Abstract
A severe outbreak of enteric and respiratory disease associated with bovine coronavirus (BCoV) infection is described. The outbreak occurred in a dairy herd of southern Italy in the first decade of September 2006, when summer temperatures were still recorded, affecting calves, heifers and adult cows, with a marked decrease in milk production. By virus isolation and RT-PCR targeting the S gene, BCoV was identified as the etiological agent of the outbreak, whereas bacteriological, parasitological and toxicological investigations failed to detect other causes of disease. BCoV strains with 99–100% nucleotide identity in the S gene were isolated from nasal, ocular and rectal swabs, thus proving the absence of separate clusters of virus on the basis of tissue tropism. Sequence analysis of the haemagglutination-esterase and spike proteins of the strain detected in one rectal sample (339/06) showed a high genetic relatedness with recent BCoV isolates (98–99% amino acid identity), with several unique amino acid substitutions in the S protein. The BCoV outbreak described in this paper presents interesting aspects: (i) the occurrence of a severe form of disease in the warmer season; (ii) the simultaneous presence of respiratory and enteric disease; (iii) the involvement of young as well as adult cattle.
Keywords: Bovine coronavirus, Warmer season, RT-PCR, Molecular analysis, ELISA
1. Introduction
Bovine coronavirus (BCoV) is a member of the genus Coronavirus within the family Coronaviridae, that includes three main antigenic groups (Enjuanes et al., 2000). Group 2 coronaviruses comprise BCoV, mouse hepatitis virus, sialodacryadenitis virus, porcine haemagglutinating encephalomyelitis virus, human coronavirus (HCoV) OC43, human enteric coronavirus (HECV) 4408 (Enjuanes et al., 2000), and the newly recognised HCoV-HKU1 (Woo et al., 2005) and canine respiratory coronavirus (CRCoV) (Erles et al., 2003, Decaro et al., 2007).
BCoV is the causative agent of severe diarrhoea in newborn calves (Snodgrass et al., 1986), winter dysentery in adult cows (Saif et al., 1991, Cho et al., 2000), and respiratory tract illness in calves and adults (Lathrop et al., 2000, Storz et al., 2000). The same virus strain could be responsible for simultaneous appearance of enteric and respiratory disease in the same animals (Chouljenko et al., 2001) as well as in both calves and adults (Tråvén et al., 2001).
Albeit characterised by low mortality, BCoV infection can cause severe economic losses, mainly due to dramatic reduction in milk production in dairy herds (Saif et al., 1998). The incidence of the disease is generally high, with outbreaks reported in most parts of the world (Saif and Heckert, 1990). The peak of incidence occurs in the winter due to the heat sensitivity showed by BCoV (Saif and Heckert, 1990). Recently, severe outbreaks of winter disease have been reported also in the warmer season (Fukutomi et al., 1999, Park et al., 2006). In this study, an outbreak of enteric and respiratory disease associated with BCoV infection is reported which occurred in the warmer season in a dairy herd of southern Italy, causing a marked decrease in milk production.
2. Materials and methods
2.1. Clinical case
The outbreak occurred in the first decade of September 2006 (mean seasonal temperature of 30 °C) in a dairy herd of Apulia region, Italy, that consisted of 80 Holstein cattle including 40 lactating cows, 20 heifers or dry cows, and 20 calves. The three groups were housed in separate paddocks, but the two facilities housing the cows and heifers were very closed. The herd had a daily milk production ranging between 800 and 900 l and was generally healthy apart from sporadic respiratory distress in 2–3-month-old calves. Vaccination against bovine viral diarrhoea virus (BVDV) was performed routinely using a modified-live virus administered at 6-month intervals. Two calves purchased from a local farm had been introduced about 20 days before the onset of clinical signs. One week before the appearance of the disease, the food administered to the lactating cows had been changed. The morning after the scheduled vaccination against BVDV, most lactating cows (25/40) displayed a severe, often bloody diarrhoea, fever (41–41.5 °C) and anorexia. Agalactia was also evident with a dramatic decrease in milk production (daily milk production below 70 l, with a 91–92% reduction of the baseline production). During the same day, all the lactating cows showed haemorrhagic diarrhoea and hyperthermia, whereas serous or catarrhal ocular and nasal discharges were observed in 27 cows within 48 h from the onset of clinical signs. After 72 h also the heifers and dry cows (20/20) were affected, displaying a temperature of 41–41.5 °C, severe diarrhoea that was haemorrhagic in few animals, ocular and nasal discharge. Simultaneously, mild cough and ocular/nasal discharge were observed in some calves (8/20), that underwent a progressive improvement of their conditions with full recover within 7–8 days. Gastroenteric and respiratory signs, together with anorexia and hypo/agalactia, persisted in lactating cows, many of which were lying down. The animals were treated with oxytetracycline (25 mg/kg of body weight) and a mild improvement of the clinical conditions in some animals was observed starting from day 7 after the onset of the disease, when gastroenteric signs became less severe, the rectal temperature regained normal values and the daily milk production increased up to 300–400 l (33–57% of the baseline production). Respiratory distress (cough and nasal discharge) persisted in heifers. After 15 days from the onset of the disease, three cows and five heifers still presented diarrhoea and serous ocular discharge, respectively, whereas the daily milk production approached standard values. However, some cows, that were infected and displayed agalactia at the end of their lactation period, did not regain any milking activity.
