Highlights
-
•
Bovine coronavirus has considerable seroprevalence in cattle across Ghana.
-
•
Sheep and goats are kept without strict separation from cattle and show seropositivity against bovine coronavirus.
-
•
Bovine coronavirus seroprevalence is positively correlated with large farm size.
-
•
Highest bovine coronavirus seroprevalence was found in Ghana´s Northern Province with prevailing arid climate.
Keywords: Bovine Coronavirus, Africa, Ghana, Global health
Abstract
Cattle, goats and sheep are dominant livestock species in sub-Saharan Africa, with sometimes limited information on the prevalence of major infectious diseases. Restrictions due to notifiable epizootics complicate the exchange of samples in surveillance studies and suggest that laboratory capacities should be established domestically. Bovine Coronavirus (BCoV) causes mainly enteric disease in cattle. Spillover to small ruminants is possible. Here we established BCoV serology based on a recombinant immunofluorescence assay for cattle, goats and sheep, and studied the seroprevalence of BCoV in these species in four different locations in the Greater Accra, Volta, Upper East, and Northern provinces of Ghana. The whole sampling and testing was organized and conducted by a veterinary school in Kumasi, Ashanti Region of Ghana. Among sampled sheep (n = 102), goats (n = 66), and cattle (n = 1495), the seroprevalence rates were 25.8 %, 43.1 % and 55.8 %. For cattle, seroprevalence was significantly higher on larger farms (82.2 % vs 17.8 %, comparing farms with >50 or <50 animals; p = 0.027). Highest prevalence was seen in the Northern province with dry climate, but no significant trend following the north-south gradient of sampling sites was detected. Our study identifies a considerable seroprevalence for BCoV in Ghana and provides further support for the spillover of BCoV to small ruminants in settings with mixed husbandry and limited separation between species.
1. Introduction
Cattle, goats and sheep are among the major livestock species in Ghana. The present numbers in 2017 based on the Food and Agriculture Organization (FAO) animal production database are estimated to range around 1.76, 6.4, and 4.6 million cattle, goats and sheep in the country, respectively. Among livestock, only chicken outnumber these species (74 million). While disease surveillance is in place, there are knowledge gaps concerning the laboratory-based prevalence of some major livestock diseases. Among these is Bovine Coronavirus (BCoV) that affects cattle and other livestock species including horses and camels.
BCoV is an enveloped plus strand RNA virus that belongs to the genus Betacoronavirus (Yang and Leibowitz, 2015; Oma et al., 2016; Pfefferle et al., 2009). While different strains may have some antigenic variability, all strains elicit cross-reactive seropositivity and thus form a single serotype (Clark, 1993; El-Ghorr et al., 1989). The virus is an important livestock pathogen causing effects on animal welfare as well as the economy (Lathrop et al., 2000a). It causes diarrhea and respiratory disease in calves, as well as winter dysentery in adult cattle (Boileau and Kapil, 2010; Ksiazek et al., 2003). Transmission of BCoV is mainly through respiratory or fecal-oral routes (Clark, 1993), infecting the respiratory (nasal, tracheal, and lung) and intestinal (villi and crypts of the ileum and colon) epithelial cells (Park et al., 2007). When infected with BCoV, within-herd transmission is generally rapid and infected animals display diverse clinical signs including diarrhea with or without blood, fever, and respiratory signs, which range from none to severe (Clark, 1993; Boileau and Kapil, 2010).
In many African countries including Ghana, livestock species live in close contact and animals serve diverse purposes such as transportation, draught power, fuel, clothing and as a source of meat and milk. Husbandry practices do not involve the same standards of species separation and hygiene as in other parts of the world. Close and sustained interaction between different animals as well as between animals and humans pose a risk of interspecies spillover of pathogens.
BCoV is characteristically a cattle virus. However, reports indicate BCoV infections also occur in small ruminants. Previous studies in Australia (Pass et al., 1982), New Zealand (Durham et al., 1979), Chile (Reinhardt et al., 1995), and Scotland (Snodgrass et al., 1980) reported BCoV infection in small ruminants. Eisa and Mohamed, 2014 also detected BCoV antigens in goats (Eisa and Mohamed, 2004) whereas Tråvén et al., detected BCoV antibodies in sheep (Tråvén et al., 1999). Recently, Gumusova et al., have also detected BCoV antibodies in goats (Gumusova et al., 2007).
Studies regarding the prevalence of BCoV and its associated risk factors are however limited in Africa, and none have been conducted in Ghana. This study evaluated the sero-prevalence of BCoV infection and assessed its associated risk factors among cattle, sheep, and goats in Ghana.
