Graphical abstract
The present study was regarding the isolation and molecular characterization of Acanthamoeba spp. isolated from air-conditioners in Malaysia. Thermo- and osmotolerance tests were employed to predict the potential pathogenicity of these Acanthamoeba isolates.
Research highlights
▶ Twenty out of 87 dust samples collected from air-conditioners were detected positive for Acanthamoeba spp. by PCR and culturing-microscopic examination. ▶ The pitfalls of Pussard and Pons morphological criteria in sub-generic classification of Acanthamoeba spp. were demonstrated: (i) species of five Acanthamoeba isolates could not be identified based on cyst morphology, and (ii) culture condition could alter cyst features greatly. ▶ Many of the air-conditioner Acanthamoeba isolates showed tolerance to high osmotic pressure and high temperature, therefore there were predicted as potential human pathogens.
Keywords: Acanthamoeba, Air-conditioners, Environmental isolates, Kuala Lumpur, Malaysia
Abstract
During a study on the quality of the indoor environment, Acanthamoeba spp. were detected in 20 out of 87 dust samples collected from air-conditioners installed in a four-story campus building located in Kuala Lumpur, Malaysia. Twenty-one cloned Acanthamoeba isolates designated as IMU1 to IMU21 were established from the positive primary cultures. Five species were identified from the 16 isolates according to the morphological criteria of Pussard and Pons; i.e. A. castellanii, A. culbertsoni, A. griffini, A. hatchetti and A. polyphaga. Species identities for the remaining five isolates (IMU4, IMU5, IMU15, IMU20 and IMU21), however, could not be determined morphologically. At genotypic characterization, these isolates were placed into T3 (IMU14); T5 (IMU16 and IMU17) and T4 (all the remaining isolates). To predict the potential pathogenicity of these Acanthamoeba isolates, thermo- and osmotolerance tests were employed; many isolates were predicted as potential human pathogens based on the outcome of these tests. This is the first time potentially pathogenic Acanthamoeba have been isolated from air-conditioners in Malaysia.
1. Introduction
Acanthamoeba spp. are free-living amoebae which are ubiquitously distributed in the nature (Marciano-Cabral and Cabral, 2003, Khan, 2006). The speciation of Acanthamoeba is mainly based on cyst structure as described by Pussard and Pons (1977). Based on this, over 20 species have been described (Visvesvara, 1991). However, polymorphism of cysts within a cloned cell line, influence of growth condition in altering cyst features, and the inability to accurately differentiate or identify Acanthamoeba spp. based on subtle differences in cyst features have made this morphological taxonomic scheme unreliable and difficult (Sawyer, 1971, Stratford and Griffiths, 1978, Walochnik et al., 2002). Other non-morphological techniques such as biochemical and molecular analyses were used to differentiate members of the genus (de Jonckheere, 1983, Khan and Paget, 2002, Kong et al., 2002, Liu et al., 2005). The most promising technique currently used is phylogenetic analysis of the complete nuclear 18S rRNA gene sequence. This approach was first used by Gast et al. in 1996, who employed molecular genotying for the taxonomic characterization of the Acanthamoeba genus (Gast et al., 1996). Today, 15 genotypes (T1 to T15) have been discovered within the genus, with a divergence of >5% between the most closely related genotypes (Stothard et al., 1998, Horn et al., 1999, Gast, 2001, Hewett et al., 2003).
The genus Acanthamoeba comprises mostly of non-pathogenic species; only a small subset of the genus is pathogenic to humans. Pathogenic Acanthamoeba isolates are recognized as the causative agents of opportunistic granulomatous amoebic encephalitis, cutaneous acanthamoebiasis, Acanthamoeba pneumonitis, and the non-opportunistic Acanthamoeba keratitis (Martinez, 1991, Rosenberg and Morgan, 2001, Marciano-Cabral and Cabral, 2003, Sukthanaa et al., 2005; mailto:fschuste@dhs.ca.gov Awwad et al., 2007). Until now, the pathogenesis for Acanthamoeba infections remain poorly understood. The ability to grow at high temperature and osmolarity, are presumed hallmarks of pathogenic Acanthamoeba. These observations may be explained by the need for Acanthamoeba trophozoite to withstand the human body temperature or the osmolarity of human tear film in order to secure a successful invasion (Walochnik et al., 2000, Khan et al., 2001, Khan, 2006). Currently, thermo- and osmotolerance tests are used to predict the pathogenic potential of environmental isolates. Under these test conditions, potentially pathogenic Acanthamoeba are able to grow at temperature ≥37 °C, and on non-nutrient agar incorporated with 1 M mannitol (Khan and Tareen, 2003, Kilic et al., 2004, Lorenzo-Morales et al., 2005a, Lorenzo-Morales et al., 2005b).
The waterborne route has frequently been cited as the method of transmitting Acanthamoeba infections; other possible sources include contaminated sand, soil and dust carried by wind in the outdoor environment (Mergeryan, 1991, Nwachuku and Gerba, 2004, Niyyati et al., 2009, Carlesso et al., 2010, Costa et al., 2010). To date, there is no direct evidence indicating that poor indoor air quality could be a source for Acanthamoeba infection. Nevertheless, contaminated indoor air handling system has been shown to be the source for numerous outbreaks of air-borne diseases such as the aspergillosis, Legionnaires’ disease caused by bacteria Legionella pneumophila, and the Severe Acute Respiratory Syndrome caused by SARS virus (O’Mahony et al., 1990, Lutz et al., 2003, Li et al., 2004). The tropical climate of Malaysia which is generally warm throughout the year has encouraged the use of air-conditioners. This could inevitably increase the risk of the building occupants to exposure of biogenic indoor air-pollutants such as cysts and trophozoites of Acanthamoeba spp. Therefore, the present study sought to detect and characterize Acanthamoeba spp. in air-conditioners in Malaysia using morphological and molecular techniques. In addition, these isolates were investigated for their pathogenic potential using thermo- and osmotolerance tests.
2. Materials and methods
Eighty-seven dust samples were collected using sterile cotton swaps from air-handler units (39 samples), vents at ceiling (37 samples), and grilles of room air-conditioners (11 samples) installed in a four-story building located in Kuala Lumpur, Malaysia. The dust particles were spread onto non-nutrient agar (NNA) plates prepared from PAS (PAGE amoebae saline) and overlaid with thin layers of living Escherichia coli. Culture plates were parafilm-sealed and incubated at ambient temperature (26 ± 2 °C) for up to two weeks. The plates were examined daily under inverted microscope for the appearance of discrete growth of Acanthamoeba. Once growth was spotted, a small piece of NNA containing the amoebae (trophozoites or cysts) was excised from the primary culture plates and inoculated onto fresh NNA plate culture plates for culture maintenance.
