Abstract
The safety and the efficacy of a modified-live (ML) canine coronavirus (CCoV) vaccine strain 257/98-3c was evaluated in 14 dogs seronegative and virus negative for CCoV. For the safety test, four dogs were inoculated, two by intramuscular and two by oronasal route, with 10 times the vaccinal dose. During the observation period (28 days) all dogs did not display any local or systemic reaction. For the efficacy test, eight dogs were vaccinated by intramuscular (four dogs—group A) or by oronasal route (four dogs—group B). Two dogs were maintained as non-vaccinated controls. In the dogs of group A, vaccinal virus was not detected in faecal samples by virus isolation (VI) and by PCR assay, while in the dogs of group B, the virus was revealed for six median days only by PCR. Twenty-eight days later, the vaccinated and control dogs were challenged with a field CCoV strain. After the challenge, the vaccinated dogs did not display clinical signs and the dogs of group A shed virus for 5.5 median days, evaluated by VI, and for 10 median days evaluated by PCR. Virus shedding was not observed, both by VI and PCR assay, in the dogs of group B. The two control dogs displayed moderate clinical signs and the virus was detected by VI for 14.5 median days starting from day 3 post-challenge (dpc 3) and by PCR assay for 23 median days starting from dpc 1.
Keywords: Canine coronavirus, Vaccine, Efficacy
1. Introduction
Coronaviruses are positive-stranded RNA viruses recently classified in the order Nidovirales, mainly on the basis of their genomic organization and their replication strategy (de Vries et al., 1997). The coronavirus genome consists of a large open reading frame (ORF) at the 5′-end which encodes the replicase gene (ORF1a and ORF1b). Downstream from the replicase gene, there are smaller ORFs which encode the structural proteins S, E, M, the nucleocapsid (N) protein and a number of presumptive non-structural proteins of largely unknown function (Luytjes, 1995).
Most research has focused on the S protein as a candidate antigen for coronavirus vaccines, since it is the major inducer of virus-neutralizing antibodies (Gebauer et al., 1991). The function of the M protein is still not clear. Although a major immunological role has been attributed to the S protein, both the amino- and the carboxy-termini of the M protein elicit strong immune responses (Enjuanes et al., 2000), inducing antibody-dependent, complement-mediated, virus neutralization (VN) (Woods et al., 1987).
The role of CCoV in inducing enteric illness in canids has been the subject of active investigation for over two decades. The virus is responsible for mild to moderate enteritis in dogs; in young pups, or in combination with other pathogens, illness may be severe with diarrhoea, vomiting, dehydration, loss of appetite and even death (Evermann et al., 1980, Appel, 1988). Shedding of CCoV in faeces occurs over a range of 6–14 days post-infection (Keenan et al., 1976, Tennant et al., 1991), however, faecal shedding in infected pups has been detected by n-PCR for periods from 37 to 150 days (Pratelli et al., 2001, Pratelli et al., 2002a). The value of CCoV vaccines in providing adequate immunity under field conditions is controversial. Although the efficacy and the duration of immunity engendered by inactivated vaccines have not been substantiated, killed CCoV vaccines have been licensed in the USA. In a recent study, the low efficacy of an inactivated CCoV vaccine in reducing the viral shedding in faeces of dogs after CCoV infection is described (Pratelli et al., 2003). A modified-live (ML) CCoV vaccine was licensed in USA in 1983, but was rapidly withdrawn due to a high rate (about 5%) of serious adverse reactions (Martin, 1985, Wilson et al., 1986). A ML CCoV vaccine, available in California and recently licensed in USA, appears to be safe and 1-year duration of immunity is claimed by the manufacturer. Unfortunately, the CCoV vaccine, when combined with canine cell-grown distemper vaccine (Rockborn strain), resulted in a high frequency of post-distemper encephalitis (Carmichael, 1997).
In the present note, we report the results of a study on the safety and efficacy of a ML CCoV vaccine in dogs, administered by intramuscular or oronasal route.
2. Materials and methods
2.1. Animals
Fourteen conventional dogs, 3 months of age were used. They were negative for CCoV antigen in the faeces, and CCoV antibodies before the experiment. Baseline body temperature and white blood cell (WBC) count were established for each dog averaging results of the 3 days before vaccination. The experimental study was performed according to the animal health and well-being regulations and was authorized by the Minister of Health of Italy (authorization no. 67/2002-C).