2.2. Sampling
Rectal swabs (or faecal samples), ocular and nasal swabs, serum and EDTA-blood samples, collected 48 h after the onset of the clinical signs from 10 lactating cows, 10 heifers and 10 calves, were used for virological, bacteriological, parasitological and serological investigations for the main pathogens of cattle. At the same time, samples of feedstuff were taken for the detection of mycotoxins. Rectal, nasal, ocular swabs and serum and EDTA-blood samples were also collected from animals that still showed clinical signs (three cows and three heifers) 15 days after the onset of the disease.
2.3. Nucleic acid extraction
Nucleic acids for (RT)-PCR assays were extracted using commercial kits. The DNeasy Tissue Kit (QIAGEN S.p.A., Milan, Italy) was used for DNA extraction from all samples, whereas RNAs were purified with the QIAamp® Viral RNA Mini Kit (QIAGEN S.p.A.) from the ocular, nasal and faecal samples and with the QIAamp® RNA Blood Mini Kit (QIAGEN S.p.A.) from blood samples.
2.4. RT-PCR for detection of BCoV RNA
Detection of BCoV RNA was carried out using SuperScript™ One-Step RT-PCR for Long Templates (Life Technologies, Invitrogen, Milan, Italy) and primers specific for the spike protein gene that are able to detect both BCoV and CRCoV RNAs (Erles et al., 2003). The following thermal protocol was used: reverse transcription at 50 °C for 30 min, inactivation of Superscript II RT at 94 °C for 2 min, 40 cycles of 94 °C for 30 s, 55 °C for 30 s, 68 °C for 30 s, with a final extension at 68 °C for 10 min. The PCR products were detected by electrophoresis through a 1.5% agarose gel and visualisation under UV light after bromide ethidium staining.
2.5. (RT)-PCR assay for screening for other bovine viral pathogens
RNA and DNA extracts from nasal and ocular swabs were tested by nested PCR for bovine respiratory syncytial virus (BRSV) (Valarcher et al., 1999) and by PCR for bovine herpesviruses types 1 (BoHV-1) (Vilcek, 1993) and 4 (BoHV-4) (Boerner et al., 1999). RNA extracts from rectal swabs were used for detection of bovine torovirus (Hoet et al., 2002), as well as rotaviruses (Gouvea et al., 1994) and caliciviruses (Jiang et al., 1999), whereas RT-PCR for bovine viral diarrhoea virus (BVDV) (Sullivan and Akkina, 1995) was carried out on RNAs purified from blood, nasal and faecal samples. RT-PCR and PCR assays were performed using SuperScript™ One-Step RT-PCR for Long Templates (Life Technologies) and LA PCR Kit Ver. 2.1 (TaKaRa Bio Inc., Shiga, Japan), respectively.
2.6. Virus isolation
The rectal, ocular and nasal swabs collected from animals that tested positive for BCoV RNA by RT-PCR were subjected to virus isolation attempts. For virus isolation, human rectal tumour (HRT-18) and Madin Darby bovine kidney (MDBK) cells were used which were grown in Dulbecco's minimal essential medium (D-MEM) added with 10% foetal calf serum. Samples were homogenised in Dulbecco's minimal essential medium (D-MEM) plus antibiotics (penicillin 5000 IU/ml, streptomycin 2500 μg/ml, amphotericin B 10 μg/ml). When the monolayers were confluent, the media were removed and the cells were washed two times with FCS-free medium plus trypsin (5 μg/ml) and inoculated with the sample homogenates. After an incubation of 60 min at 37 °C, the inocula were replaced with fresh serum-free medium plus trypsin (5 μg/ml). After 3 days of incubation at 37 °C, the inoculated cells were tested for BCoV antigen by an immunofluorescence (IF) assay using a bovine serum positive for BCoV antibodies and a rabbit anti-bovine IgG conjugated with fluorescein isothiocyanate (Sigma Aldrich srl, Milan, Italy). Each sample was passaged three times prior to be considered as negative for BCoV.
