Abstract
Postweaning multisystemic wasting syndrome (PMWS) in swine is causally associated with the newly recognised pathogen, porcine circovirus type 2 (PCV2). In this study, 3-week-old SPF PCV2-seronegative piglets were inoculated intranasally with PCV2. The effect of immunostimulation on the induction of PMWS was investigated by immunisation with keyhole limpet hemocyanin (KLH) emulsified in incomplete Freunds adjuvant. The study was terminated 5 weeks after inoculation. While disease was not observed in the age-matched controls, two out of five non-immunised PCV2-infected piglets died on postinoculation day (PID) 21, and one was euthanized on PID 25 in moribund condition. These animals had appeared lethargic with persistent fever from PID 12 onwards. The euthanized pig appeared smaller than littermates and suffered from jaundice. At postmortem examination, gastric ulceration, icterus, and liver and thymus atrophy were observed. Furthermore, histological lesions of degenerating hepatocytes and hepatitis in combination with lymphoid depletion and syncytial cells in lymph nodes were consistent with the diagnosis of PMWS. One out of five immunostimulated PCV2-infected piglets was euthanized on PID 22 with convulsions after a period with wasting. This pig was lethargic from PID 14 onwards with persistent fever from PID 8 and transient dyspnoea. No differences in clinical signs, gross pathologic or histological findings were observed for the remaining non-immunostimulated and immunostimulated PCV2-infected piglets. All 10 PCV2-inoculated piglets seroconverted to PCV2 within 14 days after inoculation. By virus isolation, quantitative polymerase chain reaction (Q-PCR), and immunostaining of cryostat sections, it was demonstrated that lymphoid tissue contained abundant PCV2 antigen. Viral DNA load in serum samples was assessed by Q-PCR. All four PMWS-affected piglets had high levels of PCV2 DNA in serum, suggesting that there was a correlation between high levels of viral DNA in serum and the development of PMWS. In conclusion, infection with PCV2 caused PMWS in SPF piglets, however, the immunostimulation did not seem to play a critical role.
Keywords: Pig-viruses, Porcine circovirus type 2 (PCV2), Postweaning multisystemic wasting syndrome (PMWS), Freunds adjuvant, Immunostimulation
1. Introduction
The small single-stranded DNA virus, porcine circovirus (PCV), has recently been recognised as a pathogen of swine. Two types of PCV have been characterised and designated PCV type 1 (PCV1) and PCV type 2 (PCV2), respectively (Allan and Ellis, 2000 and references therein). PCV1 is considered to be a non-pathogenic virus (Tischer et al., 1986, Allan et al., 1995), whereas infection of swine with PCV2 is causally associated with development of postweaning multisystemic wasting syndrome (PMWS) in weaned 5- to 12-week-old piglets (Allan et al., 1998, Ellis et al., 1998). PMWS is characterised by weight loss, dyspnoea, and jaundice combined with the pathological findings of interstitial pneumonia, generalised enlarged lymph nodes, hepatitis, and nephritis (Allan et al., 1998, Ellis et al., 1999). Typical histological lesions include degeneration and necrosis of hepatocytes, multifocal lymphohistiocytic pneumonia, and lymphocytic depletion and multinucleated giant cell formation in lymph nodes (Clark, 1997, Ellis et al., 1998). PCV2 antigen is typically localised within the multisystemic lesions in affected piglets (Clark, 1997). PMWS was first identified within high health herds in western Canada in 1991 (Clark, 1997) but has since been reported in swine throughout the world (Allan et al., 1998, Ellis et al., 1999, Onuki et al., 1999, Choi et al., 2000). Interestingly, serological surveys have shown that PCV2 was present in swine before PMWS was first reported (Magar et al., 2000b, Walker et al., 2000). Furthermore, serologic studies have revealed that many herds are seropositive to PCV2 without clinical signs of PMWS (Labarque et al., 2000, Rodriguez-Arrioja et al., 2000).
A number of experimental studies have indicated that infection with PCV2 alone is not sufficient to induce clinical PMWS. As such, several inoculation studies using PCV2 alone have resulted in asymptomatic infection with mild to moderate histopathologic lesions (Allan et al., 1999, Balasch et al., 1999, Magar et al., 2000a, Pogranichyy et al., 2000). In contrast, dual infection with PCV2 and porcine parvovirus (PPV) or porcine reproductive and respiratory syndrome virus (PRRSV) potentiates the replication and distribution of PCV2 and induces clinical disease in addition to more severe histopathological lesions (Allan et al., 2000, Kennedy et al., 2000, Krakowka et al., 2000, Harms et al., 2001). These results are in concordance with the observation of PCV2 and PPV coinfection in field cases of PMWS (Ellis et al., 2000) and suggest that the presence of another pathogen may augment the pathogenic effects of PCV2. Similarly, gnotobiotic piglets inoculated with PCV2 alone remained clinically asymptomatic (Krakowka et al., 2000) while gnotobiotic piglets inoculated with tissue homogenates from PMWS-affected piglets containing both PCV2 and PPV developed lesions typical of PMWS with severe clinical disease (Ellis et al., 1999, Krakowka et al., 2000). Recently, it has been demonstrated by Krakowka et al. (2001) that activation of the immune system is a key component of the pathogenesis of PCV2-associated PMWS in gnotobiotic pigs. In that study, immune activation was achieved by injection of keyhole limpet hemocyanin (KLH) in incomplete Freund’s adjuvant (ICFA) into gnotobiotic piglets infected with PCV2 at 1 day of age. By incorporation of bromo-desoxiuridine (BrDU), upregulation of virus production was roughly correlated to increased numbers of replicating cells. In concordance, increased amounts of infectious virus was recovered from draining lymph nodes by quantitative viral titrations. In that study, all immunised piglets developed PMWS whereas none of the non-immunised piglets inoculated with PCV2 alone developed PMWS and extensive viral replication of PCV2 was observed in PMWS-affected piglets, only (Krakowka et al., 2001). These observations also suggest why all pigs infected with PCV2 do not develop PMWS during an outbreak or an experimental infection since differences in immunological status of pigs are bound to exist both between and within herds or experimental groups. The gnotobiotic model for experimental infections cannot be directly compared to field conditions because of the immature immunological status of gnotobiotes and the obvious differences in husbandry conditions. Furthermore, under field conditions, PMWS is a problem in 5- to 12-week-old piglets indicating that the piglets are infected with PCV2 after weaning. Therefore, the objective of the present study was to investigate the significance of immunostimulation on the development of PMWS in 3-week-old SPF piglets infected with PCV2 immediately after weaning.
2. Materials and methods
2.1. Virus
The virus inoculum was prepared by three passages in gnotobiotic pigs of a 24th cell culture passage of a Canadian field isolate (1010) of PCV2. The cell culture passages were performed in PCV-free porcine kidney (PK-15) cells and confirmed to be PCV2 by nucleotide sequence analysis and reactivity with PCV2-specific mAb (Krakowka et al., 2001). The inoculum (OSU3) was a 10% (w/v) lymphoid tissue homogenate of lymphoid tissue collected from the inoculated gnotobiotic pigs. The titer of the PCV2 inoculum was 105.5 TCID50/ml as determined by titration on PCV-free PK-15 cells.
