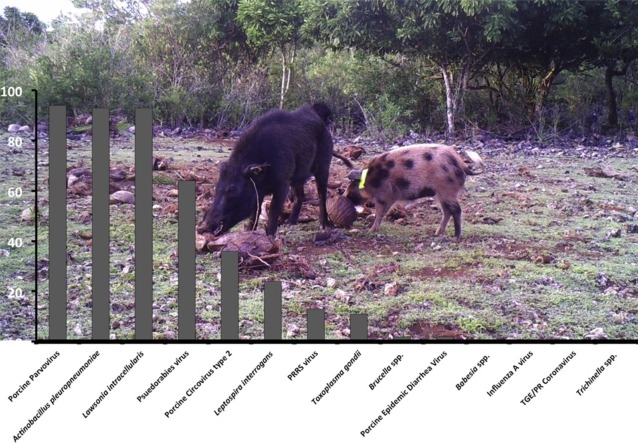
Graphical abstract
Keywords: Wild pigs, Feral pigs, Zoonoses, Pathogen, Guam, Pacific island, Parasites
Highlights
-
•
Guam has high wild pig densities with risk of pathogen transmission to people and animals.
-
•
Exposure to numerous pathogens was detected in contrast to surveys of domestic pigs.
-
•
New reports of pseudorabies virus, PRRS virus, Brucella, and Leptospira in pigs on Guam.
-
•
Highlights that domestic swine-wild pig interactions should be prevented.
-
•
Precautions are needed when handling wild pigs to minimize the pathogen transmission.
Abstract
Pigs (Sus scrofa) were introduced to Guam in the 1600’s and are now present in high densities throughout the island. Wild pigs are reservoirs for pathogens of concern to domestic animals and humans. Exposure to porcine parvovirus, transmissible gastroenteritis, and Leptospira interrogans has been documented in domestic swine but data from wild pigs are lacking. The close proximity of humans, domestic animals, and wild pigs, combined with the liberal hunting of wild pigs, results in frequent opportunities for pathogen transmission. From February–March 2015, blood, tissue and ectoparasite samples were collected from 47 wild pigs. Serologic testing found exposure to Brucella spp. (2%), Toxoplasma gondii (11%), porcine reproductive and respiratory syndrome (PRRS) virus (13%), porcine circovirus type 2 (36%), pseudorabies virus (64%), Actinobacillus pleuropneumoniae (93%), Lawsonia intracellularis (93%), and porcine parvovirus (94%). Eleven (24%) samples had low titers (1:100) to Leptospira interrogans serovars Bratislava (n = 6), Icterohaemorrhagiae (n = 6), Pomona (n = 2), and Hardjo (n = 1). Kidney samples from nine pigs with Leptospira antibodies were negative for Leptospira antigens. Numerous pigs had Metastrongylus lungworms and three had Stephanurus dentatus. Lice (Hematopinus suis) and ticks (Amblyomma breviscutatum) were also detected. No antibodies to Influenza A viruses were detected. In contrast to the previous domestic swine survey, we found evidence of numerous pathogens in wild pigs including new reports of pseudorabies virus, PRRS virus, Brucella, and Leptospira in pigs on Guam. These findings highlight that domestic swine-wild pig interactions should be prevented and precautions are needed when handling wild pigs to minimize the risk of pathogen transmission.
1. Introduction
Wild pigs are nearly globally distributed and are hosts for many parasites and bacterial and viral pathogens, some of which are transmissible to agricultural animals, wildlife, and humans (Barrios-Garcia and Ballari, 2012, Bevins et al., 2014). Numerous studies have investigated the prevalence and distribution of various pathogens in wild pigs and the risk they pose for pathogen transmission in the United States and Europe, but there are relatively few reports from Southeast Asia (Baroch et al., 2015, Hill et al., 2014, Pedersen et al., 2014, Pedersen et al., 2015, Pepin et al., 2016). Wild pig populations are increasing dramatically on several Pacific islands, yet data on pathogen exposure are limited or absent.
