Abstract
Crude theaflavin was extracted from black tea and then fractionated by HPLC into five components (initial peaks (IP), TF1, TF2A, TF2B, and TF3). The crude extract and the various fractions of theaflavin were collected and tested, individually and in combination, for antirotaviral activity. The mean effective concentration (EC50) was calculated and compared. Activity varied from the most active being the uncharacterized theaflavin-like initial peaks (IP) with an EC50 of 0.125 μg/ml to the least active being theaflavin-3 monogallate (TF2A) with an EC50 of 251.39 μg/ml. The combination of TF1+TF2A+TF2B+TF3 was more active than the sum of the activities of these four fractions individually, indicating synergism among the peaks. Only the crude extract was assayed for activity against coronavirus; the EC50 was 34.7 μg/ml.
Keywords: Tea, Theaflavin, Theaflavin gallate, Neutralization, Rotavirus, Coronavirus
1. Introduction
Gastroenteritis due to rotavirus and coronavirus infections has a significant economical impact on agriculture. Yet, despite numerous trials and decades of research on these two viruses, effective vaccines are still not available. Although research efforts have appeared promising, vaccine field trials continue to produce inadequate results or fail entirely (de Leeuw et al., 1980; Myers and Snodgrass, 1982; Snodgrass et al., 1982; Waltner-Toews et al., 1985). Following infection the only treatment available is supportive therapy such as fluid replacement. Thus, viral gastroenteritis is still largely uncontrolled and alternative methods to control infection appear necessary. One of the goals of our laboratory has been to investigate a broad spectrum antiviral agents that is not discriminatory among various serotypes and strains of viruses. Provided that these agents could be used as food additives, they would have a distinct advantage over vaccines in prevention of infection and/or disease due to their broad spectrum of activity and because most agents that cause gastroenteritis are contracted orally, usually via contaminated food and water.
The broad spectrum antiviral agents previously investigated in this laboratory included phyllosilicate clays and charcoal as viral adsorbents. Extensive research on these adsorbent materials indicates that they have high affinity adsorbing properties, although the adsorbed virus retains some infectivity in vitro. Therefore, although the use of phyllosilicate clays and charcoal are an ideal method of concentrating virus particles, they do not appear to be an effective method of eliminating the infectivity of rotavirus or coronavirus as judged from in vitro studies (Clark et al., 1998).
To pursue further the idea of using broad spectrum methods to inhibit infection, we looked for a natural substance that could neutralize the infectivity of both bovine rotavirus and bovine coronavirus. The intent was to find a substance that is active either alone or in conjunction with a phyllosilicate clay to reduce or eliminate viral infectivity. Among the chemicals considered for testing were phenol, formalin, theaflavin and theaflavin gallate derivatives. Theaflavins are extracted from black tea and were selected because they are derived from a natural source.
The oral administration of tea is reported to be used as a therapy for enteric diseases; a Japanese folk legend discusses the medicinal value of tea for curing gastroenteritis in children (Mukoyama et al., 1991). Theaflavin is thought to be responsible for the medicinal value of black tea and can be fractionated into four parts: theaflavin (TF1), theaflavin-3-monogallate (TF2A), theaflavin-3′-monogallate (TF2B), and theaflavin-3,3′ digallate (TF3) (Hara et al., 1987). TF3 has been found to have antiviral activity against influenza A and B (Green, 1949; Nakayama et al., 1990, Nakayama et al., 1993), poliovirus 1 (strain Sabin 1) coxsackie virus B3 (strain Nancy), and human rotavirus group A strains of serotypes 1–4, and 8 (Mukoyama et al., 1991).
In our studies, the crude extract of theaflavin and each of the purified derivatives were studied (individually and in combination) for their antiviral effect against rotavirus and coronavirus.
