Highlights
-
•
PDCoV infection caused severe diarrhea, virus shedding and intestinal lesion in weaned piglets.
-
•
PDCoV could induce TLR3 mRNA expression in infected Peyer's patches from weaned piglets.
-
•
PDCoV obviously induced IL-12, IFN-α, IFN-β, and PKR mRNA expression in infected Peyer's patches from weaned piglets.
Keywords: Porcine deltacoronavirus (PDCoV), Innate immune responses, Peyer’s patches, Weaned piglets
Abstract
Porcine deltacoronavirus (PDCoV) is a newly identified swine enteropathogenic coronavirus that causes watery diarrhea in piglets and results in significant economic losses to the pig industry. Currently there are no effective treatments or vaccines for PDCoV. In particular, the pathogenesis of PDCoV infection is still largely unknown. In this study, we reported that inoculating conventional weaned piglets with 1 × 109 TCID50 of the PDCoV CHN-GD-2016 strain by oral feeding could cause severe diarrhea. Virus RNA was detected in rectal swabs from 1 to 7 days post inoculation. In addition, microscopic lesions in small intestine were observed, and viral antigen also detected in the small intestines with PDCoV immunohistochemical staining. Importantly, PDCoV significantly induced mRNA expression of TLR3, IL-12, IFN-α, IFN-β, and PKR, the genes involved in modulation of the host immune responses, in infected Peyer's patches at 3 d.p.i., indicating that Peyer's patches play an important role in PDCoV immune responses in vivo. Collectively, our findings suggest that the observed gene expression profile might help explain immunological and pathological changes associated with PDCoV infection.
1. Introduction
Porcine deltacoronavirus (PDCoV), a newly identified swine enteropathogenic coronavirus, can cause acute watery diarrhea and mortality in newborn piglets. PDCoV is an enveloped, single-stranded, positive-sense RNA virus with a genome of appropriately 25-kb in length (Zhang, 2016). Compare to the complete genome of another two common swine enteropathogenic coronaviruses (PEDV, TGEV), the PDCoV CHN-GD-2016 strain (GenBank no: MF280390) shares 43.39%, 42.79% nucleotide identity with PEDV GDS01 (GenBank no: KM089829.1) and TGEV AHHF (GenBank no: KX499468.1), respectively. Since it was first detected in 2012 in Hong Kong during a molecular epidemiology study of CoVs (Woo et al., 2012), PDCoV subsequently had been reported in at least 9 states of the United States, Thailand, Vietnam, Lao PDR, South Korea, Canada, and mainland China (Leyi et al., 2014; Marthaler et al., 2014; Saeng-Chuto et al., 2017; Sunhee Lee, 2014; Wang et al., 2014; Xu et al., 2018). These molecular surveillance studies indicated that PDCoV was a common viral pathogen of pigs around the world.
The clinical symptoms in newborn piglets by PDCoV infection are similar to that by other porcine enteric pathogens such as PEDV and TGEV, include diarrhea, vomiting, dehydration and lethal (Zhang, 2016). Currently a few studies have reproduced clinical diarrheal disease by experimentally infecting gnotobiotic and conventional pigs with the isolated PDCoV strains (Chen et al., 2015; Dong et al., 2016; Kwonil et al., 2015; Ma et al., 2015; Xu et al., 2018). As expected, the 11–14 day-old gnotobiotic pigs inoculated orally with PDCoV strain OH-FD22 and OH-FD100 showed severe diarrhea, vomiting, and atrophic enteritis (Kwonil et al., 2015). In addition, all infected pigs had PEDV-like lesions characterized by thin and transparent intestinal walls and accumulation of large amounts of yellow fluid in the intestinal lumen (Kwonil et al., 2015). Further, histology analysis exhibited acute diffuse, severe atrophic enteritis and mild vacuolation of superficial epithelial cells in cecum and colon (Kwonil et al., 2015). The pathogenicity of PDCoV in conventional pigs were also confirmed with the subsequent isolation (Chen et al., 2015; Dong et al., 2016; Ma et al., 2015; Xu et al., 2018). All these studies target suckling piglets, however, there are still sporadic outbreaks of diarrhea in weaned piglets with unknown reasons.
PDCoV is an important enteropathogen in pigs, but few reports are involved in modulation of the host immune responses against PDCoV infection. Currently it is known that PDCoV infection inhibits the production of interferon-beta (IFN-β) through suppressing the activation of RIG-signaling pathway (Luo et al., 2016) in vitro. Nonstructural protein 5 (nsp5) of PDCoV was a newly identified IFN antagonist by cleaving NEMO (Zhu et al., 2017a) and destroyed subsequent type I interferon signaling by cleaving STAT2 (Zhu et al., 2017b). In addition, another protein (NS6) of PDCoV was also found to antagonize IFN-β production by interfering with binding of RIG-I/MDA5 to double-stranded RNA (Fang et al., 2018). However, little is known about the innate immune responses in vivo against PDCoV infection.
In this study, we investigated the pathogenicity of PDCoV in 33-day-old conventional weaned piglets by clinical assessment, virus shedding, histological test, and immunohistochemical study. Furthermore, to gain a better understanding about the immunological changes in vivo after PDCoV infection, we assessed the effect of PDCoV on the toll-like receptors and cytokines in Peyer's patches in inoculated-piglets by real-time PCR.
2. Materials and methods
2.1. Virus propagation in LLC-PK cells
LLC-PK cells were obtained from Wen's Foodstuffs Group Co, Ltd (Guangdong, China), and cultured in DMEM (Dulbecco' s modified eagle medium) (Hyclone, USA) supplemented with penicillin (100 U/mL), streptomycin (100 U/mL), and 10% fetal bovine serum (FBS) (BOVOGEN, Australia). The maintenance medium for PDCoV propagation was DMEM supplemented with 8 μg/mL trypsin (Gibco, USA) in 5% CO2 incubator. PDCoV CHN-GD-2016 was isolated by our laboratory (Xu et al., 2018).
Virus propagation was performed as previously described (Xu et al., 2018). Briefly, LLC-PK cells were cultured in T175 flasks, and were washed twice with maintenance medium when 90% confluent. Fifty microliters of PDCoV CHN-GD-2016 strain together with 50 mL maintenance medium were added to flask. The cell pellets and supernatants were cultured continuously at 37℃ in 5% CO2 to observe cytopathic effect (CPE). When CPE was evident in the inoculated cell monolayers (around 1 d.p.i.), the plates were fronzen at -80℃ and thawed twice. The cells and supernatants were harvested together to determine viral titers. Virus titers were calculated using the Reed-Muench method (Reed and Muench, 1938) and expressed as TCID50 per milliliter.
