Highlights
-
•
RVA vaccination program reduces the frequency and intensity of diarrhea in dairy calves.
-
•
The vaccination immune pressure can select specific genotypes in RVA field strains.
-
•
RVA genotype G10P[11] in fecal samples of calves from G6P[5] vaccinated dairy cattle herds.
Keywords: Calf, Neonatal diarrhea, Vaccine, RVA, Genotype
Abstract
Worldwide, neonatal diarrhea is one of the most important health issues affecting dairy calves, and rotavirus A (RVA) is one of its primary causes. Among the measures to mitigate the risk of diarrhea outbreaks, cow vaccination stands out as one of the most important. However, the immune pressure resulting from routine vaccination may be able to select specific G and P genotypes in RVA field strains. This study aimed to determine the frequency and intensity of neonatal diarrhea and the incidence of RVA and attempted to monitor the G and P genotypes present in the RVA strains circulating in a high milk yield cattle herd vaccinated with RVA G6P[5] strain. Fecal samples (n = 1220) from 122 Holstein heifer calves between 0–30 days old that were born from RVA-vaccinated cows were collected at 10 different time points, regardless of the presence or absence of diarrhea. The presence of RVA in fecal samples was determined by the polyacrylamide gel electrophoresis (PAGE) technique and confirmed by reverse transcription polymerase chain reaction (RT-PCR). G and P amplicons from 10 RVA-positive fecal samples from calves of different ages and collections were subjected to nucleotide sequencing. The proportion of the calves and fecal samples that were positive for RVA were 62.3% (76/122) and 8.1% (99/1220), respectively. Using sequence analysis, all 10 RVA field strains presented genotype G10P[11]. The protection of G6P[5] vaccination is clear, as this genotype was not detected in this study, and it is known that vaccination against RVA reduces the incidence of diarrhea independent of genotype involved. This result demonstrates the importance of epidemiological monitoring of RVA genotypes circulating in vaccinated dairy cattle herds to the early detection of new potential pathogenic RVA strains.
1. Introduction
Neonatal calf diarrhea (NCD) is the most common cause of calf morbidity and mortality, which can exceed 20% in certain instances (Uetake, 2013). The factors that influence the etiology of this syndrome include nutritional and sanitary management, immunological aspects, and infectious agents, such as bacteria, viruses, and protozoa (Coura et al., 2015). Bovine rotavirus A (RVA) is one of the most prevalent viral agents associated with NCD in dairy and beef cattle herds worldwide (Al Mawly et al., 2015; Coura et al., 2015).
The non-enveloped virion of RVA is composed of a triple-layered capsid that surrounds 11 dsRNA segments that encode 6 structural (VP1–VP4, VP6, and VP7) and 6 nonstructural (NSP1-NSP5/6) proteins (Greenberg and Estes, 2009). Based on their antigenic characteristics and sequence analyses of the VP6 gene, viruses within the Rotavirus genus are primarily classified into 9 distinct groups/species (A–I) (ICTV, 2017; Matthijnssens et al., 2012; Mihalov-Kovacs et al., 2015); a newly-proposed species J has been described in the bat (Banyai et al., 2017). The VP7 and VP4 genes encode 2 structural proteins responsible for the induction of the immune protective response and are used for the binary classification of RVA strains in terms of their G and P genotypes, respectively. Currently, 36 G and 51 P genotypes are recognized by the Rotavirus Classification Working Group (RCWG, 2017).
Calves aged 1 to 3 weeks have antibody levels from passive immunity decreasing to a level that is still sufficiently high to block responses to vaccines but is not sufficiently high to combat infection. Thus, this period is considered a window of opportunity for the infection of microorganisms and, consequently, the occurrence of disease (Chase et al., 2008). Therefore, the implementation of a cow vaccination program is an important strategy to protect the calves from RVA infection and consequent neonatal diarrhea.
The commercial vaccines that are available in Brazil contain inactivated RVA and other infectious agents that are implicated in NCD. There were 2 different RVA Brazilian vaccine formulations: i) the bivalent vaccine G10P[11] and G6P[1]; ii) and monovalent G6P[5] genotype, which is the most prevalent genotype worldwide (Papp et al., 2013) as well as in Brazil (Medeiros, 2016).