2. Materials and methods
2.1. Study design and area
This study employed a cross-sectional design and was conducted between January 2015 to December 2018 in five districts in four regions of Ghana. Ghana is located in the west of Africa, sharing borders with Togo to the east, Cote d'Ivoire to the west, Burkina Faso to the north and the Gulf of Guinea, to the south and lies on latitude 7.9528 and longitude -1.0307. Ghana has a tropical climate with an average annual temperature of about 26 °C and the annual rainfall of 736.6 mm/29″. Agriculture dominates the economy of Ghana and extensive farming practices in the country increase the livestock-wildlife-human interface
2.2. Ethics approval
This study was approved by the Wildlife division of the Ghana Forestry Commission (Approval number: AO4957).
2.3. Study population, sampling strategy and data collection
A total of 1498 animals aged ≥ 6 months, comprising 1328 cattle, 104 sheep and 66 goats were included in the study. Animals aged < 6 months were excluded due to the possibility of detecting maternal antibodies.
Sampling was done using a simple two-stage cluster sampling technique. The Regional Veterinary Officers of the Ministry of Food and Agriculture (MOFA), Ghana in the selected regions were contacted for information on animal populations in their respective regions prior to the study. The list provided served as the sampling frame. Prior to the study, a survey was carried out and an inclusion criteria of animal population (cattle, sheep and goats) ≥1000 for districts to be eligible for selection for the study was upheld. As a result, five (5) districts that fulfilled the criteria were randomly selected. Secondly, farms within these districts with herd size ≥100 animals were randomly selected. All cattle, sheep, and goats within the selected farms were included in the study. If a district meets the first criteria, but the individual farms fail to meet the second criteria, farms which were very close to each other were pooled together.
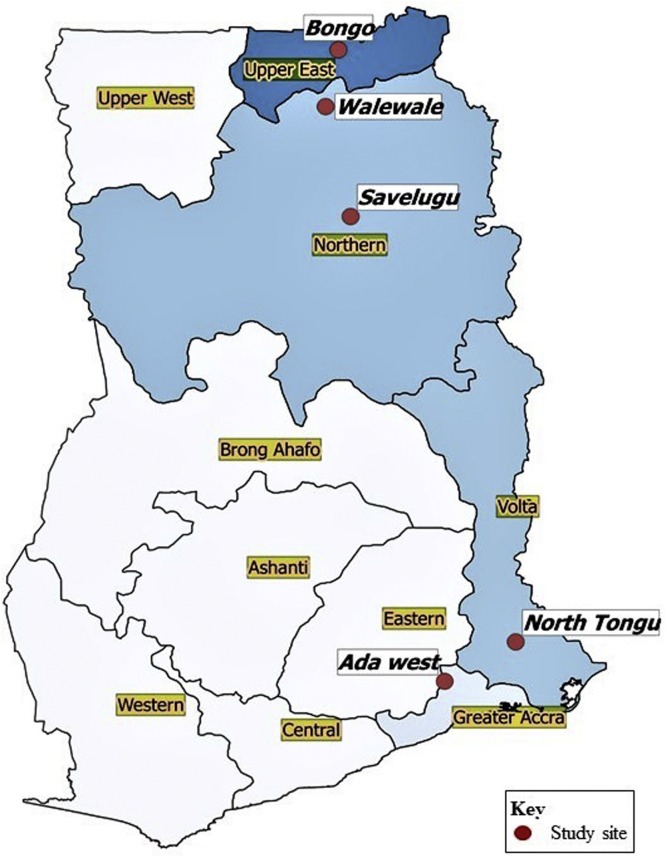
Sites included were: Bongo district in the Upper East; Savelugu and Wale wale in Northern; Ada West in Greater Accra, and North Tongu in Volta. The map with the locations for sampling in shown in Fig. 1 .
Fig. 1.
The map showing the locations for sampling.
A validated questionnaire was used to obtain data on possible risk factors of BCoV. Data collected include: age, sex, dietary changes, parturition, and lactation status of female animals. Additionally, the body score, presence of ectoparasites, and signs of infections such as fever, diarrhoea, respiratory distress, neurological disorder, and icterus were also assessed.