2.2. Piglets and experimental design
The piglets used in this experiment originated from the SPF herd at the Danish Veterinary Institute for Virus Research (DVIVR). This herd is free from PCV1 and PCV2, PRRSV, swine influenza virus (SIV), porcine respiratory coronavirus (PRCV), and a number of other microbiological agents, including mycoplasmas. Sows are routinely vaccinated against PPV. The animal experiment was carried out at the isolation facilities of DVIVR and the pigs were kept in two groups housed in separate sections of the building.
Twenty, 3-week-old colostrum-fed PCV2-seronegative piglets born from two sows were randomly divided into two groups, comprising 10 piglets each. The control piglets (group 1) were sham-inoculated intranasally with 3.5 ml Eagle’s Minimum Essential Medium (EMEM), Glasgow modification per nare (control group, nos. 1–10); the remaining 10 piglets (group 2) were inoculated intranasally with 3.5 ml virus inoculum per nare, i.e. in total 7×105.5 TCID50 PCV2 (PCV2-infected group, nos. 11–20). On postinoculation days (PIDs) 2 and 6, one-half of the piglets from the control group (nos. 6–10) and the PCV2-infected group (nos. 16–20), were intramuscularly immunised with 4.0 ml volume of 2.0 mg of KLH emulsified in ICFA in four sites, both axillas and both hips (1.0 ml per site).
Clinical signs and body temperatures were recorded daily. Blood samples were collected on PIDs 0, 3, 7, 10, 14, 21, 28 and 35 from all animals. The piglets were euthanized on PIDs 35 and 36 for control and PCV2-infected animals, respectively, or earlier if found moribund. Tissue samples of tonsil, liver, spleen, kidney, lung, thymus, and lymph nodes (mesenteric, bronchial, prescapular and superficial inguinal) were collected at necropsy for virus isolation, virus titration, cryostat sectioning, quantitative polymerase chain reaction (Q-PCR) analysis, and histopathological examination.
2.3. Serological examinations
Sera were examined for antibodies against PPV by a blocking ELISA (Madsen et al., 1997). An immunoperoxidase monolayer assay (IPMA) was carried out for the detection of antibodies against PCV1 and PCV2. In brief, monolayers of PCV-infected PK-15 cell cultures were fixed with ethyl alcohol in flat-bottomed 96-well microtiter plates. For PCV1, persistently infected PK-15 cells (ATCC CCL-33, USA) were used and for PCV2, PCV-free PK-15 cells infected with the French PCV2 isolate 48285 (Allan et al., 1998) were used. Plates with fixed PCV-infected cells were stored at −20 °C after addition of 50% glycerol in phosphate buffered saline (PBS) and washed four times in PBS containing 0.1% Tween-20 (PBST) before use. Plates were inoculated with 50 μl of five-fold dilutions at 1:50 to 1:156250 of the serum samples diluted in PBST supplemented with 5% skimmed milk powder (PBST–5% SMP) and incubated for 30 min at 37 °C. After adsorption, the plates were washed four times in PBST before addition of 50 μl peroxidase conjugated rabbit anti-swine IgG (DAKO P0164, Denmark) diluted 1:200 in PBST–5% SMP followed by incubation for 30 min at 37 °C. After the final washing in PBST, ethyl-carbazole substrate was added and the plates were left for 25 min at room temperature. Specific staining of cells indicated the presence of antibodies specific to PCV in the serum sample. The titer of a serum sample was determined as the highest dilution containing a detectable level of antibodies. Due to cross reactivity between antibodies against PCV1 and PCV2, the interpretation of the results included a comparison of the titers to PCV1 and PCV2. If the titer was highest to PCV2, the result indicated an infection with PCV2.
2.4. Virus isolation and titration
Tissue homogenate suspensions (10%, w/v) in Minimum Essential Medium with Eagle’s (MEM) salts and l-glutamine (Gibco BRL, cat. no. 31095-029, Life Technologies™) containing 0.5 mg/ml neomycin, 1.0 mg/ml streptomycin supplemented with 5% foetal calf serum (FCS) were prepared: pool 1 consisted of samples of lymph node tissue (prescapular and superficial inguinal lymph nodes), pool 2 consisted of lymphoid tissue (spleen, mesenteric and bronchial lymph nodes), pool 3 contained samples of liver, lung, and kidney, whereas pool 4 contained tonsil. Pool 4 was sterile filtrated before inoculation into cell cultures. The individual pools were examined for PCV2 by two passages in PCV-free PK-15 cells grown in MEM containing 0.05 mg/ml neomycin, 0.1 mg/ml streptomycin, and 5% FCS (PK-15 medium). The day after cell passage, cultures were incubated with 300 mM glucosamine in Hank’s salt solution for 15–25 min at 37 °C for induction of PCV DNA replication (Tischer et al., 1987). The glucosamine was removed and the monolayers washed with PK-15 medium. Fresh PK-15 medium was added before incubation for additional 2 or 3 days at 37 °C, 5% CO2. Cell cultures were fixed in ice-cold absolute ethyl alcohol at 4 °C for 45 min and peroxidase stained using a PCV2-specific monoclonal antibody (mAb), F217 (McNeilly et al., 2001) diluted 1:25 in PBST–5% SMP, as described for the IPMA. In addition, a general virus examination of 1:1 mixtures of pools 2 and 3 was carried out by two passages in primary swine kidney cells cultured in EMEM containing antibiotics and FCS as described for PK-15 medium. The cell cultures were fixed and stained for PPV, SIV, transmissible gastroenteritis virus (TGEV), hemagglutinating encephalomyelitis virus (HEV), porcine enterovirus (PEV), PRCV, classical swine fever virus (CSFV), and PCV1 and PCV2 using specific antisera.
All organ pools positive for PCV2 by virus isolation in PK-15 cells were titrated in order to determine the infectious titer of the tissue homogenates. Infectivity titrations were performed in 10-fold dilutions in 96-well microplates adding 104 PK-15 cells per well in PK-15 medium. After 3 days of incubation at 37 °C, 5% CO2, plates were fixed and stained for PCV2 as described above. The titers were calculated according to the method of Reed and Muench (1938).
