Highlights
-
•
US PEDV was highly enteropathogenic in seronegative 9-day-old nursing piglets.
-
•
Infected weaned pigs were mostly sub-clinical and required a longer incubation period prior to PEDV infection, compared to nursing pigs.
-
•
There was a lack of LGR5+ crypt stem cells and low proliferation of crypts in uninfected nursing piglets.
-
•
The numbers of Ki67+ (marker for crypt proliferation) or LGR5+ crypt cells increased remarkably in nursing piglets after PEDV infection.
-
•
Biologic mediators that promote crypt stem cell regeneration would be therapeutic targets.
Keywords: Porcine epidemic diarrhea virus, PEDV, Pathogenesis, Virus, Pig
Abstract
Our study demonstrated potential mechanisms by which porcine epidemic diarrhea virus (PEDV) infection induces greater disease severity of nursing vs. weaned conventional pigs. Twenty-six-day-old weaned [PEDV-inoculated (n = 11); mock (n = 9)] and 9-day-old nursing pigs [PEDV-inoculated (n = 9); mock (n = 11)] were inoculated orally [8.9 log10 genomic equivalents (GE)/pig] with PC21A strain or mock (MEM). Pigs were monitored for clinical signs and PEDV RNA titers in feces and serum. For pathology and immunofluorescence staining for Ki67 (marker for crypt proliferation) and LGR5 (marker for crypt stem cell), 3–4 pigs were euthanized at postinoculation days (PIDs) 1, 3 and 5. Severe watery diarrhea and atrophic enteritis with moderate to high PEDV RNA titers in feces (7.5–12.2 log10 GE/ml) and low viral RNA titers in serum (5.6–8.6 log10 GE/ml) were observed in all inoculated nursing piglets at PIDs 1–5. In contrast, weaned pigs did not show evidence of PEDV infection at PID 1. Pigs exhibited high fecal shedding titers at PIDs 2–5 and mild to severe atrophic enteritis at PIDs 3–5, indicating a longer incubation for PEDV infection. While uninoculated or inoculated 27–31-day-old pigs showed large numbers of Ki67- or LGR5-positive cells in the intestinal crypts, there was a lack of LGR5-positive cells and low proliferation of crypts in jejunum of uninoculated 10–14-day-old piglets, possibly causing a slower turnover of enterocytes; however, the number of LGR5-positive cells and proliferation of intestinal crypts increased remarkably at 3–5 days after inoculation. Biologic mediators that promote crypt stem cell regeneration would be targets to improve the intestinal epithelium renewal during PEDV infection.
1. Introduction
Porcine epidemic diarrhea virus (PEDV), a member of the genera Alphacoronavirus in the family Coronaviridae of the order Nidovirales, causes acute diarrhea, vomiting, dehydration and high mortality in seronegative nursing piglets. PEDV is enveloped and pleomorphic with a range in diameter of 95–190 nm, including the spikes (Pensaert and de Bouck, 1978). PEDV has a single-stranded positive-sense RNA genome of approximately 28 kb (excluding the poly A-tail) that encodes 4 structural proteins (spike, envelope, membrane, and nucleocapsid protein) and 4 nonstructural proteins (1a, 1b, 3a, and 3b) (Kocherhans et al., 2001). PEDV has resulted in significant economic losses, initially only in the European and Asian swine industries over the last 3 decades. Recently, however, PEDV was first reported in the United States in May 2013 in Iowa and has spread nationally (Stevenson et al., 2013). PEDV outbreaks in the US have led to significant economic impacts as a result of the high mortality among seronegative nursing piglets and decreased pork production (Cima, 2013, Stevenson et al., 2013). The US PEDV (PC21A strain) was highly enteropathogenic in experimentally infected, 10–35-day-old gnotobiotic (Gn) pigs, as evident by severe clinical disease and lesions (Jung et al., 2014).
PED is the most devastating in nursing piglets (under 3-weeks-old), causing 100% morbidity and 50–100% mortality (Saif et al., 2012, Stevenson et al., 2013). In specific-pathogen-free (SPF) pigs from 2 days to 12 weeks of age, experimentally inoculated with a wild-type Japanese PEDV strain, deaths occurred mainly in 2- to 7-day-old pigs and virus replication was higher in newborn pigs (Shibata et al., 2000). Conventional 3-week-old weaned pigs experimentally inoculated with a US PEDV strain exhibited relatively milder clinical disease (Madson et al., 2014). However, the mechanisms by which PEDV infection induces greater disease severity and deaths of nursing piglets vs. weaned pigs, have not been clearly defined (Jung and Saif, 2015).
Factors that may influence the higher susceptibility of nursing piglets to PEDV infection and slower recovery from disease include the slower turnover of enterocytes in neonatal nursing piglets (5–7 days) vs. weaned pigs (2–3 days) (Jung and Saif, 2015, Moon et al., 1973). The proliferative level of intestinal crypt cells determines the turnover time of enterocytes in pigs. The turnover rate of the intestinal epithelium may also depend on stem cells in the intestinal crypts. Intestinal stem cells consist mainly of three cell types: leucine-rich repeat-containing G protein-coupled receptor 5-positive crypt base columnar cells (LGR5-positive crypt cells), +4 cells, and Paneth cells (Sato and Clevers, 2013); however, the presence of Paneth cells in the intestine of swine is debatable (Burkey et al., 2009). Intestinal crypt stem cells may be critical for the epithelial cell renewal during PEDV infection.
The aims of our study were to characterize the comparative pathogenesis (clinical disease, fecal virus shedding, viremia, and pathology) of the wild-type US PEDV strain PC21A in nursing piglets vs. weaned pigs; and to investigate the number of Ki67- or LGR5-positive crypt cells in the small intestine of nursing piglets vs. weaned pigs, inoculated with the same dose of PC21A, to explore the basis for the age-dependent severity of PED.
2. Materials and methods
2.1. Virus
The wild-type US PEDV strain PC21A was obtained from intestinal contents of a 1-day-old, diarrheic piglet on an Ohio farm in June 2013 (Jung et al., 2014). The original sample was serially passaged 2 times in Gn pigs. The original sample and Gn pig-passaged PC21A strain were negative by RT-PCR/PCR for transmissible gastroenteritis virus/porcine respiratory coronavirus (Kim et al., 2000), porcine deltacoronavirus (Jung et al., 2015b), rotavirus groups A, B, and C (Amimo et al., 2013a, Amimo et al., 2013b), porcine enteric caliciviruses [noroviruses and sapoviruses (Sisay et al., 2013) and St-Valerien-like viruses (Wang et al., 2011)], porcine astroviruses (Chu et al., 2008, Lee et al., 2013), and porcine circovirus (Chung et al., 2005), enterovirus, kobuvirus, and bocavirus. Immune electron microscopy, using a Gn pig hyperimmune serum to PEDV, showed only PEDV particles in the original sample and Gn pig-passaged PC21A strain (Jung et al., 2014). The titer of Gn pig 2nd-passaged PC21A was 11.8 log10 genomic equivalents (GE)/ml and was used as virus inoculum after dilution in minimal essential medium (MEM).