2.7. Sequence analysis
The haemagglutinin-esterase (HE) and spike (S) genes of strain 339/06 were amplified using SuperScript™ One-Step RT-PCR for Long Templates (Life Technologies), according to the manufacturer's instructions. Eight partially overlapping fragments encompassing the ORFs 4 (HE gene) and 5 (S gene) were amplified using primer pairs listed in Table 1 . The PCR-amplified products were sequenced by Genome Express (Meylan, France) and the obtained sequences were assembled and analysed using the BioEdit software package (Hall, 1999) and the NCBIs (http://www.ncbi.nlm.nih.gov) and EMBLs (http://www.ebi.ac.uk) analysis tools. ORFs contained in the amplified genomic region were determined either with the ORF Finder tool of NCBI or on the basis of the similarity to known coronavirus proteins. The ORFs identified in this manner were translated and the predicted amino acid (aa) sequences were saved as individual files for further analyses. Phylogenetic and molecular evolutionary analyses were conducted using Mega3 (Kumar et al., 2004). Phylogenetic trees, based on the HE and S proteins of BCoV strain 339/06 were elaborated using both parsimony and neighbor-joining methods, supplying a statistical support with bootstrapping over 100 replicates.
Table 1.
Primers used for RT-PCR amplification and sequence analysis
| Primer | Sequence 5′–3′ | Sense | Positiona | Amplicon size (bp) |
|---|---|---|---|---|
| BCV-22001F | TAGACTTGAAATAGTTAAGCTTGGTG | + | 22001–22026 | 893 |
| BCV-22894R | AAATTAGCTTCACGAGCTATATATGC | − | 22868–22893 | |
| BCV-22769F | TATCGCAGCCTTACTTTTGTTAATG | + | 22769–22793 | 527 |
| BCV-23295R | CGAAAATAACAGTACGGGGGTTGACA | − | 23270–23295 | |
| BCV-23112F | TACCCTCTGGTAATTATTTAGCCATTTCA | + | 23112–23140 | 776 |
| BCV-23887R | TTCCCTTCAGTGCCATATTACGATATGT | − | 23860–23887 | |
| BCV-23510F | TATGATCCGCTACCAATTATTTTGCTTGGCA | + | 23510–23540 | 817 |
| BCV-24326R | ACAACACCAGTGTCTGTAAAATATGCA | − | 24300–24326 | |
| BCV-24182F | TAGAACTATGGCATTGGGATACAGGTGTTG | + | 24182–24211 | 1254 |
| BCV-25435R | TACACCTATCCCCTTGTAAACAAGAGTA | − | 25409–25435 | |
| BCV-25301F | ACTTAGTTGGCATAGGTGAGCACTGTTC | + | 25301–25328 | 851 |
| BCV-26151R | ACATGCTACATAATCACCACAGACAA | − | 26126–26151 | |
| BCV-26028F | TTTGTATGAAATTCAAATACCTTCAGAG | + | 26028–26055 | 877 |
| BCV-26904R | GTCTATCTGAGCTTGCGCTTCAAGAGCA | − | 26877–26904 | |
| BCV-26760F | TAAAATTCAAGCTGTTGTTAATGCAAAT | + | 26760–26787 | 1059 |
| BCV-27818R | GCTCGACCTAAATGGGTCTTATAATTAGA | − | 27791–27818 | |
Primers position is referred to the sequence of BCoV strain Mebus (accession: U00735).
2.8. Bacteriological and parasitological investigations
The samples were examined for bacterial and parasitic pathogens by standardised methods. For bacteriological investigations, the faecal samples were plated out on 5% sheep blood agar and cultured aerobically at 37 °C for 24 h to exclude the presence of aerobic pathogens. One hundred milligrams of faecal samples were resuspended in 900 μl (dilution 10−1) of fluid thioglycollate medium (FTG). The 10-fold dilutions (10−2–10−8) were subsequently plated onto 5% sheep blood agar and egg yolk agar with d-cycloserine 400 μg/ml (TSC). Bacteria were allowed to grow overnight at 37 °C in anaerobic condition. Detection of the most common enteric and respiratory parasites was achieved using zinc sulphate flotation. The Ziehl Nielsen staining was also performed on faecal samples for detection of Cryptosporidium spp.
2.9. Mycotoxicological investigations
Samples of feedstuff were analysed for the detection of mycotoxins by an external laboratory specialised in toxicological investigation (Neolac Analisi Alimentari Agrozootecniche Ecologia, Belgioso, Pavia, Italy).
2.10. Serological investigations
An ELISA test for the detection of BCoV antibodies was carried out on the sera collected from the affected animals, following the same protocol described for the detection of CRCoV antibodies (Decaro et al., 2007). In order to assess the sensitivity and specificity of the ELISA test using bovine sera, 10 sera positive and 10 sera negative for BCoV antibodies, as resulted by both virusneutralisation (VN) and haemagglutination inhibition (HI) assays (Lin et al., 2001), were tested. The cutoff value for bovine sera was defined as the mean optical density plus 3 standard deviations calculated with five foetal and five colostrum-deprived calf serum samples.