In primary cultures, Acanthamoeba cells could exist as mixed isolates. Therefore cell cloning was necessary to produce single-species cell lines. Four to six cysts were isolated from each Acanthamoeba positive culture, and respectively amplified in xenic culture condition. Cysts of the cloned cell lines were subjected to axenisation with 3% (v/v) HCl overnight. Following the treatment, cells were seeded in peptone–yeast–glucose (PYG) medium incorporated with 10% fetal bovine serum and 100 μg/ml gentamicin (Sigma) for cultivations. Acanthamoeba isolates which could proliferate well in axenic culture condition were subsequently cultured in PYG medium alone.
The Acanthamoeba genus-specific JDP1-JDP2 primer pair was employed in molecular detection of Acanthamoeba in samples (Schroeder et al., 2001). Total genomic DNA was extracted from trophozoites of Acanthamoeba using the QIAamp DNA mini kit (Qiagen, Hilden, Germany). Amplification reactions were performed according to Schroeder et al. (2001). PCR amplicons known as ASA.S1 were separated by a 1.5% agarose gel prepared in 0.5 × LB™ agarose electrophoresis buffer (Faster Better Media LLC, United States) and was incorporated with ethidium bromide. Electrophoresis was run at 250 V for 45 min using the 0.5 × LB™ agarose electrophoresis buffer as running buffer. After electrophoresis, gels were viewed under UV illumination in the chamber of BioDoc-It™ Imaging System (UVP, Cambridge, United Kingdom). Amplicon sizes were estimated by comparing with the GeneRuler 100 bp DNA ladder Plus (Fermentas Life Science, Canada).
The ASA.S1 amplicons from selected isolates were gel-purified using the QIAquick gel extraction kit (Qiagen, Hilden, Germany) prior to sequencing. The purified amplicons were sequenced directly at both strands using the amplification primers in an ABI PRISM® 3100 Genetic Analyzer (Applied Biosystems, Foster City, USA). DNA chromatograms were examined using the Chromas LITE software version 2.01 (Technelysium Pty. Ltd., Queensland, Australia). The forward and reverse sequences were pair-wise aligned using the ClustalW2 software (Labarga et al., 2007) and were manually refined to obtain a better consensus sequence. For nucleotide similarities search analyses, each consensus sequence was blasted against all eukaryotic nucleotide sequences archived in the GenBank database (Altschul et al., 1990). Nucleotide residues corresponded to primer regions were excluded from the similarities search analyses. To establish the evolutionary lineages between the present isolates with reference sequences of the 15 published genotypes, a phylogram was constructed from the alignments of the present ASA.S1 sequences and the corresponding 18S rRNA sequences of reference strains, using the neighbour-joining method of MEGA version 4 (Tamura et al., 2007). Nucleotide substitutions were calculated using the Kimura 2-parameter algorithm and the reliability of tree constructed was determined by bootstrap analysis of 1000 replicates. The DNA sequences obtained in this study have been deposited in the Genbank database with the following accession numbers: FJ970627 to FJ970647.
Axenic Acanthamoeba cell lines which were identified in pure clone by PCR-sequencing, together with the xenic clones, were characterized morphologically according to the keys of Pussard and Pons (1977). Wet smear morphology of cysts and trophozoites of Acanthamoeba isolates was determined under light microscopy. Student's t-test was used to determine the statistical differences between the mean cysts diameter of Acanthamoeba isolates which were grown in xenic condition and axenic condition respectively.
For temperature tolerance test, four sets of culture plates, each plate centrally placed with a small NNA block saturated with cysts of the respective Acanthamoeba isolates were prepared. One set of culture plates were directly incubated at 37 °C whereas another set were at 42 °C. The remaining two sets of culture plates were respectively exposed to 46 and 52 °C overnight prior to incubations at ambient temperature. All plates were examined daily for migrating and proliferating trophozoites for seven days. For osmotic tolerance test, small agar blocks containing Acanthamoeba cysts were placed centrally on NNA culture plates incorporated with 1 M D-mannitol or without the saccharide (negative control). Culture plates were incubated at ambient temperature for seven days and were similarly scored for the presence of migrating trophozoites. The experiments were repeated in triplicates.
A pathogenic strain of A. castellanii (Strain CDC:0184:V014, ATCC 50492; originally from a keratitis patient from India), was used as positive control whenever necessary.
3. Results
Utilizing microscopy and PCR approaches, Acanthamoeba spp. were detected in 20 out of the 87 dust samples collected (23%). All primary cultures containing Acanthamoeba spp. were subjected to cloning and axenisation. After the series of procedures, only 31 out of 59 cloned cell lines were successfully axenised and established into stable cultures. The clonality and identity of the established axenic cell lines were checked by direct sequencing of the ASA.S1 amplicons. Analyses on the DNA sequences revealed that 10 of the cloned isolates were mixtures as overlapping DNA sequences were obtained for them. The remaining pure clones were subjected to further characterization. They were designated as IMU1 to IMU21. Eleven different ASA.S1 sequences were amplified from the 21 axenic Acanthamoeba cell lines as indicated by multiple sequence alignment analyses. Strains that had identical gene sequences were clustered into the same group. Altogether, there were five such groups: (IMU1, IMU2, IMU6 and IMU7); (IMU4 and IMU5); (IMU10, IMU11, IMU12 and IMU13); (IMU16, IMU17); and (IMU19, IMU20 and IMU21). Nucleotide similarities search analyses indicated that most of the present ASA.S1 sequences displayed closest sequence identities (99–100%) to the 18S rRNA gene segments belonging to various Acanthamoeba spp. deposited in the GenBank database. The only exception was the ASA.S1 sequences of IMU16 and IMU17 which only gave best scores of 97% identities to the 18S rRNA gene segments of five strains of A. lenticulata (Table 1 ). In should be noted that DNA information contained within ASA.S1 amplicons is meant for genotyping and not for species identification. This is because many of the present sequences showed remarkable high sequence similarities (97–99%) with sequences belonging to different Acanthamoeba species at the same time (Table 1). Genotypic classifications of IMU1 to IMU21 were done by assembling and comparing the ASA.S1 sequences of these isolate with reference sequences encompassing all the currently established genotypes of Acanthamoeba, including selected sequences which most closely resembled the ASA.S1 sequences obtained in the study. According to the tree constructed, IMU14 was placed into T3 genotype, IMU16 and IMU17 were identified as T5 isolates and the remaining 18 isolates were assigned to T4 genotype (Fig. 1 ).
Table 1.
