impact of appropriate antimicrobial treatment on transition from ventilator-associated tracheobronchitis to ventilator-associated pneumonia. Crit Care. 2014;18(03):R129. doi: 10.1186/cc13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones R N. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51 01:S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 13.Sandiumenge A, Lisboa T, Gomez F, Hernandez P, Canadell L, Rello J. Effect of antibiotic diversity on ventilator-associated pneumonia caused by ESKAPE Organisms. Chest. 2011;140(03):643–651. doi: 10.1378/chest.11-0462. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi S, Agrawal C, Madan M, Pandey A, Chauhan H. Superbugs causing ventilator associated pneumonia in a tertiary care hospital and the return of pre-antibiotic era! Indian J Med Microbiol. 2015;33(02):286–289. doi: 10.4103/0255-0857.153566. [DOI] [PubMed] [Google Scholar]
- 15.Garnacho-Montero J, Corcia-Palomo Y, Amaya-Villar R, Martin-Villen L. How to treat VAP due to MDR pathogens in ICU patients. BMC Infect Dis. 2014;14:135. doi: 10.1186/1471-2334-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fihman V, Messika J, Hajage D et al. Five-year trends for ventilator-associated pneumonia: correlation between microbiological findings and antimicrobial drug consumption. Int J Antimicrob Agents. 2015;46(05):518–525. doi: 10.1016/j.ijantimicag.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Dennesen P J, van der Ven A J, Kessels A G, Ramsay G, Bonten M J. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am J Respir Crit Care Med. 2001;163(06):1371–1375. doi: 10.1164/ajrccm.163.6.2007020. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y Y, Wang Y, Walsh T R et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(02):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 19.van Duin D, Doi Y. Outbreak of colistin-resistant, carbapenemase-producing Klebsiella pneumoniae : are we at the end of the road? . J Clin Microbiol. 2015;53(10):3116–3117. doi: 10.1128/JCM.01399-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klompas M, Kulldorff M, Platt R. Risk of misleading ventilator-associated pneumonia rates with use of standard clinical and microbiological criteria. Clin Infect Dis. 2008;46(09):1443–1446. doi: 10.1086/587103. [DOI] [PubMed] [Google Scholar]
- 21.Ego A, Preiser J C, Vincent J L. Impact of diagnostic criteria on the incidence of ventilator-associated pneumonia. Chest. 2015;147(02):347–355. doi: 10.1378/chest.14-0610. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society; Infectious Diseases Society of America.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia Am J Respir Crit Care Med 200517104388–416. [DOI] [PubMed] [Google Scholar]
- 23.Charles M V, Easow J M, Joseph N M, Ravishankar M, Kumar S, Umadevi S. Role of appropriate therapy in combating mortality among the ventilated patients. J Clin Diagn Res. 2014;8(08):DC01–DC03. doi: 10.7860/JCDR/2014/7995.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luna C M, Aruj P, Niederman M S et al. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur Respir J. 2006;27(01):158–164. doi: 10.1183/09031936.06.00049105. [DOI] [PubMed] [Google Scholar]
- 25.Fàbregas N, Ewig S, Torres A et al. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post-mortem lung biopsies. Thorax. 1999;54(10):867–873. doi: 10.1136/thx.54.10.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirtland S H, Corley D E, Winterbauer R H et al. The diagnosis of ventilator-associated pneumonia: a comparison of histologic, microbiologic, and clinical criteria. Chest. 1997;112(02):445–457. doi: 10.1378/chest.112.2.445. [DOI] [PubMed] [Google Scholar]
- 27.Rumbak M J, Bass R L. Tracheal aspirate correlates with protected specimen brush in long-term ventilated patients who have clinical pneumonia. Chest. 1994;106(02):531–534. doi: 10.1378/chest.106.2.531. [DOI] [PubMed] [Google Scholar]