2.2. Conventional specific-pathogen-free pigs and experimental pig infection
Twenty 3-week-old, PEDV-seronegative weaned (n = 20), large white × Duroc crossbred pigs and 2 seronegative pregnant sows to acquire additional nursing piglets (n = 20), were obtained from a PEDV-free SPF (confirmed by history and seronegative sows; lack of qRT-PCR-positive fecal samples) swine herd of the Ohio State University. The SPF herd was seronegative for antibodies to PRRSV, PRCV, TGEV and porcine circovirus type 2. To mimic field conditions of nursing piglets, two sows were allowed to farrow naturally and nurse their piglets freely throughout the experiment. Twenty 9-day-old nursing piglets were randomly assigned to one of two groups and housed separately: PEDV infected (n = 9) and mock (n = 11). Additionally, twenty 26-day-old weaned pigs were randomly assigned to one of two groups with each housed separately: PEDV infected (n = 11) and Mock (n = 9). Weaned pigs were given free access to water and pelleted feed. Each experimental group of pigs was housed in a separate room in a high-security isolation facility (biosafety level 2).
Based on high pathogenicity of PC21A strain (6.8–9.0 log10 GE/pig) in 10–35-day-old Gn pigs identified in our previous study (Jung et al., 2014), nursing and weaned pigs were inoculated orally [8.9 log10 GE (≈2.9 log10 plaque forming units)/pig] (Jung et al., 2014) with the same dose of PC21A or mock inoculated with MEM. Inoculated and negative control pigs (n = 3–4/group at each time-point) were euthanized for pathological examination at an acute-stage (post-inoculation day (PID) 1), at a mid-stage (PID 3), and at a later-stage (PID 5) of infection. After PEDV inoculation, pigs were monitored for clinical signs 2–3 times daily until necropsy. Diarrhea was assessed by scoring fecal consistency. Fecal consistency was scored as follows: 0 = solid; 1 = pasty; 2 = semi-liquid; 3 = liquid, with scores of 2 or more considered diarrheic. The Institutional Animal Care and Use Committee of the Ohio State University approved all protocols related to the animal experiments in this study.
2.3. Analysis of PEDV RNA titers in fecal and serum samples
Rectal swabs and serum samples were collected from each animal throughout the experiment. Two rectal swabs were suspended in 4 ml MEM (Jung et al., 2014). The RNA was extracted from 50 μl of centrifuged (2000 × g for 30 min at 4 °C) fecal suspensions using the Mag-MAX Viral RNA Isolation Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. PEDV RNA titers in rectal swabs and serum samples were determined as described previously (Jung et al., 2014).
2.4. Gross and histological analysis and immunohistochemistry for the detection of PEDV antigen
Small (duodenum, proximal, middle and distal jejunum, and ileum) and large (cecum/colon) intestinal tissues and other major organs (lung, liver, heart, kidney, spleen, and lymph node) were examined grossly and histologically at PIDs 1, 3, and 5. Tissues were placed in 10% phosphate buffered formaldehyde (pH 7.0), dehydrated in graded alcohol, embedded in paraffin, and cut in 3-μm sections onto microscope slides, fixed and stained with hematoxylin and eosin (H&E) then analyzed for histopathological changes. The formalin-fixed, paraffin-embedded tissues were prepared and tested by immunohistochemistry (IHC) for the detection of PEDV antigen, using monoclonal antibody 6C8-1 against the spike protein of PEDV strain DR13 (provided by Dr. Daesub Song, Korea Research Institute of Bioscience and Biotechnology, Daejeon, Korea). The antibody was diluted 1:200 in PBST (phosphate-buffered saline (PBS) containing Tween 20, 0.1%). IHC was conducted as described previously (Jung et al., 2009).
2.5. Morphometric analysis
Eight pieces of formalin-fixed mid- to distal jejunum were taken from each virus-infected and control pigs for morphometric analysis. Only well-orientated, H&E- or IHC-stained jejunal sections were measured and care was taken to ensure that only transverse sections cut perpendicularly from villous enterocytes to the muscularis mucosa were included as described previously (Jung et al., 2006). Villous height, crypt depth, and number of PEDV antigen-positive cells were estimated by measuring at least 10 villi and crypts throughout the section. Mean ratios of jejunal villous height to crypt depth (VH:CD) were calculated as previously described (Jung et al., 2006).
2.6. Immunofluorescence (IF) staining for the detection of Ki67 and LGR5
To test the hypothesis that lower proliferation of intestinal crypts, or lower frequency of crypt stem cells may contribute to greater disease severity and deaths of nursing piglets vs. weaned pigs, Ki67- or LGR5-positive crypt cells were identified by IF staining in the small intestine of all pigs. Ki67 protein is a marker for proliferating crypt cells. The frozen tissues were prepared in Tissue-Tek OCT compound (Sakura, Torrance, CA, USA) and tested for the detection of Ki67 and LGR5 using monoclonal and polyclonal antibodies against human Ki67 (Dako, Glostrup, Denmark) and human LGR5 (Novus Biologicals, Littleton, CO, USA), respectively. The anti-Ki67 antibody was diluted 1:200 in PBST and incubated on the tissues at 4 °C overnight, and an anti-mouse antibody conjugated with Alexa Fluor® 488 (1:200; Invitrogen, CA, USA) was used as the detection antibody and incubated on the tissues at 37 °C for 1 h. The rabbit anti-LGR5 antibody was diluted 1:50 in PBST and incubated on the tissues at 4 °C overnight, and an anti-rabbit antibody conjugated with Alexa Fluor® 594 (Invitrogen) was used as the detection antibody and incubated on the tissues at room temperature for 1 h. The stained tissues were examined by fluorescence microscopy. Ki67- or LGR5-positive scores were computed by estimating the number of IF-positive cells in the intestinal section per microscopic area, at ×200 magnification based on the following criteria: 0, no positive cells; 1, 1–29% of Ki67- or LGR5-positive crypt epithelial cells showed staining; 2, 30–59% of Ki67- or LGR5-positive crypt epithelial cells showed staining; and 3, 60–100% of Ki67- or LGR5-positive crypt epithelial cells showed staining.
