Abstract
An infectious bronchitis virus (IBV), ck/CH/LZJ/111113, was isolated from a H120-vaccinated chicken which showed disease suspected of IBV infection. Neutralization testing showed that ck/CH/LZJ/111113 was distinct from either the Chinese predominant IBV LX4-type or Mass-type vaccine strains. Phylogenetic analysis confirmed that ck/CH/LZJ/111113 is of the 4/91 type; however, further extensive analyses of full-length genomes identified occurrence of recombination events. Therefore, ck/CH/LZJ/111113 originated from the recombination events between ck/CH/LDL/091022- and 4/91-like strains at three switch sites located upstream of the spike (S) glycoprotein gene, and the 3′ ends of S1 and nuceocapsid (N) genes, respectively. The difference of serotypes and tissue tropisms in kidneys between ck/CH/LZJ/111113 and ck/CH/LDL/091022 may have been contributed by the uptake of the S1 gene by a ck/CH/LDL/091022-like virus from a 4/91-like strain. This recombination event took place at the 3′ end of the N gene and the 3′ untranslated region may account for differences in replication efficiency in tissues of chickens inoculated by the two viruses.
Keywords: Infectious bronchitis coronavirus, Recombination event, Spike glycoprotein, Nucleocapsid tissue tropism, Replication efficiency
1. Introduction
Coronaviruses (CoVs) are a family of enveloped positive-stranded RNA viruses and found in a wide variety of animals in which they can cause respiratory, enteric, hepatic and neurological diseases of varying severities. Based on genotypical and serological characterizations, CoVs were classified into three genera, Alphacoronacirus, Betacoronavirus and Gammacoronavirus (Carstens, 2010). Avian infectious bronchitis virus (IBV) belongs to Gammacoronavirus genus and causes a highly contagious upper respiratory disease, a decrease in egg production, poor eggshell quality, reduced hatchability, nephritis and sometimes false layers in chickens.
CoVs contain an unsegmented, single-stranded, positive-sense RNA genome of 28–32 kb, with a 5′ cap and a 3′ polyA tail. In the IBV genome, similar to other CoVs, two-thirds of the 5′ region encodes the 1a and 1ab polyproteins, which are proteolytically cleaved by two virus-encoded replicase proteins (the papain-like and 3C-like proteinases) into at least 15 nonstructural proteins (nsp2–nsp16) (Ziebuhr et al., 2001). In the remaining one-third of the genome, most coronaviruses, including IBV, code four major structural proteins; the spike (S) glycoprotein, the small envelope (E) protein, the membrane (M) glycoprotein, and the nucleocapsid (N) protein. It is generally believed that the S1 subunit contains the receptor-binding activity of the S protein, carries virus-neutralizing and serotype-specific determinants, and is a determinant of cell tropism. Interspersed among the structural protein genes are two small accessory protein genes (genes 3 and 5) that vary in number and sequences among the IBVs. The 5′ and 3′ untranslated regions (UTRs) usually harbor important structural elements, which are involved in replication and/or translation (Masters, 2006).
LX4 (QX-like) and 4/91 are two of the most well characterized and important IBV serotypes that were first isolated in 1998 in China (Liu and Kong, 2004) and 1985 in France (Cook et al., 2012), respectively. Viruses of these serotypes have been detected in several European as well as Asian countries, including China, although at different frequencies (Liu and Kong, 2004, Cook et al., 2012), causing heavy economic losses. Here, we isolated and characterized an IBV strain (ck/CH/LZJ/111113) and reported data from antigenic and genomic analyses and replication efficiency in chicken tissues and showed that it represents a recombinant coronavirus infectious bronchitis virus.
2. Materials and methods
2.1. Viral isolation
In the course of our continuous surveillance activities for IBV in China in 2011, an IBV strain, designed as ck/CH/LZJ/111113, was isolated from a diseased trachea suspected of an IBV infection. The chicken flocks were vaccinated against IB with commercial live attenuated H120 vaccine and showed early signs of respiratory disease at 20 days old. The gross examination showed mild to severe tracheitis and proventriculitis.