2. Materials and methods
2.1. Extraction procedure
Theaflavin and theaflavin gallate derivatives were extracted from three sources of black tea, one sample each from the United States, India, and China. All solvents and reagent materials used were of the highest purity commercially available. Theaflavin, theaflavin monogallates, and theaflavin digallate were extracted from tea following a modified version of the method described by Roberts and Myers (1959). Tea (100 g) was added to 2.5l of water, boiled for 5 min and filtered. The filtrate was cooled below 30°C and then 20 ml of concentrated sulfuric acid was added. A precipitate formed and the mixture was left in a cool, dark place overnight. The tea was then centrifuged at 500×g for 10 min to separate the precipitate from the aqueous portion. The wet precipitate was resuspended in approximately 200 ml water and extracted four times with 150 ml ethyl acetate (EA), and centrifuged at 500×g for 10 min. EA extracts were combined and washed with 150 ml of 2.5% NaHCO3 and then with 0.1 M sulfuric acid. The extracts were centrifuged each time at 500×g to remove the aqueous layer. The EA extracts were evaporated to dryness under reduced pressure. The dry extract was reconstituted in 10 ml acetone and then precipitated with 80 ml chloroform. The precipitate was collected and centrifuged at 6500×g for 5 min. Light petroleum ether was added and the suspensions were centrifuged at 500×g for 10 min. The precipitate was dried at 37°C and the yield was calculated. Further purification was performed by high performance liquid chromatography (HPLC).
2.2. HPLC
The mobile phase for the HPLC consisted of acetonitrile, ethylacetate, and 0.05% phosphoric acid at the ratio: 21:3:76. All HPLC procedures were conducted using Waters equipment which includes: pumps (model 510), automatic sampler (WISP Model 710), column: μ Bondapak C18 (3.9×300 mm), variable UV wavelength adsorbance detector (model 481, wavelength at 278 nm), surface interface module, Professional 350, and LA50 printer. Tea extracts were reconstituted in the mobile phase at 1.0 mg/ml. The flow rate was set at 1 ml/min. The samples were analyzed for 60 min with expected retention times at approximately 14–16, 22–25, 27–30, and 32–36 min for TF1, TF2A, TF2B, and TF3, respectively (Hara et al., 1987). Fractions were collected according to their retention times and then evaporated to dryness. The precipitate was then dissolved in 1 ml of 95% ethanol to determine the concentrations. Each individual fraction was evaluated by HPLC again to verify that each sample was pure.
2.3. Calculation of the concentration of TF1, TF2A, TF2B, and TF3
The absorbance (A) of each 1 ml sample of individual peaks was obtained by a DU-65 spectrophotometer. The concentration (C) of each peak was calculated using the Lambert–Beer Law. The molecular weight (MW) and Σ are known values (Table 1 ) for each individual peak (Hara et al., 1987). The C (in μg/ml) was calculated by substituting the known values plus A into the equation below.
| Lambert–Beer Law: C=MW×A×1000Σ |
Table 1.
| Peak | MW | Σ |
|---|---|---|
| TF1 | 564.51 | 19.500 |
| TF2A | 716.63 | 29.040 |
| TF2B | 716.53 | 29.040 |
| TF3 | 868.75 | 38.580 |
2.4. Molecular modeling of TF1, TF2A, TF2B, and TF3
The compounds were modeled by drawing the structures in ISIS Draw 2.0 (MDL Information Systems, San Leandro, CA) and then importing it into HyperChem 4.5. HyperChem was used on a Pentium processor-based computer with 32 Mbytes of memory. The structure was energy minimized using the molecular mechanical MM+ method (Anonymous, 1994). The structural information was then imported to ChemPlus conformational search module for the determination of probable conformers (Anonymous, 1993). The conformational search was a random seed process that was allowed to run overnight and resulted in more than 50 iterations for theaflavin, greater than 100 iterations for each of the theaflavin monogallates, and greater than 200 iterations for the digallic ester of theaflavin.
2.5. Cell cultures and viruses
The viruses selected for study were bovine rotavirus, NCDV-Lincoln strain, (Fernelius et al., 1972) and bovine coronavirus, BCV ATCC P2 (Mebus et al., 1973; Sharpee et al., 1976). Culturing and assaying for infectivity was in the BSC-1 cell line for bovine rotavirus and the HRT-18 cell line for bovine coronavirus. These methods have been previously described (Woode et al., 1987; Storz et al., 1991).
2.6. Neutralization of infectivity assays
These assays use 5–7-day-old BSC-1 cells or 5–6-day-old HRT cells cultured in 96-well tissue culture microtiter plates, monoclonal antibody B223 E4 (MAb E4) which is made into an epitope on VP6 of B223 rotavirus (Zheng et al., 1989), gnotobiotic calf #78 (GC78) antisera to bovine coronavirus (Woode, pers. comm.), and fluorescein-labeled affinity-purified antibodies, goat anti-mouse IgG (GAM) and goat anti-bovine IgG (GAB) (Kirkegaard and Perry).