2.4. Sample collection, processing, and analysis
Ten milliliters (10 ml) of blood was collected through jugular puncture from each animal after disinfection of the site with 70 % using vacutainer tubes (Becton Dickinson, NJ, USA) with needles (18 gauge). In the field, the blood was allowed to clot before transportation to the Veterinary laboratory in the district. At the district laboratory, the samples were spun for 10 min at 1500 rpm to obtain the sera. The sera were transferred into three separate aliquots in cryotubes for each animal. The tubes were subsequently placed in liquid nitrogen to minimize antibody degradation. These processes were undertaken under sterile conditions. Upon obtaining representative samples per district, the frozen samples were transported in a cold-chain to the Kumasi Centre for Collaborative Research (KCCR) for long term storage at −70 °C prior to laboratory analysis. Sample collection and preparation in each district and transportation to the KCCR lab took an average of 5–7 days.
During laboratory analysis, frozen sera were thawed at room temperature, vortexed and aliquots of 100 μl of each sample were prepared. The aliquots were incubated at 56 °C for 30 min in warm water prior to recombinant immunofluorescence assay (IFA) as previously described (Hoye et al., 2010; El-Duah et al., 2019; Reusken et al., 2013a). Briefly, Vero B4 cells were co-transfected with pCG1 plasmids bearing Human coronavirus OC43 spike proteins. After overnight transfection, cells were harvested by treatment with trypsin to detach them in a cell culture incubator at 37 °C and re-suspended in Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen, USA) in 10 % Foetal Calf Serum (FCS). Aliquots of cells were pelleted at 300 x g for 5 min and washed twice with 1 ml phosphate buffered saline (PBS). Fifty microliters (50 μl) of cell pellets were spotted on 12 well slides by dispensing and immediately aspirating, allowing 2 s interval between spotting. The cells were fixed using ice cold acetone/methanol (1:1), dried at ambient temperature, and kept at 4 °C after drying for 20 min. To conduct the assay, 45 μl of protein-free blocking solution (Roti®-Block, Carl Roth, Karlsruhe, Germany) was first added to each of the 12 spotted fields on the slide and incubated at room temperature in a humid box for 30 min, followed by rinsing with 1X tween-free PBS. After inactivation at 56 °C for 30 min, sera to be tested were diluted 1:100 in a 1X concentration of the protein-free blocking solution. Subsequently, 30 μl of the diluted sera was applied on each of the spotted area and incubated at 37 °C for 1 h in a humid chamber, followed by rinsing with 0.1 % tween in 1X PBS. Secondary antibody detection was done by the Alexa488 fluorescent reporter-conjugated goat anti-bovine, donkey anti-sheep, and donkey anti-goat IgG antibodies for cattle, sheep, and goat BCoV IgG respectively.
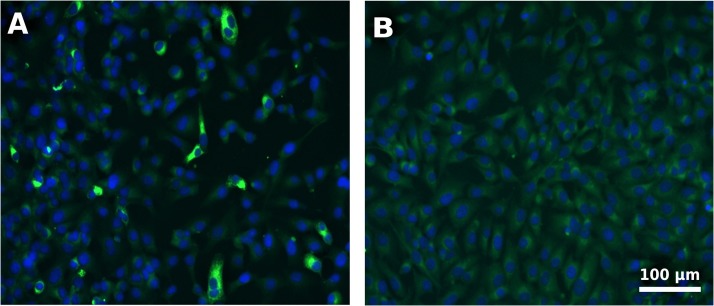
Test evaluation was done by microscopic examination under a fluorescent microscope and a positive outcome was determined by bright green cytoplasmic fluorescence as shown in Fig. 2 .
Fig. 2.
Depiction of typical BCoV IgG positive outcome (Panel A) against a negative outcome (Panel B). Cell nuclei were stained with DAPI and are shown as dark blue and the bright green impressions around the nuclei represent fluorescent antibody-antigen complexes (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
2.5. Statistical analysis
Data were presented as frequencies (percentages) and Chi square test was used to test for association where applicable. Univariate and multivariate logistic regression analysis were performed to determine the possible factors associated with BCoV sero-positivity for cattle, sheep, and goats. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM Statistical Package for the Social Sciences (SPSS) software 25 (SPSS Inc., Chicago, IL, USA), and GraphPad Prism 7 version 7.04 (GraphPad Software, Inc., La Jolla, California USA).
3. Results
The proportions of sheep, goat, and cattle were 4.4 %, 6.8 %, and 88.8 % respectively. There were more adults than weaners (74.9 % vs 25.1 %) and more female animals (71.2 %) compared to male animals (28.8 %), with 22.5 % of the females being lactating females at the time of the study. Majority of the animals had high rectal temperature (95.6 %) and 4.4 % had physical signs of disease of which 2.7 % was diarrhea, 1.7 % was respiratory distress, and 0.1 % of the animals were icteric. None of the animals had neurological disorders. Additionally, a higher proportion of the animals had ectoparasites (93.9 %), were thin (76.5 %) and have not had any dietary change (98.9 %) (Table 1 ).