2.11. GenBank accession number
GenBank accession number EF445634 was assigned to the sequenced 5.6-Kb fragment.
3. Results
3.1. Virological investigations and sequence analysis
Most rectal, ocular and nasal swabs collected from the affected animals induced a CPE at the first or second passage on HRT cells consisting of syncytial formation and subsequent lysis of the infected monolayers (Fig. 1 ). The IF assay detected the BCoV antigen in all cell cultures with evident CPE, as shown by the cytoplasmatic fluorescence observed in the infected cells. Totally, BCoV was isolated from 29 rectal, 15 ocular and 20 nasal swabs, irrespective of the age of the animals (Table 2 ). No CPE was observed in the cultures inoculated with blood samples, that did not give any detectable fluorescence signal by the IF assay.
Fig. 1.
Cytopathic effect induced on HRT cells by a BCoV strain isolated from the outbreak.
Table 2.
Detection of BCoV RNA and antibodies in affected animals
| Number | Animal | Rectal swab |
Nasal swab |
Ocular swab |
BCoV ELISA OD values | |||
|---|---|---|---|---|---|---|---|---|
| VI | PCR | VI | PCR | VI | PCR | |||
| 1 | Cowa | + | + | + | + | + | + | 0.281 |
| 2 | Cowa | + | + | + | + | + | + | 0.219 |
| 3 | Cowa | − | + | + | + | − | − | 0.417 |
| 4 | Cowa | − | + | − | + | − | − | 0.374 |
| 5 | Cowa | + | + | + | + | + | + | 0.231 |
| 6 | Cowa | + | + | + | + | − | + | 0.138 |
| 7 | Cowa | + | + | − | + | − | + | 0.344 |
| 8 | Cowa | + | + | − | + | + | + | 0.198 |
| 9 | Cowa | − | + | + | + | + | + | 0.282 |
| 10 | Cowa | + | + | − | − | − | − | 0.435 |
| 11 | Heifera | + | + | − | − | − | − | 0.301 |
| 12 | Heifera | + | + | + | + | + | + | 0.481 |
| 13 | Heifera | + | + | + | + | − | + | 0.398 |
| 14 | Heifera | + | + | + | + | + | + | 0.229 |
| 15 | Heifera | + | + | + | + | − | − | 0.180 |
| 16 | Heifera | + | + | + | + | + | + | 0.427 |
| 17 | Heifera | + | + | + | + | + | + | 0.280 |
| 18 | Heifera | + | + | + | + | − | + | 0.114 |
| 19 | Heifera | + | + | + | + | − | + | 0.461 |
| 20 | Heifera | + | + | − | + | − | + | 0.277 |
| 21 | Calfa | + | + | − | + | + | + | 0.455 |
| 22 | Calfa | + | + | + | + | + | + | 0.299 |
| 23 | Calfa | − | + | − | + | − | − | 0.300 |
| 24 | Calfa | + | + | + | + | − | + | 0.098 |
| 25 | Calfa | + | + | + | + | − | − | 0.109 |
| 26 | Calfa | + | + | + | + | − | + | 0.425 |
| 27 | Calfa | − | + | − | + | + | + | 0.397 |
| 28 | Calfa | + | + | + | + | + | + | 0.255 |
| 29 | Calfa | + | + | − | + | + | + | 0.303 |
| 30 | Calfa | + | + | − | + | − | + | 0.242 |
| 5 | Cowb | + | + | − | + | + | + | 0.358 |
| 6 | Cowb | + | + | − | + | − | + | 0.176 |
| 8 | Cowb | + | + | − | − | − | − | 0.211 |
| 11 | Heiferb | − | + | − | + | − | + | 0.374 |
| 14 | Heiferb | + | + | + | + | − | + | 0.329 |
| 16 | Heiferb | − | + | − | + | − | + | 0.477 |
VI, virus isolation; PCR, RT-PCR amplification; +, positive; −, negative.
Animal sampled at the onset of clinical signs.
Animal displaying clinical signs after 15 days from the onset of the outbreak.
By RT-PCR, the BCoV RNA was detected in 36 rectal, 28 ocular and 33 nasal swabs, whereas no blood sample tested positive for BCoV. Molecular assays did not detect other bovine viral pathogens in the tested samples.
Sequence analysis of the S-gene fragments amplified from 10 randomly chosen BCoV-positive samples showed a 99–100% nucleotide identity among the BCoV strains isolated from different animals and samples (nasal, ocular or rectal swabs) (data not shown). Consequently, the HE and S gene were PCR amplified from a single rectal swab (339/06) in order to evaluate the genetic relatedness to BCoV sequences available from the GenBank database.