Percentage of sequence similarities for present Acanthamoeba isolates against the published 18S rRNA gene sequences of specified Acanthamoeba spp. retrieved from the GenBank database.
| ASA.S1 sequences of Acanthamoeba isolates | Published 18S rRNA sequences of Acanthamoeba spp. |
|
|---|---|---|
| Most matched (%) | Other closely matched (%) | |
| IMU1, IMU2, IMU6 and IMU7 | A. castellanii CDC:0184:V014 (99) |
A. polyphaga from a pond in Germany (97) A. quina ATCC 50241 (97) |
| IMU3 | A. castellanii CDC:0184:V014 (99) |
A. polyphaga from a pond in Germany (97) A. culbertsoni Diamond (97) A. rhysodes 7AR (97) A. lugdunensis 312-2 (97) |
| IMU4 and IMU5 | A. castellanii Castellanii (99) |
A. polyphaga HC-2 (99) A. rhysodes BCM:0685:116 (99) A. culbertsoni Diamond (99) A. lugdunensis 312-2 (99) |
| IMU8 | A. castellanii CDC:0180:1 (99) |
A. culbertsoni Diamond (99) A. hatchetti 3ST (99) A. polyphaga ATCC 50372 (99) |
| IMU9 | A. castellanii CDC:0786:V042 (100) |
A. polyphaga HC-2 (99) A. rhysodes BCM:0685:116 (99) A. culbertsoni Diamond (99) A. lugdunensis 312-2 (99) A. triangularis SH621 (98) |
| IMU10, IMU11, IMU12 and IMU13 | A. culbertsoni Diamond (100) |
A. castellanii CDC:0180:1 (99) A. hatchetti 3ST (99) A. polyphaga ATCC 50372 (99) |
| IMU14 | A. griffin H37 (100) |
A. griffin S-7 (98) A. polyphaga Panola Mountain (98) A. pearcei ATCC 50435 (96) |
| IMU15 | A. hatchetti 3ST (99) |
A. culbertsoni Diamond (99) A. polyphaga ATCC 50372 (99) A. castellanii ATCC 50370 (99) |
| IMU16 and IMU17 | A. lenticulata Jc-1, CRIB56, 72/2, 7327, 45 (97) | Nil |
| IMU18 | A. triangularis SH621 (99) |
A. polyphaga ATCC 30461 (99) A. castellanii ATCC 30010 (98) A. culbertsoni Diamond (98) A. hatchetti 3ST (98) A. rhysodes 7AR (98) A. lugdunensis 312-2 (98) |
| IMU19, IMU20 and IMU21 | A. quina ATCC 50241 (99) |
A. polyphaga 5SU (99) A. palestinensis 36KL (99) A. castellanii Castellanii (98) A. hatchetti 3ST (98) |
GenBank accession numbers: A. castellanii: ATCC 30010 (EF554328), ATCC 50370 (AF534136), Castellanii (U07413), CDC:0180:1 (U07405), and CDC:0786:V042 (U07403); A. lenticulata: CRIB56 (EU377584) and 7327 (U94731); A. lugdunensis: 312-2 (AF260718); A. palestinensis 36KL (AF260719); A. pearcei ATCC 50435 (AF019053); A. polyphaga: 5SU (AF260725), ATCC30461 (AY026243), ATCC 50372 (AF534138), HC-2 (AF019056) and Panola Mountain (AF019052); A. rhysodes: 7AR (AF260720) and BCM:0685:116 (U07406). Others are listed in Fig. 1.
Fig. 1.
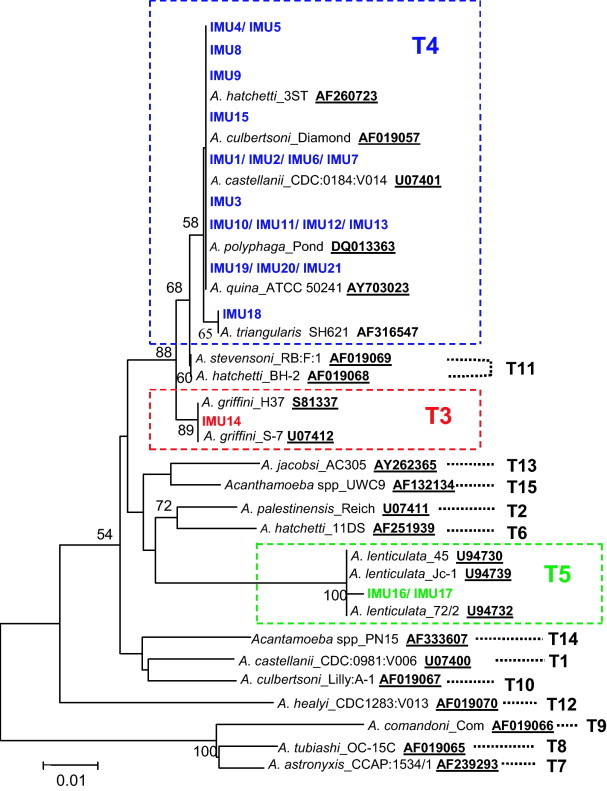
Neighbour-joining tree depicting the relationships between IMU1 to IMU21 and reference strains of Acanthamoeba representing T1 to T15 genotypes. Numbers at the nodes are percentage-bootstrapping values on 1000 replicates; only value of >50% are shown. GenBank accession numbers for reference sequences are indicated at the ends of sequence designations.
Since polymorphism of cyst shapes, sizes and number of arms could be observed within a cloned Acanthamoeba isolate, all isolates were characterized based on the average cyst morphology within the respective isolate. It was observed that all investigated isolates have cyst sizes ≤18 μm, and are therefore identified as either Group II or Group III Acanthamoeba. Isolates recognized as having Group II morphology were IMU1 to IMU9, IMU14, IMU15, and IMU18 to IMU21. Some of these isolates could be identified further to specific species based on the Pussard and Pons’ key (1977). They were A. castellanii (IMU1 to IMU3, IMU6, IMU7, IMU9 and IMU18); A. culbertsoni (IMU10 to IMU13); A. griffini (IMU14); A. lenticulata (IMU16 and IMU17), and A. polyphaga (IMU8 and IMU19) (Fig. 2 , Table 2 ). Species identity for the remaining five isolates, however, could not be determined morphologically. Cysts of IMU4 and IMU5 resembled each other. Both isolates have polygonal cysts. The cysts arms were truncated, ranging from six to seven in number. Ectocysts were wrinkled and closely attached to endocysts. These characteristics were common to both A. castellanii and A. polyphaga (Fig. 3 ). IMU15 revealed the cyst morphology of either A. hatchetti or A. polyphaga. This isolate has squarish or pentagonal endocysts. Ectocysts were wrinkled and were in close contact with the underlying endocysts, conforming to the shape of the later (Fig. 3). Cysts of IMU20 and IMU21 resembled each others. Cysts of these isolates often have pentagonal and occasionally stellate endocysts, and the ectocysts were loosely applied to endocyst, and these cysts features resembled to cysts of A. polyphaga. However, their ectocyst was rather smooth in contrast to ectocyst of A. polyphaga which was wrinkled. Therefore these three isolates could only be identified under the genus of Acanthamoeba (Fig. 3). The remaining six isolates were placed into Group III. Among these isolates, IMU10 to IMU13 were designated as A. culbertsoni whereas IMU16 and IMU17 were identified as A. lenticulata (Fig. 2).