The aim of this study was to determine the frequency and intensity of neonatal diarrhea and the incidence of RVA and to identify the RVA G and P genotypes circulating in dairy calves born from cows that are regularly vaccinated with the RVA G6P[5] strain in a high milk yield dairy cattle herd.
2. Materials and methods
2.1. Herd information
This study was conducted in a Brazilian dairy farm that is operated as a closed dairy cattle herd with 1800 Holstein cows in lactation. The cows were managed using appropriate nutritional and health practices. This high milk yield dairy cattle herd produces an average of 37 liters/day/cow. The calves are reared in individual pens on elevated floors. The colostrum intake began shortly after birth and has taken place with adequate frequency and quantity for all newborn calves.
2.2. Vaccination of cows
Sixty or 45 days before calving, the cows were vaccinated against NCD. The commercial vaccine used in this study contained inactivated rotavirus A (UK-Compton strain – G6P[5]), inactivated bovine coronavirus (Mebus strain), and E. coli (K99) adesine F5. The vaccination was performed according to the manufacturer's instructions.
2.3. Fecal samples
The fecal samples from 122 heifer calves born from RVA-vaccinated cows were collected at 10 different time points (1, 4, 7, 10, 14, 17, 21, 24, 28, and 30 days after birth), regardless of the presence or absence of diarrhea. The samples were collected between March and May 2017, and a total of 1220 fecal samples were stored at -20 °C until analysis. At the time of collection, all of the fecal samples were classified according to their consistency using the following scale: 0, normal; 1, pasty; 2, soft; 3, watery. A score of 0 or 1 was considered non-diarrheic, and scores of 2 or 3 were considered diarrheic.
2.4. Determination of the presence of RVA
The 10 fecal samples from the same animal that were collected at different time points were processed at the same time to avoid any bias. The nucleic acid extraction was performed using a combination of the phenol/chloroform/isoamyl alcohol (25:24:1) and silica/guanidinium isothiocyanate extraction methods according to Alfieri et al. (2006). The presence of RVA dsRNA in fecal samples was evaluated using polyacrylamide gel electrophoresis (PAGE) (Pereira et al., 1983) followed by silver staining (Herring et al., 1982). Fecal samples with doubtful PAGE results, as bands with low intensity or in anomalous positions, extra bands or undefined electropherotype were confirmed by reverse transcription polymerase chain reaction (RT-PCR) assay.
2.5. RVA genotyping
The presence of RVA in the fecal samples that were determined to be positive for RVA via PAGE was confirmed by RT-PCR using consensus primers to amplify a 1062 bp fragment from the VP7 gene (Gouvea et al., 1990) and a 876 bp fragment from the VP4 (VP8*) gene (Gentsch et al., 1992; Martella et al., 2006). The RT-PCR products were analyzed using electrophoresis with 2% agarose gels stained with ethidium bromide and observed under ultraviolet (UV) light. Ten RT-PCR products of good quality from the RVA-positive fecal samples from 10 calves of 7 different ages in the range of 10 to 31 days old and 7 collections, including beginning, middle, and end of the experiment, were selected for sequence analysis.
The RT-PCR products were purified using an Illustra GFX PCR DNA and Gel Band Purification Kit (GE®, Buckinghamshire, UK), quantified using a Qubit® Fluorometer (Invitrogen® Life Technologies, Eugene, OR, USA), and sequenced using an ABI3500 Genetic Analyzer sequencer with a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems®, Foster City, CA, USA).
The Phred and CAP3 software packages were used for the nucleotide (nt) quality analysis and contig assembly of the RVA sequences, respectively (http://asparagin.cenargen.embrapa.br). Sequence similarity searches were performed using the basic local alignment search tool (BLAST) software (http://blast.ncbi.nlm.nih.gov/), and the genotype identification was performed using the ROTAC2.0 tool (http://rotac.regatools.be/).
Phylogenetic trees were obtained using the neighbor-joining method with Kimura 2-parameter model in MEGA V7 software. The bootstrapping probabilities were calculated using 1000 replicates. The BioEdit software, version 7.0.8.0, was used for the construction of the nt sequence identity matrix. The gene sequences described in the present study have been deposited in the GenBank database under accession numbers: MH016179 and MH016180.