Wild pigs were introduced to Guam, an unincorporated territory of the United States located in the Marianna Island chain, in the late 1600’s and despite current liberal hunting regulations, populations continue to increase (Conry, 1988). This population increase has led to severe ecological damage and agricultural losses. The only report of swine pathogens on Guam was limited to domestic pigs (Dugies et al., 2000). The lack of data on pathogens in wild pigs is a concern as increased swine populations have led to increased pig-human interactions. Recent reports of leptospirosis in residents and tourists is one concern and although rodents tend be considered the most common reservoir of Leptospira, there is evidence of Leptospira in domestic swine on Guam (Dugies et al., 2000), and wild pigs in numerous countries have antibodies to leptospires (Jansen et al., 2007, Corn et al., 1986).
In 2015, there was an effort to decrease wild pig populations within fenced areas of two military bases on Guam to minimize the ecological damage caused by the pigs. Samples collected from these animals were used to conduct a comprehensive surveillance project on pathogen exposure of wild pigs on Guam.
2. Materials and methods
2.1. Sites
Andersen Air Force Base (AAFB) is a 4135 ha military installation in northern Guam (13.5875°N, 144.9244°E). Forested portions of the base contain high quality native habitat, some of which is included in the Guam National Wildlife Refuge. The Naval Base Guam Naval Munitions Site (NBG NMS) is centrally located on the island and covers approximately 5723 acres (13.44000°N 144.65250°E).
2.2. Sample collection
Removal of wild pigs was conducted via strategic sharp-shooting techniques conducted by well-trained shooters using suppressed 0.223 caliber rifles mounted with scopes. During removal, shooters only fired if the situation met the following criteria: 1) there was certainty that the animal would be dispatched and not escape, 2) if other animals were nearby, every animal had a high probability of being dispatched, and 3) it was safe to dispatch the animal. The shooting methods followed the American Veterinary Medical Association’s guidelines for humane euthanasia of animals (AVMA, 2013). Pigs were aged based on tooth eruption patterns and wear. Animal and sample collection procedures were reviewed and approved by UGA’s Institutional Animal Care and Use Committee (A2014 09-021).
Immediately after euthanasia, blood samples were collected via cardiocentesis and placed into ethylenediaminetetraacetic acid (EDTA) and plain tubes (Greiner Bio-one, Monroe, NC). Clotted blood was centrifuged at 1250g for 15 min and serum was removed and frozen at −20 °C until diagnostic testing. Whole blood was also frozen at −20 °C until testing. Representative ectoparasites were collected and preserved in 95% ethanol.
2.3. Diagnostic testing
Information on pathogens we screened pigs for as well as diagnostic assays and diagnostic laboratories used are listed in Table 1 . Most pigs were serologically tested for all of the pathogens listed; however, due to limited sample volume, some pigs were only tested for selected pathogens. Small sections of lung were fixed in formalin and processed for routine histology. Small sections of kidneys were also fixed in formalin and if antibodies to Leptospira were detected, they were tested for Leptospira antigens by immunohistochemistry (IHC). If any gross lesions were noted during necropsy, they were also collected in formalin for histologic examination. Pigs were not systematically examined for internal parasites, but if any were seen they were collected in formalin for identification.
Table 1.
Pathogens, diagnostic assays and diagnostic laboratories used for pathogen surveillance.