2.5. Quantitative PCR
Viral DNA levels in serum samples and samples of tonsil, liver, spleen, kidney, lung, and lymph nodes (mesenteric and bronchial) was determined by a Q-PCR method developed for the detection of both PCV1 and PCV2. Total DNA was extracted from either serum or tissue using the QIAamp® DNA minikit extraction procedure according to the manufacturers instructions (QIAGEN® GmbH, Germany), and the concentration of double-stranded DNA determined by fluorometric measurements using Picogreen (Singer et al., 1997). For serum samples, the equivalent of 0.8 μl serum was used as template for the PCR. For tissue, the equivalent of 100 ng of double-stranded DNA was used as template in the PCR. Briefly, a forward primer (5′-GATGATCTACTGAGACTGTGTGA) and a reverse primer (5′-6-FAM-AGAGCTTCTACAGCTGGGACA) conserved between PCV1 and PCV2 was used for the PCR together with two probes specific for PCV1 (5′-GCCCCCCAGGAATGGTACTCCTCXTp-3′, where ‘X’ is Texas Red) and PCV2 (5′-TCAGACCCCGTTGGAATGGTACTCCTC-Cy5-3′). The PCR contained a final concentration of 1× PCR gold buffer (Applied Biosystem, Denmark), 2.8 mM MgCl2, 0.1 mM dNTP, 0.25 μM of each primer, 0.5 μM of each probe, 0.25 U of AMP Erase (Applied Biosystems, Denmark), and 1.25 U of Ampli Taq gold DNA-polymerase (Applied Biosystems, Denmark). All reactions were carried out in triplicates on an ABI 7700 (ABI PRISM™ 7700 Sequence Detector, Applied Biosystem, Denmark). The cycling conditions were one cycle of (50 °C for 2 min and 95 °C for 5 min) and 55 cycles of (95 °C for 10 s, 60 °C for 15 s and 75 °C for 15 s) followed by a melting curve analysis added for specificity. Fluorescence data collection was performed at each annealing step of the PCR with the increase in Cy5-fluorescence being proportional to the amount of PCV2 DNA in the sample. For each separate set of PCR reactions, two internal positive controls containing different but known amounts of PCV2 viral DNA template were included. This allowed normalisation for variations in amplification efficiency between different sets of PCR reactions. Results are presented as mean values of the triplicate reactions.
2.6. Immunostaining of cryostat sections and histopathologic examinations
Tissue samples of tonsil, liver, spleen, kidney, lung and lymph nodes (mesenteric and bronchial) were collected from each piglet and immediately fixed by immersion into 4% paraformaldehyde, 22 °C for pathological examination or frozen in isopentane cooled over liquid nitrogen and stored at −20 °C for cryostat sectioning. Sections of paraffin-embedded paraformaldehyde-fixed tissue were stained with hematoxylin and eosin for morphological evaluation. The severity of lesions were scored on a ‘+’ to ‘++++’ scale. Selected blocks of tissue were stained with PCV2-specific polyclonal or monoclonal antibodies as previously described (Ellis et al., 2000). All histological and immunohistochemical evaluations were performed by individuals who were not aware of the group designation. Cryostat sections (5 μm) were fixed with acetone and stained for PCV2 antigen using the mAb F217 diluted 1:10 in PBS without Ca2+ and Mg2+ (PBS-A) supplemented with 3% skimmed milk powder for 1 h at 37 °C in a humidity chamber. Slides were washed in PBS-A and binding of the mAb was visualised by incubation for 1 h at 37 °C in a humidity chamber with FITC-conjugated goat anti-mouse-Ig (JIRL 115-095-146, USA) diluted 1:100 in PBS-A supplemented with 10% swine serum. PCV2 positive cryostat sections were scored from ‘+’ to ‘++++’ based on the number of infected cells.
3. Results
3.1. Clinical observations and gross lesions
None of the 10 uninfected control pigs showed any clinical signs of PMWS-related symptoms during the experimental period. The group of PCV2-inoculated piglets, however, appeared lethargic for a period of 2 weeks starting on PID 12. Within this period, all piglets experienced mild to more severe oedema in the eye lids. Unthriftyness and jaundice were observed for pig no. 13 (PID 22 onwards). Especially pig no. 18 appeared very lethargic and suffered from reddened conjunctivae together with swelling and dirt around the eyes. This particular pig also showed dyspnoea on PID 16. Pig no. 18 had persistent fever (determined as body temperature above 40 °C) from PID 8 onwards and piglet nos. 11–13 from PID 12 onwards. The remaining PCV2-infected piglets had fever occurring intermittently for a single or for two to four consecutive days during the experimental period. Pig nos. 11 and 12 showed inappetence on PID 20 and were found dead on PID 21. Pig no. 13 was euthanized in moribund condition on PID 25. Pig no. 18 had to be euthanized on PID 22 suffering from tremor and convulsions.
Aside from the local reactions to KLH/ICFA in piglet nos. 6–10, no gross lesions were seen in the uninfected control pigs at postmortem examination. Pig nos. 11 and 12 which died on PID 21 both showed ulceration of the stomach in the oesophageal region and generalised icterus. Pronounced thymic and liver atrophy was seen in pig no. 13 euthanized on PID 25. Pig no. 18, euthanized on PID 22, did not present any obvious gross lesions. Generally, only modestly enlarged lymph nodes were observed within the group of PCV2-infected pigs.
3.2. Serological examinations
For all pigs, decreasing antibody levels against PPV passively acquired (maternal antibodies) were detected during the experimental period indicating that no infection with PPV occurred. All PCV2-inoculated piglets seroconverted to PCV2 with high titers against PCV2 compared to PCV1. During the experimental period, the remaining two non-immunostimulated PCV2-infected pigs (nos. 14 and 15) had reached IPMA-titres of 6250 against PCV2 whereas the remaining four immunostimulated pigs (nos. 16, 17, 19, 20) had reached titers of 31 250 (Table 1 ). Thus, within the experimental period the serologic response of the immunostimulated pigs appeared increased compared to the non-immunostimulated pigs. On PIDs 14 and 21, the four pigs that died during the experiment (nos. 11–13, and 18) had low levels of antibodies against PCV2 as detected by IPMA compared to the remaining PCV2-infected pigs (Table 1). All control pigs, except pig no. 9, remained seronegative during the entire experimental period. Pig no. 9 seroconverted on PID 35 with an IPMA-titer of 50 against PCV2.
Table 1.
Determination of antibody titers against PCV2 by IPMA in serum samples from experimentally PCV2-infected piglets
| Experimental group | Pig no. | PIDa 0 | PID 3 | PID 7 | PID 10 | PID 14 | PID 21 | PID 28 | PID 35 |
| PCV2 | 11b | <50c | <50 | <50 | <50 | 50 | – | – | – |
| 12b | <50 | <50 | <50 | <50 | 50 | – | – | – | |
| 13b | <50 | <50 | <50 | 50 | 250 | 250 | – | – | |
| 14 | <50 | <50 | <50 | <50 | 250 | 6250 | 6250 | 6250 | |
| 15 | <50 | <50 | <50 | <50 | 250 | 1250 | 6250 | 6250 | |
| PCV2+immunisation | 16 | <50 | <50 | <50 | <50 | 250 | 6250 | 31250 | 6250 |
| 17 | <50 | <50 | <50 | <50 | 250 | 6250 | 31250 | 31250 | |
| 18b | <50 | <50 | <50 | 50 | 50 | <50 | – | – | |
| 19 | <50 | <50 | <50 | <50 | 250 | 6250 | 31250 | 31250 | |
| 20 | <50 | <50 | <50 | <50 | 1250 | 31250 | 31250 | 31250 | |
Postinoculation day.
Pig that died or was euthanized before termination of the experiment on PID 36.
Antibody titer against PCV2.