Fig. 2.
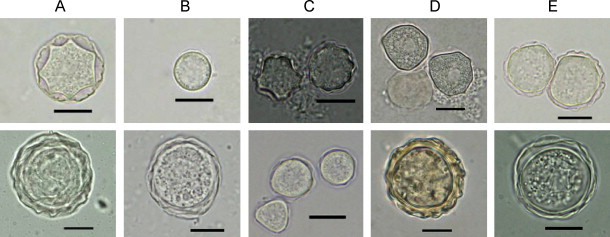
Cysts of Acanthamoeba isolates grew in xenic (top row) and axenic (bottom row) culture conditions. In xenic culture condition, cysts of respective isolates revealed the typical cysts morphology of the named species (A) A. castellanii (IMU9), (B) A. culbertsoni (IMU11), (C) A. griffin (IMU14), (D) A. lenticulata (IMU17), (E) A. polyphaga (IMU19). In contrast, axenic cysts of all isolates no longer revealed the characteristic morphologies of the respective species; they were generally bigger and round in shapes. Images were viewed under bright field microscopy. Bars represent 10 μm.
Table 2.
Morphological characteristics of Acanthamoeba isolates investigated in this study.
| Isolates | Cysts diameters |
Diagnosis (group/species) | |||
|---|---|---|---|---|---|
| Range (mean ± S.D.) | p | rS.D. (%) | Out layers (%) | ||
| IMU 1: X A |
11.48–18.04 (15.45 ± 1.67) | *** | 10.81 | Nil | II/castellanii |
| 11.46–27.97 (19.75 ± 3.33) | 16.86 | 74 | |||
| IMU 2: X A |
11.18–18.06 (15.10 ± 1.84) | *** | 12.19 | Nil | II/castellanii |
| 12.78–42.57 (20.45 ± 5.22) | 25.53 | 62 | |||
| IMU 3: X A |
11.89–18.04 (14.66 ± 1.65) | *** | 11.26 | Nil | II/castellanii |
| 11.12–22.92 (16.77 ± 1.70) | 10.14 | 22 | |||
| IMU 4: X A |
10.25–16.81 (13.90 ± 1.62) | NS | 11.65 | Nil | II/castellanii or polyphagaa |
| 11.46–22.24 (14.35 ± 1.78) | 12.40 | 32 | |||
| IMU 5: X A |
11.48–18.04 (15.51 ± 1.70) | *** | 10.96 | Nil | II/castellanii or polyphagaa |
| 15.17–31.35 (20.57 ± 3.06) | 14.88 | 80 | |||
| IMU 6: X A |
9.43–15.99 (12.93 ± 1.71) | *** | 13.23 | Nil | II/castellanii |
| 12.71–22.55 (17.04 ± 2.11) | 12.38 | 20 | |||
| IMU 7: X A |
10.25–17.22 (13.92 ± 1.51) | *** | 10.85 | Nil | II/castellanii |
| 13.30–33.21 (19.21 ± 3.79) | 19.73 | 60 | |||
| IMU 8: X A |
11.89–17.44 (14.54 ± 1.28) | *** | 8.80 | Nil | II/polyphaga |
| 13.14–34.72 (21.71 ± 4.65) | 21.42 | 76 | |||
| IMU 9: X A |
11.64–18.06 (16.40 ± 1.56) | *** | 9.51 | Nil | II/castellanii |
| 13.94–29.93 (19.56 ± 3.09) | 15.80 | 66 | |||
| IMU 10:X A |
8.61–15.99 (11.75 ± 1.58) | *** | 13.45 | Nil | III/culbertsoni |
| 13.48–24.27 (17.26 ± 2.36) | 13.67 | 30 | |||
| IMU 11: X A |
9.43–17.22 (12.96 ± 1.59) | *** | 12.27 | Nil | III/culbertsoni |
| 14.35–31.57 (22.91 ± 4.94) | 21.56 | 72 | |||
| IMU 12: X A |
8.61–17.63 (12.78 ± 2.01) | *** | 15.72 | Nil | III/culbertsoni |
| 13.82–51.90 (22.82 ± 11.17) | 48.95 | 50 | |||
| IMU 13: X A |
8.73–16.74 (11.70 ± 1.51) | *** | 12.91 | Nil | III/culbertsoni |
| 10.19–24.38 (15.88 ± 3.00) | 18.89 | 20 | |||
| IMU 14: X A |
11.07–17.22 (13.82 ± 1.40) | NS | 10.13 | Nil | II/griffini |
| 10.20–24.68 (14.40 ± 3.12) | 21.67 | 12 | |||
| IMU 15: X A |
9.59–15.99 (11.89 ± 1.57) | *** | 13.20 | Nil | II/hatchetti or polyphagaa |
| 13.53–26.65 (20.49 ± 2.67) | 13.03 | 84 | |||
| IMU16: X A |
11.48–17.63 (15.70 ± 1.31) | *** | 8.34 | Nil | III/lenticulata |
| 22.04–56.59 (32.84 ± 7.48) | 22.78 | 100 | |||
| IMU 17: X A |
11.89–18.06 (15.78 ± 1.45) | *** | 9.19 | Nil | III/lenticulata |
| 16.40–49.61 (25.72 ± 6.31) | 24.53 | 98 | |||
| IMU 18: X A |
13.46–18.19 (16.47 ± 1.46) | *** | 8.86 | Nil | II/castellanii |
| 12.13–19.55 (15.39 ± 1.47) | 9.55 | 8 | |||
| IMU 19: X A |
10.66–17.22 (14.35 ± 1.43) | *** | 9.97 | Nil | II/polyphaga |
| 11.12–27.79 (17.38 ± 2.98) | 17.15 | 30 | |||
| IMU 20: X A |
10.19–16.74 (13.34 ± 1.53) | *** | 11.47 | Nil | II/unidentified spp.b |
| 11.89–22.55 (17.49 ± 2.51) | 14.35 | 36 | |||
| IMU 21: X A |
10.66–17.22 (13.44 ± 1.49) | * | 11.09 | Nil | II/unidentified spp.b |
| 10.45–18.45 (14.34 ± 1.96) | 13.67 | 2 | |||
X = xenic culture, A = axenic culture; p = P value, rS.D. = relative standard deviation.