2.2. Vaccine
A modified-live CCoV vaccine, strain 257/98-3c, was used. The virus was originally isolated from a faecal sample of a dog with mild enteritis and was serially passaged 40 times on canine cells (A-72). The virus at the 40th passage had an infectivity titer of 104.50 TCID50/50 μl and was used throughout the study. The stock vaccine had been tested for sterility from aerobe and anaerobe bacteria, mycoplasmas, mycetes and contaminant viruses using standardized methods.
2.3. Safety test
To test the safety of the ML CCoV vaccine, four dogs (nos. 1–4) were inoculated, two by intramuscular (nos. 1 and 2) and two by oronasal route (nos. 3 and 4), with 10 times the vaccinal dose inoculated in the efficacy test. Following inoculation, dogs were individually isolated and daily observed for 28 days for signs of illness, WBC count and body temperature. For virological assays, individual faecal samples were collected from 3 days before the test through day 28. On day 28 all dogs were bled for serology.
2.4. Challenge
The field CCoV strain 144/01 was isolated from a faecal sample of a diarrhoeic pup (Marsilio et al., 2002) and was used to challenge the dogs. The virus was propagated on primary canine embryonic kidney (CEK) cells for three passages and stored at −70 °C. All dogs (immunized and control) were challenged 28 days after vaccination. Each animal received 4 ml of viral suspension (2 ml intranasally and 2 ml orally) with a titer of 105 TCID50/50 μl.
2.5. Efficacy test
After an acclimatizing period of 10 days, 10 dogs were housed separately. Eight dogs, randomly chosen, were vaccinated by intramuscular injection (four dogs—group A) or by oronasal route (four dogs—group B), each with 2 ml of ML CCoV strain 257/98-3c. Two dogs were maintained non-vaccinated. Following vaccination, the dogs were observed for any adverse local or systemic reactions and faecal samples were collected daily for 28 days post-vaccination (dpv).
Twenty-eight days later, the vaccinated and control dogs were challenged with CCoV strain 144/01. On the day of challenge, and for 28 days post-challenge (dpc), the dogs were examined clinically and virus shedding was monitored.
Because dogs are generally refractory to clinical disease after experimental infection with CCoV, protective immunity was assessed also by monitoring of virus shedding (median days of virus shedding). The experimental design is reported in Table 1 .
Table 1.
Experimental design and sampling
| Groups | Dogs | Vaccination route | Faecal sampling for VI and PCR | Serum sampling for VN and ELISA |
| A | 4 | im | Daily, for dpv 28 and dpc 28 | Weekly, for dpv 28 and dpc 28 |
| B | 4 | o/n | Daily, for dpv 28 and dpc 28 | Weekly, for dpv 28 and dpc 28 |
| Controls | 2 | no | Daily, for dpc 28 | Weekly, for dpc 28 |
im: intramuscular; o/n: oronasal; VI: virus isolation; VN: virus neutralization test; dpv: days post-vaccination; dpc: days post-challenge; no: not vaccinated.
2.6. Samples analysis
For virus isolation (VI), faecal samples were homogenized (10% w/v) in minimal essential medium (MEM). After centrifugation for 10 min at 4000×g, 200 μl of the supernatant, with antibiotics (5000 IU/ml penicillin, 2500 μg/ml streptomycin, 10 μg/ml amphotericin B), were inoculated in duplicate onto freshly trypsinised A-72 cells in 24 well-plates containing glass slides. The cells were observed daily for cytopathic effects (cpe) and, after 72 h, were fixed with cold acetone and tested by an immunofluorescence test (IF) using a CCoV monoclonal antibody (gently supplied by Dr. Gilles Chappuis, Merial, France). Each sample was considered negative if cpe or IF was not observed after three serial passages.
Additionally, a PCR test (Pratelli et al., 1999a) was used to detect CCoV in faecal samples. Briefly, reverse transcription was performed in a total reaction volume of 20 μl containing PCR buffer 1X (KCl 50 mM, Tris–HCl 10 mM, pH 8.3), MgCl2 5 mM, 1 mM of each deoxynucleotide (dATP, dCTP, dGTP, dTTP), RNase 1 U, MuLV reverse transcriptase 2.5 U, random hexamers 2.5 U. Synthesis of cDNA was carried out at 42 °C for 30 min, followed by a denaturation step at 99 °C for 5 min. The mixture was brought up to a total volume of 100 μl, containing PCR buffer 1X, MgCl2 2 mM, Amplitaq Gold DNA polymerase 2.5 U and 50 pmol of each primer, CCoV1 and CCoV2. The PCR mixture was subjected to 35 cycles (94 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min) in a DNA thermal cycler.