3.3. Virus isolations and titrations
PCV2 was isolated from all organ pools from the PCV2-infected pigs (Table 2 ). The infective titer of different tissues (pools 1–4 as previously described) from each of the PCV2-infected pigs are shown in Table 2. Tissue pools from pigs 11–13, and 18 contained the largest quantities of infectious virus. Titers ranged from 103 to 106 infectious units per gram of tissue homogenate. Tonsil tissue contained a slightly lower amount of infectious virus than the remaining three tissue pools from these pigs, however this may be a result of the sterile filtration of the tonsil tissue. In contrast, only low levels of infectious PCV2 were detected for pigs necropsied at the termination of the experiment (pig nos. 14–17, 19, and 20). This observation possibly reflects that a larger amount of PCV2 antigen was present at an earlier stage during infection and that the four pigs most likely died at the peak of infection. No differences in infectious titers were observed between tissue originating from the immunostimulated pigs compared to tissue from the non-immunostimulated pigs. PCV2 was also isolated from organ pools 1–3 originating from control pig no. 9 which had seroconverted on PID 35. PCV2 was not isolated from tissues originating from the remaining control pigs. No virus except PCV2 was detected by the general virus examination in primary swine kidney cells.
Table 2.
Results of PCV2 isolation by two passages on PK-15 cells and determination of the PCV2 infectious titer in 10% (w/v) tissue homogenates from control and experimentally PCV2-infected pigs
| Experimental group | Pig no. | PIDb | Pool 1c | PCV2 (log10 TCID50/g)a |
||
| Pool 2 | Pool 3 | Pool 4 | ||||
| PCV2 | 11d | 21 | +e (4.9f) | + (4.7) | + (5.3) | + (3.7) |
| 12d | 21 | + (5.7) | + (6.1) | + (5.5) | + (4.7) | |
| 13d | 25 | + (4.5) | + (5.7) | + (5.5) | + (3.9) | |
| 14 | 36 | + (<1.5) | + (<1.5) | + (<1.5) | + (<1.5) | |
| 15 | 36 | + (<1.5) | + (<1.5) | + (<1.5) | + (<1.5) | |
| PCV2+immunisation | 16 | 36 | + (<1.5) | + (<1.5) | + (<1.5) | + (<1.5) |
| 17 | 36 | + (<1.5) | + (<1.5) | + (<1.5) | + (<1.5) | |
| 18d | 22 | + (5.3) | + (5.9) | + (4.9) | + (2.7) | |
| 19 | 36 | + (<1.5) | + (<1.5) | + (<1.5) | + (<1.5) | |
| 20 | 36 | + (<1.5) | + (<1.5) | + (<1.5) | + (<1.5) | |
log10TCID50/g=50% tissue culture infectious dose per gram of tissue.
Postinoculation day.
Pool 1: tissue homogenate of inguinal and prescapular lymph nodes; pool 2: tissue homogenate of spleen, mesenteric and bronchial lymph nodes; pool 3: tissue homogenate of lung, liver, and kidney; pool 4: tissue homogenate of tonsils.
Pig that died or was euthanized before termination of the experiment on PID 36.
Plus (+) denotes isolation of PCV2.
The infectious titer of PCV2.
3.4. Q-PCR
High levels of PCV2 DNA were found in serum from the PCV2-infected pigs from PID 3 (Fig. 1 ). All four piglets that died prior to termination of the experiment had the highest detected levels of viral DNA load in serum indicating a correlation between the viral load and the development of PMWS. Pig nos. 11 and 12 had reached a maximum of viral DNA load on PID 14 and a similar level (above 4×109 template copies per ml of serum) was reached by pig nos. 13 and 18 on PID 21 (Fig. 1). Interestingly, the highest number of PCV2 template copies detected was observed for the two immunostimulated 18 and 20 as early as on PID 10. For pig no. 20, however, the viral DNA load rapidly declined as opposed to pig no. 18 where a very high level of viral DNA load persisted until this pig was euthanized 12 days later. Excluding pig nos. 11–13, and 18, immunostimulated pigs (nos. 16, 17, 19, and 20) generally possessed higher levels of viral DNA in serum than did the non-immunostimulated pigs (nos. 14 and 15). The Student’s t-test used to compare mean levels of viral DNA templates detected by the Q-PCR for these two groups of pigs on individual sample days did not reveal any statistically significant differences. Furthermore, comparison of the results obtained for all non-immunostimulated pigs with the results obtained for all immunostimulated pigs on PIDs 3, 7, 10, and 14 while the original groups comprising five piglets were still complete, did not reveal any statistical significant differences (Student’s t-test) in the level of PCV2 template DNA either.
Fig. 1.
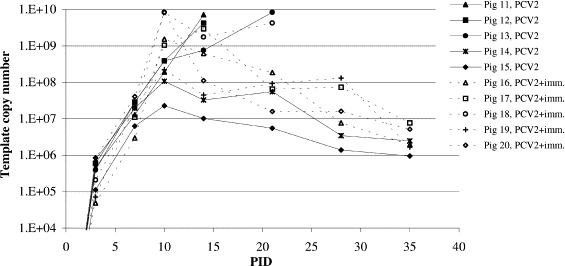
Level of PCV2 viral DNA found in serum from PCV2-infected pigs. Detection of the number of PCV2 template copies per millilitre of serum from experimentally PCV2-infected pigs by a quantitative PCR method.
None of the examined serum samples was found positive for PCV1. However, from a single of the control pigs, no. 9, PCV2 viral DNA was detected in serum from PID 21 onwards with levels of 105 number of PCV2 template copies per ml serum. DNA sequencing revealed that the PCV2 isolated from this pig was identical to the OSU3 inoculum indicating a transmission of virus from the PCV2-infected group to the control group during the experimental period. Serum from the remaining controls were consistently PCV2-negative, indicating that the virus did not spread among the remaining control pigs.
At termination of the experiment, only a low number of PCV2 template copies were detected by Q-PCR in various tissues from the PCV2-infected pigs (Table 3 , pig nos. 14–17, 19, and 20). At this point, there were no differences in the distribution of PCV2 DNA between tissues originating from immunostimulated pigs compared to non-immunostimulated pigs. For pig nos. 11–13, and 18, the load of viral DNA per cell was up to 106 higher within tissue samples at the time of death or euthanasia compared to the results for the remaining pigs euthanized at termination of the experiment (Table 3). The distribution of PCV2 DNA, however, varied between these four individual pigs with samples of tonsil, liver, and lymph nodes containing the highest template copy number. As for the remaining pigs, no apparent effect of the immunostimulation was observed when comparing the Q-PCR results obtained for tissue samples from pigs 11–13, and 18. The differences in distribution and load of viral DNA between these samples likely reflect the differences in manifestation of disease within these four pigs. A low copy number of PCV2 templates per cell were detected by Q-PCR in tissues from pig no. 9. Levels similar to the viral DNA load within samples from pigs euthanized 5 weeks after infection were observed in lymphoid (spleen and lymph nodes) and lung tissue from this pig. PCV2 was not detected within tissue samples from the remaining control pigs. The Q-PCR did not detect PCV1 in any of the tissue samples.
Table 3.
