Highlights
-
•
Unique PIV3 strain was detected from many goat farms with respiratory disease.
-
•
Virus was isolated and confirmed by RT-PCR and HA assay.
-
•
Genetic and phylogenetic analysis showed the isolate might be a new member of PIV3.
-
•
Goat derived PIV3 had pathogenicity to goats.
Keywords: PIV3, Goat, Respiratory disease, Phylogenetic analysis, Pathogenicity
Abstract
Parainfluenza virus type 3 (PIV3) is one of the most important viral respiratory pathogens for humans and for many animals, but goat infection has been rarely reported. Starting in Aug 2013, goats in the Jiangsu and Anhui provinces of eastern China suffered severe respiratory diseases. In order to identify the causative agent, numerous related pathogens were tested with RT-PCR or PCR. A unique PIV3 strain was detected in most of the clinical nasal swabs or serum samples. The virus was isolated on MDBK cells and characterized by RT-PCR, nucleotide sequence analysis and hemagglutination test. The entire M and F gene coding regions, HN, 5′-UTR-N and L gene fragments were amplified using pairs of degenerate primers. Nucleotide, amino acid sequence alignments and phylogenetic analyses based on these genes indicated that the goat-derived PIV3 strain was distinct from previously reported BPIV3 genotypes and HPIV3 strains. The novel isolate, named JS2013, might be a potentially new member of the respirovirus genus. Goats were experimentally infected with JS2013 culture. The virus-inoculated goats displayed coughing and nasal discharges that were related to respiratory diseases. Viremia and virus shedding were detected during 4–10 days post-inoculation (dpi). Virus-specific HI antibodies became positive from 14 dpi. This is the first report of the detection of PIV3 from Chinese goat herds and genetic and pathogenetic characterization of the novel goat-derived PIV3.
1. Introduction
PIV3 is an enveloped, single-stranded negative sense RNA virus within the respirovirus genus of the Paramyxoviridae family, which causes respiratory diseases in many host species (Maidana et al., 2012). The respirovirus genus includes human parainfluenza virus types 1 and 3 (HPIV1 and HPIV3, respectively), bovine parainfluenza virus type 3 (BPIV3) and Sendai virus (Fauquet et al., 2005). BPIV3 is an important pathogen associated with bovine respiratory disease complex (BRDC) which is considered the most significant disease associated with feedlot cattle worldwide (Horwood et al., 2008).
BPIV3 infection causes severe bronchopneumonia and secondary bacterial infections in instances of high stress, such as transportation, weather change and feedlot situations (Haanes et al., 1997). The main clinical signs of BPIV3 infection are coughing, anorexia, pyrexia, nasal and ocular discharges, dyspnea and sometimes diarrhea (Zhu et al., 2011). Three BPIV3 genotypes, A (BPIV3a), B (BPIV3b) and C (BPIV3c) have been described and BPIV3a and BPIV3c have been found in Chinese cattle (Zhu et al., 2011). However, reports of PIV3 infection in goat/sheep are limited and the genetic characteristics of the virus have not been determined (Elazhary et al., 1984, Lamontagne et al., 1985, Lyon et al., 1997, Taylor et al., 1975). Furthermore, nothing is known about the circulation of PIV3 in goats/sheep in China and the relationship between virus infections and clinical diseases, as well as the relationship of small ruminants’ PIV3 with BPIV3 and HPIV3.
In 2013, goats in Jiangsu and Anhui provinces of China suffered severe respiratory diseases. We used RT-PCR or PCR to test for various potential agents from clinical samples and PIV3 viral RNA was detected in most samples. Virus isolation was performed and was further confirmed by RT-PCR, sequencing and HA assay. Genetic and phylogenetic analysis was performed and suggested that the virus might be a new member of PIV3.
2. Materials and methods
2.1. Cases and pathological observations
From Aug 2013 to Mar 2014, goats of many farms in Jiangsu and Anhui provinces in eastern China suffered severe respiratory diseases, with high morbidity and varied mortality. These farms were fattening farms, which purchased weaning goats from several small backyard farms for fattening until slaughter time. Most of the diseased goats (3–6 months) showed respiratory syndrome (coughing, nasal discharges, dyspnea), anorexia emaciation and some of them suffered diarrhea. The diseased goats usually showed no obvious body temperature changes. Antibiotic treatment had no apparent effect, although a few diseased animals recovered within 10–15 days. Necropsy of the diseased goats showed mainly seroperitoneum, pathological lesions in the lung and pancreas, white punctiform tuberculum in epiploica or mesenterium and enlarged liver (Fig. 1 ).