3. Results and discussion
Seventy-six (62.3%) of the 122 heifer calves were RVA-positive according to at least 1 of the 10 fecal samples evaluated, and of these, 19 (15.6%) calves were determined to have excreted RVA on more than one occasion. Worldwide, the percentage of calves that are RVA-positive varies based on the experimental design used in each study. In Scotland and northern England (Snodgrass et al., 1986) and New Zealand (Al Mawly et al., 2015), both using commercial enzyme-linked immunosorbent assay (ELISA), rates of RVA infection of 45.9% (161/351) and 19.9% (246/1,226), respectively, were reported.
In Brazil, also using the PAGE technique, Coura et al. (2015) reported that 49.2% (33/67) of dairy heifer calves that were 0–30 days old excreted RVA during a longitudinal study, and in a transversal study only 138 of 683 (20.2%) of dairy calves 0–30 days old were found to be RVA-positive (Alfieri et al., 2006).
The percentage of RVA-positive fecal samples obtained during this study was 8.1% (99/1220). Other longitudinal studies that have been conducted in Brazil to determine the level of RVA infection in calves born from vaccinated dairy cows had percentages of RVA-positive diarrheic fecal samples that were 5.7% (49/850) (Coura et al., 2015) and 3.9% (11/281) (Rocha et al., 2017).
The distribution of the total samples and the RVA-positive fecal samples for each fecal consistency score and time point at sample collection are shown in Table 1 . The frequency of diarrhea in calves during the experiment, shown in the table, was not high. Fecal samples with scores of 0 and 1 (n = 863) represented 70.7% of the total samples, while samples with scores of 2 and 3, indicating the presence of diarrhea, comprised 17.0% (n = 208) and 12.2% (n = 149) of the total samples, respectively. In a similar study conducted in Brazil on RVA-unvaccinated dairy cattle herds, the proportion of diarrheic fecal samples (36.7%; 312/850) within the total number of samples was greater than that in this study (Coura et al., 2015).
Table 1.
Distribution of the total sample, and number of RVA-positive fecal samples by fecal consistency score, and the age of the calf at the sampling time from heifer calves born from a high milk yield dairy cattle herd vaccinated against RVA (Brazil. 2017).
| Age/ | Fecal | Score |
Total (%) | |||
|---|---|---|---|---|---|---|
| Days | Samples (n)a | 0 (normal) | 1 (pasty) |
2 (soft) |
3 (watery) |
|
| 1 | N | 23 | 44 | 32 | 23 | |
| + | – | – | – | – | – | |
| 4 | N | 25 | 74 | 16 | 7 | |
| + | – | 1 | – | – | 1 (0.8) | |
| 7 | N | 39 | 73 | 7 | 3 | |
| + | 3 | 4 | – | – | 7 (5.7) | |
| 10 | N | 40 | 55 | 16 | 11 | |
| + | 3 | 3 | 6 | 2 | 14 (11.5) | |
| 14 | N | 31 | 36 | 26 | 29 | |
| + | 6 | 4 | 3 | 6 | 19 (15.6) | |
| 17 | N | 19 | 35 | 38 | 30 | |
| + | 2 | 3 | 7 | 10 | 22 (18.0) | |
| 21 | N | 33 | 40 | 21 | 28 | |
| + | 4 | 2 | 3 | 2 | 11 (9.0) | |
| 24 | N | 45 | 47 | 18 | 12 | |
| + | 3 | 1 | 5 | 1 | 10 (8.2) | |
| 28 | N | 46 | 52 | 21 | 3 | |
| + | 2 | 3 | 2 | 1 | 8 (6.6) | |
| 30 | N | 49 | 57 | 13 | 3 | |
| + | 3 | 4 | – | – | 7 (5.7) | |
| Total | N | 350 | 513 | 208 | 149 | 1220 |
| + (%) | 26 (7.4) | 25 (4.9) | 26 (12.5) | 22 (14.8) | 99 (8.1) | |
(n) total number of fecal samples and (+) number of RVA-positive samples.