2.7. Data analysis
All values are expressed as the means ± standard error of the means (SEM) or standard deviation of the means (SDM). Fecal consistency scores, viral RNA titers (log10 transformed RNA titers) in rectal swabs and serum samples, and numbers of PEDV antigen-positive cells per villus from PEDV-infected nursing and weaned pigs were analyzed and compared by a Student’s t-test using GraphPad Prism software (GraphPad Prism Inc.). Numbers of Ki67- or LGR5-positive cells per villus from the four treatment groups (PEDV-inoculated nursing and weaned pigs and mock-inoculated nursing and weaned pigs) were analyzed and compared by one-way ANOVA using GraphPad Prism software. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Clinical observations and fecal consistency scores in PEDV-inoculated nursing piglets vs. weaned pigs
Clinical signs were first detected at PID 1 in PEDV-inoculated nursing piglets. All inoculated nursing piglets at PIDs 1–5 exhibited acute, severe watery diarrhea (all scores, 3) and/or vomiting, followed by lethargy and dehydration. However, no inoculated nursing piglets were found dead or were euthanized (moribund) due to severe clinical disease at PIDs 1–5. On the other hand, no clinical signs were detected until PID 3 in PEDV-inoculated weaned piglets. At PID 3, 3 of 8 inoculated weaned pigs exhibited mild diarrhea (all scores of 2) and/or vomiting. At PID 5, fecal samples from 4 inoculated weaned pigs appeared normal (scores, 1 or 1.5). No negative control nursing and weaned pigs showed diarrhea or other clinical signs throughout the experiment. At PIDs 1–5, fecal consistency scores in PEDV-inoculated nursing pigs were significantly higher than those in PEDV-inoculated weaned pigs (P < 0.01 at each-time point) (Fig. 1 ).
Fig. 1.
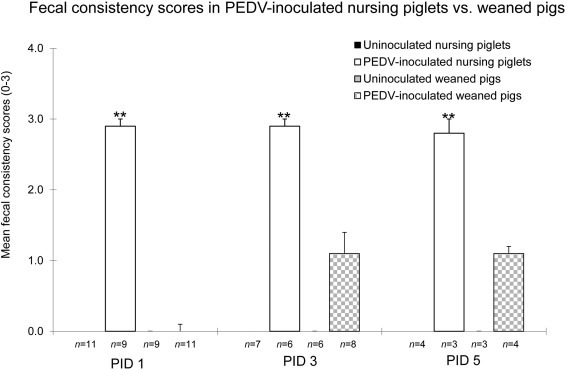
Significantly higher fecal consistency scores in PEDV-inoculated nursing pigs compared to PEDV-inoculated weaned pigs at PIDs 1-5. After PEDV inoculation, pigs were monitored for clinical signs 2–3 times daily until necropsy. Diarrhea was assessed by scoring fecal consistency. Fecal consistency was scored as follows: 0 = solid; 1 = pasty; 2 = semi-liquid; 3 = liquid, with scores of 2 or more considered diarrheic. Each bar represents the mean ± SEM. **, P < 0.01 (statistically significant differences between the PEDV-inoculated nursing and weaned pigs by Student’s t-test).
3.2. Fecal PEDV RNA titers in PEDV-inoculated nursing piglets vs. weaned pigs
By qRT-PCR, all inoculated nursing pigs tested at PIDs 1–5 had moderate to high viral RNA titers in rectal swab samples, ranging from 7.5 to 12.2 log10 GE/ml. Fecal viral RNA titers (mean titer, 11.9 log10 GE/ml) peaked at PID 1 (Fig. 2 ). Mean (± SEM) fecal viral RNA titers were 9.2 (± 0.7) and 9.3 (± 0.4) log10 GE/ml at PIDs 3 and 5, respectively. On the other hand, only 1 of 8 inoculated weaned pigs tested at PID 1 had a low fecal viral RNA titer (5.5 log10 GE/ml). At PID 2, low to high fecal viral RNA titers, ranging from 4.8 to 11.4 log10 GE/ml, were detected in 8 of 8 inoculated weaned pigs tested at PID 2. At PIDs 3 and 5, 12 of 12 inoculated weaned pigs tested had moderate to high fecal viral RNA titers, ranging from 7.7 to 12.8 log10 GE/ml. No negative control pigs had detectable viral RNA (<4.8 log10 GE/ml) in the feces throughout the experiment (Fig. 2). At PID 1, fecal shedding PEDV RNA titers in PEDV-inoculated nursing pigs were significantly higher than those in PEDV-inoculated weaned pigs (P < 0.01) (Fig. 2). At PID 3, there was no significant differences in mean fecal viral RNA titers between PEDV-inoculated nursing and weaned pigs. Conversely, at PID 5, fecal shedding PEDV RNA titers in PEDV-inoculated weaned pigs were significantly higher than those in PEDV-inoculated nursing pigs (P < 0.01) (Fig. 2).
Fig. 2.
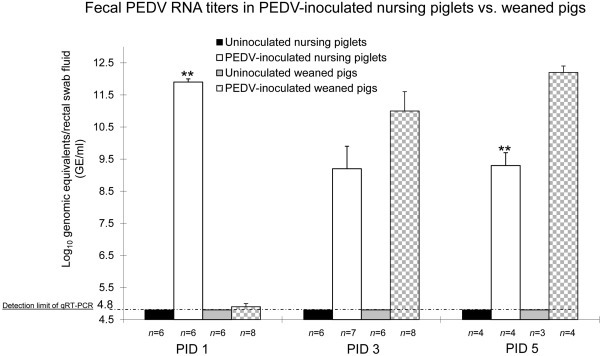
Significantly higher fecal shedding PEDV RNA titers at PID 1 but lower titers at PID 5 in PEDV-inoculated nursing pigs compared to PEDV-inoculated weaned pigs. Fecal shedding PEDV RNA titers were determined by qRT-PCR. The detection limit of qRT-PCR was 10 GE per reaction, corresponding to 4.8 log10 GE/ml of rectal swab fluid. Each bar represents the mean ± SEM. **, P < 0.01 (statistically significant differences between the PEDV-inoculated nursing and weaned pigs by Student’s t-test).