Fig. 1.
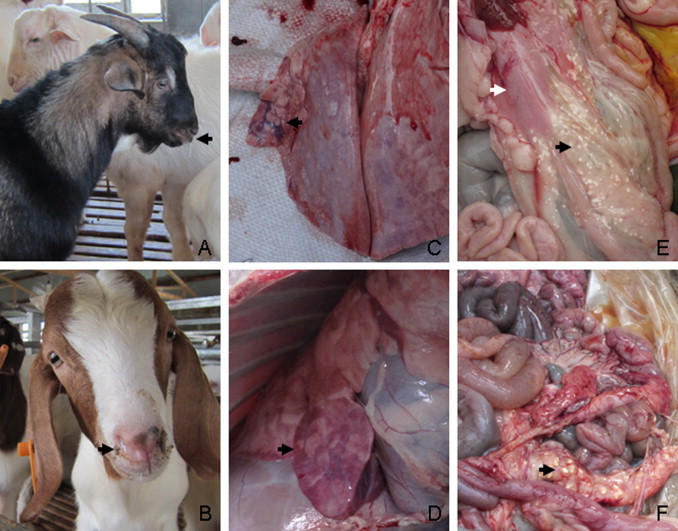
Clinical and pathological observations of diseased goats. Diseased goats with nasal discharges (A, B, black arrows); lungs with mild to moderate diffuse purple consolidation (C, D, black arrows); enlargement and hemorrhage in pancreas (E, white arrow); white punctiform tuberculum in mesenterium (E, F, black arrows).
2.2. Clinical sampling
Seventy-seven nasal swab/serum samples were collected from eleven farms in Jiangsu and Anhui provinces during Oct 2013 and Mar 2014 (Table 1 ). Samples were stored at −70 °C for RT-PCR and virus isolation. All sampled goats showed clinical signs of pneumonia when samples were collected.
Table 1.
Summary of the sampling and RT-PCR detection results.
| Farm | Location | Collection date | No. positive/total |
|
|---|---|---|---|---|
| Nasal swabs | Sera | |||
| A | Haian, Nantong, Jiangsu | 11-Oct-2013 | 0 | 1/5 |
| B | Haian, Nantong, Jiangsu | 11-Oct-2013 | 0 | 6/15 |
| C | Haian, Nantong, Jiangsu | 11-Oct-2013 | 0 | 1/5 |
| D | Haian, Nantong, Jiangsu | 4-Nov-2013 | 2/9 | 0 |
| E | Haian, Nantong, Jiangsu | 4-Nov-2013 | 4/5 | 0 |
| F | Haian, Nantong, Jiangsu | 2-Jan-2014 | 13/13 | 0 |
| G | Quanjiao, Chuzhou, Anhui | 7-Jan-2014 | 1/3 | 1/2 |
| H | Rudong, Nantong, Jiangsu | 13-Jan-2014 | 1/2 | 1/1 |
| I | Pukou, Nanjing, Jiangsu | 25-Feb-2014 | 4/6 | 4/6 |
| J | Quanjiao, Chuzhou, Anhui | 17-Mar-2014 | 2/2 | 0 |
| K | Pukou, Nanjing, Jiangsu | 19-Mar-2014 | 1/2 | 1/1 |
| Total | 28/42 | 15/35 | ||
2.3. Pathogen detection
Total RNA was extracted from nasal swabs/serum samples using TRIzol reagent (Invitrogen) according to the manufacturer's instruction. DNA was extracted from nasal swab/serum samples using phenol/chloroform.