Table 1.
Baseline characteristics of the entire animal population.
| Variables | Total (n = 1495) | Sheep (n = 66) | Goat (n = 102) | Cattle (n = 1327) |
|---|---|---|---|---|
| Type of animals | ||||
| Sheep | 66 (4.4) | – | – | – |
| Goat | 102 (6.8) | – | – | – |
| Cattle | 1327 (88.8) | – | – | – |
| Age of animals | ||||
| Weaner | 375 (25.1) | 7(10.6) | 15(14.7) | 353(26.6) |
| Adult | 1120 (74.9) | 59(89.4) | 87(85.3) | 974(73.4) |
| Sex of animals | ||||
| Male | 430 (28.8) | 4(6.1) | 13(12.7) | 413(31.1) |
| Female | 1065 (71.2) | 62(93.9) | 89(87.3) | 914(68.9) |
| Lactating females | 336 (22.5) | 1(1.5) | 8(7.8) | 327(24.6) |
| Rectal temperature | ||||
| Normal | 204 (13.6) | 16(24.2) | 32(31.4) | 204(13.6) |
| High | 1291 (86.4) | 50(75.8) | 70(68.6) | 1291(86.4) |
| Signs of disease | ||||
| No | 1430 (95.6) | 61(92.4) | 92(90.2) | 1276(96.2) |
| Yes | 66 (4.4) | 5(7.6) | 10(9.8) | 51(3.8) |
| Diarrhea | 41 (2.7) | 0(0.0) | 7(6.9) | 34(2.7) |
| Respiratory distress | 25 (1.7) | 4(6.1) | 3(2.9) | 18(1.4) |
| Icterus | 1 (0.1) | 1(1.5) | 0(0.0) | 0(0.0) |
| Neurological disorder | 0 (0.0) | 0(0.0) | 0(0.0) | 0(0.0) |
| Presence of ectoparasites | ||||
| No | 91 (6.1) | 10(15.2) | 10(9.8) | 71(5.4) |
| Yes | 1404 (93.9) | 56(84.8) | 92(90.2) | 1256(94.6) |
| Body scoring | ||||
| Emaciated | 15 (1.0) | 1(1.5) | 0(0.0) | 14(1.1) |
| Thin | 1144 (76.5) | 65(98.5) | 93(91.2) | 986(74.3) |
| Normal | 330 (22.1) | 0(0.0) | 9(8.8) | 321(24.2) |
| Moderately fat | 6 (0.4) | 0(0.0) | 0(0.0) | 6(0.5) |
| Dietary changes | ||||
| No | 1479 (98.9) | 65(98.5) | 98(96.1) | 1316(98.9) |
| Yes | 16 (1.1) | 1(1.5) | 4(3.9) | 16(1.1) |
Normal temperature for cattle: 37.8–39.5 °C; Normal temperature for sheep and goats: 38.5–40.5 °C.
For cattle, sheep and goats: Age of weaner: 6 months to 1 year; Age of adult: >1 year.
The sero-prevalence of BCoV in the entire animal population was 53.6 %. Upon stratification by sheep, goats, and cattle, the prevalence was 25.8 %, 43.1 % and 55.8 %, respectively. Cattles had significantly higher prevalence of BCoV compared to sheep and goats. Among the entire animal population, sero-positivity of BCoV was significantly associated with farms with ≥ 50 animals (75.9 % vs 24.1 %, p < 0.0001). Upon stratification by type of animal, this effect seemed to be explained by cattle (82.2 % vs 17.8 %, p = 0.027) but not sheep and goats that are normally kept in smaller groups (Table 2 ). The sero-prevalence of BCoV was highest in the Northern region followed by the Volta region (Table 3 ). Even though our sampling sites formed a north-south gradient, there was no latitude-dependent trend in seroprevalence.
Table 2.