3.3. PEDV RNA titers in serum samples of PEDV-inoculated nursing piglets vs. weaned pigs
By qRT-PCR, all inoculated nursing pigs tested at PIDs 1–5 had low to moderate viral RNA titers in serum, ranging from 5.6 to 8.6 log10 GE/ml. Peak viral RNA titers in serum (mean titer, 7.7 log10 GE/ml) were observed at PID 1 (Fig. 3 ), which coincided with peak fecal viral RNA at PID 1 (Fig. 2). On the other hand, only 1 of 8 inoculated weaned pigs tested at PID 1 had a low viral RNA titer in serum (4.1 log10 GE/ml). At PIDs 3 and 5, 11 of 12 inoculated weaned pigs had low viral RNA titers in serum, ranging from 4.5 to 6.4 log10 GE/ml. No inoculated pigs or negative controls had detectable viral RNA (<3.8 log10 GE/ml) in the prebled serum samples prior to inoculation or during the experiment (Fig. 3). At PIDs 1–5, PEDV RNA titers in serum samples of PEDV-inoculated nursing pigs were significantly higher than those in PEDV-inoculated weaned pigs (P < 0.01 at PIDs 1 and 3; P < 0.05 at PID 5).
Fig. 3.
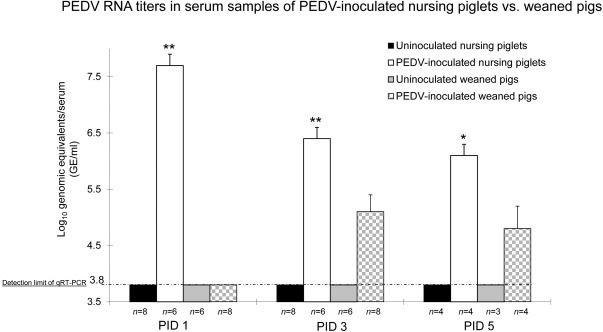
Significantly higher PEDV RNA titers in serum samples of PEDV-inoculated nursing pigs compared to PEDV-inoculated weaned pigs at PIDs 1-5. PEDV RNA titers in serum samples were determined by qRT-PCR. The detection limit of qRT-PCR was 10 GE per reaction, corresponding to 3.8 log10 GE/ml of serum sample. Each bar represents the mean ± SEM. *, P < 0.05; **, P < 0.01 (statistically significant differences between the PEDV-inoculated nursing and weaned pigs by Student’s t-test).
3.4. Gross and histologic lesions in PEDV-inoculated nursing piglets vs. weaned pigs
By macroscopic examination, all inoculated nursing pigs tested at PIDs 1–5 exhibited extensively thin and transparent intestinal walls and accumulation of large amounts of yellowish fluid in the small and large intestinal lumen, as observed previously (Debouck et al., 1981, Jung et al., 2014, Kim and Chae, 2000, Sueyoshi et al., 1995). The other internal organs appeared normal. On the other hand, there were no gross lesions evident in any inoculated weaned pigs euthanized at PIDs 1 and 3. All 4 inoculated weaned pigs tested at PID 5 had moderately thin and transparent intestinal walls in the small intestine and accumulation of large amounts of fluid in the small intestinal lumen (Fig. 4 A). Unlike the affected cecum/colon of inoculated nursing pigs, the large intestine of inoculated weaned pigs appeared normal, and solid stools were present in the lumen (Fig. 4A). The other internal organs of inoculated weaned pigs appeared normal. In inoculated nursing and weaned pigs, histologic lesions were limited to the small intestine, mainly jejunum and ileum, and included acute diffuse, severe atrophic enteritis. No histologic lesions were evident in the large intestine and other organs of the inoculated nursing and weaned pigs and negative controls.
Fig. 4.
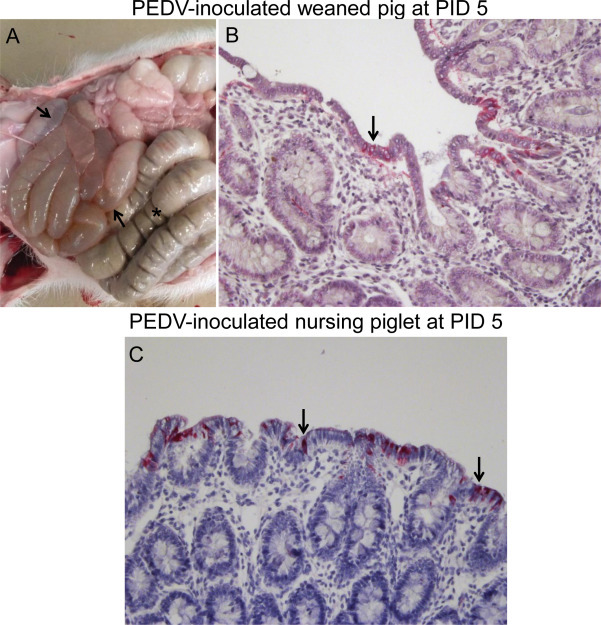
Gross lesions and immunohistochemistry for the detection of PEDV antigen in the intestine of PEDV-inoculated weaned (A and B) and nursing (C) pigs. (A) Intestine of a PEDV-inoculated weaned pig at PID 5, showing thin and transparent intestinal walls in the small intestine and accumulation of large amounts of fluid in the small intestinal lumen (arrows). Notice normal appearance of the large intestine and solid stools filled in the lumen (asterisk). (B) Colon of a PEDV-inoculated weaned pig at PID 5, showing low numbers of PEDV antigen-positive cells in the colonic epithelium. Original magnification × 200. (C) Colon of a PEDV-inoculated nursing pig at PID 5, showing moderate numbers of PEDV antigen-positive cells in the colonic epithelium. Original magnification × 200. Immunohistochemistry. Fast Red. Gill’s hematoxylin counterstaining.
3.5. VH:CD ratios in the small intestine of PEDV-inoculated nursing piglets vs. weaned pigs
To investigate the severity of atrophic enteritis in inoculated pigs, jejunal VH:CD ratios of all inoculated pigs were measured and compared with those of uninoculated negative control pigs at each time-point. Mean (± SDM) jejunal VH:CD ratios of uninoculated nursing piglets were 8.7 (± 1.4) at PID 1 (10 days of age), 7.6 (± 1.2) at PID 3 (12 days of age), and 7.1 (± 1.1) at PID 5 (14 days of age). On the other hand, mean (± SDM) jejunal VH:CD ratios of inoculated nursing piglets were 1.4 (± 0.5) at PID 1 (10 days of age), 1.2 (± 0.5) at PID 3 (12 days of age), and 1.1 (± 0.6) at PID 5 (14 days of age). At PIDs 1–5, VH:CD ratios in PEDV-inoculated nursing pigs were significantly lower than those in uninoculated nursing pigs (P < 0.01 at each-time point) (Fig. 5 A).
Fig. 5.
