Abstract
Zinc has been shown to mediate antiviral effects against certain viruses. However, the underlying mechanisms are still largely unknown. We investigated the effects of the two zinc salts, zinc chloride (ZnCl2) and zinc sulfate (ZnSO4), on infection of swine testicle (ST) cells with transmissible gastroenteritis virus (TGEV) and compared it to the effects of a control salt, magnesium sulfate (MgSO4). Virus yield reduction experiments showed that ZnCl2 and ZnSO4 did not exhibit direct virucidal effects and did not affect adsorption of TGEV to ST cells. However, ZnCl2 and ZnSO4 markedly reduced viral titers as well as TGEV RNA and viral protein synthesis when applied during virus penetration and at different time points after viral cell entry. The results of the study suggest that zinc salts do not interfere with TGEV-cell binding but that they mediate antiviral effects through inhibition of viral penetration or egress or the intracellular phase of the viral life-cycle.
Keywords: Transmissible gastroenteritis virus (TGEV), Coronavirus, Zinc, Antiviral effects
1. Introduction
Zinc (Zn) is an important trace element and, among other functions, plays a significant role in initiating and maintaining a robust immune response (Rink and Gabriel, 2000). Furthermore, Zn has been shown to exert direct inhibitory effects against a variety of viruses including human rhinovirus (Korant and Butterworth, 1976, Geist et al., 1987) herpes simplex virus (Kümel et al., 1990, Arens and Travis, 2000), human immunodeficiency virus (Haraguchi et al., 1999), hepatitis C virus (Yuasa et al., 2006), respiratory syncytial virus (Suara and Crowe, 2004), and vaccinia virus (Katz and Margalith, 1981). In swine nutrition the use of high levels of dietary Zn applied as ZnO was shown to reduce the incidence and severity of unspecific diarrhea after weaning (Zhang and Guo, 2009). However, specific effects of Zn treatment against viral diarrhea in swine have not yet been addressed.
Transmissible gastroenteritis virus (TGEV), a porcine coronavirus, is a pleomorphic, enveloped virus with a single-stranded RNA genome of positive polarity. TGEV causes symptoms of gastroenteritis in swine, a condition associated with high morbidity in animals of all ages and high mortality in suckling piglets (Schwegmann-Wessels et al., 2002, Chen et al., 2004). The appearance of a porcine respiratory coronavirus (PRCV), which is antigenically related to TGEV, has strongly decreased the risk of TGE in Europe, since neutralizing antibodies elicited by PRCV can provide cross-protection against TGEV infection. However, TGEV prevalence is still reported in different parts of the world, implying that TGEV is still threatening pig industry. As no safe and fully protective TGEV vaccine exists, an effective treatment of the disease in piglets is needed (Ren et al., 2011).
The objective of the present study was to determine if Zn salts exert an inhibitory effect on the replication of the TGEV in the naturally permissive swine testicular (ST) cells. We performed a set of virus yield reduction experiments with two Zn salts present at different time periods before and after infection, and we also determined the inhibitory effect of Zn salts on viral RNA and protein synthesis.
2. Materials and methods
2.1. Cells and viruses
Swine testicular (ST) cells were maintained in DMEM (PAN Biotech) supplemented with 10% fetal calf serum (Hyclone), and 1% penicillin/streptomycin (Biochrom).
The TGEV strain PUR-46-MAD was propagated in ST cells. All infections were done at a multiplicity of infection (MOI) of 0.1. At that MOI, virus titer reached its maximum after 24 h as determined by a multi-step growth curve (data not shown). For virus yield reduction experiments ST cells were incubated for 48 h at 37 °C after infection. Cell culture supernatants were harvested, clarified by centrifugation, and stored at −80 °C.
All viral titrations were performed with tenfold dilutions of supernatants on 96-well microtiter plates with 8 wells per dilution. After 48 h all wells were evaluated for cytopathic effect (CPE) and viral titers were calculated by the Reed and Muench method (Reed and Muench, 1938) and expressed as 50% tissue culture infection dose (TCID50) per ml.
2.2. Zinc solutions
ZnCl2 and ZnSO4 (Sigma–Aldrich) were used in all experiments. MgSO4 (Sigma–Aldrich) served as a divalent cation mock-control salt. Stock solutions of 10 mM concentration were prepared in deionized water and were sterilized by passage through a 0.22 μm pore-size syringe filter (Roth).