By sequence analysis, the HE protein of strain 339/06 showed a length of 424 aa, with a 98–99% aa identity to other BCoV strains, irrespective of the geographic origin and sample typology (nasal or faecal specimens). Analogously to BCoV reference strains, by analysis with the NetNglyc server (http://www.cbs.dtu.dk/services/NetNGlyc/), the HE of strain 339/06 displayed nine potential N-glycosylation sites. Moreover, the predicted site for neuraminidate-O-acetilesterase activity, FGDS, was detected at the N terminus. A unique aa change, S–P, was detected at position 158. The S protein, 1363 aa in length, had 21 potential N-glycosylation sites in comparison with the 19–20 N-glycosylation sites displayed by BCoV reference strains, including the recent Korean isolates (Park et al., 2006). The S protein was more related (98%) to BCoV strains isolated recently in Korea (Table 3 ). No deletions or insertions were observed in strain 339/06 compared with reference strains, as well as the aa stretch KRRSRR, responsible for the proteolytic cleavage of the S protein at residue 768 into subunits S1 and S2, was also conserved. Several unique amino acid substitutions were observed in both S1 and S2 subunits, including T257N, N/K/S499T, H525Y, D608G, R677K, E699K, R705Q, K909R, S927A, D/H1260N. The segregation pattern was confirmed by the topology of the phylogenetic trees constructed by both neighbor-joining (Fig. 2 ) and maximum parsimony (data not shown) methods.
Table 3.
Amino acid identity (%) of BCoV reference strains to isolate 339/06 in the HE and S proteins
| BCoV strain | GenBank accession number |
Aa identity (%) to isolate 339/06 |
||
|---|---|---|---|---|
| HE | S | HE | S | |
| Mebus | U00735 | U00735 | 98 | 97 |
| KWD10 | DQ016127 | AY935646 | 98 | 98 |
| KWD9 | DQ016126 | AY935645 | 98 | 98 |
| KWD8 | DQ016125 | AY935644 | 98 | 98 |
| KWD7 | DQ016124 | AY935643 | 98 | 98 |
| KWD6 | DQ016123 | AY935642 | 99 | 98 |
| KWD5 | DQ016122 | AY935641 | 99 | 98 |
| KWD4 | DQ016121 | AY935640 | 98 | 98 |
| KWD3 | DQ016120 | AY935639 | 98 | 98 |
| KWD2 | DQ016119 | AY935638 | 98 | 98 |
| KWD1 | DQ016118 | AY935637 | 99 | 98 |
| F15 | NA | D00731 | ND | 97 |
| KCD10 | DQ389651 | DQ389641 | 99 | 98 |
| KCD9 | DQ389650 | DQ389640 | 98 | 98 |
| KCD8 | DQ389649 | DQ389639 | 99 | 98 |
| KCD7 | DQ389648 | DQ389638 | 98 | 97 |
| BCoV-LUN | AF391542 | DQ389637 | 99 | 98 |
| BCoV-ENT | AF391541 | DQ389636 | 99 | 98 |
| BCV-Vaccine | NA | M64668 | ND | 97 |
| L9 | S69264 | M64667 | 98 | 97 |
| OK-0514-3 | AF058944 | AF058944 | 99 | 98 |
| LSU-94LSS-051-2 | AF058943 | AF058943 | 99 | 98 |
| LY-138 | AF058942 | AF058942 | 99 | 97 |
| Quebec | AF220295 | AF220295 | 98 | 96 |
| Alpaca | DQ915164 | DQ915164 | 99 | 97 |
| DB2 | DQ811784 | DQ811784 | 99 | 98 |
NA, not available; ND, not done.
Fig. 2.
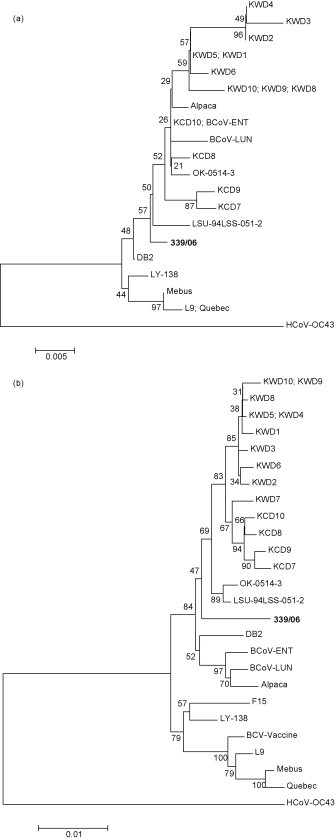
Neighbor-joining trees based on spike (a) and haemagglutinin-esterase (b) proteins of bovine coronaviruses. Accession numbers of the strains used for phylogeny are reported in Table 3. HCoV OC43 (accession number NC_005147) was used as outgroup. Bootstrap values were calculated and are indicated at each node. The scale bars represent 0.5 (a) or 1 (b) substitutions per 100 sequence positions.