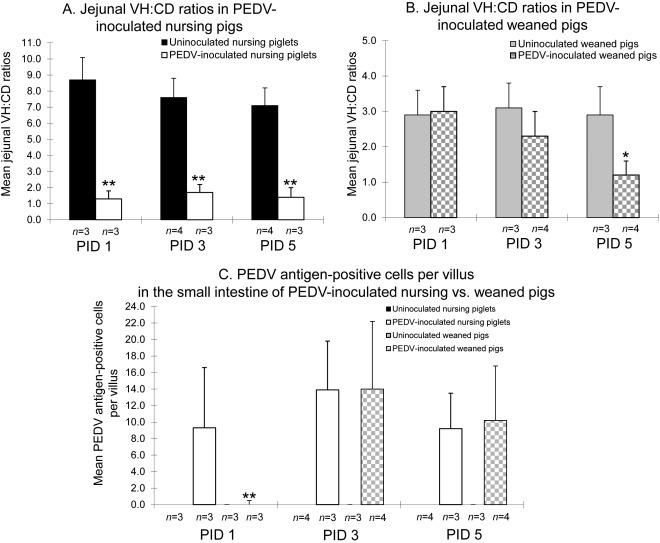
Mean ratios of jejunal villous height to crypt depth (VH:CD) in PEDV-inoculated nursing (A) and weaned (B) pigs. (C) Mean PEDV antigen-positive cells per villus in the small intestine of PEDV-inoculated nursing piglets vs. weaned pigs. Eight pieces of formalin-fixed mid- to distal jejunum were taken from each virus-infected and control pigs for morphometric analysis. Only well-orientated, H&E- or IHC-stained jejunal sections were measured. Villous height, crypt depth, and number of PEDV antigen-positive cells were estimated by measuring at least 10 villi and crypts throughout the section. Each bar represents the mean ± SEM. *, P < 0.05; **, P < 0.01 (statistically significant differences between the PEDV-inoculated nursing and weaned pigs by Student’s t-test).
Mean (± SDM) jejunal VH:CD ratios of uninoculated weaned pigs were 2.9 (± 0.7) at PID 1 (27 days of age), 3.1 (± 0.7) at PID 3 (29 days of age), and 2.9 (± 0.8) at PID 5 (31 days of age). On the other hand, mean (± SDM) jejunal VH:CD ratios of inoculated weaned pigs were 3.0 (± 0.7) at PID 1 (27 days of age), 2.3 (± 0.7) at PID 3 (29 days of age), and 1.2 (± 0.4) at PID 5 (31 days of age). Mean VH:CD ratios in PEDV-inoculated weaned pigs were slightly lower at PID 3 and significantly lower at PID 5 (P < 0.05), compared to uninoculated weaned pigs (Fig. 5B).
3.6. PEDV antigen-positive cells per villus in the small intestine of PEDV-inoculated nursing piglets vs. weaned pigs
Under our IHC conditions, mean (± SDM) PEDV antigen-positive cells per jejunal villus of inoculated nursing pigs were 9.3 (± 7.3) at PID 1, 13.9 (± 5.9) at PID 3, and 9.2 (± 4.3) at PID 5. On the other hand, mean (± SDM) PEDV antigen-positive cells per jejunal villus of inoculated weaned pigs were 0.0 (± 0.5) at PID 1, 14.0 (± 8.2) at PID 3, and 10.2 (± 6.6) at PID 5. At PID 1, significantly higher PEDV antigen-positive cells per jejunal villus in PEDV-inoculated nursing pigs were detected compared to PEDV-inoculated weaned pigs (P < 0.01) (Fig. 5C). There were no significant differences in mean numbers of PEDV antigen-positive cells per jejunal villus between PEDV-inoculated nursing and weaned pigs at PIDs 3 and 5 (Fig. 5C). Occasionally, PEDV antigen was detected in the cecal and/or colonic epithelial cells of inoculated nursing or weaned pigs (Fig. 4B and C), which did not coincide with the lack of gross and histologic lesions in the large intestine of the inoculated weaned pigs. No other internal organs of the inoculated pigs showed IHC-positive staining. No PEDV antigen-positive cells were detected in the negative control pigs.
3.7. Ki67-positive crypt epithelial cells in the small intestine of PEDV-inoculated nursing piglets vs. weaned pigs
In frozen jejunal tissues of the uninoculated nursing pigs at PIDs 1–5, small to moderate numbers of Ki67-positive cells were detected in the small intestinal crypts (Fig. 6 A and E). Ki67 protein was expressed and localized in the nuclei of crypt epithelial cells by IF staining (Fig. 6A–D). PEDV-inoculated nursing pigs tested at PIDs 1–5 exhibited large numbers of Ki67-positive cells in the crypts of the small intestine (Fig. 6B and E). Unlike nursing piglets, uninoculated or PEDV-inoculated weaned pigs tested at PIDs 1–5 exhibited large numbers of Ki67-positive cells in the crypt cell layers of the small intestine (Fig. 6C–E). At PIDs 1–5, mean Ki67-positive scores in the small intestine of uninoculated nursing pigs were significantly lower than those in the other groups (P < 0.01 at each-time point). However, there were no significant differences in mean Ki67-positive scores among the other three groups at PIDs 1–5.
Fig. 6.
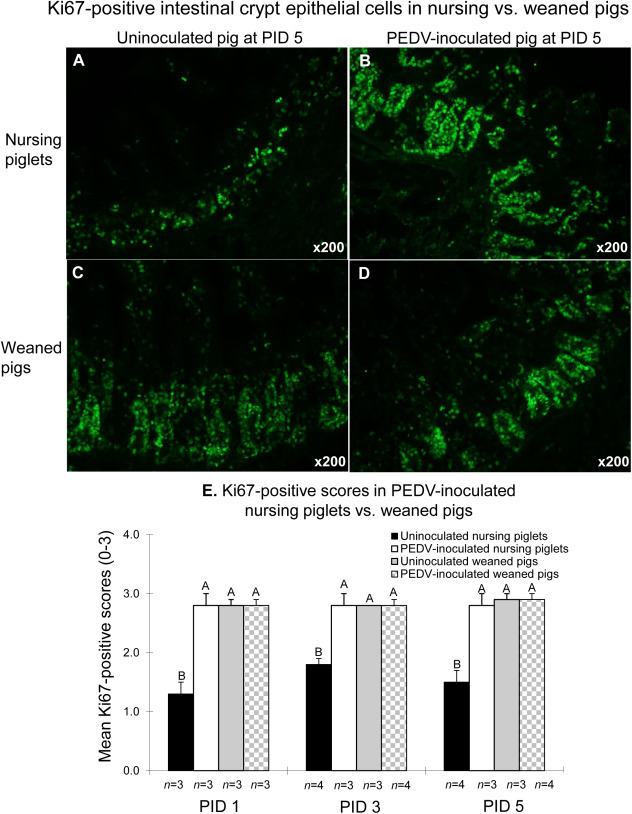
Detection of Ki67 protein by immunofluorescence (IF) staining in the small intestine of uninoculated (A) or PEDV-inoculated (B) nursing piglets compared to uninoculated (C) or PEDV-inoculated (D) weaned pigs at PID 5. (E) Mean Ki67-positive scores in the small intestine of PEDV-inoculated nursing piglets vs. weaned pigs. Ki67-positive scores were computed by estimating the number of IF-positive cells in the intestinal section per microscopic area, at ×200 magnification based on the following criteria: 0, no positive cells; 1, 1–29% of Ki67-positive crypt epithelial cells showed staining; 2, 30–59% of Ki67-positive crypt epithelial cells showed staining; and 3, 60–100% of Ki67-positive crypt epithelial cells showed staining. Each bar represents the mean ± SDM. Different letters denote significant differences among groups at each time-point (ANOVA test, P < 0.05).