Sero-prevalence of BCoV and its association with farm density.
| Variables | Sero-prevalence | Farm with < 50 animals | Farm with ≥ 50 animals | p-value |
|---|---|---|---|---|
| Total | <0.0001 | |||
| Positive | 801(53.6) | 193(24.1) | 608(75.9) | |
| Negative | 694(46.4) | 241(34.7) | 453(65.3) | |
| Sheep | NA | |||
| Positive | 17(25.8) | 17(100.0) | 0(0.0) | |
| Negative | 49(74.2) | 49(100.0) | 0(0.0) | |
| Goat | NA | |||
| Positive | 44(43.1) | 44(100.0) | 0(0.0) | |
| Negative | 58(56.9) | 58(100.0) | 0(0.0) | |
| Cattle | 0.027 | |||
| Positive | 740(55.8) | 132(17.8) | 608(82.2) | |
| Negative | 587(44.2) | 134(22.8) | 453(77.2) | |
Table 3.
The sero-prevalence of BCoV among the entire animal population stratified by regions.
| Region | Negative | Positive | p-value |
|---|---|---|---|
| Upper East | 191(54.7) | 158(45.3) | 0.002 |
| Greater Accra | 142(47.3) | 158(52.7) | |
| Volta | 198(43.3) | 259(56.7) | |
| Northern | 163(41.9) | 226(58.1) |
There was no statistically significant association between the possible risks factors assessed and BCoV sero-positivity among all animals with the exception of dietary change, where a significantly lower odd of BCoV was observed among cattle with recent dietary change [OR = 0.08, 95 % CI (0.01-0.61), p = 0.015] (Table 4 ). Multivariate logistic regression identified both effect to be independent (farm size [OR = 1.39, 95 % CI (1.04–1.87), p = 0.025]; dietary change [OR = 0.07, 95 % CI (0.01-0.58), p = 0.013]) in cattle (Table 5 ).
Table 4.
Possible risk factors for BCoV sero-positivity.
| Variables | Sheep | Goat | Cattle | |||
|---|---|---|---|---|---|---|
| Age of animals | OR(95 % CI) | p-value | OR(95 % CI) | p-value | OR(95 % CI) | p-value |
| Weaner | 1 | 1 | 1 | |||
| Adult | 2.23(0.25–20.02) | 0.474 | 0.32(0.10–1.02) | 0.054 | 1.18(0.93–1.51) | 0.175 |
| Sex of animals | ||||||
| Male | 1 | 1 | 1 | |||
| Female | 0.32(0.04–2.47) | 0.274 | 1.25(0.38–4.12) | 0.716 | 1.16(0.92–1.46) | 0.219 |
| Lactating females | 9.00(0.35–231.83) | 0.185 | 2.35(0.53–10.42) | 0.261 | 1.25(0.97–1.62) | 0.080 |
| Rectal temperature | ||||||
| Normal | 1 | 1 | 1 | |||
| High | 0.47(0.14–1.58) | 0.223 | 0.55(0.24–1.29) | 0.171 | 0.91(0.65–1.28) | 0.606 |
| Signs of disease | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 0.70(0.07–6.77) | 0.761 | 0.30(0.06–1.48) | 0.138 | 1.14(0.65–2.01) | 0.654 |
| Diarrhea | – | NA | 0.20(0.02–1.74) | 0.145 | 1.14(0.57–2.27) | 0.716 |
| Respiratory distress | 0.96(0.09–9.89) | 0.972 | 0.65(0.06–7.42) | 0.730 | 0.99(0.39–2.53) | 0.986 |
| Icterus | 0.92(0.04–23.75) | 0.962 | – | NA | – | NA |
| Neurological disorder | – | NA | – | NA | – | NA |
| Presence of ectoparasites | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 0.27(0.07–1.10) | 0.068 | 1.15(0.31–4.37) | 0.833 | 0.86(0.53–1.40) | 0.555 |
| Body scoring | ||||||
| Normal | – | NA | 1 | 1 | ||
| Emaciated | 1 | – | 1.06(0.36–3.12) | 0.919 | ||
| Thin | 0.11(0.004–2.86) | 0.185 | 0.94(0.24–3.74) | 0.934 | 0.99(0.77–1.28) | 0.098 |
| Moderately fat | – | NA | – | 1.59(0.29–8.79) | 0.597 | |
| Dietary changes | ||||||
| No | 1 | 1 | 1 | |||
| Yes | 9.00(0.35–231.83) | 0.185 | 0.14(0.01–2.60) | 0.185 | 0.08(0.01–0.61) | 0.015 |
Table 5.