*p ≤ 0.05; ***p ≤ 0.001; NS = Not significant.
Species of isolate could not be identified as it resembled closely to morphology of the mentioned species.
Species of isolate could not be identified morphologically.
Fig. 3.
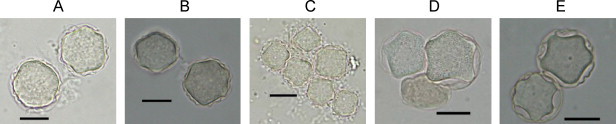
Cysts of Acanthamoeba isolates that could not be determined morphologically. (A) IMU4, (B) IMU5, (C) IMU15, (D) IMU20, (E) IMU21. Images were viewed under bright field microscopy. Bars represent 10 μm.
Cysts morphological parameters for IMU1 to IMU21 are presented in Table 2. In general, cyst diameters of all Acanthamoeba isolates cultured in xenic condition were in agreement with the morphological grouping by Pussard and Pons, and cysts that grew in this condition were less variable in cysts diameters (ranged from 8.34% up to 15.72% of relative standard deviation). However, in axenic liquid medium, cysts of almost all isolates were much larger and variable in diameter (ranging from 9.55% up to 48.95% of relative standard deviation). Student's t-test showed that all Acanthamoeba isolates grown under axenic culture condition, except IMU4 and IMU14, have significantly larger mean diameters of cysts as compared to those grown under xenic culture condition. Outliers, defined by cyst diameter >18 μm, were observed for Acanthamoeba cysts grown under axenic culture condition, whereas none were observed under xenic culture condition. Indeed, some cysts grown in the former condition even achieved extreme diameters of more than 40–50 μm.
Growth rates of Acanthamoeba isolates at different temperatures were graded qualitatively by visual observation rather than through enumeration of organisms using a hematocytometer. Quantification was not practical because trophozoites of some cell lines adhered rather strongly to substrates of agar plates, and these made cell enumeration based on sample aliquots inaccurate. At 37 °C incubation, all isolates were able to grow at this temperature, however IMU1, IMU2, IMU3, IMU7, IMU15, IMU16 and IMU21 grew slower compared to other isolates. At 42 °C incubation, none except IMU17 could produce growth. To investigate the effects of higher temperatures on their viability, cysts of each isolate were exposed to 46 °C and 52 °C overnight prior to incubations at lower temperature (26 ± 2 °C) which favored excystation. The test revealed that cysts of all isolates could tolerate temperature up to 52 °C.
In osmotolerance test, IMU4, IMU5, IMU10, IMU13, IMU14 and IMU21 showed good osmotolerance to 1 M D-mannitol, as evident by indistinguishable growth profiles and cell features in cultures that grew in the presence or absence of the saccharide. On the other hand, IMU3, IMU18, IMU9 and IMU20 showed partial tolerance towards osmotic stress with 1 M D-mannitol; these isolates excysted infrequently and trophozoites that emerged were relatively small and nearly rounded in shape. There remaining 10 isolates were very sensitive towards 1 M D-mannitol. In the presence of the saccharide, no excystation was observed for these isolates.
4. Discussion
In the present study, grilles at air-handler yielded the most number of Acanthamoeba isolations (13 samples out of the 20 Acanthamoeba positive samples), and five out of the six nominal species of this genus were isolated from these sites. The air-handler is the indoor component of the central conditioner. When the centralized air-conditioner is operating, warmer indoor air is drawn from the rooms or zones to be conditioned. Most of the return air is sent directly to the air-handler machine through the return air-ducts which are installed above the ceiling. Several exhaust fans mounted on the concrete wall facing the grilles of the air-handler extract fresh air from the outdoors into the air-handler room. Inside the room, the fresh outdoor air together with some indoor air which is returned via the corridors surrounding the air-handler room, will penetrate the grilles of air-handler and flow into the machine by negative pressure. Inside the air-handler, all air is mixed. The mixed air is then filtered through fiber filter which helps to remove most of the air-borne solid particles. The filtered air is then blown at the evaporation coil to be instantly cooled; excess moisture within the air is condensed and drained away. The conditioned air is then delivered back to the respective zone through air-supply ducts (Mull, 1997, Oughton et al., 2002).
It is postulated that Acanthamoeba spp. detected on grilles at air-handler originated from indoors via return air and fresh outdoor air that passed through the grilles. The high occurrence and diversity of Acanthamoeba spp. obtained from these places are attributed to availability of food for the amoebae. Microorganisms like algae, bacteria, fungi, and protozoa are probably present within the air particles in the return air and fresh air, and these would be retained at the grilles. These microorganisms would accumulate and proliferate here and serve as food source for other free-living organisms such as the Acanthamoeba spp. It is possible that fresh outdoor air could carry additional Acanthamoeba into the indoor air. On the other hand, fresh air has some beneficial role. It is needed to dilute microorganisms and pollutants in the air-conditioned space to improve indoor air-quality (Ananthanarayanan, 2005).
There were six Acanthamoeba positive samples detected in dusts collected from vents at ceilings. These vents carry cooled air from the air handler. They are connected to the air-handler through the air supply ducts. Acanthamoeba cells detected at these sites could have originated from the air-handler. It is postulated that the fiber filter of the air-handler failed to retain all air-borne solid particles, especially the microorganisms. Microorganisms such as Acanthamoeba spp. would possibly transfer to the respective vents by air flow. Less Acanthamoeba spp. were detected at the vents compared to that at air-handlers. This is possibly due to the distance that air needed to travel from the air-handlers to the vents, allowing dilution of the Acanthamoeba cells carried within the air. Since conditioned air was recirculated in an enclosed environment, these pollutants would in time, accumulate indoors. Therefore, it is postulated that the use of air-conditioners could increase the exposure risk of building occupants to air-borne Acanthamoeba infections.
There was only one Acanthamoeba positive sample detected from a room air-conditioner in the present study. The use of room air-conditioners is limited to small rooms and small compartments; their functions are to complement the insufficient cool air supplied by the central air-conditioners to these places. Room air-conditioners in the present building were rather clean as less dust accumulated at the grills and bodies of the machines. The frequent cleaning of these machines certainly could help to prevent the growth of Acathamoeba spp. at these machines. This prediction was supported by our preliminary study conducted in a separate building. In that study, a higher rate of Acanthamoeba spp. isolations were made from grilles of room air-conditioners that were poorly maintained and were covered by thick dust.
The present study clearly demonstrated the pitfalls of using cyst morphology to classify Acanthamoeba at species level. As many as five Acanthamoeba isolates could not be identified with confidence based on morphological characteristics. Furthermore, the present study clearly showed that culture conditions (xenic vs. axenic) could greatly distort the typical cyst features of Acanthamoeba isolates. Our findings on the inconsistency of cyst features influenced by culture conditions concur with those reported previously (Sawyer, 1971, Stratford and Griffiths, 1978).