3.2. Bacteriological, parasitological and mycotoxicological investigations
From the faecal samples, Lactobacillus spp. but no β-haemolytic E. coli was isolated in aerobic conditions, whereas C. perfringens and C. sporogenes were isolated in anaerobic conditions from dilutions 10−2 to 10−4.
Parasitological investigations failed to identify common parasites of cattle. Mycotoxins detected in the feedstuff, including T2 and deoxynivalenol (DON) toxins, were significantly below the threshold values assessed for the bovine species.
3.3. Serological investigations
Analysis of foetal and colostrum-deprived calf sera gave an optical density cut-off value of 0.042. The ELISA test for BCoV antibodies was found to be sensitive, as positive results were obtained by all VN and HI positive sera. The relative specificity was 80% considering that two VN and HI negative sera gave OD values exceeding the cut-off.
The results of the BCoV ELISA test carried out on the 30 and 6 sera taken from the affected cattle at the onset of the disease and 15 days later, respectively, are shown in Table 2. All tested animals showed high OD values, ranging from 0.098 to 0.481 that were indicative of coronavirus infection. A slight increase in antibody titres was observed in animals samples 15 days after the onset of clinical signs.
4. Discussion
In this study, we have reported a severe outbreak of BCoV infection occurring in the warmer season and involving the entire cattle herd irrespective of the age. However, lactating cows were severely affected by haemorrhagic diarrhoea, respiratory distress and hypogalactia. Winter dysentery, the most severe clinical form of BCoV infection, occurs in adult cattle in the winter, due to predisposing events such as cold temperatures and drinking cold water (White et al., 1989). In the described outbreak, the lactating cows were affected by both enteric and respiratory signs during the warmer season. In fact, the first decade of September 2006 was particularly warm in our region (Apulia), with maximum temperatures reaching 34 °C, about 10 °C higher than the seasonal mean temperature registered 1 year before (http://www.meteo.it/). Other predisposing causes were investigated, including the presence of mycotoxins in feedstuff and the administration of a BVD modified-live vaccine. Since mycotoxins detected in the feedstuff were largely below the toxicity minimal levels, an essential role in triggering the severity of BCoV disease may be ascribed to BVD vaccination. However, any reversion to virulence of the vaccine virus could be ruled out as it was not detected in the samples collected from vaccinated animals, probably as consequence of strong immunity due to previous administrations of the vaccine. Therefore, it could be speculated that the vaccination action rather than the vaccine stressed the animals, thus leading to an increase in virulence of a BCoV strain. Such a strain may have circulated latently in the herd causing only mild respiratory signs in calves and the BVD vaccination may have represented the main factor triggering the onset of severe disease in heifers and adult cows. The appearance of clinical signs nearly simultaneously (in few hours) in all lactating cows reinforces the hypothesis of an increase in virulence of a BCoV strain already infecting the animals rather than of a rapid spread of the infection due to vaccine-driven immunosuppression. The high BCoV antibody titres detected by ELISA in the bovine sera during the acute phase of the disease provide further support to the former hypothesis.
To our knowledge, there are only two reports of BCoV severe diarrhoea in adult cattle in the warmer season, both occurring in East Asia (Fukutomi et al., 1999, Park et al., 2006). In the outbreak described in South Korea, the occurrence of winter dysentery in adult cows has been associated to continued exposure to BCoV due to high prevalence of BCoV infection in that country (Park et al., 2006). This hypothesis cannot be verified for the Italian outbreak since the real prevalence of BCoV infection in Italy is unknown due to the lack of published results. Unlike the previous reports, the Italian outbreak was characterised by the simultaneous occurrence of gastroenteritis and respiratory distress. Although both enteric and respiratory signs were observed in the same animals, no obvious genetic differences were detected in the HE and S genes between strains isolated from the faeces and those isolated from the respiratory tract. This finding is in agreement with recent observations that BCoV strains currently circulating possess both enteric and respiratory tropisms (Jeong et al., 2005, Liu et al., 2006), whereas other authors had found genetic markers distinguishing respiratory from enteric strains circulating in the previuos years (Chouljenko et al., 2001, Gelinas et al., 2001a, Gelinas et al., 2001b, Hasoksuz et al., 2002). Therefore, our results support the hypothesis that recent BCoVs may be diverging over the time from an enteric tropism to a dual respiratory and enteric tropism (Jeong et al., 2005).
In conclusion, the outbreak described in this paper highlights the need for in-depth epidemiological and molecular investigations on BCoV infection in Italian cattle herds.