Detection of the number of PCV2 template copies per cell by a quantitative PCR method in various tissues from control and experimentally PCV2-infected pigs
| Experimental group | Pig no. | PIDa | PCV2 template copy number per cell (×102) |
||||||
| Tonsil | Liver | Spleen | Kidney | Lung | Bronchial lymph node | Mesenteric lymph node | |||
| PCV2 | 11b | 21 | 2020 | 122160 | 2730 | 80 | 790 | 2920 | 565260 |
| 12b | 21 | 303480 | 39230 | 2320 | 80 | 830 | 1360 | 4200 | |
| 13b | 25 | 2590 | 710 | 2730 | 40 | 830 | 1170 | 303480 | |
| 14 | 36 | <10 | <10 | <10 | <10 | <10 | 20 | <10 | |
| 15 | 36 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | |
| PCV2+immunisation | 16 | 36 | <10 | <10 | <10 | <10 | <10 | <10 | 10 |
| 17 | 36 | 20 | <10 | <10 | <10 | <10 | 10 | 10 | |
| 18b | 22 | 2590 | 460 | 710 | 150 | 710 | 256630 | 1280 | |
| 19 | 36 | <10 | <10 | <10 | <10 | <10 | 10 | 10 | |
| 20 | 36 | <10 | <10 | <10 | <10 | <10 | 10 | <10 | |
Postinoculation day.
Pig that died or was euthanized before termination of the experiment on PID 36.
3.5. Immunostaining of cryostat sections
Immunostaining of cryostat sections revealed an abundance of PCV2 antigen in tissues collected from pigs 11–13, and 18 which died during the experimental period (Table 4 ). For the remaining PCV2-infected pigs, lymphoid tissues, i.e. spleen, tonsils, and lymph nodes contained the highest amount of viral antigen (Table 4). PCV2 antigen was also found at a low level in lung, spleen, and lymph nodes from control pig no. 9. PCV2 was not detected by immunostaining of cryostat sections within tissues from any of the remaining control pigs.
Table 4.
Results of immunofluorescent staining for PCV2 antigen in cryostat sections of various tissues from control and experimentally PCV2-infected pigsa
| Experimental group | Pig no. | PIDb | Tonsils | Liver | Spleen | Kidney | Lung | Bronchial lymph node | Mesenteric lymph node |
| PCV2 | 11c | 21 | ++ | ++++ | ++++ | +++ | ++++ | ++++ | ++++ |
| 12c | 21 | + | +++ | ++++ | ++ | ++++ | ++++ | +++ | |
| 13c | 25 | ++++ | ++++ | +++ | ++ | ++++ | ++++ | ++++ | |
| 14 | 36 | ++ | + | ++ | − | + | +++ | ++ | |
| 15 | 36 | + | + | + | − | − | ++ | ++ | |
| PCV2+immunisation | 16 | 36 | ++ | + | ++ | − | − | ++ | ++ |
| 17 | 36 | +++ | − | ++ | + | + | ++ | +++ | |
| 18c | 22 | ++++ | +++ | ++++ | ++ | ++++ | ++++ | ++++ | |
| 19 | 36 | ++ | + | + | − | − | ++ | ++ | |
| 20 | 36 | ++ | − | ++ | + | + | +++ | ++ | |
The level of antigen detected in each tissue section was scored from a ‘+’ indicating low levels increasing to ‘++++’ indicating abundant levels of PCV2 antigen; ‘–’ no PCV2 antigen detected.
Postinoculation day.
Pig that died or was euthanized before termination of the experiment on PID 36.
3.6. Histological findings
No significant histological lesions were found in the sham-inoculated control pigs (nos. 1–10). In contrast, three (nos. 11–13) of five pigs in the infected non-immunostimulated group had moderate to severe (3+ to 4+) lymphoid depletion in lymphoid organs including thymus, spleen, lymph nodes, and tonsils (Fig. 2 ). This was associated with the presence of copious amounts of PCV2 antigen in mononuclear phagocytes and syncytial cells. These pigs also had moderate to severe (3+ to 4+) hepatic atrophy associated with non-suppurative cholangiohepatitis. Many Kupffer cells and mononuclear phagocytes in these atrophic livers contained PCV2 antigen (Fig. 3 ). The remaining two pigs, nos. 14 and 15, in this group had mild (1+ to 2+) lymphoid depletion in lymph nodes. These pigs also had mild (1+ to 2+) non-suppurative cholangiohepatitis without significant hepatic atrophy. Two pigs, nos. 14 and 15, in the non-immunostimulated group had mild non-suppurative interstitial nephritis, and two pigs, nos. 13 and 14, had mild (1+) peribronchiolar thickening or hyperplasia of bronchus-associated lymphoid tissue. In the infected immunostimulated group, three pigs (nos. 16, 18, and 20) had mild (1+ to 2+) lymphoid depletion in one lymph node. Pig no. 18 also had moderate (2+ to 3+) lymphoid depletion in the thymus, spleen, and tonsil. In addition, this piglet (no. 18) which showed CNS signs clinically had multifocal non-suppurative encephalitis. These lesions did not contain PCV2 antigen. All immunostimulated pigs had mild (1+ to 2+) non-suppurative cholangiohepatitis without hepatic atrophy and three of these pigs (nos. 16–18) had mild (1+ to 2+) peribronchiolar thickening and/or hyperplasia of bronchus-associated lymphoid tissue.
Fig. 2.
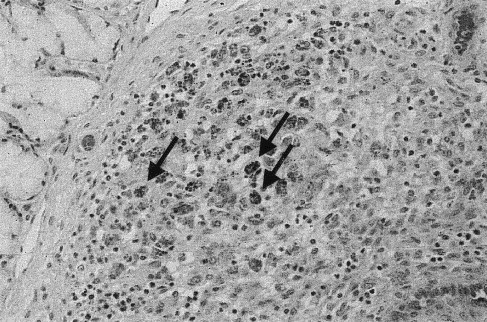
Lymphoid depletion in tonsil from a PCV2-infected non-immunostimulated pig. High power of germinal centre in a tonsil from a PMWS-affected pig with lymphoid depletion showing typical PCV inclusion bodies in macrophages (arrows).
Fig. 3.
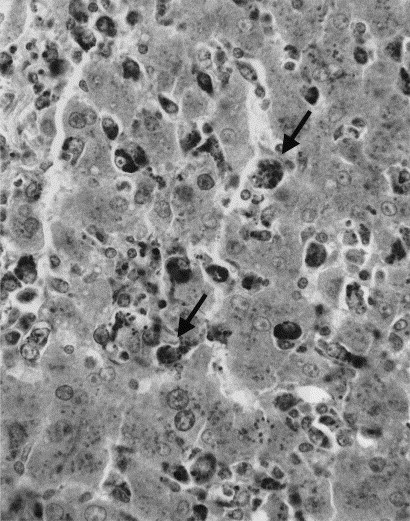
Hepatic atrophy in a PCV2-infected non-immunostimulated pig. Immunohistochemical staining of a section of liver which demonstrates the distribution of the capsid structural protein of PCV2 in the liver of a PMWS-affected pig. Viral antigen is present in the cytoplasm of Kupffer cells and infiltrating macrophages (arrows) as both multiple viral inclusion bodies and as diffusely distributed cytoplasmic product. Hepatocytes are largely devoid of viral antigen.