2.2. Pigs
Thirty-three-day-old crossbred (Duroc × Landrace × Big White) conventional weaned piglets were procured from Wen's Foodstuffs Group Co, Ltd (Guangdong, China). All pigs were maintained in our animal facility with food and water ad libitum for a minimum of 7 days before the experimentation. The animal study was approved by the Institutional Animal Care and Use Committee of the Sun Yat-sen University (Guangdong, China) and animals were treated in accordance with the regulations and guidelines of this committee.
2.3. Experimental infection with the PDCoV CHN-GD-2016 strain in conventional weaned piglets
Sixteen conventional weaned piglets were randomly divided into two groups (8 piglets/group) and were housed in two separate rooms. Prior to inoculation, weaned piglets were confirmed negative for the major procine enteric viruses (PDCoV, PEDV, TGEV, PRoV) by testing the rectal swabs on day −1 as previously described (Xu et al., 2018). On day 0, weaned piglets in group 1 were orally inoculated with 10 mL of maintenance medium and served as uninfected controls. Weaned piglets in group 2 were orally challenged with 10 mL of maintenance medium containing a total of 1 × 109 TCID50 of the PDCoV CHN-GD-2016 strain. After infection, weaned piglets were observed daily for clinical signs of vomiting, diarrhea, lethargy, body weight. Diarrhea severity was scored with the following criteria: 0=normal, 1=soft (cowpie), 2=liquid with some solid content, 3=watery with no solid content.
Rectal swabs collected from each piglet on 1 d.p.i. to 7 d.p.i. and were submerged into 1 mL sterile 1 × PBS (pH 7.4) immediately after collection. Four weaned piglets from each group were randomly selected for necropsy at 3 d.p.i., and the remaining weaned piglets were necropsied at 7 d.p.i. At necropsy, the fresh Peyer's patches were collected and stored at −80 °C until further use, and the fresh jejunum were collected and fixed by 10% formalin for histopathology and immunohischemistry analysis.
2.4. Histological and immunohistochemical staining
Histological and Immunohistochemical staining were performed as previously described (Xu et al., 2018). Briefly, tissue samples of jejunum of the piglets from the challenged and control groups were separated and routinely fixed in 10% formalin for 36 h at room temperature, and then dehydrated in graded ethanol, embedded in paraffin, cut in 5-μm sectioned, and mounted onto glass slides. After the sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin (H&E), the slides were examined and analyzed with conventional light microscopy. Sections (5 μm) of formalin-fixed paraffin-embedded tissues were placed onto positively charged glass slides and the slides were air dried for 120 min at 60 °C. The tissue sections were deparaffinized, and then rinsed and incubated with target retrieval solution (Servicebio, China). After being blocked with 1% BSA (Solarbio, China), the sections were incubated with PDCoV specific rabbit antisera (Wen's Foodstuffs Group Co., Ltd, China) (1:100) as the primary antibody for 12 h at 4 °C. They were then incubated with peroxidase-labeled goat anti-rabbit IgG secondary antibody (KPL, USA) (1:200) for 50 min at room temperature, and the samples were finally visualized with a 3, 3'-diaminobenzidine (DAB) chromogen kit (Dako, Denmark). Hematoxylin was used for counterstaining. Tissues of piglets from negative control groups were used as negative samples. The immunohistochemistry slides were evaluated by a veterinary pathologist according to the evaluation system of histology and immunohistochemistry by Jung et al., 2014 (Jung et al., 2014).
2.5. RNA isolation and real-time RT-PCR analysis
Viral RNA shedding in piglet feces after infection with PDCoV was detected as previously described (Xu et al., 2018). Briefly, total RNA was prepared from the supernatants of rectal swab from each piglet using a RNeasy kit (Magen, China) per the manufacturer' instruction, and was treated with DNase I. Two μg of total RNA was used for cDNA synthesis by reverse transcription using RT-PCR kit (TaKaRa, Dalian). The specific primers for the membrane (m) gene of PDCoV (sense: 5′-ATCGACCACATGGCTCCAA-3′; antisense: 5′-CAGCTCTTGCCCATGTAGCTT-3′), and probe (5′-FAM-CACACCAGTCGTTAAGCATGGCAAGCT-BHQ-3′) were designed with reference to the previous publication (Marthaler et al., 2014) and synthesized by Invitrogen Company (Shanghai, China). The real-time PCR assay was carried out with an Applied Biosystem 7500 instrument (Life Technologies, USA). The PCR was performed in a 20-μL volume containing 1 μL of cDNA, 10 μL of Thunderbird Probe qPCR Mix, 0.04 μL 50 × Rox reference dye (TOYOBO, Shanghai), 0.2 μmol/L of probe, and a 0.3 μmol/L of each gene-specific primer. The thermal cycling parameters were as follows: 95 °C for 20 s; 40 cycles of 95 °C for 3 s, 60 °C for 30 s. And the m gene was amplified to generate the standard curve from PDCoV CHN-GD-2016 strain using the specific primers (sense: 5′-ATGTCTGACGCAGAAGAGTG-3′; antisense: 5′-TTACATATACTTATACAGGCGAGC-3′) that were designed with reference to the published sequence (GenBank, Accession no: MH715491), and the PCR products were cloned into the pMD19-T (TaKaRa, Dalian). The known plasmid concentration was 10-fold serially diluted for generating a standard curve in each plate. The quantity of PDCoV viral RNA in tested samples was calculated based on the cycle threshold (Ct) values for the standard curve.