| Pathogen type | Pathogen | Assaya | Diagnostic laboratoryb | No tested | No. positive (%) |
|---|---|---|---|---|---|
| Virus | Influenza A virus (IAV) | bELISA | SCWDS | 47 | 0 |
| Porcine circovirus type 2 virus (PCV-2) | ELISA | ISUVDL | 44 | 16 (36.4) | |
| Porcine epidemic diarrhea virus (PEDV) | ELISA | ISUVDL | 44 | 1 (2.3) | |
| Porcine parvovirus (PPV) | HI | UGAVDL | 46 | 16 (36.4) | |
| Porcine reproductive and respiratory syndrome (PRRS) | ELISA | UGAVDL | 46 | 6 (13) | |
| Psuedorabies virus (PRV) | ELISA | UGAVDL | 45 | 29 (64.4) | |
| Transmissible gastroenteritis (TGE)/Porcine respiratory coronavirus (PRCV) | ELISA | ISUVDL | 44 | 0 | |
| Bacteria | Actinobacillus pleuropneumoniae serotypes (APP) | ELISA | ISUVDL | 44 | 41 (93.2) |
| Brucella spp. | Card testc | UGAVDL | 46 | 1 (2.2) | |
| Lawsonia intracellularis | ELISA | ISUVDL | 44 | 41 (93.2) | |
| Leptospira interrogans | MAT, IHC | UGAVDL | 46 | 11 (23.9) | |
| Parasites | Babesia spp. | PCRd | SCWDS | 47 | 0 |
| Toxoplasma gondii | MATe | USDA | 47 | 5 (10.6) | |
| Trichinella spp. | ELISAe | USDA | 47 | 0 | |
bELISA: blocking ELISA (commercially available from IDEXX Laboratories); ELISA: Enzyme linked immunosorbent Assay; HI: Hemagglutination Inhibition; IHC: Immunohistochemistry; MAT: Modified Agglutination Test; PCR: Polymerase Chain Reaction. Detailed assay methods are available from UGA VDL and ISU VDL unless indicated.
ISUVDL: Iowa State University Veterinary Diagnostic Laboratory; SCWDS: Southeastern Cooperative Wildlife Disease Study; UGA VDL: University of Georgia Veterinary Diagnostic Laboratory; USDA: United States Department of Agriculture.
The positive sample on the screening card test was sent to the National Center for Veterinary Laboratories (Aimes IA) for confirmatory testing using the Fluorescent Polarization Assay (FPA).
PCR protocols provided in Shock et al. (2014).
Assay details are provided in Hill et al. (2014).
2.4. Statistical analyses
Body weights were compared between male and female swine using a two-sample t-test. Prevalence of pathogen exposures was compared between males and females and between adult and juvenile swine using Fisher’s exact test. All testing assumed a two-sided alternative hypothesis and P < 0.05 was considered statistically significant. Analyses were performed using commercially available statistical software (Stata version 13.1, StataCorp LP, College Station, TX).
3. Results
From the two sites, a total of 47 wild pigs were sampled including six (12.8%) juveniles and 41 (87.2%) adults; 18 (38.3%) were males and 29 (61.7%) were females. Weight (kilograms) was recorded for 32 individuals: 5 juveniles and 27 adults. All juveniles weighed <11.3 kgs and the mean ± SD weight of the 12 adult males (47.1 ± 16.5) was significantly greater than that of the 15 adult females (25.4 ± 4.1; P < 0.001).
All pigs were positive for at least one of the pathogens included in the study. Exposure to or infection with influenza A virus (IAV), Brucella, porcine epidemic diarrhea virus, Babesia spp., transmissible gastroenteritis/porcine respiratory coronavirus and Trichinella spp. was rare or absent (Table 1). Based on antibody testing, a high percentage of pigs were exposed to Actinobacillus pleuropneumonia (APP; 93%), Lawsonia intracellularis (93%), and porcine parvovirus (94%). Exposure to multiple serotypes of APP was also common (Table 2 ). Based on MAT testing, antibodies to four Leptospira serovars were detected in 11 pigs (Table 1, Table 3 ), with some individuals having antibodies to more than one serovar. Kidney samples from nine pigs with Leptospira antibodies were negative for Leptospira antigens by IHC.
Table 2.
Results for Actinobacillus pleuropneumoniae serotype exposure in wild pigs in Guam.
| Actinobacillus pleuropneumoniae serotypes | No. tested | No. positive (%) |
|---|---|---|
| Serotypes 1-2-9-11 | 44 | 25 (56.8) |
| Serotypes 10-12 | 44 | 12 (27.3) |
| Serotypes 3-6-8-15 | 44 | 38 (86.4) |
| Serotypes 4-5-7 | 44 | 32 (72.7) |
Table 3.