Previous studies have indicated that the presence of RVA infection in feces from diarrheic calves is significantly higher than that in non-diarrheic feces (Al Mawly et al., 2015; Bartels et al., 2010; Chitambar et al., 2011). Forty-eight of the 357 (13.4%) diarrheic fecal samples evaluated were RVA-positive. On average, the frequency of RVA-positive samples obtained from diarrheic feces in calves worldwide ranges between 5.5 and 57.9% (Alfieri et al., 2006; Badaracco et al., 2012; Barreiros et al., 2004; Brito et al., 2000; Buzinaro et al., 2009; Caruzo et al., 2010; Chitambar et al., 2011; Falcone et al., 1999; Malik et al., 2012; Silva et al., 2012). In this study, dsRNA to from the RVA was identified in 5.9% (51/863) of the fecal samples with scores of 0 or 1 and in 12.5% (26/208) and 14.8% (22/149) of the fecal samples with scores of 2 and 3, respectively. Although RVA was more frequent in diarrheic calves, the overall frequency of RVA diagnosis was lower than that observed in unvaccinated herds (Rocha et al., 2017).
The distribution of the fecal samples according to the age of the calves reveals that during the first and fourth weeks, the frequency of diagnosis of RVA was 2.2% (8/366) and 6.8% (25/366), respectively. However, in the second and third weeks, the frequency of RVA-positive fecal samples was higher, with the rate for the second week reaching 13.5%. This finding suggests the occurrence of a decrease in passive immunity, corroborating the findings of previous studies that suggest that passive immunity protects calves during their first week and that their own natural resistance is not initiated until 4 weeks of age (Alfieri et al., 2006); suggesting the high susceptibility of these animals to disease during the period between the first and fourth week. Meganck et al. (2014) and Coura et al. (2015) reported that diarrhea caused by RVA is most frequent in 1- to 3-week-old calves, and Alfieri et al. (2006) suggested that animals from dairy cattle herds were most susceptible to RVA infection at 2 to 3 weeks of age.
In order to reduce the occurrence of neonatal diarrhea, the dairy farm utilizes vaccination with a commercial vaccine containing the RVA UK-Compton strain with the genotype G6P[5]. This vaccine is frequently used in dairy and beef cattle herds both from Brazil and other countries around the world mainly in countries of the Northern Hemisphere such as Canada, the USA, and the European Union. To determine the G and P genotypes of RVA strains present in the fecal samples collected during this study, 10 RVA-positive fecal samples were selected from 10 calves of different ages and collections for nt sequence analysis of the VP7 and VP4 amplicons. The analysis enabled the identification of the genotypes, which were G10P[11] in all of the wild-type RVA strains with a high (99.3 to 100%) nt similarity between them.
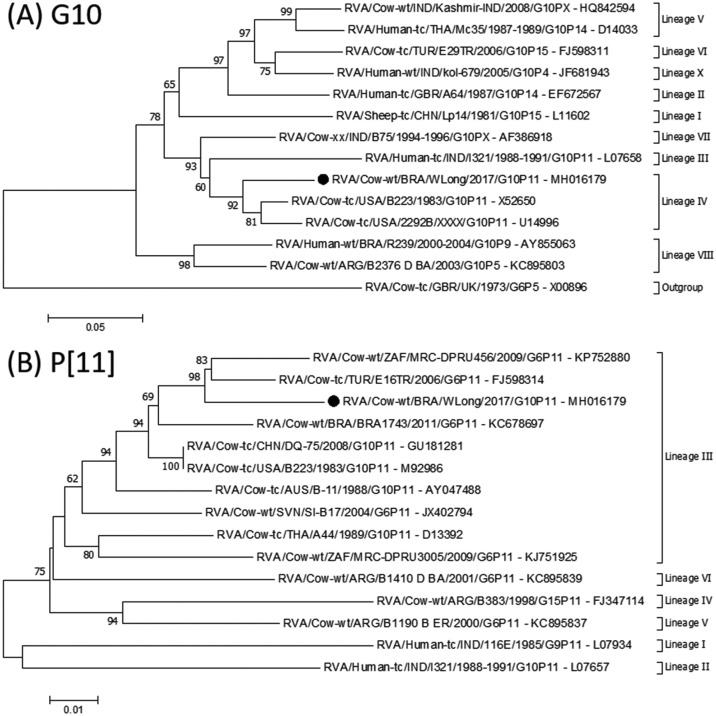
Viruses with the G10 and P[11] genotypes are not included in the RVA vaccine used by the dairy farm evaluated in this study. The sequence obtained of VP7 gene of RVA had the highest (94%) nt similarity with that of the prototype B223 strain (GenBank accession number: X52650 (Xu et al., 1991)), which belongs to G10 lineage IV (Fig. 1 A), and the VP4 gene had the highest similarity (96.1%) to that from the Turkish E16TR strain (GenBank accession number: FJ598314 (unpublished data)) of P[11] lineage III (Fig. 1B).