To analyze immunological changes in piglets with PDCoV infection, total RNA was prepared from Peyer's patches using a RNeasy kit (Magen, China) per the manufacturer' instruction, and was treated with DNase I. Two μg of total RNA was used for cDNA synthesis by reverse transcription using RT-PCR kit (TaKaRa, Dalian). The specific primers for porcine IL-1β (sense: 5′-AACGTGCAGTCTATGGAGT-3′; antisense: 5′-GAACACCACTTCTCTCTTCA-3′), IL-6 (sense: 5′-CTGGCAGAAAACAACCTGAACC-3′; antisense: 5′-TGATTCTCATCAAGCAGGTCTCC-3′), IL-12p35 (sense: 5′-CGTGCCTCGGGCAATTATA-3′; antisense: 5′-CGCAGGTGAGGTCGCTAGTT-3′), TNF-α (sense: 5′-AACCTCAGATAAGCCCGTCG-3′; antisense: 5′-ACCACCAGCTGGTTGTCTTT-3′), TLR-3 (sense: 5′-CAAAACCAGCAACACGACTTTC-3′; antisense: 5′-AATCATTACCAATCACACTTAAGCTGTTA-3′), IFN-α (sense: 5′-TCTCATGCACCAGAGCCA-3′; antisense: 5′−CCTGGACCACAGAAGGGA-3′), IFN-β (sense: 5′-AGTGCATCCTCCAAATCGCT-3′; antisense: 5′-GCTCATGGAAAGAGCTGTGGT-3′), PKR (sense: 5′-AAAGCGGACAAGTCGAAAGG-3′; antisense: 5′-TCCACTTCATTTCCATAGTCTTCTGA-3′), OAS (sense: 5′-GAGCTGCAGCGAGACTTCCT-3′; antisense: 5′-TGCTTGACAAGGCGGATGA-3′), Mx1 (sense: 5′-GGCGTGGGAATCAGTCATG-3′; antisense: 5′-AGGAAGGTCTATGAGGGTCAGATCT-3′), and glyceraldehydes-3-phosphate dehydrogenase (GAPDH; sense: 5′−CCTTCCGTGTCCCTACTGCCAAC-3′; antisense: 5′-GACGCCTGCTTCACCACCTTCT-3′) were designed with reference to previous publications (Borca et al., 2008; Huang et al., 2014) and synthesized by Sangon Company (Shanghai, China). The real-time PCR assay was carried out with an Applied Biosystem 7500 instrument (Life Technologies, USA). The PCR was performed in a 20-μL volume containing 1 μL of cDNA, 10 μL of 2 × SYBR green Premix Ex Taq (TaKaRa, Dalian), and a 0.4 μM of each gene-specific primer. The thermal cycling parameters were referring to the previous study (Borca et al., 2008), and were as follows: 95 °C for 30 s; 40 cycles of 95 °C for 3 s, 60 °C for 30 s; and 1 cycle of 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s, 60 °C for 15 s. The final step was to obtain a melt curve for the PCR products to determine the specificity of the amplification. The GAPDH gene was utilized as the reference gene. Expression levels of genes were calculated relative to the expression of the GAPDH gene and expressed as fold increase or decrease relative to the control samples.
2.6. Statistical analysis
Statistical comparisons were performed using GraphPad Prism software. The significance of the differences between the treatment group and control in the mRNA expressions (cytokines, toll-like receptor, and antiviral molecules) was determined by the ANOVA and Mann-Whitney accordingly.
3. Results
3.1. PDCoV was highly pathogenic to weaned piglets
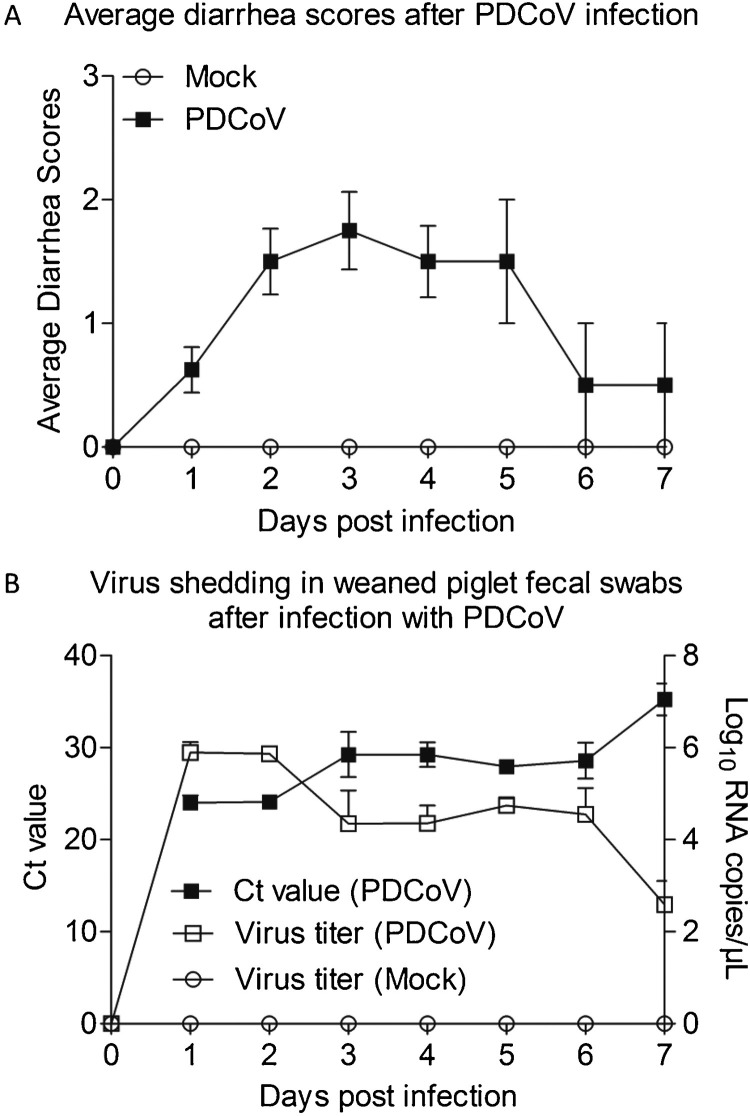
To evaluate the pathogenicity of PDCoV in weaned piglets, we experimentally infected 33-day old conventional weaned piglets with PDCoV CHN-GD-2016. As shown in Fig. 1 A, the weaned piglets inoculated with CHN-GD-2016 at a dose of 1 × 109 TCID50/head via oral feeding showed acute and severe watery diarrhea from 1 d.p.i. to 7 d.p.i., as compared with controls. To confirm the presence of PDCoV, virus RNA was detected by qRT-PCR in fecal swabs collected from orally inoculated piglets from 1 d.p.i. to 7 d.p.i., and no PDCoV RNA was detected in the negative control piglets during the study (Fig. 1B). Taken together, these results demonstrated that PDCoV was highly pathogenic to the weaned piglets.
Fig. 1.
Reproduction of watery diarrhea and fecal viral shedding in weaned piglets inoculated with PDCoV CHN-GD-2016 strainviaoral feeding.