In the present study, all the Acanthamoeba isolates investigated were genotyped using DNA sequencing with the JDP1–JDP2 primers. However, this approach is limited by a drawback; the amount of DNA information obtained was insufficient to discriminate isolates closely related to T3, T4 and T11 genotypes (Schroeder et al., 2001). The better way to differentiate Acanthamoeba genotypes is probably to sequence the complete 18S rRNA genes; however, this is not possible in the current study due to financial constraint. To date, the taxonomic status of Acanthamoeba genus is still evolving. The decision on whether to classify Acanthamoeba isolates by genotypes or specific names is still debated. Although publications and DNA sequences deposited into GenBank database reflect the current trend on the use of molecular genotyping of Acanthamoeba spp., the value of morphological characteristics should not be entirely excluded. Morphological classification of Acanthamoeba spp. should be preserved, at least until a globally standardized scheme is established.
Eighteen of the air-conditioned isolates characterized in the current study are categorized into the T4 genotype. Many previous studies similarly reported that T4 is the most prevalent genotype in their environment samples (Fuerst et al., 2003, Maghsood et al., 2005). The greater abundance of T4 isolates in our environment samples probably reflects their better adaptation to limited growth condition relative to isolates from other genotypes. On the other hand, the isolations of two T5 Acanthamoeba isolates from central air-conditioners could signify that these sampling sites have high moisture content. Our postulation was deduced from other studies which reported the frequent isolations of this rare Acanthamoeba genotype from aquatic environment (Lorenzo-Morales et al., 2005a, Lorenzo-Morales et al., 2005b, Rivera and Adao, 2008, Huang and Hsu, 2010).
All 21 air-conditioner isolates of Acanthamoeba investigated in the present study were able to grow at 37 °C; one isolate (IMU17) can even survive at 42 °C. These observations have led us to postulate that all present Acanthamoeba isolates can survive and grow in human bodies which have an average body temperature of ±37 °C, and are therefore considered as potential human pathogenic isolates. Other studies have demonstrated that a higher environmental temperature would favor the growth of thermotolerant Acanthamoeba. It is believed that such isolates could have evolved through natural selection to adapt to the heat stress in their niche (Rivera et al., 1993, Gianinazzi et al., 2009). However, unlike these findings, our study did not show such association. The site of Acanthamoeba isolations in the present study, i.e. the grilles at air-handler units, the vents at ceilings and the grilles of room air-conditioners are either at low temperature (∼25 °C during office hour, with an operating duration of 10.5 h per day, from Monday to Friday each week), or at ambient temperature (>30 °C, during non-office hours). However, the present study could not entirely rule out the possible influence of niche heat stress on the better survival of thermotolerant Acanthamoeba in air-conditioners. The origin of the air-conditioner Acanthamoeba isolates investigated in the present study could not be identified. As mentioned earlier, they could be from outdoor fresh air or from indoors, including building occupants. It is known that the climate of Malaysia is generally warm; the highest outdoor temperature being recorded in Kuala Lumpur could even achieve 37 °C (National Environment Agency, 2009). Therefore, it is likely that a higher proportion of Acanthamoeba isolates in the Malaysian environment, particularly in Kuala Lumpur are naturally thermotolerant. This assumption may help to explain the isolations of large numbers of thermotolerant air-conditioner Acanthamoeba isolates in the present study. On the other hand, cysts of all Acanthamoeba isolates retained their viability after overnight exposure to temperature up to 52 °C. The remarkable tolerance and resilience of Acanthamoeba cysts to extreme growth temperature probably contribute to the ubiquity, abundance and persistent occurrence of these free-living amoebae in diverse environments. This physiological characteristic may also enable the cyst to serve as the ideal vehicle for transmitting Acanthamoeba infection from environment to human, and their persistence in the human host.
In osmotolerance test, IMU4, IMU5, IMU10, IMU13, IMU14 and IMU21 are high osmotolerant Acanthamoeba, and thus could be regarded as potential human keratitis isolates. Although the precise measurement of osmolarity given by 1 M mannitol in non-nutrient agar culture plates used in the present study has not been determined, a previous study indicated that it was 0.25 osmolar (250 mOsmol/L), in contrast to 0.025 osmolar in culture plates without the saccharide (Khan and Tareen, 2003). Tear film osmolarity in normal human subjects has previously been measured by several research groups (between 1978 and 2004); the cumulative average value was ∼300 mOsmol/L (lowest was 283.3 ± 11.3 as determined by Isekei et al., and the highest was 318 ± 31 as reported by Benjamin and Hill) (Benjamin and Hill, 1983, Iskeleli et al., 2002, Tomlinson et al., 2006). Although most studies on Acanthamoeba pathogenicity and osmotolerance find good association using 1 M mannitol, future investigation into this aspect should use higher concentrations of mannitol, to better mimic the osmolarity of the human tear film.
The present study is the first demonstrating the presence of potential human pathogenic Acanthamoeba in indoor unit of air-conditioners in Malaysia. Our finding was complementary to the findings by el Sibae in Egypt, who managed to detect Acanthamoeba polyphaga in cooling towers; the outdoor units of air-conditioning systems (el Sibae, 1993). The air-conditioner is essential in commercial as well as residential settings for comfort. Its wide use is attributed to the increase in climatic temperature due to global warming, and as a symbol of development and modern lifestyles. Our findings should alert us on the risk of acquiring Acanthamoeba infections through the use of poorly maintained air-conditioners. In addition, although environmental Acanthamoeba spp. feed on surrounding bacteria, fungal and other free-living organisms, they could be host for certain endosymbionts such as the pathogenic Legionella pneumophila, Parachlamydia and mimivirus, and indirectly become agents of transmission for these pathogens. Therefore, it is important to understand the interactions between Acanthamoeba spp. and the surrounding microorganisms, especially the pathogenic microbes. In order to decrease the risk of building occupants being exposed to both pathogenic Acanthamoeba spp. and their endosymbionts, regularly cleaning and decontamination of air-conditioners using effective Acanthamoeba cidal cleansing solutions are essential (Srikanth and Berk, 1993).
Acknowledgement
This study was supported by Research Grant No. IMU 086/05 from the International Medical University.