4. Discussion
The present study was performed to examine the development of PMWS in 3-week-old SPF PCV2-seronegative piglets. Piglets of this age were used because under field conditions, PMWS primarily affects weaned pigs 5–12 weeks of age (Allan et al., 1998, Ellis et al., 1998), suggesting that infection with PCV2 occurs when the level of maternal antibodies is low around weaning at 3–5 weeks of age. The results obtained within the present study describes for the first time the reproduction of clinical PMWS in colostrum-fed SPF pigs experimentally infected with PCV2 alone irrespective of whether the pigs were immunostimulated or not.
Four of 10 pigs infected with PCV2 developed severe PMWS characteristic clinical disease and died prior to termination of the experiment at PID 35. In accordance with field observations (Clark, 1997, Harding, 1997), the observed clinical signs of PMWS varied among individual pigs. A period with persistent fever was observed for each of the four PMWS-affected pigs prior to development of clinical disease. The remaining PCV2-infected pigs experienced transient fever, yet, none of these pigs displayed overt clinical signs of infection. Quantification of PCV2 DNA by PCR demonstrated that the four PMWS-affected pigs contained extremely high levels of PCV2 DNA in serum. These data suggest a direct association between an increased load of viral DNA and clinical PMWS in a PCV2-infected pig. The highest amount of viral DNA detected in serum was observed at PID 10 for two individual pigs. Interestingly, one of these pigs developed PMWS and died whereas the level of PCV2 DNA detected in serum from the other pig rapidly declined and this pig survived until termination of the experiment. Thus, the potential pathogenic effects of an increased level of viral DNA needs further investigation.
Sera from the PMWS-affected pigs contained a lower level of PCV2-specific antibodies compared to the non-diseased pigs. This observation could be explained by the presence of large amounts of PCV2 antigen binding a substantial amount of virus-specific antibodies that were no longer detectable by serologic examinations. Furthermore, a dramatic reduction in the number of B lymphocytes observed in PCV2-infected pigs with wasting disease syndrome has been reported (Shibahara et al., 2000, Segales et al., 2001) that may also explain the low level of PCV2-specific antibodies in the PMWS-affected piglets. In addition, the elimination of B cells may result in compromised production of antibodies towards other agents and by that immunosupression and inability to respond to concurrent infections.
Gross and histological lesions in the pigs with clinical PMWS were similar to those previously reported in both naturally acquired and experimentally induced disease (Ellis et al., 1998, Ellis et al., 1999, Allan et al., 1999, Balasch et al., 1999, Rosell et al., 1999). Immunostaining of cryostat sections demonstrated an abundance of PCV2 antigen in various tissues from the diseased PCV2-infected pigs at PIDs 21, 22, and 25. In contrast, lower amounts of PCV2 antigen primarily within lymphoid tissues was found 5 weeks after infection in non-diseased pigs. These results were comparable with immunohistochemical staining of tissues. The results of virus isolation were in accordance with the results for immunofluorescence staining and PCV2 was isolated from tissue homogenates from all PCV2-infected piglets. A marked difference between diseased and non-diseased pigs was observed with large amount of infectious PCV2 demonstrated in tissue homogenates from PMWS-affected pigs. A similar pattern of PCV2 distribution was obtained by Q-PCR analysis of tissues. Five weeks after infection low PCV2 template copy numbers were detected, whereas high numbers were detected in various tissues from the diseased pigs at PIDs 21, 22, and 25. Thus, in the four PCV2-infected pigs that developed PMWS extensive viral replication led to high amounts of PCV2 antigen widely distributed in various tissues. However, since the diseased pigs died or were euthanized 10–14 days before termination of the experiment, there was no data concerning the amount and distribution pattern of PCV2 antigen at this point in tissues from pigs that did not develop clinical disease. So, the observed difference between diseased and non-diseased pigs regarding virus load may also reflect a time dependence.
In the present study, development of clinical PMWS was demonstrated in both immunostimulated and non-immunostimulated PCV2-infected SPF pigs for the first time. In contrast to previous studies in neonatal gnotobiotic pigs (Krakowka et al., 2001), immunostimulation of weanling SPF PCV2-infected pigs had little apparent effect and was not required for the development of clinical PMWS. In fact, three out of five non-immunostimulated pigs compared to one out of five immunostimulated pigs developed severe clinical signs. There were no significant gross pathological differences between non-immunostimulated and immunostimulated PCV2-infected pigs although histologic lesions were generally more severe in the non-immunostimulated pigs. Five weeks after infection, PCV2 was isolated from all tissue pools from all PCV2-infected pigs with similar titers. Furthermore, there were no indications of any immunostimulatory effect upon the distribution of PCV2 antigen detected by immunofluorescence or Q-PCR within tissue samples originating from non-immunostimulated pigs compared to immunostimulated pigs. From PIDs 7 to 10, there was a tendency for increasing PCV2 template copy numbers detected by Q-PCR in serum from immunostimulated pigs compared to non-immunostimulated pigs but this difference was not statistically significant. Three to 5 weeks after infection, the levels of PCV2 template copy number in serum were also slightly increased in the immunostimulated pigs compared to the non-immunostimulated pigs. Again, there were no statistically significant differences in the load of viral DNA between the two experimental groups. All pigs infected with PCV2 seroconverted between PIDs 10 and 14, and by PID 28, all immunostimulated pigs had higher antibody titers than the non-immunostimulated pigs. None of the non-immunostimulated reached this level of antibodies within the experimental period. Thus, although immunostimulation was not essential for the development of PMWS, it did apparently influence the immune response to the virus.
While immunostimulation was non-essential for development of clinical PMWS in the present study, it was absolutely essential in the previous study by Krakowka et al. (2001). The immature immunological status of 1-day-old gnotobiotic pigs used by Krakowka et al. (2001) may explain why these pigs were more susceptible to immunomodulation as further stimulation of the more mature immune system of 3-week-old SPF pigs may have little effect on whether they will develop PMWS or not upon infection with PCV2. Furthermore, another difference between the immunostimulation experiment of Krakowka et al. (2001) and the present experiment is the fact that the SPF piglets were exposed to external factors and other environmental parameters than the gnotobiotic piglets which were held in biologically secure isolators. Whether such “additional immune activation” is critical in the disease process is, however, not known at present.