(A) Average diarrhea scores after PDCoV infection. (B) Ct values of group PDCoV inoculation weaned piglet fecal swabs and viral RNA shedding in fecal swabs after PDCoV inoculation or mock inoculation.
3.2. Histopathological and immunohistochemistry results of weaned piglets infected with PDCoV
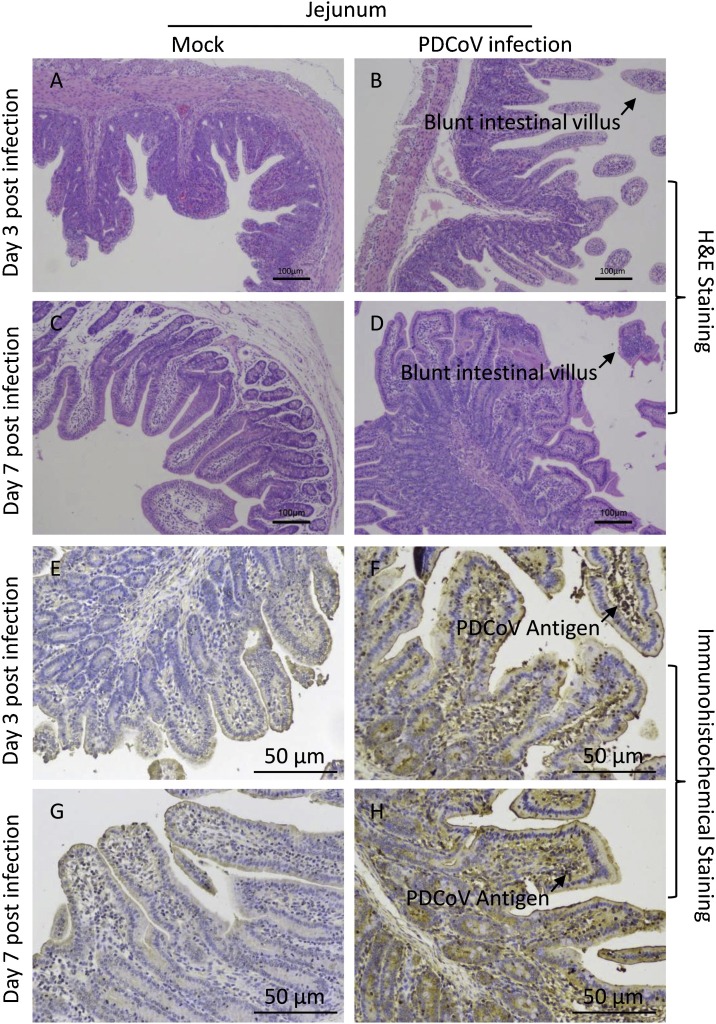
To determine the histological changes in weaned piglets infected with the PDCoV, piglets were necropsied at 3 d.p.i. and 7 d.p.i. As shown in Fig. 2 B&D, blunt intestinal villus was observed, while that in negative control was normal (Fig. 2A&C). In addition, PDCoV antigen was detected in the cytoplasm of the villous enterocytes of the PDCoV-challenged piglets by immunohistochemical analysis (Fig. 2F&H), but no PDCoV antigen was detected in negative control (Fig. 2E&G). Taken together, these results indicate that PDCoV could cause intestinal lesion in weaned piglets.
Fig. 2.
Intestinal changes in weaned piglets inoculated with PDCoV strain CHN-GD-2016.
(A&C) H&E-stained jejunum tissue section of control piglets at 3 d.p.i. and 7 d.p.i. (B&D) Hematoxylin and eosin (H&E)-stained jejunum tissue section of PDCoV-challenged piglets at 3 d.p.i. and 7 d.p.i. (Blunt intestinal villus was indicated by arrows). (E&G) Immunohistochemically stained jejunum tissue section of control piglets at 3 d.p.i. and 7 d.p.i. (F&H) Immunohistochemically stained jejunum tissue section of PDCoV-challenged piglets at 3 d.p.i. and 7 d.p.i.
3.3. PDCoV induces the mRNA expressions of TLR3 in Peyer's patches
Toll-like receptor 3 (TLR3) is modulated rapidly in response to RNA virus infection (Xie et al., 2018). To determine whether PDCoV could induce TLR3 mRNA expression, we examined the mRNA expressions of TLR3 in Peyer's patches from PDCoV-infected piglets by real-time PCR assays. We observed that TLR3 expression was changed by PDCoV infection (Fig. 3 ). Up to five fold upregulation of TLR3 expression was observed at 3 d.p.i. (p < 0.01), as compared to that of control, indicating that PDCoV induce TLR3 mRNA expression in vivo.
Fig. 3.
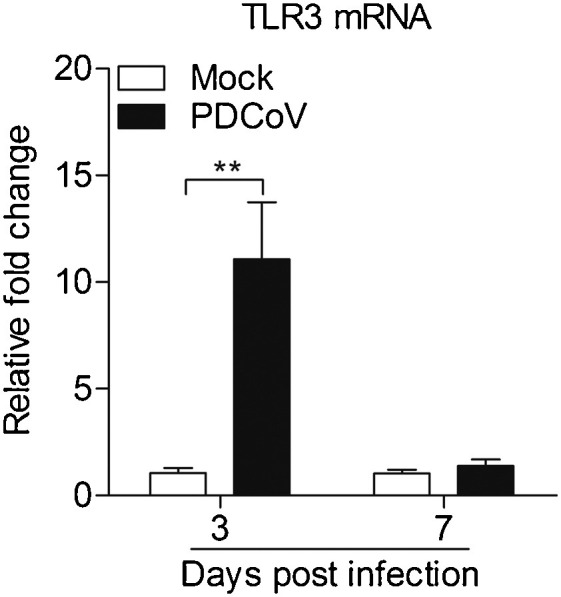
Expressions of toll-like receptor 3 in Peyer's patches from weaned piglets after infection with PDCoV strain CHN-GD-2016 at 3 d.p.i. and 7 d.p.i.
The mRNA expressions of TLR3 in Peyer's patches from weaned piglets after infection with PDCoV strain CHN-GD-2016 were examined with qRT-PCR using specific primers at 3 d.p.i. and 7 d.p.i. The mRNA expression level of TLR3 was calculated in relation to the expression level of GAPDH. Data are represented as mean ± SD, n = 4. **stands for p < 0.01.