Crude theaflavin was assayed for its ability to inactivate both bovine rotavirus and coronavirus. In addition to the crude extract, the component parts of the extract including the initial peaks and peaks TF1, TF2A, TF2B, and TF3 (individual and combined) were assayed and compared for their ability to neutralize the infectivity of bovine rotavirus. All the original work used theaflavins extracted from the U.S. source of tea. Crude theaflavin extractions were later made from Indian and Chinese black tea and compared to the U.S. source for inactivation activity against bovine rotavirus.
Neutralization of infectivity assays has been previously described for rotavirus (Woode et al., 1987) and for coronavirus (Hussain et al., 1991). Briefly, theaflavin was made into two-fold dilutions in microtiter plates and then an equal volume of virus was added. For bovine rotavirus 200–400 immunofluorescent focus-forming units (IFFU) of virus were used, and for bovine coronavirus, 100 TCID50. The theaflavin plus virus was incubated for 1.5 h before being transfered to the microtiter plates of cultured BSC-1 or HRT cells (100 μl/well). At least three wells per dilution were used for rotavirus samples and at least four wells per dilution for coronavirus samples. BSC-1 cells were used at 6–7 days of age and were washed with SF/MEM prior to use. HRT-18 cells were used at 5–6 days of age and were washed with SF/RPMI prior to use.
Microtiter plate(s) of BSC-1 rotavirus-infected cells were incubated at 37°C in the presence of 5% CO2 for 20 h and HRT-18 coronavirus-infected cells were incubated under the same conditions but for 4 days. Post-incubation, plates were fixed with 80% acetone and dried for approximately 3 h. The plates were rehydrated for at least 10 min in PBS prior to staining. BSC-1 plates were first stained with MAb E4 and then with GAM (1 h each). HRT-18 plates were first stained with GC78 and then GAB (1 h each). Each stain was washed off using PBS. Plates were read by counting IFFU in the case of rotavirus and by counting fluorescent wells in the case of coronavirus for each dilution. The neutralizing activity of theaflavin was expressed as the mean effective concentrations in μg/ml that inhibited 50% of viral infection (EC50). The EC50 was calculated by applying a linear equation using power of regression (Microsoft Excel) for bovine rotavirus and the method of Reed and Muench for bovine coronavirus (Reed and Muench, 1938).
3. Results
3.1. Extraction and HPLC
The crude theaflavin extract was measured to quantitate the yield of each extraction with the Indian source of tea yielding the highest quantity of crude extract. Each of the extracts was then examined by HPLC. The chromatograms of the crude extracts showed the same four peaks: TF1, TF2A, TF2B, and TF3 at the expected retention times of 14–16, 22–25, 27–30, and 32–36 min and in addition, multiple peaks prior to peaks TF1 through TF3 which we refer to as initial peaks (Fig. 1 ). The initial peaks are suspected to be theaflavin-like substances but as of now they remain uncharacterized. The U.S. source of tea was selected for additional studies. After fractionation of each of the peaks TF1 through TF3 of this source, each individual fraction was evaluated by HPLC again to verify that each sample was pure. Proposed chemical structures of TF1, TF2A, TF2B, and TF3 are shown in Fig. 2 (Takino et al., 1965).
Fig. 1.
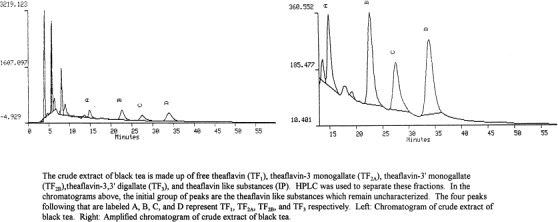
Chromatograms of crude extract of black tea.
Fig. 2.