Multivariate analysis of potential risk factors of BCoV sero-positivity in cattle.
| Variables | aOR (95 % CI) | p-value |
|---|---|---|
| Farm density | ||
| <50 animals | 1 | |
| ≥ 50 animals | 1.39(1.04–1.87) | 0.025 |
| Body scoring | ||
| Normal | 1 | |
| Emaciated | 1.01(0.34–3.00) | 0.983 |
| Thin | 0.91(0.70–1.20) | 0.509 |
| Moderately fat | 1.78(0.32–9.97) | 0.513 |
| Dietary changes | ||
| No | 1 | |
| Yes | 0.07(0.01–0.58) | 0.013 |
| Lactating females | 1.27(0.98–1.65) | 0.067 |
4. Discussion
BCoV is a ubiquitous infection and BCoV-specific antibodies have been detected in cattle populations in numerous countries (Hasoksuz et al., 2002; Kapil et al., 1990; Lathrop et al., 2000b; Yavru et al., 2016). BCoV shares recent common ancestry with human coronavirus OC43 (HCoV-OC43) (Vijgen et al., 2006) and the two are serologically closely related to the extent that HCoV-OC43 is often used as a proxy in serological testing as previously described where specific proteins of BCoV were not available for serological testing or when National legislation restricts the use of certain livestock pathogens (Reusken et al., 2013b; Meyer et al., 2014). In most African countries such as Ghana, diverse farm animals live in close contact that poses a risk of cross-species infection. Indeed, we found seropositivity against BCoV not only among cattle (55.8 %) but also among sheep and goats at 25.8 % and 43.1 % prevalence rates, respectively. This prevalence was predominant among animals from the Volta region of Ghana. Cattle presented with significantly higher prevalence of BCoV compared to sheep and goats.
Varying prevalence rates of BCoV have been reported in different countries. A study by Alkan et al. reported prevalence ranging from 4.4 to 100.0% among cattle in Turkey (Alkan et al., 2003). Another study by Gumusova et al. in northern Turkey reported a sero-prevalence of 98.43 % in cattle. Yavru et al. (2016) and O'Connor et al. (O’Connor et al., 2001) reported a sero-prevalence of 94 % among 184 calves and their mothers in Burdur, Turkey and 90 % among 852 animals from 3 Ontario feedlots, respectively based on enzyme linked immunosorbent assay (ELISA) method.
Bidokhti et al. (2009); Hasoksuz et al. (2005), and Yildirim et al. (2008) also reported a sero-prevalence of 82–86 %, 54.5 %, and 26.3 %, respectively among cattle. The discrepancies in the prevalence rates compared to that of this present study could be attributed, at least in part, to differences in geographical location, different management systems, source population size, method employed for BCoV antibody detection, and the samples size used in the different investigations. In addition, the higher prevalence rate among cattle could be due to the fact that a higher proportion of the animals were cattle as well as the tropism of BCoV to cattle
Few studies have been conducted to evaluate the prevalence of BCoV among small ruminants. The most recent study was conducted by Gumusova et al. in 2007 in Turkey. In their study, they evaluated the sero-prevalence of BCoV in goats by employing commercially available competitive ELISA kits and reported a BCoV sero-prevalence of 41.1 % (Gumusova et al., 2007). In a previous study, Eisa and Mohamed reported detection of BCoV antigens in goats (Eisa and Mohamed, 2004). Additionally, Tråvén et al., in a study among 218 sheep from 40 flocks in different parts of Sweden, reported that 19 % of the sheep were positive for BCoV antibodies (Tråvén et al., 1999). Prior to these recent studies, there had been reports of BCoV infection in small ruminants in Australia (Pass et al., 1982), New Zealand (Durham et al., 1979), Chile (Reinhardt et al., 1995), and Scotland (Snodgrass et al., 1980). Our finding in sheep and goats, thus, provides update information of spillover of BCoV from cattle.
Granted the contagious nature of BCoV, it is imperative that factors that influence exposure and the determinants of BCoV infection be identified which would assist in the development of apt control and preventive measures against BCoV and other infectious diseases.
Though there was no statistically significant association between the possible risks factors assessed and BCoV sero-positivity, we found BCoV sero-positivity to be significantly associated with farms with higher cattle density. This finding is in harmony with studies by Beaudeau et al. (2010); Hägglund et al. (2006), and Ohlson et al. (2010) who reported that large herd size is a risk factor for BCoV infections in dairy cattle. This may be due to poor biosecurity especially among farms with larger herd size in Ghana, and also due to the close contact between animals in these farms which could potentiate the transmission of BCoV compared to farms with small herd size (Beaudeau et al., 2010; Ohlson et al., 2010).
Infectious diseases surveillance can be greatly enhanced by research studies, as often there is close collaboration between governmental and academic institutions. Restrictions in the movement of samples to prevent the spread of notifiable livestock diseases create a demand for domestic laboratory capacities. Through this study we hope to demonstrate the value of capacity building in field-based research.