- 28.Blot F, Raynard B, Chachaty E, Tancrède C, Antoun S, Nitenberg G. Value of gram stain examination of lower respiratory tract secretions for early diagnosis of nosocomial pneumonia. Am J Respir Crit Care Med. 2000;162(05):1731–1737. doi: 10.1164/ajrccm.162.5.9908088. [DOI] [PubMed] [Google Scholar]
- 29.Scholte J B, van Dessel H A, Linssen C F et al. Endotracheal aspirate and bronchoalveolar lavage fluid analysis: interchangeable diagnostic modalities in suspected ventilator-associated pneumonia? J Clin Microbiol. 2014;52(10):3597–3604. doi: 10.1128/JCM.01494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugin J, Auckenthaler R, Mili N, Janssens J P, Lew P D, Suter P M.Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid Am Rev Respir Dis 1991143(5 Pt 1):1121–1129. [DOI] [PubMed] [Google Scholar]
- 31.Kollef M H, Bock K R, Richards R D, Hearns M L. The safety and diagnostic accuracy of minibronchoalveolar lavage in patients with suspected ventilator-associated pneumonia. Ann Intern Med. 1995;122(10):743–748. doi: 10.7326/0003-4819-122-10-199505150-00002. [DOI] [PubMed] [Google Scholar]
- 32.Papazian L, Thomas P, Garbe Let al. Bronchoscopic or blind sampling techniques for the diagnosis of ventilator-associated pneumonia Am J Respir Crit Care Med 1995152(6, Pt 1):1982–1991. [DOI] [PubMed] [Google Scholar]
- 33.Bello G, Pennisi M A, Di Muzio F et al. Clinical impact of pulmonary sampling site in the diagnosis of ventilator-associated pneumonia: a prospective study using bronchoscopic bronchoalveolar lavage. J Crit Care. 2016;33:151–157. doi: 10.1016/j.jcrc.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Fagon J Y, Chastre J, Wolff M et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med. 2000;132(08):621–630. doi: 10.7326/0003-4819-132-8-200004180-00004. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Nieto J M, Torres A, Garcia-Cordoba F et al. Impact of invasive and noninvasive quantitative culture sampling on outcome of ventilator-associated pneumonia: a pilot study. Am J Respir Crit Care Med. 1998;157(02):371–376. doi: 10.1164/ajrccm.157.2.97-02039. [DOI] [PubMed] [Google Scholar]
- 36.Rello J, Vidaur L, Sandiumenge A et al. De-escalation therapy in ventilator-associated pneumonia. Crit Care Med. 2004;32(11):2183–2190. doi: 10.1097/01.ccm.0000145997.10438.28. [DOI] [PubMed] [Google Scholar]
- 37.Souweine B, Veber B, Bedos J P et al. Diagnostic accuracy of protected specimen brush and bronchoalveolar lavage in nosocomial pneumonia: impact of previous antimicrobial treatments. Crit Care Med. 1998;26(02):236–244. doi: 10.1097/00003246-199802000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Canadian Critical Care Trials Group.A randomized trial of diagnostic techniques for ventilator-associated pneumonia N Engl J Med 2006355252619–2630. [DOI] [PubMed] [Google Scholar]
- 39.Berton D C, Kalil A C, Teixeira P J. Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst Rev. 2014;10(10):CD006482. doi: 10.1002/14651858.CD006482.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez-Lerma F; ICU-Acquired Pneumonia Study Group.Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit Intensive Care Med 19962205387–394. [DOI] [PubMed] [Google Scholar]
- 41.Iregui M, Ward S, Sherman G, Fraser V J, Kollef M H. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122(01):262–268. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 42.Kollef M H, Sherman G, Ward S, Fraser V J. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115(02):462–474. doi: 10.1378/chest.115.2.462. [DOI] [PubMed] [Google Scholar]
- 43.Andersson M E, Olofsson S, Lindh M. Comparison of the FilmArray assay and in-house real-time PCR for detection of respiratory infection. Scand J Infect Dis. 2014;46(12):897–901. doi: 10.3109/00365548.2014.951681. [DOI] [PubMed] [Google Scholar]