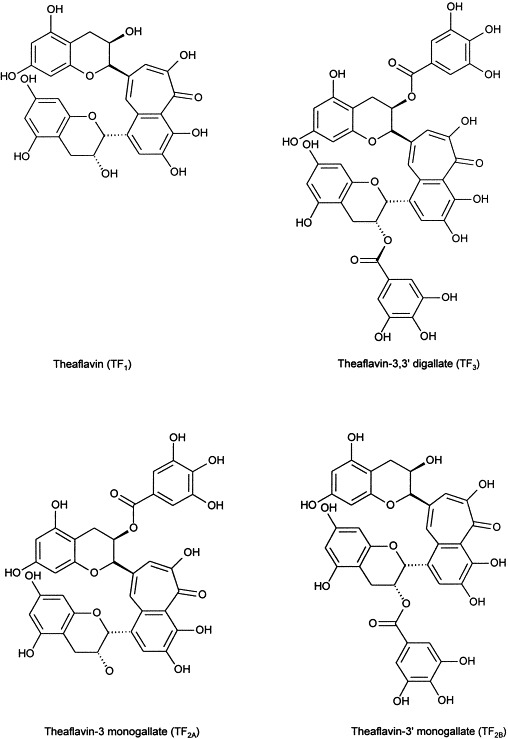
Chemical structures of TF1, TF2A, TF2B, TF3.
3.2. Molecular modeling
The structures were modeled and the possible conformations were examined. It was found in the conformational search of TF1 that the hydroxylated bynzopyranyl (HBP) functional groups were commonly found parallel to one another and perpendicular to the benzocyloheptenone (BCH) ring structure. An additional gallic acid group attached through an ester bond on one of the HBP functional groups formed the TF2A and TF2B compounds (Fig. 3 ). In reviewing the results of the conformational search for TF2A, it was noted that the two HBP groups would form a conformer with their orientation parallel to one another and the gallic ester group would lie parallel to the BCH group (Fig. 3). This was similar to the three-dimensional structure of TF1, except for the placement of the gallic ester group. However, when the conformers of TF2B were reviewed the parallel conformation of the HBP functional groups was found but the placement of gallic ester was not along the BCH ring structure. The lowest energy conformer of the TF3 had both gallic esters parallel over the BCH ring system. The orientation of the gallic esters is interdependent on the orientation of the HBP functional groups which were also found parallel to one another and perpendicular to the main ring system (Fig. 3).
Fig. 3.
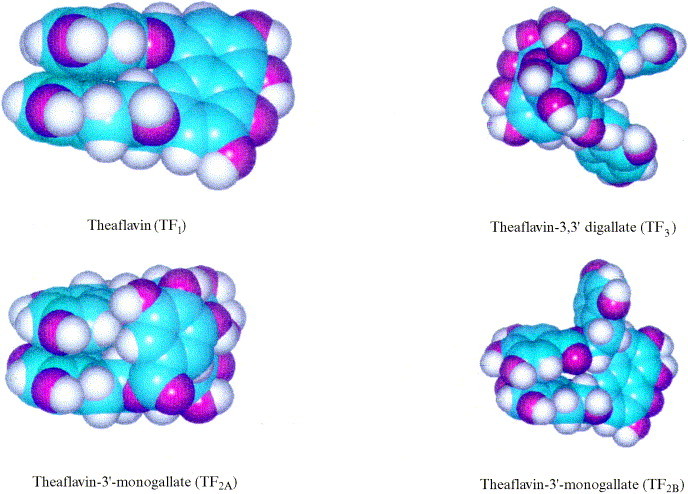
Proposed molecular structures of TF1, TF2A, TF2B, TF3.
3.3. Neutralization assays
All the theaflavin and gallate derivatives were shown to have inactivation activity (in vitro) against bovine rotavirus. By separating the theaflavins by HPLC and testing their activity we found that the most active components were the uncharacterized initial peaks with an EC50 of 0.125 μg/ml. All individual fractions have at least some antiviral activity with TF1 being most active. Our results indicate that there is synergism between the component fractions TF1, TF2A, TF2B, and TF3 which have an activity level that is greater when combined and when any one fraction is tested individually. The order of activity, from highest to lowest, is as follows: uncharacterized initial peaks > crude theaflavin extract >TF1+TF2A+TF2B+TF3>TF1>TF3>TF2B>TF2A (Table 2 ).
Table 2.
Rotavirus neutralizing activity of theaflavin extract (U.S. source)
| Sample tested | EC50 (μg/ml) |
|---|---|
| Crude extract | 0.353 |
| Free theaflavin (TF1) | 0.943 |
| Theaflavin-3 monogallate (TF2A) | 251.39 |
| Theaflavin-3′ monogallate (TF2B) | 5.07 |
| Theaflavin-3,3′ digallate (TF3) | 5.51 |
| Theaflavin like substances/initial peaks | 0.125 |
| TF1+TF2A+TF2B+TF3 | 0.404 |
Limited studies were carried out to assess the activity of the crude theaflavin extract against bovine coronavirus; results of the assays conclude the mean EC50 for coronavirus is 34.7 μg/ml (standard error 9 μg/ml).