2.3. Toxicity of Zn salts for uninfected cells
Zn cytotoxicity was assessed using the MTT method (3-(4,5)-dimethylthiazol-2-yl-2-5-diphenyl-tetrazolium bromide). Different concentrations (10–500 μM) of Zn salts dissolved in the culture medium were added to 90% confluent ST cell monolayers in 96-well plates for 48 h. MTT (Sigma–Aldrich), which is cleaved by active mitochondria, was added to each well for 4 h, followed by the addition of dimethyl sulfoxide containing 0.6% acetic acid and 10 g SDS (Sigma–Aldrich). The reduction of MTT to colored formazan was assessed by measuring the absorbance of the sample on a microplate reader (Tecan) at 590 nm. All experiments were done in triplicate. The percentage of cytotoxicity was calculated for each concentration as [ODt/ODc) × 100], where ODt and ODc correspond to the absorbance of treated and control cells, respectively. The CC50 value was defined as the concentration of each salt that reduced the absorbance of treated cells by 50% when compared to untreated control cells and was calculated by regression analysis of dose–response curves generated from the data.
2.4. Virus yield reduction experiments
The effect of Zn on the amount of virus generated from infected ST cells was examined by applying ZnCl2, ZnSO4, and MgSO4 in four different non cytotoxic concentrations (10, 50, 100, and 200 μM) and at different treatment periods before or after TGEV infection. After each treatment, cells were washed and kept in growth medium. After incubation for 48 h, supernatants were collected and virus titers were determined.
To asses direct virucidal effects of Zn salts and its effects on virus adsorption when present during a cell-free preincubation period, equal volumes of virus and Zn or control salt were co-incubated in Opti-MEM I (Gibco) for 2 h at a pH of 7.2 in a total volume of 500 μl at 37 °C. The mixture was then used to infect ST cells for 1 h at 37 °C. Control wells were similarly infected with virus diluted in Opti-MEM I without addition of salts.
To determine the influence of Zn present during an early stage of virus infection, ST cells were washed with ice-cold PBS and infected with TGEV for 1 h at 4 °C. At this temperature virus can only attach to the cell surface but not penetrate into the cells. Non-adsorbed virus was removed by washing with PBS, and DMEM containing Zn or control salt was added for 4 h post-infection (hpi) at 37 °C.
For the examination of Zn treatment effects at a later stage of infection after multiple replication cycles, ST cells were infected with TGEV for 1 h at 37 °C. Cells were washed and kept under growth medium for 22 h. DMEM containing Zn or control salt was then added for 4 h at 37 °C.
Finally, the influence of a long-term Zn treatment on TGEV-infected ST cells was determined. After infection with TGEV for 1 h at 37 °C, non-adsorbed virus was removed by washing with PBS, and DMEM containing Zn or control salt was added for 48 hpi at 37 °C.
2.5. Real-time PCR quantification of viral RNA synthesis
For the relative quantification of viral RNA synthesis, ST cells were infected with TGEV for 1 h and then treated with Zn or control salt at a concentration of 100 μM for additional 4 h. Cells were harvested after 48 h, and total RNA was isolated using the Invisorb Spin Cell RNA Mini Kit (Invitek). RNA was eluted in 50 μl RNase-free water and treated with Amplification Grade DNase I (Invitrogen) according to the manufacturer's instructions. Total RNA was reverse transcribed using the RevertAidTM First Strand cDNA Synthesis Kit (Fermentas) with oligo(dT) and random hexamer primers. Using the iCycler iQ5 real-time PCR detection system (Bio-Rad) and the SensiMixPlus SYBR Kit (Quantace) quantitative real-time PCR was performed in a reaction volume of 20 μl. The relative expression of the TGEV S protein gene (forward, 5′-GTATTGGGATTATGCT-3′; reverse, 5′-GGTGGTGGTAGTAGGT-3′) was determined by normalizing against the porcine β-actin gene (forward, 5′-GGACTTCGAGCAGGAGATGG-3′; reverse, 5′-GCACCGTGTTGGCGTAGAGG-3′).