- 44.Crotty M P, Meyers S, Hampton N et al. Impact of antibacterials on subsequent resistance and clinical outcomes in adult patients with viral pneumonia: an opportunity for stewardship. Crit Care. 2015;19:404. doi: 10.1186/s13054-015-1120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crotty M P, Meyers S, Hampton N et al. Epidemiology, co-infections, and outcomes of viral pneumonia in adults: an observational cohort study. Medicine (Baltimore) 2015;94(50):e2332. doi: 10.1097/MD.0000000000002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azadeh N, Sakata K K, Brighton A M, Vikram H R, Grys T E. FilmArray Respiratory Panel Assay: comparison of nasopharyngeal swabs and bronchoalveolar lavage samples. J Clin Microbiol. 2015;53(12):3784–3787. doi: 10.1128/JCM.01516-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Micek S T, Chew B, Hampton N, Kollef M H. A case-control study assessing the impact of nonventilated hospital-acquired pneumonia on patient outcomes. Chest. 2016;150(05):1008–1014. doi: 10.1016/j.chest.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J H, Lam H Y, Yip C C et al. Clinical evaluation of the new high-throughput Luminex NxTAG Respiratory Pathogen Panel assay for multiplex respiratory pathogen detection. J Clin Microbiol. 2016;54(07):1820–1825. doi: 10.1128/JCM.00517-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers B B, Shankar P, Jerris R C et al. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med. 2015;139(05):636–641. doi: 10.5858/arpa.2014-0257-OA. [DOI] [PubMed] [Google Scholar]
- 50.Subramony A, Zachariah P, Krones A, Whittier S, Saiman L. Impact of multiplex polymerase chain reaction testing for respiratory pathogens on healthcare resource utilization for pediatric inpatients. J Pediatr. 2016;173:196–20100. doi: 10.1016/j.jpeds.2016.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oboho I K, Tomczyk S M, Al-Asmari A M et al. 2014 MERS-CoV outbreak in Jeddah--a link to health care facilities. N Engl J Med. 2015;372(09):846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cercenado E, Marín M, Burillo A, Martín-Rabadán P, Rivera M, Bouza E. Rapid detection of Staphylococcus aureus in lower respiratory tract secretions from patients with suspected ventilator-associated pneumonia: evaluation of the Cepheid Xpert MRSA/SA SSTI assay . J Clin Microbiol. 2012;50(12):4095–4097. doi: 10.1128/JCM.02409-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leone M, Malavieille F, Papazian L et al. Routine use of Staphylococcus aureus rapid diagnostic test in patients with suspected ventilator-associated pneumonia . Crit Care. 2013;17(04):R170. doi: 10.1186/cc12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kunze N, Moerer O, Steinmetz N, Schulze M H, Quintel M, Perl T. Point-of-care multiplex PCR promises short turnaround times for microbial testing in hospital-acquired pneumonia--an observational pilot study in critical ill patients. Ann Clin Microbiol Antimicrob. 2015;14:33. doi: 10.1186/s12941-015-0091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vincent J L, Brealey D, Libert N et al. Rapid diagnosis of infection in the critically ill, a multicenter study of molecular detection in bloodstream infections, pneumonia, and sterile site infections. Crit Care Med. 2015;43(11):2283–2291. doi: 10.1097/CCM.0000000000001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banerjee R, Teng C B, Cunningham S A et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis. 2015;61(07):1071–1080. doi: 10.1093/cid/civ447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker T, Dumadag S, Lee C J et al. Clinical impact of laboratory implementation of verigene BC-GN microarray-based assay for detection of gram-negative bacteria in positive blood cultures. J Clin Microbiol. 2016;54(07):1789–1796. doi: 10.1128/JCM.00376-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dodémont M, De Mendonça R, Nonhoff C, Roisin S, Denis O. Evaluation of Verigene Gram-Positive Blood Culture Assay performance for bacteremic patients. Eur J Clin Microbial Infect Dis. 2015;34:473–477. doi: 10.1007/s10096-014-2250-4. [DOI] [PubMed] [Google Scholar]