Viral isolation was performed as previously described (Liu and Kong, 2004). Blind passages were performed until the characteristic embryo changes appeared, such as dwarfing, stunting, or curling of the embryos, which were observed between 2 and 7 days post-inoculation, based on a previous report. In addition, the IBV strain ck/CH/LDL/091022 (Sun et al., 2011) was used in this study. The embryo-propagated viral stocks of ck/CH/LDL/091022 and ck/CH/LZJ/111113 were produced by inoculating the virus into embryonated SPF chicken eggs via the allantoic cavity and collecting the infectious allantoic fluid 72 h post-inoculation as previously described (Liu and Kong, 2004).
2.2. Eggs and chicks
Fertile White Leghorn SPF chicken eggs were obtained from the Harbin Veterinary Research Institute, as were the White Leghorn SPF chicks. The birds were maintained in isolators with negative pressure, and food and water were provided ad libitum.
2.3. Virus-neutralization tests
The titers of the two viruses were determined by inoculation at 10-fold dilutions into groups of five 10-day-old embryonated chicken eggs. The 50% embryo infectious dose (EID50) was calculated by the method of Reed and Muench (1938). The β VN method with constant virus and diluted serum was employed in SPF chicken embryos for serotyping. The R-values were calculated by the formula of Archetti and Horsfall (1950).
2.4. Sequencing and phylogenic analysis of IBV strains ck/CH/LDL/091022 and ck/CH/LZJ/111113
For complete genomic sequencing, viral RNA was extracted from 400 μl of ck/CH/LDL/091022 and ck/CH/LZJ/111113 viral stocks, respectively, as described previously (Liu et al., in press), using TRIzol reagent according to the manufacturer's instructions. Fourteen overlapped PCR fragments spanning the entire viral genome were amplified using specific primer sets (Liu et al., in press). The 3′ and 5′ ends of the viral genomes were confirmed by rapid amplification of cDNA ends using a 3′/5′ RACE kit (TaKaRa) according to the manufacturer's instructions. The sense and antisense primers used for amplifying the 3′ and 5′ ends of the genome had been designed as previously described (Liu et al., in press). The PCR products were cloned into the pMD-18T vector (TaKaRa) following the manufacturer's instructions, and each fragment of the viral genome was sequenced at least five times and the consensus sequence was determined. Prediction of the ORFs was conducted using Vector NTI Advanced 10 (Invitrogen), and the sequences were analyzed using Lasergene DNAStar (version 7, Lasergene Corp, Madison, WI, USA). The pairwise nucleotide identity was determined using Vector NTI Advanced 10 and multiple sequence alignments were generated using Clustal-W (Thompson et al., 1997).
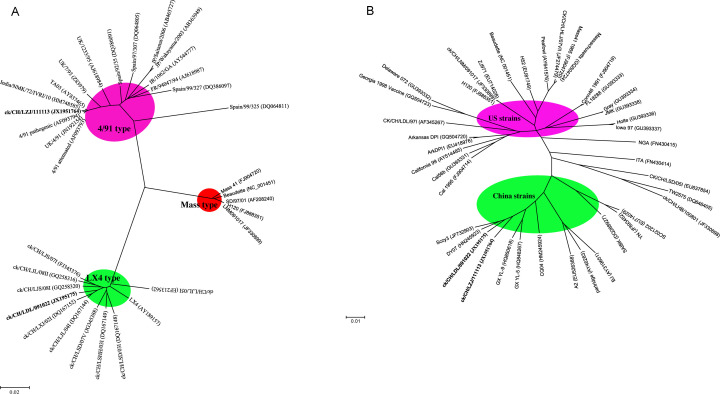
A total of 30 or 39 IBV reference strains for which the S1 or entire genomic sequences were available in the GenBank database were selected for phylogenetic analysis. The selected avian coronavirus reference strains and their accession numbers are shown in Fig. 1A and B, respectively.
Fig. 1.