References
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan P.N. 3rd ed. Tata McGraw-Hill Publishing Co. Ltd.; New Delhi: 2005. Basic Refrigeration and Air Conditioning. p. 255. [Google Scholar]
- Awwad S.T., Petroll W.M., McCulley J.P., Cavanagh H.D. Updates in Acanthamoeba keratitis. Eye Contact Lens. 2007;33:1–8. doi: 10.1097/ICL.0b013e31802b64c1. [DOI] [PubMed] [Google Scholar]
- Benjamin W.J., Hill R.M. Human tears: osmotic characteristics. Invest. Ophthalmol. Vis. Sci. 1983;24:1624–1626. [PubMed] [Google Scholar]
- Carlesso A.M., Artuso G.L., Caumo K., Rott M.B. Potentially pathogenic acanthamoeba isolated from a hospital in Brazil. Curr. Microbiol. 2010;60:185–190. doi: 10.1007/s00284-009-9523-7. [DOI] [PubMed] [Google Scholar]
- Costa A.O., Castro E.A., Ferreira G.A., Furst C., Crozeta M.A., Thomaz-Soccol V. Characterization of acanthamoeba isolates from dust of a public hospital in Curitiba, Paraná, Brazil. J. Eukaryot. Microbiol. 2010;57:70–75. doi: 10.1111/j.1550-7408.2009.00453.x. [DOI] [PubMed] [Google Scholar]
- de Jonckheere J.F. Isoenzyme and total protein analysis by agarose isoelectric focusing, and taxonomy of the genus Acanthamoeba. J. Eukaryot. Microbiol. 1983;30:701–706. [Google Scholar]
- el Sibae M.M. Detection of free-living amoeba (Acanthamoeba polyphagia) in the air conditioning systems. J. Egypt. Soc. Parasitol. 1993;23:687–690. [PubMed] [Google Scholar]
- Fuerst P.A., Booton G.C., Visvesvara G.S., Byers T.J. Genotypic identification of non-keratitis infections caused by the opportunistically pathogenic ameba genus Acanthamoeba. J. Eukaryot. Microbiol. 2003;50:512–513. doi: 10.1111/j.1550-7408.2003.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Gast R.J., Ledee D.R., Fuerst P.A., Byers T.J. Subgenus systematics of Acanthamoeba: four nuclear 18S rDNA sequence types. J. Eukaryot. Microbiol. 1996;43:498–504. doi: 10.1111/j.1550-7408.1996.tb04510.x. [DOI] [PubMed] [Google Scholar]
- Gast R.J. Development of an Acanthamoeba-specific reverse dot-blot and the discovery of a new ribotype. J. Eukaryot. Microbiol. 2001;48:609–615. doi: 10.1111/j.1550-7408.2001.tb00199.x. [DOI] [PubMed] [Google Scholar]
- Gianinazzi C., Schild M., Wüthrich F., Müller N., Schürch N., Gottstein B. Potentially human pathogenic Acanthamoeba isolated from a heated indoor swimming pool in Switzerland. Exp. Parasitol. 2009;121:180–186. doi: 10.1016/j.exppara.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Hewett M.K., Robinson B.S., Monis P.T., Saint C.P. Identification of a new Acanthamoeba 18S rRNA gene sequence type, corresponding to the species Acanthamoeba jacobsi Sawyer, Nerad and Visvesvara, 1992 (Lobosea: Acanthamoebidae) Acta Protozool. 2003;42:325–329. [Google Scholar]
- Horn M., Fritsche T.R., Gautom R.K., Schleifer K.H., Wagner M. Novel bacterial endosymbionts of Acanthamoeba spp. related to the Paramecium caudatum symbiont Caedibacter caryophilus. Environ. Microbiol. 1999;1:357–367. doi: 10.1046/j.1462-2920.1999.00045.x. [DOI] [PubMed] [Google Scholar]
- Huang S.W., Hsu B.M. Isolation and identification of Acanthamoeba from Taiwan spring recreation areas using culture enrichment combined with PCR. Acta Trop. 2010;115:282–287. doi: 10.1016/j.actatropica.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Iskeleli G., Karakoç Y., Aydin O., Yetik H., Uslu H., Kizilkaya M. Comparison of tear-film osmolarity in different types of contact lenses. CLAO J. 2002;28:174–176. doi: 10.1097/01.ICL.0000024117.46518.A4. [DOI] [PubMed] [Google Scholar]
- Khan N.A., Jarroll E.L., Paget T.A. Acanthamoeba can be differentiated by the polymerase chain reaction and simple plating assays. Curr. Microbiol. 2001;43:204–208. doi: 10.1007/s002840010288. [DOI] [PubMed] [Google Scholar]
- Khan N.A., Paget T.A. Molecular tools for speciation and epidemiological studies of Acanthamoeba. Curr. Microbiol. 2002;44:444–449. doi: 10.1007/s00284-001-0050-4. [DOI] [PubMed] [Google Scholar]
- Khan .N.A., Tareen N.K. Genotypic, phenotypic, biochemical, physiological and pathogenicity-based categorisation of Acanthamoeba strains. Folia Parasitol. (Praha) 2003;50:97–104. doi: 10.14411/fp.2003.017. [DOI] [PubMed] [Google Scholar]
- Khan N.A. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol. Rev. 2006;30:564–595. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- Kilic A., Tanyuksel M., Sissons J., Jayasekera S., Khan N. Isolation of Acanthamoeba isolates belonging to T2, T3, T4 and T7 genotypes from environmental samples in Ankara, Turkey. Acta Parasitol. 2004;49:246–252. [Google Scholar]
- Kong H.H., Shin J.Y., Yu H.S., Kim J., Hahn T.W., Hahn Y.H., Chung D.I. Mitochondrial DNA restriction fragment length polymorphism (RFLP) and 18S small-subunit ribosomal DNA PCR-RFLP analyses of Acanthamoeba isolated from contact lens storage cases of residents in southwestern Korea. J. Clin. Microbiol. 2002;40:1199–1206. doi: 10.1128/JCM.40.4.1199-1206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarga A., Valentin F., Anderson M., Lopez R. European bioinformatics institute. Nucleic Acids Res. 2007;35:W6–W11. doi: 10.1093/nar/gkm291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Huang X., Yu I.T.S., Wong T.W., Qian H. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air. 2004;15:83–95. doi: 10.1111/j.1600-0668.2004.00317.x. [DOI] [PubMed] [Google Scholar]
- Liu H., Moon E.K., Yu H.S., Jeong H.J., Hong Y.C., Kong H.H., Chung D.I. Evaluation of taxonomic validity of four species of Acanthamoeba: A. divionensis, A. paradivionensis, A. mauritaniensis, and A. rhysodes, inferred from molecular analyses. Korean J. Parasitol. 2005;43:7–13. doi: 10.3347/kjp.2005.43.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Morales J., Lindo J.F., Martinez E., Calder D., Figueruelo E., Valladares B., Ortega-Rivas A. Pathogenic Acanthamoeba strains from water sources in Jamaica, West Indies. Ann. Trop. Med. Parasitol. 2005;99:751–758. doi: 10.1179/136485905X65215. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Morales J., Monteverde-Miranda C.A., Jiménez C., Tejedor M.L., Valladares B., Ortega-Rivas A. Evaluation of Acanthamoeba isolates from environmental sources in Tenerife, Canary Islands, Spain. Ann. Agric. Environ. Med. 2005;12:233–236. [PubMed] [Google Scholar]
- Lutz B.D., Jin J., Rinaldi M.G., Wickes B.L., Huycke M.M. Outbreak of invasive Aspergillus infection in surgical patients, associated with a contaminated air-handling system. Clin. Infect. Dis. 2003;37:786–793. doi: 10.1086/377537. [DOI] [PubMed] [Google Scholar]
- Maghsood A.H., Sissons J., Rezaian M., Nolder D., Warhurst D., Khan N.A. Acanthamoeba genotype T4 from the UK and Iran and isolation of the T2 genotype from clinical isolates. J. Med. Microbiol. 2005;54:755–759. doi: 10.1099/jmm.0.45970-0. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F., Cabral G. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 2003;16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A.J. Infection of the central nervous system due to Acanthamoeba. Rev. Infect. Dis. 1991;13:S399–S402. doi: 10.1093/clind/13.supplement_5.s399. [DOI] [PubMed] [Google Scholar]
- Mergeryan H. The prevalence of Acanthamoeba in the human environment. Rev. Infect. Dis. 1991;13:S390–S401. doi: 10.1093/clind/13.supplement_5.s390. [DOI] [PubMed] [Google Scholar]
- Mull T.E. McGraw-Hill Co. Inc.; New York: 1997. HVAC Principles and Applications Manual. p. 528. [Google Scholar]
- National Environment Agency, Singapore Government. The Climate of Malaysia. 2009. http://app2.nea.gov.sg/asiacities_malaysia.aspx.