Acknowledgement
This work was supported by Deutsche Forschungsgemeinschaft under a Grant to Y. A. S. and C. D. (DR 772/12-1).
References
- Alkan F., Bilge-Dağalp S., Can Şahna K., Özgünlük İ. Sığırlarda coronavirus enfeksiyonunun epidemiyolojisi. Ankara Üniv Vet Fak Derg. 2003;50:59–64. [Google Scholar]
- Beaudeau F., Björkman C., Alenius S., Frössling J. Spatial patterns of bovine corona virus and bovine respiratory syncytial virus in the Swedish beef cattle population. Acta Vet. Scand. 2010;52(1):33. doi: 10.1186/1751-0147-52-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidokhti M.R., Tråvén M., Fall N., Emanuelson U., Alenius S. Reduced likelihood of bovine coronavirus and bovine respiratory syncytial virus infection on organic compared to conventional dairy farms. Vet. J. 2009;182(3):436–440. doi: 10.1016/j.tvjl.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau M.J., Kapil S. Bovine coronavirus associated syndromes. Veterinary Clinics: Food Animal Practice. 2010;26(1):123–146. doi: 10.1016/j.cvfa.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. Bovine coronavirus. Br. Vet. J. 1993;149(1):51–70. doi: 10.1016/S0007-1935(05)80210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham P., Stevenson B., Farquharson B. Rotavirus and coronavirus associated diarrhoea in domestic animals. N. Z. Vet. J. 1979;27:30–32. doi: 10.1080/00480169.1979.34595. [DOI] [PubMed] [Google Scholar]
- Eisa M., Mohamed A. Role of enteric pathogens in enteritis in lambs, goat kids and children and their zoonotic importance. Vet Med J Giza. 2004;52:41–59. [Google Scholar]
- El-Duah P., Sylverken A., Owusu M., Yeboah R., Lamptey J., Frimpong Y.O. Potential intermediate hosts for coronavirus transmission: No evidence of clade 2c coronaviruses in domestic livestock from Ghana. Trop. Med. Infect. Dis. 2019;4(1):34. doi: 10.3390/tropicalmed4010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghorr A., Snodgrass D., Scott F., Campbell I. A serological comparison of bovine coronavirus strains. Arch. Virol. 1989;104(3–4):241–248. doi: 10.1007/BF01315546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumusova O., Yazici Z., Albayrak H., Çakiroglu D. First report of bovine rotavirus and bovine coronavirus seroprevalance in goats in Turkey. Vet glasnik. 2007;61:75–79. [Google Scholar]
- Hägglund S., Svensson C., Emanuelson U., Valarcher J., Alenius S. Dynamics of virus infections involved in the bovine respiratory disease complex in Swedish dairy herds. Vet. J. 2006;172(2):320–328. doi: 10.1016/j.tvjl.2005.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasoksuz M., Hoet A.E., Loerch S.C., Wittum T.E., Nielsen P.R., Saif L.J. Detection of respiratory and enteric shedding of bovine coronaviruses in cattle in an Ohio feedlot. J. Vet. Diagn. Investig. 2002;14(4):308–313. doi: 10.1177/104063870201400406. [DOI] [PubMed] [Google Scholar]
- Hasoksuz M., Kayar A., Dodurka T., Ilgaz A. Detection of respiratory and enteric shedding of bovine coronaviruses in cattle in Northwestern Turkey. Acta Vet. Hung. 2005;53(1):137–146. doi: 10.1556/AVet.53.2005.1.13. [DOI] [PubMed] [Google Scholar]
- Hoye B.J., Munster V.J., Nishiura H., Klaassen M., Fouchier R.A. Surveillance of wild birds for avian influenza virus. Emerg Infect Dis. 2010;16(12):1827. doi: 10.3201/eid1612.100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapil S., Trent A.M., Goyal S.M. Excretion and persistence of bovine coronavirus in neonatal calves. Arch. Virol. 1990;115(1–2):127–132. doi: 10.1007/BF01310629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Lathrop S.L., Wittum T.E., Brock K.V., Loerch S.C., Perino L.J., Bingham H.R. Association between infection of the respiratory tract attributable to bovine coronavirus and health and growth performance of cattle in feedlots. Am. J. Vet. Res. 2000;61(9):1062–1066. doi: 10.2460/ajvr.2000.61.1062. [DOI] [PubMed] [Google Scholar]