Leptospira interrogans serovar exposure of wild pigs in Guam.
| Serovars | No. tested | No. positive (%) |
|---|---|---|
| L. bratislava | 46 | 6 (13) |
| L. canicola | 46 | 0 (0) |
| L. grippotyphosa | 46 | 0 (0) |
| L. hardjo | 46 | 1 (2.2) |
| L. icterohaemorrhagiae | 46 | 6 (13) |
| L. pomona | 46 | 2 (4.3) |
Juvenile pigs had a significantly higher prevalence of antibodies to porcine circovirus type 2 (6/6 [100%]) compared with adults (10/38 [26.3%]; P = 0.001). In contrast, there was a significantly lower prevalence of antibodies to L. intracellularis and pseudorabies virus in juveniles (4/6 [66.7%] vs. 37/38 [97.4%]; P = 0.045 and 1/6 [16.7%] vs. 28/39 [71.8%]; P = 0.017, respectively). There was no difference in prevalence between males and females for any pathogen.
Only 38 pigs were examined for ectoparasites; lice (Hematopinus suis) and ticks (Amblyomma breviscutatum) were found on 12 (32%) and seven (18%) pigs, respectively. Lung samples from 32 pigs were examined histologically and eight (25%) were positive for Metastrongylus lungworms. Stephanurus dentatus were found in abdominal lesions from three pigs and one pig had a liver abscess with intralesional nematode larvae which could not be identified.
4. Discussion
Our results indicate that wild pigs on Guam are exposed to multiple pathogens of zoonotic and agricultural importance. Guam has many free-range pig operations which have an increased risk of domestic pig-wild pig interactions. Previous work on Guam was restricted to domestic pigs and in contrast to our data, domestic pigs were exposed to relatively few pathogens (Duguies et al., 2000). Clinically, several diseases were noted, but the only pathogen detected by serologic testing was parvovirus (7/14, 50%). Although domestic pigs were not serologically tested for APP or L. intracellularis, porcine pleuropneumonia and proliferative enteritis were noted. Domestic pigs tested negative for antibodies to pseudorabies virus (n = 65), porcine reproductive respiratory syndrome virus (n = 16), Brucella spp. (n = 66), Leptospira spp. (n = 52), swine influenza virus (n = 61), transmissible gastroenteritis virus (n = 27), Mycoplasma hyopneumoniae (n = 70), and Trichinella spp. (n = 53) while we found serologic evidence of the first four pathogens in wild pigs (Duguies et al., 2000).
Porcine pleuropneumonia, caused by APP, is a highly infectious disease that is economically important for domestic swine. Although known to occur on Guam among domestic pigs, prevalence was unknown (Duguies et al., 2000, Bossé et al., 2002). Clinical disease and mortality resulting from infection with one or more APP serotypes can vary geographically; however, serotype 2 has been consistently associated with morbidity and mortality in domestic pigs (Baroch et al., 2015). Over half of the wild pigs from Guam had antibodies for serotypes 1-2-9-11. Reproductive disease in domestic pigs due to porcine parvovirus (PPV) has been reported on Guam (Duguies et al., 2000, Ruiz-Fons et al., 2006). In addition to reproductive failure due to PPV infection, coinfection with porcine circovirus type 2 (PCV2) in domestic pigs can result in mortalities (Ellis et al., 2000). We report in wild pigs the first evidence of PCV2 on Guam; surveillance and vaccination for these pathogens may be warranted. L. intracellularis has been documented worldwide in domestic and wild pigs and is the etiologic agent of proliferative enteropathy (Chiriboga et al., 1999). The high prevalence of these three pathogens in wild pigs, indicates that transmission to domestic pigs is a risk.
Exposure to Leptospira interrogans and Toxoplasma gondii, two important zoonoses, was common in wild pigs on Guam. Leptospirosis is a growing concern in Guam (Mason et al., 1998). While exposure to several serotypes was detected, prevalence is likely underestimated because antigens from serotypes readily available for testing in our US laboratory were used rather than those likely present in Guam/other Pacific nations. Regardless, exposure of pigs does not implicate them in human cases due to the diverse reservoir-range, but suggests that proper PPE should be utilized when in contact with pigs. Domestic pigs have not been previously tested for T. gondii but evidence of T. gondii was noted in goats (Duguies et al., 2000). This parasite has a wide host range and can pose a risk to native avian fauna that do not have an evolutionary history with the parasite (Work et al., 2000). In addition, T. gondii is zoonotic; due to the popularity of wild pig hunting for sport and population management, hunters in Guam should properly butcher and cook wild pig meat (Hill et al., 2014, Conry, 1988).