PIV3-specific RT-PCR amplification was carried out with Easyscript one-step RT-PCR supermix (Transgen, Bio, Inc.) in a 20 μl reaction mixture containing 2× R-Mix buffer, 20 pM of each primer (MF1/MR1, Table 2 ), 0.5 μl of E-Mix and 4 μl extracted RNA. The reaction was run in a thermocycler (Mjmini, BIO-RAD) with the following program: 45 °C 40 min for reverse transcription; denaturation at 94 °C for 5 min, 35 cycles composed of denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s and extension at 72 °C for 45 s; and was terminated with a final extension of 10 min at 72 °C. Amplification products were detected by electrophoresis in 1.2% agarose gels.
Table 2.
Primers used for PIV3 detection and gene amplification.
| Primers | Sequences (5′–3′) | Location (to NM09) | Target | Expected products size (bp) | Annealing temperature (°C) |
|---|---|---|---|---|---|
| MF1 | AGTGATCTAGATGATGATCCA | 3960–3980 | M | 329 | 50 |
| MR1 | GTTATTGATCCAATTGCTGT | 4269–4288 | |||
| HNF | GCCAATACAGAGAATGACTCATGA | 7210–7233 | HN | 674 | 54 |
| HNR | CTCTTTGTGTTTTGCCWGGACA | 7862–7883 | |||
| NF | AGGTRAGGGRGAAGARATCCT | 78–98 | 5′-UTR-N | 881 | 54 |
| NR | AGAGCTGCCATTCTRGTYTCAAT | 936–958 | |||
| LF | AGAGARGTBATGGATGATCTD | 11,079–11,099 | L | 545 | 54 |
| LR | GAYTCRCCTGGTTCYTGATTC | 11,603–11,623 | |||
| MF2 | AGCATCASMARCTCYRRAATMT | 3738–3759 | M | 1053 | 52 |
| MR2 | CTATTGYYTRATYTTYCCGACYCCT | 4766–4790 | |||
| FF1 | CTCATTACCTGGTGAATTCA | 4721–4740 | M-F | 1481 | 53 |
| FR1 | ACAAATGCATATCKTGGYAC | 6182–6201 | |||
| FF2 | ACTTCRACAGTTGAYCAATA | 5798–5817 | F-HN | 1297 | 53 |
| FR2 | GTCTTGTGTTTATTCCYGAYTG | 7073–7094 |
Other related pathogens, ovine respiratory syncytial virus (ORSV) (Eleraky et al., 2001), peste des petits ruminants virus (PPRV) (Mao et al., 2010), pestivirus (Vilcek et al., 1994), foot and mouth disease virus (FMDV) (Xu et al., 2013), bovine coronavirus (Amer et al., 2013) and Mycoplasma (Bascunana et al., 1994, McAuliffe et al., 2003) were also tested by previously reported methods.
2.4. PIV3 isolation
Positive nasal swab samples were centrifuged at 12,000 rpm for 20 min, the supernatants were filtered through 0.22 μm filter (Millipore) and inoculated onto Madin–Darby bovine kidney (MDBK) cell monolayers cultured in 24-well cell culture plates. Positive serum samples were used for inoculation directly after centrifugation at 12,000 rpm for 20 min. The MDBK cultures were observed for 5–7 days. Cell cultures were harvested and passaged 4 more times. Each viral stock was stored at −70 °C for RT-PCR detection. Virus isolation was confirmed by RT-PCR using primers MF1/MR1 and HNF/HNR, and the isolates were further identified by hemagglutination test and genetic analysis.
2.5. Hemagglutination (HA) test
HA test was performed with 0.25% guinea pig erythrocyte suspension in 96-well V-bottom plates. Equal volumes (0.05 mL) of serial twofold dilutions of the virus isolates and erythrocytes were mixed, shaken and allowed to sediment for 45 min at 37 °C. The end point was the last well in which the guinea pig erythrocytes did not agglutinate.
2.6. Genetic and phylogenetic analysis
To confirm the specificity of JS2013, the HN, 5′-UTR-N and L gene fragments and entire M and F gene coding regions were amplified from positive samples using the degenerate primers listed in Table 2, purified with a purification kit (Axygen Bio, Inc.), cloned into pMD18-T vector (Takara Bio, Inc.), and transformed into Escherichia coli DH5α competent cells. Three positive clones for each PCR products were sequenced. Each sequence was determined in both directions.