3.4. PDCoV induces mRNA expressions of IL-12, but not IL-1β, IL-6 and TNF-α in Peyer's patches
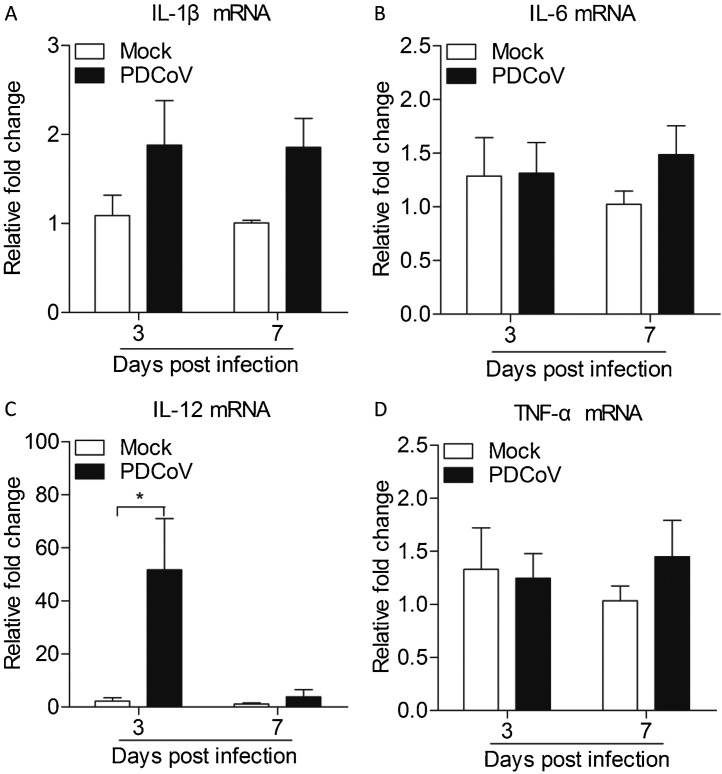
After recognition of viral nucleic acids, TLRs transmit signals to activate nuclear factor-κB (NF-κB), which is mainly involved in pro-inflammatory induction (Wu and Chen, 2014). To examine the effect of PDCoV on pro-inflammatory cytokines, we examined the mRNA expressions of IL-1β, IL-6, IL-12 and TNF-α in Peyer's patches from PDCoV-infected weaned piglets by real-time PCR assays. We found that the mRNA expressions of IL-1β, IL-6 and TNF-α of PDCoV-infected Peyer's patches were similar to that of mock-infected Peyer's patches at 3 d.p.i. and 7 d.p.i. (Fig. 4 A&B&D). However, PDCoV could increase the mRNA expressions of IL-12 in Peyer's patches at 3 d.p.i. (p < 0.05) as compared to the control (Fig. 4C).
Fig. 4.
Expressions of pro-inflammatory cytokines in Peyer's patches from weaned piglets after infection with PDCoV strain CHN-GD-2016 at 3 d.p.i. and 7 d.p.i.
The mRNA expressions of IL-1β (A), IL-6 (B), IL-12 (C) and TNF-α (D) in Peyer's patches from weaned piglets after infection with PDCoV strain CHN-GD-2016 were examined with qRT-PCR using specific primers at 3 d.p.i. and 7 d.p.i. The mRNA expression levels of these cytokines were calculated in relation to the expression level of GAPDH. Data are represented as mean ± SD, n = 4. *stands for p < 0.05.
3.5. PDCoV induces an antiviral response in Peyer's patches
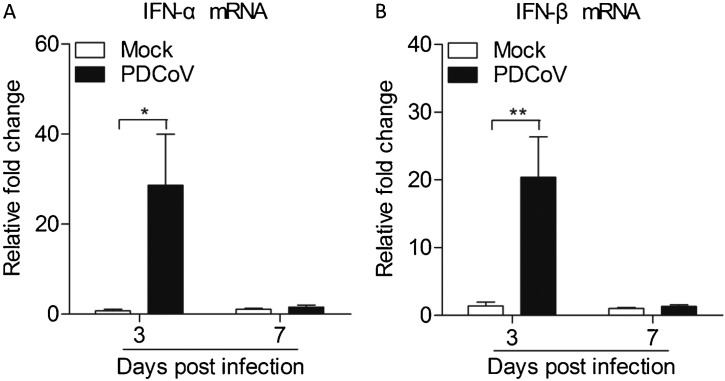
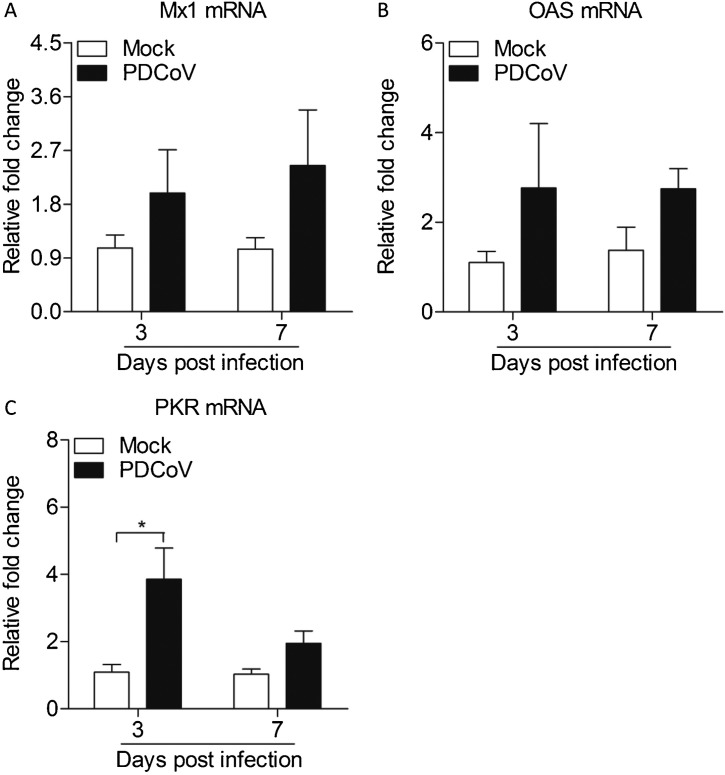
Since TLR3 was induced by PDCoV, we attempted to assess the expressions of type I interferon in Peyer's patches. As expected, PDCoV could increase the mRNA expressions of IFN-α (p < 0.05) and IFN-β (p < 0.01) in Peyer's patches from PDCoV infected weaned piglets at 3 d.p.i. IFNs production converges on the induction of IFN-stimulated genes (ISGs) (Xie et al., 2018). Consistent with the type I interferon results, PDCoV could increase the mRNA expressions of PKR (p < 0.05) in Peyer's patches from weaned piglets infected with PDCoV at 3 d.p.i., but not other antiviral effector genes like OAS and Mx1, indicating that PKR plays a critical role against PDCoV infection (Fig. 5, Fig. 6 ).