Acknowledgements
We thank Donato Narcisi, Carlo Armenise and Arturo Gentile for their excellent technical assistance. We also acknowledge Dr. Vito Loconte for anamnestic data and sample collection.
References
- Boerner B., Weigelt W., Buhk H.J., Castrucci G., Ludwig H. A sensitive and specific PCR/Southern blot assay for detection of bovine herpesvirus 4 in calves infected experimentally. J. Virol. Methods. 1999;83:169–180. doi: 10.1016/s0166-0934(99)00117-2. [DOI] [PubMed] [Google Scholar]
- Cho K.O., Halbur P.G., Bruna J.D., Sorden S.D., Yoon K.J., Janke B.H., Chang K.O., Saif L.J. Detection and isolation of coronavirus from feces of three herds of feedlot cattle during outbreaks of winter dysentery-like disease. J. Am. Vet. Med. Assoc. 2000;217:1191–1194. doi: 10.2460/javma.2000.217.1191. [DOI] [PubMed] [Google Scholar]
- Chouljenko V.N., Lin X.Q., Storz J., Kousoulas K.G., Gorbalenya A.E. Comparison of genomic and predicted amino acid sequences of respiratory and enteric bovine coronaviruses isolated from the same animal with fatal shipping pneumonia. J. Gen. Virol. 2001;82:2927–2933. doi: 10.1099/0022-1317-82-12-2927. [DOI] [PubMed] [Google Scholar]
- Decaro N., Desario C., Elia G., Mari V., Lucente M.S., Cordioli P., Colaianni M.L., Martella V., Buonavoglia C. Serological and molecular evidence that canine respiratory coronavirus is circulating in Italy. Vet. Microbiol. 2007;121:225–230. doi: 10.1016/j.vetmic.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes L., Brian D., Cavanagh D., Holmes K., Lai M.M.C., Laude H., Masters P., Rottier P., Siddell S., Spaan W.J.M., Taguchi F., Talbot P. Family Coronaviridae. In: van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carstens E.B., Estes M.K., Lemon S.M., Maniloff J., Mayo M.A., McGeoch D.J., Pringle C.R., Wickner R.B., editors. Virus Taxonomy, Classification and Nomenclature of Viruses. Academic Press; New York: 2000. pp. 835–849. [Google Scholar]
- Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutomi T., Tsunemitsu H., Akashi H. Detection of bovine coronaviruses from adult cows with epizootic diarrhea and their antigenic and biological diversities. Arch. Virol. 1999;144:997–1006. doi: 10.1007/s007050050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas A.M., Boutin M., Sasseville A.M., Dea S. Bovine coronaviruses associated with enteric and respiratory diseases in Canadian dairy cattle display different reactivities to anti-HE monoclonal antibodies and distinct amino acid changes in their HE, S and ns4. 9 protein. Virus Res. 2001;76:43–57. doi: 10.1016/S0168-1702(01)00243-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas A.M., Sasseville A.M., Dea S. Identification of specific variations within the HE, S1, and ORF4 genes of bovine coronaviruses associated with enteric and respiratory diseases in dairy cattle. Adv. Exp. Med. Biol. 2001;494:63–67. doi: 10.1007/978-1-4615-1325-4_9. [DOI] [PubMed] [Google Scholar]
- Gouvea V., Santos N., Timenetsky Mdo C. Identification of bovine and porcine rotavirus G types by PCR. J. Clin. Microbiol. 1994;32:1338–1340. doi: 10.1128/jcm.32.5.1338-1340.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Hasoksuz M., Sreevatsan S., Cho K.O., Hoet A.E., Saif L.J. Molecular analysis of the S1 subunit of the spike glycoprotein of respiratory and enteric bovine coronavirus isolates. Virus Res. 2002;84:101–109. doi: 10.1016/S0168-1702(02)00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoet A.E., Cho K.O., Chang K.O., Loerch S.C., Wittum T.E., Saif L.J. Enteric and nasal shedding of bovine torovirus (Breda virus) in feedlot cattle. Am. J. Vet. Res. 2002;63:342–348. doi: 10.2460/ajvr.2002.63.342. [DOI] [PubMed] [Google Scholar]
- Jeong J.H., Kim G.Y., Yoon S.S., Park S.J., Kim Y.J., Sung C.M., Shin S.S., Lee B.J., Kang M.I., Park N.Y., Koh H.B., Cho K.O. Molecular analysis of S gene of spike glycoprotein of winter dysentery bovine coronavirus circulated in Korea during 2002–2003. Virus Res. 2005;108:207–212. doi: 10.1016/j.virusres.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Huang P.W., Zhong W.M., Farkas T., Cubitt D.W., Matson D.O. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J. Virol. Methods. 1999;83:145–154. doi: 10.1016/s0166-0934(99)00114-7. [DOI] [PubMed] [Google Scholar]