The nucleotide sequences and corresponding predicted amino acid sequences were edited by Editseq (DNASTAR Inc., Madison, WI) and subjected to MegAlign (DNASTAR Inc., Madison, WI) and Blast analyses. Multiple sequence alignment was done by using Clustal X 1.83 (Thompson et al., 1997), together with other reference sequences of previously identified BPIV3 and human parainfluenza virus type 3 (HPIV3). After determining the percentages of sequence identity among different PIV 3 strains, phylogenetic trees were generated with the distance-based neighbor-joining (NJ) method by using MEGA 4.0.2 software (Tamura et al., 2007). The robustness of the phylogenetic trees was determined by bootstrap resampling analysis carried out on 1000 replicates.
2.7. Infection of goats with JS2013
Eight goats were randomly divided into two groups of four each and housed separately. Group CC was intranasally inoculated with JS2013 (3 mL/goat, HA titer of 256). Group NC was inoculated with PBS and used as negative control. After challenge, all animals were monitored for 35 days. Rectal temperatures and clinical signs were observed daily and clinical observations were recorded. The serum samples were collected from all animals at 0, 4, 7, 10, 14, 21, 28 and 35 dpi. Nasal swabs were taken at 0, 4, 7, 10, 14, 21 dpi. RNA was extracted from serum and nasal swab samples at 0, 4, 7, 10, 14, 21 dpi; RT-PCR was performed as mentioned above (Section 2.3) to detect viremia and virus shedding. Hemagglutination inhibition (HI) assay was performed to determine PIV3-specific antibodies in serum samples of 0, 14, 21, 28 and 35 dpi.
2.8. Hemagglutination inhibition (HI) assay
For the HI assay, serial two-fold dilutions of sera were allowed to react with 4 HA units of PIV3 for 30 min at 37 °C, followed by addition of guinea pig erythrocytes. After 40 min incubation at 37 °C, HI titers were calculated as the highest serum dilution that inhibited 4HA unit antigen. If HI titer was less than or equal to 3 log2, it was considered negative; if HI titer was more than or equal to 4 log2, it was considered positive.
2.9. Ethical approval
The collection of nasal and serum samples and the artificial infection experiment were performed in strict accordance with the guidelines of Jiangsu Province Animal Regulations (Government Decree No. 45).
3. Results
3.1. Field sample detection
M gene fragments were detected in 43 of 77 (56%) nasal swab/serum samples by RT-PCR, and all tested farms had positive samples (Table 1). The amplified products were purified, cloned and sequenced. Blast search revealed that the sequences shared the highest identity of 82% with Japanese BN-CE strain of bovine PIV3. All amplified M gene products from the different farms we tested had 100% identity with each other, indicating they might be the same PIV3 strain.
In addition, ovine respiratory syncytial virus was detected in 5/77 samples (farm A: 2, C: 1 and G: 2). Mycoplasma ovipneumoniae was detected in 7/77 samples (farm D: 3, E: 2 and I: 2). PPRV, bovine coronavirus, pestivirus and FMDV were negative.
3.2. Virus isolation
After culture and passage in MDBK cells, one virus isolate was obtained from a nasal swab sample (isolation from serum samples was not successful). The isolate uniformly produced cytopathic effect from the 2nd passage. The 329 bp M gene and 674 bp HN gene fragments were amplified from virus cultures by RT-PCR (data not shown). In addition, these two genes of different passages did not change (100% identity). The 3rd, 4th and 5th passages of isolated viral stock showed titers of 32, 128 and 256 by hemagglutination test, respectively. These results suggested successful viral isolation and amplification; the novel virus was named JS2013.
3.3. Nucleotide and amino acid sequence analysis
To confirm the specificity of JS2013, the entire M and F gene coding regions, HN, 5′-UTR-N and L gene fragments were amplified and sequenced (GenBank accession No. M: KJ850331, HN: KJ767525; N: KJ843145; F: KM279938; L: KJ843144). Blast research and MegAlign analysis indicated that the M, F, HN, N and L sequences shared the highest nucleotide identity of 80.2% with SF strain, 77% with SF strain, 80% with BN-CE strain, 82.5% with Q5592 strain and 83.5% with Q5592 strain, respectively. They possessed lower identity with HPIV3 sequence. In brief, they showed nucleotide identity of 77.5–80.2%, 72.5–76.9%, 74.1–80%, 78.4–82.5% and 80.7–83.5% with the selected representative sequences of different BPIV3 genotypes and HPIV3 strains, respectively (Table 3 ). The F gene possessed the lowest identity and L gene was the highest with other compared sequences (Table 3).