Fig. 5.
Expressions of type I interferon in Peyer's patches from weaned piglets after infection with PDCoV strain CHN-GD-2016 at 3 d.p.i. and 7 d.p.i.
The mRNA expressions of IFN-α (A) and IFN-β (B) in Peyer's patches from weaned piglets after infection with PDCoV strain CHN-GD-2016 were examined with qRT-PCR using specific primers at 3 d.p.i. and 7 d.p.i. The mRNA expression level of these cytokines were calculated in relation to the expression level of GAPDH. Data are represented as mean ± SD, n = 4. **stands for p < 0.01, *stands for p < 0.05.
Fig. 6.
Expressions of antiviral molecules in Peyer's patches from weaned piglets after infection with PDCoV strain CHN-GD-2016 at 3 d.p.i. and 7 d.p.i.
The mRNA expressions of Mx1 (A), OAS (B), and PKR (C) in Peyer's patches from weaned piglets after infection with PDCoV strain CHN-GD-2016 were examined with qRT-PCR using specific primers at 3 d.p.i. and 7 d.p.i. The mRNA expression level of these molecules were calculated in relation to the expression level of GAPDH. Data are represented as mean ± SD, n = 4. *stands for p < 0.05.
4. Discussion
Since the first report of PDCoV in pigs in 2012 in Hong Kong (Woo et al., 2012), this novel swine enteric coronavirus has been widely detected and isolated in pig farms in different countries (Leyi et al., 2014; Saeng-Chuto et al., 2017; Sunhee Lee, 2014; Xu et al., 2018). Although a few studies have demonstrated PDCoV was highly pathogenic to newborn piglets (Chen et al., 2015; Xu et al., 2018), there are no published papers reporting the pathogenicity of PDCoV in weaned piglets and the effect of PDCoV infection on innate immune responses in vivo is still unclear. In the present study, we reported that PDCoV was highly pathogenic to weaned piglets and obviously induced innate immune responses in vivo.
As a widespread swine pathogen, the pathogenicity of PDCoV in weaned piglets is still unknown. We infected the 33-day-old weaned piglets with the PDCoV CHN-GD-2016 strain via oral feeding. PDCoV causing severe diarrhea in weaned piglets strongly suggested that PDCoV posed a huge threat to weaned piglets like newborn piglets in pig farms. Interestingly, unlike in newborn piglets, PDCoV infection did not cause vomiting and death in weaned piglets at a high dose (1 × 109 TCID50/head) in 7 days, indicating that newborn piglets were more susceptible to PDCoV infection than weaned piglets. This phenomenon demonstrated that PDCoV infection is another age-dependent disease severity like PEDV and TGEV (Moon et al., 1975; Isao et al., 2000). Furthermore, we collected fecal swabs from the PDCoV-challenged piglets and detected the viral fecal shedding by real-time PCR. PDCoV RNA was detected from 1 d.p.i. to 7 d.p.i., while no RNA was detected in the negative control piglets during the study, indicating that the infection with PDCoV by fecal-oral contamination might be the major mode of transmission for diarrhea in pig farms. In addition, microscopic lesions were obviously observed in jejunums of the 33-day-old piglets at necropsy at 3 d.p.i. and 7 d.p.i., similar to observations in PDCoV infected newborn piglets (Xu et al., 2018). What is more, no microscopic lesions were observed in any other organs of PDCoV-challenged weaned piglets (data not shown), indicating that intestinal tract might be the target organ of PDCoV. Consistent with the histopathological results, the PDCoV antigen was detected in the cytoplasm of the villous enterocytes of the PDCoV-challenged weaned piglets by immunohistochemical analysis. Together, all these results confirmed that the PDCoV could also cause enteric diseases in weaned piglets.
It might not fully reflect the body's immune status because the immune system of the neonatal suckling piglets is fragile and immature when virus infection occurs. And we found that PDCoV did not activate T lymphocytes of Peyer's patches from newborn piglets (data not shown). In addition, PDCoV was highly pathogenic to the weaned piglets. Taken together, we attempted to turn to pigs with more mature immune system like weaned piglets to study the impact of PDCoV infection on the immune system. Innate immunity is the first line of host defense against a wide variety of pathogenic infections (Li et al., 2013). Pathogen associated pathogen-associated molecular patterns (PAMPs) sensed by host pattern recognition receptors (PRRs) are essential portion of the innate immune response in hosts against viral infection. Several classes of PRRs have been identified: Toll-like receptors (TLRs), retinoic acid inducible gene I (RIG-I)-like receptors (RLRs), Nod-like receptors (NLRs) and C-type lectin receptors (Iborra and Sancho, 2015; Mosser and Suresh, 2013). Of these PRRs, TLRs and RLRs play a critical role in recognition of RNA virus infection (Akira and Takeuchi, 2009). TLR3, which localize on the endosomal membrane, recognize double-stranded RNA (dsRNA) (Lena et al., 2001). It was reported that PEDV infection induces NF-κB activation through the TLR3 pathways in porcine intestinal epithelial cells (Cao et al., 2015). The small intestinal mucosa is thought to be the primary site for defense against enteropathogen (Yuan et al., 2018). And Peyer's patches serve as the primary inductive sites for intestinal immunity (Fang and Vazquenz-Torres, 2000), which occupy most of the area in the ileum (Van Kruiningen et al., 2002). In the present study, we found that the mRNA expression of TLR3 was induced in Peyer's patches from PDCoV-infected weaned piglets, indicating that PDCoV could active innate immune response in pigs. After recognition of viral nucleic acids, TLRs could recruit downstream kinases that phosphorylate downstream adaptor proteins to transmit signals to activate transcription factors IFN regulatory factor 3 (IRF3), IRF7 and nuclear factor-κB (NF-κB), which are mainly involved in IFN and pro-inflammatory genes induction (Wang et al., 2018). Our results demonstrate that PDCoV infection could induce the expression of IL-12 in Peyer's patches at 3 d.p.i., which might be the result of TLR3 activation. However, PDCoV did not affect the expression of other pro-inflammatory cytokine (IL-1β, IL-6 and TNF-α) in vivo. It seems that the body will activate cellular immunity against PDCoV infection at the later stage. In addition, we also found that PDCoV could increase the mRNA expressions of IFN-α and IFN-β in Peyer's patches at 3 d.p.i. IFN-α/β as important cytokines induced by virus induction could establish an anti-viral state in infected cells and neighboring non-infected cells, and also regulate the adaptive immune response (Xie et al., 2018). It is known that IFNs production trigger the induction of IFN-stimulated genes (ISGs) against virus infection (Lenschow et al., 2007), such as dsRNA activated protein kinase R (PKR) (Williams, 1999), 2′-5′-oligoadenylate synthetase (OAS) (Sanfilippo et al., 2018) and Mx proteins (Zhou et al., 2018). In this study, we found that PDCoV could increase the mRNA expressions of PKR in Peyer's patches. Interestingly, other ISGs like OAS and Mx1 did not increase upon PDCoV infection, indicating that the body restrain the PDCoV RNA transfer mainly by PKR. In addition, all results shown that innate immunity occurred only at 3 d.p.i., but not 7 d.p.i., indicating that virus might have overcome the innate immunity to infect the body at 7 d.p.i. Since PDCoV induced innate immune response in vivo, several important questions are raised. For example, What’s the viral protein of PDCoV inducing the expressions of these cytokines in vivo? And what’s the exact underlying mechanism? How to overcome the innate immune response to cause damage when PDCoV infection occurs? More efforts will be required to elucidate the molecular mechanisms underlying the pathogenesis of PDCoV infection.