2.6. Relative quantification of viral protein synthesis by ELISA
As for the determination of viral RNA synthesis, cells were infected with TGEV for 1 h followed by the addition of Zn salts or MgSO4 at different concentrations for 4 h. At 48 h after infection, cell culture supernatants were harvested, and an indirect ELISA was used to determine the relative amount of viral proteins accumulated extracellularly compared to untreated cells. A feline anti-TGEV polyclonal antiserum (Natutec) was applied at 1:256 dilution to bind viral proteins followed by an alkaline phosphatase-labeled sheep anti-feline IgG (Rockland) at a dilution of 1:20,000. The absorbance of the substrate 1,2 phenylendiamine (Sigma–Aldrich) was measured on a microplate reader (Tecan) at 492 nm. A sample was considered positive if the optical density value was higher than the mean background + 2 standard deviations.
2.7. Statistics
SPSS software for Windows version 12.0 (SPSS Inc.) was used to analyze data. The differences between groups were compared by one-way analysis of variance. Differences with P < 0.05 were considered significant.
3. Results and discussion
We investigated the effects of two zinc salts, ZnCl2 and ZnSO4, on infection of ST cells with TGEV and compared it to the effects of a control salt, MgSO4.
As a first step, cytotoxic effects of treatment with both Zn salts and MgSO4 were examined. Both Zn salts caused cytotoxic effects in a concentration-dependent fashion only at high concentrations when applied for 48 h, while no such effect was observed for MgSO4. The 50% cytotoxic concentration (CC50) for ST cells treated with ZnCl2 and ZnSO4 for 48 h was calculated to be 321 μM and 343 μM, respectively. When Zn salts were only present for 4 h, no cytotoxicity was observed even at the highest concentration of 500 μM (data not shown).
For the examination of Zn effects on viral replication, different non-cytotoxic concentration of Zn salts were applied to ST cells infected with a low MOI of 0.1, in order to more closely reflect natural infection, where multiple infection cycles occur.
Pretreatment of TGEV with Zn salts under cell-free conditions before infection did not affect the virus yield (Fig. 1 a), which indicates that Zn salts do not inactivate TGEV directly and fail to inhibit receptor binding. For other viruses, Zn was shown to block virus adsorption through deposition onto the virion surface (Kümel et al., 1990) or through deposition on the target cells (Suara and Crowe, 2004). These mechanisms seem to be irrelevant for TGEV as suggested by our results, which may be due to differences in the viral glycoproteins and their receptors, and/or target cells.
Fig. 1.
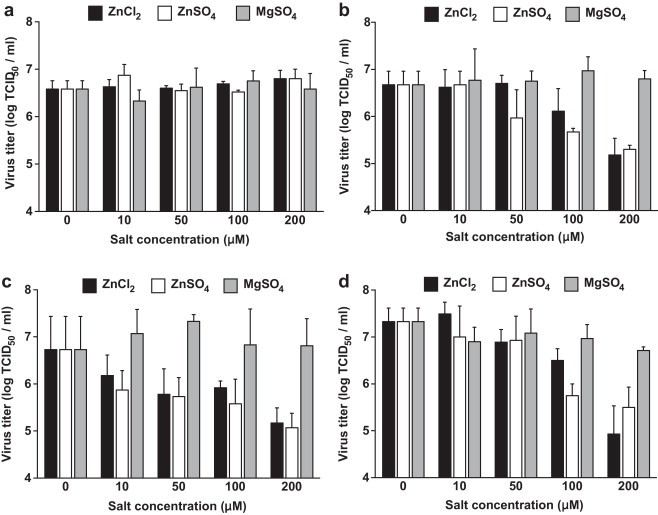
Determination of virus yields (TCID50 assay) following different Zn salt treatment periods. Cell-free preincubation of Zn salts with TGEV (a), Zn salt treatment for 1–4 hpi (b), for 22–24 hpi (c), for 1–48 hpi (d). Virus titer values indicate the mean values and SEM of three independent experiments.
The addition of Zn salts to ST cells at an early stage of infection (1–4 hpi), when TGEV had been allowed to bind to its receptors but not to enter the cells, led to a significant reduction of viral titers (Fig. 1b). The mean virus titer in the mock-treated cultures was 106.7 TCID50/ml (SEM = 0.28 log10 TCID50/ml) compared to 105.2 TCID50/ml (SEM = 0.36 log10 TCID50/ml) for ZnCl2 and 105.3 TCID50/ml (SEM = 0.08 log10 TCID50/ml) for ZnSO4 at a concentration of 200 μM. This corresponds to a more than 25-fold reduction in viral titers for Zn treatment.