2.7. Serological tests
Blood samples were collected from the 10 dogs for serological tests on the day of vaccination and, later, on dpv 7, 14, 21 and 28.
Additional samples were collected on the day of challenge and at 7, 14, 21 and 28 dpc. ELISA and virus neutralization tests were carried out as described (Elia et al., 2002, Pratelli et al., 2002b); VN titers are expressed as geometric means and OD values as median values.
2.8. Statistical analysis
Given the small size of the comparison groups and the non-normal distribution of data, as verified by the Kolmogorov–Smirnov test, the non-parametric Mann–Whitney U-test was used to compare the duration of CCoV shedding, the VN titers and the ELISA OD values after vaccination and after challenge. A P-value<0.05 was considered as significant.
3. Results
3.1. Safety
Following intramuscular or oronasal inoculation of 10 times the vaccinal dose, all dogs remained normal and alert throughout the observation period and no local or generalized reactions to the vaccine were observed. WBC counts, performed daily in order to evaluate total leukocyte, remained normal.
Virus isolation and PCR assay performed on the faecal samples of the two dogs inoculated by intramuscular route were negative. The faecal samples of the two dogs inoculated by oronasal route were PCR positive for 6 days, starting from dpv 2 to 7. Serologic tests (VN and ELISA) carried out at dpv 28 indicated that all dogs had developed CCoV antibody titers (Table 2 ).
Table 2.
Results of the safety test of the CCoV vaccine in dogs
| Dogs | Route | Virus shedding (days) |
Antibodies (dpv 28) |
||
| VI | PCR | VN | ELISA | ||
| 1 | im | 0 | 0 | 4 | 0.152 |
| 2 | im | 0 | 0 | 8 | 0.164 |
| 3 | o/n | 0 | 6 | 8 | 0.190 |
| 4 | o/n | 0 | 6 | 8 | 0.215 |
im: intramuscular; o/n: oronasal; VI: virus isolation; VN: virus neutralization titer; dpv: days post-vaccination.
3.2. Efficacy in dogs vaccinated by intramuscular route (group A)
After vaccination, VI and PCR of the faecal samples were negative in all the dogs (Table 3 a). By the VN test, at dpv 28 all dogs had slight increases (1:4) in VN antibodies titers. By the ELISA test, the highest antibody values were observed at dpv 28 (OD=0.143) (Fig. 1 ).
Table 3.
Results of the efficacy test in dogs after CCoV vaccination (a) and CCoV challenge (b)
| Group | Dogs | Virus shedding |
||||||||||
| VI |
PCR |
|||||||||||
| Onseta | Duration | Meanb | Onseta | Duration | Meanb | |||||||
| (a) CCoV vaccination | ||||||||||||
| Group A | 1 | nd | nd | |||||||||
| 2 | nd | nd | ||||||||||
| 3 | nd | nd | ||||||||||
| 4 | nd | nd | ||||||||||
| Group B | 1 | nd | 2 | 6 | 6 | |||||||
| 2 | nd | 2 | 6 | |||||||||
| 3 | nd | 2 | 6 | |||||||||
| 4 | nd | 2 | 5 | |||||||||
| Group | Dogs |
Virus shedding |
|
|
|
|
|
|||||
| VI |
PCR |
|||||||||||
| Onsetc | Duration | Meanb | Onsetc | Duration | Meanb | |||||||
| (b) CCoV challenge | ||||||||||||
| Group A | 1 | 4 | 6 | 5.5 | 2 | 11 | 10 | |||||
| 2 | 4 | 5 | 2 | 8 | ||||||||
| 3 | 4 | 5 | 2 | 9 | ||||||||
| 4 | 4 | 7 | 2 | 11 | ||||||||
| Group B | 1 | nd | nd | |||||||||
| 2 | nd | nd | ||||||||||
| 3 | nd | nd | ||||||||||
| 4 | nd | nd | ||||||||||
| Control | 1 | 3 | 14 | 14.5 | 1 | 22 | 23 | |||||
| Control | 2 | 3 | 15 | 1 | 24 | |||||||
Group A: dogs vaccinated by intramuscular route; Group B: dogs vaccinated by oronasal route; VI: virus isolation; nd: not detected.