4. Discussion
Theaflavins are polyphenolic compounds extracted from black tea; their precursors are found in the unfermented green tea leaf and their concentration varies according to genetics, season, and growing conditions. As expected, we found that the source of tea varies widely in yield of overall crude extract and thus quantity of individual fractions which should be considered when purchasing tea. According to our data, the activity of the crude extract and the theaflavin and theaflavin gallate derivatives is constant in individual samples but there is a large variation among samples as a result of the source of tea used. The results of this study support that theaflavin and theaflavin gallate derivatives have inactivation activity (in vitro) against both rotavirus and coronavirus. Neutralization activity varies among the crude extract, the uncharacterized initial peaks and each of the individual fractions. An explanation of the differences in effectiveness of the theaflavin compounds could be due to conformational differences. The simplest structure was TF1 which also had the greatest effect on infectivity of the individual fractions (Table 2). The theaflavin monogallate compounds are very similar in their structure (the same functional groups, same molecular weights) but have significant differences in their effect on infectivity. It is suggested since the compounds differ only by the placement of the gallic ester group that a conformation could be responsible for the difference in the effects on infectivity. Steric interactions may restrict access to the BCH group in TF2A and thus reduce its effect on neutralizing infectivity. The conformations of TF1, TF2B, and TF3 had conformers that left the functional groups of the BCH ring structure sterically accessible while the gallic ester on TF2A would wrap around the BCH structure which could reduce accessibility to this functional group. Since the initial peaks appear to have such great activity, future work should include defining their chemical composition.
Black teas are consumed in large amounts by people worldwide for pleasure and according to both Japanese folk legends and anecdotal data from Egypt and India, black teas are used for the cure of gastroenteritis. Scientific data indicate that theaflavins have both bactericidal (Vibrio cholerae, Salmonella typhi, Staphylococcus aureus) and virucidal (influenza virus, vaccinia virus, herpes simplex virus, coxsackie virus, poliovirus-1, and human rotavirus) activity. Is drinking a cup of tea in fact therapeutic for rotavirus infection? We of course cannot extrapolate from our in vitro studies to answer that. However, according to our extractions, one 8 ounce cup (240 ml) of brewed tea (U.S. source, 2.5 gm of tea leaves) contains approximately 0.375 mg of crude theaflavin extract, which is equal to 15.6 μg/ml. There is 0.35 μg/ml of crude theaflavin required for one EC50 in vitro and thus approximately 45 times that amount in each cup of tea. This would be expected to vary according to the source of tea; different sources of tea vary in their amount of theaflavin concentration (unpublished data).
Acknowledgements
This research was funded by a grant from the Texas Advanced Technology Program, Grant number 999902-004.
References
- Anonymous, 1993. ChemPlus: Extension for HyperChem. Hypercube, Ont., Canada, pp. 95–127
- Anonymous, 1994. HyperChem: Computational Chemistry. Hypercube, Ont., Canada, pp. 1–285
- Clark, K.J., Sarr, A.B., Grant, P.G., Phillips, T.D., Woode, G.N., 1998. In vitro studies on the use of clay, clay minerals, and charcoal to adsorb bovine rotavirus and bovine coronavirus. Vet. Microbiol., 63, 137–146 [DOI] [PMC free article] [PubMed]
- de Leeuw P.W., Ellens D.J., Talmon F.P., Zimmer G.N., Kommerij R. Rotavirus infections in calves: Efficacy of oral vaccination in endemically infected herds. Res. Vet. Sci. 1980;29:142–147. doi: 10.1016/S0034-5288(18)32654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernelius A.L., Ritchie A.E., Classick L.G., Norman J.O., Mebus C.A. Cell culture adaptation and propagation of a reovirus-like agent of calf diarrhea from a field outbreak in Nebraska. Arch. Virusforsch. 1972;37:114–130. doi: 10.1007/BF01241157. [DOI] [PubMed] [Google Scholar]