Similar results were observed when Zn salts were added in a later stage of infection (22–24 hpi) after several infection cycles. Virus yields were also significantly reduced at 100 and 200 μM concentrations of each Zn salt (Fig. 1c). The mean virus titer at 200 μM in the mock-treated cultures was 106.7 TCID50/ml (SEM = 0.72 log10 TCID50/ml) compared to 105.2 TCID50/ml (SEM = 0.32 log10 TCID50/ml) for ZnCl2 and 105.1 TCID50/ml (SEM = 0.36 log10 TCID50/ml) for ZnSO4 corresponding to a more than 30-fold reduction in viral titers for Zn treatment.
These results suggest that Zn interferes with stages of the viral replication cycle that follow the adsorption of TGEV to its receptor. When added at an early stage of infection (1–4 hpi), Zn might partly block or delay the initial cell entry of TGEV, which is mediated through receptor mediated endocytosis and acid dependent fusion (Schwegmann-Wessels et al., 2002). The same could be valid for the entry of newly produced virus, when Zn is added in a later stage of infection (22–24 hpi) after multiple replication cycles. Additionally or alternatively, Zn could also impair stages of viral replication that follow the release of TGEV into the cytoplasm. A possible intracellular target for Zn-mediated inhibition is the viral RNA polymerase which was reportedly inhibited by increasing intracellular Zn ions in case of the SARS-Coronavirus (SARS-CoV) and other viruses (te Velthuis et al., 2010). In our study, the relative amount of viral S-protein RNA detectable in infected cells was significantly reduced by Zn treatment for 1–4 hpi without previous inhibition of virus penetration as shown in Fig. 2 . In case of ZnCl2 treatment, the reduction was more than 40-fold and in case of ZnSO4 it was more than 16-fold compared to the viral RNA levels in the untreated wells. We also found the relative amount of viral proteins released from infected cells to be reduced by more than 40% for the early-stage treatment without previous inhibition of virus penetration (Fig. 3 ). This reduction of viral proteins could be a result of the inhibition of previous stages of viral replication, but again there is also evidence that Zn could directly inhibit the proteolytic cleavage of viral polypeptides (Korant and Butterworth, 1976). Potential points of action against TGEV might be a chymotrypsin-like protease that is responsible for the cleavage of polyproteins into functional proteins, and which is highly conserved in coronaviruses (Kuo et al., 2009), or a papain-like protease which, in case of the SARS-CoV, was shown to be potently inhibited by Zn ions (Han et al., 2005).
Fig. 2.
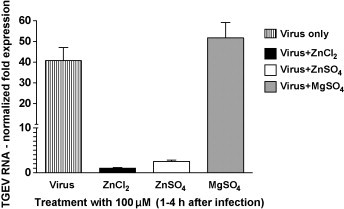
Effects of Zn salt treatment on viral RNA synthesis. The relative expression of the TGEV S protein gene was determined by normalizing values against the porcine β-actin gene.
Fig. 3.
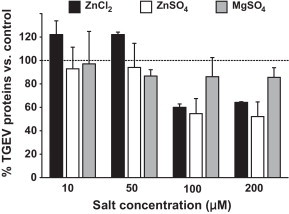
Influence of Zn salts on viral protein synthesis. Values represent means and SEM and were normalized for the average TGEV protein expression in untreated wells.
The highest reduction in virus yields was observed when Zn was added at 1 hpi following the virus entry and treatment was continued for the whole infection time of 48 h (Fig. 1d). The mean virus titer in the mock-treated wells was 107.3 TCID50/ml (SEM = 0.28 log10 TCID50/ml) compared to 104.9 (SEM = 0.43 log10 TCID50/ml) for ZnCl2 at 200 μM (P < 0.05), and 105.5 (SEM = 0.43 log10 TCID50/ml) for ZnSO4 at 200 μM (P < 0.05). In case of ZnCl2 treatment this parallels a more than 250-fold reduction and in case of ZnSO4 a more than 60-fold reduction. Presumably the repeated inhibition of one or more viral replication steps could potentiate the antiviral effects of Zn treatment. The observation that the virus yield reduction achieved with the long-term treatment did not reach the potential amount of the combined early and late short-term treatment, however, might be explained by the fact that the long-term treatment was initiated without prior inhibition of initial virus penetration as is was the case with the early short-term treatment (1–4 hpi), so that this initial reduction is missing.