Several pathogens were not detected or were rare in wild pigs. No evidence of exposure to Trichinella spp., IAV, or coronaviruses associated with enteric and respiratory disease was found; one pig had antibodies to porcine epidemic diarrhea virus. Previously, Trichinella was absent from domestic pigs, but because of the zoonotic potential of this parasite, surveillance and education campaigns to ensure pork is thoroughly cooked (which would also kill any T. gondii present) should continue. Swine influenza, a zoonosis, is relatively common in domestic pigs worldwide, but exposure of wild pigs is not ubiquitous and varies geographically (Smith et al., 2009). While prevalence was high in a study in southern China (Luo et al., 2013), in Korea, the US and Spain, seroprevalence was generally low (<20%) and varied seasonally; it is possible that our sample size was insufficient to detect exposure in Guam (Hall et al., 2008, Corn et al., 2009, Feng et al., 2014). Enteric and respiratory coronaviruses, which have been reported in several Asian countries, are of significant concern due to high morbidity and mortality in naïve pig populations (Song and Park, 2012). Porcine epidemic disease virus is clinically similar to transmissible gastroenteritis; both are coronaviruses that can lead to acute diarrhea in all age groups of swine, often resulting in poor body condition or mortalities (Lee, 2015). One wild pig had antibodies to Brucella; the species could not be distinguished based on serologic testing. Serology indicates if Brucella is present in a population, but culture and/or advanced molecular assays are required to confirm species (Leiser et al., 2013). Brucellosis, a zoonotic disease, has been documented in wild pig populations globally (Leiser et al., 2013).
Lice (H. suis) were common on Guam, similar to studies on domestic and wild pigs worldwide (Girişgin et al., 2009). H. suis can transmit swine poxvirus and classical swine fever virus but there is no evidence that these viruses are present on Guam. Amblyomma breviscutatum were found on several pigs but little is known about this tick species; it is believed to have been historically present on Guam and other islands in greater Micronesia (Vander Velde and Vander Velde, 2013). Currently, there are no data on pathogens associated with A. breviscutatum. Our testing method for helminth parasites was limited due to time and importation restrictions; we only examined small sections of lung tissue (or lesions if noted). Confirmed infections with Metastrongylus lungworms and S. dentatus were relatively common, and likely under-recognized because of testing method. Both Metastrongylus lungworms and S. dentatus, previously reported in domestic pigs on Guam, can impact domestic pig health (Duguies et al., 2000).
5. Conclusions
Our study highlights the importance of surveillance efforts for pathogens transmitted by wild pigs on Guam as many of the pathogens circulating in wild pigs can cause disease in domestic swine. We detected serologic evidence for multiple zoonotic pathogens, necessitating simple yet effective preventative measures. Wearing appropriate PPE, practicing good hygiene, washing all utensils and surfaces that have come into contact with butchered wild pigs, and thoroughly cooking meat prior to consumption may assist in preventing infection. Management of wild pigs is often controversial as they are seen as a food source for hunters or in some cases may be considered native species. The wild pig populations on Guam are not native, harbor pathogens of importance to domestic swine and people, and very importantly, cause major damage to the environment leading to economic losses for farmers and extreme habitat destruction for native species of wildlife.
Acknowledgements
Primary financial support for this project came from the US Department of the Navy (N40192-14-R-8000 and N40192-14-R-8005). Additional financial support was provided by the wildlife management agencies of the Southeastern Cooperative Wildlife Disease Study member states through the Federal Aid to Wildlife Restoration Act (50 Stat. 917) and by the US Department of the Interior Cooperative Agreement G11AC20003. The authors thank the personnel at the diagnostic laboratories for diagnostic support and R.L. Poulson for comments on this manuscript.