In summary, our results revealed that PDCoV infection caused severe diarrhea, virus shedding and intestinal lesion in weaned piglets. Remarkably, inoculation of weaned piglets with PDCoV obviously induced TLR3, IL-12, IFN-α, IFN-β, and PKR mRNA expression in infected Peyer's patches. These findings have provided insights for further studies of the molecular mechanism underlying host response against PDCoV infection.
Author contributions
YCC and ZCX conceived and designed the experiments; ZCX and HLZ performed the experiments; ZCX and HLZ analyzed the data; YCC, SJH, QFZ, YPD, LC, and CYX contributed reagents/materials/analysis tools; ZCX wrote the paper. YCC checked and finalized the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that they have no conflict interest.
Ethical approval
The animal study was supervised by the Institutional Animal Care and Use Committee of Sun Yat-sen University (IACUC DD-17-0403) and used in accordance with regulation and guidelines of this committee.
Acknowledgements
This work was supported by the National Key Research and Development Program, China (2016YFD0500101).
References
- Akira S., Takeuchi O. Innate immunity to virus infection. Immunol. Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borca M.V., Gudmundsdottir I., Fernandez-Sainz I.J., Holinka L.G., Risatti G.R. Patterns of cellular gene expression in swine macrophages infected with highly virulent classical swine fever virus strain Brescia. Virus. Res. 2008;138:89–96. doi: 10.1016/j.virusres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Cao L., Ge X., Gao Y., Ren Y., Ren X., Li G. Porcine epidemic diarrhea virus infection induces NF-kappaB activation through the TLR2, TLR3 and TLR9 pathways in porcine intestinal epithelial cells. J. Gen. Virol. 2015;96:1757–1767. doi: 10.1099/vir.0.000133. [DOI] [PubMed] [Google Scholar]
- Chen Q., Gauger P., Stafne M., Thomas J., Arruda P., Burrough E., Madson D., Brodie J., Magstadt D., Derscheid R., Welch M., Zhang J. Pathogenicity and pathogenesis of a United States porcine deltacoronavirus cell culture isolate in 5-day-old neonatal piglets. Virology. 2015;482:51–59. doi: 10.1016/j.virol.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N., Fang L., Yang H., Liu H., Du T., Fang P., Wang D., Chen H., Xiao S. Isolation, genomic characterization, and pathogenicity of a Chinese porcine deltacoronavirus strain CHN-HN-2014. Vet. Microbiol. 2016;196:98–106. doi: 10.1016/j.vetmic.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F.C., Vazquenz-Torres A. Cellular routes of invasion by enteropathogens. Curr. Opin. Microbiol. 2000;3:54–59. doi: 10.1016/s1369-5274(99)00051-x. [DOI] [PubMed] [Google Scholar]
- Fang P., Fang L., Ren J., Hong Y., Liu X., Zhao Y., Wang D., Peng G., Xiao S. Porcine deltacoronavirus accessory protein NS6 antagonizes interferon Beta production by interfering with the binding of RIG-I/MDA5 to double-stranded RNA. J. Virol. 2018;92:712–718. doi: 10.1128/JVI.00712-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Zhang Q., Guo X.K., Yu Z.B., Xu A.T., Tang J., Feng W.H. Porcine reproductive and respiratory syndrome virus nonstructural protein 4 antagonizes beta interferon expression by targeting the NF-kappaB essential modulator. J. Virol. 2014;88:10934–10945. doi: 10.1128/JVI.01396-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iborra S., Sancho D. Signalling versatility following self and non-self sensing by myeloid C-type lectin receptors. Immunobiology. 2015;220:175–184. doi: 10.1016/j.imbio.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isao Shibataa, Tsuda T., Masahumi Mori, Masaaki Ono, Sueyoshib Masuo, Urunoa Katsuyoshi. Isolation of porcine epidemic diarrhea virus in porcine cell cultures and experimental infection of pigs of different ages. Vet. Microbiol. 2000;72:173–182. doi: 10.1016/S0378-1135(99)00199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Wang Q., Scheuer K.A., Lu Z., Zhang Y., Saif L.J. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg. Infect. Dis. 2014;20:662–665. doi: 10.3201/eid2004.131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwonil Jung, Hu Hui, Eyerly Bryan, Lu Zhongyan, Juliet Chepngeno, Saif Linda J. Pathogenicity of 2 porcine deltacoronavirus strains in gnotobiotic pigs. Emerg. Infect. Dis. 2015;21:650–654. doi: 10.3201/eid2104.141859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena Alexopoulou, Holt A.C., Medzhitov Ruslan, Flavell Richard A. Recognition of double-stranded RNA and activation of NF-kB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Lenschow D.J., Lai C., Frias-Staheli N., Giannakopoulos N.V., Lutz A., Wolff T., Osiak A., Levine B., Schmidt R.E., Garcia-Sastre A., Leib D.A., Pekosz A., Knobeloch K.P., Horak I., Virgin H.W.t. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc. Natl. Acad. Sci. U S A. 2007;104:1371–1376. doi: 10.1073/pnas.0607038104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyi Wang, Byrum B., Zhang Yan. Porcine Coronavirus HKU15 detected in 9 US States, 2014. Emerg. Infect. Dis. 2014;20:1594–1595. doi: 10.3201/eid2009.140756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wang Y., Li X., Li X., Cao H., Zheng S.J. Critical roles of glucocorticoid-induced leucine zipper in infectious bursal disease virus (IBDV)-induced suppression of type I Interferon expression and enhancement of IBDV growth in host cells via interaction with VP4. J. Virol. 