Relationships among the ck/CH/LZJ/111113, ck/CH/LDL/091022 and reference strains illustrated by a maximum likelihood phylogeny unrooted tree, based on S1 nucleotides 1–1626 (A) and the full-length of genomic sequences (B), respectively.In (A), the strains ck/CH/LZJ/111113, ck/CH/LDL/091022 are shown in bold. The genotypes of 4/91-, LX4- and mass-type strains were shown in pink, blue and red, respectively. In (B), the US and China strains were shown in pink and blue, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
2.5. Accession number
The genomic sequences of the IBV strains ck/CH/LDL/091022 and ck/CH/LZJ/111113 have been submitted to the GenBank database and have been assigned the accession numbers JX195175 and JX195176, respectively.
2.6. Experimental design
Ninety, 1-day-old SPF White Leghorn chicks were housed in different isolators and divided into three groups, each containing 30 birds. Chickens in groups 1 and 2 were inoculated with either ck/CH/LDL/091022 or ck/CH/LZJ/111113 (1 × 106 EID50 per chick, respectively) by oculonasal administration at 15 days of age with a dose of log106 EID50 per chick. Birds in group 3 were mock-inoculated with sterile allantoic fluid and served as the control. Ten birds from each group were killed humanely at 4 and 7 days post-inoculation. Tissue samples were collected from the trachea, lung, kidney, Harderian gland, thymus, spleen, bursa of Fabricius, cecal tonsil, proventriculus, duodenum, liver, pancreas, small intestine, large intestine, rectum and caecum for viral RNA detection by real-time RT-PCR. The remaining birds in each group were examined daily for signs of infection for 30 days post-inoculation. Blood samples were collected from all birds in each treatment group at 4, 8, 12, 16, 20 and 24 days post-inoculation. Serum samples were assayed using a commercial total antibody ELISA (IDEXX Corporation, Westbrook, ME, USA) according to the manufacturer's instructions. Each sample was tested in triplicate.
2.7. Real-time RT-PCR
The homogenized tissue samples were used for quantitative real-time RT-PCR as described previously (Jones et al., 2011). One-step real-time RT-PCR reactions were performed using One Step PrimeScript® RT-PCR kit (TaKaRa) on a LightCycler® 480 real-time PCR system (Roche, Basel, Switzerland). The data were analyzed using LightCycler® 480 Software Version 1.5. All real-time RT-PCR products were confirmed by electrophoresis on a 2.0% agarose gel and visualized using ethidium bromide.
3. Results
3.1. Serotype of IBV by cross virus neutralization tests
The ck/CH/LZJ/111113 was serologically characterized with the Chinese predominant LX4-type strain, ck/CH/LDL/091022, and the vaccine strain, H120. Cross virus neutralization tests were performed using anti-sera against strains ck/CH/LZJ/111113, ck/CH/LDL/091022 and H120 to determine the antigenic relationship among the three strains. End-points were calculated by Reed and Muench method (1938). The R value for strains ck/CH/LZJ/111113 and ck/CH/LDL/091022, and ck/CH/LZJ/111113 and H120 were 28.42 and 29.73%, respectively, which indicated that ck/CH/LZJ/111113 is distantly related to strains ck/CH/LDL/091022 and H120. Thus, strain ck/CH/LZJ/111113 represents a new serotype that is antigenically distinct from LX4 and Mass serotypes of IBVs.
3.2. Phylogenetic analysis of the strain ck/CH/LZJ/111113
The S1 gene of ck/CH/LZJ/111113 had 99.6 and 99.5% identity with pathogenic and attenuated UK/4/91 strains, respectively (Fig. 1A). These figures are very similar to the comparison of a recent Indian 4/91-type isolate, India/NMK/72/IVRI/10. Thus, the S1 sequence of ck/CH/LZJ/111113 was clearly of the 4/91 type. However, the sequence was markedly different from those of 4/91 from position 1453 in the S1 gene to the end of the S1 gene, from which position it was closely related to a LX4-type virus, ck/CH/LDL/091022.