3.8. LGR5-positive crypt epithelial cells in the small intestine of PEDV-inoculated nursing piglets vs. weaned pigs
No LGR5-positive cells were detected in uninoculated nursing pigs tested at PIDs 1–5 (Fig. 7 A and E). In frozen jejunal tissues of the inoculated nursing pigs at PIDs 3 and 5, but not PID 1, moderate to large numbers of LGR5-positive crypt cells were detected in the small intestine (Fig. 7B and E). Under our IHC conditions tested, LGR5 protein was detected on the apical surface or in the cytoplasm of intestinal crypt epithelial cells (Fig. 7B–D). On the other hand, regardless of infection status, weaned pigs exhibited moderate to large numbers of LGR5-positive cells in the crypts of the small intestine at PIDs 1–5 (Fig. 7C–E), which coincided with high Ki67-positve scores at these time-points (Fig. 6E).
Fig. 7.
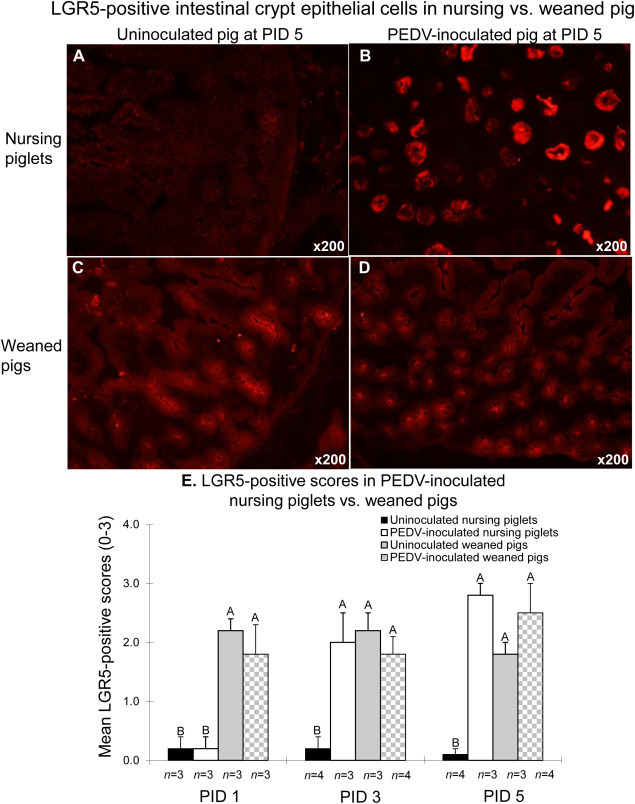
Detection of LGR5 protein by immunofluorescence (IF) staining in the small intestine of uninoculated (A) or PEDV-inoculated (B) nursing piglets compared to uninoculated (C) or PEDV-inoculated (D) weaned pigs at PID 5. (E) Mean LGR5-positive scores in the small intestine of PEDV-inoculated nursing piglets vs. weaned pigs. LGR5-positive scores were computed by estimating the number of IF-positive cells in the intestinal section per microscopic area, at ×200 magnification based on the following criteria: 0, no positive cells; 1, 1–29% of LGR5-positive crypt epithelial cells showed staining; 2, 30–59% of LGR5-positive crypt epithelial cells showed staining; and 3, 60–100% of LGR5-positive crypt epithelial cells showed staining. Each bar represents the mean ± SDM. Different letters denote significant differences among groups at each time-point (ANOVA test, P < 0.05).
4. Discussion
Our study demonstrates the comparative pathogenesis of the US PEDV strain PC21A and Ki67- or LGR5-positive intestinal crypt cells in nursing piglets vs. weaned pigs to explore the basis for the age-dependent severity of PEDV diarrhea, lesions and deaths. Our study confirmed that the US PEDV PC21A is highly enteropathogenic in conventional, seronegative nursing piglets, as evident by severe watery diarrhea and atrophic enteritis with moderate to high fecal shedding titers (7.5–12.2 log10 GE/ml) and viral RNA in serum (5.6–8.6 log10 GE/ml) in all inoculated 9–12-day-old pigs at PIDs 1–5. In contrast, during the acute stage of infection, conventional seronegative, 26-day-old weaned pigs appeared resistant to PC21A strain, as manifested by little clinical disease, fecal virus shedding, and gross and histologic intestinal lesions at PID 1. Thereafter, however, it was notable that the inoculated weaned pigs exhibited high fecal viral shedding titers at PIDs 2–5 and mild to severe atrophic enteritis at PIDs 3–5. A longer incubation period of PEDV was required for the weaned pigs to show fecal virus shedding (by 1 more day) or lesions (by 2 more days).
In our study, mean PEDV RNA titers peaked at PID 1 in rectal swab samples of PEDV-inoculated, 9-day-old nursing piglets and then decreased moderately at PIDs 3 and 5. Decreased fecal shedding PEDV titers at PIDs 3 and 5 might coincide with extensively reduced sites of viral replication following the substantially decreased numbers of enterocytes lining the epithelium of severely atrophied villi at these time-points (Fig. 5A). Regardless of the infection stage, all inoculated nursing piglets at PIDs 1–5 commonly exhibited high fecal consistency scores (all scores, 3). Based on these observations, PEDV-infected nursing piglets are mostly clinical and shed large amounts of viruses (viral RNA) in the diarrheic feces during the early to later stages of infection. For the clinical period (PIDs 1–5), severe atrophic enteritis observed in the inoculated pigs was mostly accompanied by severe watery diarrhea and high fecal shedding viral RNA titers.