Days post-vaccination.
Median days.
Days post-challenge.
Fig. 1.
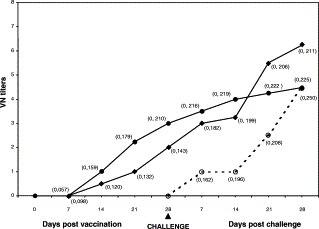
Serological responses in dogs after CCoV vaccination and CCoV challenge: (◆) dogs vaccinated by intramuscular route; (•) dogs vaccinated by oronasal route; (○) unvaccinated dogs. VN titers are expressed as the reciprocal (log2) of the geometric means. ELISA OD median values are shown in brackets.
After the challenge, the vaccinated dogs displayed no clinical signs and they shed virus for 5.5 days (median value) from dpc 4 to 10. By PCR, CCoV was detected for 10 days (median value), starting from dpc 2 to 12 (Table 3b). Antibody titers in the vaccinated dogs, as evaluated by VN test, increased progressively to 1:64 (three dogs) and 1:128 (one dog) (geometric mean=75.9) at dpc 28. ELISA test showed progressively increasing OD values, reaching the highest values at dpc 28 (OD=0.211) (Fig. 1).
3.3. Efficacy in dogs vaccinated by oronasal route (group B)
After vaccination, VI from faecal samples was negative, while the PCR assay revealed CCoV in the samples for 6 days (median value) from dpv 2 to 7 (Table 3a). By VN test, antibodies reached values of 1:8 between dpv 21 and 28. By ELISA test, antibodies reached the highest value at dpv 28 (OD=0.210) (Fig. 1).
After challenge, the vaccinated dogs did not have any clinical signs or virus shedding by either VI or PCR assays (Table 3b). However, there was seroconversion in all dogs by both VN and ELISA tests, with VN titers 1:16 (two dogs) and 1:32 (two dogs) (geometric mean=22.4) and OD=0.225, at 28 dpc (Fig. 1).
3.4. Unvaccinated dogs (controls)
The two control dogs inoculated with the field strain developed hyperthermia from dpc 2 to 4 and mild diarrhoea from dpc 3 to 6. In the faecal samples, virus was isolated, respectively, from dpc 3 to 16–17 (median value=14.5). By PCR, virus was detected, respectively, from dpc 1 to 22–24 (median value=23) (Table 3b).
Antibodies by VN test increased at 28 dpc (geometric mean=22.4). By ELISA test the highest OD values were reached at dpc 28 (OD median value=0.250) (Fig. 1).
3.5. Statistical analysis
The length of CCoV shedding as well as the antibody levels detected by VN and by ELISA tests after vaccination showed statistically significant differences between groups A and B. The P-value of the Mann–Whitney U-test was 0.029 for all comparisons.
Statistically significant differences were observed after CCoV challenge, between groups A and B, regarding the length of CCoV shedding, as detected by VI and by PCR, and in regarding the VN antibody titers (the P-value of the Mann–Whitney U-test was 0.029 in all cases).
Differences in length of viral shedding between group A and control dogs (P=0.133) and between group B and control dogs (P=0.067) were not significant.
4. Discussion
In the present study we report the safety and the efficacy of a ML CCoV vaccine in dogs. No local or systemic adverse reactions were observed in the vaccinated dogs. After vaccination, infectious virus was not detected in faecal samples by VI or CCoV nucleic acid by the PCR assay in dogs inoculated by the intramuscular route (group A). However, virus nucleic acid was revealed by PCR for six median days in the dogs inoculated by the oronasal route (group B), suggesting low viral titers or viral reminds in the samples (Pratelli et al., 1999a). In general, the efficacy of the vaccine appeared satisfactory. Considering that the protection against coronavirus infections has been generally correlated with the presence of specific antibodies in the mucosal surface (Ogra et al., 1980, Saif, 1996, Murphy, 1999), a significant difference was observed between dogs of groups A (intramuscular vaccination) and B (oronasal vaccination) (P=0.029).
It should be stressed that no CCoV shedding, after challenge, was observed by either VI or PCR in the dogs vaccinated by oronasal route (group B).