The ck/CH/LZJ/111113 and ck/CH/LDL/091022 sequences were assembled into one contiguous sequence to represent the entire viral genomes. The sequences of 27,675 and 27,673 nucleotides were obtained from ck/CH/LZJ/111113 and ck/CH/LDL/091022, respectively, including the poly-A tail at the 3′ end. The genomes of both viruses were similar overall in their coding capacities and genomic organizations to those of other IBVs. In a phylogenetic analysis using the full-length genomic sequences, both ck/CH/LZJ/111113 and ck/CH/LDL/091022 were clustered into the same group (Fig. 1B).
3.3. Recombinant analyses of ck/CH/LZJ/111113 genome
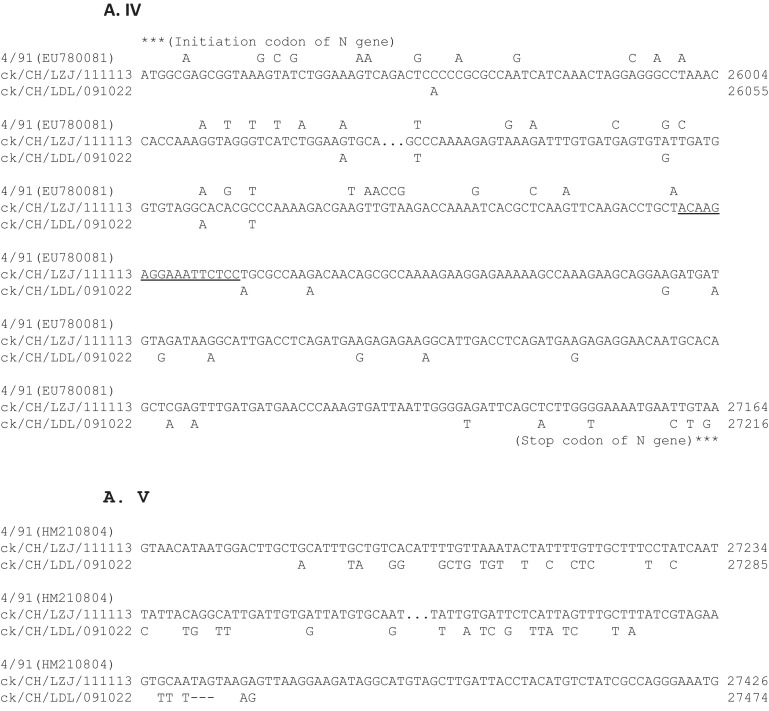
Because no full-length genomic sequence of 4/91-type was available, we pairwise compared the nsp 13, S, M, N and 3′ UTR of the 4/91 strain with those of ck/CH/LZJ/111113 and ck/CH/LDL/091022. As shown in Fig. 2 , the nsp 13 of the ck/CH/LZJ/111113 strain bears a much greater nucleotide sequence identity to that of ck/CH/LDL/091022 (96.3%) than to 4/91, though the ck/CH/LZJ/111113 1–1453 in the S1 gene showed an identical sequence to that of 4/91. It worth noting that in agreement to the nsp 13, strain ck/CH/LZJ/111113 1453–3492 in spike, M and 1–1015 in the N genes shared 99.3, 99.0 and 94.9% identities to those of ck/CH/LDL/091022, respectively, in contrast to the 84.8, 91.3 and 86.9% to 4/91. To our surprise, ck/CH/LZJ/111113 from 1032 to the end of the N gene and 3′ UTR again have identical sequences to those of 4/91 and were different from those of ck/CH/LDL/091022 (91.3 and 75.2%, respectively). In addition, ck/CH/LZJ/111113 and ck/CH/LDL/091022 had a 96% nucleotide identity; however, this figure was up to 97.2% when the 4/91-like sequences in the 5′ ends of S1 and N were excluded. These data strongly suggested that ck/CH/LZJ/111113 arose from a homologous RNA recombinant event from multiple template switches (at least three switches) between 4/91- and ck/CH/LDL/091022-like viruses. Here, we investigated the presence of two switch sites at the 3′ ends of the S1 and N genes, respectively (Fig. 2), implying that template switches occurred within the S1 and N genes. Due to the unavailability of the sequences between nsp 13 and the spike genes in the 4/91 virus, we could not identify the exact switch site before the spike gene; however, by comparing the sequence with that of ck/CH/LDL/091022, we could predict that this site may tend to lie immediately upstream of the S glycoprotein gene because ck/CH/LZJ/111113 and ck/CH/LDL/091022 shared highly identical sequences in this region.