In contrast, PEDV-inoculated, 26-day-old weaned pigs inoculated with the same dose of PEDV that infected 100% of nursing piglets, were mostly sub-clinical (9/12 pigs tested) at PIDs 3–5, while they exhibited mild to severe atrophic enteritis and high fecal shedding PEDV RNA titers. This observation confirms that sub-clinical PEDV-infected weaned pigs are able to shed a large amount of viruses (viral RNA) to infect other naïve pigs (Madson et al., 2014). It was notable that at PIDs 3–5, the inoculated weaned pigs (11/12 pigs tested) also showed low viral RNA titers in the sera. None of the PEDV-inoculated weaned pigs tested at PID 1 showed clinical disease (0/8 pigs tested), gross and histologic intestinal lesions (0/8 pigs tested), and PEDV antigen-positive cells in the intestine (0/8 pigs tested). The low frequency of fecal virus shedding (1/8 pigs tested) coincided with the similar low rate of detection of viral RNA in the serum samples of the corresponding pigs (1/8 pigs tested) and low fecal shedding RNA titer (mean, 4.9 log10 GE/ml) at this time-point and the finding that weaned pigs did not show evidence of PEDV infection at PID 1 (delayed onset). This indicates that weaned pigs failed to show evidence of US PEDV strain PC21A infection at PID 1. Significantly greater innate immune responses in ileum and blood at PID 1 may have contributed to the early resistance of weaned pigs to PEDV infection, compared to nursing piglets (Annamalai, T., Jung, K., Saif, L.J., unpublished). As observed in PEDV-inoculated nursing piglets at PID 1, high fecal shedding PEDV RNA titers were mostly accompanied by moderate viral RNA titers in serum. Overall, rather than the severity of clinical disease, the level of viremia in PEDV-infected pigs may relate to the severity of atrophic enteritis and structural alteration of tight and adherens junctions in the jejunal and ileal villous epithelium in a manner dependent on the extent of PEDV replication (reflected by fecal viral RNA shedding titer) in the intestine (Jung et al., 2015a, Jung et al., 2015b). The 2 sows and all weaned pigs used in our study were confirmed negative for PEDV antibodies by ELISA.
Relative to the 9–14-day-old nursing pigs tested in our study, 26–31-day-old weaned pigs had anatomically a more developed cecum and colon. Although the related mechanisms were unclear, gross and histologic lesions were limited to the small intestine of PEDV-inoculated nursing and weaned pigs, but not the large intestine. At PIDs 1–5, however, the large intestine of inoculated nursing piglets was mostly accumulated with large amounts of yellowish fluid, which coincided with the high frequency of severe watery diarrhea for the period. Large amounts of diarrheic fluid filled the small intestine of PEDV-inoculated weaned pigs at PID 5, but stools in the large intestinal lumen appeared solid. By pathological examinations, the structurally normal large intestine of PEDV-infected weaned pigs may contribute to reabsorption of the large amount of water accumulated or released from the small intestinal lumen, thereby maintaining hydration and leading to a rapid recovery from disease in weaned pigs. The normal large intestine of PEDV-inoculated weaned pigs also coincided with the frequent lack of diarrhea (9/12 pigs tested) at PIDs 3 and 5.
Our study, for the first time, revealed localization of Ki67- or LGR5-positive crypt cells in the small intestine of nursing and weaned pigs, with or without PEDV infection. Intestinal stem cells located in the villous crypts replace the apoptotic (normal process) or necrotic (virus-induced) enterocytes shed at the villous tips and are essential for maintenance of the intestinal epithelium (Saif et al., 2012). The turnover rate of the intestinal epithelium may depend on the stem cells and their proliferative levels in the intestinal crypt. The turnover of enterocytes was slower in neonatal nursing piglets vs. weaned pigs (Moon et al., 1973). Our study suggests that a lack of LGR5-positive crypt stem cells and lower numbers of proliferating crypt cells in the small intestine of uninoculated nursing piglets might lead to the slower turnover of enterocytes in nursing piglets vs. weaned pigs, possibly also influencing the slower recovery from disease in nursing piglets. After PEDV inoculation, however, the number of LGR5-positive crypt stem cells and proliferation of intestinal crypt cells in nursing piglets increased remarkably at PIDs 3 and 5, likely leading to replacement of necrotic enterocytes shed from infected villi. On the other hand, the uninoculated weaned pigs tested at PIDs 1–5 exhibited high numbers of proliferating intestinal crypt cells and LGR5-positive crypt stem cells, likely contributing to the rapid turnover rate of enterocytes. The high proliferation rates and LGR5-positive scores in the intestinal crypts were maintained after PEDV inoculation, ultimately influencing the rapid recovery from disease in weaned pigs.
In conclusion, the high mortality of PEDV-infected, seronegative nursing piglets is likely associated with extensive dehydration as a result of severe villous atrophy. Partially based on our data, in infected nursing piglets, there are increased proliferation of crypt cells and numbers of LGR5+ crypt stem cells in the intestine, reorganization of the damaged intestinal epithelium, and migration of mature enterocytes to the tips of villi which may be not sufficient to prevent severe dehydration in nursing piglets. This process is essential for efficient digestion and adsorption of milk or water. The rapid dehydration of PEDV-infected nursing piglets in the field limits recovery from the disease through the naturally occurring epithelial cell renewal by crypt stem cells. Pharmacologic or biologic mediators such as epidermal growth factor that promote stem cell regeneration or maturation would be interesting targets to use to try to shorten the time for epithelial cell renewal and reduce PEDV death losses among nursing pigs from dehydration.
Conflict of interest
Neither of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Acknowledgements
We thank Dr. Juliette Hanson, Andrew Wright, Megan Strother, and Ronna Wood for assistance with animal care; and Xiaohong Wang, Bryan Eyerly and John Blakenship for technical assistance. Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University. This work was supported by a grant from the OARDC SEEDS, Grant # OAOH1536 (Jung K, PI).
Contributor Information
Kwonil Jung, Email: jung.221@osu.edu.
Linda J. Saif, Email: saif.2@osu.edu.