- 59.Beal S G, Ciurca J, Smith G et al. Evaluation of the nanosphere verigene gram-positive blood culture assay with the VersaTREK blood culture system and assessment of possible impact on selected patients. J Clin Microbiol. 2013;51(12):3988–3992. doi: 10.1128/JCM.01889-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alby K, Daniels L M, Weber D J, Miller M B. Development of a treatment algorithm for streptococci and enterococci from positive blood cultures identified with the Verigene Gram-positive blood culture assay. J Clin Microbiol. 2013;51(11):3869–3871. doi: 10.1128/JCM.01587-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McMullen A R, Wallace M A, Pincus D H, Wilkey K, Burnham C A. Evaluation of the Vitek MS Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry System for identification of clinically relevant filamentous fungi. J Clin Microbiol. 2016;54(08):2068–2073. doi: 10.1128/JCM.00825-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzalez M D, Weber C J, Burnham C A. Rapid identification of microorganisms from positive blood cultures by testing early growth on solid media using matrix-assisted laser desorption ionization-time of flight mass spectrometry. Diagn Microbiol Infect Dis. 2016;85(02):133–135. doi: 10.1016/j.diagmicrobio.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 63.Wilen C B, McMullen A R, Burnham C A. Comparison of sample preparation methods, instrumentation platforms, and contemporary commercial databases for identification of clinically relevant mycobacteria by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2015;53(07):2308–2315. doi: 10.1128/JCM.00567-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McElvania TeKippe E, Burnham C A. Evaluation of the Bruker Biotyper and VITEK MS MALDI-TOF MS systems for the identification of unusual and/or difficult-to-identify microorganisms isolated from clinical specimens. Eur J Clin Microbiol Infect Dis. 2014;33(12):2163–2171. doi: 10.1007/s10096-014-2183-y. [DOI] [PubMed] [Google Scholar]
- 65.Pence M A, McElvania TeKippe E, Wallace M A, Burnham C A. Comparison and optimization of two MALDI-TOF MS platforms for the identification of medically relevant yeast species. Eur J Clin Microbiol Infect Dis. 2014;33(10):1703–1712. doi: 10.1007/s10096-014-2115-x. [DOI] [PubMed] [Google Scholar]
- 66.Branda J A, Rychert J, Burnham C A et al. Multicenter validation of the VITEK MS v2.0 MALDI-TOF mass spectrometry system for the identification of fastidious gram-negative bacteria. Diagn Microbiol Infect Dis. 2014;78(02):129–131. doi: 10.1016/j.diagmicrobio.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 67.Demarco M L, Burnham C A. Diafiltration MALDI-TOF mass spectrometry method for culture-independent detection and identification of pathogens directly from urine specimens. Am J Clin Pathol. 2014;141(02):204–212. doi: 10.1309/AJCPQYW3B6JLKILC. [DOI] [PubMed] [Google Scholar]
- 68.Verroken A, Defourny L, le Polain de Waroux O et al. Clinical impact of MALDI-TOF MS identification and rapid susceptibility testing on adequate antimicrobial treatment in sepsis with positive blood cultures. PLoS One. 2016;11(05):e0156299. doi: 10.1371/journal.pone.0156299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perez K K, Olsen R J, Musick W L et al. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch Pathol Lab Med. 2013;137(09):1247–1254. doi: 10.5858/arpa.2012-0651-OA. [DOI] [PubMed] [Google Scholar]
- 70.Mather C A, Werth B J, Sivagnanam S, SenGupta D J, Butler-Wu S M. Rapid detection of vancomycin-intermediate Staphylococcus aureus by matrix-assisted laser desorption ionization-time of flight mass spectrometry . J Clin Microbiol. 2016;54(04):883–890. doi: 10.1128/JCM.02428-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Youn J H, Drake S K, Weingarten R A, Frank K M, Dekker J P, Lau A F. Clinical performance of a matrix-assisted laser desorption ionization-time of flight mass spectrometry method for detection of certain blaKPC-containing plasmids. J Clin Microbiol. 2016;54(01):35–42. doi: 10.1128/JCM.01643-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kannaiah S, Amster-Choder O. Methods for studying RNA localization in bacteria. Methods. 2016;98:99–103. doi: 10.1016/j.ymeth.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 73.Poppert S, Essig A, Stoehr B et al. Rapid diagnosis of bacterial meningitis by real-time PCR and fluorescence in situ hybridization. J Clin Microbiol. 2005;43(07):3390–3397. doi: 10.1128/JCM.43.7.3390-3397.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koncan R, Parisato M, Sakarikou C et al. Direct identification of major Gram-negative pathogens in respiratory specimens by respiFISH® HAP Gram (-) Panel, a beacon-based FISH methodology. Eur J Clin Microbiol Infect Dis. 2015;34(10):2097–2102. doi: 10.1007/s10096-015-2458-y. [DOI] [PubMed] [Google Scholar]