Fig. 2.
Analysis of recombination among IBV ck/CH/LDL/091022, 4/91 and ck/CH/LZJ/111113 strains. (A) Multiple sequence alignments surrounding numbered regions in A. The numbers on the right of each alignment showed the nucleotide positions in genome of each virus. The sequences of ck/CH/LZJ/111113 are listed and the only nucleotides differing from those of ck/CH/LZJ/111113 are depicted. The region where the template switches (switch site) have taken place is underlined. The deleted nucleotides are indicated by -. NA represents unavailable sequence. (B) Percentages of nucleotide sequence identity among ck/CH/LZJ/111113, ck/CH/LDL/091022 and 4/91.Percentages of nucleotide sequence identity of gene fragments before and after the switch sites in S1 and N genes are also indicated.
3.4. Virulence studies
Clinical signs were observed in all ck/CH/LDL/091022-infected chicks from days 3–14 post-inoculation and in all ck/CH/LZJ/111113-infected chicks from days 4 to 18 post-inoculation. The clinical signs included listlessness, huddling, ruffled feathers and dark, shrunken combs. The clinical signs were more severe in ck/CH/LZJ/111113-infected chicks than those infected with ck/CH/LDL/091022. Two of the 10 chicks died: one on day 4 and a second on day 7, in the ck/CH/LDL/091022-infected group during the experiment; however, no chicks died in ck/CH/LZJ/111113-infected group. Gross lesions in the organs of the dead chicks were confined mainly to the kidneys. No overt disease was observed in chicks of the control group. In addition, chickens inoculated with ck/CH/LZJ/111113 and ck/CH/LDL/091022 were negative for IBV antibodies until 8 days post-inoculation. At each time point from 8 days post-inoculation onwards, the ck/CH/LZJ/111113-inoculated chickens had comparable antibody titers with those of ck/CH/LDL/091022.
3.5. Analysis of IBV-RNA in tissues of inoculated chickens by real-time PCR
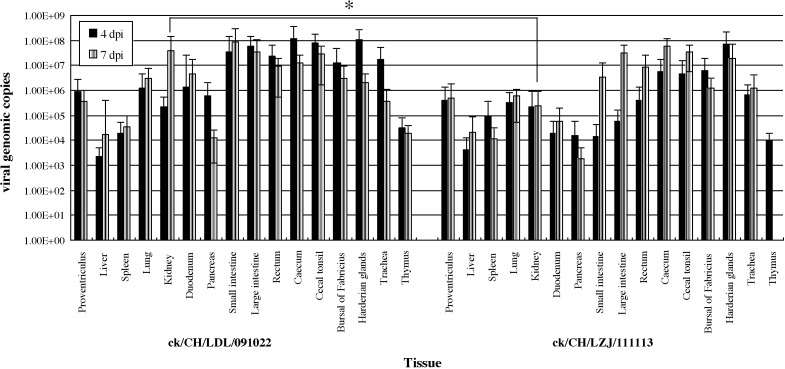
As illustrated in Fig. 3 , all tissues investigated had detectable viral RNA on days 4 and 7 post-inoculation, respectively. Overall, the replication efficiency of ck/CH/LDL/091022 in the chicken tissues was higher than that of ck/CH/LZJ/111113. However, both strains showed a somewhat high affinity to the gastrointestinal tract, as reflected by the high viral genomic copies detected by real-time RT-PCR. Furthermore, ck/CH/LDL/091022 had a strong affinity to the kidney, compared to that of ck/CH/LZJ/111113. No IBV-derived RNA was detected in the tissues of the control chickens.
Fig. 3.