References
- Amimo J.O., Vlasova A.N., Saif L.J. Detection and genetic diversity of porcine group A rotaviruses in historic (2004) and recent (2011 and 2012) swine fecal samples in Ohio: predominance of the G9P[13] genotype in nursing piglets. J. Clin. Microbiol. 2013;51:1142–1151. doi: 10.1128/JCM.03193-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amimo J.O., Vlasova A.N., Saif L.J. Prevalence and genetic heterogeneity of porcine group C rotaviruses in nursing and weaned piglets in Ohio, USA and identification of a potential new VP4 genotype. Vet. Microbiol. 2013;164:27–38. doi: 10.1016/j.vetmic.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey T.E., Skjolaas K.A., Minton J.E. Board-invited review: porcine mucosal immunity of the gastrointestinal tract. J. Anim. Sci. 2009;87:1493–1501. doi: 10.2527/jas.2008-1330. [DOI] [PubMed] [Google Scholar]
- Chu D.K., Poon L.L., Guan Y., Peiris J.S. Novel astroviruses in insectivorous bats. J. Virol. 2008;82:9107–9114. doi: 10.1128/JVI.00857-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.B., Chan W.H., Chaung H.C., Lien Y., Wu C.C., Huang Y.L. Real-time PCR for quantitation of porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 in naturally-infected and challenged pigs. J. Virol. Methods. 2005;124:11–19. doi: 10.1016/j.jviromet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Cima G. Viral disease affects U.S. pigs: porcine epidemic diarrhea found in at least 11 states. J. Am. Vet. Med. Assoc. 2013;243:30–31. [PubMed] [Google Scholar]
- Debouck P., Pensaert M., Coussement W. The pathogenesis of an enteric infection in pigs, experimentally induced by the coronavirus-like agent, Cv-777. Vet. Microbiol. 1981;6:157–165. [Google Scholar]
- Jung K., Saif L.J. Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015 doi: 10.1016/j.tvjl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Kim J., Ha Y., Choi C., Chae C. The effects of transplacental porcine circovirus type 2 infection on porcine epidemic diarrhoea virus-induced enteritis in preweaning piglets. Vet. J. 2006;171:445–450. doi: 10.1016/j.tvjl.2005.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Renukaradhya G.J., Alekseev K.P., Fang Y., Tang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus modifies innate immunity and alters disease outcome in pigs subsequently infected with porcine respiratory coronavirus: implications for respiratory viral co-infections. J. Gen. Virol. 2009;90:2713–2723. doi: 10.1099/vir.0.014001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Wang Q., Scheuer K.A., Lu Z., Zhang Y., Saif L.J. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg. Infect. Dis. 2014;20:662–665. doi: 10.3201/eid2004.131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Eyerly B., Annamalai T., Lu Z., Saif L.J. Structural alteration of tight and adherens junctions in villous and crypt epithelium of the small and large intestine of conventional nursing piglets infected with porcine epidemic diarrhea virus. Vet. Microbiol. 2015 doi: 10.1016/j.vetmic.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Hu H., Eyerly B., Lu Z., Chepngeno J., Saif L.J. Pathogenicity of 2 porcine deltacoronavirus strains in gnotobiotic pigs. Emerg. Infect. Dis. 2015;21:650–654. doi: 10.3201/eid2104.141859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim O., Chae C. In situ hybridization for the detection and localization of porcine epidemic diarrhea virus in the intestinal tissues from naturally infected piglets. Vet. Pathol. 2000;37:62–67. doi: 10.1354/vp.37-1-62. [DOI] [PubMed] [Google Scholar]
- Kim L., Chang K.O., Sestak K., Parwani A., Saif L.J. Development of a reverse transcription-nested polymerase chain reaction assay for differential diagnosis of transmissible gastroenteritis virus and porcine respiratory coronavirus from feces and nasal swabs of infected pigs. J. Vet. Diagn. Invest. 2000;12:385–388. doi: 10.1177/104063870001200418. [DOI] [PubMed] [Google Scholar]
- Kocherhans R., Bridgen A., Ackermann M., Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes. 2001;23:137–144. doi: 10.1023/A:1011831902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.H., Jeoung H.Y., Park H.R., Lim J.A., Song J.Y., An D.J. Phylogenetic analysis of porcine astrovirus in domestic pigs and wild boars in South Korea. Virus Genes. 2013;46:175–181. doi: 10.1007/s11262-012-0816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madson D.M., Magstadt D.R., Arruda P.H., Hoang H., Sun D., Bower L.P., Bhandari M., Burrough E.R., Gauger P.C., Pillatzki A.E., Stevenson G.W., Wilberts B.L., Brodie J., Harmon K.M., Wang C., Main R.G., Zhang J., Yoon K.J. Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs. Vet. Microbiol. 2014;174:60–68. doi: 10.1016/j.vetmic.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Moon H.W., Norman J.O., Lambert G. Age dependent resistance to transmissible gastroenteritis of swine (TGE): I. Clinical signs and some mucosal dimensions in small intestine. Can. J. Comp. Med. 1973;37:157–166. [PMC free article] [PubMed] [Google Scholar]
- Pensaert M.B., de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L.J., Pensaert M.P., Sestak K., Yeo S.G., Jung K. Coronaviruses. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J., Stevenson G.W., editors. Diseases of Swine. Wiley-Blackwell, Iowa State University; 2012. pp. 501–524. [Google Scholar]
- Sato T., Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- Shibata I., Tsuda T., Mori M., Ono M., Sueyoshi M., Uruno K. Isolation of porcine epidemic diarrhea virus in porcine cell cultures and experimental infection of pigs of different ages. Vet. Microbiol. 2000;72:173–182. doi: 10.1016/S0378-1135(99)00199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisay Z., Wang Q., Oka T., Saif L. Prevalence and molecular characterization of porcine enteric caliciviruses and first detection of porcine kobuviruses in US swine. Arch. Virol. 2013;158:1583–1588. doi: 10.1007/s00705-013-1619-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G.W., Hoang H., Schwartz K.J., Burrough E.R., Sun D., Madson D., Cooper V.L., Pillatzki A., Gauger P., Schmitt B.J., Koster L.G., Killian M.L., Yoon K.J. Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Invest. 2013;25:649–654. doi: 10.1177/1040638713501675. [DOI] [PubMed] [Google Scholar]
- Sueyoshi M., Tsuda T., Yamazaki K., Yoshida K., Nakazawa M., Sato K., Minami T., Iwashita K., Watanabe M., Suzuki Y. An immunohistochemical investigation of porcine epidemic diarrhoea. J. Comp. Pathol. 1995;113:59–67. doi: 10.1016/S0021-9975(05)80069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Scheuer K., Ahang Z., Gebreyes W.A., Molla B.Z., Hoet A.E., Saif L.J. Characterization and prevalence of a new porcine calicivirus in Swine, United States. Emerg. Infect. Dis. 2011;17:1103–1106. doi: 10.3201/eid1706.101756. [DOI] [PMC free article] [PubMed] [Google Scholar]