- Green R.H. Inhibition of multiplication of influenza virus by extracts of tea. Proc. Soc. Exp. Biol. Med. 1949;71:84–85. doi: 10.3181/00379727-71-17089p. [DOI] [PubMed] [Google Scholar]
- Hara Y., Matsuzaki T., Suzuki T. Angiotensin I converting enzyme inhibiting activity of tea component. Nippon Nokeikagaku kaishi. 1987;61:803–808. [Google Scholar]
- Hussain K.A., Storz J., Kousoulas K.G. Comparison of bovine coronavirus (BCV) antigens: Monoclonal antibodies to the spike glycoprotein distinguish between vaccine and wild-type strains. Virology. 1991;183:442–445. doi: 10.1016/0042-6822(91)90163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebus C.A., Stair E.L., Rhodes M.B., Twiehaus M.J. Neonatal calf diarrhea: Propagation, attentuation, and characteristics of a coronavirus-like agent. Am. J. Vet. Res. 1973;34:145–150. [PubMed] [Google Scholar]
- Mukoyama A., Ushijima H., Nishimura S., Koike H., Toda M., Hara Y., Shimamura T. Inhibition of rotavirus and enterovirus infections by tea extracts. Jpn. J. Med. Sci. Biol. 1991;44:181–186. doi: 10.7883/yoken1952.44.181. [DOI] [PubMed] [Google Scholar]
- Myers L.L., Snodgrass D.R. Colostral and milk antibody titers in cows vaccinated with a modified live rotavirus–coronavirus vaccine. J. Am. Vet. Med. Assoc. 1982;181:486–488. [PubMed] [Google Scholar]
- Nakayama M., Toda M., Okubo S., Shimamura T. Inhibition of influenza virus infection by tea. Lett. Appl. Microbiol. 1990;11:38–40. [Google Scholar]
- Nakayama M., Suzuki K., Toda M., Okubo S., Hara Y., Shimamura T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antiviral. Res. 1993;21:289–299. doi: 10.1016/0166-3542(93)90008-7. [DOI] [PubMed] [Google Scholar]
- Reed I.J., Muench H. A simple method in estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Roberts, E.A.H., Myers, M., 1959. The phenolic substances of manufactured tea. VI. The preparation of theaflavin and of theaflavin gallate. J. Sci. Food Agric., 176–179
- Sharpee R.L., Mebus C.A., Bass E.P. Characterization of a calf diarrheal coronavirus. Am. J. Vet. Res. 1976;37:1031–1041. [PubMed] [Google Scholar]
- Snodgrass D.R., Nagy L.K., Sherwood D., Campbell I. Passive immunity in calf diarrhea: Vaccination with K99 antigen of enterotoxigenic Escherichia coli and rotavirus. Infect. Immun. 1982;37:586–591. doi: 10.1128/iai.37.2.586-591.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J., Herrler G., Snodgrass D.R., Hussain K.A., Zhang X.M., Clark M.A., Rott R. Monoclonal antibodies differentiate between the haemagglutinating and the receptor-destroying activities of bovine coronavirus. J. Gen. Virol. 1991;72:2817–2820. doi: 10.1099/0022-1317-72-11-2817. [DOI] [PubMed] [Google Scholar]
- Takino Y., Ferretti A., Flanagan V., Gianturco M., Vogel M. The structure of theaflavin, a polyphenol of black tea. Tetrahedron Lett. 1965;45:4019–4025. [Google Scholar]
- Waltner-Toews, D., Martin, S.W., Meek, A.H., McMillan, I., 1985. Crouch C.F. A field trial to evaluate the efficacy of a combined rotavirus–coronavirus/Escherichia coli vaccine in dairy cattle. Can. J. Comp. Med. 49, 1–9 [PMC free article] [PubMed]
- Woode G.N., Zheng S.L., Rosen B.I., Knight N., Gourley N.E., Ramig R.F. Protection between different serotypes of bovine rotavirus in gnotobiotic calves: Specificity of serum antibody and coproantibody responses. J. Clin. Microbiol. 1987;25:1052–1058. doi: 10.1128/jcm.25.6.1052-1058.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S.L., Woode G.N., Melendy D.R., Ramig R.F. Comparative studies of the antigenic polypeptide species VP4, VP6, and VP7 of three strains of bovine rotavirus. J. Clin. Microbiol. 1989;27:1939–1945. doi: 10.1128/jcm.27.9.1939-1945.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