- 75.Douglas I S, Price C S, Overdier K H et al. Rapid automated microscopy for microbiological surveillance of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2015;191(05):566–573. doi: 10.1164/rccm.201408-1468OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burnham C A, Frobel R A, Herrera M L, Wickes B L. Rapid ertapenem susceptibility testing and Klebsiella pneumoniae carbapenemase phenotype detection in Klebsiella pneumoniae isolates by use of automated microscopy of immobilized live bacterial cells . J Clin Microbiol. 2014;52(03):982–986. doi: 10.1128/JCM.03255-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.May A K, Brady J S, Romano-Keeler J et al. A pilot study of the noninvasive assessment of the lung microbiota as a potential tool for the early diagnosis of ventilator-associated pneumonia. Chest. 2015;147(06):1494–1502. doi: 10.1378/chest.14-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dutta R, Hines E L, Gardner J W, Boilot P. Bacteria classification using Cyranose 320 electronic nose. Biomed Eng Online. 2002;1:4. doi: 10.1186/1475-925X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Schee M P, Paff T, Brinkman P, van Aalderen W M, Haarman E G, Sterk P J. Breathomics in lung disease. Chest. 2015;147(01):224–231. doi: 10.1378/chest.14-0781. [DOI] [PubMed] [Google Scholar]
- 80.Hanson C W, III, Thaler E R. Electronic nose prediction of a clinical pneumonia score: biosensors and microbes. Anesthesiology. 2005;102(01):63–68. doi: 10.1097/00000542-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 81.Bos L D, Martin-Loeches I, Kastelijn J B et al. The volatile metabolic fingerprint of ventilator-associated pneumonia. Intensive Care Med. 2014;40(05):761–762. doi: 10.1007/s00134-014-3260-5. [DOI] [PubMed] [Google Scholar]
- 82.Schnabel R, Fijten R, Smolinska A et al. Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Sci Rep. 2015;5:17179. doi: 10.1038/srep17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Filipiak W, Beer R, Sponring A et al. Breath analysis for in vivo detection of pathogens related to ventilator-associated pneumonia in intensive care patients: a prospective pilot study. J Breath Res. 2015;9(01):16004. doi: 10.1088/1752-7155/9/1/016004. [DOI] [PubMed] [Google Scholar]
- 84.Huang A M, Newton D, Kunapuli A et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis. 2013;57(09):1237–1245. doi: 10.1093/cid/cit498. [DOI] [PubMed] [Google Scholar]
- 85.Parta M, Goebel M, Thomas J, Matloobi M, Stager C, Musher D M. Impact of an assay that enables rapid determination of Staphylococcus species and their drug susceptibility on the treatment of patients with positive blood culture results. Infect Control Hosp Epidemiol. 2010;31(10):1043–1048. doi: 10.1086/656248. [DOI] [PubMed] [Google Scholar]
- 86.Spellberg B, Srinivasan A, Chambers H F. New societal approaches to empowering antibiotic stewardship. JAMA. 2016;315(12):1229–1230. doi: 10.1001/jama.2016.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kollef M H, Micek S T. Rational use of antibiotics in the ICU: balancing stewardship and clinical outcomes. JAMA. 2014;312(14):1403–1404. doi: 10.1001/jama.2014.8427. [DOI] [PubMed] [Google Scholar]
- 88.Kollef M H, Bassetti M, Burnham J, Dimopoulos G, Garnacho-Montero J, Lipman J, Luyt C E, Nicolau D P, Postma M J, Torres A, Welte T G, Wunderink R. Intensive Care Med. 2017 doi: 10.1007/s00134-017-4682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