- Lathrop S.L., Wittum T.E., Loerch S.C., Perino L.J., Saif L.J. Antibody titers against bovine coronavirus and shedding of the virus via the respiratory tract in feedlot cattle. Am. J. Vet. Res. 2000;61(9):1057–1061. doi: 10.2460/ajvr.2000.61.1057. [DOI] [PubMed] [Google Scholar]
- Meyer B., Müller M.A., Corman V.M., Reusken C.B., Ritz D., Godeke G.J. Antibodies against MERS coronavirus in dromedaries, United Arab Emirates, 2003 and 2013. Emerg Infect Dis. 2014;20(4):552–559. doi: 10.3201/eid2004.131746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor A., Martin S.W., Nagy E., Menzies P., Harland R. The relationship between the occurrence of undifferentiated bovine respiratory disease and titer changes to bovine coronavirus and bovine viral diarrhea virus in 3 Ontario feedlots. Can. J. Vet. Res. 2001;65(3):137. [PMC free article] [PubMed] [Google Scholar]
- Ohlson A., Heuer C., Lockhart C., Tråvén M., Emanuelson U., Alenius S. Risk factors for seropositivity to bovine coronavirus and bovine respiratory syncytial virus in dairy herds. Vet. Rec. 2010;167(6):201–207. doi: 10.1136/vr.c4119. [DOI] [PubMed] [Google Scholar]
- Oma V.S., Tråvén M., Alenius S., Myrmel M., Stokstad M. Bovine coronavirus in naturally and experimentally exposed calves; viral shedding and the potential for transmission. Virol. J. 2016;13(1):100. doi: 10.1186/s12985-016-0555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Kim G., Choy H., Hong Y., Saif L., Jeong J. Dual enteric and respiratory tropisms of winter dysentery bovine coronavirus in calves. Arch. Virol. 2007;152(10):1885–1900. doi: 10.1007/s00705-007-1005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass D., Penhale W., Wilcox G., Batey R. Intestinal coronavirus-like particles in sheep with diarrhoea. Vet. Rec. 1982;111:106–107. doi: 10.1136/vr.111.5.106. [DOI] [PubMed] [Google Scholar]
- Pfefferle S., Oppong S., Drexler J.F., Gloza-Rausch F., Ipsen A., Seebens A. Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229E in bats, Ghana. Emerging Infect. Dis. 2009;15(9):1377. doi: 10.3201/eid1509.090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt G., Zamora J., Tadich N., Polette M., Aguilar M., Riedermann S. Diagnosis of coronavirus in sheep in Valdidia province, X region, Chile. Arch. Med. Vet. 1995;27:129–132. [Google Scholar]
- Reusken C., Ababneh M., Raj V., Meyer B., Eljarah A., Abutarbush S. 2013. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Serology in Major Livestock Species in an Affected Region in Jordan, June to September 2013. [DOI] [PubMed] [Google Scholar]
- Reusken C.B., Haagmans B.L., Müller M.A., Gutierrez C., Godeke G.-J., Meyer B. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect. Dis. 2013;13(10):859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D., Herring J., Reid H., Scott F., Gray E. Virus infections in cattle and sheep in Scotland 1975–1978. Vet. Rec. 1980;106:193–195. doi: 10.1136/vr.106.9.193. [DOI] [PubMed] [Google Scholar]
- Tråvén M., Carlsson U., Lundén A., Larsson B. Serum antibodies to bovine coronavirus in Swedish sheep. Acta Vet. Scand. 1999;40:69–74. doi: 10.1186/BF03547042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgen L., Keyaerts E., Lemey P., Maes P., Van Reeth K., Nauwynck H. Evolutionary history of the closely related group 2 coronaviruses: porcine hemagglutinating encephalomyelitis virus, bovine coronavirus, and human coronavirus OC43. J. Virol. 2006;80(14):7270–7274. doi: 10.1128/JVI.02675-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Leibowitz J.L. The structure and functions of coronavirus genomic 3′ and 5′ ends. Virus Res. 2015;206:120–133. doi: 10.1016/j.virusres.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavru S., Yapici O., Kale M., Sahinduran S., Pehlivanoglu F., Albay M.K. Bovine coronavirus (BoCV) infection in calves with diarrhoea and their dams. Acta Sci. Vet. 2016;44:1–7. [Google Scholar]
- Yildirim Y., Dagalp S.B., Tan M.T., Kalaycioglu A.T. Seroprevalence of the rotavirus and corona virus infections in cattle. J. Anim. Vet. Adv. 2008;7(10):1320–1323. [Google Scholar]