Viral genome RNA copies in tissues from chickens inoculated with ck/CH/LZJ/111113 and ck/CH/LDL/091022, respectively. Viral RNA copy number was measured by real-time RT-PCR in tissues from three chickens on days 4 and 7 post-inoculation, respectively. Results represent the mean of three independent experiments with three replicates per experiment and each bar is the mean ± SD. The data were analyzed using SAS (SAS, 1996); *P < 0.05.
4. Discussion
In 2011, we conducted routine surveillance of IBV in China and isolated and identified an IBV strain from an H120-vaccinated chicken, designed as ck/CH/LZJ/111113. Serological data suggested that ck/CH/LZJ/111113 possessed distinct characteristics setting it apart from the predominant LX4-type strain in China (Liu and Kong, 2004) and the mass-type vaccine strain. Phylogenetic analysis of the ck/CH/LZJ/111113 strain showed that it was mostly related to pathogenic UK 4/91 and the recently isolated Indian virus strain India/NMK/72/IVRI/10, which strongly suggested that this isolate was of the 4/91 type. However, the clearly divergent 3′ end of the S1 gene, compared to that of 4/91, implicated that a recombinant event may have occurred during the origination and evolution of the virus. Further proof of multiple recombinant events was found by extensive analysis of the full-length genomic sequences.
Coronaviruses are known to efficiently recombine, especially within the spike gene, to modulate pathogenesis and even the host range. This has been well documented in IBV, which was clearly shown to be responsible for the emergence of several new serotypes or variants (Jackwood et al., 2010), and contributed to the outbreaks of diseases in chickens. The recombination events from which the ck/CH/LZJ/111113 virus resulted can be explained by two models. In the most simple scenario, the recombination may have involved only two parental viral strains with RNA replication initiating in a ck/CH/LDL/091022-like template of either negative or positive polarity and then switching of the polymerase–nascent cRNA complex to a 4/91-like virus template, followed by double switches at the 3′ ends of the S1 and N genes, respectively. The first switch may have occurred in the vicinity of the ORF 1/S boundary, presumptively upstream of the spike glycoprotein gene, but the exact site for this is currently unknown because of the sequence of 4/91 at this position is unavailable. In this scenario, the ck/CH/LDL/091022- and 4/91-like viruses must be both circulating in the animal breeding facilities for long periods, in which chickens were infected simultaneously and recombination events occurred. Alternatively, a more complicated scheme can be envisaged. In this scheme, a ck/CH/LDL/091022-4/91 hybrid arose from a single switch at either of the three or from two switches at two sites and spread among the chicken flocks and in turn engaged in additional recombination events with another 4/91-like virus. IBV circulates extensively in chicken flocks, either broiler or layer hens, and even in the vaccinated flocks in many countries, in some cases, with more than one type of strain. Conceivably, this and the long persistence of IBV in chickens could raise the odds of occurrences of double or triple infections.
It is believed that the conditions for recombination amongst the IBV strains in the field are as follows: an extremely large number of chickens, most maintained at high density; ease of spread of the virus; and serotype co-circulation, including proof of co-infection with more than one serotype in a given flock. This is true in the case of ck/CH/LZJ/111113. China has an extremely large number of chickens and most of the chickens in different districts in China are maintained at a high density. LX4-type IBV isolates are found to be the predominant IBV type circulating in both laying hens and broilers in China. Though only small numbers of 4/91-like viruses have been isolated in chicken flocks in China (Han et al., 2011), however, 4/91 live vaccine are commonly used during the breeding period, the vaccines must certainly be implicated as a source for 4/91-like sequences. The LX4- or 4/91-like sources of genetic material were probably a strain that naturally infects these affected flocks, as found in ck/CH/LZJ/111113 in this study. The natural recombination event demonstrated here could suggest that the continuous use of live vaccines may actually contribute to natural recombination and IBV-associated disease.