2013;87:1221–1231. doi: 10.1128/JVI.02421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Fang L., Dong N., Fang P., Ding Z., Wang D., Chen H., Xiao S. Porcine deltacoronavirus (PDCoV) infection suppresses RIG-I-mediated interferon-beta production. Virology. 2016;495:10–17. doi: 10.1016/j.virol.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhang Y., Liang X., Lou F., Oglesbee M., Krakowka S., Li J. Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. mBio. 2015;6 doi: 10.1128/mBio.00064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler D., Raymond L., Jiang Y., Collins J., Rossow K., Rovira A. Rapid detection, complete genome sequencing, and phylogenetic analysis of porcine deltacoronavirus. Emerg. Infect. Dis. 2014;20:1347–1350. doi: 10.3201/eid2008.140526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H.W., Kemeny L.J., Lamberts G., Stark S.L., Booth G.D. Age-dependent resistance to transmissible gastroenteritis of swine: III. Effects of epithelial cell kinetics on coronavirus production and on atrophy of intestinal villi. Vet. Pathol. 1975;12:434–445. doi: 10.1177/0300985875012005-00610. [DOI] [PubMed] [Google Scholar]
- Mosser D.M., Suresh R. Pattern recognition receptors in innate immunity, host defense, and immunopathology. Adv. Physiol. Educ. 2013;37:284–291. doi: 10.1152/advan.00058.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938;27:3. [Google Scholar]
- Saeng-Chuto K., Lorsirigool A., Temeeyasen G., Vui D.T., Stott C.J., Madapong A., Tripipat T., Wegner M., Intrakamhaeng M., Chongcharoen W., Tantituvanont A., Kaewprommal P., Piriyapongsa J., Nilubol D. Different lineage of porcine deltacoronavirus in Thailand, Vietnam and Lao PDR in 2015. Transbound. Emerg. Dis. 2017;64:3–10. doi: 10.1111/tbed.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilippo C., Pinzone M.R., Cambria D., Longo A., Palumbo M., Di Marco R., Condorelli F., Nunnari G., Malaguarnera L., Di Rosa M. OAS Gene family expression Is associated with HIV-related neurocognitive disorders. Mol. Neurobiol. 2018;55:1905–1914. doi: 10.1007/s12035-017-0460-3. [DOI] [PubMed] [Google Scholar]
- Sunhee Lee C.L. Complete genome characterization of korean porcine deltacoronavirus strain KOR/KNU14-04/2014. Genome Announc. 2014;26 doi: 10.1128/genomeA.01191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kruiningen Herbert J., West A.B., Freda Benjamin J., Holmes Kimberly.A. Distribution of Peyer’s patches in the Distal Ileum. Inflamm. Bowel. Dis. 2002;8:180–185. doi: 10.1097/00054725-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Wang L., Byrum B., Zhang Y. Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerg. Infect. Dis. 2014;20:1227–1230. doi: 10.3201/eid2007.140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Fu M., Liu Y., Wang Y., Li X., Cao H., Zheng S.J. Gga-miR-155 enhances type I interferon expression and suppresses infectious burse disease virus replication via targeting SOCS1 and TANK. Front. Cell. Infect. Microbiol. 2018;8:55. doi: 10.3389/fcimb.2018.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B.R. PKR; A sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H., Bai R., Teng J.L., Tsang C.C., Wang M., Zheng B.J., Chan K.H., Yuen K.Y. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Chen Z.J. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 2014;32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- Xie S., Chen X.X., Qiao S., Li R., Sun Y., Xia S., Wang L.J., Luo X., Deng R., Zhou E.M., Zhang G.P. Identification of the RNA pseudoknot within the 3’ end of the porcine reproductive and respiratory syndrome virus genome as a pathogen-associated molecular pattern to activate antiviral signaling via RIG-I and toll-like receptor 3. J. Virol. 2018;92 doi: 10.1128/JVI.00097-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Zhong H., Zhou Q., Du Y., Chen L., Zhang Y., Xue C., Cao Y. A highly pathogenic strain of porcine deltacoronavirus caused watery diarrhea in newborn piglets. Virol. Sin. 2018;33:131–141. doi: 10.1007/s12250-018-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C., Zhang E., Huang L., Wang J., Yang Q. Oral administration of inactivated porcine epidemic diarrhea virus activate DCs in porcine Peyer’s patches. BMC. Vet. Res. 2018;14:239. doi: 10.1186/s12917-018-1568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Porcine deltacoronavirus: overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus Res. 2016;226:71–84. doi: 10.1016/j.virusres.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Chen J., Zhang X.M., Gao Z.C., Liu C.C., Zhang Y.N., Hou J.X., Li Z.Y., Kan L., Li W.L., Zhou B. Porcine Mx1 protein inhibits classical swine fever virus replication by targeting nonstructural protein NS5B. J. Virol. 2018;92 doi: 10.1128/JVI.02147-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Fang L., Wang D., Yang Y., Chen J., Ye X., Foda M.F., Xiao S. Porcine deltacoronavirus nsp5 inhibits interferon-beta production through the cleavage of NEMO. Virology. 2017;502:33–38. doi: 10.1016/j.virol.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Wang D., Zhou J., Pan T., Chen J., Yang Y., Lv M., Ye X., Peng G., Fang L., Xiao S. Porcine deltacoronavirus nsp5 antagonizes type I interferon signaling by cleaving STAT2. J. Virol. 2017;91 doi: 10.1128/JVI.00003-17. [DOI] [PMC free article] [PubMed] [Google Scholar]