Fig. 1.
Phylogenetic tree of the partial VP7 gene of RVA of the G10 genotype (A) and partial VP4 gene of RVA of the P[11] genotype (B) of the RVA/Cow-wt/BRA/WLong/2017/G10P[11] strain, both represented by a black circle. The trees were constructed using the neighbor-joining method and the Kimura 2-parameter model for nucleotide substitution. The numbers adjacent to the nodes represent the percentage of bootstrap support (1000 replicates) for the clusters. Bootstrap values less than 60% are not shown. The scale bars at the bottom of the trees represent the number of nt substitutions per site.
A putative vaccine breakthrough associated with heterotypic RVA infection in newborn calves was described in Turkey, where the emergence of an RVA strain with the genotype G8P[5] was reported in a cattle herd vaccinated with the G6P[5] RVA strain, which demonstrated a failure of heterologous protection (Karayel et al., 2017). The occurrence of emerging heterologous RVA genotypes is a phenomenon has been previously observed in Brazilian dairy and beef cattle herds after the administration of a RV vaccine (Medeiros et al., 2015; Rocha et al., 2017). Similar occurrences have also been described in humans in Brazil and other countries around the world (Banyai et al., 2012; Cowley et al., 2013; Doro et al., 2014; Khandoker et al., 2018; Santos et al., 2018).
However, depending on other factors, such as the type of cattle (beef or dairy herds), the method of cow and calf management (intensive or extensive), and the inclusion of vaccination for neonatal diarrhea in the health program, different results can be found between studies. In France, Kaplon et al. (2013) evaluated beef cattle herds subject to extensive management and found no differences in the frequencies of diarrhea and RVA-positive fecal samples between vaccinated and unvaccinated herds; the majority of the genotyped RVA-positive diarrheic fecal samples were found to have the same genotype (G6P[5]) as that of the vaccine strain.
Among the measures used to mitigate the risk of neonatal diarrhea outbreaks, the vaccination of cow herds appears to be the most important. In humans, the World Health Organization recommends the inclusion of the RVA vaccination in all national immunization programs (WHO, 2009). Several studies have demonstrated that vaccination does not totally eliminate the risk of infection, but it certainly reduces both the frequency and severity of the episodes of neonatal diarrhea caused by RVA infection in calves (Kaplon et al., 2013; Parreno et al., 2004). Considering that the immune pressure exerted by routine vaccination may have the potential to select strains containing specific G and P genotypes, it is important to monitor the genotypes present in the RVA strains that are circulating among and within vaccinated cattle herds (Cashman et al., 2010).
In summary, in this longitudinal study evaluating 1220 fecal samples (122 calves), the low occurrence of RVA in diarrheic fecal samples from calves born from regularly RVA G6P[5]-vaccinated cows was determined. In addition, the genotype G6P[5], present in the RVA vaccine strain used on the farm, was not identified in any RVA field strains identified in calves in this study. Additionally, the presence of the RVA G10P[11] genotype was verified, which was different from the vaccine strain, reinforcing the vaccine protection. The presence of RVA in vaccinated herds may occurs due to immune pressure, individual factors, management procedures, nutrition, and environmental variations. The constant monitoring of circulating RVA strains in neonatal diarrhea in dairy cattle herds is important to identify the emergence of new RVA strains, highlighting those with higher virulence and/or zoonotic potential.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank the following Brazilian Institutes for financial support: the National Council of Scientific and Technological Development (CNPq), the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES), and the Araucária Foundation (FAP/PR). Alfieri, A.A. and Alfieri, A.F are recipients of CNPq fellowships.