The sera of dogs from group A showed high OD values in the ELISA test, confirming that this test is substantially more sensitive than the VN test in detecting antibodies to CCoV (Pratelli et al., 2002b). After CCoV challenge, evaluation of the reduction of challenge virus shed in faeces revealed a decrease in virus in the vaccinated dogs, compared with the control dogs.
In the dogs vaccinated by oronasal route (group B) high antibody values were detected by the ELISA test. Protection from CCoV infection was complete since challenge virus was never detected by VI or by PCR assay. It seems plausible that the protection observed in the vaccinated groups might be modulated by the production of mucosal IgA (Ogra et al., 1980, Murphy, 1999).
We do not have data on the production of faecal IgA in dogs after intramuscular or oronasal administration of ML CCoV vaccine. However, it may be speculated that dogs vaccinated by the intramuscular route had low levels of faecal IgA which provided partial protection to challenge, but insufficient to confer complete protection as observed in TGEV pigs (Saif, 1996).
While the immune mechanisms of the protection from CCoV infection are still not clear, we can hypothesize that a ML vaccine inoculated by the oronasal route might generate a strong IgA production in the gut conferring a complete protection from the infection. The results of this study are interesting for several reasons. CCoV infection alone is considered of minor clinical relevance, but in the past, dual infections between CCoV and CPV2 (Pratelli et al., 1999b) and CCoV and CAdV1 (Pratelli et al., 2001) have been observed in dogs that had severe clinical illness due to the enhanced pathogenicity of dual infections. Polymicrobial infections are common in high density populations, such as unvaccinated kennels.
An additional epidemiological consideration is related to recent findings where CCoV shedding in the faeces of infected pups occurred over a period of 37 days (Pratelli et al., 2001). It, therefore, seems likely that immunization of dogs against CCoV would have positive epidemiological effects.
Our studies suggest that effective vaccines which are able to induce truly protective and long-lived immunity to infection should be sought to control the spread of CCoV in high risk dog populations.
Acknowledgements
This work was supported by grants from Ministry of University, Italy (project: Enteriti virali del cane).
References
- Appel M.J.G. Does canine coronavirus augment the effect of subsequent parvovirus infection? Vet. Med. 1988;83:360–366. [Google Scholar]
- Carmichael, L.E., 1997. Vaccines for dogs. In: Pastoret, P.-P., Blancou, J., Vannier, P., Verschueren, C. (Eds.), Veterinary Vaccinology. Elsevier, New York, pp. 326–335.
- de Vries A.A.F., Horzinek M.C., Rottier P.J.M., de Groot R.J. The genome organization of the Nidovirales: similarities and differences between arteri-, toro-, and coronaviruses. Semin. Virol. 1997;8:33–47. doi: 10.1006/smvy.1997.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia G., Decaro N., Tinelli A., Martella V., Pratelli A., Buonavoglia C. Evaluation of antibody response to canine coronavirus infection in dogs by Western blotting analysis. New Microbiol. 2002;25:275–280. [PubMed] [Google Scholar]
- Enjuanes, L., Brian, D., Cavanagh, D., Holmes, K., Lai, M.M.C., Laude, H., Masters, P., Rottier, P., Siddell, S., Spaan, W.J.M., Taguchi, F., Talbot, P., 2000. Coronaviridae. In: van Regenmortel, M.H.V., Fauquet, C.M., Bishop, D.H.L., Carstens, E.B., Estes, M.K., Lemon, S.M., Maniloff, J., Mayo, M.A., McGeoch, D.J., Pringle, C.R., Wickner, R.B. (Eds.), Virus Taxonomy, Classification and Nomenclature of Viruses. Academic Press, New York, pp. 835–849.
- Evermann J.F., Foreyt W., Maag-Miller L., Leathers C.W., McKeirnan A.J., LeaMaster B. Acute hemorrhagic enteritis associated with canine coronavirus and parvovirus in a captive coyote population. J. Am. Vet. Med. Assoc. 1980;177:784–786. [PubMed] [Google Scholar]
- Gebauer F., Posthumus W.A.P., Correa I., Suñé C., Sánchez C.M., Smerdou C., Sanchez C.M., Lenstra J.A., Meloen R.H., Enjuanes L. Residues involved in the formation of the antigenic sites of the S protein of transmissible gastroenteritis coronavirus. Virology. 1991;183:225–238. doi: 10.1016/0042-6822(91)90135-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K.P., Jervis H.R., Marchwicki R.H., Binn L.N. Intestinal infection of neonatal dogs with canine coronavirus 1–71: studies by virologic, histologic, histochemical and immunofluorescent techniques. Am. J. Vet. Res. 1976;37:247–256. [PubMed] [Google Scholar]
- Luytjes, W., 1995. Coronavirus gene expression: genome organization and protein expression. In: Siddell, S.G. (Ed.), The Coronaviridae. Plenum Press, New York, pp. 33–49.