References
- [AVMA] American Veterinary Medical Association . edition. AVMA; Schaumburn, IL: 2013. Guidelines for the Euthanasia of Animals; p. 102. [Google Scholar]
- Baroch J.A., Gagnon C.A., Lacouture S., Gottschalk M. Exposure of feral swine (Sus scrofa) in the United States to selected pathogens. Can. J. Vet. Res. 2015;79:74–78. [PMC free article] [PubMed] [Google Scholar]
- Barrios-Garcia M.N., Ballari S.A. Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biol. Invasions. 2012;14:2283–2300. [Google Scholar]
- Bevins S.N., Pedersen K., Lutman M.W., Gidlewski T., Deliberto T.J. Consequences associated with the recent range expansion of nonnative feral swine. BioScience. 2014 doi: 10.1093/biosci/biu015. biu015. [DOI] [Google Scholar]
- Bossé J.T., Janson H., Sheehan B.J., Beddek A.J., Rycroft A.N., Kroll J.S., Langford P.R. Actinobacillus pleuropneumoniae: pathobiology and pathogenesis of infection. Microbes Infect. 2002;4:225–235. doi: 10.1016/s1286-4579(01)01534-9. [DOI] [PubMed] [Google Scholar]
- Chiriboga A.E.N., Guimarães W.V., Vanetti M.C.D., Araújo E.F. Detection of Lawsonia intracellularis in faeces of swine from the main producing regions in Brazil. Can. J. Microbiol. 1999;45:230–234. doi: 10.1139/w98-234. [DOI] [PubMed] [Google Scholar]
- Conry P.J. Management of feral and exotic game species on Guam. Trans. West. Sect. Wildl. Soc. 1988;24:26–30. [Google Scholar]
- Corn J.L., Swiderek P.K., Blackburn B.O., Erickson G.A., Thiermann A.B., Nettles V.F. Survey of selected diseases in wild swine in Texas. J. Am. Vet. Med. Assoc. 1986;189:1029–1032. [PubMed] [Google Scholar]
- Corn J.L., Cumbee J.C., Barfoot R., Erickson G.A. Pathogen exposure in feral swine populations geographically associated with high densities of transitional swine premises and commercial swine production. J. Wildl. Dis. 2009;45:713–721. doi: 10.7589/0090-3558-45.3.713. [DOI] [PubMed] [Google Scholar]
- Duguies M., Nusbaum S., Saville P. ADAP Project. ADAP Animal Health Survey for Guam, Northern Marianas Islands, Palau, Federated States of Micronesia, and American Samoa; ADAP; Honolulu (HI): 2000. Animal Health Survey for Guam 1999; pp. 2000–2025. [Google Scholar]
- Ellis J.A., Bratanich A., Clark E.G., Allan G., Meehan B., Haines D.M., Harding J., West K.H., Krakowka S., Konoby C., Hassard L. Coinfection by porcine circoviruses and porcine parvovirus in pigs with naturally acquired postweaning multisystemic wasting syndrome. J. Vet. Diagn. Invest. 2000;12:21–27. doi: 10.1177/104063870001200104. [DOI] [PubMed] [Google Scholar]
- Feng Z., Baroch J.A., Long L.P., Xu Y., Cunningham F.L., Pedersen K., Lutman M.W., Schmit B.S., Bowman A.S., DeLiberto T.J., Wan X.F. Influenza A subtype H3 viruses in feral swine, United States, 2011–2012. Emerg. Infect. Dis. 2014;20:843. doi: 10.3201/eid2005.131578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girişgin O., Girişgin A.O., Sönmez F., Akyol C.V. Occurrence of Haematopinus suis Linnaeus, 1758 (Insecta, Anopluridae) on a wild boar (Sus scrofa) Turk. J. Vet. Anim. Sci. 2009;33:529–530. [Google Scholar]
- Hall J.S., Minnis R.B., Campbell T.A., Barras S., DeYoung R.W., Pabilonia K., Avery M.L., Sullivan H., Clark L., McLean R.G. Influenza exposure in United States feral swine populations. J. Wildl. Dis. 2008;44:362–368. doi: 10.7589/0090-3558-44.2.362. [DOI] [PubMed] [Google Scholar]
- Hill D.E., Dubey J.P., Baroch J.A., Swafford S.R., Fournet V.F., Hawkins-Cooper D., Pyburn D.G., Schmit B.S., Gamble H.R., Pedersen K., Ferreira L.R. Surveillance of feral swine for Trichinella spp. and Toxoplasma gondii in the USA and host-related factors associated with infection. Vet. Parasitol. 2014;205:653–665. doi: 10.1016/j.vetpar.2014.07.026. [DOI] [PubMed] [Google Scholar]