Table 3.
The nucleotide and deduced amino acid identities of JS2013 compared to different BPIV3 and HPIV3 isolates.
| Nucleotide identity (%) |
Amino acid identity (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BPIV3a |
BPIV3b | BPIV3c | HPIV3 | BPIV3a |
BPIV3b | BPIV3c | HPIV3 | |||
| SF | BN-CE | Q5592 | SD0835 | GP | SF | BN-CE | Q5592 | SD0835 | GP | |
| M | 80.2 | 79.6 | 79.7 | 77.5 | 77.8 | 89.5 | 87.7 | 88.6 | 88.9 | 88.6 |
| HN | 78.5 | 80 | 77.9 | 77 | 74.1 | 86.6 | 87.5 | 88.4 | 84.4 | 77.7 |
| Na | 81 | 80.7 | 82.5 | 81.1 | 78.4 | 92.2 | 92.6 | 92.9 | 90.4 | 87.2 |
| Fa | 76.9 | 76.4 | 76.6 | 74.9 | 72.5 | 79.1 | 78.2 | 77.6 | 77.5 | 75.2 |
| L | 81.7 | 80.7 | 83.5 | 82 | 82.4 | 95.6 | 95 | 95 | 95 | 93.9 |
Only the coding region was analyzed.
The corresponding amino acid sequences had higher identities than their nucleotide sequences (Table 3). Similarly, they had lower identity with HPIV3 sequences. Among them, the L protein was the highest (93.9–95.6%), while F had the lowest (75.2–79.1%), indicating that F was more variable amongst different strains.
3.4. Phylogenetic analysis
Phylogenetic reconstructions were compiled using a 1056 nucleotide region of M, a 674 nucleotide region of HN, a 848 nucleotide region of N and a 1623/1626 nucleotide region of F, respectively. Phylogenetic analysis based on M and F gene sequences showed similar grouping results, while HN- and N-based phylogenetic trees had similar structures, in which JS2013 located between BPIV3 and HPIV3 clusters. All of them showed that JS2013 formed a unique branch, which clearly differed from HPIV3 and other reported BPIV3 genotypes (Fig. 2 ). The results, together with nucleotide and amino acid alignment results, indicated that JS2013 might represent a novel member of the respirovirus genus in the Paramyxoviridae family.
Fig. 2.
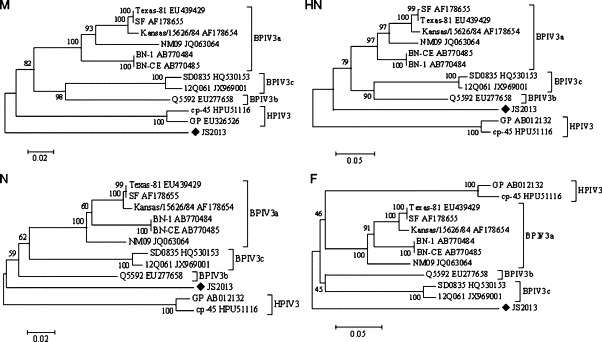
Neighbor-joining unrooted phylogenetic trees based on the M, N, HN and F gene sequences. Numbers at nodes indicate bootstrap percentages obtained after 1000 replicates. Scale bar indicates genetic distance. Our isolate JS2013 is marked with ◆. GenBank accession numbers of the sequences used in the phylogenetic tree are as follows: BPIV3 reference strains: SF, AF178655; Kansas/15626/84, AF178654; BN-CE, AB770485; BN-1, AB770484; Texas-81, EU439429; NM09, JQ063064; Q5592, EU277658; SD0835, HQ530153; 12Q061, JX969001 and HPIV3 reference strains: cp-45, HPU51116; GP, AB012132.
3.5. JS2013 showed pathogenicity in goats
Goats were intranasally infected with JS2013 to preliminarily assess the pathogenicity of JS2013. After challenge, rectal temperatures of all animals remained normal, with no significant differences observed between the inoculated and non-treated groups (data not shown).