References
- Al Mawly J., Grinberg A., Prattley D., Moffat J., Marshall J., French N. Risk factors for neonatal calf diarrhoea and enteropathogen shedding in New Zealand dairy farms. Vet. J. 2015;203:155–160. doi: 10.1016/j.tvjl.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri A.A., Parazzi M.E., Takiuchi E., Medici K.C., Alfieri A.F. Frequency of group A rotavirus in diarrhoeic calves in Brazilian cattle herds, 1998-2002. Trop. Anim. Health Prod. 2006;38:521–526. doi: 10.1007/s11250-006-4349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaracco A., Garaicoechea L., Rodriguez D., Uriarte E.L., Odeon A., Bilbao G., Galarza R., Abdala A., Fernandez F., Parreno V. Bovine rotavirus strains circulating in beef and dairy herds in Argentina from 2004 to 2010. Vet. Microbiol. 2012;158:394–399. doi: 10.1016/j.vetmic.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Banyai K., Laszlo B., Duque J., Steele A.D., Nelson E.A., Gentsch J.R., Parashar U.D. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: insights for understanding the impact of rotavirus vaccination programs. Vaccine. 2012;30(Suppl 1):A122–130. doi: 10.1016/j.vaccine.2011.09.111. [DOI] [PubMed] [Google Scholar]
- Banyai K., Kemenesi G., Budinski I., Foldes F., Zana B., Marton S., Varga-Kugler R., Oldal M., Kurucz K., Jakab F. Candidate new rotavirus species in Schreiber’s bats, Serbia. Infect. Genet. Evol. 2017;48:19–26. doi: 10.1016/j.meegid.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiros M.A., Alfieri A.F., Medici K.C., Leite J.P., Alfieri A.A. G and P genotypes of group A rotavirus from diarrhoeic calves born to cows vaccinated against the NCDV (P[1],G6) rotavirus strain. J. Vet. Med. 2004;51:104–109. doi: 10.1111/j.1439-0450.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- Bartels C.J., Holzhauer M., Jorritsma R., Swart W.A., Lam T.J. Prevalence, prediction and risk factors of enteropathogens in normal and non-normal faeces of young Dutch dairy calves. Prev. Vet. Med. 2010;93:162–169. doi: 10.1016/j.prevetmed.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito W.M.E.Dd., Munford V., Villaça A.M., Caruzo T.A.R., Rácz M.-L. Characterization of mixed infections with different strains of bovine rotavirus in an outbreak of diarrhea in dairy herds in Goiás, Brazil. Braz. J. Microbiol. 2000;31:140–145. [Google Scholar]
- Buzinaro M.G., Samara S.I., Pereira E.A.S., Fuentes D.B., Oliveira M.C.S. Ocorrência dos Genótipos G e P de Rotavirus do grupo Aem Bezerros de Rebanhos de Corte no Estado de São Paulo, Brasil. Arquivos do Instituto Biológico. 2009;76:99–105. [Google Scholar]
- Caruzo T.A., Brito W.M., Munford V., Racz M.L. Molecular characterization of G and P-types bovine rotavirus strains from Goias, Brazil: high frequency of mixed P-type infections. Memórias do Instituto Oswaldo Cruz. 2010;105:1040–1043. doi: 10.1590/s0074-02762010000800014. [DOI] [PubMed] [Google Scholar]
- Cashman O., Lennon G., Sleator R.D., Power E., Fanning S., O’Shea H. Changing profile of the bovine rotavirus G6 population in the south of Ireland from 2002 to 2009. Vet. Microbiol. 2010;146:238–244. doi: 10.1016/j.vetmic.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Chase C.C., Hurley D.J., Reber A.J. Neonatal immune development in the calf and its impact on vaccine response. Vet. Clin. North Am. Food Anim. Pract. 2008;24:87–104. doi: 10.1016/j.cvfa.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitambar S.D., Arora R., Kolpe A.B., Yadav M.M., Raut C.G. Molecular characterization of unusual bovine group A rotavirus G8P[14] strains identified in western India: emergence of P[14] genotype. Vet. Microbiol. 2011;148:384–388. doi: 10.1016/j.vetmic.2010.08.027. [DOI] [PubMed] [Google Scholar]