Taken together, the data reported in this report show that Zn salts specifically inhibit the replication of the coronavirus TGEV in a concentration dependent manner. Clearly, further studies are indicated to elucidate the exact mechanisms and the in vivo relevance of those findings. Concerning the potential in vivo application, the present results suggest that Zn salts even if used for a short-term treatment might be therapeutically efficient in early as well as in later stages of coronavirus infections in animals as well as in humans.
Acknowledgements
The authors would like to thank Prof. K. Osterrieder for critically reading the manuscript. This study was funded by the Deutsche Forschungsgemeinschaft (DFG), Grant FOR 438 and SFB 852/1. This study was also funded by the National Natural Science Foundation of China (No. 31101796).
References
- Arens M., Travis S. Zinc salts inactivate clinical isolates of herpes simplex virus in vitro. J. Clin. Microbiol. 2000;38:1758–1762. doi: 10.1128/jcm.38.5.1758-1762.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Huang W., Lin Z., Zhou Z., Yu H., Zhu D. Development of a novel real-time RT-PCR assay with LUX primer for the detection of swine transmissible gastroenteritis virus. J. Virol. Methods. 2004;122:57–61. doi: 10.1016/j.jviromet.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geist F.C., Bateman J.A., Hayden F.G. In vitro activity of zinc salts against human rhinoviruses. Antimicrob. Agents Chemother. 1987;31:622–624. doi: 10.1128/aac.31.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y.S., Chang G.G., Juo C.G., Lee H.J., Yeh S.H., Hsu J.T., Chen X. Papain-like protease2 (PLP2) from severe acute respiratory syndrome coronavirus (SARS-CoV): expression, purification, characterization, and inhibition. Biochemistry. 2005;44:10349–10359. doi: 10.1021/bi0504761. [DOI] [PubMed] [Google Scholar]
- Haraguchi Y., Sakurai H., Hussain S., Anner B.M., Hoshino H. Inhibition of HIV-1 infection by zinc group metal compounds. Antiviral Res. 1999;43:123–133. doi: 10.1016/s0166-3542(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Katz E., Margalith E. Inhibition of vaccinia virus maturation by zinc chloride. Antimicrob. Agents Chemother. 1981;19:213–217. doi: 10.1128/aac.19.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B.D., Butterworth B.E. Inhibition by zinc of rhinovirus protein cleavage: interaction of zinc with capsid polypeptides. J. Virol. 1976;18:298–306. doi: 10.1128/jvi.18.1.298-306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümel G., Schrader S., Zentgraf H., Daus H., Brendel M. The mechanism of the antiherpetic activity of zinc sulphate. J. Gen. Virol. 1990;71(Pt 12):2989–2997. doi: 10.1099/0022-1317-71-12-2989. [DOI] [PubMed] [Google Scholar]
- Kuo C.J., Liu H.G., Lo Y.K., Seong C.M., Lee K.I., Jung Y.S., Liang P.H. Individual and common inhibitors of coronavirus and picornavirus main proteases. FEBS Lett. 2009;583:549–555. doi: 10.1016/j.febslet.2008.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent end points. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Ren X., Meng F., Yin J., Li G., Li X., Wang C., Herrler G. Action mechanisms of lithium chloride on cell infection by transmissible gastroenteritis coronavirus. PLoS One. 2011;6:e18669. doi: 10.1371/journal.pone.0018669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink L., Gabriel P. Zinc and the immune system. Proc. Nutr. Soc. 2000;59:541–552. doi: 10.1017/s0029665100000781. [DOI] [PubMed] [Google Scholar]
- Suara R.O., Crowe J.E., Jr. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob. Agents Chemother. 2004;48:783–790. doi: 10.1128/AAC.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwegmann-Wessels C., Zimmer G., Laude H., Enjuanes L., Herrler G. Binding of transmissible gastroenteritis coronavirus to cell surface sialoglycoproteins. J. Virol. 2002;76:6037–6043. doi: 10.1128/JVI.76.12.6037-6043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa K., Naganuma A., Sato K., Ikeda M., Kato N., Takagi H., Mori M. Zinc is a negative regulator of hepatitis C virus RNA replication. Liver Int. 2006;26:1111–1118. doi: 10.1111/j.1478-3231.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- Zhang B., Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br. J. Nutr. 2009;5:687–693. doi: 10.1017/S0007114509289033. [DOI] [PubMed] [Google Scholar]