Overall, the body of evidence in favor of a recombinant origin for the S1, partial N genes and 3′ UTR of ck/CH/LDL/091022, together with its distinct antigenic and tropism profiles, argue in favor of setting it apart from other known IBVs as novel strains. However, a recombination event itself is unlikely to be the only reason directly responsible for these distinctions between ck/CH/LZJ/111113 and ck/CH/LDL/091022 because only a few mutations were found in other parts of the genomes. Because the S glycoprotein gene is involved in host cell attachment and contains viral neutralizing epitopes, recombination of the S glycoprotein gene can result in the emergence of new strains or serotypes of the virus as well as new viruses with distinct characteristics such as pathogenicity and tissue tropism. This may likely to be the actual reason for the change in serotype and tissue tropism for the ck/CH/LZJ/111113 strain compared to ck/CH/LDL/091022.
In the CoV genome, as most RNA viruses, the 5′ and 3′ UTRs usually harbor important structural elements that are involved in replication and/or translation (Masters, 2006). In IBV, the 3′ UTR binds to the N protein which is essential for synthesis of negative-strain viral RNA. Perhaps the acquisition of the 3′ end of the N gene and the 3′ UTR from 4/91 by ck/CH/LZJ/111113 can alter the viral replication efficiency, as observed in this study. Hence, this alternation may in turn affect pathogenicity. However, further investigations using reverse genetic system should provide further insight into this issue and increase our understanding of IBV pathogenesis.
Acknowledgments
This work was supported by grants from the China Agriculture Research Systerm (no. CARS-41-K12) and Special Fund for Agro-scientific Research in the Public Interest (no. 201203056).
References
- Archetti I., Horsfall F.L. Persistent antigenic variation of influenza A viruses after incomplete neutralization in ova with heterologous immune serum. J. Exp. Med. 1950;92:441–462. doi: 10.1084/jem.92.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses. Arch. Virol. 2010;155:133–146. doi: 10.1007/s00705-009-0547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.K.A., Jackwood M., Jones R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41:239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- Han Z., Sun C., Yan B., Zhang X., Wang Y., Li C., Zhang Q., Ma Y., Shao Y., Liu Q., Kong X., Liu S. A 15-year analysis of molecular epidemiology of avian infectious bronchitis coronavirus in China. Infect. Genet. Evol. 2011;11:190–200. doi: 10.1016/j.meegid.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood M.W., Boynton T.O., Hilt D.A., McKinley E.T., Kissinger J.C., Paterson A.H., Robertson J., Lemke C., McCall A.W., Williams S.M., Jackwood J.W., Byrd L.A. Emergence of a group 3 coronavirus through recombination. Virology. 2010;398:98–108. doi: 10.1016/j.virol.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.M., Ellis R.J., Cox W.J., Errington J., Fuller C., Irvine R.M., Wakeley P.R. Development and validation of RT-PCR tests for the detection and S1 genotyping of infectious bronchitis virus and other closely related gammacoronaviruses within clinical samples. Transbound Emerg. Dis. 2011;58:411–420. doi: 10.1111/j.1865-1682.2011.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Kong X. A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinated flocks in China. Avian Pathol. 2004;33:321–327. doi: 10.1080/0307945042000220697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., Shao, Y., Ma, H., Sun, C., Zhang, X., Li, C., Han, Z., Yan, B., Kong, X., Liu, S. Comparative analysis of four Massachusetts type infectious bronchitis coronavirus genomes reveals a novel Massachusetts type strain and evidence of natural recombination in the genome. Infection, Genet. Evol., in press. [DOI] [PMC free article] [PubMed]
- Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Sun C., Han Z., Ma H., Zhang Q., Yan B., Shao Y., Xu J., Kong X., Liu S. Phylogenetic analysis of infectious bronchitis coronaviruses newly isolated in China, and pathogenicity and evaluation of protection induced by Massachusetts serotype H120 vaccine against QX-like strains. Avian Pathol. 2011;40:43–54. doi: 10.1080/03079457.2010.538037. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr J., Theil V., Gorbalenya A.E. The autolytic release of a putative RNA virus transcription factor from its polyprotein precursor involves two paralogus papin-like proteinase that cleave the same peptide bond. J. Biol. Chem. 2001;276:33220–33232. doi: 10.1074/jbc.M104097200. [DOI] [PMC free article] [PubMed] [Google Scholar]