- Coura F.M., Freitas M.D., Ribeiro J., de Leme R.A., de Souza C., Alfieri A.A., Facury Filho E.J., de Carvalho A.U., Silva M.X., Lage A.P., Heinemann M.B. Longitudinal study of Salmonella spp., diarrheagenic Escherichia coli, Rotavirus, and Coronavirus isolated from healthy and diarrheic calves in a Brazilian dairy herd. Trop. Anim. Health Prod. 2015;47:3–11. doi: 10.1007/s11250-014-0675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley D., Donato C.M., Roczo-Farkas S., Kirkwood C.D. Novel G10P[14] rotavirus strain, northern territory, Australia. Emerg. Infectious Diseases J. 2013;19:1324–1327. doi: 10.3201/eid.1908.121653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doro R., Laszlo B., Martella V., Leshem E., Gentsch J., Parashar U., Banyai K. Review of global rotavirus strain prevalence data from six years post vaccine licensure surveillance: is there evidence of strain selection from vaccine pressure? Infection. Genet. Evol. 2014;28:446–461. doi: 10.1016/j.meegid.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone E., Tarantino M., Di Trani L., Cordioli P., Lavazza A., Tollis M. Determination of bovine rotavirus G and P serotypes in italy by PCR. J. Clin. Microbiol. 1999;37:3879–3882. doi: 10.1128/jcm.37.12.3879-3882.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J.R., Glass R.I., Woods P., Gouvea V., Gorziglia M., Flores J., Das B.K., Bhan M.K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Glass R.I., Woods P., Taniguchi K., Clark H.F., Forrester B., Fang Z.Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H.B., Estes M.K. Rotaviruses: from pathogenesis to vaccination. Gastroenterology. 2009;136:1939–1951. doi: 10.1053/j.gastro.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A.J., Inglis N.F., Ojeh C.K., Snodgrass D.R., Menzies J.D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J. Clin. Microbiol. 1982;16:473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICTV . 2017. International Committee on Taxonomy of Viruses. [Google Scholar]
- Kaplon J., Fremy C., Bernard S., Rehby L., Aho S., Pothier P., Ambert-Balay K. Impact of rotavirus vaccine on rotavirus genotypes and caliciviruses circulating in French cattle. Vaccine. 2013;31:2433–2440. doi: 10.1016/j.vaccine.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayel I., Feher E., Marton S., Coskun N., Banyai K., Alkan F. Putative vaccine breakthrough event associated with heterotypic rotavirus infection in newborn calves, Turkey, 2015. Vet. Microbiol. 2017;201:7–13. doi: 10.1016/j.vetmic.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandoker N., Thongprachum A., Takanashi S., Okitsu S., Nishimura S., Kikuta H., Yamamoto A., Sugita K., Baba T., Kobayashi M., Hayakawa S., Mizuguchi M., Ushijima H. Molecular epidemiology of rotavirus gastroenteritis in Japan during 2014-2015: characterization of re-emerging G2P[4] after rotavirus vaccine introduction. J. Med. Virol. 2018;90:1040–1046. doi: 10.1002/jmv.25067. [DOI] [PubMed] [Google Scholar]
- Malik Y.S., Sharma K., Vaid N., Chakravarti S., Chandrashekar K.M., Basera S.S., Singh R., Minakshi Prasad G., Gulati B.R., Bhilegaonkar K.N., Pandey A.B. Frequency of group A rotavirus with mixed G and P genotypes in bovines: predominance of G3 genotype and its emergence in combination with G8/G10 types. J. Vet. Sci. 2012;13:271–278. doi: 10.4142/jvs.2012.13.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V., Ciarlet M., Banyai K., Lorusso E., Cavalli A., Corrente M., Elia G., Arista S., Camero M., Desario C., Decaro N., Lavazza A., Buonavoglia C. Identification of a novel VP4 genotype carried by a serotype G5 porcine rotavirus strain. Virology. 2006;346:301–311. doi: 10.1016/j.virol.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Matthijnssens J., Otto P.H., Ciarlet M., Desselberger U., Van Ranst M., Johne R. VP6-sequence-based cutoff values as a criterion for rotavirus species demarcation. Arch. Virol. 2012;157:1177–1182. doi: 10.1007/s00705-012-1273-3. [DOI] [PubMed] [Google Scholar]