- Niyyati M., Lorenzo-Morales J., Rahimi F., Motevalli-Haghi A., Martín-Navarro C.M., Farnia S., Valladares B., Rezaeian M. Isolation and genotyping of potentially pathogenic Acanthamoeba strains from dust sources in Iran. Trans. R. Soc. Trop. Med. Hyg. 2009;103:425–427. doi: 10.1016/j.trstmh.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Nwachuku N., Gerba C.P. Health effects of Acanthamoeba spp. and its potential for waterborne transmission. Rev. Environ. Contam. Toxicol. 2004;180:93–131. doi: 10.1007/0-387-21729-0_2. [DOI] [PubMed] [Google Scholar]
- O’Mahony M.C., Stanwell-Smith R.E., Tillett H.E., Harper D., Hutchison J.G., Farrell I.D. The Stafford outbreak of Legionnaires’ disease. Epidemiol. Infect. 1990;104:361–380. doi: 10.1017/s0950268800047385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oughton D., Hodkinson S., Martin P.L., Faber Ó., Kell J. Butterworth-Heinemann; Oxford: 2002. Faber and Kell's Heating and Air-conditioning of Buildings. p. 720. [Google Scholar]
- Pussard M., Pons R. Morphologies de la paroi kystique et taxonomie du genre Acanthamoeba (Protozoa Amoebida) Protistologica. 1977;13:57–610. [Google Scholar]
- Rivera W.L., Adao D.E. Identification of the 18S-ribosomal-DNA genotypes of Acanthamoeba isolates from the Philippines. Ann. Trop. Med. Parasitol. 2008;102:671–677. doi: 10.1179/136485908X337544. [DOI] [PubMed] [Google Scholar]
- Rivera F., Ramirez E., Bonilla P., Calderon A., Gallegos E., Rodriguez S., Ortiz R., Zaldívar B., Ramírez P., Durán A. Pathogenic and free-living amoebae isolated from swimming pools and physiotherapy tubs in Mexico. Environ. Res. 1993;62:43–52. doi: 10.1006/enrs.1993.1087. [DOI] [PubMed] [Google Scholar]
- Rosenberg A.S., Morgan M.B. Disseminated acanthamoebiasis presenting as lobular panniculitis with necrotizing vasculitis in a patient with AIDS. J. Cutan. Pathol. 2001;28:307–313. doi: 10.1034/j.1600-0560.2001.028006307.x. [DOI] [PubMed] [Google Scholar]
- Sawyer T.K. Acanthamoeba griffini, a New Species of Marine Amoeba. J. Eukryot. Microbiol. 1971;18:650–654. [Google Scholar]
- Schroeder J.M., Booton G.C., Hay J., Niszl I.A., Seal D.V., Markus M.B., Fuerst P.A., Byers T.J. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoebae from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 2001;39:1903–1911. doi: 10.1128/JCM.39.5.1903-1911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S., Berk S.G. Stimulatory effect of cooling tower biocides on amoebae. Appl. Environ. Microbiol. 1993;59:3245–3249. doi: 10.1128/aem.59.10.3245-3249.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard D.R., Schroeder-Diedrich J.M., Awwad M.H., Gast R.J., Ledee D.R., Rodriguez-Zaragoza S., Dean C.L., Fuerst P.A., Byers T.J. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J. Eukaryot. Microbiol. 1998;45:45–54. doi: 10.1111/j.1550-7408.1998.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford M.P., Griffiths A.J. Variations in the properties and morphology of cysts of Acanthamoeba castellanii. J. Gen. Microbiol. 1978;108:33–37. [Google Scholar]
- Sukthanaa Y., Riguntib M., Siripantha C., Kusolsukc T., Chintrakarnd C., Kulpaditharomd B. An exotic sinusitis. Trans. Roy. Soc. Trop. Med. Hyg. 2005;99:555–557. doi: 10.1016/j.trstmh.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Khanal S., Ramaesh K., Diaper C., McFadyen A. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest. Ophthalmol. Vis. Sci. 2006;47:4309–4315. doi: 10.1167/iovs.05-1504. [DOI] [PubMed] [Google Scholar]
- Visvesvara G.S. Classification of Acanthamoeba. Rev. Infect. Dis. 1991;13:S369–S372. doi: 10.1093/clind/13.supplement_5.s369. [DOI] [PubMed] [Google Scholar]
- Walochnik J., Obwaller A., Aspöck H. Correlations between morphological, molecular biological, and physiological characteristics in clinical and nonclinical isolates of Acanthamoeba spp. Appl. Environ. Microbiol. 2000;66:4408–4413. doi: 10.1128/aem.66.10.4408-4413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walochnik J., Michel R., Aspöck H. Discrepancy between morphological and molecular biological characters in a strain of Hartmannella vermiformis Page 1967 (Lobosea, Gymnamoebia) Protistology. 2002;2:185–188. [Google Scholar]