The finding of the present study also differs from most previous studies where co-infection with either PPV or PRRSV has been required to induce clinical PMWS (Allan et al., 2000, Kennedy et al., 2000, Krakowka et al., 2000). In that respect, however, the current study support the very recent findings by Bolin et al. (2001) and Harms et al. (2001) that infection of caesarean-derived colostrum-deprived (CD/CD) pigs with PCV2 alone may result in development of clinical PMWS. This apparent inconsistency between different studies on the requirement for co-infection or co-factors in inducing clinical PMWS parallel in many ways the situation from the field. Antibodies against PCV are widespread within swine herds in both Europe and North America (Dulac and Afshar, 1989, Hines and Lukert, 1995, Tischer et al., 1995, Labarque et al., 2000, Rodriguez-Arrioja et al., 2000). It seems reasonable to believe that PCV infection is ubiquitous throughout the world. Infection of a herd with PCV2, however, often results in subclinical infection only (Labarque et al., 2000, Rodriguez-Arrioja et al., 2000). It is therefore critical to identify co-factors that have an influence upon the development of PMWS-related problems. Differences in immunological status among herds and age of pigs at the time of PCV2 infection might still be part of the answer. Immunological differences are probably more pronounced in the field than under experimental conditions due to larger variability of management conditions and presence of other infectious agents. For example, such differences may explain why countries such as Denmark, despite having a high PCV2 seroprevalence, have not yet experienced severe outbreaks of PMWS as reported from other countries (Segales et al., 1997, Allan et al., 1998, Ellis et al., 1999, Ladekjær-Mikkelsen et al., 2001). As well, there could be as yet unrecognised differences in virulence among PCV2 isolates that are circulating in various pig populations. Introduction of more pathogenic PCV2 strains could then potentially dramatically increase the rate of PMWS-related problems in countries that as yet have not reported PCV2-associated disease. Another important unexplored co-factor could be genetic variability in pig populations. Some pigs may simply be more susceptible to infection with PCV2 and or may be more permissive for viral replication, and, therefore, may be more at the risk of developing PMWS.
Further examination of material from PMWS-affected pigs and pigs without clinical symptoms is necessary to determine whether the Q-PCR could serve as diagnostic tool. By qualitative PCR analysis of various tissues from randomly tested diseased pigs with a wide variety of clinical signs and lesions, Hamel et al. (2000) detected PCV2 in more than half the tested individuals. If a certain level of PCV2 DNA is critical for the development of PMWS, the Q-PCR could discriminate between subclinical and clinical PCV2 infection and allow a definitive diagnosis of PMWS.
In conclusion, this study for the first time demonstrated the development of clinical PMWS by infection of 3-week-old SPF pigs with PCV2 alone. The effect of immunostimulation of the PCV2-infected pigs was found not to be essential for the development of clinical disease.
Acknowledgements
This work was partly funded by the European Union (Project QLK2-CT-1999-00445) and (Canadian) Natural Sciences and Engineering Research Council—Collaborative Research Opportunities Grant 234281-00.
References
- Allan G.M., Ellis J.A. Porcine circoviruses: a review. J. Vet. Diagn. Invest. 2000;12:3–14. doi: 10.1177/104063870001200102. [DOI] [PubMed] [Google Scholar]
- Allan G.M., McNeilly F., Cassidy J.P., Reilly G.A.C., Adair B., Ellis W.A., McNulty M.S. Pathogenesis of porcine circovirus experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet. Microbiol. 1995;44:49–64. doi: 10.1016/0378-1135(94)00136-k. [DOI] [PubMed] [Google Scholar]
- Allan G.M., McNeilly F., Kennedy S., Daft B., Clarke E.G., Ellis J.A., Haines D.M., Meehan B.M., Adair B.M. Isolation of porcine circovirus-like viruses from piglets with a wasting disease in the United States of America and Europe. J. Vet. Diagn. Invest. 1998;10:3–10. doi: 10.1177/104063879801000102. [DOI] [PubMed] [Google Scholar]
- Allan G.M., Kennedy S., McNeilly F., Foster J.C., Ellis J.A., Krakowka S., Meehan B.M., Adair B.M. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J. Comp. Pathol. 1999;121:1–11. doi: 10.1053/jcpa.1998.0295. [DOI] [PubMed] [Google Scholar]
- Allan G.M., McNeilly F., Ellis J., Krakowka S., Meehan B., McNair I., Walker I., Kennedy S. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch. Virol. 2000;145:2421–2429. doi: 10.1007/s007050070031. [DOI] [PubMed] [Google Scholar]
- Balasch M., Segalés J., Rosell C., Domingo M., Mankertz A., Urniza A., Plana-Durán J. Experimental inoculation of conventional pigs with tissue homogenates from pigs with post-weaning multisystemic wasting syndrome. J. Comp. Pathol. 1999;121:139–148. doi: 10.1053/jcpa.1999.0310. [DOI] [PubMed] [Google Scholar]
- Bolin S.R., Stoffregen W.C., Nayar G.P.S., Hamel A.L. Postweaning multisystemic wasting syndrome induced after experimental inoculation of cesarean-derived, colostrum-deprived piglets with type 2 porcine circovirus. J. Vet. Diagn. Invest. 2001;13:185–194. doi: 10.1177/104063870101300301. [DOI] [PubMed] [Google Scholar]
- Choi C., Chae C., Clark E.G. Porcine post-weaning multisystemic wasting syndrome in Korean pig: detection of porcine circovirus 2 infection by immunohistochemistry and polymerase chain reaction. J. Vet. Diagn. Invest. 2000;12:151–153. doi: 10.1177/104063870001200209. [DOI] [PubMed] [Google Scholar]
- Clark, E.G., 1997. Post-weaning multisystemic syndrome. In: Proceedings of the American Association of Swine Practitioners, pp. 499–501.
- Dulac G.C., Afshar A. Porcine circovirus antigens in PK-15 cell line (ATCC CCL-33) and evidence of antibodies in Canadian pigs. Can. J. Vet. Res. 1989;53:431–433. [PMC free article] [PubMed] [Google Scholar]
- Ellis J., Hassard L., Clark E., Harding J., Alaan G., Willson P., Strokappe J., Martin K., McNeilly F., Meehan B., Todd D., Haines D. Isolation of circovirus from lesions of piglets with postweaning multisystemic wasting syndrome. Can. Vet. J. 1998;39:44–51. [PMC free article] [PubMed] [Google Scholar]
- Ellis J.A., Krakowka S., Lairmore M., Haines D., Bratanich A., Clark E., Allan G., Konoby C., Hassard L., Meehan B., Martin K., Harding J., Kennedy S., McNeilly F. Reproduction of lesions of postweaning multisystemic wasting syndrome in gnotobiotic piglets. J. Vet. Diagn. Invest. 1999;11:3–14. doi: 10.1177/104063879901100101. [DOI] [PubMed] [Google Scholar]
- Ellis J.A., Bratanich A., Clark E.G., Allan G., Meehan B., Haines D.M., Harding J., West K.H., Krakowka S., Konoby C., Hassard L., Martin K., McNeilly F. Coinfection by porcine circoviruses and porcine parvovirus in pigs with naturally acquired postweaning multisystemic wasting syndrome. J. Vet. Diagn. Invest. 2000;12:21–27. doi: 10.1177/104063870001200104. [DOI] [PubMed] [Google Scholar]
- Hamel A.L., Lin L.L., Sachvie C., Grudeski E., Nayar G.P.S. PCR detection and characterization of type-2 porcine circovirus. Can. J. Vet. Res. 2000;64:44–52. [PMC free article] [PubMed] [Google Scholar]
- Harding, J., 1997. Post-weaning multisystemic wasting syndrome (PMWS): preliminary epidemiology and clinical presentation. In: Proceedings of the American Association of Swine Practitioners, p. 503.