- Medeiros T.N.S. Universidade Estadual de Londrina; 2016. Bovine Neonatal Diarrhea: Epidemiology and Molecular Characterization of G (VP7) and P (VP4) Genotypes of Rotavirus a, Brazil, 2006–2015. [Google Scholar]
- Medeiros T.N.S., Lorenzetti E., Alfieri A.F., Alfieri A.A. Phylogenetic analysis of a G6P[5] bovine rotavirus strain isolated in a neonatal diarrhea outbreak in a beef cattle herd vaccinated with G6P[1] and G10P[11] genotypes. Arch. Virol. 2015;160:447–451. doi: 10.1007/s00705-014-2271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meganck V., Hoflack G., Opsomer G. Advances in prevention and therapy of neonatal dairy calf diarrhoea: a systematical review with emphasis on colostrum management and fluid therapy. Acta Vet. Scand. 2014;56:75. doi: 10.1186/s13028-014-0075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalov-Kovacs E., Gellert A., Marton S., Farkas S.L., Feher E., Oldal M., Jakab F., Martella V., Banyai K. Candidate new rotavirus species in sheltered dogs, Hungary. Emerg. Infectious Diseases J. 2015;21:660–663. doi: 10.3201/eid2104.141370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp H., Laszlo B., Jakab F., Ganesh B., De Grazia S., Matthijnssens J., Ciarlet M., Martella V., Banyai K. Review of group A rotavirus strains reported in swine and cattle. Vet. Microbiol. 2013;165:190–199. doi: 10.1016/j.vetmic.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreno V., Bejar C., Vagnozzi A., Barrandeguy M., Costantini V., Craig M.I., Yuan L., Hodgins D., Saif L., Fernandez F. Modulation by colostrum-acquired maternal antibodies of systemic and mucosal antibody responses to rotavirus in calves experimentally challenged with bovine rotavirus. Vet. Immunol. Immunopathol. 2004;100:7–24. doi: 10.1016/j.vetimm.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H.G., Azeredo R.S., Leite J.P., Candeias J.A., Racz M.L., Linhares A.C., Gabbay Y.B., Trabulsi J.R. Electrophoretic study of the genome of human rotaviruses from Rio de Janeiro, São Paulo and Para, Brazil. J. Hyg. 1983;90:117–125. doi: 10.1017/s0022172400063919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RCWG . 2017. Rotavirus Classification Working Group. Newly Assigned Genotypes – June 30th 2017. [Google Scholar]
- Rocha T.G., Silva F.D., Gregori F., Alfieri A.A., Buzinaro M.D., Fagliari J.J. Longitudinal study of bovine rotavirus group A in newborn calves from vaccinated and unvaccinated dairy herds. Trop. Anim. Health Prod. 2017;49:783–790. doi: 10.1007/s11250-017-1263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos V.S., Nobrega F.A., Soares M.W.S., Moreira R.D., Cuevas L.E., Gurgel R.Q. Rotavirus genotypes circulating in Brazil before and after the national rotavirus vaccine program: a review. Pediatr. Infect. Dis. J. 2018;37:e63–e65. doi: 10.1097/INF.0000000000001770. [DOI] [PubMed] [Google Scholar]
- Silva F.D.F., Gregori F., Gonçalves A.C.S., Samara S.I., Buzinaro M.G. Molecular characterization of group A bovine rotavirus in southeastern and central-western Brazil, 2009-2010. Pesquisa Veterinária Brasileira. 2012;32:237–242. [Google Scholar]
- Snodgrass D.R., Terzolo H.R., Sherwood D., Campbell I., Menzies J.D., Synge B.A. Aetiology of diarrhoea in young calves. Vet. Rec. 1986;119:31–34. doi: 10.1136/vr.119.2.31. [DOI] [PubMed] [Google Scholar]
- Uetake K. Newborn calf welfare: a review focusing on mortality rates. Anim. Sci. J. 2013;84:101–105. doi: 10.1111/asj.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO World health organisation. Rotavirus vaccines: an update. Wkly. Epidemiol. Rec. 2009;84:533–540. [PubMed] [Google Scholar]
- Xu L., Harbour D., McCrae M.A. Sequence of the gene encoding the major neutralization antigen (VP7) of serotype 10 rotavirus. J. Gen. Virol. 1991;72(Pt 1):177–180. doi: 10.1099/0022-1317-72-1-177. [DOI] [PubMed] [Google Scholar]