- Kumar S., Tamura K., Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Lathrop S.L., Wittum T.E., Brock K.V., Loerch S.C., Perino L.J., Bingham H.R., McCollum F.T., Saif L.J. Association between infection of the respiratory tract attributable to bovine coronavirus and health and growth performance of cattle in feedlots. Am. J. Vet. Res. 2000;61:1062–1066. doi: 10.2460/ajvr.2000.61.1062. [DOI] [PubMed] [Google Scholar]
- Lin X., O’Reilly K.L., Burrell M.L., Storz J. Infectivity-neutralizing and hemagglutinin-inhibiting antibody responses to respiratory coronavirus infections of cattle in pathogenesis of shipping fever pneumonia. Clin. Diagn. Lab. Immunol. 2001;8:357–362. doi: 10.1128/CDLI.8.2.357-362.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Hagglund S., Hakhverdyan M., Alenius S., Larsen L.E., Belak S. Molecular epidemiology of bovine coronavirus on the basis of comparative analyses of the S gene. J. Clin. Microbiol. 2006;44:957–960. doi: 10.1128/JCM.44.3.957-960.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.J., Jeong C., Yoon S.S., Choy H.E., Saif L.J., Park S.H., Kim Y.J., Jeong J.H., Park S.I., Kim H.H., Lee B.J., Cho H.S., Kim S.K., Kang M.I., Cho K.O. Detection and characterization of bovine coronaviruses in fecal specimens of adult cattle with diarrhea during the warmer seasons. J. Clin. Microbiol. 2006;44:3178–3188. doi: 10.1128/JCM.02667-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J., Heckert R.A. Enteropathogenic coronaviruses. In: Saif L.J., Theil K.W., editors. Viral diarrheas of man and animals. 1st ed. CRC Press; Boca Raton, FL: 1990. pp. 185–252. [Google Scholar]
- Saif L.J., Brock K.V., Redman D.R., Kohler E.M. Winter dysentery in dairy herds: electron microscopic and serological evidence for an association with coronavirus infection. Vet. Rec. 1991;128:447–449. doi: 10.1136/vr.128.19.447. [DOI] [PubMed] [Google Scholar]
- Saif L.J., Redman D.R., Brock K.V., Kohler E.M., Heckert R.A. Winter dysentery in adult dairy cattle: detection of coronavirus in the faeces. Vet. Rec. 1998;123:300–301. doi: 10.1136/vr.123.11.300. [DOI] [PubMed] [Google Scholar]
- Snodgrass D.R., Terzolo H.R., Sherwood D., Campbell I., Menzies J.D., Synge B.A. Aetiology of diarrhoea in young calves. Vet. Rec. 1986;119:31–34. doi: 10.1136/vr.119.2.31. [DOI] [PubMed] [Google Scholar]
- Storz J., Purdy C.W., Lin X., Burrell M., Truax R.E., Briggs R.E., Frank G.H., Loan R.W. Isolation of respiratory bovine coronavirus, other cytocidal viruses, and Pasteurella spp. from cattle involved in two natural outbreaks of shipping fever. J. Am. Vet. Med. Assoc. 2000;216:1599–1604. doi: 10.2460/javma.2000.216.1599. [DOI] [PubMed] [Google Scholar]
- Sullivan D.G., Akkina R.K. A nested polymerase chain reaction assay to differentiate pestiviruses. Virus Res. 1995;38:231–239. doi: 10.1016/0168-1702(95)00065-x. [DOI] [PubMed] [Google Scholar]
- Tråvén M., Naslund K., Linde N., Linde B., Silvan A., Fossum C., Hedlund K.O., Larsson B. Experimental reproduction of winter dysentery in lactating cows using BCV—comparison with BCV infection in milk-fed calves. Vet. Microbiol. 2001;81:127–151. doi: 10.1016/S0378-1135(01)00337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valarcher J.F., Bourhy H., Gelfi J., Schelcher F. Evaluation of a nested reverse transcription-PCR assay based on the nucleoprotein gene for diagnosis of spontaneous and experimental bovine respiratory syncytial virus infections. J. Clin. Microbiol. 1999;37:1858–1862. doi: 10.1128/jcm.37.6.1858-1862.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek S. Detection of the bovine herpesvirus-1 (BHV-1) genome by PCR. J. Virol. Methods. 1993;41:245–247. doi: 10.1016/0166-0934(93)90132-b. [DOI] [PubMed] [Google Scholar]
- White M.E., Schukken Y.H., Tanksley B. Space-time clustering of, and risk factors for, farmer-diagnosed winter dysentery in dairy cattle. Can. Vet. J. 1989;30:948–951. [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y., Wong B.H., Poon R.W., Cai J.J., Luk W.K., Poon L.L., Wong S.S., Guan Y., Peiris J.S., Yuen K.Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