- Jansen A., Luge E., Guerra B., Wittschen P., Gruber A.D., Loddenkemper C., Schneider T., Lierz M., Ehlert D., Appel B., Stark K. Leptospirosis in urban wild boars, Berlin, Germany. Emerg. Infect. Dis. 2007;13:739–742. doi: 10.3201/eid1305.061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Porcine epidemic diarrhea virus: an emerging and re-emerging epizootic swine virus. Virol. J. 2015;12(1):193. doi: 10.1186/s12985-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser O.P., Corn J.L., Schmit B.S., Keim P.S., Foster J.T. Feral swine brucellosis in the United States and prospective genomic techniques for disease epidemiology. Vet. Microbiol. 2013;166:1–10. doi: 10.1016/j.vetmic.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Luo J., Dong G., Li K., Lv Z., Huo X., He H. Exposure to swine H1 and H3 and avian H5 and H9 influenza A viruses among feral swine in Southern China, 2009. J. Wildl. Dis. 2013;49:375–380. doi: 10.7589/2012-03-079. [DOI] [PubMed] [Google Scholar]
- Mason R.J., Fleming P.J.S., Smythe L.D., Dohnt M.F., Norris M.A., Symonds M.L. Leptospira interrogans antibodies in feral pigs from New South Wales. J. Wildl. Dis. 1998;34:738–743. doi: 10.7589/0090-3558-34.4.738. [DOI] [PubMed] [Google Scholar]
- Pedersen K., Quance C.R., Robbe-Austerman S., Piaggio A.J., Bevins S.N., Goldstein S.M., Gaston W.D., DeLiberto T.J. Identification of Brucella suis from feral swine in selected states in the USA. J. Wildl. Dis. 2014;50:171–179. doi: 10.7589/2013-09-235. [DOI] [PubMed] [Google Scholar]
- Pedersen K., Pabilonia K.L., Anderson T.D., Bevins S.N., Hicks C.R., Kloft J.M., Deliberto T.J. Widespread detection of antibodies to Leptospira in feral swine in the United States. Epidemiol. Infect. 2015;143:2131–2136. doi: 10.1017/S0950268814003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin K.M., Davis A.J., Beasley J., Boughton R., Campbell T., Cooper S.M., Gaston W., Hartley S., Kilgo J.C., Wisely S.M., Wyckoff C. Contact heterogeneities in feral swine: implications for disease management and future research. Ecosphere. 2016;7:1–11. [Google Scholar]
- Ruiz-Fons F., Vicente J., Vidal D., Höfle U., Villanúa D., Gauss C., Segalés J., Almería S., Montoro V., Gortázar C. Seroprevalence of six reproductive pathogens in European wild boar (Sus scrofa) from Spain: the effect on wild boar female reproductive performance. Theriogenology. 2006;65(4):731–743. doi: 10.1016/j.theriogenology.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Shock B.C., Moncayo A., Cohen S., Mitchell E.A., Williamson P.C., Lopez G., Garrison L.E., Yabsley M.J. Diversity of piroplasms detected in blood-fed and questing ticks from several states in the United States. Ticks Tick Borne Dis. 2014;5:373–380. doi: 10.1016/j.ttbdis.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Smith G.J., Vijaykrishna D., Bahl J., Lycett S.J., Worobey M., Pybus O.G., Ma S.K., Cheung C.L., Raghwani J., Bhatt S., Peiris J.M. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- Song D., Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44:167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Velde N., Vander Velde B. Known and potential ticks and tick-borne pathogens of Micronesia. Micronesica. 2013;2013(1):1–26. [Google Scholar]
- Work T.M., Massey J.G., Rideout B.A., Gardiner C.H., Ledig D.B., Kwok O.C.H., Dubey J.P. Fatal toxoplasmosis in free-ranging endangered ‘Alala from Hawaii. J. Wild. Dis. 2000;36:205–212. doi: 10.7589/0090-3558-36.2.205. [DOI] [PubMed] [Google Scholar]