- Marsilio F., Pratelli A., Elia G., Ricci L. Enterite da coronavirus del cane: caratterizzazione del virus isolato. Veterinaria. 2002;2:1–4. [Google Scholar]
- Martin M.L. Canine coronavirus enteritis and a recent outbreak following modified-live virus vaccination. Comp. Cont. Educ. Pract. Vet. 1985;7:1013–1017. [Google Scholar]
- Murphy, B.R., 1999. Mucosal immunity to viruses. In: Ogra, P.L., Mestecky, J., Lamm, M.E., Strober, W., Bienenstock, J., McGhee, J.R. (Eds.), Mucosal Immunology. Academic Press, New York, pp. 695–707.
- Ogra P.L., Fishaut M., Gallagher M.R. Viral vaccination via the mucosal route. Rev. Infect. Dis. 1980;2:352–369. doi: 10.1093/clinids/2.3.352. [DOI] [PubMed] [Google Scholar]
- Pratelli A., Tempesta M., Greco G., Martella V., Buonavoglia C. Development of a nested PCR for the detection of canine coronavirus. J. Virol. Meth. 1999;80:11–15. doi: 10.1016/S0166-0934(99)00017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Tempesta M., Roperto F.P., Sagazio P., Carmichael L.E., Buonavoglia C. Fatal coronavirus infection in puppies following canine parvovirus 2b infection. J. Vet. Diagn. Invest. 1999;11:550–553. doi: 10.1177/104063879901100615. [DOI] [PubMed] [Google Scholar]
- Pratelli A., Martella V., Elia G., Tempesta M., Guarda F., Capucchio M.T., Carmichael L.E., Buonavoglia C. Severe enteric disease in an animal shelter associated with dual infections by canine adenovirus type 1 and canine coronavirus. J. Vet. Med. B. 2001;48:385–392. doi: 10.1046/j.1439-0450.2001.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Elia G., Martella V., Tinelli A., Decaro N., Marsilio F., Buonavoglia D., Tempesta M., Buonavoglia C. M gene evolution of canine coronavirus in naturally infected dogs. Vet. Rec. 2002;151:758–761. [PubMed] [Google Scholar]
- Pratelli A., Elia G., Martella V., Palmieri A., Cirone F., Tinelli A., Corrente M., Buonavoglia C. Prevalence of canine coronavirus (CCoV) antibodies in dogs in Bari, Italy, by an enzyme-linked immunosorbent assay. J. Virol. Meth. 2002;102:67–71. doi: 10.1016/S0166-0934(01)00450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A., Tinelli A., Decaro N., Cirone F., Elia G., Roperto S., Tempesta M., Buonavoglia C. Efficacy of an inactivated canine coronavirus vaccine in pups. New Microbiol. 2003;26:151–155. [PubMed] [Google Scholar]
- Saif L.J. Mucosal immunity: an overview and studies of enteric and respiratory coronavirus infections in a swine model of enteric disease. Vet. Immunol. Immunopathol. 1996;54:163–169. doi: 10.1016/S0165-2427(96)05702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant B.J.R., Gaskell M., Kelly D.F., Carter S.C., Gaskell C.J. Canine coronavirus infection in dog following oronasal inoculation. Res. Vet. Sci. 1991;51:11–18. doi: 10.1016/0034-5288(91)90023-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.B., Holladay J.A., Cave J.A. A neurologic syndrome associated with use of a canine coronavirus–parvovirus vaccine in dogs. Comp. Cont. Educ. Pract. Vet. 1986;8:117–122. [Google Scholar]
- Woods R.D., Wesley R.D., Kapke P.A. Complement-dependent neutralization of transmissible gastroenteritis virus by monoclonal antibodies. Adv. Exp. Med. Biol. 1987;218:493–500. doi: 10.1007/978-1-4684-1280-2_64. [DOI] [PubMed] [Google Scholar]