As shown in Table 4 , three out of the four virus-inoculated goats (D5, D7 and D8) displayed coughing and mild to severe nasal discharges during 4–10 dpi. Viremia was detected in all goats of group CC during 4–10 dpi. Virus shedding was confirmed by RT-PCR from nasal swabs and M gene sequencing indicated that it was same as the originally infected virus. In addition, virus specific HI antibodies were positive from 14 dpi; 3/4 goats of CC group showed ≥2 fold change of HI titers between 0 and 14 dpi, and all goats showed ≥2 fold HI titers increase in 28 dpi.
Table 4.
Clinical signs, viremia and HI antibody detection at different time points post-infection.
| Clinical observations (coughing/nasal discharges) |
Viremia/virus shedding |
HI antibody (log2) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 10 | 14 | 0 | 4 | 7 | 10 | 14 | 21 | 0 | 14 | 21 | 28 | 35 | ||
| NC | D1 | – | – | – | – | – | – | – | – | – | – | – | 2 | 2 | 3 | 3 | 2 |
| D2 | – | – | – | – | – | – | – | – | – | – | – | 2 | 2 | 2 | 3 | 2 | |
| D3 | – | – | – | – | – | – | – | – | – | – | – | 3 | 2 | 3 | 2 | 3 | |
| D4 | – | – | – | – | – | – | – | – | – | – | – | 2 | 3 | 2 | 3 | 3 | |
| CC | D5 | – | +/+a | ++/+ | – | – | – | +/+ | +/+ | +/+ | – | – | 2 | 4 | 4 | 4 | 4 |
| D6 | – | – | – | – | – | – | +/− | +/+ | – | – | – | 3 | 4 | 4 | 5 | 4 | |
| D7 | – | −/+ | +/++ | +/+ | – | – | +/+ | +/+ | +/+ | – | – | 2 | 4 | 5 | 4 | 4 | |
| D8 | – | +/++ | ++/+++ | +/+ | – | – | +/+ | +/+ | +/+ | – | – | 1 | 6 | 5 | 5 | 5 | |
Coughing/nasal discharges: –, normal; +, mild; ++, moderate; +++, severe.
4. Discussion
Respiratory diseases have been causing major economic loss to feedlot goat farms. In feedlot cattle, BRDC is considered the most significant disease, and BPIV3 plays an important role in BRDC development. Viral infection plus stress conditions and other secondary or co-infections result in severe clinical observations (Haanes et al., 1997, Maidana et al., 2012). However, there are few reports about related goat viral pathogens. Most previous reports regarding PIV3 in goat/sheep were published in the 1970s–1980s and only serological tests were done; thus, related virus genome characteristics and virus isolations had not been identified (Afshar and Terlecki, 1979, Elazhary et al., 1984, Kennedy-Stoskopf et al., 1983, Lamontagne et al., 1985, Lyon et al., 1997, Taylor et al., 1975). In the present cases, the goats were imported from different places and transport stress might trigger disease development. The diseased goats were treated with different antibiotics and did not show significant changes in clinical signs. The diseased and dead animals showed similar pathological characteristics. In the primary laboratory examination, several potential pathogens were tested. Most of the samples (56%) showed PIV3 positive results, and sequencing results indicated that viruses from different farms shared 100% identity, indicating the contribution of this novel PIV3 to this clinical disease. ORSV and M. ovipneumoniae were also detected from some samples, indicating different co-infection situations in different farms and this might result in the difference of recovery rate between farms; however, ORSV and M. ovipneumoniae were not the major causes of the goat infections. PIV3 was successfully isolated from nasal swabs and was intranasally infected into goats to assess its pathogenicity. From 4 to 10 dpi, viremia and virus shedding could be detected in all virus-inoculated goats; and 3/4 goats displayed coughing, with mild to moderate nasal discharges. Virus-specific HI antibodies were positive from 14 dpi (Table 4). These results further confirmed the goat-derived PIV3 strain had pathogenicity in goats. Of course, other potential unknown pathogens might also be present, requiring further studies.