- Harms P.A., Sorden S.D., Halbur P.G., Bolin S.R., Lager K.M., Morozov I., Paul P.S. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syndrome virus. Vet. Pathol. 2001;38:528–539. doi: 10.1354/vp.38-5-528. [DOI] [PubMed] [Google Scholar]
- Hines R.K., Lukert P.D. Porcine circovirus: a serological survey of swine in the United States. Swine Health Prod. 1995;2:71–73. [Google Scholar]
- Kennedy S., Moffett D., McNeilly F., Meehan B., Ellis J., Krakowka S., Allan G.M. Reproduction of lesions of postweaning multisystemic wasting syndrome by infection of conventional pigs with porcine circovirus type 2 alone or in combination with porcine parvovirus. J. Comp. Pathol. 2000;122:9–24. doi: 10.1053/jcpa.1999.0337. [DOI] [PubMed] [Google Scholar]
- Krakowka S., Ellis J.A., Meehan B., Kennedy S., McNeilly F., Allan G.A. Viral wasting syndrome of swine: experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection with porcine circovirus 2 and porcine parvovirus. Vet. Pathol. 2000;37:254–263. doi: 10.1354/vp.37-3-254. [DOI] [PubMed] [Google Scholar]
- Krakowka S., Ellis J.A., McNeilly F., Ringler S., Rings D.M., Allan G. Activation of the immune system is the pivotal event in the production of wasting disease in pigs infected with porcine circovirus-2 (PCV-2) Vet. Pathol. 2001;38:31–42. doi: 10.1354/vp.38-1-31. [DOI] [PubMed] [Google Scholar]
- Labarque G.G., Nauwynck H.J., Mesu A.P., Pensaert M.B. Seroprevalence of porcine circovirus types 1 and 2 in the Belgian pig population. Vet. Q. 2000;22:234–236. doi: 10.1080/01652176.2000.9695065. [DOI] [PubMed] [Google Scholar]
- Ladekjær-Mikkelsen A.-S., Nielsen J., Storgaard T., Bøtner A., Allan G., McNeilly F. Transplacental infection with PCV-2 associated with reproductive failure in a gilt. Vet. Rec. 2001;148:759–760. [PubMed] [Google Scholar]
- Madsen E.S., Madsen K.G., Nielsen J., Jensen M.H., Lei J.C., Have P. Detection of antibodies against porcine parvovirus nonstructural protein NS1 may distinguish between vaccinated and infected pigs. Vet. Microbiol. 1997;54:1–16. doi: 10.1016/s0378-1135(96)01270-9. [DOI] [PubMed] [Google Scholar]
- Magar R., Larochelle R., Thibault S., Lamontagne L. Experimental transmission of porcine circovirus type 2 (PCV2) in weaned pigs: a sequential study. J. Comp. Pathol. 2000;123:258–269. doi: 10.1053/jcpa.2000.0413. [DOI] [PubMed] [Google Scholar]
- Magar R., Muller P., Larochelle R. Retrospective serological survey of antibodies to porcine circovirus type 1 and type 2. Can. J. Vet. Res. 2000;64:184–186. [PMC free article] [PubMed] [Google Scholar]
- McNeilly F., McNair I., Mackie D.P., Meehan B.M., Kennedy S., Moffett D., Ellis J., Krakowka S., Allan G.M. Production, characterisation and application of monoclonal antibodies to porcine circovirus 2. Arch. Virol. 2001;146:909–922. doi: 10.1007/s007050170124. [DOI] [PubMed] [Google Scholar]
- Onuki A., Abe K., Togashi K., Kawashima K., Taneichi A., Tsunemitsu H. Detection of porcine circovirus from lesions of a pig with wasting disease in Japan. J. Vet. Med. Sci. 1999;61:1119–1123. doi: 10.1292/jvms.61.1119. [DOI] [PubMed] [Google Scholar]
- Pogranichyy R.M., Yoon K.-J., Harms P.A., Swenson S.L., Zimmerman J.J., Sorden S.D. Characterization of immune response of young pigs to porcine circovirus type 2 infection. Viral Immunol. 2000;13:143–153. doi: 10.1089/vim.2000.13.143. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Rodriguez-Arrioja G.M., Segalés J., Balasch M., Rosell C., Quintana J., Folch J.M., Plana-Durán J., Mankertz A., Domingo M. Serum antibodies to porcine circovirus type 1 and type 2 in pigs with and without PMWS. Vet. Rec. 2000;146:762–764. doi: 10.1136/vr.146.26.762. [DOI] [PubMed] [Google Scholar]
- Rosell C., Segalés J., Plana-Durán J., Balasch M., Rodríguez-Arrioja G.M., Kennedy S., Allan G.M., McNeilly F., Latimer K.S., Domingo M. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J. Comp. Pathol. 1999;120:59–78. doi: 10.1053/jcpa.1998.0258. [DOI] [PubMed] [Google Scholar]
- Segalés J., Sitjar M., Domingo M., Dee S., Del Pozo M., Noval R., Sacristan C., De las Heras A., Ferro A., Latimer K.S. First report of post-weaning multisystemic wasting syndrome in pigs in Spain. Vet. Rec. 1997;141:600–601. [PubMed] [Google Scholar]
- Segalés J., Alonso F., Rosell C., Pastor J., Chianini F., Campos E., López-Fuertes L., Quintana J., Rodríguez-Arrioja G., Calsamiglia M., Pujols J., Domínguez J., Domingo M. Changes in peripheral blood leukocyte populations in pigs with natural postweaning multisystemic wasting disease (PMWS) Vet. Immunol. Immunopathol. 2001;81:37–44. doi: 10.1016/s0165-2427(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Shibahara T., Sato K., Ishikawa Y., Kadota K. Porcine circovirus induces B lymphocyte depletion in pigs with wasting disease syndrome. J. Med. Vet. Sci. 2000;62:1125–1131. doi: 10.1292/jvms.62.1125. [DOI] [PubMed] [Google Scholar]
- Singer V.L., Jones L.J., Yue S.T., Haugland R.P. Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation. Anal. Biochem. 1997;249:228–238. doi: 10.1006/abio.1997.2177. [DOI] [PubMed] [Google Scholar]
- Tischer I., Mields W., Wolff D., Vagt M., Griem W. Studies on epidemiology and pathogenicity of porcine circovirus. Arch. Virol. 1986;91:271–276. doi: 10.1007/BF01314286. [DOI] [PubMed] [Google Scholar]
- Tischer I., Peters D., Rasch R., Pociuli S. Replication of porcine circovirus: induction by glucosamine and cell cycle dependence. Arch. Virol. 1987;96:39–57. doi: 10.1007/BF01310989. [DOI] [PubMed] [Google Scholar]
- Tischer I., Bode L., Peters D., Pociuli S., Germann B. Distribution of antibodies to porcine circovirus in swine populations of different farms. Arch. Virol. 1995;140:737–743. doi: 10.1007/BF01309961. [DOI] [PubMed] [Google Scholar]
- Walker I.W., Konoby C.A., Jewhurst V.A., McNair I., McNeilly F., Meehan B.M., Cottrell T.S., Ellis J.A., Allan G.M. Development and application of a competitive enzyme-linked immunosorbent assay for the detection of serum antibodies to porcine circovirus type 2. J. Vet. Diagn. Invest. 2000;12:400–405. doi: 10.1177/104063870001200502. [DOI] [PubMed] [Google Scholar]