Three BPIV3 genotypes have been described. BPIV3a was relatively older and widespread. BPIV3b and BPIV3c were found in Australia (Horwood et al., 2008) and Chinese cattle (Zhu et al., 2011), respectively. Blast searches of the 329 bp M region of JS2013 showed the highest identity of only 82% with Japanese BN-CE strain, indicating that JS2013 was distinct from the previously reported BPIV3 strains. Phylogenetic analysis based on this region gave the same result (data not shown). It was hypothesized that JS2013 might represent a potentially new group of PIV3. To confirm this hypothesis, several pairs of degenerate primers were designed to amplify the entire M and F coding region, partial N, HN and L gene fragments. The sequences were used for genetic and phylogenetic analysis. Similarly, the highest nucleotide identities of M, F, N, HN and L genes with reported strains in Genbank were only 76.9–83.5% (Table 3). The new PIV3 strain shared slightly higher identity with BPIV3 than HPIV3. As shown in Table 3, the F and HN genes and proteins possessed relatively lower identities while the L gene and protein had the highest identity with other sequences, suggesting that F and HN were highly varied among different strains. Considering that HN protein was an immunodominant protein of PIV3, HN-based serological methods and vaccine should be developed in the future.
Zhu et al. had proposed the use of PIV3 M and HN genes as appropriate targets for genotyping (Zhu et al., 2011). F gene was also used for other paramyxovirus genotyping. Therefore, M, F, HN and N genes were used for phylogenetic analysis in this study and they showed similar grouping results (Fig. 2): JS2013 was clearly differed from HPIV3 and other reported BPIV3 genotypes. The results presented here strongly indicate that JS2013 might represent a novel member of PIV3 in the respirovirus genus and the name caprine parainfluenza virus type 3 is proposed (Fig. 2). Full-length genome sequencing is currently underway for detailed understanding the genomic characteristics of this new virus.
To our knowledge, this is the first report about the detection and isolation of PIV3 in Chinese goat flocks with respiratory diseases. A broader epidemiological investigation of PIV3 in goats should be carried out and serological methods should be established for detecting specific antibodies against PIV3 in goat as well as sheep herds. The pathogenicity of this new isolate needs further detailed studies and related preventative techniques should be developed. This would be a good beginning for detailed research on respiratory diseases of goat/sheep in China.
Acknowledgments
This work was supported by the Special Fund for Independent innovation of Agricultural Science and Technology in Jiangsu province (CX(14)2090). We thank Dr. Kevin Coombs (Professor, Department of Medical Microbiology, University of Manitoba, Canada) for his proof reading of the manuscript.
References
- Afshar A., Terlecki S. Experimental infection of goats with parainfluenza virus type 3. Zentralbl Veterinarmed B. 1979;26:641–651. doi: 10.1111/j.1439-0450.1979.tb00858.x. [DOI] [PubMed] [Google Scholar]
- Amer H.M., Abd El Wahed A., Shalaby M.A., Almajhdi F.N., Hufert F.T., Weidmann M. A new approach for diagnosis of bovine coronavirus using a reverse transcription recombinase polymerase amplification assay. J. Virol. Methods. 2013;193:337–340. doi: 10.1016/j.jviromet.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascunana C.R., Mattsson J.G., Bolske G., Johansson K.E. Characterization of the 16S rRNA genes from Mycoplasma sp. strain F38 and development of an identification system based on PCR. J. Bacteriol. 1994;176:2577–2586. doi: 10.1128/jb.176.9.2577-2586.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazhary M.A., Silim A., Dea S. Prevalence of antibodies to bovine respiratory syncytial virus, bovine viral diarrhea virus, bovine herpesvirus-1, and bovine parainfluenza-3 virus in sheep and goats in Quebec. Am. J. Vet. Res. 1984;45:1660–1662. [PubMed] [Google Scholar]
- Eleraky N.Z., Kania S.A., Potgieter L.N. The ovine respiratory syncytial virus F gene sequence and its diagnostic application. J. Vet. Diagn. Invest. 2001;13:455–461. doi: 10.1177/104063870101300601. [DOI] [PubMed] [Google Scholar]
- Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A. Elsevier Academic Press; San Diego: 2005. Virus Taxonomy: VIIIth Report of the International Committee on Taxonomy of Viruses. [Google Scholar]
- Haanes E.J., Guimond P., Wardley R. The bovine parainfluenza virus type-3 (BPIV-3) hemagglutinin/neuraminidase glycoprotein expressed in baculovirus protects calves against experimental BPIV-3 challenge. Vaccine. 1997;15:730–738. doi: 10.1016/s0264-410x(96)00231-9. [DOI] [PubMed] [Google Scholar]
- Horwood P.F., Gravel J.L., Mahony T.J. Identification of two distinct bovine parainfluenza virus type 3 genotypes. J. Gen. Virol. 2008;89:1643–1648. doi: 10.1099/vir.0.2008/000026-0. [DOI] [PubMed] [Google Scholar]
- Kennedy-Stoskopf S., Narayan O., Hirsch R.L. Immunosuppression in goats inoculated with parainfluenza type 3 virus. Am. J. Vet. Res. 1983;44:2302–2306. [PubMed] [Google Scholar]
- Mao L., Dou Y.X., Zhai J.J., Wang Y.C., Gong W., Cai X.P. Development of RT-PCR for detection of peste des petits ruminants virus. Chin. Vet. Sci. 2010;40:593–597. (in Chinese) [Google Scholar]
- Lamontagne L., Descôteaux J.P., Roy R. Epizootiological survey of parainfluenza-3, reovirus-3, respiratory syncytial and infectious bovine rhinotracheitis viral antibodies in sheep and goat flocks in Quebec. Can. J. Comp. Med. 1985;49:424–428. [PMC free article] [PubMed] [Google Scholar]
- Lyon M., Leroux C., Greenland T., Chastang J., Patet J., Mornex J.F. Presence of a unique parainfluenza virus 3 strain identified by RT-PCR in visna-maedi virus infected sheep. Vet. Microbiol. 1997;57:95–104. doi: 10.1016/s0378-1135(97)00104-1. [DOI] [PubMed] [Google Scholar]
- Maidana S.S., Lomonaco P.M., Combessies G., Craig M.I., Diodati J., Rodriguez D., Parreno V., Zabal O., Konrad J.L., Crudelli G., Mauroy A., Thiry E., Romera S.A. Isolation and characterization of bovine parainfluenza virus type 3 from water buffaloes (Bubalus bubalis) in Argentina. BMC Vet. Res. 2012;8:83. doi: 10.1186/1746-6148-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe L., Hatchell F.M., Ayling R.D., King A.I., Nicholas R.A. Detection of Mycoplasma ovipneumoniae in Pasteurella-vaccinated sheep flocks with respiratory disease in England. Vet. Rec. 2003;153:687–688. doi: 10.1136/vr.153.22.687. [DOI] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Taylor W.P., Momoh M., Okeke A.N. Antibodies to parainfluenza 3 virus in cattle, sheep and goats from northern Nigeria. Vet. Rec. 1975;97:183. doi: 10.1136/vr.97.10.183. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek S., Herring A.J., Herring J.A., Nettleton P.F., Lowings J.P., Paton D.J. Pestiviruses isolated from pigs, cattle and sheep can be allocated into at least three genogroups using polymerase chain reaction and restriction endonuclease analysis. Arch. Virol. 1994;136:309–323. doi: 10.1007/BF01321060. [DOI] [PubMed] [Google Scholar]
- Xu L., Hurtle W., Rowland J.M., Casteran K.A., Bucko S.M., Grau F.R., Valdazo-Gonzalez B., Knowles N.J., King D.P., Beckham T.R., McIntosh M.T. Development of a universal RT-PCR for amplifying and sequencing the leader and capsid-coding region of foot-and-mouth disease virus. J. Virol. Methods. 2013;189:70–76. doi: 10.1016/j.jviromet.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Zhu Y.M., Shi H.F., Gao Y.R., Xin J.Q., Liu N.H., Xiang W.H., Ren X.G., Feng J.K., Zhao L.P., Xue F. Isolation and genetic characterization of bovine parainfluenza virus type 3 from cattle in China. Vet. Microbiol. 2011;149:446–451. doi: 10.1016/j.vetmic.2010.11.011. [DOI] [PubMed] [Google Scholar]


